Abstract
The parabrachial nucleus (PBN) integrates interoceptive and exteroceptive information to control various behavioral and physiological processes including breathing, emotion, and sleep/wake regulation through the neural circuits that connect to the forebrain and the brainstem. However, the precise identity and function of distinct PBN subpopulations are still largely unknown. Here, we leveraged molecular characterization, retrograde tracing, optogenetics, chemogenetics, and electrocortical recording approaches to identify a small subpopulation of neurotensin-expressing neurons in the PBN that largely project to the emotional control regions in the forebrain, rather than the medulla. Their activation induces freezing and anxiety-like behaviors, which in turn result in tachypnea. In addition, optogenetic and chemogenetic manipulations of these neurons revealed their function in promoting wakefulness and maintaining sleep architecture. We propose that these neurons comprise a PBN subpopulation with specific gene expression, connectivity, and function, which play essential roles in behavioral and physiological regulation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-022-00994-8.
Keywords: Parabrachial nucleus, Neurotensin, Anxiety, Sleep, Wakefulness, Breathing, Neural circuit, Freezing, Medulla
Introduction
Understanding how the nervous system senses and integrates external and internal stimuli and adjusts the physiological and behavioral responses accordingly is a central question in neuroscience. Often, this is confounded by the close vicinity of diverse neuronal types with distinct gene expressions and connectivity in the same brain region. The parabrachial nucleus (PBN) is one of the pontine regions containing heterogeneous neurons that are subject to a variety of interoceptive and exteroceptive stimuli and control different vital behavioral and physiological processes [1–7]. Over the years, extensive studies have revealed that PBN neurons receive intensive afferent inputs from the brainstem, and project to many regions throughout the brain, including the amygdala, lateral hypothalamus, basal forebrain, thalamus, and medulla [8–12], which are consistent with the heterogeneous functions of the PBN in critical homeostatic processes, including breathing, sleep-wake regulation, emotion, taste, water balance, pain and itch, and thermoregulation [2–9, 13–19]. More recently, modern genetic and neurogenetic approaches have been applied to dissect the PBN subpopulations that made significant progress in our understanding of PBN functions [2–5, 7, 9, 13]. For instance, neurons expressing calcitonin gene-related peptide (CGRP) are one of the most studied populations in the lateral PBN [4, 5, 7–9, 19]. Functional manipulation and neural circuit tracing experiments have revealed that neurons in the PBN have diverse functions and circuit connectivity, indicating that various kinds of information are processed via different subsets within the PBN to maintain homeostasis [1, 4]. Therefore, further studies on the precise identity and function of PBN subpopulations are critical for understanding the organizational principles of brain circuits.
To identify the molecular identities and functions of PBN neuronal subpopulations, we carried out a screen on the Allen Brain Atlas [20] for molecular markers that are specifically expressed in PBN subsets. One of the markers strongly expressed in the lateral PBN is neurotensin (Nts), a neuropeptide that has been implicated in the regulation of breathing, sleep promotion, social behavior, locomotor activity, and feeding [21–29]. However, the function of Nts neurons in the PBN remained largely unknown. In this study, we integrated molecular characterization, circuit tracing, optogenetics, chemogenetics, and electrocortical recording approaches to pinpoint the functions of NtsPBN neurons in the control of behavior and physiology. Our results show that these NtsPBN neurons are a small subpopulation, largely localized to the external lateral PBN, that projects to the forebrain but not to the medulla. Optogenetic and chemogenetic manipulation experiments showed that these neurons are critical for freezing and anxiety-like behavior, and in turn regulate breathing responses. Furthermore, these Nts-expressing neurons are important in maintaining wakefulness, distinct from the functions of other brainstem Nts-expressing neurons [26].
Material and Methods
Animals
All procedures were carried out in accordance with the animal care standards in the National Institutes of Health (NIH) guidelines and approved by the University Committee on Use and Care of Animals at the University of Michigan. The animals were maintained under a 12-h light/dark cycle (lights on at 06:00) with ad libitum access to food and water. All mice were on a C57BL/6J genetic background. Nts-IRES-Cre mice (JAX #017525) and Ai14 mice (JAX #007908) have been previously reported [30, 31]. Both genders were used.
Stereotaxic Surgery
All stereotaxic injections were performed on adult mice under anesthesia with isoflurane (5% induction, 1.5% maintenance) using a stereotaxic instrument (David Kopf Instruments). The body temperature was maintained at ~35°C using a feedback-controlled heating pad (Physitemp, TCAT-2LV).
For viral injection, 300 nL–400 nL AAVs were stereotactically injected into the PBN. The AAVs used in this study were from Addgene (AAV5-EF1α-DIO-hChR2(H134R)-eYFP, Addgene#20298-AAV5, Lot V60009, Titer: 3×1013 vg/mL; AAV5- EF1α-DIO-EYFP, Addgene #27056-AAV5, Lot V29488, Titer: 1.3×1013 vg/mL; AAV2-hsyn-DIO-hM3Dq-mCherry, Addgene #44361-AAV2, Lot V48120, Titer: 2.6×1013 vg/mL; and AAV2-hsyn-DIO-hM4Di-mCherry, Addgene #44362-AAV2, Lot V45281, Titer: 5.3×1012 vg/mL), the Salk Institute (AAV9-EF1α-DO-hChR2(H134R)-mCherry-WPRE-pA, Titer: 7.62×1012vg/mL), and the University of North Carolina Vector Core (AAV5-CAG-Flex-GFP, Lot AV4531C, Titer: 4.5×1012 vg/mL and AAV2-hsyn-DIO-mCherry, Lot AV4753B, Titer: 3.1×1012 vg/mL). Experiments were performed 4–12 weeks after stereotaxic injection.
For retrograde tracing, 50 nL–100 nL Cholera Toxin Subunit B-647 (Invitrogen; C34778) and 50 nL–100 nL 40 mmol/L fast blue (Polysciences; 17740-1) were stereotactically injected into the ipsilateral central nucleus of the amygdala (CeA) and pre-Bötzinger complex (preBötC). Brain samples were collected 7 days after the surgery for analysis. Coordinates for stereotaxic injections (in mm): PBN: AP –5.30, ML ± 1.50, DV 2.55 from brain surface; preBötC: AP –6.90, ML ± 1.30, DV 4.95 from brain surface; CeA: AP –1.20, ML ± 2.90, DV 4.90 from bregma.
Histology and Immunostaining
Mice were deeply anesthetized by isoflurane and transcardially perfused with 10 mL–20 mL phosphate-buffered saline (PBS), followed by 10 mL 4% paraformaldehyde (PFA). The brains were harvested and post-fixed overnight in 4% PFA at 4°C, followed by cryopreservation for 48 h in 30% sucrose in PBS at 4°C. The processed tissue was embedded in optimum cutting temperature (OCT) compound and coronal serial sections were cut at 30 μm on a freezing cryostat (Leica cryostat, CM3050 S).
For immunostaining, sections were briefly washed with PBS with 0.1% Tween20 (PBST), followed by permeabilization with 0.3% PBS Triton X-100 for 30 min, and blocking by 2% bovine serum albumin in PBST for 1 h. Sections were incubated with the primary antibody, goat anti-ChAT (Millipore, #AB144P, 1:300 dilution), overnight at 4°C. Sections were washed with PBST and incubated with the secondary antibody Alexa Fluor 488-conjugated donkey anti-goat (Life Technologies, #A11055, 1:400 dilution) for 2 h at room temperature. Following 3 washes with PBST, sections were stained with DAPI (Invitrogen, D1306) and mounted with prolonged gold antifade reagent (Invitrogen, P36930) with coverslips. Fluorescent images were captured by a Nikon A1 confocal microscope.
