Abstract
INTRODUCTION
This meta-analysis aimed to determine the comparative effectiveness of timed alarm device-assisted urotherapy vs. standard urotherapy alone in managing pediatric daytime urinary incontinence (pDUI).
METHODS
A systematic literature search was performed in December 2021, with an update search in July 2022. Comparative studies assessing the pDUI treatment effectiveness of timed alarm device-assisted urotherapy vs. urotherapy alone were identified and evaluated according to Cochrane collaboration recommendations. The assessed outcome includes pDUI complete response and adherence rates. Relative risk (RR ) with 95% confidence intervals (CI) was extrapolated. A random-effects model was used to pool effect estimates. Heterogeneity was assessed with sensitivity and subgroup analysis performed according to study design and comparative group characteristics. GRADE criteria were used to assess evidence certainty. (PROSPEROCRD 42022299173).
RESULTS
Four studies (three randomized controlled trials [RCTs] and one retrospective cohort) with 635 cases were included. The pooled effect estimates of pDUI complete response showed no differences between intervention groups (RR 1.20, 95% CI 0.81, 1.76). Pooled effect estimates for treatment adherence were generated from two studies, which showed significantly better adherence for the timed-alarm device group (RR 2.97, 95% CI 1.46, 6.06). Significant interstudy heterogeneity was noted; the source is likely from the study design and comparator device characteristics. The quality of evidence was assessed to be of very low certainty.
CONCLUSIONS
Based on very low certainty evidence, timed alarm device-assisted urotherapy does not seem to have the advantage of complete treatment response over standard urotherapy alone in managing pDUI; however, a timed-alarm device is likely able to improve urotherapy treatment adherence.
INTRODUCTION
According to the International Children’s Continence Society (ICCS), daytime urinary incontinence (DUI) is defined as intermittent involuntary urine leakage during the daytime wake period among children aged five years old or older.1 A recent ICCS standardization document for the treatment of DUI recommends that treatment modalities be tailored according to the individual child’s condition.2 Given that the majority (>65%) of the DUI etiology in children is determined to be functional,3 urotherapy is considered the primary intervention after organic and concomitant medical morbidities have been ruled out.2 Specifically, according to some studies, behavioral modification (timed voiding, avoidance of urine holding, and optimizing voiding posture) treated 40–45% of DUI in children.4,5
Timed alarm devices, such as alarm watches, are being suggested to enhance pediatric (p) DUI treatment.2,6 Notably, the suggested mechanism of action for the timed alarm device is timed voiding reminders of school-age children.7 Prior studies have shown the superiority of urotherapy with a timed alarm device over standard urotherapy alone;8,9 however, a recent study has shown no difference in treatment outcomes.10 Due to inconsistent reported evidence, this systematic review and meta-analysis aimed to determine the comparative effectiveness of timed alarm device-assisted urotherapy vs. standard urotherapy alone in managing DUI among children.
METHODS
The meta-analysis protocol was made in consultation with a topic expert and review methodologist, and subsequently registered priori at the PROSPERO registry CRD 42022299173. The meta-analysis was conducted according to the Cochrane Collaboration recommendation and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.11,12
Identification and evaluation of the literature
A comprehensive literature search with no language restriction was carried out initially in December 2021; an update search was conducted in July 2022 to identify published medical literature of human studies on the use of any timed alarm device in the management of pDUI. The databases used were MEDLINE, EMBASE, Scopus, and PubMed, while Googlescholar and Clinicaltrial.gov were searched for grey literature and trial registry for unpublished data. The platform/database-specific search strategies are detailed in the Appendix (available at cuaj.ca). In addition, relevant Cochrane reviews and studies that met our inclusion criteria were cross-referenced for potentially eligible records.
This meta-analysis included comparative studies, such as randomized controlled trials (RCTs, prospective and retrospective cohorts) that compare clinical outcomes of the use of timed alarm device-assisted urotherapy vs. standard urotherapy alone or with other non-timed devices in the management of pDUI. Excluded studies were non-comparative trials, reviews, commentaries, non-assessment of clinical outcome response rate, and adult population studies. The primary outcome considered in this meta-analysis was the post-intervention response rate, specifically complete response, which according to ICCS is defined as a 100% reduction in wet days per week.1,2 The secondary outcome assessed was treatment adherence, defined by the individual studies.
