Abstract
Solitary fibrous tumor (SFT), previously termed hemangiopericytoma, is a rare primary intracranial tumor. SFT is classified into grades I, II, and III with different prognoses; grade III tumor has malignant characteristics with a high probability of recurrence and extracranial metastasis. We report the case of a 63-year-old female patient admitted to the Vietnam National Cancer Hospital with headache, dizziness, nausea, ataxia, and loss of balance. Computed tomography showed a markedly enhanced tumor, without calcification, located in the posterior fossa close to the tentorium cerebelli. No changes in the adjacent bone were seen. Magnetic resonance imaging revealed a lobular extra-axial tumor with prominent flow voids, a finding that has been seen frequently in these tumors. The tumor was resected following an initial diagnosis of SFT. Postoperative histology indicated a grade III SFT according to the World Health Organization 2021 classification. SFT is often misdiagnosed as meningioma, as they have some imaging features in common. However, we believe that there are some characteristic magnetic resonance imaging features that help to distinguish between these tumors, as well as playing an essential role in SFT grading and potentially guiding the best therapeutic decision.
Keywords: Hemangiopericytoma, Magnetic resonance imaging, Rare tumor
Introduction
Solitary fibrous tumor (SFT) is a rare mesenchymal brain neoplasm that accounts for less than 1% of all primary intracranial tumors [1]. SFT often affects adults in their fourth to fifth decade of life, with a slight predominance in males [2]. It was first included in the World Health Organization classification of central nervous system tumors in 2002. In 2013, both SFT and hemangiopericytoma (HPC) were discovered to express a NAB2–STAT6 gene fusion as well as nuclear overexpression of STAT6 detected by immunohistochemistry; therefore, SFT and HPC were combined into a single disease entity known as SFT/HPC in 2016 [3]. However, in the 2021 version of the World Health Organization classification of central nervous system tumors, SFT/HPC is simply referred to as SFT under the mesenchymal neoplasm group [[1], [2], [3], [4]]. The term HPC has become obsolete, emphasizing the biological similarities between the 2 tumor types [4]. Because SFT is a rare intracranial tumor, few studies have investigated it and precise treatment regimens for it are lacking. Therefore, the treatment of SFT remains challenging. The common approach remains radical surgery to remove the tumor, with or without radiotherapy and adjuvant chemotherapy after surgery. Prognostic factors, including age, tumor location, tumor size, histology, adjuvant therapy, and especially, the extent of resection, have been shown to be closely associated with the likelihood of recurrence and metastasis as well as the survival time of patients. In this article, we describe a rare intracranial HPC.
Case report
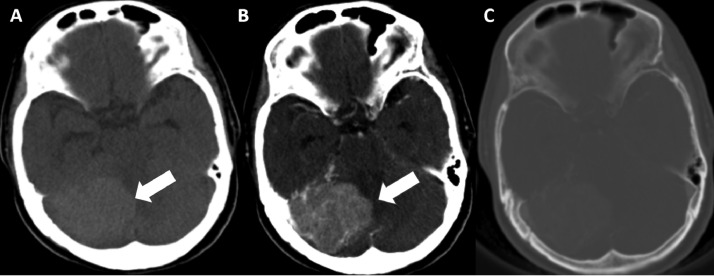
A 63-year-old female patient with no remarkable medical history was admitted to the hospital because of headache, dizziness, nausea, ataxia, and loss of balance persisting for several months. Head computed tomography (CT) showed a tumor located in the posterior fossa, adjacent to the right cerebellar hemisphere and attached to the tentorium cerebelli, with increased attenuation in nonenhanced CT compared to gray matter, indicating high cellularity. No intratumoral calcification was found. Postcontrast CT showed the vividly enhanced tumor and mild edema of the surrounding cerebral tissue, with the mass effect causing compression of the fourth ventricle. No invasion or changes in adjacent bone were observed (Fig. 1).
Fig. 1.
Preoperative enhanced head computed tomography (CT) with contrast. (A) Nonenhanced CT shows a mass (arrow) adjacent to the tentorium cerebellum, hyperdense compared to grey matter, with no calcification. (B) Postcontrast CT reveals a vivid homogeneously enhanced mass (arrow) without a clear dural tail. (C) Axial noncontrast enhanced CT image does not show bone changes.