RNA in situ Hybridization
Brains were harvested from wild-type mice after deep anesthesia and directly frozen into OCT. Serial coronal sections were cut at 16 μm, fixed in 4 % PFA, and dehydrated in an ethanol series. This was followed by a pretreatment reagent (Advanced Cell Diagnostics). Multiplex fluorescent in situ assays were applied according to the ACD RNAscope protocols. The probes used were: Nts (Cat# 420441), Oprm1 (Cat# 493251), Calca (Cat# 417961), and Slc17a6 (Cat# 319178).
Optogenetics
For optogenetic activation, channelrhodopsin-2 expressing vector AAV5-EF1α-DIO-hChR2(H134R)-eYFP or AAV9-EF1α-DO-hChR2(H134R)-mCherry was stereotactically injected into the PBN. An optic fiber cannula (200 μm) with a 1.25 mm ceramic ferrule (Thorlabs) was implanted 200 μm–500 μm above the injection site immediately after virus injection and stabilized to the skull surface by Metabond (Parkell). After 3–4 weeks for recovery, the cannula was connected to a 473 nm laser of 10 mW power for activation at 10-ms intervals.
Chemogenetics
For chemogenetic manipulation, AAV2-hsyn-DIO-hM3Dq-mCherry or AAV2-hsyn-DIO-hM4Di-mCherry was stereotactically injected into the bilateral PBN. After 3–4 weeks for recovery, clozapine-n-oxide (CNO; 1 mg/kg, Tocris Bioscience, Cat# 4936) was injected intraperitoneally to activate the chemogenetic receptors 30 min before the behavioral assays. Saline was injected as the control.
Breathing Monitoring and Analysis
In the awake condition, individual mice were placed in a whole-body plethysmography chamber (Buxco) at room temperature (22°C) and with room air input by a Bias flow regulator (Buxco) at 1 L/min. Mice were allowed to acclimate to the chamber for 30 min before plethysmography data were collected by a flow transducer (Buxco) connected to Powerlab system (AD instruments) via a 4 Strain-Gage Preamplifier (Buxco Max II).
In the anesthetized condition, the procedure was performed as previously described [32]. In brief, mice were injected with 1.6 g/kg urethane (Sigma) and then placed on a stereotaxic frame with a heating pad. The trachea was cannulated and connected to an intratracheal tube. Airflow was detected by a flow-head connected to the Powerlab system (AD instruments). All breathing data were collected and analyzed by Labchart (AD instruments).
Breathing was monitored under awake and anesthetized conditions before, during, and after 5-s laser stimulation at 5 Hz, 10 Hz, 20 Hz, or 30 Hz.
Open-Field Test
Before all the tests for anxiety-like and locomotor behaviors, mice were habituated to the room for the test for 2–3 days. In the open-field test, on the day of the experiment, mice were intraperitoneally injected with CNO (1 mg/kg). 30 min later, mice were placed in the center of a 50×50 cm2 open-field chamber and allowed to move freely. Motor activity was recorded by an overhead camera (Logitech) for 10 min. The experiments with saline treatment were performed one week after the CNO treatment. The center of the arena was labeled as the 25 × 25 cm2 area in the center of the open field. The distance traveled and time spent in the center were analyzed using MatLab software [33].
Locomotor Activity Test
Mice were placed in the center of a 24 × 24 cm2 arena at the beginning of the experiment. Locomotor activity was recorded by an overhead camera (Logitech) for 9 min: 3 min each before, during, and after the laser was turned on for photoactivation. The distance traveled was analyzed using MatLab software [33]. Freezing was defined as the absence of visible movement for at least 2 consecutive seconds.
Elevated Plus Maze
Mice were placed in the center zone of an elevated plus maze, with two 50-cm-long arms, and faced the open arm 30 min after intraperitoneal injection of CNO (1 mg/kg). Then the mice were allowed to freely move for 10 min, and their movements were recorded by an overhead camera. The experiments with saline treatment were performed one week after the CNO treatment. Data were analyzed using MatLab software [33] and validated with manual scoring.
Sleep-Wake Recording and Analysis
Mice were implanted with EEG/EMG recording electrodes three weeks after viral injection. Two stainless steel screws (Pinnacle Technology, Inc., #8212) were inserted into the skull 1 mm anterior to bregma and 1.5 mm lateral to the midline and the other over the parietal area of the right hemisphere. Two EMG wires (Phoenix Wire Inc.) were placed into the neck musculature [34]. The ends of the EEG/EMG electrodes were connected to a 4-pin header (Pemco Inc., HWS12398) that was secured to the skull using dental cement (Stoelting Co.). After recovery in an individual cage for at least one week after the surgery, sleep/wake states and breathing were measured simultaneously during the light phase for at least 3 h. EEG/EMG electrodes were connected to recording cables for data collection. The signals were amplified, digitized at a sampling rate of 1000 Hz, and filtered (EEG, 0.1 Hz–100 Hz band-pass; EMG, 25 Hz high-pass) by Powerlab (AD Instruments). Spectral heatmaps were generated via fast Fourier transform, and brain state in 5-s epochs was scored as wakefulness, NREM, and REM using the published criteria [27, 34] using custom-written MatLab software. After automatic scoring, stages were examined and manually corrected. Before chemogenetics experiments, all mice were allowed to habituate to the environment and saline injection 2–3 times. Before optogenetics recording, all mice were acclimated in the chamber for 2–3 days, and during the recording, mice were connected to a laser controlled by a Master-8 pulse stimulator (AMPI). Each activation trial was set at a 20 Hz pulse for 2 min, and the inter-trial interval was 15 min–25 min. There were 6–8 sessions for each optogenetic manipulation experiment.
Quantification and Statistical Analysis
All statistical analysis was processed using GraphPad Prism 8. All data are presented as the mean ± SEM. The appropriate statistical test (Student’s t-test, or one- or two-way analysis of variance) was applied. Statistical significance was set as follows: *P <0.05; **P <0.01; ***P <0.001; ns, no significant difference. All data details in the figures are noted in the figure legend.
