Abstract
Introduction
Remdesivir seems to reduce the risk of hospitalization and improve clinical outcome in hospitalized patients with COVID-19.
Objectives
To compare the clinical outcome of COVID-19 hospitalized patients treated with remdesivir plus dexamethasone versus dexamethasone alone, according to their vaccination status.
Material and methods
A retrospective observational study was carried out in 165 patients hospitalized for COVID-19 from October 2021 to January 2022. Multivariate logistic regression, Kaplan–Meier and the log-rank tests were used to evaluate the event (need for ventilation or death).
Results
Patients treated with remdesivir plus dexamethasone (n = 87) compared with dexamethasone alone (n = 78) showed similar age (60 ± 16, 47–70 vs. 62 ± 37, 51–74 years) and number of comorbidities: 1 (0–2) versus 1.5 (1–3). Among 73 fully vaccinated patients, 42 (47.1%) were in remdesivir plus dexamethasone and 31 (41%) in dexamethasone alone. Patients treated with remdesivir plus dexamethasone needed intensive care less frequently (17.2% vs. 31%; p = 0.002), high-flow oxygen (25.3% vs. 50.0%; p = 0.002) and non-invasive mechanical ventilation (16.1% vs. 47.4%; p < 0.001). Furthermore, they had less complications during hospitalization (31.0% vs. 52.6%; p = 0.008), need of antibiotics (32.2% vs. 59%; p = 0.001) and radiologic worsening (21.8% vs. 44.9%; p = 0.005). Treatment with remdesivir plus dexamethasone (aHR, 0.26; 95% CI: 0.14–0.48; p < 0.001) and vaccination (aHR 0.39; 95% CI: 0.21–0.74) were independent factors associated with lower progression to mechanical ventilation or death.
Conclusions
Remdesivir in combination with dexamethasone and vaccination independently and synergistically protects hospitalized COVID-19 patients requiring oxygen therapy from progression to severe disease or dead.
Keywords: Vaccination, Remdesivir, COVID-19, Hospitalization, Dexamethasone
Abstract
Introducción
Remdesivir parece reducir el riesgo de hospitalización y mejorar el resultado clínico en pacientes hospitalizados con COVID-19.
Objetivos
Comparar el desenlace clínico de pacientes hospitalizados con COVID-19 tratados con remdesivir más dexametasona vs. dexametasona sola, según su estado de vacunación.
Material y métodos
Se realizó un estudio observacional retrospectivo en 165 pacientes hospitalizados por COVID-19 desde octubre de 2021 hasta enero de 2022. Se consideró como evento la necesidad de ventilación o muerte.
Resultados
Los pacientes tratados con remdesivir más dexametasona (n = 87) en comparación con dexametasona sola (n = 78) mostraron una edad similar (60 ± 16, 47-70 vs. 62 ± 37, 51-74 años) y número de comorbilidades: 1 (0-2) vs. 1,5 (1-3). Entre 73 pacientes completamente vacunados, 42 (47,1%) estaban en remdesivir más dexametasona y 31 (41%) en dexametasona sola. Los pacientes tratados con remdesivir más dexametasona necesitaron cuidados intensivos con menos frecuencia (17,2 vs. 31%; p = 0,002), oxígeno de alto flujo (25,3 vs. 50%; p = 0,002) y ventilación mecánica no invasiva (16,1 vs. 47,4%, p < 0,001). Además, tuvieron menos complicaciones durante la hospitalización (31 vs. 52,6%; p = 0,008), necesidad de antibióticos (32,2 vs. 59%; p = 0,001) y empeoramiento radiológico (21,8 vs. 44,9%; p = 0,005). El tratamiento con remdesivir más dexametasona (aHR, 0,26; IC 95% 0,14-0,48; p < 0,001) y la vacunación (aHR 0,39; IC 95% 0,21-0,74>) fueron factores independientes asociados con una menor progresión a ventilación mecánica o muerte.
Conclusiones
Remdesivir en combinación con dexametasona protegieron de forma independiente y sinérgica a los pacientes hospitalizados con COVID-19 que requieren oxigenoterapia de la progresión a la enfermedad grave o la muerte.
Palabras clave: Vacunación, Remdesivir, COVID-19, Hospitalización, Dexametasona
Introduction
After more than two years since the outbreak of the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection and a titanic vaccination effort, coronavirus disease 2019 (COVID-19) continues to be a major public health problem worldwide. Although a complete vaccination schedule seems to reduce to a certain extent the transmission of the virus and notably improves the prognosis of patients who require hospitalization,1, 2 high transmissibility of the new variants of SARS-CoV-2 is leading our hospitals and intensive care units (ICUs) to new all-time highs. Unfortunately, and despite having received the complete vaccination schedule, an excessively high percentage of patients still succumbs to the disease.3 For this reason, and until effective drugs are available for the treatment of COVID-19, it is important to continue investigating the usefulness of currently available anti-viral treatments.
Remdesivir has broad-spectrum antiviral action against many single-stranded RNA viruses including the SARS-CoV-2 and the Middle East respiratory syndrome coronavirus (MERS-CoV). Remdesivir shows antiviral activity by binding and blocking the action of more than one target on the SARS-CoV-2, such as the membrane protein (Mprotein), the RNA-dependent RNA polymerase (RDRP), and the main protease (Mprotease).4 Early administration of remdesivir in patients requiring oxygen therapy has been associated with a reduction in clinical progression, duration of the disease4, 5, 6 and mortality.7 However, it is still questionable whether remdesivir reduces the mortality. In fact, the World Health Organization COVID-19 Solidarity Trial for COVID-19 Treatments (SOLIDARITY) did not find a reduction of mortality in patients treated with remdesivir. For this reason, the WHO guidelines did not add remdesivir in their indications for the treatment of COVID-19. Nonetheless, these results could have been influenced by the timing remdesivir was administered. Recently, it has been observed that an early administration of remdesivir (<5 days from the onset of symptoms) would increase its benefits in terms of reducing the need for ventilation and the number of deaths.8 In addition, administered in combination with dexamethasone, it could increase its benefits in patients who require oxygen therapy and in whom a strong inflammatory response has already been established.9 Furthermore, among non-hospitalized patients, early administration of remdesivir has been associated with an 87% reduction in hospitalization/death compared to placebo.10
On the other hand, clinical trials, and most studies with remdesivir, have been carried out with the initial coronavirus variants. Since mutations of coronavirus mostly affect the S protein and not the RNA polymerase, it is expected that the efficacy of remdesivir will be preserved for new variants.11, 12 While new studies are needed to confirm this issue, the role that vaccination may play in the therapeutic efficacy and convenience of using remdesivir in severely COVID-19 hospitalized patients should be further explored.