The retrieved records from the databases were imported into a systematic review software, Covidence app.13 Once duplicate records were removed, unique records were independently evaluated by two of the three reviewers (MR, NM, MEC). Records that either reviewer flagged were retrieved for full-text and were further reviewed to determine whether they met the inclusion criteria. The full-text review was performed independently by two other reviewers (MEC and NB) who were knowledgeable in the principles of critical appraisal. The risk of bias, quality of the design, execution, and data analysis of studies were assessed according to Cochrane Collaborative recommendations using risk of bias for RCTs and ROBINS-I for non-RCT comparative studies.14,15 Differences in the assessment were resolved through consensus.
Data extraction, synthesis, and measures of treatment effect
One reviewer extracted and summarized the study characteristics and outcome assessment of the included studies and these were counter-verified by another (LKA). The RevMan5 program from www.Cochrane.org was used to report the data outcome extracted from the studies.16 Dichotomous data of the treatment response rate per intervention group were extrapolated as risk ratios (RR ) with 95% confidence intervals (95% CI). Effect estimates were pooled using the inverse variance (IV) method with the random-effects model. The random-effects model meta-analyses were chosen to provide a more conservative estimate by considering both the estimates of between-study variation (i.e., study heterogeneity) and the small study sample size.12,17 Intention-to-treat analysis was applied to each study, with all dropouts considered non-responders and non-treatment adherents. When reported by the studies, adverse events were summarized with detailed descriptive analysis.
Assessment of heterogeneity, subgroup analysis, publication bias, and GRADE criteria
The Chi-squared statistical test for heterogeneity and the overlap of CIs on the forest plot assessed the heterogeneity between different studies. A p-value of 0.10 was used to show heterogeneity, and the I2 statistic of >40% was used to identify substantial between-study variations.12 The source of heterogeneity among the study characteristics was then determined by considering the clinical and methodological characteristics of the included studies. Subgroup analysis was performed according to the study design and comparator device. A funnel plot was generated to assess the possibility of publication bias. Finally, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE ) criteria was used to assess the certainty of the synthesized evidence from the meta-analysis.18
RESULTS
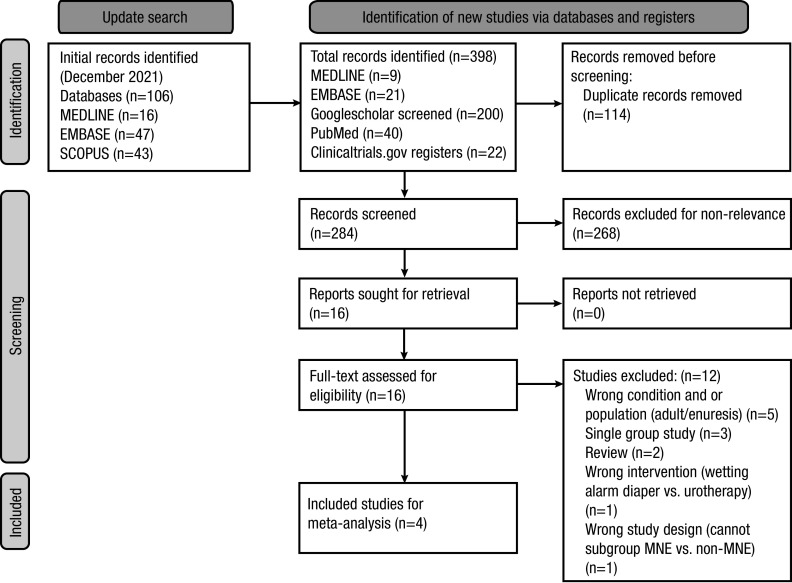
The initial literature search from December 2021 retrieved 106 records. An update search on July 2022 retrieved 292 from the same databases, PubMed, and additional 200 records screened from Googlescholar and registered trials from clinicaltrials.gov. From the total of 398 records, 114 duplicates were removed, and 284 records were screened for relevance. Subsequently, 268 records were excluded based on the relevance of the studies. The full-text article was retrieved for the 16 studies. Upon full-text review, 12 studies were excluded based on various reasons detailed in Figure 1.
