Abstract
Objectives
Vaccine effectiveness against transmission (VET) of SARS-CoV-2-infection can be estimated from secondary attack rates observed during contact tracing. We estimated VET, the vaccine-effect on infectiousness of the index case and susceptibility of the high-risk exposure contact (HREC).
Methods
We fitted RT-PCR-test results from HREC to immunity status (vaccine schedule, prior infection, time since last immunity-conferring event), age, sex, calendar week of sampling, household, background positivity rate and dominant VOC using a multilevel Bayesian regression-model. We included Belgian data collected between January 2021 and January 2022.
Results
For primary BNT162b2-vaccination we estimated initial VET at 96% (95%CI 95–97) against Alpha, 87% (95%CI 84–88) against Delta and 31% (95%CI 25–37) against Omicron. Initial VET of booster-vaccination (mRNA primary and booster-vaccination) was 87% (95%CI 86–89) against Delta and 68% (95%CI 65–70) against Omicron. The VET-estimate against Delta and Omicron decreased to 71% (95%CI 64–78) and 55% (95%CI 46–62) respectively, 150–200 days after booster-vaccination. Hybrid immunity, defined as vaccination and documented prior infection, was associated with durable and higher or comparable (by number of antigen exposures) protection against transmission.
Conclusions
While we observed VOC-specific immune-escape, especially by Omicron, and waning over time since immunization, vaccination remained associated with a reduced risk of SARS-CoV-2-transmission.
Keywords: Vaccine Effectiveness, mRNA-vaccine, Viral-vector vaccine, SARS-CoV-2, Alpha Variant of Concern, Delta Variant of Concern, Omicron Variant of Concern, Transmission, Infection-acquired immunity, Vaccine-induced immunity, Infectiousness, Susceptibility
1. Introduction
In an effort to reduce the spread and severity of COVID-19, Belgium started the roll-out of a large-scale vaccination campaign at the end of 2020. After prioritizing nursing home residents, healthcare professionals and persons with a high risk of severe COVID-19, the general population, from the oldest to the youngest, was invited to be vaccinated. For primary vaccination, four different vaccines were used: two mRNA-vaccines (BNT162b2, mRNA-1273) and two viral-vector vaccines (Ad26.COV2.S, ChAdOx1). By September 2021, all adults residing in Belgium had received an invitation to complete their primary schedule. Booster-vaccination and, for immunocompromised persons, additional doses were offered from September onwards using almost exclusively mRNA-vaccines. The administration of booster-vaccines continued into 2022.
From January 2021 to January 2022, three periods (waves) with high COVID-19 incidences were observed in Belgium: March-April 2021 (associated with the Alpha variant of concern), October-December 2021 (Delta) and January 2022 (Omicron). Throughout this period, public health authorities continued tracing and testing of high-risk exposure contacts (HREC). Originally set up in May 2020, contact tracing relied on index cases (any person with a laboratory-confirmed SARS-CoV-2-infection) to report HREC (any persons with whom they had close contact; e.g. direct physical contact or more than 15 min contact at <1.5 m). The main indicators and performance metrics of Belgium’s contact tracing during 2021 can be consulted elsewhere [1].
In a study performed in July 2021 [2], we described high initial vaccine effectiveness (VE) against transmission of SARS-CoV-2-infection during high-risk exposure. Vaccines reduced susceptibility of HREC (VEs) and infectiousness of breakthrough cases (VEi). The combined effect (reduced risk of transmission, VET) was estimated at 92% when both persons in a high-risk exposure contact completed BNT162b2 primary-vaccination. Primary-vaccination (typically two doses) outperformed partial (single) dose vaccination and mRNA-vaccines outperformed viral-vector vaccines. In a follow-up study [3], including data until December 2021, we investigated vaccine effectiveness over time during periods of Alpha and Delta-dominance. Vaccine-induced protection, both VEs and VEi, decreased over time. This process is typically referred to as waning. Older age was associated with lower initial VEi and faster waning. For the period of Delta-dominance, we observed reduced VEs and especially reduced VEi compared to the period of Alpha-dominance. Waning and Delta’s ability to escape vaccine-induced immunity reduced VET against Delta to 61% (CI 60–63), 150–200 days after BNT162b2 primary-vaccination. We also quantified the protective effect of a prior infection and found what appeared to be good cross-neutralization from Alpha to Delta. Hybrid immunity (documented prior infection and vaccination) was associated with the largest and most durable reduction in transmission. Belgium’s neighboring countries also reported significantly reduced Delta-transmission after vaccination, lower however than the estimate against Alpha-transmission [4], [5].
From January 2022 onwards, the majority of the Belgian adult population was booster-vaccinated and Omicron became dominant. Both events affected SARS-CoV-2-transmission.. Omicron led to a rapid increase in the number of infections through its transmissibility [6], ability to escape immunity [7], [8] and high propensity for reinfection [9], while booster-vaccination increased the levels of neutralizing antibodies [10]. In this study we aim to investigate Alpha, Delta and Omicron SARS-CoV-2-transmission during high-risk exposure by immunity status (primary vaccination, booster-vaccination or infection) and time since immunization of HREC and index case.
2. Methods
2.1. Data included
We included data on high-risk exposure events from 26 January 2021 to 10 January 2022. During this period, the testing strategy required HREC to be tested twice: a first PCR-test as soon as possible and a second test (when the first test was negative), 7 days after the last contact or as soon as symptoms appeared. A single negative test sufficed during the summer holidays (July-August 2021) and from 2 December 2021 for persons who completed primary-vaccination and if there had been no contact with the index case in the last three days. If asymptomatic, no testing was necessary if HREC tested positive for SARS-CoV-2 in the last 180 days. We included test results of first and second tests from unvaccinated persons and persons who completed primary or booster-vaccination. Persons who received a single dose of a two-dose vaccine schedule (incomplete vaccination) or a mixed (heterologous) primary-vaccination schedule were excluded.
Belgium’s genomic surveillance of COVID-19 applies a baseline random sampling approach aiming to cover 5–10% of all RT-PCR positive samples, which allows for the monitoring of circulating VOCs [11]. Time periods during which at least 80% of sequenced samples were identified as a certain VOC were used as period-proxies for VOC-dominance. The period from 26 January 2021 to 18 June 2021 was defined as the period-proxy for Alpha the period from 15 July 2021 to 22 December 2021 as period-proxy for Delta and 3 January 2022 to 10 January 2022 as period-proxy for Omicron. High-risk exposure events in transition periods were excluded.