In this context, our study aims to evaluate the efficacy of remdesivir associated with dexamethasone compared to dexamethasone alone on the treatment of hospitalized COVID-19 patients who require oxygen therapy, at the time of the sixth wave of COVID-19 in Spain. At this time, the delta and omicron variants were dominant in a population with more than 80% of complete vaccination schedule.13
Methods
Study design, participants, setting and eligibility
This retrospective observational cohort study developed in Murcia (Spain) between October 2021 and January 2022 included 165 consecutive adult patients admitted to the Reina Sofia Hospital and Los Arcos del Mar Menor Hospital for a clinical syndrome consistent with acute COVID-19 and a positive SARS-CoV-2 PCR test within 10 days after symptom onset. Patients were included consecutively and distributed into two groups: patients receiving Remdesivir plus dexamethasone (n = 87) or dexamethasone alone (n = 78).
In accordance with the Spanish Agency of Medicines (AEMPS),14 all COVID-19 adult patients requiring non-invasive supplemental O2-therapy were eligible for treatment with remdesivir and dexamethasone. Patients eligible for remdesivir treatment were also required to have a COVID-19 symptom duration of no more than 10 days. Exclusion criteria were requirement of high-flow nasal cannula, or non-invasive ventilation, invasive mechanical ventilatory support, vasoactive drugs, ECMO, transaminase elevations greater than 5-fold and those with an eGFR lower than 30 mL/min.
Remdesivir was administered by IV, at a dose of 200 mg on the first day and then 100 mg for a total of 5 days. Dexamethasone was administered at a dose of 6 mg/day for 10 days. The two drugs in the combination group were started on the same day. All patients received the same supportive standard of care. In particular, prophylaxis with low molecular weight heparin at therapeutic dosage was set when necessary. Specific antibiotic therapy was carried out in the presence of bacterial infection or empirically when an infection was suspected. All active comorbidities were treated according to their respective guidelines. Based on the degree of hypoxemia, patients received O2-therapy via nasal cannula, venturi mask, high-flow nasal cannula, or non-invasive ventilation (NIV).
Patients were also differentiated by their vaccination status. Vaccinated patients included those with ‘complete vaccination schedule’ that met the following criteria: (1) for Pfizer vaccine, the minimum period between the two doses was ≥19 days and ≥7 days after the second dose; (2) for Moderna vaccine, the minimum period between doses was 25 days and 14 days after the second dose; (3) for AstraZeneca vaccine, the minimum period between doses was 21 days and 14 days after the second dose; and (4) for Janssen vaccine, the minimum period was 14 days before infection. Details of COVID-19 vaccination, including dates and location, vaccine product, and lot number, were ascertained through a systematic process including patient or proxy interview and source verification. Sources of documentation included vaccination cards, hospital records, vaccine state registries, and vaccine records requested from clinics and pharmacies. Vaccine doses were classified as administered if source documentation was identified or if the patient or proxy reported a vaccine dose with a plausible date and location of vaccination.
Demographic, clinical and laboratory data were collected by trained personnel through standardized participant (or proxy) interviews and medical record reviews. The study conformed principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines and was approved by the local Ethics Committee (‘Comité Ético de Investigación Clínica del Hospital General Universitario Reina Sofía de Murcia’).
Laboratory analysis
Upper respiratory specimens were collected from enrolled patients, refrigerated at 4 °C, and shipped to a central laboratory at Reina Sofia and Los Arcos del Mar Menor Hospitals (Murcia, Spain).2, 15 Reverse transcriptase-polymerase chain reaction (RT-RCP) was routinely used to confirm diagnosis. Detection of IgG antibodies to SARS-CoV-2 in human serum was made with the SARS-CoV-2 IgG assay, a chemiluminescent microparticle immunoassay (CMIA). The Alinity i system calculates the calibrator mean chemiluminescent signal from 3 calibrator replicates and stores the result. Results are reported by dividing the sample result by the stored calibrator result. The default result unit for the SARS-CoV-2 IgG assay is Index (S/C). Determination of Ferritin and CRP were analyzed with an ADVIA® system and a Dimension Xpand Plus® system (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA), respectively. D-dimer was analyzed with the ACL-TOP 500 CTS® system (Werfen, Barcelona, Spain).
COVID-19 severity classification
We collected data on severity for patients hospitalized with COVID-19. These outcome data were collected until the earlier of hospital discharge or 35 days after hospital admission. The primary classification of disease severity was a binary measure that divided patients into those who experienced death or invasive or non-invasive mechanical ventilation (progression to high disease severity) and those who did not progress (no progression to high disease severity).
As a secondary assessment, we classified COVID-19 severity using a modified version of the World Health Organization COVID-19 Clinical Progression Scale, a commonly used ordinal scale for assessing COVID-19 severity that ranges from uninfected (level 0) and infected but asymptomatic (level 1) to death (level 6). We classified severity according to the highest ordinal level that the patient experienced during the first 35 days of hospitalization. In this analysis of hospitalized patients, the highest severity level experienced could range from level 3 to 6, including hospitalized with standard supplemental oxygen (level 3), with high-flow nasal cannula or noninvasive ventilation (level 4), with invasive mechanical ventilation (level 5) and in-hospital death (level 6).