Figure 1.
PRISMA 2020 flow diagram for systematic reviews, which included searches of databases and registers only. Adapted from Page MJ, et al. BMJ 2021;372:n71. For more information, visit: http://www.prisma-statement.org/.
CHARACTERISTICS OF THE INCLUDED STUDIES
Four studies ( three RCTs and one retrospective cohort) with 635 cases (timed alarm device=232, control/comparator= 403) were included for the meta-analysis.8–10,19 Two studies were from Denmark,8,9 one from the U.K.,19 and one from Australia.10 One study compared the timed alarm device (watch)-assisted urotherapy to a control group of standard urotherapy with a similar watch device but was not set for a specific time.10 One study compared another device that set the alarm when urine contacted the sensor in the diaper,19 while two other studies used a timer watch and compared it to standard urotherapy alone.8,9 All enrolled patients ranging from 5–15 years old in the included studies. The followup period ranged from 3–24 months; most studies had a three-month treatment assessment. All studies reported the treatment response as complete dryness, and two studies further adapted the ICCS definition of response and partial response.8,10 Treatment adherence was assessed by the same two studies.8,10 Table 1 details the included studies’ detailed characteristics.
Table 1.
Detailed study characteristics of included studies
| Author (year) | Country | Study method | Population | Intervention description | Comparison description | Outcome comparison | Followup duration | Timed alarm | Comparator | Outcome (success) | Remarks | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Halliday (1987) | U.K. | RCT |
|
Flat plastic wetting sensor alarm (Headingley Scientific Instruments, Leeds) does not go off with a buzzer when urine is in contact with the sensor; instead, the set alarm buzzes almost every 2 hours | Flat plastic wetting sensor alarm (Headingley Scientific Instruments, Leeds) goes off with a buzzer when urine is in contact with the sensor | Success rate is defined as 6 consecutive weeks without daytime wetting | Treatment up to 3 months, followup within 1 month after stopping treatment | 22/19 (2 drop out) | 22/20 (2 drop out) | 13 | 16 |
|
| Hagstroem (2008) | Denmark | Retrospective cohort |
|
Standard urotherapy with timer watch | Standard urotherapy alone | The response is defined as a complete daytime continence for 14 days | 24 months | 60 | 230 | 42 | 126 |
|
| Hagstroem (2010) | Denmark | RCT |
|
Timer assisted- watch with 7 alarms (Triax, Nike Inc., Oregon) | Standard urotherapy alone, daily fluid intake at least 1200 ml per day, timed voiding every 2 hrs until bedtime | Response defined as ICCS of full response of complete daytime continence | 12 weeks for assessment of treatment response | 30 | 28 | 9 | 0 |
|
| Caldwell (2021) | Australia | RCT |
|
Urotheraphy similar to control group and personalized alarm watch to vibrate at approximately 2-hr intervals during the day and locked to prevent tamperin |
|
Response by 3 months, complete response defined as 100% reduction | 3 months | 120/116 | 123/110 | 26 | 19 |
|
DUI: daytime urinary incontinence; ICCS: International Children’s Continence Society; IVP: intravenous pyelogram; KUB: kidney-ureter-bladder; RCT: randomized controlled trial; SD: standard deviation; VCUG: voiding cystourethrogram.
TREATMENT EFFECT
The pooled effect estimates of complete response showed no between-group differences (RR 1.20, 95% CI 0.81, 1.76). Subgroup analysis was performed according to the study design. Pooled effect estimates from RCTs showed no between-group difference (RR 1.27, 95% CI 0.59, 2.71). Subgroup analysis considering only the studies compared with standard urotherapy also showed no between-group differences (RR 1.40, 95% CI 0.92, 2.12) (Figure 2). Among the RCTs, there was a noted significant inter-study heterogeneity; however, when the subgroup was analyzed according to comparative group characteristics, inter-study heterogeneity was not significant. The source of heterogeneity was likely from the study design, with comparator device characteristics as a confounder.
Figure 2A.