HREC with a potential negative duration of exposure were excluded: when the HREC was tested earlier than the index case or, if symptoms were reported, when symptom-onset was earlier in HREC. In order to exclude a possible ‘within-index case clustering’-effect and a possible superspreader-effect we randomly selected three HREC for inclusion and excluded the others HREC for index cases with more than three HREC.
2.2. Model variables
Person-level data on personal characteristics (age, sex), test-results (result of the test, sampling and testing date), vaccination (vaccine brand and date of vaccination) and contact tracing (date of symptom onset, date of last contact, household-membership) were linked by a pseudonymized National Registry Number.
Primary-vaccination was considered effective 14 days after the second dose of ChAdOx1/ mRNA-1273, seven days after the second dose of BNT162b2 and 21 days after a single dose of Ad26.COV2.S. Booster-vaccination was considered effective 7 days after administration of the booster-vaccine [12]. We chose to include the four brands used for primary-vaccination and two types of prime-boost schedules (1) mRNA-vaccines followed by a mRNA-booster-dose (mRNA + Booster) and (2) viral-vector-vaccines followed by a mRNA-booster dose (VV + Booster). We included two different prime-boost schedules as persistence of humoral responses has been reported to differ by schedule [13]. Prior SARS-CoV-2-infection was defined as a documented positive RT-PCR or antigenic test more than 90 days prior to the date of sampling. Time to last immunity-conferring event was defined as the time between sampling (after high-risk exposure) and prior infection or vaccination, whichever was most recent. Hybrid immunity refers to vaccinated persons with a prior infection, irrespective of the sequence of prior infection and vaccination.
Sex and age at sampling were obtained from the National Registry. Age groups were 0–2, 3–5, 6–8, 9–11, 12–18, 18–24, 25–44, 45–64 and 65 to 84 years old. As we could only include a small number of persons aged 85 years and older, these were excluded from the analysis. VE was not estimated for persons younger than 12 years as this age group was only eligible for vaccination (with a pediatric vaccine) late in the study period. We included a dummy variable to indicate if the index case and HREC were part of the same household. We included whether the test was a first test or a second test (in combination with the test result of the first test). Calendar time, the week during which the sample was taken, was included as a random effect in the model. Finally, the background exposure was included as the positivity rate (centered 7-day moving average) of all PCR and antigenic tests of the province of residency of the HREC at the sampling date.
2.3. The model
We estimated Vaccine Effectiveness against transmission (), susceptibility () and infectiousness () as:
To obtain the probability of a positive test () we fitted a multilevel Bayesian regression-model to the test results of the HREC. The probability of a positive test was a function of characteristics of the index and HREC (age, sex, household, immunity ()), the dominant VOC, background exposure and the calendar week.
2.4. Immunity: The effect of vaccination and prior Covid-19 infection
The effect of immunity () was included for the index case (effect on infectiousness) and the HREC (effect on susceptibility) as an initial effect () in interaction with waning ( and the VOC ().
Prior infection and vaccination () were included as a factor with 14 factor-levels (2*7: yes / no prior infection and unvaccinated / Ad26.COV2.S / ChAdOx1 / BNT162b2 / mRNA-1273 / VV + Booster / mRNA + Booster). is included as a linear spline over 50-day periods since the last immunity-conferring event with a single knot at 150 days. Splines with multiple knots were explored, but these resulted in comparable functions. The spline’s coefficients are determined by age group, sex, VOC and the combination of vaccination and prior infection.
One way to interpret the model is to look at its three levels. The first level represents a baseline for transmissibility (infectiousness/susceptibility) defined by age, sex (of index case and HREC), household, VOC, background exposure and calendar week. The second level represents the initial effect of the vaccination/prior infection (first 50 days after last immunity-conferring event). The third level represents the waning of this initial effect. Note that variables such as age, sex, vaccination (brand)/prior infection are included on all three levels. The model allows age to be associated with changes in susceptibility, changes in vaccine effectiveness and faster or slower waning.
We reported 95% credible intervals as CI. The Bayesian model was fitted using the R-package nimble. Code for the model and the priors used can be found in the supplementary material. Females aged 45–64 years old without a prior infection were used as reference category in this paper. Whenever VE is reported without additionally mentioning sex, age group and prior infection, it refers to females aged 45 to 64 years of age without prior infection. BNT162b2 and mRNA + Booster were most frequently administered in Belgium and are therefore used as reference in this study.
3. Results
3.1. Hrec and index cases included
Over the study period, 1 761 574 test results were available. Results were excluded because of missing variables for the HREC (N = 7 102), missing variables for the index case (N = 208 352), sampling during transition periods between VOC-dominance (N = 73 694), incomplete or mixed primary schedule (N = 117 319), second tests in fully vaccinated persons during summer (N = 21 618), an index case or HREC aged 85 years or older (N = 11 726), more than 3 HREC per index case (N = 222 780) and misclassification (e.g. testing of HREC before testing of index case, N = 121 709). We included 1 134 400 test results, of which 266 862 were positive (23.5%), from 413 363 index cases and 703 057 HREC in the analysis.
The number of included HREC and index cases by prior infection, vaccine brand, VOC and age can be found in Table 1 and Table 2 respectively. Descriptive statistics on the temporal evolution of the unadjusted secondary attack rate in HREC are provided in the supplementary material.
Table 1.
Number of included High-Risk Exposure Contacts by prior infection, age group (at the time of high-risk exposure contact) and vaccination. The positivity rate of the first test of the HREC is presented in brackets (%). HREC < 12 years not presented. Belgian contact tracing, 26/01/2021–10/01/2022.