We also evaluated in-hospital treatments administered for severe COVID-19 during admission (corticosteroids, dexamethasone 20 mg bolus, tocilizumab, baricitinib, or low molecular weight heparin prophylaxis (LMWH)) according to a binary category of no COVID-19 treatments versus 1 or more.
Statistical analysis
A descriptive analysis of patients’ characteristics was carried out using frequency tables for categorical variables. Mean and standard deviation (SD) were used for continuous variables. Differences in categorical variables between patients receiving remdesivir plus dexamethasone versus those receiving dexamethasone alone were assessed through the chi-squared test or Fisher test, and t-student tests for continuous variables.
Among patients hospitalized with COVID-19, the association between progression to non-invasive/invasive mechanical ventilation or death and treatment with remdesivir plus dexamethasone was calculated using multivariable Cox regression adjusted for the variables that had p < 0.1 in the univariate analysis: vaccination, comorbidities, chronic kidney disease, cardiovascular disease, age, SOFA (Sepsis related Organ Failure Assessment) score and remdesivir use. The presence of collinearity was evaluated, and those variables that were collinear were not included in the analysis.
To assess the impact of antibody levels on clinical evolution a logarithmic transformation of the antibody titer was made.
The association between just death and remdesivir use was calculated with multivariable logistic regression analysis evaluating the odds of vaccination among patients with COVID-19 who died versus those that survived.
Kaplan–Meier curves were built to compare time to disease progression between patients in the remdesivir plus dexamethasone group and those in the dexamethasone alone group. Nonparametric (log-rank) tests were used to compare event-free survival functions in the 2 study groups.
P < 0.05 was considered statistically significant. The statistical analysis was conducted using IBM SPSS Statistics software, version 23.0 (IBM Corp., Armonk, New York) and R free software.
Results
Clinical and biological characteristics of COVID-19 patients
A total of 165 COVID-19 patients mostly infected with the SARS-CoV-2 delta (B.1.617.2) variant were included in the study. Patients who were not infected with delta were infected with omicron (B.1.1.529). The data on the type of variant are estimates considering epidemiological data since sequencing has not been performed in all patients. When 90% of patients were admitted, at that time, the dominant variant was delta.
The basal characteristics of patients are shown in Table 1 . Patients’ mean age was 61 years, 53.9% were men, 81% were Spaniards, and 75.1% had one or more comorbidities. Patients treated with remdesivir plus dexamethasone (n = 87) compared with dexamethasone alone (n = 78) showed similar age (60 ± 16 vs. 62 ± 37 years) and number of comorbidities: 1 (0–2) versus 1.5 (1–3). However, patients treated with remdesivir were less likely to have cardiovascular disease (14.9% vs. 29.5%, p = 0.038) and chronic kidney disease (3.4% vs. 15.4%, p = 0.017).
Table 1.
Patients’ characteristics and clinical evolution according to treatment received.
| Dexamethasone alone (n = 78) | Remdesivir and dexamethasonea (n = 87) | p | |
|---|---|---|---|
| Demographics and clinical background | |||
| Male sex, n (%) | 47 (60.3) | 42 (48.3) | 0.166 |
| Age, years, mean (SD, range) | 62.3 ± 17.4 (47–70) | 60.0 ± 16.2 (51–74) | 0.396 |
| Age, years range, n (%) | |||
| <40 | 12 (15.4) | 11 (12.6) | 0.672 |
| 40–59 | 18 (23.1) | 25 (28.7) | |
| 60–79 | 34 (43.6) | 40 (46.0) | |
| ≥80 | 14 (17.9) | 11 (12.6) | |
| Origin not Spain (%) | 10 (13.0) | 19 (21.8) | 0.201 |
| Complete vaccinationa | 32 (41) | 42 (47.1) | 0.528 |
| Chronic medical conditionsb, median (IQR) | 1.5 [1.0, 3.0] | 1.0 [0.0, 2.0] | 0.094 |
| Hypertension, n (%) | 41 (52.6) | 39 (44.8) | 0.403 |
| Type 2 Diabetes, n (%) | 22 (28.2) | 24 (27.6) | 1.000 |
| Obesity (body mass index ≥30), n (%) | 25 (32.1) | 24 (27.6) | 0.648 |
| Current tobacco use, n (%) | 9 (11.5) | 8 (9.2) | 0.180 |
| Cardiovascular disease, n (%) | 23 (29.5) | 13 (14.9) | 0.038 |
| Chronic kidney disease, n (%) | 12 (15.4) | 3 (3.4) | 0.017 |
| Pulmonary disease, n (%) | 14 (17.9) | 17 (19.5) | 0.951 |
| Liver disease, n (%) | 7 (9.0) | 2 (2.3) | 0.123 |
| Dementia, n (%) | 5 (6.4) | 2 (2.3) | 0.357 |
| Immunocompromised conditionc, n (%) | 4 (5.1) | 3 (3.4) | 0.883 |
| COVID-19 status at admission | |||
| Days from 1st symptom to admission (mean ± SD) | 6.4 ± 3.9 | 5.4 ± 2.7 | 0.047 |
| CURB-65, n (%) | |||
| 0 | 17 (21.8) | 13 (14.9) | 0.335 |
| 1 | 23 (29.5) | 37 (42.5) | |
| 2 | 28 (35.9) | 29 (33.3) | |
| 3 | 9 (11.5) | 8 (9.2) | |
| 4 | 1 (1.3) | 0 (0.0) | |
| Chest radiology, n (%) | |||