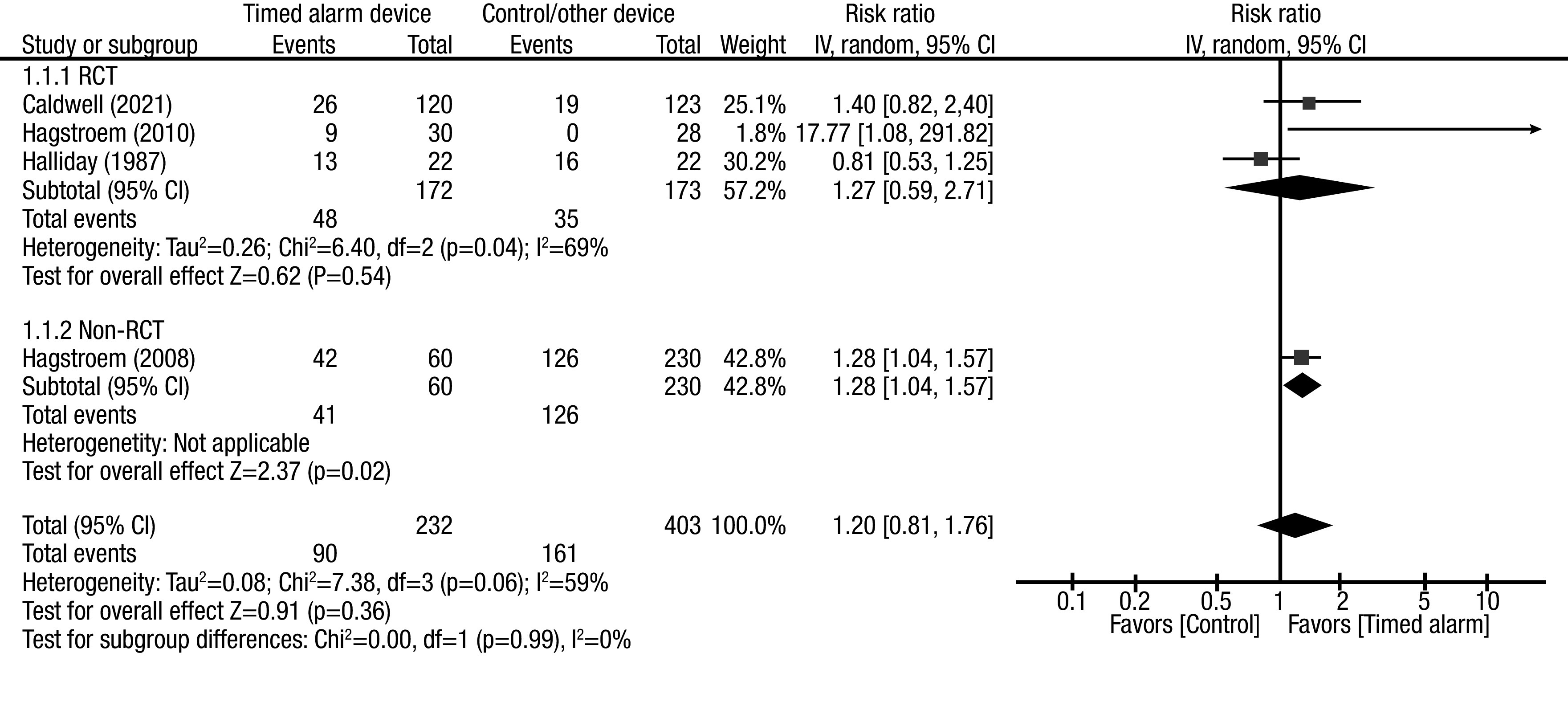
Forest plot pooled effect estimates for outcome of complete response rate (CRR); comparison: timed alarm vs. control/ other device; subgroup: study design (RCTs and non-RCTs). Statistical method: Inverse variance with random-effect model (relative risk [RR] and 95% confidence interval [CI]). RCT: randomized controlled study.
Figure 2B.
