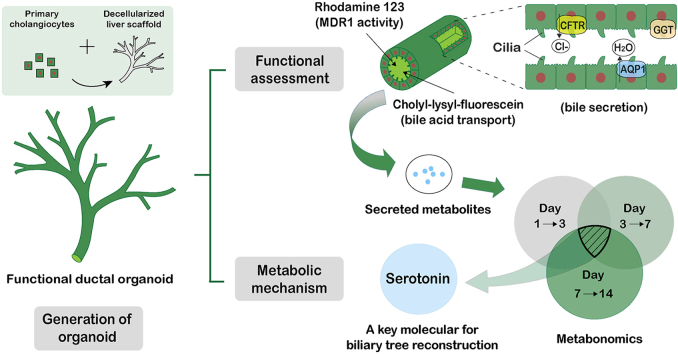
Abstract
Developing functional ductal organoids (FDOs) is essential for liver regenerative medicine. We aimed to construct FDOs with biliary tree networks in rat decellularized liver scaffolds (DLSs) with primary cholangiocytes isolated from mouse bile ducts. The developed FDOs were dynamically characterized by functional assays and metabolomics for bioprocess clarification. FDOs were reconstructed in DLSs retaining native structure and bioactive factors with mouse primary cholangiocytes expressing enriched biomarkers. Morphological assessment showed that biliary tree-like structures gradually formed from day 3 to day 14. The cholangiocytes in FDOs maintained high viability and expressed 11 specific biomarkers. Basal-apical polarity was observed at day 14 with immunostaining for E-cadherin and acetylated α-tubulin. The rhodamine 123 transport assay and active collection of cholyl-lysyl-fluorescein exhibited the specific functions of bile secretion and transportation at day 14 compared to those in monolayer and hydrogel culture systems. The metabolomics analysis with 1075 peak pairs showed that serotonin, as a key molecule of the tryptophan metabolism pathway linked to biliary tree reconstruction, was specifically expressed in FDOs during the whole period of culture. Such FDOs with biliary tree networks and serotonin expression may be applied for disease modeling and drug screening, which paves the way for future clinical therapeutic applications.
Keywords: Cholangiocyte, Decellularized scaffold, Ductal organoid, Extracellular matrix
Graphical abstract
Highlights
-
•
A functional ductal organoid with a biliary tree network was generated.
-
•
A long-term culture for at least 14 days was available.
-
•
Functions of bile secretion, transportation and excretion were exhibited.
-
•
The dynamic metabolic features were demonstrated using metabolomics.
1. Introduction
End-stage liver disease is a fatal condition associated with high mortality, and the only curative treatment is liver transplantation. However, liver transplantation, as an effective treatment option, remains limited due to the shortage of donor livers [1,2]. Functional bioengineered livers are considered a replacement for liver grafts [[3], [4], [5]]. Transplantation of hepatocyte-populated grafts into rodents and large animal models has been reported, but it did not support the functions involved in bile transportation and circulating xenobiotic substance excretion due to the lack of a biliary network [[6], [7], [8]]. The biliary tree significantly contributes to the structural and functional integrity of the liver, and an incomplete biliary tree results in severe liver damage as a posttransplant complication [9,10]. A recent study showed that the functions of liver grafts for transplantation were improved with biliary epithelial continuity, indicating that a functional biliary tree network is required for a bioengineered liver graft [11]. Thus, it is of great interest to construct a bioengineered liver with a functional ductal organoid (FDO2), which provides a promising alternative to the donor shortage for end-stage liver disease treatment.
Many efforts have been made in FDO generation in tissue engineering to reproduce the anatomical and functional features of native biliary trees [[12], [13], [14], [15]]. The primary cholangiocyte is a preferred cell source for FDO generation compared to induced pluripotent stem cells and embryonic stem cells, owing to the stable phenotypes and mature functions that closely resemble those in native livers [16,17]. Previous studies have mainly focused on the self-assembly of primary cholangiocytes to develop a biliary structure by spheroid generation or cyst formation in hydrogel (HG3) [12,13,18,19]. Although primary cholangiocytes in existing culture systems can form proliferative spheroid-like structures possessing specific biomarkers with epithelial polarity, a biliary network is not observed, indicating that the bile duct function in bile transportation outside the liver is incomplete. Recent studies indicated that fabricated synthetic and biological scaffolds can support the generation of bioengineered bile ducts by seeding cholangiocytes [[12], [13], [14]]. However, such scaffolds for extrahepatic bile duct reconstruction are far from reproducing the complex geometry and functions of the intrahepatic bile duct. Decellularization is a mature technique in tissue engineering, with cell components removed and the extracellular matrix (ECM4) retained, which supports cell adhesion and proliferation [5,20,21]. Recent studies have shown that decellularized whole liver scaffolds with ECM components and topologic structures resembling the native liver provide the potential for the implantation of various liver cells, including endothelial cells and hepatocytes [6,8,22], indicating that they are potentially ideal scaffolds for cholangiocyte repopulation for the generation of intrahepatic FDOs.
In this study, we presented a method to generate an FDO using rat decellularized whole liver scaffolds with mouse primary cholangiocyte retrograde transfusion through extrahepatic bile ducts. Compared with previous reports, the FDO constructed in our study formed a continuous biliary tree-like network structure after long-term culture in vitro. Functions similar to the native biliary tree were characterized by the polarity and the dynamic transportation of rhodamine 123 and cholyl-lysyl-fluorescein (CLF5) compared to cholangiocytes cultured in monolayer and HG systems. Metabolomic analysis of the culture medium at different time points displayed the specific expressed substances in FDO generation and revealed the metabolic pathways linked to biliary functions.
2. Materials and methods
2.1. Animals
6-8-week-old healthy Sprague-Dawley rats and 6-8-week-old healthy Balb/c mice were purchased from the Experimental Animal Center in the First Affiliated Hospital of Zhejiang University School of Medicine and Shengyuan Life Science (Zhejiang Anji) Co., Ltd. A constant 12 h light/dark cycle and a temperature- and humidity-controlled environment were provided, with free access to food and water. All animal experiments were approved by the Animal Health Ethics Committee of the First Affiliated Hospital of Zhejiang University (No. 2016–207 and No. 2021–1401) and were performed in accordance with the standards in the "Guide to the Care and Use of Animals".