Results
NtsPBN Neurons are a Subset of Excitatory Neurons in the External Lateral PBN (PBel)
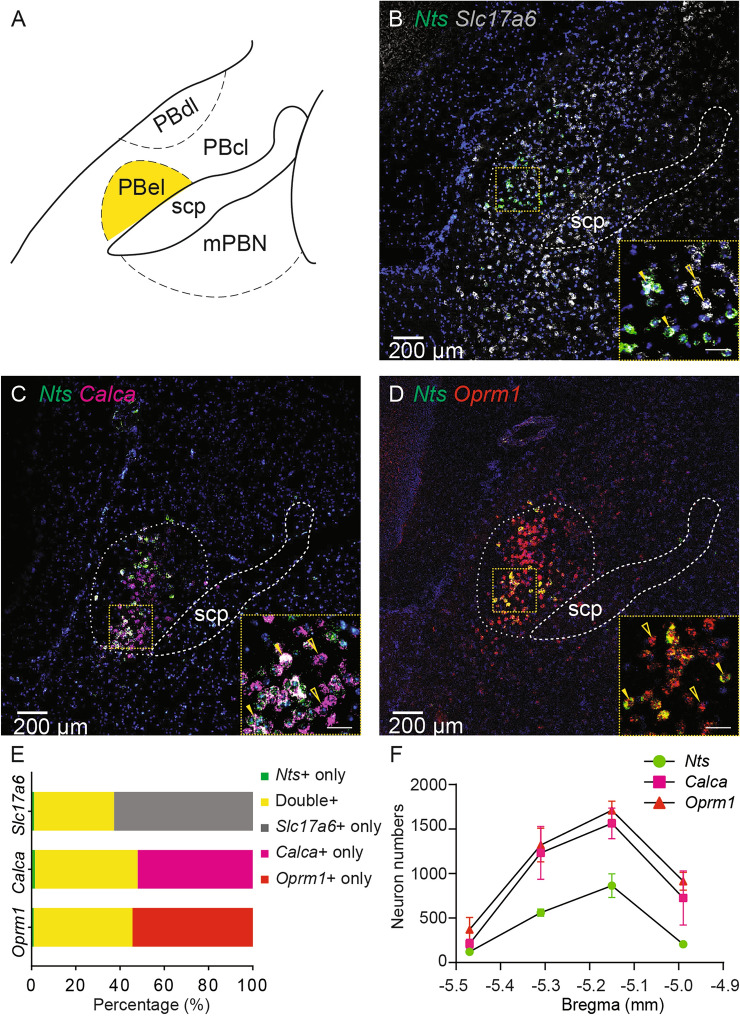
To characterize the gene expression of Nts in the PBN, we examined the Nts mRNA transcript and its co-localization with other known markers of PBN neurons, namely, Slc17a6, Calca, and Oprm1, using multiplex single-molecule RNA in situ hybridization. First, we found that Nts mRNA was largely expressed in the PBel, in that the majority of Nts-expressing neurons (90.7% ± 2.0%, n = 3) were located in the PBel (Fig. 1A–D). Next, almost all Nts-positive neurons (97.2% ± 1.1%; n = 3) in the PBel co-expressed vesicular glutamate transporter 2 (Vglut2 or Slc17a6), a marker of glutamatergic excitatory neurons (Fig. 1B and E; 36.1% ± 5.3% Slc17a6 and Nts double positive, 1.1% ± 0.6% Nts-positive, 62.8% ± 5.8% Slc17a6-positive, n = 3). Conversely, only about one-third of Slc17a6-positive neurons in the PBel (36.6% ± 5.5%, n = 3) expressed Nts (Fig. 1B and E). These results suggest that Nts-positive neurons are a small subset of excitatory neurons in the PBel. Last, we examine the co-localization of Nts with other markers which is well charaterized in this region in previous studies [4, 5, 7–9, 16, 17, 19]. We found that Nts expression largely overlapped with calcitonin gene-related peptide (CGRP or Calca) (Fig. 1C and E; 46.5% ± 4.6% Calca and Nts double-positive, 1.5% ± 0.8% Nts-positive, 52.0% ± 5.3% Calca-positive, n = 3), as almost all (97.1% ± 1.2%) Nts neurons expressed Calca, while about half (47.2% ± 5.0%) of Calca neurons expressed Nts (Fig. 1C, E, and F). Similarly, almost all (98.0% ± 0.6%) Nts neurons expressed the mRNA of the mu-opioid receptor (Oprm1), while nearly half (45.1% ± 2.4%) of Oprm1 neurons expressed Nts (Fig. 1D–F; 44.7% ± 2.3% Oprm1 and Nts double-positive, 1.0% ± 0.3% Nts-positive, 54.4% ± 2.6 % Oprm1-positive, n = 3). Altogether, these results suggest that Nts neurons in the PBel are a small group of excitatory neurons, which belong to a subset of Calca neurons or Oprm1 neurons.
Fig. 1.
Molecular characterization of NtsPBN neurons. A Schematic of PBN subdivisions. PBel, external lateral parabrachial nucleus; PBcl, central lateral parabrachial nucleus; PBdl, dorsal lateral parabrachial nucleus; mPBN, medial parabrachial nucleus; Scp, Superior cerebellar peduncles. B–D. Multiplex in situ hybridization (RNAscope) in the PBN region of a wild-type mouse (n = 3) probed for Nts (green, in B–D), Slc17a6 (white, in B), Calca (magenta, in C), and Oprm1 (red, in D). Insets, higher magnification of the boxed regions showing representative neurons that express only one marker (open arrowheads) or co-express both markers (filled arrowheads). Scale bars, 200 μm (insets, 50 μm). E Quantification of co-localization of Nts and other PBN markers. Data are shown as the mean of 3 mice. F Quantification and distribution of PBN neurons positive for Nts (green), CGRP (magenta), or Oprm1 (red) across the rostro-caudal extent from −5.40 to −5.02 mm to Bregma. n = 3. Data are shown as the mean ± SEM.
Activation of NtsPBN Neurons Regulates Breathing in Freely-Behaving but not in Anesthetized Mice
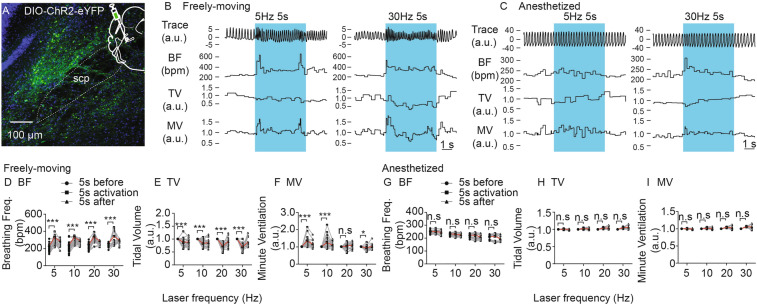
The PBN is an important nucleus in the breathing control network [12, 35–37]. Neurons with endogenous activity correlated to different phases of breathing have been found in the PBN [38]. Furthermore, manipulation of PBN neurons has been shown to regulate breathing [4, 16, 17]. To explore whether NtsPBN neurons play a role in the control of breathing, we activated these neurons using an optogenetics approach in freely moving and anesthetized mice while recording their breathing responses. The adeno-associated viral vector (AAV) with a Cre-dependent channelrhodopsin (ChR2) expressing the construct (AAV-DIO-Chr2-eYFP) was stereotactically injected into the PBN of Nts-Cre mice, and a fiber-optic cannula was implanted above the injection site. The construct was specifically expressed in the NtsPBN neurons after 4–12 weeks (Fig. 2A and S1). Animals were placed in a whole-body plethysmography chamber to record breathing. Mice exhibited an acute change in breathing behavior during photostimulation, including an increase in breathing frequency (Fig. 2B and D) and a decrease in tidal volume (Fig. 2B and E). As a result, the minute ventilation showed a significant increase at low frequencies (5 and 10 Hz) but a decrease at a high frequency (30 Hz) (Fig. 2F). The changes in breathing were not found in Nts-Cre mice injected with a control construct (AAV-DIO-GFP) (Fig. S2). To further investigate the mechanism underlying breathing control, we tested the breathing response in urethane-anesthetized mice through intratracheal intubation after tracheotomy. Surprisingly, activation of these neurons had no effect at all on breathing frequency, tidal volume, or minute ventilation (Fig. 2C, G–I). These results demonstrated that NtsPBN neurons are sufficient for breathing regulation specifically in freely-moving mice.
Fig. 2.
