Abstract
Objective
The purpose of this study was to evaluate the effectiveness of middle meningeal artery embolization (MMAE) in elderly high-risk patients with symptomatic chronic subdural hematoma (CSDH) in terms of reduction in hematoma volume and recurrence rate.
Methods
We retrospectively reviewed data prospectively collected from nine patients who underwent 13 MMAE for CSDH between June 2017 and May 2022. The volume of the subdural hematoma was measured using a computer-aided volumetric analysis program. Hematoma volume changes during the follow-up period were analyzed and clinical outcomes were evaluated.
Results
The mean follow-up period was 160 days (range, 46−311 days). All procedures were technically successful and there were no procedure-related complications. Of the 13 MMAE, 84% (11 out of 13 hemispheres) showed mean 88% of reduction on follow-up volumetric study with eight cases of complete resolution. There was one refractory case with MMAE which had been performed multiple burr-hole trephinations, for which treatment was completed by craniotomy and meticulous resection of multiple pseudomembranes. There was no recurrent case during the follow-up period, except for refractory case.
Conclusions
MMAE for CSDH in selected high-risk elderly patients and relapsed patients might be effective. Despite the small cohort, our findings showed a high rate of complete resolution with no complications. Further prospective randomized trials are warranted to evaluate its usefulness as a primary treatment option for CSDH.
Keywords: Chronic subdural hematoma, Embolization, Middle meningeal artery, Craniotomy
INTRODUCTION
Chronic subdural hematoma (CSDH) is one of the most common neurosurgical diseases that primarily affects the elderly population, with an estimated incidence of 10/100,000 [6]. Surgical evacuation of hematoma, conducted using burr hole trephination and drainage, is currently considered the gold standard for symptomatic CSDH. However, elderly patients, who comprise the majority of CSDH patients, have a high risk of perioperative complications (up to 32%) for surgical intervention due to their old age and various comorbidities [2]. In addition, the recurrence rate of surgical treatment is reported as 10–30% at 1–8 week intervals [1,8,9].
Middle meningeal artery embolization (MMAE) is a technique that has recently emerged as an alternative or adjunctive to surgical procedures [3,10]. Previous studies already reported favorable radiological and clinical outcomes of MMAE for chronic subdural hemorrhage. Therefore, we evaluated its effect on the reduction of hematoma volume using three-dimensional computer-assisted volumetric reconstruction analysis and clinical outcomes in elderly patients with surgical risk factors and recurrent CSDH.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board of Korea University (IRB No. 2022GR0333). We retrospectively reviewed a prospectively collected dataset of 13 hemispheres from nine patients who underwent MMAE for CSDH between June 2017 and May 2022.
The patient group was selected based on the following criteria:
Among elderly patients >60 years old with:
a. Chronic or mixed-type non-acute subdural hematoma with evident hemispheric compression or mass effect with symptoms
or
b. Asymptomatic subdural hematoma but increasing amount of hematoma on serial follow-up imaging
And those who meet at least one of the following three lists
1) Who was being with relapsed hematoma after surgical evacuation
2) Who had comorbidities that increase the risk of surgery (e.g. heart failure, severe liver cirrhosis)
3) Who was taking anticoagulant or antiplatelet agents Exclusion criteria of MMAE were as follows:
Mixed type or chronic subdural hematoma with
1) Minimal or no compressing effect without symptoms
or
2) Life threatening mass effect
or
3) Decreased consciousness
Clinical data included demographics, clinical presentation, comorbidities, use of anti-platelet or anticoagulant medications, primary or post-evacuation status, and hospitalization days after MMAE. Radiological data included changes in the hematoma volume over time on follow-up non-contrast brain computed tomography (CT). Clinical outcomes were measured using the modified Rankin Scale at the last clinical follow-up.