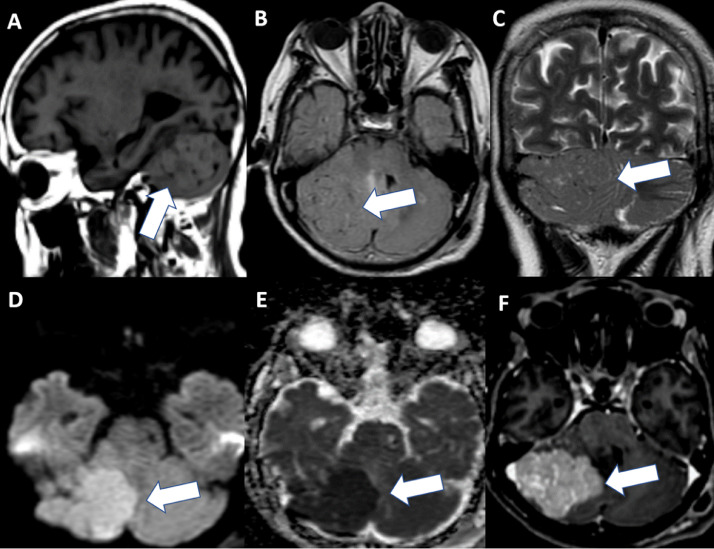
Magnetic resonance imaging (MRI) showed a well-defined lobulated extra-axial mass, 50 × 45 × 41 mm in size, attached to the tentorium cerebelli with a narrow base. The tumor appeared as isointense on T1W and T2W/FLAIR compared to cortical gray matter, with marked enhancement and without the dural tail sign on postcontrast T1W. The corresponding apparent diffusion coefficient (ADC) maps showed strongly restricted diffusion (minimum ADC value = 0.45 × 10−3 mm2/s). In addition, the flow-void sign observed on T2W/FLAIR corresponded to intratumoral vessels (Fig. 2). Other features, such as the dural tail sign, calcification, cyst formation or necrosis, and adjacent bone destruction, bone erosion, or hyperostosis, were not detected.
Fig. 2.
Preoperative magnetic resonance imaging. (A-C) Axial images of T1WI, FLAIR, and T2W (A: T1WI; B: FLAIR; and C: T2W) show a mass adjacent to the right cerebellum. The lesion appears isointense on T1WI and T2W/FLAIR compared to cortical gray matter. Note the flow-void sign in the tumor (indicated by arrow). (D, E) Diffusion-weighted imaging and apparent diffusion coefficient (ADC) indicate that tumor (arrow) was strongly restricted diffusion, with the minimum ADC on the ADC map being 0.45 × 10−3 mm2/s. (F) Contrast-enhanced T1WI shows homogeneous and vivid enhancement of the tumor (arrow) without the dural tail sign.
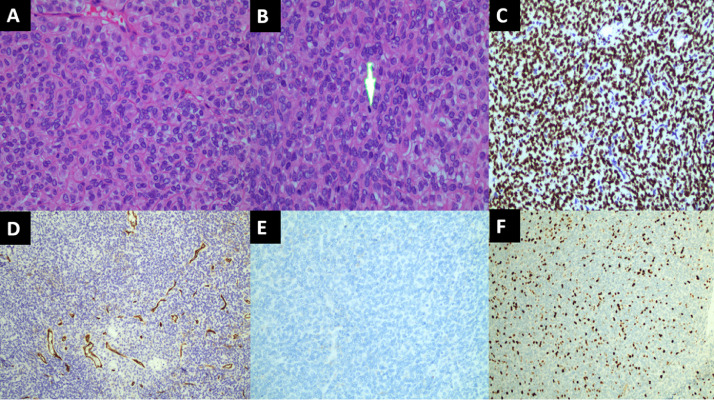
The patient underwent surgical resection of the tumor after an initial diagnosis of SFT. Hematoxylin and eosin staining of tumor sections revealed diffuse proliferation of spindle cells with round or ovoid nuclei. Mitotic activity was also noted (5 or more in 10 high-power fields). Immunohistochemistry examination results were positive for CD34 (+), STAT6 (++), and Bcl2 (+) and negative for EMA. Ki-67 staining showed a proliferative index of 30% (Fig. 3). These findings led to a diagnosis of SFT grade III according to the WHO classification.