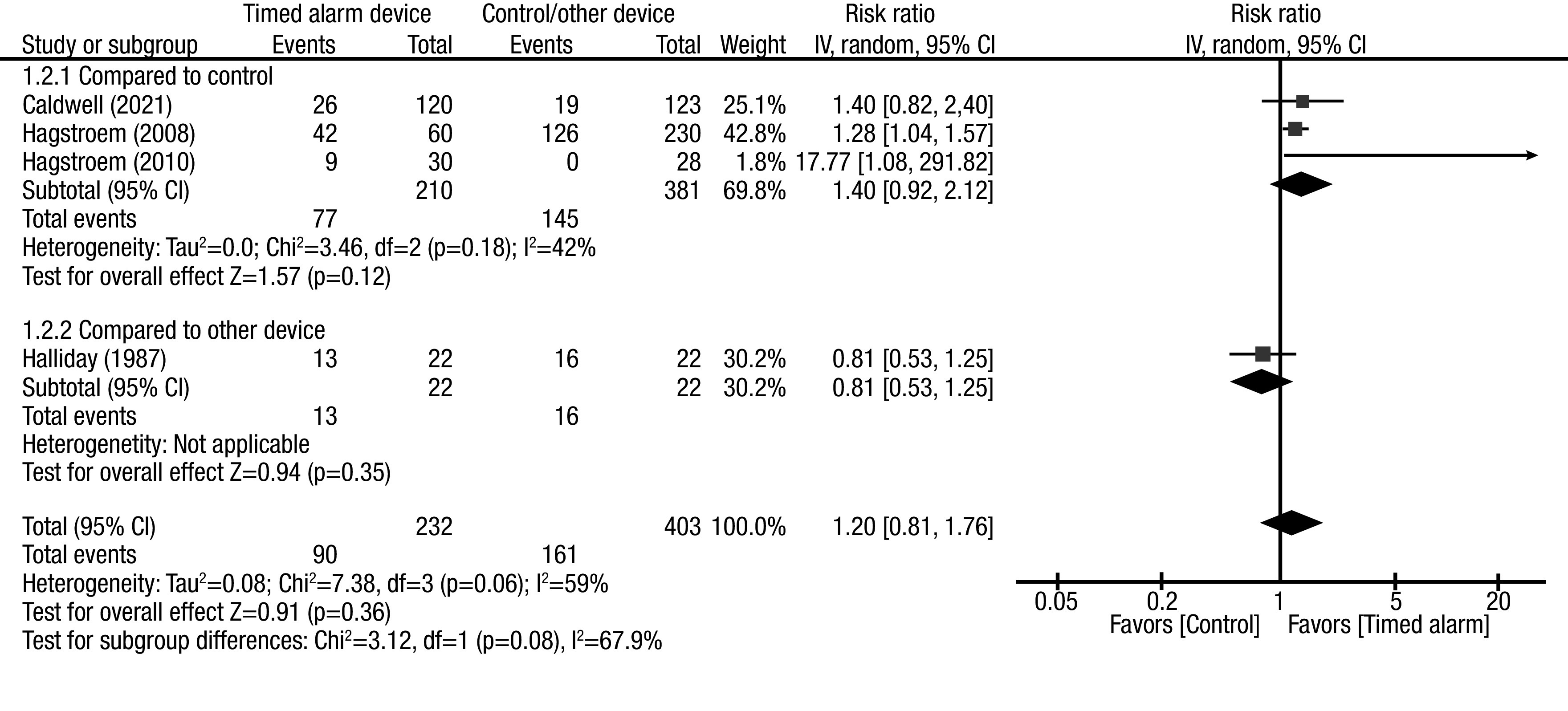
Forest plot pooled effect estimates for outcome of complete response rate (CRR); comparison: timed alarm vs. control/ other device; subgroup: study design (control and other device). Statistical method: Inverse variance with random-effect model (relative risk [RR] and 95% confidence interval [CI]).
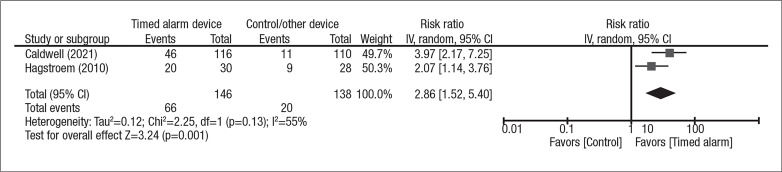
Pooled effect estimates for treatment adherence were generated from two studies, which showed significantly better adherence with the timed alarm device vs. the comparator group (RR 2.97, 95% CI 1.46, 6.06) (Figure 3). Inter-study heterogeneity was borderline significant; when the analysis was performed according to per-protocol analysis without assuming lost to followup patients as non-adherent, the heterogeneity became insignificant (Supplementary Figure 1; available in the Appendix at cuaj.ca).