Procedure of middle meningeal artery embolization
All procedures were performed under local anesthesia and systemic heparinization, maintaining an ACT of over 1.5 times the initial baseline ACT value. Placement of a 6 Fr Envoy guiding catheter (Codman Neuro, Raynham, MA, US) in the proximal external carotid artery was followed by the placement of a microcatheter with an inner diameter of 0.017” in the common stem of middle meningeal artery. Superselection of the middle meningeal artery major branch (usually the frontal and squamosal branches) and microangiography were used to confirm the embolization safety zone for dangerous anastomosis with intracranial vessels. Particle embolic materials with polyvinyl alcohol (PVA Embolization Particles, Bearing nsPVA, Merit Medical, Utah, US) and Gelfoam (Nexsphere, Next Biomedical, Incheon, KOR) were used in this study. The preferred PVA particle size was 150–250 µm and the gelfoam particle size was >150 µm to prevent shunting into the venous system and cranial nerve ischemia. The particles were dissolved in a solution of contrast medium with saline in a 3:1 ratio. The selected major branch of the middle meningeal artery was embolized with PVA particles first and then with gelfoam particles. Strong resistance after several injections of the particles was considered an indication of sufficient embolization and occlusion of the selected middle meningeal artery. The microcatheter was then safely removed and discarded. A new microcatheter was introduced into another major branch and the same procedure was performed. Finally, angiography was used to confirm completion of embolization of the middle meningeal artery.
Hematoma volume measurement
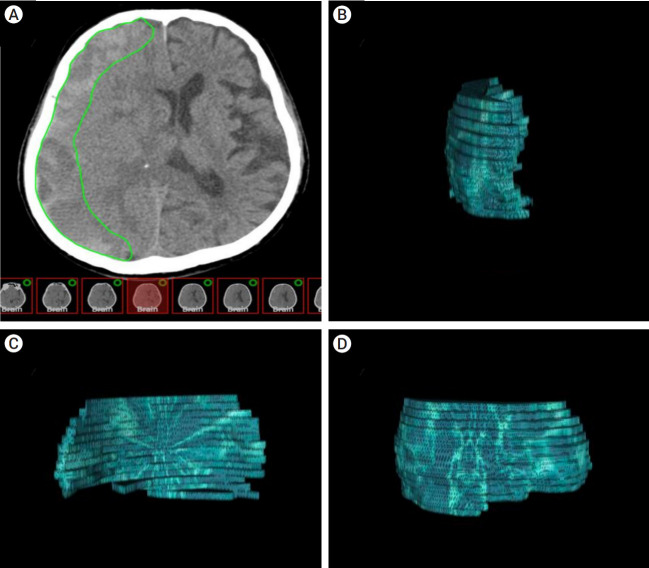
Subdural hematoma volume was calculated using OsiriX software (an open-source DICOM viewer, OsiriX Lite, v.12.5.2 32bit, Pixmeo, Geneva, Switzerland), a computer-aided volumetric analysis program. The hematoma area on each slice was semi-automatically delineated using the OsiriX grow region and repulsor tools (Fig. 1A). We defined blood on CT within the range of 40–80 HU. Special attention was paid to avoid the inclusion of the cranium and areas of calcification in volume calculations. After accurately defining the hemorrhage, the volume was calculated using OsiriX software, and the results were recorded in cubic centimeters. These areas were defined as regions of interest (Fig. 1B–D). The outcome data were analyzed based on non-contrast brain CT for complete, partial, same, and worse outcomes after MMAE, along with procedural complications. If the hematoma volume was <10 ml, resolution was defined as complete. Partial resolution was defined as an SDH volume of >10 ml [11].
Fig. 1.