Fig. 3.
Histologic findings. (A, B) Hematoxylin and eosin staining (400×) shows a high density of tumor spindle cells, with 10 high-power fields (400×) showing mitosis (indicated by arrow). (C-F) Immunohistochemistry examination of STAT6 (C, 200×), and CD34 (D, 100×), and Bcl2 (not shown) were positive. Ki-67 staining (F, 100×) showed a proliferative index of 30%. EMA (E, 200×) was negative.
Discussion
SFT, previously termed HPC, is a rare extra-axial brain tumor of mesenchymal origin, accounting for less than 1% of all primary intracranial tumors [1]. Patients have nonspecific clinical symptoms depending on the location, size, and invasion of the tumor. On imaging, the tumor usually presents as an extra-axial lobular lesion with a well-defined margin, slightly increased attenuation on CT, iso- to hyperintense on T1W and T2W/ FLAIR, which may be related to the corresponding histopathological profile due to the relatively high tumor cell density. SFT often shows heterogeneous intensity due to necrosis, a cyst component, and intratumoral vessels. It has a rich blood supply from the branches of the internal carotid and vertebral arteries. Hence, the tumor is usually moderately to strongly enhanced with the intratumoral flow-void sign represented by vascular channels with low signal on all sequences, demonstrating high vascular flow [5]. Pang et al. [5] found that the flow-void sign was quite common in SFT (in 80% of 20 cases).
Preoperative embolization is recommended as a safe method of reducing tumor size and the risk of bleeding during surgery. Cystic and necrosis are common features and may cause SFT to be misdiagnosed as other tumors, especially cystic meningioma [1]. Differential diagnoses with meningioma is always indicated because they have similar imaging features, but the prognosis and follow-up management differ. Compared to meningioma, SFT is often more aggressive and has a higher risk of local recurrence and metastasis, with a rate of up to 20%, requiring closer and more prolonged post-treatment follow-up [6]. Overall, lobular borders, rare calcification, a narrow base attachment, presentation of the flow-void sign, and rare hyperostosis of adjacent bone are more frequent in SFT than in meningioma [1,2,5]. Instead of hyperostosis, SFT tends to cause bone erosion. Chiechi et al. [7] found that while nearly 60% of SFT cases had bone erosion none had bone hyperostosis. Another feature that can help distinguish between these tumors is the dural tail sign. This finding is relatively common in meningioma, observed in 52%-78% of cases [7], but is less common in SFT. Pang et al. [5] observed the dural tail sign only in 1 case out of 15, while in Chiechi et al.’s [8] study, 8 out of 14 cases showed this sign. In addition, the application of advanced MRI, including diffusion-weighted imaging (DWI), also helps to differentiate between SFT and meningioma. Although SFT exhibits dense infiltration of tumor cells on histopathology, it also includes many vascular spaces because of abundant intratumoral vessels. In contrast, meningiomas are firmly bound by desmosomal attachments and intercellular cell junctions. Therefore, meningiomas may be exposed to less extracellular space and more intracellular space than SFT, leading to restricted water diffusion [9]. However, differential diagnosis is unfeasible if using DWI alone. Chen et al. [9] showed that with a cutoff nADC (mean tumor area ADC/normal white matter ADC) value of 1.15, the sensitivity and specificity of differentiation between SFT and meningioma were 75% and 60.42%, respectively. However, the ADC value depends considerably on meningioma pathology and SFT grade. ADC values do not differ significantly between angiomatous meningioma and SFT; the former has higher ADC values even compared to grade 1 SFT/HPC [10,11]. In our case, strongly restricted diffusion of SFT with a minimum ADC of 0.45 × 10−3 mm2/s represents high cellularity and corresponds to the histopathology of grade III high-grade tumor with dense infiltration of tumor cells, increased mitotic number, enlarged nuclei, and high nuclear/cytoplasmic ratio. In addition, DWI with the measurement of ADC values can be useful in SFT grading. Mama et al. [12] reported that all grade II SFTs/HPCs had higher ADC values than grade III (range 1.26-1.50 × 10−3 mm2/s and 0.638-0.833 × 10−3 mm2/s, respectively). Conventional MRI also showed signs suggestive of SFT malignancy, including a tumor with an irregular shape, indistinct margin, lobular border, and necrosis and bone destruction [13,14]. However, previous studies of SFT were hampered by small sample sizes, and only a few studied the role of advanced MRI in SFT grading and differential diagnosis with other tumors.
