Abstract
Clinical outcomes for intermediate or high-risk pulmonary emboli (PE) remain sub-optimal, with limited improvements in survival for the past 15 years. Anticoagulation alone results in slow thrombus resolution, persistent right ventricular (RV) dysfunction, patients remaining at risk of haemodynamic decompensation and increased likelihood of incomplete recovery. Thrombolysis elevates risk of major bleeding and is thus reserved for high-risk PE. Thus, a huge clinical need exists for an effective technique to restore pulmonary perfusion with minimal risk and avoidance of lytic therapy. In 2021, large bore suction thrombectomy (ST) was introduced in Asia for the first time and this study assessed the feasibility and short-term outcomes of Asian patients undergoing ST for acute PE. 40 consecutive patients (58% male, mean age of 58.3 ± 16.6 years) with intermediate (87.5%) or high-risk PE (12.5%) were enrolled in this prospective registry. 20% had prior VTE, 42.5% had contraindications to thrombolysis, and 10% failed to respond to thrombolysis. PE was idiopathic in 40%, associated with active cancer in 15% and post-operative status in 12.5%. Procedural time was 124 ± 30 min. Emboli were aspirated in all patients without the need for thrombolytics, resulting in a 21.4% reduction in mean pulmonary arterial pressures and 123% increase TASPE-PASP ratio, a prognostic measure of RV-arterial coupling. (both p < 0.001) Procedural complications were 5% and 87.5% patients survived to discharge without symptomatic VTE recurrence during 184 days of mean follow-up. ST affords an effective reperfusion option for PE without thrombolytics, normalises RV overload and provides excellent short-term clinical outcomes.
Keywords: Pulmonary embolism, Thrombectomy, Suction, Catheter
Key points
Large bore suction thrombectomy is an effective minimally invasive reperfusion therapy for patients with intermediate or high-risk pulmonary embolism (PE), without the use of thrombolytics.
Significant reductions in mean pulmonary arterial pressures and heart rates were achieved, resulting in normalisation of right ventricular (RV) strain, size and improvement in RV-pulmonary arterial coupling and stroke volumes.
95% of PE patients who did not require cardiopulmonary resuscitation, survived to discharge without VTE recurrence during 184 days of mean follow-up.
Ongoing multi-centre randomized controlled trials comparing suction thrombectomy to routine care will confirm the benefits of early catheter-based reperfusion therapy for PE.
Introduction
Clinical outcomes for patients diagnosed with intermediate or high-risk pulmonary emboli (PE) remain sub-optimal, with limited improvements in survival rates for the past 10 years [1, 2]. Anticoagulation alone for PE results in slow thrombus resolution, persistent right ventricular (RV) dysfunction, patients remaining at risk of haemodynamic decompensation and increased the likelihood of incomplete recovery [2–4]. Systemic thrombolysis is associated with elevated risk of major bleeding and is thus reserved for high-risk PE [3, 5]. Thus, a huge clinical need exists for an effective technique to restore pulmonary perfusion with minimal risk and avoidance of lytic therapy.
Whilst effective, surgical embolectomy is extremely invasive and in clinical practice, reserved as treatment of last resort [5]. Catheter-directed thrombolysis or low-dose thrombolytic therapy incurs a smaller risk of bleeding but would still be contraindicated in a sizeable percentage of PE patients due to bleeding risk and co-morbidities [6]. In 2018, a large bore, catheter-based suction thrombectomy (ST) system (FlowTriever, Inari Medical, CA, USA) attained regulatory approval for the treatment of PE without the need for the use of thrombolytics, with promising early results [7]. In late 2021, the FlowTriever catheters were introduced into Singapore. This prospective registry enrolled consecutive PE patients from 5 different Singaporean hospitals who underwent ST for acute PE. This study examined the feasibility, procedural and short-term clinical outcomes of ST.