2.2. Preparation of rat decellularized liver scaffold (DLS6)
The whole process was completed on a sterile operating table. Sprague-Dawley rats weighing 200–250 g were anaesthetized by isoflurane inhalation for euthanasia. The midline of the abdomen was incised to expose the liver. The portal vein and extrahepatic bile duct were cannulated with a cannula, and 5 ml of heparin-physiological saline (100 U/ml) was perfused into the liver through the portal vein. The perihepatic ligament and connective tissue were removed, and the liver was dissociated from the body. The isolated liver was placed in a sterile basin, and the portal vein catheter was connected to the peristaltic pump hose, with liquid perfusion at a speed of 30 ml/min. During the preparation of the rat DLS, we successively perfused 1% (v/v) Triton X-100 for 1h to disrupt the DNA-protein, lipid-protein and protein-protein interactions. Then we applied 0.1% (w/v) SDS for 1h to remove the nuclear remnants and cytoplasmic proteins from dense tissues, and PBS for 3h to wash out the decellularization detergent and protect DLS from any residue-baring cytotoxicity, until the DLS was completely transparent. The scaffold was transferred to a sterile 10 cm petri dish, perfused with 20 ml of PBS solution containing 1% penicillin/streptomycin, and stored at 4 °C.
2.3. Preparation of gelatine methacryloyl solution
Gelatine methacryloyl (Engineering for Life of Yongqinquan, Suzhou, China) was dissolved in PBS at 37 °C according to the manufacturer's instructions, and 0.25% (w/v) lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) (Engineering for Life of Yongqinquan, Suzhou, China) was added to the solution as a photoinitiator. The gelatine methacryloyl solution was sterilized with a 0.22 μm sterile filter at 37 °C protected from light.
2.4. Preparation of decellularized ECM (dECM7) hydrogel (HG)
The rat DLS was cut into pieces and placed in a centrifuge tube, and washed with 30 ml of 75% alcohol at 4 °C for 24 h. The alcohol was discarded later, and PBS solution containing 1% penicillin/streptomycin was added to wash for 24 h. The DLS was freeze-dried for 24 h and dissolved in a pepsin solution prepared by 0.5 M acetic acidto digest for 48 h at 37 °C. NaOH (10 mol/l) was used to neutralize the pH to 7.4, and 1/10 vol (10X) PBS was added for dissolution. The solution was freeze dried again for 48 h to produce lyophilized dECM powder. The lyophilized powder was added to the preprepared gelatine methacryloyl solution at a concentration of 10 mg/ml and mixed with a pipette to produce the dECM-HG.
2.5. Identification of decellularized liver scaffolds (DLSs)
DLS samples were formalin-fixed, paraffin-embedded and cut into 5-μm thick sections for H&E staining and immunofluorescence staining. For immunofluorescence staining, DLS samples were deparaffinized, hydrated, and neutralized peroxidase activity with 3% hydrogen peroxide for 10 min. The sections were antigenically repaired and blocked with 5% bovine serum albumin for 30 min and then incubated with anti-collagen I, anti-collagen III, anti-collagen IV, anti-fibronectin and anti-laminin (Abcam, Cambridge, UK) overnight at 4 °C. Goat anti-rabbit IgG (H + L), DyLight® 488 (Abcam, Cambridge, UK) and goat anti-mouse IgG (H + L), AF™ 488 (Invitrogen, Carlsbad, CA, USA) were used as secondary antibodies and incubated with DLS sections.
The native liver, DLS, and dECM lyophilized powder (n = 3 each) were weighed to 10 mg and evaluated with concentrations of rat glycosaminoglycan (GAG8), hepatocyte growth factor (HGF9), epidermal growth factor (EGF10), and transforming growth factor beta (TGF-β11) according to the steps of the ELISA kit (Elisalab, Wuhan, China).
2.6. Isolation of primary cholangiocytes
The whole process was completed in an ultraclean workbench. Balb/c mice weighing 15–25 g were anaesthetized by isoflurane inhalation for euthanasia. The gallbladder and extrahepatic bile ducts were isolated, removed and placed in a sterile 10 cm dish containing ice-cold DMEM/F12 Medium (Gibco, Grand Island, NY). Surrounding connective tissues were mechanically stripped, and the blood cells were washed away. Collagenase IV (1 g/l, Gibco, Grand Island, NY) solution prepared in DMEM/F12 Medium and prewarmed at 37 °C was used for preliminary digestion for 5 min in a 37 °C, 5% CO2 incubator to peel off the connective tissue again. The bile duct was cut into pieces and digested with prewarmed 0.5 g/l collagenase I and 4.2 g/l Dispase (Gibco, Grand Island, NY) solution in DMEM/F12 Medium at 37 °C for 15 min. The suspension was filtered through a 100 μm cell strainer, and the undigested tissue was digested again. The collected cell suspension was washed by centrifugation and the primary cholangiocytes were aggregated as precipitations.
2.7. Primary cholangiocyte culture in the dECM-HG
Primary cholangiocytes were resuspended in dECM-HG solution to a cell density of 2 × 106/ml with careful pipetting. The dECM-HG with primary cholangiocytes was dropped into a 24-well plate. Each dECM-HG construct was a 30 μl droplets-formed dome structure with a diameter of 5 mm. The 24-well plate was inverted to solidify under the irradiation of ultraviolet light at 405 nm for 10 s, and 500 μl of primary cholangiocyte culture medium was added to each well. The cells were cultured in an incubator (37 °C, 5% CO2), and the medium was replaced every 2 days. The density and growth status of the cells were observed under a microscope every day.
2.8. Identification of primary cholangiocytes
The isolated cholangiocytes were characterized with specific biomarkers by flow cytometry. Primary cholangiocytes were collected, counted, and permeabilized using a fixation/permeabilization kit (BD Cytofix/Cytoperm). Then, cholangiocytes were resuspended with Pharmingen Stain Buffer (BD Pharmingen) and incubated with anti-cytokine 7 (CK712) and anti-cytokine 19 (CK1913) (Abcam, Cambridge, UK) overnight at 4 °C. After incubation with goat anti-rabbit IgG (H + L) CF™ 647 and goat anti-rabbit IgG (H + L) DyLight® 488 (Abcam, Cambridge, UK) at 4 °C for 30 min in the dark, cholangiocytes were washed and resuspended in PBS and detected with a FACS Aria III (BD Biosciences).
The mRNA of native bile duct tissue and primary cholangiocytes was extracted using the RNeasy Mini Kit (QIAGEN, Hilden, Germany). The expression levels of specific gene including CK7, CK19, SRY-box transcription factor 9 (SOX914), gamma-glutamyl transpeptidase (GGT15), cystic fibrosis transmembrane conductance regulator (CFTR16) and aquaporin 1 (AQP117) were assessed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR18) according to the steps of HiScript III RT SuperMix for qPCR (Vazyme Biotech Co., Ltd.) and ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.).