| VOC | Prior Infection | Age Group | Unvaccinated | Ad26.COV2.S | ChAdOx1 | BNT162b2 | mRNA-1273 | VV + Booster | mRNA + Booster |
|---|---|---|---|---|---|---|---|---|---|
| Alpha | Yes | [12,18) | 855 (0.08) | / | / | / | / | / | / |
| [18,25) | 1344 (0.06) | / | / | 91 (0.03) | / | / | / | ||
| [25,45) | 3032 (0.07) | / | / | 247 (0.03) | 20 (0.1) | / | / | ||
| [45,65) | 1938 (0.06) | / | / | 204 (0.02) | 18 (0.11) | / | / | ||
| [65,85) | 237 (0.08) | / | / | 34 (0.03) | / | / | / | ||
| No | [12,18) | 27,645 (0.26) | / | / | 74 (0.07) | / | / | / | |
| [18,25) | 25,624 (0.23) | / | 10 (0) | 630 (0.04) | 30 (0) | / | / | ||
| [25,45) | 64,020 (0.22) | 15 (0) | 56 (0.07) | 2567 (0.06) | 209 (0.03) | / | / | ||
| [45,65) | 48,944 (0.23) | 20 (0.1) | 36 (0.06) | 2540 (0.07) | 238 (0.01) | / | / | ||
| [65,85) | 11,070 (0.28) | / | / | 793 (0.07) | 91 (0.01) | / | / | ||
| Delta | Yes | [12,18) | 1063 (0.12) | / | / | 1227 (0.03) | 21 (0) | / | / |
| [18,25) | 843 (0.13) | 193 (0.03) | 76 (0.07) | 1514 (0.04) | 129 (0.02) | / | 20 (0) | ||
| [25,45) | 2273 (0.14) | 273 (0.04) | 671 (0.05) | 5939 (0.06) | 913 (0.04) | 70 (0) | 196 (0.04) | ||
| [45,65) | 803 (0.1) | 343 (0.03) | 1035 (0.05) | 2996 (0.04) | 395 (0.03) | 82 (0.01) | 139 (0.01) | ||
| [65,85) | 57 (0.07) | 17 (0.12) | 157 (0.02) | 450 (0.04) | 37 (0) | 54 (0) | 99 (0.04) | ||
| No | [12,18) | 17,886 (0.33) | 26 (0.23) | / | 26,519 (0.09) | 187 (0.06) | / | 34 (0.06) | |
| [18,25) | 8389 (0.33) | 2313 (0.17) | 720 (0.14) | 17,773 (0.11) | 1472 (0.08) | 145 (0.1) | 210 (0.08) | ||
| [25,45) | 21,777 (0.34) | 3941 (0.21) | 8841 (0.18) | 86,736 (0.16) | 12,188 (0.12) | 1512 (0.12) | 2622 (0.1) | ||
| [45,65) | 9672 (0.34) | 4831 (0.22) | 16,032 (0.19) | 47,488 (0.17) | 6085 (0.12) | 1605 (0.13) | 2451 (0.1) | ||
| [65,85) | 1088 (0.4) | 186 (0.22) | 5343 (0.18) | 13,613 (0.21) | 1155 (0.13) | 2167 (0.12) | 3286 (0.13) | ||
| Omicron | Yes | [12,18) | 725 (0.49) | / | / | 818 (0.31) | 18 (0.06) | / | / |
| [18,25) | 337 (0.47) | 29 (0.45) | / | 562 (0.35) | 52 (0.42) | 48 (0.19) | 88 (0.25) | ||
| [25,45) | 1150 (0.46) | 77 (0.43) | 57 (0.42) | 1875 (0.37) | 258 (0.36) | 256 (0.29) | 830 (0.3) | ||
| [45,65) | 406 (0.44) | 54 (0.31) | 140 (0.27) | 697 (0.27) | 119 (0.29) | 383 (0.19) | 638 (0.23) | ||
| [65,85) | 29 (0.34) | / | / | 31 (0.23) | / | 59 (0.15) | 147 (0.14) | ||
| No | [12,18) | 3773 (0.57) | / | / | 10,695 (0.46) | 101 (0.49) | / | 50 (0.34) | |
| [18,25) | 1364 (0.53) | 202 (0.43) | 25 (0.4) | 4465 (0.4) | 408 (0.35) | 540 (0.3) | 701 (0.29) | ||
| [25,45) | 4513 (0.53) | 415 (0.51) | 403 (0.48) | 15,732 (0.45) | 2058 (0.46) | 2747 (0.37) | 8157 (0.4) | ||
| [45,65) | 1923 (0.49) | 335 (0.49) | 1241 (0.43) | 6742 (0.4) | 874 (0.43) | 4901 (0.32) | 7609 (0.31) | ||
| [65,85) | 203 (0.52) | 19 (0.79) | 75 (0.4) | 316 (0.43) | 32 (0.28) | 1389 (0.26) | 3097 (0.25) |
Table 2.
Number of included index cases (included once per tested High-Risk Exposure Contact) by prior infection, age group (at the time of high-risk exposure contact) and vaccination. The positivity rate of the first test of the HREC reported by the index case is presented in brackets (%). Index cases < 12 years not presented. Belgian contact tracing, 26/01/2021–10/01/2022.
| VOC | Prior Infection | Age Group | Unvaccinated | Ad26.COV2.S | ChAdOx1 | BNT162b2 | mRNA-1273 | VV + Booster | mRNA + Booster |
|---|---|---|---|---|---|---|---|---|---|
| Alpha | Yes | [12,18) | 477 (0.09) | / | |||||