Methods
Study population and settings
Singapore is a multi-ethnic Asian country with a population of 5.4 million. Healthcare is provided by a government-run publicly funded universal healthcare system combined with a significant private healthcare sector. Consecutive patients from 2 public hospitals (National University Hospital and Ng Teng Fong General Hospital) and 3 private hospitals (Mount Elizabeth Orchard, Mount Elizabeth Novena and Gleneagles Hospitals) with intermediate or high-risk PE, according to European Society of Cardiology (ESC) risk stratification system, who underwent ST, were enrolled in a prospective procedural registry [5]. In the 2 public hospitals, institutional PE response teams and treatment algorithms had been implemented since 2017 [8].
Large bore suction thrombectomy.
The operators are all interventional cardiologists with sub-speciality interests in pulmonary hypertension, structural heart disease, coronary and peripheral intervention, cardiac electrophysiology and intensive care, bringing a range of different skillsets to the team. ST were performed with at least 2 operators working in tandem after case discussion, consensus decision making and obtaining informed consent. This new procedure was approved by the respective institutional medical boards with results regularly audited to ensure safe performance. All procedures were conducted in the cardiac catheterisation laboratories, with conscious sedation and local anaesthesia. General anaesthesia was avoided whenever possible. Anticoagulation was uninterrupted and systemic thrombolysis was not a contraindication for ST. Right common femoral venous access was attained under ultrasonic guidance, with or without micropuncture access kits to allow for passage of 8Fr short venous sheaths. Heparin boluses were given to maintain an activated clotting time (ACT) of more than 250 s. The pulmonary artery was accessed with a 6 Fr pigtail catheter (Boston Scientific, MA, USA) and invasive pulmonary arterial pressures were measured. A stiff exchange length guidewire (Amplatz Super Stiff, Boston Scientific, MA, USA) was positioned distally in the target lung. The short femoral venous sheath was exchanged for a larger 24Fr introducer sheath. (Intri, Inari Medical, CA, USA) The FlowTriever (preferably the 24Fr) catheter was passed into each lung whereby ST was performed. Selective pulmonary angiography was performed using the FlowTriever after a few suction attempts to determine residual clot burden and plan subsequent catheter positioning. Typically, less than 60 mls of contrast was used in each case. When available, the blood removed were passed through a microfilter (FlowSaver, Inari Medical, CA, USA) and returned via the femoral introducer sheath to minimise blood loss. PA pressures were re-measured at the end of the procedure before all catheters were removed. Haemostasis was achieved by suture mediated closure devices (Perclose, Abbott, IL, USA) or figure of 8 subcutaneous suture held tight by a retention device. (FlowStasis, Inari Medical, CA, USA)
Patient management and follow-up
Figure of 8 sutures were released the following morning. Patients were recovered in high-dependency units overnight and discharged to home or the general ward if well. Patients were discharged on uninterrupted anticoagulation for at least 6 months, with regular 3 monthly review in the outpatient clinic. At the discretion of the admitting physicians, transthoracic echocardiography may be performed before and/or after ST.
Outcome measurements
The primary endpoint was changes in mean PA pressures following ST. Secondary endpoints included changes in heart rate, systemic blood pressure and echocardiographic parameters (in a subset of patients) namely PA systolic pressure (PASP), tricuspid annular systolic plane systolic plane excursion (TAPSE), right ventricle to left ventricle (RV:LV) ratio and right ventricular outflow tract velocity time integral.(RVOT VTI) The TAPSE/PASP ratio, which reflects right ventricular-pulmonary arterial coupling, is a validated prognostic marker of 30 day mortality in PE, with an optimal cut off value of 0.4 mm/mmHg for predicting adverse outcomes in acute PE patients [9]. The RVOT VTI is a significant predictor of low cardiac index, with values of less than 9.5 cm associated with increased risk of PE-related mortality [10]. RV:LV ratio was obtained using the RV and LV internal diameter in diastole at the level of tip of the atrioventricular valve leaflets from the apical 4 chamber view. Pulmonary artery systolic pressures (PASP) were estimated by the modified Bernoulli principle utilising tricuspid regurgitation velocities and adding the estimated right atrial pressure. RVOT VTI was obtained utilising pulse wave doppler at the level of the right ventricular outflow adjacent to the pulmonary valve from the parasternal short axis view. TAPSE was measured on M-mode images as the difference in RV basal motion from peak systole to end-diastole.