2.9. Functional ductal organoids (FDOs) generation
DLS was perfused with 10 ml of primary cholangiocyte culture medium through the extrahepatic bile duct in a new sterile dish. Primary cholangiocytes were resuspended in culture medium at a density of 106/ml and seeded into DLS via slowly retrograde infusion through the extrahepatic bile duct. The DLS seeded with cholangiocytes was cultured in an incubator (37 °C, 5% CO2) for 20 min, and the cell suspension in the dish was collected and infused again, which was repeated 3 times. Then, 25 ml of primary cholangiocyte culture medium was added for recirculating perfusion in the incubator at 5% CO2 and 37 °C for 14 days to generate a FDO.
2.10. Morphological and histological assessment of FDO
FDOs were observed under a phase contrast microscope for morphological assessment, and the viability of cholangiocytes inside was assessed by staining with Calcein AM/PI Cell Viability/Cytotoxicity Detec (KeyGEN, Nanjing, China) following the instructions.
The immunohistochemical and immunofluorescence with anti-CK7, anti-CK19, anti-epithelial cell adhesion molecule (EpCAM19) (Abcam, Cambridge, UK), anti-acetylated α-tubulin (Sigma-Aldrich, St. Louis, Missouri), and anti-E-cadherin (Thermo Scientific, Waltham, MA) were performed as described above.
2.11. Tight junction and barrier function assessment
The immunofluorescence with anti-tight junction protein 1(ZO120) were performed as described above to evaluate the tight junction in FDO.
For the dynamic evaluation of barrier function of FDO, 5 μM cholyl-lysyl-fluorescein (CLF) (Corning, Midland, MI) was retrogradely injected into the biliary tree of DLS and FDO (n = 3 each) via the cannula in extrahepatic bile duct, and the leakage of CLF in surrounding was dynamically observed with a Zeiss laser-scanning confocal microscope (LSM 710/780).
2.12. Rhodamine 123 transport assay
The function of the multidrug resistance-1 (MDR121) transporter was detected by a rhodamine 123 assay (Sigma-Aldrich, St. Louis, Missouri). Samples in the monolayer system, dECM-HG system, and the FDO (n = 3 each) were pretreated of 10 μM verapamil (Sigma-Aldrich, St. Louis, Missouri) for 30 min at 37 °C, and then were incubated with 100 μM rhodamine 123 for 5 min. The samples were washed for 3 times to remove rhodamine 123 and were incubated with the culture medium for another 30 min at 37 °C. Images were acquired with a Zeiss laser-scanning confocal microscope (LSM 710/780).
2.13. Cholyl-lysyl-fluorescein (CLF) transport assay
The samples in the monolayer system, dECM-HG system, and the FDOs (n = 3 each) were dipped in the 5 μM CLF (Corning, Midland, MI) at 37 °C for 40 min to dynamically evaluate whether the bile acids in the surrounding tissues can be transported into the lumen of biliary tree in FDO. Images were acquired using a Zeiss laser-scanning confocal microscope (LSM 710/780).
2.14. Gamma-glutamyl transpeptidase (GGT) activity
The GGT activity of DLSs and FDOs (n = 3 each) was measured using the GGT activity detection kit (Solarbio, Beijing, China) at a wavelength of 405 nm according to the manufacturer's instructions. The activity unit refers to light absorbance.
2.15. Metabolism analysis
The culture supernatant of ductal organoids from days 1, 3, 7, and 14 was metabolite labeled, LC-UV normalized and LC-MS analyzed for metabolite peak pair analysis. Raw data were imputed with minimum values, mean-normalized, and then log-transformed. A Student's t-test was used to compare the relative expression level of metabolites in medium obtained at different time points. Fold change (FC22) ≥ 2 and p < 0.05 were set as the cutoff of differential metabolite analysis. Metabolic pathway enrichment analysis and pathway topology analysis were conducted using the MetaboAnalyst 5.0 computational platform.
2.16. Statistical analysis
All measurement data in this study were done using GraphPad Prism 8.0.1 statistical software. All results were analyzed by a one-way analysis of variance (ANOVA) or a Student's t-test. The data are expressed as mean ± standard error of mean (SEM23) with n ≥ 3. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001.
3. Results
3.1. Ductal organoids were generated with decellularized liver scaffolds and primary cholangiocytes
During the process of decellularization, the rat liver gradually turned translucent from brown by consistent perfusion, and the intact liver capsule was reserved at the end of decellularization (Fig. 1A). The route of oil red O solution retrogradely injected via the extrahepatic bile duct showed the integrity of the network structures of the decellularized liver scaffold (DLS) (Fig. 1B). H&E staining revealed that the cell components were completely removed and an empty honeycomb structure was retained after decellularization (Fig. 1C). The ELISA results showed that the content of GAG associated with tissue moisture and viscosity, and growth factors, including HGF, EGF, and TGF-β, showed inapparent differences in DLS, freeze-dried dECM and native liver (Fig. 1D). Immunofluorescence confirmed that basement membrane proteins, including collagen I, collagen IV, laminin, and fibronectin, were efficiently retained in DLS (Fig. 1E).
Fig. 1.
Characterization of the decellularized liver scaffolds (DLSs). (A) Process of decellularization of native liver. (B) Intrahepatic bile duct tree morphology shown by oil red perfusion. (C) Images of H&E-stained paraffin slides of (a) native liver and (b) DLS. Scale bars represent 50 μm. (D) Comparison of growth factor content (EGF, HGF, and TGF-β) and GAG content in native liver, DLS and freeze-dried dECM. n = 3 per group. (E) Immunofluorescence of matrix components (collagen, laminin, and fibronectin) in DLS. Scale bars represent 50 μm.