Effect of optogenetic activation of NtsPBN neurons on breathing. A A brain slice of the mouse PBN region showing expression of the AAV-DIO-ChR2-eYFP construct. A schematic of stereotactic viral injection is shown in the top right corner. scp, superior cerebellar peduncle. Scale bar, 100 μm. B, C Representative examples showing the effects of different photostimulation conditions (5 Hz and 30 Hz) on breathing traces (Trace), breathing frequency (BF), tidal volume (TV), and minute ventilation (MV) under freely-moving (B) and anesthetized (C) conditions. Blue, 5-s light ON phase. D–F Quantification of BF (D), TV (E), and MV (F) from 5 s before, during, and after laser activation at different frequencies in freely-moving mice (n = 15–25, from 5 mice). G–I Quantification of the BF (G), TV (H), and MV (I) from 5 s before, during, and after laser activation at different frequencies in anesthetized mice (n = 15–25 from 5 mice). Data are shown as the mean ± SEM. *P <0.05, **P <0.01, ***P <0.001, paired t-test.
To examine the function of Nts-negative neurons in the PBN, similar optogenetic experiments were carried out on Nts-Cre mice injected with a Cre-off AAV construct (AAV-DO-Chr2-mCherry) into the PBN (Fig. S3). Photoactivation of Nts-negative neurons in freely-behaving mice induced a dramatic increase in breathing frequency at all tested laser frequencies (Fig. S3A, C, and I). Contrary to the decreased tidal volume seen after activation of NtsPBN neurons, the tidal volume exhibited a significant increase starting at 20 Hz (Fig. S3D and J). As a result, the minute ventilation increased up to 2-fold at 5 Hz laser stimulation, and the magnitude of this increase was 3-fold when the laser stimulation increased to 30 Hz (Fig. S3E and K). In the anesthetized condition, activation of Nts-negative neurons triggered elevated tidal volume at certain laser frequencies (Fig. S3G and N) which is distinct from the lack of effect by the activation of Nts-expressing neurons (Fig. 2H). Furthermore, activation of Nts-negative neurons induced a sigh, an augmented large breath that is essential for life [39, 40], at all laser frequencies (Fig. S3P).
In summary, activation of NtsPBN neurons affects breathing only in freely-moving conditions, but not in an anesthetized setting. In contrast, Nts-negative neurons in the PBN induce more pronounced changes in breathing phenotypes under both conditions. These results thus suggest that the PBel contains neuronal subpopulations with distinct functions in regulating breathing.
NtsPBN Neurons Project to the Forebrain but not the Brainstem
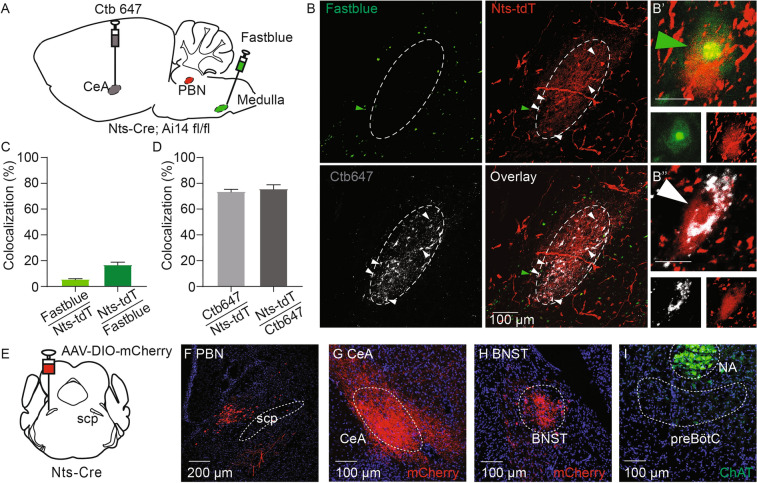
The PBN is known as a critical information relay hub that has diverse connections and projection patterns [1], which may explain the differences in breathing control between NtsPBN and non-NtsPBN subpopulations. To test this hypothesis, we first examined the connectivity of PBel neurons by dual retrograde tracing from the forebrain and the medulla in Nts-Cre; Ai14fl/fl mice, in which the Nts-Cre lineage neurons were labeled by tdTomato (Fig. 3A). The PBel has strong projections to the CeA [4, 5], a limbic region important for behavioral control, and the PBN also provides synaptic inputs to the ventral respiratory column, a key breathing control region in the medulla, to form and adapt the breathing pattern [12]. Retrograde tracers [Alexa Fluor 647 cholera toxin B subunit (CTB-647) and fast blue] were stereotactically injected into the CeA and the ventral respiratory column of the Nts-Cre; Ai14fl/fl mice, respectively (Fig. S4). Interestingly, the CTB-647-labeled neurons (projecting to the CeA) were at the center of the PBel, while the fast blue-labeled neurons (projecting to the medulla) were at the edge of the PBel (Fig. 3B), with only 1.2% ± 0.8% of neurons (n = 3) labeled by both tracers in the intermediate zone. These results indicate that PBel contains heterogeneous neuronal populations that have distinct anatomical locations and different projection patterns to the forebrain and the medulla. We then analyzed the retrograde labeling pattern of Nts-Cre lineage neurons (tdTomato+). A small portion of NtsPBN neurons was labeled by fast blue (5.7% ± 1.0%; n = 3) whereas Nts was expressed in only 16.9% ± 2.7% of all fast blue-labeled cells in the PBN (Fig. 3C). Surprisingly, the majority of NtsPBN neurons were labeled by CTB-647 (73.8% ± 2.2%; n = 3), while Nts was expressed in 75.8% ± 3.8% of all CTB-647-labeled neurons in the PBN (Fig. 3D). Together, these results reveal the distinct connectivity pattern of neurons in the PBN to the forebrain and the medulla.
Fig. 3.
Connectivity of NtsPBN neurons. A Schematic of the retrograde tracing strategy. Retrograde tracers Ctb647 and Fast blue are respectively injected into the CeA and the preBötC of Nts-Cre; Ai14fl/fl mice. The PBN region is used for colocalization analysis. B PBN brain slice labeled by Fast blue (pseudocolored as green), Ctb647 (white), and tdTomato (red, Nts-tdT). Please note that the dotted circle shows the Ctb647 labeling and Nts neurons at the center of the PBel. White arrowheads, Nts neurons co-labeled by Ctb647 injected into the CeA; green arrowheads, Nts neurons co-labeled by Fast blue injected into the preBötC. White dashed circle represents the area labeled by Ctb. Scale bar, 100 μm. B’, A representative neuron with co-localization of Nts-tdT and Fast blue. Scale bar, 5 μm. B’’, A representative neuron with co-localization of Nts-tdT and Ctb647. Scale bar, 5 μm. C Quantification of co-localization of Fast blue and Nts-tdT (n = 3). Data are shown as the mean ± SEM. D Quantification of co-localization of Ctb647 and Nts-tdT (n = 3). Data are shown as the mean ± SEM. E Schematic of viral injection into the PBN. F A brain slice of the PBN showing the expression of viral vector (mCherry). Scale bar, 200 μm. scp, superior cerebellar peduncle. G, H Slices of forebrain regions showing the mCherry-positive terminals of PBN Nts neurons in CeA (G) and BNST (H). BNST, bed nuclei of the stria terminalis; CeA, central amygdala nucleus. Scale bars, 100 μm. I Slice of the medulla showing the absence of mCherry-positive terminals of PBN Nts neurons in the preBötC region. NA is labeled by ChAT immunostaining. preBötC, pre-Bötzinger complex; NA, nucleus ambiguus. Scale bar, 100 μm.