The hematoma model was reconstructed using OsiriX software (an open-source DICOM viewer, OsiriX Lite, v.12.5.2 32bit, Pixmeo, Geneva, Switzerland). (A) Axial CT-scan showing a right-sided subdural hematoma (SDH). Volumetric measurements were performed by hand-tracing the hematoma form in each slice, as shown in green. (B) 3D frontal aspect reconstruction of computer-assisted volumetric measurement of single-sided SDH. (C) 3D medial aspect reconstruction of computer-assisted volumetric measurement of single-sided SDH. (D) 3D lateral aspect reconstruction of computer-assisted volumetric measurement of single-sided SDH. CT, computed tomography
RESULTS
Thirteen procedures were performed on 13 hemispheres from nine patients. The mean age was 77.33 years-old (range, 64–86 years). All patients in the embolization treatment group had a variable history of comorbidities, and seven patients were taking antithrombotic agents. The clinical characteristics of the embolization treatment groups are summarized in Table 1. Of the 13 hemispheres, eight underwent embolization treatment as the primary therapy because of their medical comorbidities or antithrombotic agents. Five hemispheres were treated for recurrence after prior surgical evacuation. Among these five, two hemispheres underwent MMAE just before craniotomy as adjunctive therapy to reduce the recurrence rate after surgery. The mean follow-up period was 160 days (range, 46–311 days). All procedures were technically successful and there were no procedure-related complications. The average length of hospital stay after MMAE for all patients was 5.3 days. However, in patients number 2 and 7, the period of stay in hospital was extended due to clinical issues related to other departments such as orthopedic surgery for pelvic fracture, rehabilitation for stroke; excluding these patients, the mean hospitalization was 2.8 days. All patients’ clinical outcomes were favorable, showing improved modified Rankin Scale scores.
Table 1.
Summary of clinical information from patients participating in the study
| Patient No. | Hemisphere No. | Side | Sex/Age | Symptom | Indication for MMAE | Comorbidity | Antithrombotic agent | Previous surgical evacuation before MMAE | Additional treatment | Hospitalization days after MMAE | Initial mRS | mRS at their last clinical follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Right | M/74 | HA | Hemispheric compression with relapsed hematoma after surgical evacuation | DM | Anti-PLT | Y | N | 2 | 2 | 0 |
| 2 | 2 | Right | F/86 | Lower limb motor weakness | Mass effect with comorbidities and anti-PLT use | CVD, Prostate Ca. | Anti-PLT | Y | Burr-hole | 17 | 4 | 0 |
| 3 | 3 | Left | F/73 | Upper limb motor weakness, Paresthesia | Hemispheric compression with anti-PLT use | CVD | Anti-PLT | N | N | 3 | 3 | 1 |
| 4 | 4 | Right | M/82 | HA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anti-PLT & coagulant use | HTN, CAD, Renal Ca. | Anti-PLT, Anti-coagulant | N | N | 2 | 2 | 0 |
| 4 | 5 | Left | M/82 | SA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anti-PLT & coagulant use | SA | SA | SA | SA | SA | SA | SA |
| 5 | 6 | Right | M/82 | HA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anti-PLT use | HTN, DM, CVD | Anti-PLT | N | N | 3 | 2 | 0 |
| 5 | 7 | Left | M/82 | SA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anti-PLT use | SA | SA | SA | SA | SA | SA | SA |
| 6 | 8 | Right | M/64 | HA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anticoagulant use | HF, Arrythmia, Heart valve disease | Anti-coagulant | N | N | 2 | 2 | 0 |
| 6 | 9 | Left | M/64 | SA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anticoagulant use | SA | SA | SA | SA | SA | SA | SA |
| 7 | 10 | Right | M/80 | HA | Increasing amount of subdural hematoma on serial follow-up imaging with comorbidities and anti-PLT use | HTN, DM, CVD, CKD | Anti-PLT | N | N | 11 | 2 | 0 |
| 8 | 11 | Right | M/76 | HA, General weakness | Hemispheric compression with relapsed hematoma after surgical evacuation | DM | None | Y | N | 3 | 2 | 0 |
| 8 | 12 | Left | M/76 | SA | Hemispheric compression with relapsed hematoma after surgical evacuation | SA | SA | SA | Craniotomy | SA | SA | SA |
| 9 | 13 | Left | M/79 | HA | Hemispheric compression with relapsed hematoma after surgical evacuation | HTN, HLD | None | Y | N | 5 | 2 | 1 |
MMAE, middle meningeal artery embolization; mRS, modified Rankin Scale; HA, headache; DM, diabetes mellitus; PLT, platelet; CVD, cerebrovascular disease; SA, same above; HTN, hypertension; HF, heart failure; CKD, chronic kidney disease; HLD, hyperlipidemia
Volumetric analysis revealed a decrease in the hematoma volume over time in all patients. The solitude effect of MMAE could not be addressed in patient number two and the left hemisphere lesion of patient number eight due to subsequent surgery, as described below. Nevertheless, the rest of the hemispheres (84.6%) showed mean 88% of reduction on follow-up volumetric study, indicating the effectiveness of MMAE for CSDH. A total of eight hemispheres (61.5%) acquired complete hematoma resorption and they were not treated with subsequent surgery. Serial monthly image follow-ups demonstrated that early volume reduction occurred within 3 months in patients with complete resorption (Fig. 2).