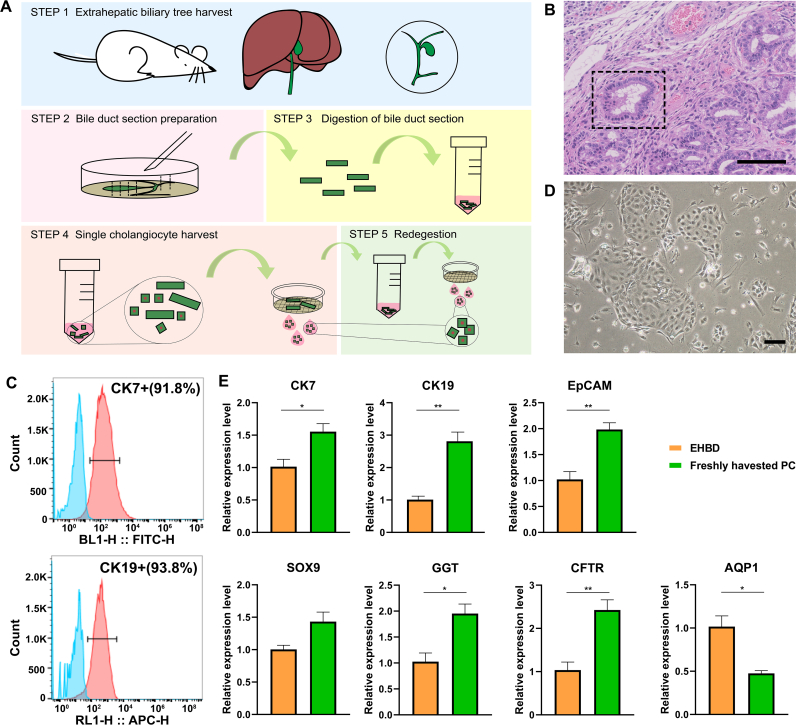
Primary cholangiocytes were efficiently isolated from mouse extrahepatic bile ducts and gallbladders (Fig. S1), represented schematically in Fig. 2A. Flow cytometry analyses revealed that 91.8% of isolated cells expressed CK7, and 93.8% of cells expressed CK19 (Fig. 2C). Morphologic assessment showed that cholangiocytes were cuboidal or columnar in the bile duct in vivo and exhibited polygonal adherent cells in vitro (Fig. 2B, D). The results of qRT-PCR showed that the expression level of bile duct intrinsic markers (CK7, CK19, EpCAM) and functional markers (GGT and CFTR) were significantly higher in freshly harvested primary cholangiocytes than those in extrahepatic bile ducts (p < 0.01), and SOX9 were similarly expressed in these two groups (p = 0.0553). The extrahepatic bile ducts expressed AQP1 in a significant higher level than freshly harvested primary cholangiocytes (p < 0.05) (Fig. 2E).
Fig. 2.
Characterization of isolated primary cholangiocytes. (A) Schematic representation of the method used for primary cholangiocyte isolation. (B) Images of H&E-stained paraffin slides of the extrahepatic bile duct. Black lines indicate the positions of cholangiocytes. Scale bars represent 50 μm. (C) Flow cytometry for isolated cells. (D) Morphology by phase contrast microscopy of adherent-well cholangiocytes. Scale bars represent 100 μm. (E) Expression levels of cholangiocyte-related marker genes displayed by qRT-PCR of the extrahepatic bile duct (EHBD24) and freshly harvested primary cholangiocytes (PC25s). n = 3 per group. *: p < 0.05, **: p < 0.01.
Primary cholangiocytes were cultured in the monolayer adherent culture system, liver dECM-HG, and DLS, respectively (Fig. S2A). The morphological results of phase contrast microscopy showed that in the monolayer adherent culture system, primary cholangiocytes gradually expanded into island-like cell clusters and formed a confluent layer with circles from day 1 to day 7, but massively died at day 14 (Fig. 3A a). In liver dECM-HG, primary cholangiocytes gradually expanded intoproliferative spheroids and cysts from day 1 to day 14 (Fig. 3A b). In DLS, primary cholangiocytes seeded via bile duct were distributed to the retaining ductal structures and adhered to the inner side of the lumen, and a continuous single layer was gradually generated on the basement membrane (Fig. 3A c). Cell viability staining confirmed that primary cholangiocytes with high activity were evenly distributed within the lumen and gradually formed a continuous bile duct network structure in DLS (Fig. 3B c). In contrast, the network structures were not observed in the monolayer and dECM-HG culture systems, with floating dead cells sustained with prolonged cultivation (Fig. 3B a, b). The coexpression of specific biomarkers CK7, CK19, and EpCAM of cholangiocytes in these three systems at day 14 was detected by qRT-PCR analysis (Fig. 3C) and immunofluorescence staining (Fig. 4A, B, C).
Fig. 3.
Morphological characteristics and gene expression level of primary cholangiocytes in three culture systems. (A) Morphology of primary cholangiocytes (PCs) by phase contrast microscopy in (a) monolayer adherent culture (2D), (b) dECM hydrogel (HG), and (c) functional ductal organoid (FDO). Scale bars represent 100 μm. (B) Cell viability staining of PCs in (a) 2D, (b) HG, and (c) FDO. Calcein AM (green) staining represented living cells and PI (red) staining represented dead cells, respectively. Scale bars represent 100 μm. (C) Gene expression levels of cholangiocyte-specific markers displayed by qRT-PCR of freshly harvested PCs and PCs cultured in 2D, HG, and FDO. Standardized with freshly harvested PC. n = 3. **: p < 0.01, ****: p < 0.0001.
Fig. 4.
Protein expression in three culture systems. (A) (B) (C) Immunofluorescence of specific markers of cholangiocytes in monolayer adherent culture (2D), dECM hydrogel (HG), and functional ductal organoid (FDO). Scale bars represent 50 μm. (D) H&E staining and immunohistochemistry of specific markers of cholangiocytes in FDO at different time points. Scale bars represent 100 μm.
H&E staining and immunohistochemistry showed the process of ductal organoids generation with CK7, CK19, and EpCAM expressing in DLS. The primary cholangiocytes gathered in the retained bile duct lumen of DLS and adhered to the ductal basal layer from day 1 to day 3, and gradually formed the biliary tree-like structure with cell-cell tight junctions from day 3 to day 14 (Fig. 4D). The 3D network structure formed by primary cholangiocytes in DLS was observed at day 14 (Fig. S3A), with high metabolic capacity and proliferation potential (Figs. S3B and C).