| No infiltrates | 4 (5.1) | 5 (5.7) | 0.329 |
| Unilateral infiltrates | 4 (5.1) | 10 (11.5) | |
| Bilateral infiltrates | 70 (89.7) | 72 (82.8) | |
| SARS-CoV-2 antibody status at admissiond | |||
| SARS-CoV-2 S antibody positive, n (%) | 30 (66.7) | 38 (52.8) | 0.197 |
| SARS-CoV-2 N antibody positive, n (%) | 15 (33.3) | 18 (25.0) | 0.445 |
| Log10 SARS-CoV-2 N antibody UI/ml (mean ± SD) | −0.44 ± 0.9 | −0.45 ± 0.81 | 0.948 |
| Log10 SARS-CoV-2 S antibody UI/ml (mean ± SD) | 2.85 ± 1.46 | 2.55 ± 1.37 | 0.278 |
| Treatments | |||
| Dexamethasone boluses (20 mg/d), n (%) | 43 (55.1) | 36 (41.4) | 0.108 |
| Tocilizumab, n (%) | 20 (25.6) | 18 (20.7) | 0.569 |
| Baricitinib, n (%) | 0 (0.0) | 7 (8.0) | 0.030 |
| LMHW use (%) | 78 (100) | 86(98) | 1 |
| Antibiotics (%) | 46 (59.0) | 28 (32.2) | 0.001 |
| Clinical evolution | |||
| Hospital mean stay (mean ± SD) | 11.1 ± 7.3 | 10.1 ± 6.3 | 0.347 |
| Complications during hospitalizatione, n (%) | 41 (52.6) | 27 (31.0) | 0.008 |
| Radiological worsening | 35 (44.9) | 19 (21.8) | 0.005 |
| ICU admission, n (%) | 31 (40.3) | 15 (17.2) | 0.002 |
| CRP levels, mg/dl median (IQR) | 9.75 [4.9, 13.5] | 7.9 [3.8, 14.8] | 0.297 |
| D-dimer levels, ng/mL, median (IQR) | 1,152 [686, 2445] | 827 [555, 1267] | 0.006 |
| Higher ferritin levels, ng/mL, median (IQR) | 537 [278, 1048] | 438 [268, 849] | 0.253 |
| SaO2/FiO2mmHg (mean ± SD) | 254 ± 135 | 317 ± 113 | 0.002 |
| PaO2/FiO2less than 200 mmHg, n (%) | 29 (46.8) | 18 (22.2) | 0.004 |
| Need for high-flow oxygen, n (%) | 39 (50.0) | 22 (25.3) | 0.002 |
| Need for ventilation, n (%) | 37 (47.4) | 14 (16.1) | <0.001 |
| Need for non-invasive ventilation, n (%) | 37 (47.4) | 14 (16.1) | <0.001 |
| Need for invasive ventilation, n (%) | 10 (12.8) | 5 (5.7) | 0.191 |
| Death, n (%) | 11 (14.1) | 2 (2.3) | 0.012 |
| Time from admission to eventf, days (mean ± SD) | 5.47 (5.38) | 7.75 (5.00) | 0.006 |
| WHO ordinal scale adapted, n (%) | |||
| 2 | 24 (24.0) | 24 (28.6) | 0.006 |
| 3 | 35 (35.0) | 44 (52.4) | |
| 4 | 23 (23.0) | 12 (14.3) | |
| 5 | 8 (8.0) | 4 (4.8) | |
| 6 | 10 (10.0) | 0 (0.0) | |
LMWH: low molecular weight heparin; ICU: intensive care unit; SOFA: sepsis related organ failure assessment.
Complete vaccination when met the following criteria: for Pfizer vaccine, the minimum period between the two doses must be ≥19 days and the period after the 2nd dose to entail risk ≥7 days. For Moderna vaccine, a minimum period between doses of 25 days and a period after the 2nd dose of 14 days. For AstraZeneca vaccine, a minimum period between doses of 21 days and a period after the 2nd dose of 14 days. Finally, for Janssen vaccine, a period of 14 days after vaccination.
Chronic medical conditions included the following: cardiovascular disease, neurologic disease, pulmonary disease, hepatic disease, endocrine disease, kidney disease, hematologic disease, malignancy and immunosuppression.
Immunocompromised condition included active solid cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months), active hematologic cancer (such as leukemia, lymphoma, or myeloma), HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive medication, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease (Crohn disease or ulcerative colitis).
SARS-CoV2 serology was determined in 117 patients (72 in remdesivir plus dexamethasone and 45 in dexamethasone alone).
Complications during hospitalization: deep vein thrombosis or pulmonary thromboembolism, bacterial infection, acute renal failure, heart failure, stroke, acute myocardial infarction and elevated transaminases.
Event: need for ventilation or death.
The median days from symptom onset to remdesivir prescription was 5.41 ± 2.67 days; 41 patients (47.1%) received remdesivir in the first 5 days from symptom onset. In the dexamethasone alone cohort, the median days from symptom onset to treatment was 6.45 ± 3.92 days.
Among 73 fully vaccinated patients, 49 (67.1%) received the RNAm vaccines (BNT162b2 or the mRNA-1273 vaccine); 6 (8.2%) Ad26.COV2-S and 12 (16.4%) ChAdOx1-S vaccine. These patients were distributed equally between patients treated with remdesivir plus dexamethasone (n = 42, 47.1%) and dexamethasone alone (n = 31, 41%).
Hospitalized patients receiving remdesivir progressed to severe COVID-19 less frequently
Compared remdesivir plus dexamethasone with patient treated with dexamethasone alone, patients treated with remdesivir plus dexamethasone needed ICU care less frequently (17.2% vs. 31%; p = 0.002), high flow oxygen (25.3% vs. 50.0%; p = 0.002) and non-invasive mechanical ventilation (16.1% vs. 47.4%; p < 0.001). Furthermore, they had less complications during hospitalization (31.0% vs. 52.6%; p = 0.008), less need to use antibiotics (32.2% vs. 59%; p = 0.001) and less radiologic worsening (21.8% vs. 44.9%; p = 0.005).