| [18,25) | 637 (0.07) | / | / | 23 (0.04) | / | / | / | ||
| [25,45) | 1640 (0.09) | / | / | 85 (0.12) | / | / | / | ||
| [45,65) | 877 (0.11) | / | / | 50 (0.06) | / | / | / | ||
| [65,85) | 152 (0.11) | / | / | 29 (0) | / | / | / | ||
| No | [12,18) | 22,267 (0.16) | / | ||||||
| [18,25) | 28,313 (0.18) | / | / | 150 (0.07) | / | / | / | ||
| [25,45) | 77,560 (0.25) | / | 13 (0) | 637 (0.1) | 54 (0.11) | / | / | ||
| [45,65) | 51,411 (0.29) | 15 (0.2) | / | 547 (0.11) | 38 (0.03) | / | / | ||
| [65,85) | 9383 (0.3) | / | / | 173 (0.11) | 11 (0.09) | / | / | ||
| Delta | Yes | [12,18) | 836 (0.1) | 324 (0.07) | / | ||||
| [18,25) | 644 (0.15) | 51 (0.1) | 44 (0.05) | 449 (0.06) | 33 (0.15) | / | / | ||
| [25,45) | 1630 (0.18) | 118 (0.08) | 225 (0.09) | 1847 (0.13) | 238 (0.12) | / | 41 (0.15) | ||
| [45,65) | 481 (0.21) | 44 (0.07) | 306 (0.11) | 820 (0.1) | 81 (0.07) | / | 23 (0.04) | ||
| [65,85) | 26 (0.15) | / | 33 (0.06) | 99 (0.11) | / | / | 21 (0.05) | ||
| No | [12,18) | 26,154 (0.19) | 29 (0.28) | / | 8775 (0.13) | 44 (0.09) | 15 (0.33) | ||
| [18,25) | 13,722 (0.22) | 3914 (0.11) | 1696 (0.1) | 13,921 (0.11) | 918 (0.1) | 60 (0.02) | 84 (0.21) | ||
| [25,45) | 35,898 (0.33) | 4822 (0.23) | 12,006 (0.2) | 73,643 (0.2) | 7868 (0.18) | 489 (0.21) | 871 (0.22) | ||
| [45,65) | 12,458 (0.36) | 5556 (0.22) | 16,645 (0.2) | 37,161 (0.2) | 3558 (0.15) | 536 (0.16) | 924 (0.18) | ||
| [65,85) | 1265 (0.35) | 139 (0.28) | 3669 (0.25) | 9264 (0.26) | 517 (0.19) | 547 (0.19) | 905 (0.23) | ||
| Omicron | Yes | [12,18) | 645 (0.4) | 577 (0.29) | / | / | |||
| [18,25) | 356 (0.42) | 37 (0.32) | 11 (0.27) | 566 (0.27) | 45 (0.22) | 30 (0.27) | 49 (0.24) | ||
| [25,45) | 1463 (0.45) | 68 (0.43) | 63 (0.38) | 1835 (0.37) | 275 (0.37) | 103 (0.38) | 372 (0.36) | ||
| [45,65) | 472 (0.47) | 42 (0.31) | 119 (0.39) | 647 (0.39) | 85 (0.44) | 113 (0.25) | 234 (0.35) | ||
| [65,85) | 16 (0.5) | / | / | 20 (0.35) | / | 19 (0.05) | 45 (0.27) | ||
| No | [12,18) | 4084 (0.48) | / | 12,955 (0.41) | 93 (0.33) | 54 (0.31) | |||
| [18,25) | 1809 (0.43) | 299 (0.31) | 54 (0.19) | 6646 (0.31) | 530 (0.27) | 415 (0.21) | 454 (0.26) | ||
| [25,45) | 6562 (0.51) | 546 (0.5) | 691 (0.43) | 22,388 (0.43) | 3063 (0.42) | 1698 (0.42) | 4406 (0.41) | ||
| [45,65) | 2148 (0.53) | 446 (0.5) | 1689 (0.42) | 7453 (0.44) | 939 (0.45) | 2471 (0.37) | 3290 (0.37) | ||
| [65,85) | 222 (0.44) | 22 (0.64) | 52 (0.44) | 289 (0.45) | 25 (0.56) | 655 (0.39) | 1231 (0.37) |
3.2. Impact of the VOC on initial effectiveness
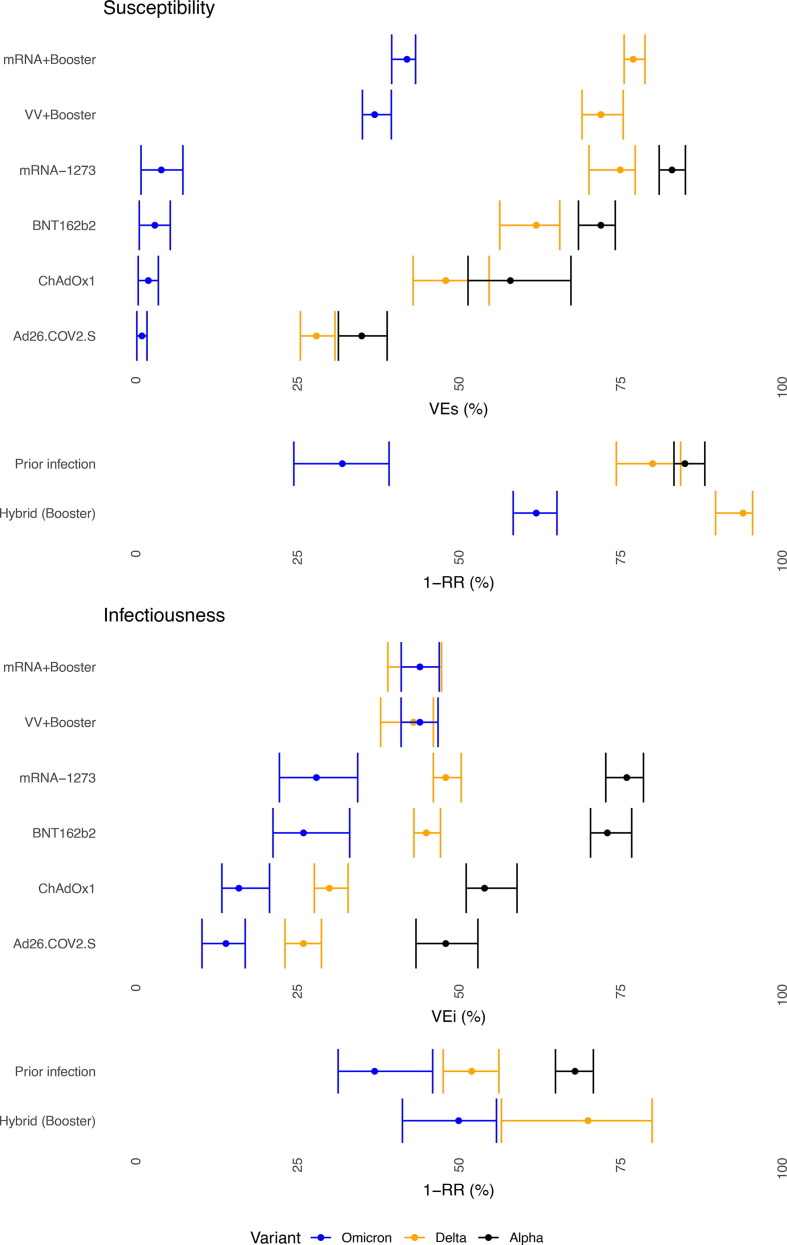
We observed significant differences in VEi and VEs estimates by VOC, with a decrease from Alpha to Delta and the lowest estimates for Omicron. The reduction was most pronounced in VEs against Omicron. We estimated VEs close to 0 after primary-vaccination and around 37–42% after booster-vaccination against Omicron. Hybrid immunity resulted in the highest effectiveness against susceptibility to an Omicron-infection. VEi-estimates dropped significantly from Alpha to Delta. Omicron was associated with a further decrease of VEi-estimates for primary-vaccination. For booster-vaccination, VEi against Delta and Omicron was comparable and estimated around 35% (Fig. 1 ).
Fig. 1.
Initial, 0–50 days after vaccination, effectiveness VEs (susceptibility, upper) and VEi (infectiousness, lower) by VOC by vaccine and prior infection, females, 45–64 years, Belgian contact tracing, 26/01/2021 – 10/01/2022.