3.2. Ductal organoids highly expressed stemness and functional genes
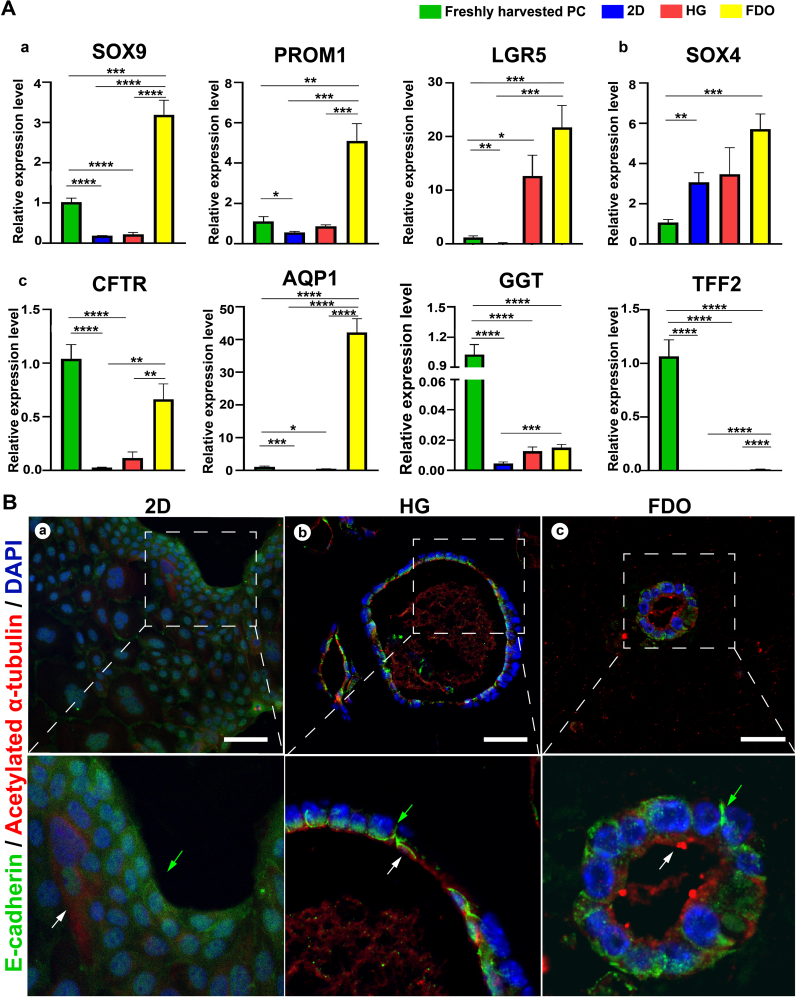
The qRT-PCR results showed that compared with freshly harvested primary cholangiocytes, FDOs significantly upregulated the somatic stem cell markers including SOX9, leucine rich repeat containing G protein coupled receptor 5 (LGR526), and prominin 1 (PROM127) at day 14, and the expression levels were significantly higher than those in monolayer and dECM-HG culture systems (p < 0.01) (Fig. 5A a). In addition, the significant decreased expression level of trefoil factor 2 (TFF228) (an extrahepatic bile duct regional-specific marker) and the significantly increased expression level of SRY-box transcription factor 4 (SOX429) (an intrahepatic bile duct regional-specific marker) were observed in FDOs after 14-day culture compared with freshly harvested primary cholangiocytes (p < 0.001) (Fig. 5A b), indicating the differentiation potential of extrahepatic cholangiocytes to intrahepatic cholangiocytes.
Fig. 5.
Characterization of gene expression level and polarity in three culture systems. (A) Gene expression level of (a) stemness, (b) regional characterization, and (c) function in monolayer culture system (2D), dECM hydrogel (HG), and functional ductal organoid (FDO) by qRT-PCR. Standardized with freshly harvested primary cholangiocytes. n = 3 per group. *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001. (B) Epithelial polarity in 2D, HG, and FDO characterized by immunofluorescence staining. Scale bars represent 50 μm.
The expression of CFTR and AQP1, which are closely related to bile secretion and bile flow modulation, was significantly higher in FDOs than in the other two culture systems (p < 0.01) (Fig. 5A c). In addition, the expression level of CFTR in FDOs was similar to that in freshly harvested primary cholangiocytes (p = 0.0825), and the expression AQP1 was significantly higher in FDOs than freshly harvested primary cholangiocytes, while both CFTR and AQP1 in the two control groups were significantly decreased (p < 0.001) (Fig. 5A c). GGT is critical for the promotion of bile-salt-independent bile flow by the degradation of glutathione. The gene expression of GGT was generally decreased after long-term culture in vitro according to the qRT-PCR results, but the expression level was higher in FDOs than that in monolayer culture systems (p < 0.01) and in dECM-HG (p = 0.5243) (Fig. 5A c). In addition, FDO exhibited high GGT activity compared with DLS as a control (p < 0.05) (Fig. S4C).
3.3. Ductal organoids exhibited significant epithelial polarity and functions of bile secretion, transportation and excretion
The immunofluorescent staining of acetylated α-tubulin on the apical side of the lumenindicated the primary cilia facing the lumen of biliary tree structures in FDOs, and the E-cadherin expression on the basal side was observed (Fig. 5B c). This result revealed the significant epithelial polarity of cholangiocytes in FDOs, which is considered the structural basis for bile duct function. Although polarity was also observed in cholangiocyte cysts cultured in dECM-HG, some of these cysts were incompletely polar (Fig. 5B b). No polarity was observed in adherent monolayer cultures (Fig. 5B a).
The MDR130-dependent transport of rhodamine 123 revealed the secretory function of FDOs. Rhodamine 123 is a fluorescent substrate for the cholangiocyte surface glycoprotein MDR1, which can be transported to the bile duct lumen through the MDR1 gene product P-glycoprotein and can be prevented by verapamil, an MDR1 inhibitor. In FDOs, rhodamine 123 was efficiently transported into the bile duct-like structures with incubation for 30 min (Fig. 6A c). With pretreatment of verapamil, rhodamine 123 failed to be transported into the lumen and was diffusely distributed throughout the surroundings (Fig. 6A f). The aggregation of rhodamine 123 into cholangiocyte cysts were observed in the dECM-HG and was inhibited by verapamil (Fig. 6A b, e). In contrast, rhodamine 123 did not aggregate in adherent monolayer cultures (Fig. 6A a, d). The dynamic detection of cholyl-lysyl-fluorescein (CLF) as a bile acid analogue revealed apical sodium-dependent bile acid transporter (ASBT) activity. In monolayer adherent culture conditions, CLF was not detected (Fig. 6B a). In the dECM-HG, the CLF in the lumen was completely expelled and no fluorescence signal was detected in the lumen after incubation for 40 min, revealing the active export of CLF from the lumen of cholangiocyte cysts. (Fig. 6B b). However, CLF transport in dECM-HG was quite different from that in native bile duct trees. In FDOs, CLF gradually accumulated into the bile duct-like structures over time, which was similar to the process as bile acid drained into the lumen of the biliary tree in vivo (Fig. 6B c). These results confirmed the functions of biliary secretion and transport in FDOs. Additionally, the immunofluorescent staining with ZO1 indicated the tight junction of cholangiocytes in FDO at day 14 (Fig. S4A). The CLF imported through the bile duct remained confined within the biliary tree of FDOs, while that in DLS diffused to the surroundings, revealing the barrier function against bile acid in FDOs (Fig. S4B).
Fig. 6.