With the concern that the Cre-dependent reporter also labels neurons that express Nts only during development, we next traced the projection processes of the adult NtsPBN neurons by injecting an AAV-DIO-mCherry reporter vector to the PBN of adult Nts-Cre mice (Fig. 3E, F). The regions with the most robust innervation were the CeA (Fig. 3G) and the bed nucleus of the stria terminalis (BNST) (Fig. 3H), which are involved in controlling behaviors and emotions such as fear [5, 41–44]. The processes of NtsPBN neurons were also observed in other regions, including the parasubthalamic nucleus, the ventral posteromedial nucleus of the thalamus, parvocellular, and the midbrain reticular nucleus (Fig. S5A–C), but not in the substantia innominata and the insular cortex (Fig. S5D and E), which are innervated by CGRPPBN neurons [4]. This difference in their projection patterns confirms that the Nts neurons are a subset of CGRPPBN neurons. In contrast to the forebrain, there was a complete absence of mCherry signal in the ventral respiratory column in the medulla, including the preBötC (Fig. 3I), the inspiratory rhythm generator with critical functions in the control of breathing patterns [39, 45]. Very sparse signals were found in the nucleus of the solitary tract (NTS) (Fig. S5F).
To investigate the projection of Nts-negative PBel neurons, we next traced the mCherry-positive processes in Nts-Cre mice injected with the Cre-off construct AAV-DO-Chr2-mCherry in the PBN. We found that in addition to the regions that receive projections from NtsPBN neurons (Fig. S6E–H), Nts-negative neurons also send robust projections to the medullary regions, including the preBötC and the NTS (Fig. S6B and C), and forebrain regions, such as the ventromedial hypothalamic nucleus (Fig. S6D). These results are consistent with the robust response in breathing when the Nts-negative neurons were activated.
Together, these results suggested that the PBN contains neuronal subsets with distinct projections to the forebrain and the medulla, and their cell bodies have a stereotypic distribution in the PBel. Among them, NtsPBN neurons are the neurons at the center of the PBel that preferentially project to the forebrain.
NtsPBN Neurons are Anxiety-Promoting Neurons that Reduce Locomotor Activity
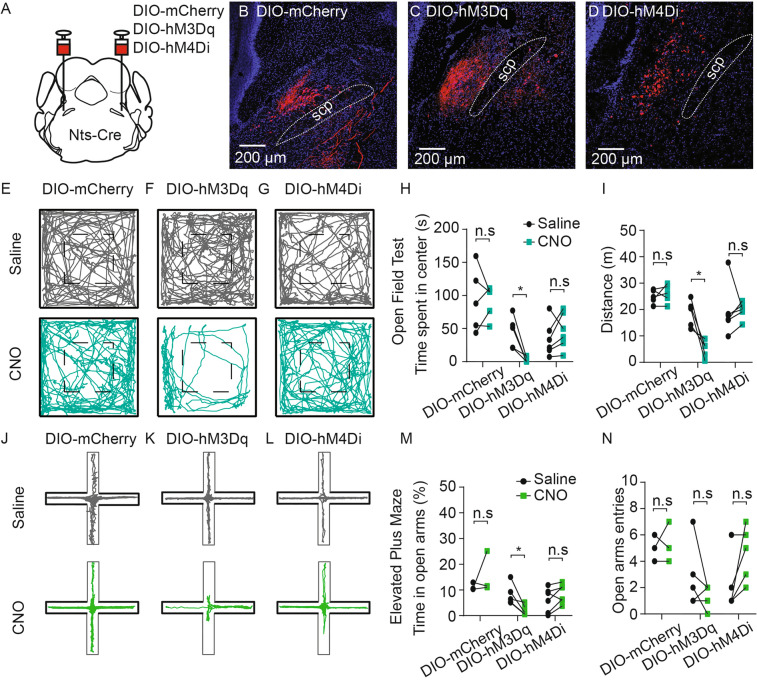
Given the robust projection pattern of NtsPBN neurons to the forebrain emotional control regions, we next manipulated NtsPBN neurons and set out to test the effect on behavioral responses (Fig. S7). First, we optogenetically activated NtsPBN neurons at 10 Hz for 3 min while the mouse was in an open field arena. Interestingly, photo-activation induced complete immobility of the mice (Fig. S7E-H and Video S1). This response is consistent with the function of the amygdala, where the robust innervation of NtsPBN neurons was found, in regulating freezing behavior [5]. When we activated the Nts-negative neurons, the mice exhibited reduced movement and increased freezing time (Fig. S7I–L), but these effects were less dramatic than the activation of NtsPBN neurons. Intriguingly, once the laser was turned off, the mice continued to have similar or more pronounced immobility (Fig. S7I–L), which is distinct from the immediate recovery observed when the NtsPBN neurons were activated (Fig. S7E–H). Next, we chemogenetically manipulated NtsPBN neurons by injecting the modified excitatory muscarinic G-protein-coupled receptor (AAV-DIO-hM3Dq-mCherry) bilaterally into the PBN of Nts-Cre mice. AAV-DIO-mCherry was injected as a control (Fig. 4A). The viral construct was expressed specifically in the PBN (Fig. 4B–D). Mice received an intraperitoneal injection of either 1 mg/kg CNO or saline 30 min before being subjected to an open field test or an elevated plus maze test. In the open field test, compared to the control trials with saline injection, CNO-induced activation of NtsPBN neurons triggered a dramatically reduced distance traveled (Fig. 4F and I). Furthermore, animals spent significantly less time at the center of the field (Fig. 4H). In the elevated plus maze, CNO-induced activation of NtsPBN neurons caused a significant reduction of the time spent in the open arms (Fig. 4K and M). None of these responses were found in Nts-Cre mice injected with the control construct AAV-DIO-mCherry (Fig. 4E, H, I, J, M, and N), or the inhibitory construct AAV-DIO-hM4Di-mCherry followed by CNO treatment (Fig. 4G–I and L–N).
Fig. 4.
Activation of NtsPBN neurons induces anxiety-like behavior and decreases locomotion. A Schematic of bilateral viral injection into the PBN of Nts-Cre mice with a Cre-dependent AAV construct with DIO-mCherry, DIO-hM3Dq-mCherry, and DIO-hM4Di-mCherry. B–D Brain slices of the PBN showing the expression of DIO-mCherry (B), DIO-hM3Dq (C), and DIO-hM4Di (D). scp, superior cerebellar peduncle. Scale bars, 200 μm. E-G Representative track plots in the open-field area by mice expressing DIO-mCherry (E), DIO-hM3Dq (F), or DIO-hM4Di (G) with the injection of saline (upper) or CNO (lower) in a 10-min recording. H Quantification of the time spent in the center during the open field test for DIO-mCherry (n = 5), DIO-hM3Dq (n = 5), and DIO-hM4Di groups (n = 6) with saline (black) or CNO treatment (green). I Quantification of the distance traveled during the open field test for the DIO-mCherry (n = 5), DIO-hM3Dq (n = 5), and DIO-hM4Di groups (n = 6) with saline (black) or CNO treatment (green). J–L Representative track plots in the elevated plus maze of mice expressing DIO-mCherry (J), DIO-hM3Dq (K), or DIO-hM4Di (L) with the injection of saline (upper) or CNO (lower) in a 10-min recording. M Quantification of the percentage of time spent in the open arms during the elevated plus maze test for DIO-mCherry (n = 3), DIO-hM3Dq (n = 5), and DIO-hM4Di groups (n = 6) with saline (black) and CNO treatment (green). N Quantification of open arm entries during the elevated plus maze test for DIO-mCherry (n = 3), DIO-hM3Dq (n = 5), and DIO-hM4Di groups (n = 6) with saline (black) and CNO treatment (green). Data are shown as the mean ± SEM; *P <0.05. n. s., not significantly different, paired t-test.