Statistical analysis
Summary statistics were presented as mean ± SD for parametric data and median with inter-quartile range for non-parametric data. Comparisons of clinical and hemodynamic characteristics before and after the index procedure were conducted with paired Student’s t-tests or the Fisher exact test. A p value of < 0.05 was considered to indicate statistical significance.
Results
Patient demographics
40 consecutive patients were recruited with a mean age of 58.3 ± 16.6 years of which 57.5% were male. 20% of patients had a prior history of venous thromboembolism. (VTE) 32.5% were inpatients when they developed symptoms of PE. (Table 1) The cause of PE was idiopathic in 40%, related to cancer in 20%, immobility or post stroke in 7.5% and temporally associated with recent COVID19 infection or vaccination in 7.5%. Hypertension was the most common co-morbidity, affecting 40% of this cohort, with 25% being diabetic and 15% having ongoing oncological treatments. PE was diagnosed by computed tomography pulmonary angiography (CTPA) in all patients and the mean RV/LV ratio on CTPA was 1.4 ± 0.3. Cardiac biomarkers, namely high sensitivity troponin or NT-proBNP were elevated in 95% of patients. In all but 1 patient, PE were bilateral. According to the ESC PE risk stratification algorithm, 12.5% were considered to have high-risk PE, 82.5% had intermediate-high risk and 5% had intermediate-low risk PE. 4 (10%) patients had already failed to respond to systemic thrombolysis and a further 42.5% (recent surgery in 8 patients, recent stroke in 2, anaemia in 3, gastrointestinal bleed in 3, haemoptysis in 1 and haematuria in 1) had contraindications to thrombolysis. Of the 5 high-risk patients, 3 had experienced cardiac arrest requiring cardiopulmonary resuscitation (CPR), of which one patient was having ongoing chest compressions with a mechanical chest compression device during ST. Two of them had received peri-resuscitation thrombolysis with 50 mg of alteplase.
Table 1.
Patient Demographics
| Male gender | 57.5% | Prior VTE | 20% |
|---|---|---|---|
| Mean age | 58.3 ± 16.6 years (range − 20 to 85 years) |
Status at PE onset | 32.5% inpatient |
| Mean RV/LV ratio on CT scan | 1.4 ± 0.3 | Elevated cardiac biomarkers (troponin or NT-proBNP) | 95% |
| Cause of PE | Co-morbidities | ||
| Idiopathic | 40% | Hypertension | 40% |
| Cancer | 20% | Active cancer | 15% |
| Surgery | 12.5% | Systolic heart failure | 5% |
| Immobility | 5% | Stroke | 7.5% |
| Stroke | 2.5% | Diabetes Mellitus | 25% |
| COVID-19 infection / vaccination | 7.5% | Chronic kidney disease | 5% |
| Obesity (BMI > 30 kg/m2) | 17.5% | ||
| None | 25% | ||
| PE location | 97.5% bilateral | ESC PE risk | 12.5% high risk |
| 2.5% unilateral + intracardiac clot in transit | 82.5% intermediate-high | ||
| 5% intermediate-low | |||
| Failure to respond to thrombolysis | 10% | Contraindications to systemic thrombolysis | 42.5% |
| Skin to skin procedural times | 123.8 ± 29.9 | Device dwell times | 90.6 ± 31.5 mins |
| Clot aspirated | 100% | VTE recurrence during follow-up | 0% |
ESC – European Society of Cardiology; PE - pulmonary embolism; VTE- venous thromboembolism
Procedural results
The mean skin-to-skin procedural times were 123.8 ± 29.9 min and FlowTriever dwell times were 90.6 ± 31.5 min. Clots were aspirated in all patients. (Fig. 1) Median blood loss in the first 10 cases was 250 mls (IQR 200 to 300 mls) which decreased to 50 mls (IQR 20 to 100mls) in the subsequent 30 cases, with the introduction of a cell saver that allowed for return of micro-filtered blood. Overall, there were significant decreases in heart rate, pulmonary arterial pressures and increase in oxygen saturation. (Table 2) Mean pulmonary arterial pressures decreased by 26.7%. Systolic blood pressure increased significantly by 19% in high-risk PE patients. (p < 0.01) There were no cases with significant haemodynamic instability during the ST procedure that required initiation of CPR, intubation and mechanical ventilation or temporary pacing. In 1 case described above, the patient was having ongoing CPR before the ST procedure commenced.