3.3. Booster-vaccination
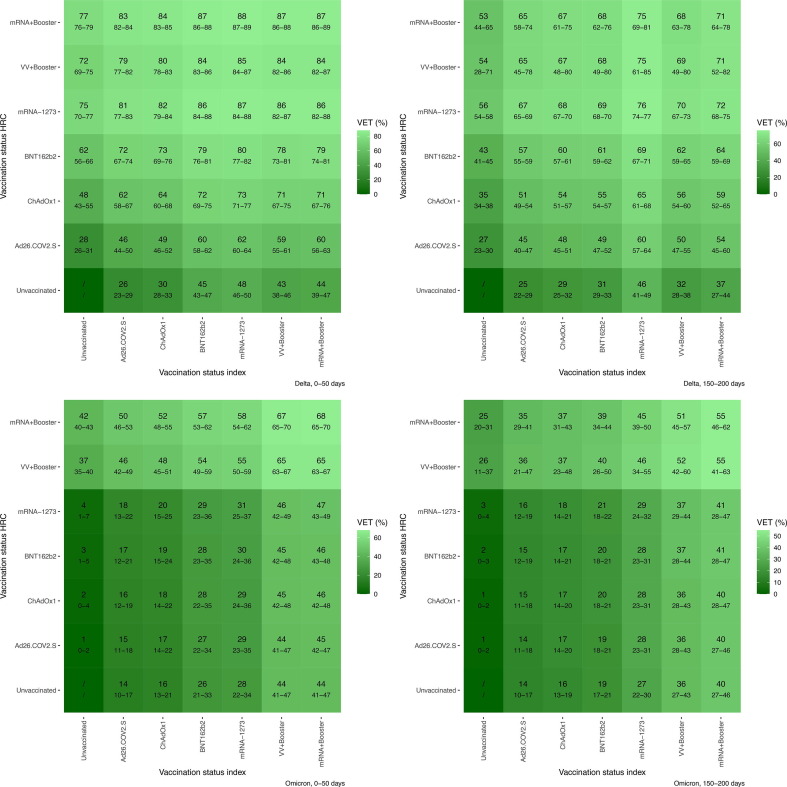
Booster-vaccination significantly increased VET-estimates against Delta after primary-vaccination with a viral-vector-vaccine. For example: primary-vaccination of both index and HREC with ChAdOx1 resulted in an initial VET-estimate of 64% (CI 60–68). After booster-vaccination, this increased to 84% (CI 82–86). If primary-vaccination consisted of mRNA-vaccines, booster-vaccination resulted in initial VET-estimates of 87% (CI 86–89) (compared to VET of 79% (CI 76–81) for primary vaccination with BNT162b2). Booster-vaccination considerably increased initial VET-estimates against Omicron. Initial VET-estimates for all primary schedules were low; these were 18% (CI 14–22) and 28% (CI 23–35) for ChAdOx1 and BNT162b2 respectively, which increased to 65–68% after booster-vaccination (Fig. 2 ).
Fig. 2.
VET-estimates for females aged 45–64 years old without prior infection by VOC (upper = Delta, lower = Omicron) and time since vaccination (left = 0–50 days, right = 150–200 days). Belgian contact tracing, 26/01/2021 – 10/01/2022. The first column presents VE-estimates against susceptibility (protection of the HREC by vaccination against exposure from an unvaccinated index case), the lowest row presents VE-estimates against infectiousness (protection of unvaccinated HREC through reduced infectiousness of an index case by vaccination).
3.4. Waning of the initial effectiveness
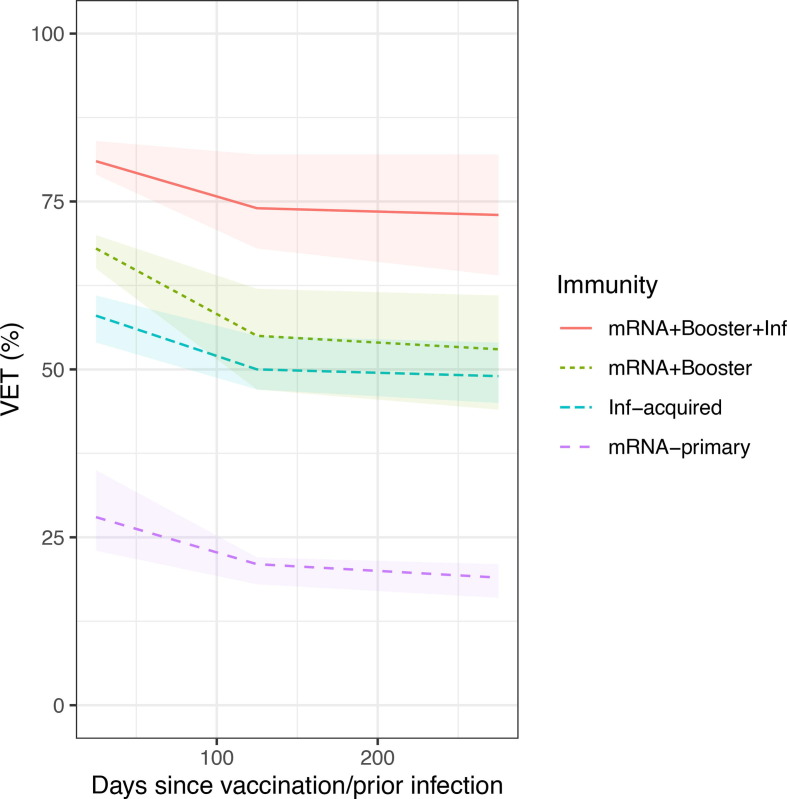
We observed waning of VET-estimates, driven mainly by waning of VEs-estimates (see also Supplementary Fig. 3-7), with faster waning during the initial period (first 150 days), followed by slower waning in the subsequent period. For immunity that was vaccine-induced only, slightly faster waning was observed compared to infection-acquired immunity (Fig. 3 ).
Fig. 3.
VET-estimates against Omicron over time since vaccination/prior infection by immunity status (mRNA + Booster without and with prior infection, BNT162b2 primary-vaccination and infection-acquired immunity without vaccination), females 45–64 years, Belgian contact tracing, 26/01/2021 – 10/01/2022.
4. Discussion
In this study we investigated protection by primary-vaccination, booster-vaccination and prior infection against transmission of Alpha, Delta and Omicron-infection during high-risk exposure. While vaccine-induced and infection-acquired immunity of either index case or HREC decreased the risk, VOC-specific immune-escape and waning of immunity increased the risk of SARS-CoV-2 transmission.