Therefore, these results suggest that activation of NtsPBN neurons triggers freezing, reduces locomotor activity, and promotes anxiety-like behaviors.
NtsPBN Neurons Promote Wakefulness
Neurotensin and the neurons that express it have been implicated in regulating sleep-wake states. Infusion of neurotensin peptide into the fourth ventricle promotes non-rapid eye movement (NREM) sleep and reduces both wakefulness and rapid eye movement (REM) sleep [26], which is consistent with the role of neurotensin-expressing neurons in the CeA, the ventrolateral periaqueductal gray, and multiple medullary regions in promoting NREM sleep [26, 27, 29]. In contrast, the neurotensinergic neurons in the lateral hypothalamic area are sufficient in inducing wakefulness [46]. To examine the function of Nts-expressing neurons in the PBN in sleep-wake regulation, we first combined an optogenetics strategy with sleep recording by electroencephalography (EEG) and electromyography (EMG). Nts-Cre mice with AAV-DIO-Chr2-eYFP injected into the PBN were implanted with EEG/EMG electrodes. Activation of NtsPBN neurons induced wakefulness throughout the 2-min light activation period (Fig. S8B). The distribution of sleep and wake went back to the baseline once the laser was turned off (Fig. S8B). When the Nts-negative neurons were activated, mice exhibited similar induced wakefulness when the laser was on (Fig. S8C). However, the wakefulness state continued after the laser was turned off (Fig. S8C).
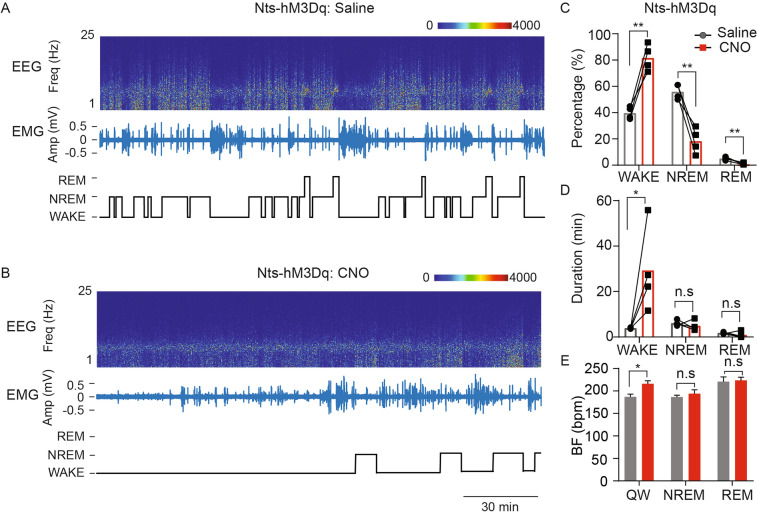
To further investigate how the sustained activation of NtsPBN neurons over an extended time could impact sleep-wake states, we studied the function of NtsPBN neurons in sleep-wake regulation using chemogenetic activation. First, CNO treatment of wild-type mice implanted with EEG/EMG electrodes showed that there was no change in sleep-wake distribution, bout duration, and transitions, compared to saline treatment (Fig. S8D–F). We next implanted EEG/EMG electrodes into Nts-Cre mice that were stereotactically injected with the excitatory chemogenetic receptor construct (AAV-DIO-hM3Dq-mCherry) into the PBN. After 3–4 days of acclimation, animals were injected with 1 mg/kg CNO 30 min before a 3-h recording of sleep-wake states (Fig. 5A, B). Compared to saline-treated trials (Fig. 5A; Nts-hM3Dq: Saline), the Nts-hM3Dq: CNO trials exhibited significantly increased wakefulness, decreased NREM sleep, and the elimination of REM sleep (Fig. 5C). The total increased wakefulness was caused by the dramatically increased time in which the animal was awake in each wakefulness episode (Fig. 5D). Furthermore, the transitions from wakefulness to NREM sleep were significantly reduced (Fig. S8G), suggesting that activation of NtsPBN neurons promotes and extends the wakefulness of the animals. Consistent with the optogenetic activation, the breathing frequency was also increased during wakefulness (Fig. 5E). These results thus elucidate that PBN neurotensinergic neurons are sufficient to promote wakefulness.
Fig. 5.
NtsPBN neurons promote wakefulness. A, B EEG power spectrum, EMG trace, and sleep/wake scoring showing the 3-h sleep-wake states of an Nts-Cre mouse with hM3Dq expression (Nts-hM3Dq) in the Nts neurons injected with saline (A) or CNO (B). C–E Quantification of sleep/wake distribution (C), average bout duration (D), and breathing frequency during different states (E) for Nts-Cre mice (n = 4) with hM3Dq expression (Nts-hM3Dq) in the Nts neurons injected with saline (grey) or CNO (red). Data are shown as the mean ± SEM. *P <0.05, **P <0.01, paired t-test.
NtsPBN Neuronal Activity is Required for Maintaining Sleep Architecture
To further examine the role of NtsPBN neurons in regulating sleep-wake states, we next acutely silenced these neurons using chemogenetics. Nts-Cre mice injected with the inhibitory chemogenetic receptor construct (AAV-DIO-hM4Di-mCherry) were subjected to sleep-wake recording for 3 h after either CNO treatment or saline. Interestingly, mice in the CNO-treated (Nts-hM4Di: CNO) trials exhibited dramatically decreased wakefulness and increased NREM sleep compared to the saline-treated trials (Nts-hM4Di: Saline) (Fig. 6A–C); this was caused by the changes in the duration of each episode of wakefulness and NREM, respectively (Fig. 6D). In contrast to the chemogenetic activation, silencing these neurons had no effect on breathing frequency in all states (Fig. 6E). These results suggest that NtsPBN neurons are required for maintaining normal sleep architecture.
Fig. 6.
NtsPBN neuronal activity is required for normal sleep architecture. A, B EEG power spectrum, EMG trace, and sleep/wake scoring showing the 3-h sleep-wake states of an Nts-Cre mouse with hM4Di expression (Nts-hM4Di) in the Nts neurons injected with saline (A) or CNO (B). C–E Quantification of sleep/wake distribution (C), average bout duration (D), and breathing frequency during different states (E) for Nts-Cre mice (n = 4) with hM4Di expression (Nts-hM4Di) in the Nts neurons injected with saline (grey) or CNO (blue). Data are shown as the mean ± SEM. *P <0.05, **P <0.01. paired t-test.
Taken together, our results suggest that NtsPBN neurons play a critical role in the control of sleep-wake states.
Discussion
The PBN is a critical pontine relay station that contains heterogeneous neurons with functions in multiple physiological and behavioral processes. Our results here showed that neurotensinergic neurons are a small subset of PBN neurons that project preferentially to the forebrain. Activation of these neurons induces freezing and anxiety-like behaviors, which in turn regulates breathing. Furthermore, these neurons are essential for promoting wakefulness and maintaining normal sleep architecture. Their function in the control of behaviors is distinct from Nts-negative neurons. These results thus elucidate the anatomical, connectivity, and functional heterogeneity of PBN neurons.