Figure 3.
Forest plot pooled effect estimates for outcome of treatment adherence; comparison: timed alarm vs. control; subgroup: none. Statistical method: Inverse variance with random-effect model (relative risk [RR] and 95% confidence interval [CI]).
Among the included studies, only one reported a safety concern of using timed alarm devices, which was described as tolerable to the families and had no reported significant adverse effects.10
Study quality, risk of bias, publication bias, and GRADE criteria
Based on the risk of bias 2 tool, the included RCTs were assessed as having some concerns and a high risk of bias (Table 2). Most of the concerns for risk of bias were due to a lack of detailed information on the randomization process and allocation. The non-RCT retrospective study included was assessed according to ROBINS-I as having serious to critical risk of bias, which was due to bias from confounder and selection of participants to the intervention.
Table 2.
Study quality assessment according to risk of bias tool
| Author (year) | Study design | ROBINS-I | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bias due to confounding | Bias in selection of participants into the study | Bias in measurement of interventions | Bias due to departures from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias | ||
| Halliday (1987) | RCT | ||||||||
| Hagstroem (2008) | Retrospective cohort | Serious | Serious | Low | Serious | Moderate | Moderate | Moderate | Serious-critical |
| Hagstroem (2010) | RCT | ||||||||
| Caldwell (2021) | RCT | ||||||||
| ROB-RCT | |||||||||
| Author (year) | Study design | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Other potential bias | Overall bias | |
| Halliday (1987) | RCT | Some concern | Low concern | Low concern | Low concern | Low concern | Low concern | Some concern | |
| Hagstroem (2008) | Retrospective cohort | ||||||||
| Hagstroem (2010) | RCT | Some concern | Some concern | Low concern | Some concern | Low concern | Some concern | Some concern | |
| Caldwell (2021) | RCT | Low concern | Low concern | Some concern | Low concern | Low concern | Low concern | Some concern | |
RCT: randomized controlled trial.
Publication bias based on the generated funnel plot showed a likelihood of a small study effect (Supplementary Figure 2; available in the Appendix at cuajca.). Specifically, the small sample-sized RCT gave significantly higher effect estimates for the timed alarm device. Based on GRADE criteria, some to high concerns of risk of bias, significant heterogeneity, and the possibility of publication bias have downgraded the evidence as very low certainty.18
DISCUSSION
Standard urotherapy is recommended as the first-line management of pDUI.2 Furthermore, timed voiding is an integral part of standard urotherapy that aims to reduce urinary incontinence by preventing overflow incontinence and improving bladder control among toilet-trained children.2 Although in the management of adult DUI, timed voiding was assessed to be highly effective (with an 80% complete response rate),20 this was reported to be less effective in pDUI, as most cases are functional and non-organic.2,5
Using a timed alarm device as a regular reminder for timed voiding has been postulated to increase compliance among pediatric patients.2,8 This meta-analysis finding supports such postulation, as we showed approximately three times improved treatment adherence among patients with timed alarm devices compared to standard urotherapy alone.
Despite the improved adherence, we found no significant difference in the pooled effect estimates for the overall complete response rate between the treatment groups. Standard urotherapy is highly effective in treating functional pDUI; however, as suggested by the ICCS position statement on pDUI, when refractory to standard urotherapy, pDUI patients need further adjunctive pharmacological management and need to be evaluated for neurogenic or anatomic etiology.2 Another plausible explanation for the noted equivocal complete response rate between the two intervention groups could be due to the placebo effect of the control device. Among the studies that used comparative devices, the control groups in Cadwell et al10 and Halliday et al19 had better overall complete response rates compared to studies in which only standard urotherapy alone was used without placebo/another device as control.5,8
Limitations
Even with a sensitive search strategy and an extensive search for evidence, the inherent limitation of this systematic review and meta-analysis is the limited amount of available comparative studies that assess the differential effectiveness of timed alarm devices vs. standard urotherapy alone. Although RCTs were included, the methodological quality of these studies was assessed have concern for risk of bias. Moreover, a significant inter-study variability and the possibility of publication bias were noted, which further limited the certainty of the generated evidence. Based on the GRADE criteria, the evidence from available literature was determined to be very low to be able to generate recommendations; however, from a clinical perspective, with the recognized low to no adverse effect of a timed alarm device, clinicians may consider adding these to standard urotherapy among pDUI patients identified as refractory due to poor compliance. Furthermore, future studies may consider identifying the pDUI subgroup that could benefit from adding a timed alarm device.