Patients treated with dexamethasone alone accounted for 74.1% (40/51) of cases with disease progression to mechanical ventilation or death. The composite of patients with mechanical ventilation or death was 14 of 87 (16.1%) in patients treated with remdesivir plus dexamethasone and 40 of 78 (51.3%) in patients treated with dexamethasone alone (absolute difference of −35.2%; 95% CI, −16.9 to −28.4%; p < 0.001). The event of mechanical ventilation or death in patients hospitalized for COVID-19 was associated with a higher SOFA score (aOR, 1.45; 95% CI, 1.11–1.89; p = 0.006) and inversely with vaccination (aOR, 0.253; 95% CI, 0.11–0.57; p = 0.001) and treatment with remdesivir plus dexamethasone (aOR, 0.219; 95% CI, 0.1–0.48; p < 0.001). Death occurred in 2 of 87 (2.3%) patients in the remdesivir plus dexamethasone group and in 11 of 78 (14.1%) patients in the dexamethasone alone group (OR, 0.143; 95% CI, 0.031–0.669; p = 0.005).
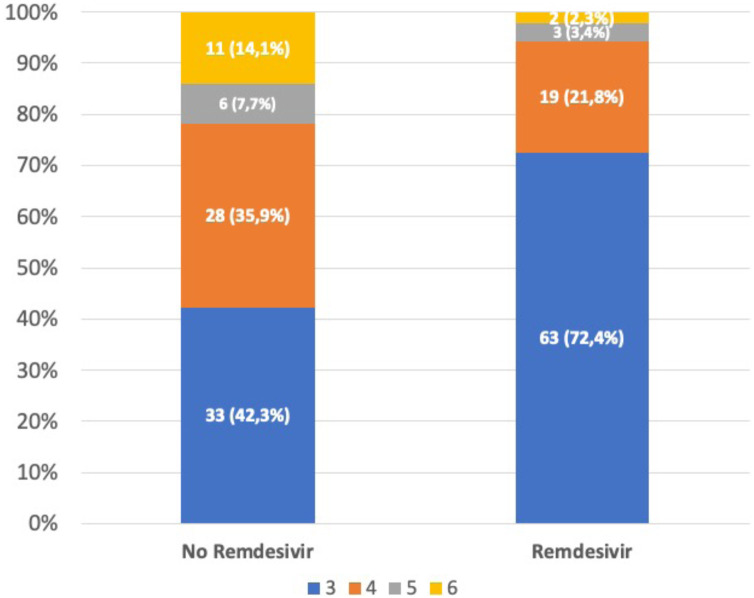
According to the modified World Health Organization COVID-19 Clinical Progression Scale, the highest level (4, 5 or 6) of disease severity was experienced significantly less frequently in patients treated with remdesivir plus dexamethasone than in those treated with dexamethasone alone (27.6% vs. 57.7%; p < 0.001) (Fig. 1 ).
Fig. 1.
Highest severity level experienced on the WHO COVID-19 Clinical Progression Scale during the first 35 days of hospitalization among patients treated with just dexamethasone or remdesivir plus dexamethasone. The highest level (4, 5 or 6) of disease severity was significantly lower among patients treated with remdesivir (27.6% vs. 57.7%; p < 0.001).
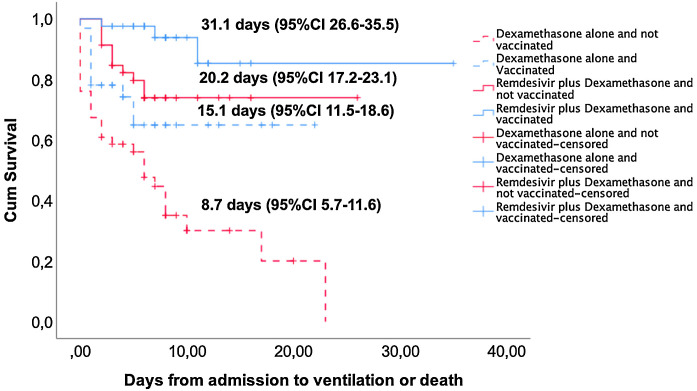
Kaplan–Meier curves show that unvaccinated patients treated with dexamethasone alone had the shortest mean event-free survival (event: need for mechanical ventilation or death): 8.7 days (95% CI: 5.7–11.6) compared to 15.1 days (95% CI: 11.5–18.6) in vaccinated patients treated with dexamethasone alone, 20.2 days (95% CI: 17.2–23.1) in unvaccinated patients treated with remdesivir plus dexamethasone, and 31.1 days (95% CI: 26.6–35.5) in vaccinated patients treated with remdesivir plus dexamethasone (Fig. 2 ). Patients treated with remdesivir plus dexamethasone had significantly higher event-free survival rate at day 35 than those treated with dexamethasone alone (83.9% vs. 48.7%, p < 0.001). Complete vaccination status versus no vaccination was also associated with higher event-free survival rate at day 35 both in the remdesivir plus dexamethasone (92.7% vs. 76.1%; p = 0.033) and in the dexamethasone alone (68.8% vs. 34.8%; p = 0.003) group.
Fig. 2.
Kaplan–Meier and log-rank tests for event (need for ventilation or death)-free survival according to the type of treatment and vaccination status. Mean time (days) to event is indicated for each group, p < 0.001 (log-rank).