Our results showed that NtsPBN neurons are a small subpopulation of excitatory neurons in the PBN, which are co-localized with other known molecular markers for PBel, such as Calca and Oprm1. Importantly, the NtsPBN neurons are a subset of these neuronal populations in PBel, with projections preferentially to the forebrain; this is distinct from the medulla-projecting neurons that are located peripherally to the NtsPBN neurons. Although we found that ~6 % of PBN Nts-Cre lineage neurons showed uptake of the retrograde tracer injected into the ventral medulla, this may be caused by the developmental expression of Nts-Cre in these neurons, because there was a complete absence of Nts projections in the same medullary regions when an anterograde tracing construct was injected into the PBN of adult Nts-Cre mice. In contrast, the Nts-negative neurons sent robust projections to this region. Our results here are also consistent with the recent report that different projections of Oprm1 neurons are seen in the central and the peripheral PBel [17]. Therefore, these results collectively demonstrate that PBN contains heterogeneous subpopulations with distinct molecular identity, anatomical location, and connectivity, in that the Nts-positive neurons are centrally located in the PBel with projection to the limbic system, whereas the neurons that send projections to the medulla are mostly Nts-negative and located peripherally to the Nts-positive neurons.
The PBN contains neurons that are important in breathing control. Our results showed that activation of NtsPBN neurons induced fast and shallow breathing when mice were conscious, but not when they were anesthetized. Furthermore, inhibition of these neurons did not affect breathing. Together with the results that they robustly projected to the forebrain, but not the medullary regions, these results suggested that altered breathing after the activation of NtsPBN neurons is likely a consequence of the induced emotional state through their targets in the limbic system, rather than the result of direct regulation through the medullary projection. In contrast, activation of Nts-negative neurons in the PBN that directly project to the medullary breathing control regions, e.g., the preBötC and the NTS, resulted in dramatically elevated ventilation through increased breathing frequency and tidal volume. A recent study suggested a local circuit between the PBNOprm1 neurons with divergent projections to the forebrain and the medulla [17]. Thus, the NtsPBN neurons could also regulate breathing through this local circuit to the medulla-projecting neurons in the peripheral PBel, which may be repressed in the anesthetized condition. Further investigations into the possible heterogeneity of the medulla-projecting Nts-negative neurons are needed to elucidate the function of the PBN in breathing control.
Activation of NtsPBN neurons by optogenetics and chemogenetics is sufficient to induce freezing and anxiety-like behaviors, which is consistent with their robust projection to the limbic system, including the CeA and the BNST, which are essential in the control of fear and anxiety [4, 42, 44, 47, 48]. Indeed, previous studies reported that activation of PBel neurons enhances anxiety-like behavior and arousal through their projection to the CeA or the BNST [5, 9, 49]. Interestingly, activation of NtsPBN neurons induced an immediate and almost complete immobilization that lasted the entire period of photo-activation. This effect was eliminated once the laser was turned off. In contrast, activation of Nts-negative neurons induced a modest but long-lasting response, that continued even when the laser was turned off. These results showed that discrete PBN subpopulations have different functions in regulating anxiety and locomotion behaviors.
Many brain regions that respond to stress stimuli have been shown to promote wakefulness [50]. In this study, we found that activation of NtsPBN neurons not only induced anxiety-like behavior, but also increased wakefulness by triggering arousal, prolonging the wakefulness time, and preventing the initiation of sleep. These findings in sleep-wake states are consistent with the report that activation of PBNCGRP neurons, of which the NtsPBN neurons are a subset, promotes wakefulness [9]. Because the NtsPBN neurons increased the levels of stress and anxiety, the elevated wakefulness could be the result of the salience condition. Sleep disturbance, including insomnia, has been found in pathological conditions with increased fear and anxiety, including anxiety disorders, posttraumatic stress disorders, and depression [51, 52]. Perhaps altered NtsPBN neurons contribute to these pathological diseases.
Our results demonstrated that silencing NtsPBN neurons increases NREM and decreases wakefulness, suggesting an essential role of these neurons in maintaining normal sleep architecture. The spontaneous activity of NtsPBN neurons likely is what maintains a wakefulness state. Many neurotensin-expressing neuronal groups have been reported to function in sleep-wake regulation. For instance, the neurotensinergic neurons in the sublaterodosal tegmental nucleus, the lateral periaqueductal gray, and the CeA, promote NREM sleep [26, 27, 29]. In contrast, the neurotensinergic neurons in the lateral hypothalamic area promote wakefulness when activated [46]. Our results here demonstrate that Nts neurons in the PBN promote wakefulness. Therefore, understanding whether and how Nts-expressing neurons in different brain regions are connected as a circuitry of neurotensinergic neurons to regulate sleep-wake states would provide important insight into the evolution and organization of the sleep-wake circuit.
Neurotensin has previously been suggested as an NREM-promoting neuropeptide, in that when the peptide is infused into the fourth ventricle, it promotes NREM sleep and reduces both wakefulness and REM sleep [26], which is consistent with the function of many Nts-expressing neurons in various brain regions in promoting NREM [26, 27, 29]. However, activation of the Nts neurons in the PBN inhibits NREM and promotes wakefulness. Besides neurotensin, NtsPBN neurons also release other neurotransmitters, such as glutamate and CGRP, as most, if not all, of these neurons, express Slc17a6 and Calca. The precise functions of each neurotransmitter released by these neurons in sleep-wake states and other behaviors are important for understanding the molecular mechanisms underlying the functions of PBN neurons.
In summary, our study demonstrates that a small group of neurotensinergic neurons in the PBN control breathing, anxiety-like behavior, and sleep-wake functions, differently from Nts-negative neurons in the PBN. Further investigation is needed to identify the molecular markers for other PBN neuronal types and their functions to comprehensively understand the role of the PBN in controlling behavioral and physiological functions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Giancarlo Vanini for advice on the EEG/EMG implantation surgery, and Dr. Roger Cone for the setups for elevated plus maze and open field test. We also thank Dr. Xuenan Wang for her help with EEG/EMG data analysis. This work was supported by the University of Michigan startup funds and the NIH Grants R01 AT011652 and R01 HL156989.
Conflict of interest
The authors claim that there are no conflicts of interest.