Fig. 1.
Images of thrombi aspirated for patients, placed on an anatomical representation of the pulmonary arteries
Table 2.
Effects of suction thrombectomy on haemodynamics and echocardiographic parameters
| All patients (n = 40) | Pre thrombectomy | Post thrombectomy | P value |
|---|---|---|---|
| BP (mmHg) | 115 ± 20 / 75 ± 18 | 121 ± 18 / 79 ± 12 | 0.21 |
| h (bpm) | 96 ± 17 | 87 ± 17 | < 0.01 |
| SaO2 (%) | 94 ± 6 | 98 ± 2 | < 0.01 |
| PA pressure (mmHg) | 47 ± 14 /16 ± 6 | 33 ± 9 / 15 ± 5 | < 0.001 |
| Mean PA pressure (mmHg) | 28.8 ± 6.1 | 22.1 ± 5.1 | < 0.001 |
| Intermediate Risk (n = 35) | |||
| BP (mmHg) | 117 ± 19 / 76 ± 18 | 122 ± 19 / 80 ± 12 | 0.32 |
| h (bpm) | 94 ± 16 | 87 ± 17 | < 0.01 |
| SaO2 (%) | 94 ± 6 | 98 ± 2 | < 0.001 |
| High Risk (n = 5) | |||
| BP (mmHg) | 96 ± 9 / 68 ± 11 | 114 ± 9 / 77 ± 9 | < 0.01 |
| h (bpm) | 112 ± 11 | 87 ± 17 | < 0.01 |
| Patients with transthoracic echocardiography performed pre- and post-thrombectomy (n = 17) | |||
| RV:LV ratio | 1.2 ± 0.2 | 0.7 ± 0.2 | < 0.001 |
| PASP (mmHg) | 51.1 ± 11.5 | 32.1 ± 12.1 | < 0.001 |
| TAPSE (mm) | 15.8 ± 4.9 | 19.8 ± 2.4 | < 0.001 |
| TAPSE:PASP ratio (mm/mmHg) | 0.31 ± 0.11 | 0.69 ± 0.19 | < 0.001 |
| RVOT VTI (cm) | 6.5 ± 1.3 | 13.0 ± 2.5 | < 0.001 |
Fig. 2.
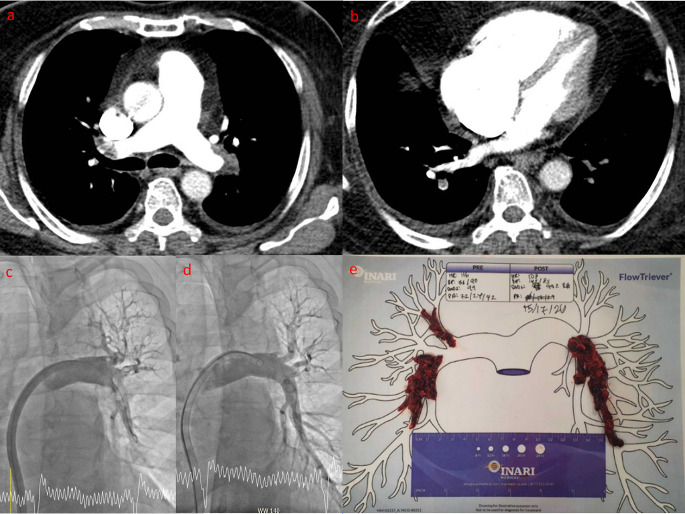
Representative example of a patient with intermediate risk pulmonary embolism (PE) undergoing suction thrombectomy. (ST) Panel a – computed tomography pulmonary angiography showing bilateral PE. Panel b – Note the RV dilatation and the RV:LV ratio exceeding 1. Panel c – Intraprocedural contrast angiography of the right pulmonary artery showing thrombus in the right interlobar artery with diminished perfusion to the lower lobe. Panel d – angiography repeated after ST confirming reduced thrombus burden and improved lower lobe perfusion. Panel e – thrombus aspirated during this case
Clinical outcomes
There were no intraprocedural deaths. Overall, 87.5% of patients (n = 35) survived to discharge. None of the 3 patients who experienced cardiopulmonary arrest survived to discharge and succumbed to multi-organ failure including hypoxic ischaemic encephalopathy, despite maximal supportive measures. 1 patient with intermediate-high risk PE died from systemic melioidosis which was responsible for acute and chronic PE. There was only 1 procedural-related death which occurred in a patient with intermediate-high risk PE. Copious amounts of thrombi were aspirated from both lungs and 1 h after ST, developed reperfusion injury, requiring initiation of extra-corporeal membrane oxygenation. (ECMO) Pulmonary haemorrhages were excluded by repeat CTPA and despite maximal supportive measures, died 3 days later. Thus, the survival to discharge rates for high risk and intermediate risk PE were 40% and 95%. There was only one other procedural complication which was a femoral haematoma that was treated conservatively.