Functional characterization in three culture systems. (A) Fluorescence images of rhodamine 123 secretion in (a) monolayer adherent culture (2D), (b) dECM hydrogel (HG), and (c) functional ductal organoid (FDO), and the inhibited secretion with pretreatment of verapamil in (d) 2D, (e) HG, and (f) FDO. Scale bars represent 100 μm. (B) Fluorescence images of CLF distribution in (a) adherent culture (2D), (b) dECM hydrogel (HG), and (c) FDO confirming bile acid transfer. Scale bars represent 100 μm.
Taken together, these results demonstrated that FDOs with a continuous bile duct network was generated in DLSs using primary cholangiocytes, with barrier function and the capacity for biliary secretion, transport, and excretion.
3.4. Serotonin in the tryptophan metabolism pathway was linked to biliary tree remodeling in functional ductal organoids
To further determine the metabolic characteristics in FDO generation, we analyzed the components of FDO culture medium at several time points by metabolomics. A total of 1075 metabolites were identified, and 278 metabolites were accurately identified by positive identification (CIL Library) and high confidence putative identification (LI Library) (Supplementary Table 1). Principal component analysis (PCA31) was used to assess the separation of FDO metabolites at different time points. The quality control (QC32) samples were tightly clustered, indicating good instrument stability throughout the dansylation LC-MS analysis (Fig. S5A). This PCA model (the first principal component, 32.1%, and the second principal component, 21.9%) showed that the metabolites of FDOs were well separated with a gradual trend at days 1, 3, 7, and 14 (Fig. 7A), indicating significant metabolic profile changes in FDOs during prolonged culture. The metabolite clustering of the four groups in the heatmap revealed the dynamic metabolite variation over time (Fig. 7B). Pairwise comparison with standard as log2(FC) ≥ 2 or log2(FC) ≤ (−2), p < 0.05 highlighted the differences between groups at the different time points. Compared with day 1, there were 90, 91, 98 differential metabolites with increased expression and 26, 46, 47 differential metabolites with decreased expression at day 3, day 7, and day 14, respectively (Fig. 7C). Venn diagram analysis showed 35 differential metabolites were overlapped between day 1 to day 3 and day 1 to day 7; 13 differential metabolites were overlapped between day 1 to day 3 and day 1 to day 14; 24 differential metabolites were overlapped between day 1 to day 7 and day 1 to day 14 (Fig. 7D). The enrichment analysis of the overlapping differential metabolites above, showed that these metabolites were mainly located on arginine and proline metabolism, glutathione metabolism and arginine biosynthesis pathways (Fig. S5B).
Fig. 7.
Metabolomics analysis for medium supernatant in functional ductal organoids (FDOs) at different time points. (A) PCA score plots and (B) clustering analysis by heatmap for metabolites in FDOs at different time points. (C) Representative metabolites between different time points with a log2(FC) ≥ 2 or a log2(FC) ≤ (−2) and p <0.05 for FDO by volcano plots. (D)(E) Venn diagram comparing the metabolites exhibiting significant changes as a function of different time points. (F) Standardized measurements of the differential metabolite serotonin at different time points by LC/MS. n = 4. **: p < 0.01, ***: p < 0.001, ****: p < 0.0001. (G) Overview of pathway analysis based on the differential metabolites at different time points.
The differential metabolites during day 1 to day 3, day 3 to day 7, and day 7 to day 14 were performed for further revealing the continuous biological process changes in FDO construction. Only 1 metabolite decreased significantly and 5 metabolites increased significantly from day 3 to day 7; 34 metabolites decreased and 5 metabolites increased from day 7 to day 14 (Fig. 7C). This result revealed that, in general, the metabolic profile of FDOs underwent significant changes from day 1 to day 3 and from day 7 to day 14. a Venn diagram analysis of the three processes, including day 1 to day 3, day 3 to day 7, and day 7 to day 14, showed that only 1 metabolite (serotonin) overlapped (Fig. 7E), which is closely related to regulation of biliary tree growth in vivo [23,24]. A significant increase in serotonin levels was observed from day 1 to day 7, and a significant decrease occurred from day 7 to day 14 (Fig. 7F). Enrichment and pathway analysis showed that the differential metabolic pathways, including alanine, aspartate and glutamate metabolism, arginine and proline metabolism, and tryptophan metabolism (Fig. 7G), impacted FDO generation throughout the culture, indicating the relevance of biological activities such as the urea cycle, energy metabolism, serotonin synthesis, cell migration, and cell proliferation. The lysine degradation pathway related to the metabolism of L-lysine in the liver was specific from day 1 to day 3. Arginine biosynthesis and D-glutamine and D-glutamate metabolism were specific metabolic pathways observed from day 7 to day 14 (Fig. 7G), indicating the relevance of biological activities such as the urea cycle, neuroendocrine hormone metabolism, and amino acid metabolism.
4. Discussion
The generation of an FDO with a biliary tree network has significant implications for the reconstruction of bioengineered livers. The primary cholangiocyte is considered a preferred cell source in biliary tree reconstruction due to the effective maintenance of native cell physiology [16,17]. Some recent studies have reported that biliary organoids can be generated by primary cholangiocytes with natural or synthetic materials, but the complex network structures that determine the functions of the biliary tree were not observed [12,13,18]. Recently, Roos, F. constructed a human branching cholangiocyte organoids using Matrigel, revealing their potential in mimicking tubular development and their application to disease modeling [25]. However, compared to the material reported in previous studies [[12], [13], [14], [15],25], DLS provided liver-specific microenvironments as we characterized previously by proteomic analysis [26], including ECM components and mechanical stresses. In this study, we developed an FDO using primary cholangiocytes to reconstruct the intrahepatic biliary tree in a DLS. The native network structures in the DLS provided a structural basis for reconstruction of the complex intrahepatic bile duct network, and the membrane proteins locating on the luminal surface promoted the adhesion, self-assembly, and polarization of cholangiocytes. Considering that long-term culture with maintenance of functionality is a key element in FDO generation [27], we used a 3D circulating perfusion system (Fig. S2B) as described in our previous work [26], to significantly prolong the available culture time to more than 14 days compared with existing studies [28].