Differential characteristics between patients with or without clinical events
As expected, among patients with less favorable clinical evolution, complete vaccination schedule was less frequent than in patients with a more favorable outcome (24.1% vs. 54.1%, p < 0.001). Besides, patients with unfavorable outcome showed lower PaO2/FiO2 at admission (417.9 ± 66.2 mmHg vs. 367.9 ± 110.5 mmHg, p = 0.002), higher SOFA score (2.0 ± 1.9 vs. 1.04 ± 1.2, p < 0.001), and higher frequency of bilateral infiltrates (98.1% vs. 80.2%, p = 0.007). Consequently, higher frequency of high-dose dexamethasone treatment (87% vs. 28.8%, p < 0.001) was required in these patients. Besides, all parameters related with clinical evolution, including hospital mean stay, complications, radiological worsening, ICU admission, highest D-dimer and ferritin levels, and lowest SaO2/FiO2 were worse in patients requiring ventilation or who died during the follow-up (see Table 2 for details).
Table 2.
Characteristics of patients according to their outcome.
| No events (N = 111) | Ventilation or death (N = 54) | p | |
|---|---|---|---|
| Demographics and clinical background | |||
| Male sex, n (%) | 56 (50.5) | 33 (61.1) | 0.262 |
| Age, years (DE and range) | 61.8 (16.7, 47–73) | 59.5 (16.7, 49–68) | 0.415 |
| Age, years range, n (%) | |||
| <40 | 14 (12.6) | 9 (16.7) | 0.527 |
| 40–59 | 26 (23.4) | 17 (31.5) | |
| 60–79 | 53 (47.7) | 21 (38.9) | |
| ≥80 | 18 (16.2) | 7 (13.0) | |
| Complete vaccinationa | 60 (54.1) | 13 (24.1) | 0.001 |
| Origin not Spain (%) | 17 (15.5) | 12 (22.2) | 0.395 |
| Chronic medical conditionsb, median (IQR) | 1.0 [0.0, 2.5] | 1.0 [1.0, 3.0] | 0.427 |
| Hypertension, n (%) | 56 (50.5) | 24 (44.4) | 0.577 |
| Type 2 Diabetes, n (%) | 34 (30.6) | 12 (22.2) | 0.345 |
| Obesity (body mass index ≥30), n (%) | 28 (25.2) | 21 (38.9) | 0.105 |
| Current tobacco use, n (%) | 10 (9.0) | 7 (13.0) | 0.382 |
| Cardiovascular disease, n (%) | 21 (18.9) | 15 (27.8) | 0.275 |
| Chronic kidney disease, n (%) | 9 (8.1) | 6 (11.1) | 0.733 |
| Pulmonary disease, n (%) | 20 (18.0) | 11 (20.4) | 0.880 |
| Liver disease, n (%) | 5 (4.5) | 4 (7.4) | 0.685 |
| Dementia, n (%) | 5 (4.5) | 2 (3.7) | 1.000 |
| Immunocompromised conditionc, n (%) | 3 (2.7) | 4 (7.4) | 0.320 |
| COVID-19 status at admission | |||
| Days from symptom onset to admission (mean ± SD) | 5.9 ± 3.2 | 5.8 ± 3.6 | 0.739 |
| CURB-65, n (%) | |||
| 0 | 20 (18.0) | 10 (18.5) | 0.202 |
| 1 | 43 (38.7) | 17 (31.5) | |
| 2 | 40 (36.0) | 17 (31.5) | |
| 3 | 8 (7.2) | 9 (16.7) | |
| 4 | 0 (0.0) | 1 (1.9) | |
| SOFA >2, n (%) | 30 (27.3) | 30 (56.6) | 0.001 |
| Chest radiology, n (%) | |||
| No infiltrates | 9 (8.1) | 0 (0.0) | 0.007 |
| Unilateral infiltrates | 13 (11.7) | 1 (1.9) | |
| Bilateral infiltrates | 89 (80.2) | 53 (98.1) | |
| SARS-CoV-2 antibody status at admissiond | |||
| SARS-CoV-2 antibody positive, n (%) | 52 (62.7) | 16 (47.1) | 0.178 |
| SARS-CoV-2 N antibody positive, n (%) | 24 (28.9) | 9 (26.5) | 0.968 |
| Log10 SARS-CoV-2 N antibody UI/ml (mean ± SD) | −0.35 ± 0.76 | −0.69 ± 0.98 | 0.082 |
| Log10 SARS-CoV-2 S antibody UI/ml (mean ± SD) | 2.86 ± 1.35 | 2.14 ± 1.43 | 0.019 |
| Treatments | |||
| Remdesivir, n (%) | 73 (65.8) | 14 (25.9) | <0.001 |
| Dexamethasone boluses (20 mg/d), n (%) | 32 (28.8) | 47 (87.0) | <0.001 |
| Tocilizumab, n (%) | 17 (15.3) | 21 (38.9) | 0.001 |
| Baricitinib, n (%) | 6 (5.4) | 1 (1.9) | 0.515 |
| LMHW use (%) | 108 (98) | 54 (100) | 1 |
| Clinical evolution | |||
| Hospital mean stay (mean ± SD) | 8.2 ± 5.1 | 15.5 ± 7.2 | <0.001 |
| Complications during hospitalizatione, n (%) | 38 (34.2) | 30 (55.6) | 0.015 |
| Radiological worsening | 27 (24.3) | 27 (50.0) | <0.001 |
| ICU admission, n (%) | 3 (2.7) | 43 (81.1) | <0.001 |
| CRP levels, mg/dl, median (IQR) | 8.3 [3.9, 12.9] | 9.6 [4.9, 17.6] | 0.100 |
| D-dimer levels, ng/mL median (IQR) | 750 [484, 1238] | 1542 [1071, 3901] | <0.001 |
| Ferritin levels, ng/mL median (IQR) | 469 [263, 838] | 650 [328,1332] | 0.054 |
| SaO2/FiO2mmHg (mean ± SD) | 349 ± 89 | 153 ± 87 | <0.001 |
| Need for high-flow oxygen, n (%) | 15 (13.5) | 46 (85.2) | <0.001 |
| Need for ventilation, n (%) | 0 (0.0) | 51 (94.4) | <0.001 |
| Need for non-invasive mechanical ventilation, n (%) | 1 (0.9) | 50 (92.6) | <0.001 |
| Need for invasive mechanical ventilation, n (%) | 0 (0.0) | 15 (27.8) | <0.001 |
| Death, n (%) | 0 (0.0) | 13 (24.1) | <0.001 |
| Time from admission to event, days (mean ± SD) | 8.16 (5.07) | 3.61 (4.38) | <0.001 |
LMHW: low molecular weight heparin; ICU: intensive care unit; SOFA: sepsis related organ failure assessment.