References
- 1.Chiang MC, Bowen A, Schier LA, Tupone D, Uddin O, Heinricher MM. Parabrachial complex: a hub for pain and aversion. J Neurosci. 2019;39:8225–8230. doi: 10.1523/JNEUROSCI.1162-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu O, Iwai Y, Kondoh K, Misaka T, Minokoshi Y, Nakajima KI. SatB2-expressing neurons in the parabrachial nucleus encode sweet taste. Cell Rep. 2019;27:1650–1656. doi: 10.1016/j.celrep.2019.04.040. [DOI] [PubMed] [Google Scholar]
- 3.Jarvie BC, Chen JY, King HO, Palmiter RD. Satb2 neurons in the parabrachial nucleus mediate taste perception. Nat Commun. 2021;12:224. doi: 10.1038/s41467-020-20100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen AJ, Chen JY, Huang YW, Baertsch NA, Park S, Palmiter RD. Dissociable control of unconditioned responses and associative fear learning by parabrachial CGRP neurons. eLife. 2020;9:e59799. doi: 10.7554/eLife.59799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD. Elucidating an affective pain circuit that creates a threat memory. Cell. 2015;162:363–374. doi: 10.1016/j.cell.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geerling JC, Kim M, Mahoney CE, Abbott SBG, Agostinelli LJ, Garfield AS, et al. Genetic identity of thermosensory relay neurons in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol. 2016;310:R41–R54. doi: 10.1152/ajpregu.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41:280–293. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota S, Kaur S, VanderHorst VG, Saper CB, Chamberlin NL. Respiratory-related outputs of glutamatergic, hypercapnia-responsive parabrachial neurons in mice. J Comp Neurol. 2015;523:907–920. doi: 10.1002/cne.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, et al. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron. 2017;96:1153–1167. doi: 10.1016/j.neuron.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi R, Corsetti G, Rodella L, Tredici G, Gioia M. Supraspinal connections and termination patterns of the parabrachial complex determined by the biocytin anterograde tract-tracing technique in the rat. J Anat. 1998;193(Pt 3):417–430. doi: 10.1046/j.1469-7580.1998.19330417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan PJ, Ross SI, Campos CA, Derkach VA, Palmiter RD. Oxytocin-receptor-expressing neurons in the parabrachial nucleus regulate fluid intake. Nat Neurosci. 2017;20:1722–1733. doi: 10.1038/s41593-017-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, et al. A central neural circuit for itch sensation. Science. 2017;357:695–699. doi: 10.1126/science.aaf4918. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Shi L, Chen J, Jun S, Hao Y, Wang S, et al. A neural circuit mechanism controlling breathing by leptin in the nucleus tractus solitarii. Neurosci Bull. 2022;38:149–165. doi: 10.1007/s12264-021-00742-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Kim DI, Oh TG, Pao GM, Kim JH, Palmiter RD, et al. Neural basis of opioid-induced respiratory depression and its rescue. Proc Natl Acad Sci USA. 2021;118:e2022134118. doi: 10.1073/pnas.2022134118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Ye M, Pao GM, Song SM, Jhang J, Jiang H, et al. Divergent brainstem opioidergic pathways that coordinate breathing with pain and emotions. Neuron. 2022;110:857–873. doi: 10.1016/j.neuron.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez E, Ryu D, Zhao S, Han BX, Wang F. Identifying parabrachial neurons selectively regulating satiety for highly palatable food in mice. eNeuro 2019, 6: ENEURO.0252–ENEURO.0219.2019. [DOI] [PMC free article] [PubMed]
- 19.Campos CA, Bowen AJ, Roman CW, Palmiter RD. Encoding of danger by parabrachial CGRP neurons. Nature. 2018;555:617–622. doi: 10.1038/nature25511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder LE, Leinninger GM. Role of central neurotensin in regulating feeding: Implications for the development and treatment of body weight disorders. Biochim Biophys Acta BBA Mol Basis Dis. 2018;1864:900–916. doi: 10.1016/j.bbadis.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE. Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci. 2000;20:8452–8461. doi: 10.1523/JNEUROSCI.20-22-08452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wimersma Greidanus TB, Schijff JA, Noteboom JL, Spit MC, Bruins L, van Zummeren M, et al. Neurotensin and bombesin, a relationship between their effects on body temperature and locomotor activity? Pharmacol Biochem Behav. 1984;21:197–202. doi: 10.1016/0091-3057(84)90214-4. [DOI] [PubMed] [Google Scholar]
- 24.Russjan E, Kaczyńska K. Beneficial effects of neurotensin in murine model of hapten-induced asthma. Int J Mol Sci. 2019;20:5025. doi: 10.3390/ijms20205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morin-Surun MP, Marlot D, Kessler JP, Denavit-Saubie M. The excitation by neurotensin of nucleus tractus solitarius neurons induces apneustic breathing. Brain Res. 1986;384:106–113. doi: 10.1016/0006-8993(86)91225-4. [DOI] [PubMed] [Google Scholar]
- 26.Kashiwagi M, Kanuka M, Tatsuzawa C, Suzuki H, Morita M, Tanaka K, et al. Widely distributed neurotensinergic neurons in the brainstem regulate NREM sleep in mice. Curr Biol. 2020;30:1002–1010. doi: 10.1016/j.cub.2020.01.047. [DOI] [PubMed] [Google Scholar]
- 27.Ma C, Zhong P, Liu D, Barger ZK, Zhou L, Chang WC, et al. Sleep regulation by neurotensinergic neurons in a thalamo-amygdala circuit. Neuron. 2019;103:323–334. doi: 10.1016/j.neuron.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Normandeau CP, Ventura-Silva AP, Hawken ER, Angelis S, Sjaarda C, Liu X, et al. A key role for neurotensin in chronic-stress-induced anxiety-like behavior in rats. Neuropsychopharmacology. 2018;43:285–293. doi: 10.1038/npp.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong P, Zhang Z, Barger Z, Ma C, Liu D, Ding X, et al. Control of non-REM sleep by midbrain neurotensinergic neurons. Neuron. 2019;104:795–809. doi: 10.1016/j.neuron.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Leinninger GM, Opland DM, Jo YH, Faouzi M, Christensen L, Cappellucci LA, et al. Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14:313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Li SB, Wang X, Phillips CD, Schwarz LA, Luo L, et al. Brain circuit of claustrophobia-like behavior in mice identified by upstream tracing of sighing. Cell Rep. 2020;31:107779. doi: 10.1016/j.celrep.2020.107779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Li H, Han R. An open-source video tracking system for mouse locomotor activity analysis. BMC Res Notes. 2020;13:48. doi: 10.1186/s13104-020-4916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oishi Y, Takata Y, Taguchi Y, Kohtoh S, Urade Y, Lazarus M. Polygraphic recording procedure for measuring sleep in mice. J Vis Exp 2016: e53678. [DOI] [PMC free article] [PubMed]
- 35.Bachmutsky I, Wei XP, Kish E, Yackle K. Opioids depress breathing through two small brainstem sites. eLife 2020, 9: e52694. [DOI] [PMC free article] [PubMed]
- 36.Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol. 2004;143:215–233. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol. 2004;143:167–175. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Song G, Yu Y, Poon CS. Cytoarchitecture of pneumotaxic integration of respiratory and nonrespiratory information in the rat. J Neurosci. 2006;26:300–310. doi: 10.1523/JNEUROSCI.3029-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, et al. The peptidergic control circuit for sighing. Nature. 2016;530:293–297. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li P, Yackle K. Sighing. Curr Biol. 2017;27:R88–R89. doi: 10.1016/j.cub.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Gross CT, Canteras NS. The many paths to fear. Nat Rev Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- 42.Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 2016;537:97–101. doi: 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 44.Goode TD, Ressler RL, Acca GM, Miles OW, Maren S. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. eLife 2019, 8: e46525. [DOI] [PMC free article] [PubMed]
- 45.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naganuma F, Kroeger D, Bandaru SS, Absi G, Madara JC, Vetrivelan R. Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLoS Biol. 2019;17:e3000172. doi: 10.1371/journal.pbio.3000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatry. 2015;77:859–869. doi: 10.1016/j.biopsych.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai YQ, Wang W, Paulucci-Holthauzen A, Pan ZZ. Brain circuits mediating opposing effects on emotion and pain. J Neurosci. 2018;38:6340–6349. doi: 10.1523/JNEUROSCI.2780-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, et al. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci. 2013;33:7627–7640. doi: 10.1523/JNEUROSCI.0173-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 52.Khazaie H, Ghadami MR, Masoudi M. Sleep disturbances in veterans with chronic war-induced PTSD. J Inj Violence Res. 2016;8:99–107. doi: 10.5249/jivr.v8i2.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.