Conclusion
SFT, previously termed HPC, is a rare extra-axial intracranial tumor. Its typical imaging features include lobular border, heterogeneous signal, rare calcification, presentation of flow-void sign, and strong postcontrast enhancement, with or without the dural tail sign, and it causes bone erosion instead of bone hyperostosis. CT and MRI play a vital role in its diagnosis as well as in grading, thereby contributing to the coordination of effective treatment and patient monitoring.
Author's contributions
Ta HN, Vu LM, and Nguyen MD: Case file retrieval and case summary preparation. Ta HN, Vu LM, and Nguyen MD: preparation of manuscript and editing. All authors read and approved the final manuscript.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
Our institution does not require ethical approval for reporting individual cases or case series.
Patient consent
Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Footnotes
Acknowledgments: None to declare.
Competing Interests: The authors declare that they have no competing interests.
References
- 1.Chikasue T, Uchiyama Y, Tanoue S, Komaki S, Sugita Y, Abe T. Intracranial solitary fibrous tumor/hemangiopericytoma mimicking cystic meningioma: a case report and literature review. Radiol Case Rep. 2021;16(7):1637–1642. doi: 10.1016/j.radcr.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen-Inbar O. Nervous system hemangiopericytoma. Can J Neurol Sci. 2020;47(1):18–29. doi: 10.1017/cjn.2019.311. [DOI] [PubMed] [Google Scholar]
- 3.Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014;184(4):1209–1218. doi: 10.1016/j.ajpath.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pang H, Yao Z, Ren Y, Liu G, Zhang J, Feng X. Morphologic patterns and imaging features of intracranial hemangiopericytomas: a retrospective analysis. Onco Targets Ther. 2015;8:2169–2178. doi: 10.2147/OTT.S85971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang K, Mei F, Wu S, Tan Z. Hemangiopericytoma: incidence, treatment, and prognosis analysis based on SEER database. Biomed Res Int. 2020;2020 doi: 10.1155/2020/2468320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiechi M.V., Smirniotopoulos J.G., Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996;17(7):1365–1371. [PMC free article] [PubMed] [Google Scholar]
- 8.Wen M, Jung S, Moon K-S, Pei J, Lee K-H, Jin S-G, et al. Immunohistochemical profile of the dural tail in intracranial meningiomas. Acta Neurochir (Wien) 2014;156(12):2263–2273. doi: 10.1007/s00701-014-2216-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen T, Jiang B, Zheng Y, She D, Zhang H, Xing Z, et al. “Differentiating intracranial solitary fibrous tumor/hemangiopericytoma from meningioma using diffusion-weighted imaging and susceptibility-weighted imaging. Neuroradiology. 2020;62(2):175–184. doi: 10.1007/s00234-019-02307-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Yin B, Geng D-Y, Li Y, Zhang B-Y, Peng W-J. Comparison of ADC values of intracranial hemangiopericytomas and angiomatous and anaplastic meningiomas. J Neuroradiol. 2014;41(3):188–194. doi: 10.1016/j.neurad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Kanazawa T, Minami Y, Jinzaki M, Toda M, Yoshida K, Sasaki H. Preoperative prediction of solitary fibrous tumor/hemangiopericytoma and angiomatous meningioma using magnetic resonance imaging texture analysis. World Neurosurg. 2018;120:e1208–e1216. doi: 10.1016/j.wneu.2018.09.044. [DOI] [PubMed] [Google Scholar]
- 12.Mama N., Ben Abdallah A., Hasni I., Kadri K., Arifa N., Ladib M., et al. MR imaging of intracranial hemangiopericytomas. J Neuroradiol. 2014;41(5):296–306. doi: 10.1016/j.neurad.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Bai L-C, Luo T-Y, Zhu H, Xu R. MRI features of intracranial anaplastic hemangiopericytoma. Oncol Lett. 2017;13(5):2945–2948. doi: 10.3892/ol.2017.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiyama M., Sakai H., Onoue H., Miyazaki Y., Abe T. Imaging intracranial haemangiopericytomas: study of seven cases. Neuroradiology. 2004;46(3):194–197. doi: 10.1007/s00234-003-1157-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.