Complete vaccination when met the following criteria: for Pfizer vaccine, the minimum period between the two doses must be ≥19 days and the period after the 2nd dose to enter risk ≥7 days. For Moderna vaccine, a minimum period between doses of 25 days and a period after the 2nd dose of 14 days. For AstraZeneca vaccine, a minimum period between doses of 21 days and a period after the 2nd dose of 14 days. Finally, for Janssen vaccine, a period of 14 days after vaccination.
Chronic medical conditions included the following: cardiovascular disease, neurologic disease, pulmonary disease, hepatic disease, endocrine disease, kidney disease, hematologic disease, malignancy and immunosuppression.
Immunocompromising conditions included active solid organ cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in the past 6 months), active hematologic cancer (such as leukemia, lymphoma, or myeloma), HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive medication, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease (Crohn disease or ulcerative colitis).
SARS-CoV2 serology was determined in 117 patients (83 in no events and 34 in events).
Complications during hospitalization: deep vein thrombosis or pulmonary thromboembolism, bacterial infection, acute renal failure, heart failure, stroke, acute myocardial infarction and elevated transaminases.
In contrast, no significant differences in the positivity for SARS-CoV-2 antibody (28.9% vs. 26.5%, p = 0.968) or SARS-CoV-2 S antibody titer (10,569 ± 16,221 vs. 5720 ± 13,143; p = 0.125) were detected between patients with worse or better clinical outcome.
Remdesivir treatment and vaccination are independent variables associated with a favorable outcome in COVID-19 hospitalized patients
Table 3 shows the Cox regression analysis including age, SOFA score, cardiovascular and chronic kidney diseases, vaccination status, and treatment with remdesivir. Both treatment with remdesivir plus dexamethasone (aHR, 0.26; 95% CI 0.14–0.48; p < 0.001) and vaccination status (aHR 0.39; 95% CI 0.21–0.74) were independent factors associated with lower progression to mechanical ventilation or death.
Table 3.
Cox regression of factors associated with ventilation or death.
| HR | 95% CI | p | aHR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Age | 0.97 | 0.95–0.99 | 0.03 | |||
| SOFA score | 1.20 | 0.98–1.47 | 0.06 | |||
| Cardiovascular disease | 2.50 | 1.04–6.02 | 0.04 | |||
| Chronic kidney disease | 2.67 | 0.91–7.81 | 0.07 | |||
| Comorbidities number | 1.14 | 0.85–1.53 | 0.37 | |||
| Vaccinated | 0.51 | 0.24–1.05 | 0.07 | 0.39 | 0.21–0.74 | 0.004 |
| Remdesivir | 0.27 | 0.14–0.51 | <0.001 | 0.26 | 0.14–0.48 | <0.001 |
HR: hazard ratio; aHR: adjusted hazard ratio; SOFA score: sepsis related organ failure assessment.
Discussion
The usefulness of remdesivir in the treatment of patients hospitalized for COVID-19 has been the subject of extensive literature in the last 2 years, and the results continue to be contradictory. This is probably due to the heterogeneity of the studies in terms of type of patient, timing of remdesivir administration, use of concomitant drugs, virus variants and vaccination status, among others.1, 3, 16 Although the SOLIDARITY trial did not find a reduction of mortality in patients treated with remdesivir,3 more recent results seem to indicate that an early administration of remdesivir (<5 days from the onset of symptoms) would increase its benefits in terms of reducing the need for ventilation and the number of deaths.8, 17 Although the number of patients included in our study is limited, the data support that treatment with remdesivir plus dexamethasone is associated with lower risk of progression to severe disease in hospitalized patients for COVID-19 requiring supplemental oxygen therapy, irrespective of their vaccination status or if they were infected with the new variants delta and omicron. Data show that treatment with remdesivir plus dexamethasone significantly reduces the need for ventilation and mortality at day 35 in comparison to treatment with dexamethasone alone. These results confirm those of Marrone et al.9 showing in a comparable prospective study that patients treated with remdesivir plus dexamethasone had greater and faster improvement of inflammatory and respiratory parameters and lower mortality at day 30, compared with dexamethasone alone. However, in contrast with studies that circumscribe the utility of remdesivir if administered in the first 3–5 days of symptoms onset,8, 17 in our study clinical benefit of remdesivir were observed even if introduced beyond the ninth day, although the sample size or the higher severity of patients from our series compared to others10 could have influenced these results.
In our study, patients treated with remdesivir did not show differences in terms of the maximum peak of C-reactive protein and ferritin inflammatory markers. However, these patients had lower levels of D-dimer, an inflammatory marker that has been associated with increased risk of thrombosis and worse prognosis in COVID-19.18 Although the mechanisms underlying this association are largely unknown, remdesivir could contribute to reduce virus replication and, in combination with dexamethasone, could jointly modulate the inflammatory response observed in SARS-COV2 infection, the so-called ‘cytokine storm’ related to the severity of the disease.19 In fact, patients treated with remdesivir in our series had less radiological worsening, lower probability of developing ARDS (had fewer complications), less high-flow oxygen or mechanical ventilation requirements and ICU admission compared with dexamethasone alone.