Biologically functional FDOs are important for metabolite clearance in bioengineered livers and further therapeutic applications. Barrier function, bile component secretion and excretion of metabolic waste products are considered the key functions of a biliary tree in an FDO. The integrity of biliary tree structures with tight junctions and the epithelial polarity of cholangiocytes determines the barrier function, which protects the surrounding tissue against bile leakage and prevents the cytotoxic effects that lead to severe biliary fibrosis and liver failure [10]. The modulation of bile synthesis and secretion is also an important function of the biliary tree. Active bile secretion and excretion allow the transport of bile acid and the excretion of drug molecules and metabolites from hepatocytes through the biliary tree [9,10]. Recent studies have proven the secretion and transport potential in cholangiocyte cysts and spheroids [12,18,19], but such functions in biliary organoids with network structures have not been directly demonstrated [28]. In this study, with the gradual formation of mature network structures, barrier function of FDOs appeared as confining the CLF within the lumen of biliary tree, indicating that the FDO possessed the potential to prevent bile leakage from the network. The upregulated gene expression related to the water transportation and exchange of anions revealed the potential of FDO to modulate bile composition and bile flow. The gradual accumulation ofrhodamine 123 and CLF into the lumen of the network in FDOs was highly similar to the process of bile secretion and collection into the lumen and excretion through the native biliary tree. This result suggests that the FDO constructed in our study possess the potential to excrete harmful bile components and drugs metabolized by hepatocytes [9,10] and may be of great significance for further application of biliary trees in disease modeling, drug screening, and clinical therapeutic applications. Interestingly, the FDOs appeared to undergo a degree of dedifferentiation in addition to functional maturation of the biliary tree after long-term culture. The Wnt/β-catenin signaling pathway is closely related to the regulation of cholangiocyte expansion and polarity [29,30]. The upregulation of stemness genes involved in this pathway and the downregulation of regionally characteristic genes of the extrahepatic bile duct indicate that FDO exhibited high proliferation and differentiation potential after long-term culture in vitro [11,19]. At the same time, intrahepatic bile duct-specific genes were upregulated, indicating that the residual intrahepatic microenvironment of the DLS may promote the development from dedifferentiated cholangiocytes to intrahepatic cholangiocytes [11]. Such an FDO with both biliary tree function and differentiation potential can be used as an effective substitute for damaged intrahepatic bile ducts, thus laying the foundation for the construction of a completely bioengineered liver and its application in the treatment of end-stage liver disease.
Clarification of the mechanism in biliary tree reconstruction is crucial for the further application of FDOs. In previous studies, the mechanisms of bile duct development have been partially shown by transcriptomics [11,19], but the dynamic variation of metabolic signatures during bile duct remodeling is unclear. To further studied the potential regulatory mechanisms based on recent reports [12,14,22], we investigated the metabolic mechanisms linked to functional biliary tree remodeling in DLS with metabolomics analysis of culture supernatants for the first time. We found that serotonin was consistently presented as a differential metabolite during the whole culture period and is thought to be a key metabolite in FDO remodeling regulation. According to earlier reports, serotonin can be synthesized de novo by cholangiocytes in vivo and regulate the growth of the biliary tree in an autocrine or paracrine way by crosstalk between Ca2+ and cAMP/PKA signaling [24,31], and it plays proproliferative or antiproliferative roles in liver regeneration by binding to different receptors [23]. Therefore, the dynamic changes in serotonin reflected the various bioprocesses in FDO generation and maturity. In this study, mature tubular structure with significant polarity of cholangiocytes appeared after day 7, which indicated that FDO development shifted from proliferation (seen in the first 7 days) to the formation of mature functional structures. Based on the autocrine properties of serotonin, the increasing serotonin concentration was considered to be secreted by the proliferative cholangiocytes in the first 7 days and was depleted to regulate the growth and proliferation of cholangiocytes after day 7 [23], thereby avoiding the hyperproliferation of cholangiocytes and keeping the biliary tree in structural and functional homeostasis [32]. The tryptophan metabolic pathway (which involves serotonin) may be the key pathway affecting the construction of FDOs [24,33]. Although in-depth investigation of how serotonin and tryptophan metabolism affects the reconstruction of functional biliary trees is still needed, our study provides insights to further elucidate the precise metabolic regulatory mechanism of the functional reconstruction of biliary trees and lays the basis for disease modeling and alternative therapies in end-stage liver diseases, and we hope to provide a specific and detailed mechanism in the near future with appropriate studies.
In summary, FDOs with biliary tree networks were stable for functional long-term culture, providing a basis for further elucidation of the mechanism in bile duct remodeling by dynamically revealing metabolic signatures. It may be used for disease modeling (such as drug-induced bile duct injury, vanishing bile duct syndrome, and cholangiocarcinoma), and drug screening for evaluating the potency and toxic effects on bile duct, providing a basis for the safety and effectiveness of these drugs in clinical application. Constructing a complete bioengineered liver based on such an FDO requires the coculture of hepatocytes and nonparenchymal cells and the in-depth mechanism of regulation in biliary tree reconstruction should be further studied by multi-omics. However, the generation of FDO with biliary networks in this study paves the way for future clinical therapeutic applications.
5. Conclusion
An FDO with biliary tree network structures that exhibited bile secretion, transportation and excretion functions was generated in DLSs with primary cholangiocytes, and serotonin in the tryptophan metabolism pathway was considered a key molecule for biliary tree reconstruction in FDO generation by metabolomics analysis. The FDO may be applied for disease modeling and drug screening, and is of great significance for the generation of functional bioengineered livers, which lays the foundation for future use in clinical therapy.
Funding
This work was supported by the National Key R&D Program of China (2022YFA1104102, 2022YFA1104603, 2022YFC2304801), the National Natural Science Foundation of China (81830073, 82272426), the National Provincial special support program for high-level personnel recruitment (Ten-thousand Talents Program, 2018RA4016) and Zhejiang Public Welfare Project (LY21H030007, LGF21H200006).
Data availability
The raw data required to reproduce these findings can be shared by the authors upon request.
Ethics approval and consent to participate
Animal subjects were purchased from the Experimental Animal Center in the First Affiliated Hospital of Zhejiang University School. A constant 12 h light/dark cycle and a temperature- and humidity-controlled environment were provided, with free access to food and water. All animal experiments were approved by the Animal Health Ethics Committee of the First Affiliated Hospital of Zhejiang University (No. 2016–207 and No. 2021–1401) and were performed in accordance with the standards in the "Guide to the Care and Use of Animals".
CRediT authorship contribution statement
Jiaxian Chen: Conceptualization, Methodology, Validation, Writing – original draft. Shiwen Ma: Methodology, Validation, Formal analysis, Writing – original draft. Hui Yang: Methodology, Validation, Formal analysis, Writing – original draft. Xi Liang: Formal analysis, Software. Heng Yao: Formal analysis, Software. Beibei Guo: Methodology, Validation. Deying Chen: Investigation, Data curation. Jing Jiang: Investigation, Data curation. Dongyan Shi: Resources, Investigation. Jiaojiao Xin: Resources, Investigation. Keke Ren: Validation. Xingping Zhou: Methodology. Yun Li: Methodology. Lei Geng: Conceptualization, Supervision. Jun Li: Conceptualization, Supervision, Writing – review & editing, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
FDO: functional ductal organoid.