Fig. 2.
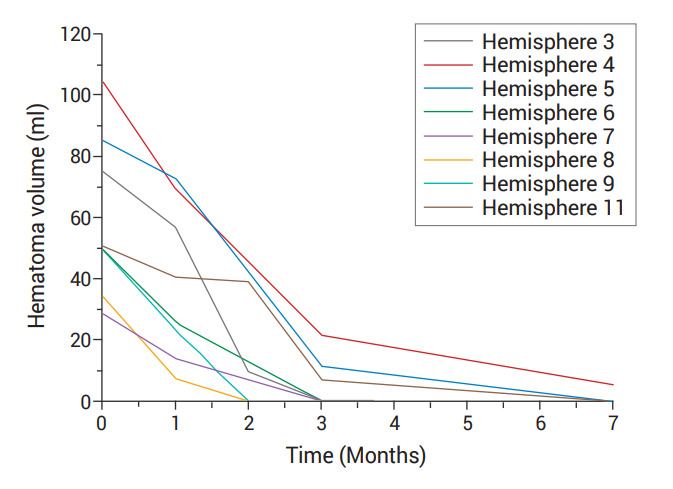
Pattern of volume reduction. This graph indicates changes in hematoma volume after performing MMAE in each patient who showed complete resorption. MMAE, middle meningeal artery embolization
Patient two was initially scheduled for a combined treatment strategy to reduce the possibility of recurrence and enhance post-treatment mobility according to comorbidities and preoperative neurological symptoms. Patient eight had bilateral lesions and underwent two burr-hole surgeries for the right side and three burrhole surgeries for left side lesions at institutions outside of our hospital. Even after these procedures, the hematoma recurred and bilateral MMAE was performed. The right-side lesion showed no hematoma expansion after embolization; however, the left-side lesion (hemisphere number 12) demonstrated increased hematoma volume with mass effect in 1 month. Therefore, craniotomy and hematoma evacuation with multilayered pseudomembrane resection were performed on the left side. Serial CT follow-up showed a decreased hematoma volume in both hemispheres.
DISCUSSION
MMAE for CSDH with significant hematoma volume and mass effect on the brain cortex in elderly patients (with surgical risk factors or recurrent hematoma after surgery) might be effective in hematoma volume reduction with good clinical outcomes and minimal complications in this small cohort.