4.1. Increased immune-escape by omicron
We previously described immune-escape by Delta (3). The period of Omicron-dominance, however, was associated with additional escape from both infection-acquired and vaccine-induced immunity. Protection by prior infection was limited: 32% (CI 24–39). Protection by primary-vaccination was even lower: VEs 3% (CI 1–5, BNT162b2). This is considerably lower than the VEs-estimate against Delta (62% (CI 56–66)) and Alpha (73% (CI 60–80)). VEi was less impacted by Omicron: 20% (CI 17–27, BNT162b2 primary-vaccination only). This however was again lower than VEi-estimates against Delta and Alpha (36% (CI 32–41) and 63% (CI 55–70) respectively). VEi represents lower infectiousness of breakthrough infections compared to infections in unvaccinated persons and has been linked to accelerated viral clearance [14], [15], [16], [17], reduced viral shedding [14], [18] and severity of symptoms [19].
Omicron’s immune-escape, including the high probability of re-infection, has been described previously [9], [20] and linked to mutations mainly in the spike protein [21]. The inclusion of Alpha, Delta and Omicron within the same transmission-model highlighted how the immune-escape increased from Alpha to Delta to Omicron. The magnitude of the escape was different for susceptibility and infectiousness (e.g. reduction of infectiousness after vaccination was better maintained against Omicron compared to susceptibility). The emergence of Omicron has challenged the generalizability of past observations. For example, the observation that ‘natural immunity showed roughly similar effect sizes regarding protection against reinfection across different SARS-CoV-2 variants’ [22], no longer holds true. Only little protection was offered by primary-vaccination or prior infection only. It is important to note that we could only include a short time period for Omicron as the mandatory testing of HREC ended shortly after Omicron became dominant. Consequently, protection against Omicron by prior infection excludes prior Omicron-infection. Both the Alpha and Delta periods were long enough (greater than90 days) to include such observations. Future studies should include additional details on prior infection or on the sequence of prior infections (for multiple prior infections). When evaluating the impact of Omicron, all characteristics should be taken into account. We previously also reported immune-escape with respect to symptomatic infection and hospitalization [23] and a lower intrinsic severity within Belgian hospitalized COVID-19 patients compared to Delta [24].
4.2. Waning
All VE-estimates waned, but the rate of waning was higher for VEs compared to VEi (see also Supplementary Fig. 4-7). Vaccine-induced immunity seemed to wane faster than immunity that was (partially) infection-acquired. While we observed waning, the effect was small compared to the VOC-specific decrease in relative risk reduction. A Danish study, with a similar study period, also reported that VOCs made a larger contribution to the increased transmission between vaccinated persons compared to waning [25].
The low VEs against Omicron, the larger impact of Delta on VEi compared to VEs and the faster waning of VEs compared to VEi possibly all reflect the underlying biological mechanisms that differentiate VEs from VEi. While, in both cases, the mechanisms consist of several components, to some extent, VEs reflects neutralizing antibodies as, e.g. T cells cannot prevent host cells from initially becoming infected, [26], [27], [28] while cellular immunity, e.g. increased neutralized breadth of memory B cells and T cell mediated viral clearance after vaccination [29], [30], affects VEi.
4.3. Booster-vaccination & hybrid immunity
Our findings illustrate that booster-vaccination can be used to restore waning immunity as well as to compensate for lower VET-estimates associated with less effective primary-vaccination schedules. Irrespective of the schedule used for primary-vaccination, we estimated VET above 80% against Delta after booster-vaccination. Against Omicron, booster-vaccination was necessary to considerably increase the low VET-estimates associated with primary-vaccination (e.g. 28% for BNT162b2). Other studies that compared two to three doses also attributed effectiveness against Omicron mostly to the third dose [10], [27]. Serological data and studies on infectious viral load offer further insight: Puhach et al. reported reduced viral load after booster-vaccination, but not after primary-vaccination for Omicron [14]. Hybrid immunity did not outperform booster-vaccination against Omicron, as it did against Delta, when the number of antigen exposures was equal. Hybrid immunity associated with booster-vaccination did offer most protection (see also Supplementary Figure 6), but was typically associated with four antigen exposures. Future research should investigate how differences in routes of exposure affect the different immunological layers (e.g. IgA) [31] in addition to between VOC cross-neutralization.
4.4. Transmission
A meta-analysis found that the secondary attack rate (SAR) associated with household transmission of Alpha, Delta and wild-type SARS-CoV2 is lower for vaccinated compared to unvaccinated index cases [32]. While less pronounced, we found this to still hold true for Omicron and reported significant VEi- and VET-estimates after primary and booster-vaccination. Studies from Denmark [33], Norway [34], US [6], [35] and England [36] have also reported a limited, but significant effect of vaccines on transmission of Omicron. The significant relative risk reduction by vaccination needs to be separated from the high absolute risk of Omicron-transmission. Our study and the previously cited studies all reported high SAR for Omicron (US, Norway and Denmark in the range 30–50%, England notably lower: 15%), higher than the SAR associated with previous VOCs [32].
4.5. Limitations
Since the capacity of both contact tracing and testing were exceeded during January 2022, mandatory testing of vaccinated, asymptomatic HREC was discontinued and we could only include a short period of Omicron-dominance. Testing was still mandatory for the period until 10 January 2022 and contact tracing partially scaled to the higher Omicron incidences: a 27% higher case-incidence was associated with a 15% increase in the number of index cases contacted compared to the week of peak Delta incidence (week 47 2021). Possible reduced case-finding associated with high circulation of Omicron, was unlikely to already affect VE-estimates obtained for the first week of Omicron-dominance. Incomplete case-finding prior to and over the study period however remains a possible bias for our VE-estimates. Despite high capacity and free RT-PCR-tests, Belgian seroprevalence studies report underdiagnosis of SARS-CoV-2-infections [37].
Our conclusions are constrained to what is allowed by the underlying model. For example, since we included calendar week as a random effect, it was hard to compare transmissibility between periods of VOC-dominance. Given the vaccination strategy with its age-specific roll-out and the specific temporal dominance of VOCs, VOC-specific waning might be confounded with age-specific waning. It is also unclear if such a term is needed for VEs. Studies on neutralizing antibodies have reported similar decay rates against different variants [38]. There seems to be more consensus on the necessity to allow for waning differences between infection-acquired and vaccine-induced immunity [39]. Our model did not include brand-age interactions for the waning coefficients (brand and age were included separately). A diminished IgG response in male versus female and older versus younger recipients has been reported specifically for BNT162b2 [40].