Besides, a notable point in our study is that patients treated with remdesivir and dexamethasone had fewer bacterial infections and, therefore, a lower need for antibiotic use. This important result, not previously reported, justifies by itself the use of this combined therapy in patients who need oxygen therapy. The control of viral replication together with the modulation of the inflammatory response could have a beneficial effect in this regard.
Another important contribution of our study is that, for the first time, it is demonstrated that remdesivir plus dexamethasone improve the results of dexamethasone alone for the new SARS-CoV2 variants both for vaccinated and unvaccinated patients. While in other studies vaccination was an exclusion criteria for remdesivir treatment,10 comparable proportion of fully vaccinated patients were included in both groups of our study. In this setting, although vaccination protected patients from unfavorable outcome, remdesivir independently improved results in both groups of patients. It is noteworthy, as shown in Fig. 2, that there are statistically significant differences in the four treatment levels and that adding remdesivir to dexamethasone in both vaccinated and unvaccinated patients achieves a very significant improvement in clinical evolution. Therefore, this treatment should be recommended in all patients requiring oxygen therapy regardless of their vaccination status.
One of the main limitations of this study is that it is a retrospective design, so some measurements may not have been collected. However, all admitted patients followed a strict protocol where the same variables were evaluated, so it was rare for measurements to be lost. We rectified the selection bias that the study design may introduce by choosing all the patients who entered consecutively. In addition, a sample calculation was made to be as accurate as possible. With an alpha risk of 0.05 and beta risk of 0.20, and at least 73 subjects were required in each group.
Despite patients treated with dexamethasone alone presented slightly higher frequency of comorbidities in our series, remdesivir in combination with dexamethasone, administered in the early days of symptoms onset in COVID-19 patients requiring oxygen therapy, is an independent favorable prognostic factor associated with lower progression to severe disease and mortality. Therefore, the use of this combination therapy could be considered an effective treatment in these patients with COVID-19 who require oxygen therapy, regardless of their vaccination status, so it should be considered as a treatment option.
Ethical approval
This research work has been approved by the Ethics and Research Committee of the Hospital General Universitario Reina Sofía.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Authors state no conflict of interest.
References
- 1.Ghazy R.M., Ashmawy R., Hamdy N.A., Elhadi Y.A.M., Reyad O.A., Elmalawany D., et al. Efficacy and effectiveness of SARS-CoV-2 vaccines: a systematic review and meta-analysis. Vaccines. 2022;10:350. doi: 10.3390/vaccines10030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernal E., García-Villalba E., Pons E., Vicente M.R., Tomás C., Minguela A., et al. Role of vaccination and anti-SARS-CoV-2 antibodies in the clinical outcome of hospitalized COVID-19 patients. Med Clin (Barc) 2023 doi: 10.1016/j.medcli.2022.12.018. S0025-7753(23)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dessie Z.G., Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan F.I., Kang T., Ali H., Lai D. Remdesivir strongly binds to RNA-dependent RNA polymerase, membrane protein, and main protease of SARS-CoV-2: indication from molecular modeling and simulations. Front Pharmacol. 2021;12:710778. doi: 10.3389/fphar.2021.710778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., et al. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Solidarity Trial Consortium. Pan H., Peto R., Henao-Restrepo A.-M., Preziosi M.-P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for Covid-19 – Interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falcone M., Suardi L.R., Tiseo G., Barbieri C., Giusti L., Galfo V., et al. Early use of remdesivir and risk of disease progression in hospitalized patients with mild to moderate COVID-19. Clin Ther. 2022;44:364–373. doi: 10.1016/j.clinthera.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrone A., Nevola R., Sellitto A., Cozzolino D., Romano C., Cuomo G., et al. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of COVID-19 patients requiring supplemental O2 therapy: a prospective controlled non-randomized study. Clin Infect Dis. 2022;75:e403–e409. doi: 10.1093/cid/ciac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takashita E., Kinoshita N., Yamayoshi S., Sakai-Tagawa Y., Fujisaki S., Ito M., et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. N Engl J Med. 2022;386:995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vangeel L., Chiu W., De Jonghe S., Maes P., Slechten B., Raymenants J., et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. Antiviral Res. 2022;198:105252. doi: 10.1016/j.antiviral.2022.105252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministerio de Sanidad – Profesionales – Cuadro de mando resumen de datos de vacunación s.f.
- 14.Informe de Posicionamiento Terapéutico de remdesivir (Veklury®) en el tratamiento de la COVID-19 s.f.:12.
- 15.García de Guadiana-Romualdo L., Martínez Martínez M., Rodríguez Mulero M.D., Esteban-Torrella P., Hernández Olivo M., Alcaraz García M.J., et al. Circulating MR-proADM levels, as an indicator of endothelial dysfunction, for early risk stratification of mid-term mortality in COVID-19 patients. Int J Infect Dis. 2021;111:211–218. doi: 10.1016/j.ijid.2021.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh A.K., Venkatraman S., Reshetnyak E., Rajan M., An A., Chae J.K., et al. Association between city-wide lockdown and COVID-19 hospitalization rates in multigenerational households in New York City. PLoS ONE. 2022;17:e0266127. doi: 10.1371/journal.pone.0266127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Vidal C., Alonso R., Camon A.M., Cardozo C., Albiach L., Agüero D., et al. Impact of remdesivir according to the pre-admission symptom duration in patients with COVID-19. J Antimicrob Chemother. 2021;76:3296–3302. doi: 10.1093/jac/dkab321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Lyu X., Li D., Wang L., Wang Y., Zou W., et al. A cohort study of 676 patients indicates D-dimer is a critical risk factor for the mortality of COVID-19. PLoS ONE. 2020;15:e0242045. doi: 10.1371/journal.pone.0242045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manjili R.H., Zarei M., Habibi M., Manjili M.H. COVID-19 as an acute inflammatory disease. J Immunol. 2020;205:12–19. doi: 10.4049/jimmunol.2000413. [DOI] [PMC free article] [PubMed] [Google Scholar]