CSDH presents as a severe headache, focal neurological lateralizing signs, or mental status change due to the mass effect. It occurs mainly in the elderly population because age-related brain atrophy renders cortical veins prone to shear injury and bleeding caused by minor head trauma. Over time, the hematoma develops pseudomembranes containing macrophages and giant capillaries that are connected to the termini of the middle meningeal arterioles. This vascularity is considered to be the main pathophysiology of hematoma expansion, mixed chronology of hematoma nature, and high recurrence rate after surgery [4]. The mainstay of treatment for CSDH is surgery, conducted using burrhole trephination. However, the recurrence rate after surgery is reportedly up to 30% [12]. Elderly patients, who comprise the majority of the CSDH patient population, are at greater risk of surgical complications due to their comorbidities [5,7]. The MMAE was introduced as an alternative to surgical treatment for CSDH to reduce perioperative complications for elderly patients at high risk. In addition, the vulnerability to recurrence due to the antithrombotic agents that elderly people often take can be reduced by this minimally invasive procedure. In the current study, the mean age of the cohort was 77.33 years old. The patients had many comorbidities and were taking antithrombotic agents for their medical conditions. The majority of cases showed significant hematoma volume reduction (11 out of 13, 84.6%) and favorable clinical outcomes (100%) without recurrence during the follow-up period (except for hemisphere number 12, 92.3%). Considering the pathophysiology of CSDH, MMAE seems to block the connection between the giant capillaries of pseudomembranes and the MMA terminus of the dura. This blockage interrupts the blood supply to the pseudomembrane, prevents capillary rupture, reduces neoangiogenesis from the pseudomembrane, and eventually enhances the resolution of hematoma and resorption. This effect is believed to have reduced the recurrence rate. The hematoma volume reduction effect appeared to be initiated in the early postoperative stage. By calculating the hematoma volume using the OsiriX program with 3-D reconstruction, we were able to obtain relatively objective hematoma volume data (Table 2). This data demonstrated that significant volume reduction began within 1 month of treatment. Within 2 or 3 months, more volume reduction continued until complete resorption (Fig. 2). These results support the use of MMAE as a means to resolve CSDH in patients who are difficult to treat surgically, and consequently increases the likelihood of favorable functional outcomes. Two patients underwent additional surgery, one underwent burr-hole trephination according to the treatment strategy, and the other underwent large craniotomy due to late hematoma expansion even after meticulous embolization. These two cases indicate that surgery is more beneficial for cases with a high-volume mass effect that require prompt resolution, and there was no recurrence during the follow-up period. Cases that had been refractory to surgery would benefit from MMAE in addition to the surgical procedure. That is, it might suggest the efficacy and safety of MMAE in upfront treatment, in the treatment of recurrence, and as a prophylactic therapy before performing surgical intervention. There were no complications after MMAE in any of the patients. In particular, no hemorrhagic complications were seen in patients taking antithrombotic agents. In addition, MMAE offers several advantages over conventional surgical treatment for CSDH. Unlike surgical treatment, which requires postoperative bed rest for several days due to placement of a subdural drain, patients are usually ambulatory within a few hours after MMAE, and for patients with minor symptoms, such as significant headache only, it is possible for them to be discharged home after a short period of observation.
Table 2.
Summary of preprocedural and postprocedural information with the radiological outcome. Changes in hematoma volume (ml) for each hemisphere with time after MMAE are shown
| Patient No. | Hemisphere No. | Location | Midline shift (mm) before MMAE | Maximum thickness (mm) before MMAE | Pre-Embo Volume (ml) | Post-Embo Volume (ml) 1 month | Post-Embo Volume (ml) 2 months | Post-Embo Volume (ml) 3 months | Post-Embo Volume (ml) 7 months | Final radiological outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Right | Y (7.44) | 14.96 | 110.32 | 75.81 | NA | 23.33 | NA | Partial |
| 2 | 2 | Right | Y (9.88 mm) | 25.16 | 170.08 | 102.43 | 58.22 | NA | 37.50 | Partial |
| 3 | 3 | Left | N | 15.48 | 75.31 | 57.10 | 9.75 | NA | 0.11 | Complete |
| 4 | 4 | Right | Y (2.28 mm) | 19.37 | 105.01 | 69.73 | 45.90 | NA | 5.15 | Complete |
| 4 | 5 | Left | SA | 13.28 | 85.40 | 73.17 | 42.72 | NA | 0.00 | Complete |
| 5 | 6 | Right | N | 15.59 | 50.28 | 25.92 | NA | 0.00 | Complete | |
| 5 | 7 | Left | SA | 10.41 | 28.69 | 14.1 | NA | 0.00 | Complete | |
| 6 | 8 | Right | N | 7.72 | 34.51 | 7.38 | 0.00 | Complete | ||
| 6 | 9 | Left | SA | 15.32 | 50.19 | 23.21 | 0.00 | Complete | ||
| 7 | 10 | Right | Y (3.35 mm) | 20.29 | 106.69 | 102.76 | NA | 32.52 | NA | Partial |
| 8 | 11 | Right | Y (4.68 mm) | 14.58 | 50.75 | 40.49 | 39.29 | NA | 0.00 | Complete |
| 8 | 12 | Left | SA | 14.45 | 108.72 | 133.78 | 49.41 | 46.93 | 28.52 | Partial |
| 9 | 13 | Left | N | 12.78 | 47.99 | 36.01 | 34.87 | 34.54 | NA | Partial |
MMAE, middle meningeal artery embolization
The main limitation of this study was that it was a single-center, small patient population, nonrandomized study, in which there was no comparable control group. In particular, it was difficult to distinguish the net effect of MMAE on CSDH in our study because there were also many reported cases CSDH could be resolved with spontaneous hematoma resorption over time. Nevertheless, this study might be meaningful because it indicates that MMAE can be an alternative or an adjuvant treatment for elderly patients who do not have favorable conditions for conventional surgical treatment.