HG: hydrogel.
ECM: extracellular matrix.
CLF: cholyl-lysyl-fluorescein.
DLS: decellularized liver scaffold.
dECM: decellularized extracellular matrix.
GAG: glycosaminoglycan.
HGF: hepatocyte growth factor.
EGF: epidermal growth factor.
TGF-β: transcriptional growth factor beta.
CK7: cytokine 7.
CK19: cytokine 19.
SOX9: SRY-box transcription factor 9.
GGT: gamma-glutamyl transpeptidase.
CFTR: cystic fibrosis transmembrane conductance regulator.
AQP1: aquaporin 1.
RT-PCR: reverse transcription-polymerase chain reaction.
EpCAM: epithelial cell adhesion molecule.
ZO1: tight junction protein 1.
MDR1: multidrug resistance-1.
FC: fold change.
SEM: standard error of mean.
EHBD: extrahepatic bile duct.
PC: primary cholangiocyte.
LGR5: leucine rich repeat containing G protein coupled receptor 5.
PROM1: prominin 1.
TFF2: trefoil factor 2.
SOX4: SRY-box transcription factor 4.
MDR1: Multidrug Resistance Protein 1
PCA: principal component analysis.
QC: quality control.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.03.012.
Contributor Information
Lei Geng, Email: geng97927@163.com.
Jun Li, Email: lijun2009@zju.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Li J., et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J. Hepatol. 2021;75(5):1104–1115. doi: 10.1016/j.jhep.2021.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Kim W.R., et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N. Engl. J. Med. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Struecker B., Raschzok N., Sauer I.M. Liver support strategies: cutting-edge technologies. Nat. Rev. Gastroenterol. Hepatol. 2014;11(3):166–176. doi: 10.1038/nrgastro.2013.204. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., et al. A decade of progress in liver regenerative medicine. Biomaterials. 2018;157:161–176. doi: 10.1016/j.biomaterials.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q., Li L., Li J. Stem cells with decellularized liver scaffolds in liver regeneration and their potential clinical applications. Liver Int. 2015;35(3):687–694. doi: 10.1111/liv.12581. [DOI] [PubMed] [Google Scholar]
- 6.Uygun B.E., et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010;16(7):814–820. doi: 10.1002/hep.24067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baptista P.M., et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 8.Yang W., et al. A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation. Biomaterials. 2018;177:52–66. doi: 10.1016/j.biomaterials.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Boyer J.L., Soroka C.J. Bile formation and secretion: an update. J. Hepatol. 2021;75(1):190–201. doi: 10.1016/j.jhep.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Banales J.M., et al. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019;16(5):269–281. doi: 10.1038/s41575-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampaziotis F., et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science. 2021;371(6531):839–846. doi: 10.1126/science.aaz6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampaziotis F., et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017;23(8):954–963. doi: 10.1038/nm.4360. [DOI] [PubMed] [Google Scholar]
- 13.Tysoe O.C., et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 2019;14(6):1884–1925. doi: 10.1038/s41596-019-0168-0. [DOI] [PubMed] [Google Scholar]
- 14.Mazari-Arrighi E., et al. Construction of functional biliary epithelial branched networks with predefined geometry using digital light stereolithography. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121207. [DOI] [PubMed] [Google Scholar]
- 15.Chen C., et al. Bioengineered bile ducts recapitulate key cholangiocyte functions. Biofabrication. 2018;10(3) doi: 10.1088/1758-5090/aac8fd. 034103-034103. [DOI] [PubMed] [Google Scholar]
- 16.Shiota J., Samuelson L.C., Razumilava N. Hepatobiliary organoids and their applications for studies of liver Health and disease: are we there yet? Hepatology. 2021;74(4):2251–2263. doi: 10.1002/hep.31772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsee A., et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 2021;28(5):816–832. doi: 10.1016/j.stem.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soroka C.J., et al. Bile-derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology. 2019;70(3):871–882. doi: 10.1002/hep.30470. [DOI] [PubMed] [Google Scholar]
- 19.Rimland C.A., et al. Regional differences in human biliary tissues and corresponding in vitro-derived organoids. Hepatology. 2021;73(1):247–267. doi: 10.1002/hep.31252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., et al. Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022;10:15–31. doi: 10.1016/j.bioactmat.2021.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomofuji K., et al. Liver ductal organoids reconstruct intrahepatic biliary trees in decellularized liver grafts. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121614. [DOI] [PubMed] [Google Scholar]
- 23.Marzioni M., et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128(1):121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Kyritsi K., et al. Modulation of the tryptophan hydroxylase 1/monoamine oxidase-A/5-hydroxytryptamine/5-hydroxytryptamine receptor 2A/2B/2C Axis regulates biliary proliferation and liver fibrosis during cholestasis. Hepatology. 2020;71(3):990–1008. doi: 10.1002/hep.30880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos F.J.M., et al. Human branching cholangiocyte organoids recapitulate functional bile duct formation. Cell Stem Cell. 2022;29(5):776–794. doi: 10.1016/j.stem.2022.04.011. e13. [DOI] [PubMed] [Google Scholar]
- 26.Guo B., et al. Functionalized vascular structure in bioengineered liver identified with proteomics. ACS Biomater. Sci. Eng. 2020;6(11):6394–6404. doi: 10.1021/acsbiomaterials.0c01353. [DOI] [PubMed] [Google Scholar]
- 27.Garreta E., et al. Tissue engineering by decellularization and 3D bioprinting. Mater. Today. 2017;20(4):166–178. doi: 10.1016/j.mattod.2016.12.005. [DOI] [Google Scholar]
- 28.Chen Y., et al. Repopulation of intrahepatic bile ducts in engineered rat liver grafts. Technology. 2019;7(1–2):46–55. doi: 10.1142/S2339547819500043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huch M., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494(7436):247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell J.O., Monga S.P. Wnt/β-Catenin signaling in liver development, homeostasis, and pathobiology. Annu. Rev. Pathol. 2018;13:351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aloia L., et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat. Cell Biol. 2019;21(11):1321–1333. doi: 10.1038/s41556-019-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang J.H., et al. Serotonin protects mouse liver from cholestatic injury by decreasing bile salt pool after bile duct ligation. Hepatology. 2012;56(1):209–218. doi: 10.1002/hep.25626. [DOI] [PubMed] [Google Scholar]
- 33.Renzi A., et al. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology. 2013;57(3):1130–1141. doi: 10.1002/hep.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data required to reproduce these findings can be shared by the authors upon request.