In addition to limitations by the underlying model, we were further limited by the data that was included. For HREC, VE against outcomes other than infection could not be estimated. Clinical presentation has been reported to impact transmission. Higher SARs were observed in households with symptomatic rather than asymptomatic index cases [32]. Symptomatic prior infections have been associated with more pronounced protection [25]. We tried to limit misclassification (transmission from HREC to index case) by use of the dates available to us and further included the background positivity rate as a second possible source of infection. Misclassification however remains possible and background positivity rate is only a crude measure for other possible sources of infection.
Mandatory and repeated testing of HREC avoided bias associated with differences in testing behavior, but as most epidemiological studies, we cannot exclude a bias introduced by differences in risk behavior (e.g. within household physical distancing) between vaccinated an unvaccinated persons. Part of the data used in this study has previously been used for the estimation of VET against Alpha (2) and Delta (3). Given that we expanded (e.g. smaller age groups were included) and refitted the model with more data, some VET-estimates differ slightly (some percentage points) from our previous studies, but in general, estimates were robust.
5. Conclusion
Vaccination and prior infection reduced susceptibility and infectiousness. The extent to which they did, decreased over time and was affected by the dominant VOC. Delta increased both susceptibility and infectiousness after breakthrough infection, but the latter effect was largest. Omicron further increased infectiousness, but in contrast to Delta, its main immune-escape was associated with susceptibility. Susceptibility after primary-vaccination or prior infection only, remained high for Omicron. A higher number of antigen-exposures, either through booster-vaccination or hybrid immunity, improved protection. In conclusion, we report significant VET-estimates for SARS-CoV-2-infection during a period of Alpha, Delta and Omicron-dominance (January 2021 to January 2022).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement & Role of the funding source
This study was supported by the Belgian Federal and Regional Authorities through funding for the LINK-VACC project and organizing and financing of contact tracing. The funding source had no role in the study design, collection, analysis, interpretation, writing of the report or decision to submit the paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.03.069.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Proesmans K, Hancart S, Braeye T, Klamer S, Robesyn E, Djiena A, et al. COVID-19 contact tracing in Belgium: main indicators and performance, January – September 2021. Arch Public Health. 2022 Apr 13;80(1):118. [DOI] [PMC free article] [PubMed]
- 2.Braeye T., Cornelissen L., Catteau L., Haarhuis F., Proesmans K., De Ridder K., et al. Vaccine effectiveness against infection and onwards transmission of COVID-19: Analysis of Belgian contact tracing data, January-June 2021. Vaccine. 2021 Sep 15;39(39):5456–5460. doi: 10.1016/j.vaccine.2021.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braeye T, Catteau L, Brondeel R, van Loenhout J, Proesmans K, Cornelissen L, et al. VACCINE EFFECTIVENESS AGAINST ONWARD TRANSMISSION OF SARS-COV2-INFECTION BY VARIANT OF CONCERN AND TIME SINCE VACCINATION, BELGIAN CONTACT TRACING, 2021. Vaccine [Internet]. 2022 Apr 12 [cited 2022 Apr 17]; Available from: https://www.sciencedirect.com/science/article/pii/S0264410X22004418. [DOI] [PMC free article] [PubMed]
- 4.de Gier B., Andeweg S., Joosten R., ter Schegget R., Smorenburg N., van de Kassteele J., et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Eurosurveillance. 2021 Aug 5;26(31):2100640. doi: 10.2807/1560-7917.ES.2021.26.31.2100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eyre D.W., Taylor D., Purver M., Chapman D., Fowler T., Pouwels K.B., et al. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N Engl J Med. 2022 Feb 24;386(8):744–756. doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JM, Nakayama JY, O’Hegarty M, McGowan A, Teran RA, Bart SM, et al. SARS-CoV-2 B.1.1.529 (Omicron) Variant Transmission Within Households — Four U.S. Jurisdictions, November 2021–February 2022. Morb Mortal Wkly Rep. 2022 Mar 4;71(9):341–6. [DOI] [PMC free article] [PubMed]
- 7.Dejnirattisai W., Shaw R.H., Supasa P., Liu C., Stuart A.S., Pollard A.J., et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2022 Jan 15;399(10321):234–236. doi: 10.1016/S0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogt A.C.S., Augusto G., Martina B., Chang X., Nasrallah G., Speiser D.E., et al. Increased Receptor Affinity and Reduced Recognition by Specific Antibodies Contribute to Immune Escape of SARS-CoV-2 Variant Omicron. Vaccines. 2022 May;10(5):743. doi: 10.3390/vaccines10050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulliam J.R.C., van Schalkwyk C., Govender N., von Gottberg A., Cohen C., Groome M.J., et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. 2022 Mar;15;376(6593):eabn4947 doi: 10.1126/science.abn4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. cited 2022 Feb 5. Available from: 2022 Jan 21 doi: 10.1001/jama.2022.0470. [DOI] [Google Scholar]
- 11.Sciensano’s COVID-19 dashboard: genomic surveillance [Internet]. Available from: https://datastudio.google.com/embed/reporting/c14a5cfc-cab7-4812-848c-0369173148ab/page/urrUC.