CONCLUSIONS
MMAE chosen with narrow indications as a treatment modality for refractory, recurrent, or high-risk CSDH might be effective in hematoma volume reduction and improvement of clinical outcomes. The patients had a very low complication rate and required a short hospital stay. In the future, prospective, large-scale comparative studies will be needed to warrant its effectiveness as a general treatment modality for CSDH.
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, Yarascavitch B, et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014 Mar;259(3):449–57. doi: 10.1097/SLA.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 2.Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015 Nov;123(5):1209–15. doi: 10.3171/2014.9.JNS141550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 2018 Mar;286(3):992–9. doi: 10.1148/radiol.2017170053. [DOI] [PubMed] [Google Scholar]
- 4.Bokka S, Trivedi A. Histopathological study of the outer membrane of the dura mater in chronic sub dural hematoma: its clinical and radiological correlation. Asian J Neurosurg. 2016 Jan-Mar;11(1):34–8. doi: 10.4103/1793-5482.154979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borger V, Vatter H, Oszvald Á, Marquardt G, Seifert V, Güresir E. Chronic subdural haematoma in elderly patients: a retrospective analysis of 322 patients between the ages of 65-94 years. Acta Neurochir (Wien) 2012 Sep;154(9):1549–54. doi: 10.1007/s00701-012-1434-x. [DOI] [PubMed] [Google Scholar]
- 6.Foreman P, Goren O, Griessenauer CJ, Dalal SS, Weiner G, Schirmer CM. Middle meningeal artery embolization for chronic subdural hematomas: cautious optimism for a challenging pathology. World Neurosurg. 2019 Jun;126:528–9. doi: 10.1016/j.wneu.2019.03.160. [DOI] [PubMed] [Google Scholar]
- 7.Kim SO, Jung SI, Won YS, Choi CS, Yang JY. A comparative study of local versus general anesthesia for chronic subdural hematoma in elderly patients over 60 years. Korean Journal of Neurotrauma. 2013;9(2):47–51. [Google Scholar]
- 8.Liu W, Bakker NA, Groen RJM. Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg. 2014 Sep;121(3):665–73. doi: 10.3171/2014.5.JNS132715. [DOI] [PubMed] [Google Scholar]
- 9.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 2001 Aug;41(8):371–81. doi: 10.2176/nmc.41.371. [DOI] [PubMed] [Google Scholar]
- 10.Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. 2019 Feb;122:613–9. doi: 10.1016/j.wneu.2018.11.167. [DOI] [PubMed] [Google Scholar]
- 11.Tiwari A, Dmytriw AA, Bo R, Farkas N, Ye P, Gordon DS, et al. Recurrence and coniglobus volumetric resolution of subacute and chronic subdural hematoma post-middle meningeal artery embolization. Diagnostics (Basel) 2021 Feb;11(2):257. doi: 10.3390/diagnostics11020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008 Dec;63(6):1125–9. doi: 10.1227/01.NEU.0000335782.60059.17. discussion 1129. [DOI] [PubMed] [Google Scholar]