CONCLUSIONS
Based on the available, very low-certainty evidence, timed alarm device-assisted urotherapy does not seem to have the advantage of complete treatment response over standard urotherapy alone in managing pDUI; however, a timed alarm device was determined to improve treatment adherence to timed voiding. Future studies may consider identifying a specific pDUI subgroup that may render a complete DUI treatment response for timed alarm devices.
Supplementary Information
Footnotes
Appendix available at cuaj.ca
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn. 2016;35:471–81. doi: 10.1002/nau.22751. [DOI] [PubMed] [Google Scholar]
- 2.Chang SJ, Van Laecke E, Bauer SB, et al. Treatment of daytime urinary incontinence: A standardization document from the International Children’s Continence Society. Neurourol Urodyn. 2017;36:43–50. doi: 10.1002/nau.22911. [DOI] [PubMed] [Google Scholar]
- 3.von Gontard A, Niemczyk J, Weber M, et al. Specific behavioral comorbidity in a large sample of children with functional incontinence: Report of 1001 cases. Neurourol Urodyn. 2015;34:763–8. doi: 10.1002/nau.22651. [DOI] [PubMed] [Google Scholar]
- 4.van Gool JD, de Jong TP, Winkler-Seinstra P, et al. Multicenter, randomized controlled trial of cognitive treatment, placebo, oxybutynin, bladder training, and pelvic floor training in children with functional urinary incontinence. Neurourol Urodyn. 2014;33:482–7. doi: 10.1002/nau.22446. [DOI] [PubMed] [Google Scholar]
- 5.Allen HA, Austin JC, Boyt MA, et al. Initial trial of timed voiding is warranted for all children with daytime incontinence. Urology. 2007;69:962–5. doi: 10.1016/j.urology.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Buckley BS, Sanders CD, Spineli L, et al. Conservative interventions for treating functional daytime urinary incontinence in children. Cochrane Database Syst Rev. 2019;9:CD012367. doi: 10.1002/14651858.CD012367.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Laecke E, Wille S, Vande Walle J, et al. The daytime alarm: A useful device for the treatment of children with daytime incontinence. J Urology. 2006;176:325–7. doi: 10.1016/S0022-5347(06)00303-X. [DOI] [PubMed] [Google Scholar]
- 8.Hagstroem S, Rittig S, Kamperis K, et al. Timer watch assisted urotherapy in children: A randomized controlled trial. J Urology. 2010;184:1482–8. doi: 10.1016/j.juro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Hagstroem S, Rittig N, Kamperis K, et al. Treatment outcome of day-time urinary incontinence in children. Scand J Urol of Urol Nephrol. 2008;42:528–33. doi: 10.1080/00365590802098367. [DOI] [PubMed] [Google Scholar]
- 10.Caldwell PH, Kerr M, Hamilton S, et al. An alarm watch for daytime urinary incontinence: A randomized controlled trial. Pediatrics. 2022;149:e2021053863. doi: 10.1542/peds.2021-053863. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [Google Scholar]
- 13.Software CSR. Covidence systematic review software. Veritas Health Innovation; Melbourne: 2018. [Google Scholar]
- 14.Sterne JA, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016. p. 355i4919. [DOI] [PMC free article] [PubMed]
- 16.The Cochrane Collaboration. Manager R. Version 5.4. The Nordic Cochrane Centre Copenhagen; Denmark: 2020. [Google Scholar]
- 17.Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Halliday S, Meadow S, Berg I. Successful management of daytime enuresis using alarm procedures: A randomly controlled trial. Arch Disease Child. 1987;62:132–7. doi: 10.1136/adc.62.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostaszkiewicz J, Johnston L, Roe B. Timed voiding for the management of urinary incontinence in adults. Cochrane Database Syst Rev. 2004;2004:CD002802. doi: 10.1002/14651858.CD002802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.