- 12.Glanville D. COVID-19 vaccines: key facts [Internet] European Medicines Agency. 2020 https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts [cited 2021 Dec 7]. Available from: [Google Scholar]
- 13.Liu X, Munro APS, Feng S, Janani L, Aley PK, Babbage G, et al. Persistence of immunogenicity after seven COVID-19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK: three month analyses of the COV-BOOST trial. J Infect [Internet]. 2022 Apr 9 [cited 2022 Apr 15]; Available from: https://www.sciencedirect.com/science/article/pii/S0163445322002006. [DOI] [PMC free article] [PubMed]
- 14.Puhach O, Adea K, Hulo N, Sattonnet P, Genecand C, Iten A, et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022 Apr 8;1–1. [DOI] [PubMed]
- 15.Kissler S.M., Fauver J.R., Mack C., Tai C.G., Breban M.I., Watkins A.E., et al. Viral Dynamics of SARS-CoV-2 Variants in Vaccinated and Unvaccinated Persons. N Engl J Med. 2021 Dec 23;385(26):2489–2491. doi: 10.1056/NEJMc2102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singanayagam A., Hakki S., Dunning J., Madon K.J., Crone M.A., Koycheva A., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis [Internet] 2021 Oct 29 [cited 2021 Dec 22];0(0). doi: 10.1016/S1473-3099(21)00648-4. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00648-4/fulltext Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung J., Kim J.Y., Park H., Park S., Lim J.S., Lim S.Y., et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Netw Open. 2022 May 24;5(5):e2213606. doi: 10.1001/jamanetworkopen.2022.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regev-Yochay G, Amit S, Bergwerk M, Lipsitch M, Leshem E, Kahn R, et al. Decreased infectivity following BNT162b2 vaccination: A prospective cohort study in Israel. Lancet Reg Health – Eur [Internet]. 2021 Aug 1 [cited 2022 Apr 26];7. Available from: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(21)00127-7/fulltext. [DOI] [PMC free article] [PubMed]
- 19.Passaretti C.L., Priem J.S., Agner T.G., McCurdy L. Reducing the rates of household transmission: The impact of COVID-19 vaccination in healthcare workers with a known household exposure. Vaccine. 2022 Feb 23;40(9):1213–1214. doi: 10.1016/j.vaccine.2022.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster-elicited serum, but evades most convalescent serum and therapeutic antibodies. Sci Transl Med. 0(0):eabn8543. [DOI] [PMC free article] [PubMed]
- 21.Zhang L., Li Q., Liang Z., Li T., Liu S., Cui Q., et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. 2022 Dec;11(1):1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilz S., Theiler-Schwetz V., Trummer C., Krause R., Ioannidis J.P.A. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022 Jun;1(209) doi: 10.1016/j.envres.2022.112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braeye T, Loenhout J van, Brondeel R, Stouten V, Hubin P, Billuart M, et al. COVID-19 Vaccine effectiveness against symptomatic infection and hospitalization in Belgium, July 2021-APRIL 2022 [Internet]. medRxiv; 2022 [cited 2022 May 28]. p. 2022.05.09.222746Available from: https://www.medrxiv.org/content/10.1101/2022.05.09.22274623v1. [DOI] [PMC free article] [PubMed]
- 24.Van Goethem N., Chung P.Y.J., Meurisse M., Vandromme M., De Mot L., Brondeel R., et al. Clinical Severity of SARS-CoV-2 Omicron Variant Compared with Delta among Hospitalized COVID-19 Patients in Belgium during Autumn and Winter Season 2021–2022. Viruses. 2022 Jun;14(6):1297. doi: 10.3390/v14061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michlmayr D., Hansen C.H., Gubbels S.M., Valentiner-Branth P., Bager P., Obel N., et al. Observed protection against SARS-CoV-2 reinfection following a primary infection: A Danish cohort study among unvaccinated using two years of nationwide PCR-test data. Lancet Reg Health Eur. 2022 Sep;20 doi: 10.1016/j.lanepe.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Yang M., Peng Y., Liang Y., Wei J., Xing L., et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol. 2022 Feb;7:1–11. doi: 10.1038/s41564-021-01051-2. [DOI] [PubMed] [Google Scholar]
- 27.Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine–elicited human sera. Science [Internet]. 2022 Jan 18 [cited 2022 Feb 6]; Available from: https://www.science.org/doi/abs/10.1126/science.abn7591. [DOI] [PMC free article] [PubMed]
- 28.Wherry E.J., Barouch D.H. T cell immunity to COVID-19 vaccines. Science. 2022 Aug 19;377(6608):821–822. doi: 10.1126/science.add2897. [DOI] [PubMed] [Google Scholar]
- 29.Kotaki R, Adachi Y, Moriyama S, Onodera T, Fukushi S, Nagakura T, et al. SARS-CoV-2 Omicron-neutralizing memory B cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci Immunol. 7(70):eabn8590. [DOI] [PMC free article] [PubMed]
- 30.Vardhana S., Baldo L., Morice W.G., Wherry E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci Immunol. 2022 May 20;7(71):eabo1303. doi: 10.1126/sciimmunol.abo1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates TA, McBride SK, Leier HC, Guzman G, Lyski ZL, Schoen D, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol [Internet]. 2022 Jan 25 [cited 2022 Jan 30]; Available from: https://www.science.org/doi/abs/10.1126/sciimmunol.abn8014. [DOI] [PMC free article] [PubMed]
- 32.Madewell Z.J., Yang Y., Longini I.M., Halloran M.E., Dean N.E. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020 Dec 1;3(12):e2031756. doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyngse F.P., Kirkeby C.T., Denwood M., Christiansen L.E., Mølbak K., Møller C.H., et al. Household transmission of SARS-CoV-2 Omicron variant of concern subvariants BA.1 and BA.2 in Denmark. Nat Commun. 2022 Sep;30;13(1):5760 doi: 10.1038/s41467-022-33498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jalali N., Brustad H.K., Frigessi A., MacDonald E.A., Meijerink H., Feruglio S.L., et al. Increased household transmission and immune escape of the SARS-CoV-2 Omicron compared to Delta variants. Nat Commun. 2022 Sep 29;13(1):5706. doi: 10.1038/s41467-022-33233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan S.T., Kwan A.T., Rodríguez-Barraquer I., Singer B.J., Park H.J., Lewnard J.A., et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the Omicron wave. Nat Med. 2023 Feb;29(2):358–365. doi: 10.1038/s41591-022-02138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen H., Tessier E., Turner C., Anderson C., Blomquist P., Simons D., et al. Comparative transmission of SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants and the impact of vaccination: national cohort study, England. Epidemiol Infect. 2023 Mar 20:1–20. doi: 10.1017/S0950268823000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog S.A., Bie J.D., Abrams S., Wouters I., Ekinci E., Patteet L., et al. Seroprevalence of IgG antibodies against SARS-CoV-2 – a serial prospective cross-sectional nationwide study of residual samples, Belgium, March to October 2020. Eurosurveillance. 2022 Mar 3;27(9):2100419. doi: 10.2807/1560-7917.ES.2022.27.9.2100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanshylla K., Tober-Lau P., Gruell H., Münn F., Eggeling R., Pfeifer N., et al. Durability of omicron-neutralising serum activity after mRNA booster immunisation in older adults. Lancet Infect Dis. 2022 Apr 1;22(4):445–446. doi: 10.1016/S1473-3099(22)00135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Israel A., Shenhar Y., Green I., Merzon E., Golan-Cohen A., Schäffer A.A., et al. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines. 2022 Jan;10(1):64. doi: 10.3390/vaccines10010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keshavarz B, Richards NE, Workman LJ, Patel J, Muehling LM, Canderan G, et al. Trajectory of IgG to SARS-CoV-2 After Vaccination With BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison With Natural Infection. Front Immunol [Internet]. 2022 [cited 2022 Apr 16];13. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2022.850987. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.