Median length of hospital stay was 6 days (IQR of 3 to 10 days), and ICU stay was 2 days (IQR of 1 to 3 days). For patients developed PE outside of the hospital and was admitted acutely, the median hospital and ICU stay were only 4 and 2 days respectively. 60% of patients who underwent lower limb doppler scans had evidence of deep venous thrombi, all of which were treated medically. 74% were discharged on direct acting oral anticoagulants, 17% on low molecular weight heparins and the remainder on warfarin. During 184 ± 109 days of follow-up, there were no further mortality or VTE recurrences. All 35 patients who survived to discharge completed 30 days follow-up. There was no additional mortality between discharge and 30 days post-procedure.
Echocardiographic outcomes
17 patients underwent transthoracic echocardiography before and after ST. There were significant reductions in RV:LV ratio, PASP and TASPE whilst TASPE:PAP ratio and RVOT VTI increased significantly. (Table 2). A TASPE/PASP ratio cut-off of less than 0.4 mm/mmHg predicted adverse outcomes in acute PE. Pre-ST, 15 out of 17 (88%) had a TASPE/PASP ratio of less than 0.4 mm/mmHg compared to 2 out of 17 (12%) post ST. RVOT VTI of less than 9.5 cm was an adverse prognostic indicator, correlating with low cardiac index in acute PE. No patient had a RVOT VTI of more than 9.5 cm before ST whereas all patients had RVOT VTI of more than 9.5 cm after ST.
Discussion
In our cohort of 40 consecutive Asian patients with acute PE, ST successfully aspirated clot in all patients with minimal blood loss and avoidance of thrombolytics. Acutely, there was a significant reduction in RV overload as evident by a fall in pulmonary arterial pressures and RV size. Cardiac output and RV-PA coupling significantly improved as evident by a significant fall in heart rate and a concomitant increase in arterial blood pressure, oxygen saturations, TASPE:PASP ratio and RVOT VTI. Survival to discharge rates exceeded 94% in patients who had not experienced cardiac arrest, with procedural complication rates of 5%. (1 femoral haematoma and 1 procedural-related mortality due to reperfusion lung injury)
Results from a prospective, European registry of 1001 acute PE patients published in 1997 revealed that in-hospital mortality was 11% for intermediate risk PE and 33% for higher risk PE [11]. In the larger International Cooperative Pulmonary Embolism Registry (ICOPER) registry which reported clinical outcomes of PE patients in 1999, the 3 month all-cause mortality rate was 15.1% for haemodynamically stable patients and 58.3% for those with haemodynamically unstable PE [12]. As recent as 2012, the Italian Pulmonary Embolism Registry (IPER) reported 31.8% in-hospital all-cause mortality for patients with haemodynamically unstable PE [13]. In these series, patients were anticoagulated with heparin and as many as 48% of patients in 1 cohort received early thrombolysis. The findings of the PEITHO study does not support routine thrombolysis for non-massive PE due to the excess bleeding risk of systemic thrombolysis with 100 mg alteplase without any significant reduction in PE related mortality. Furthermore, as many as 32% of PE patients have contraindications to thrombolysis, 13% may fail to respond to thrombolysis and residual pulmonary obstruction with or without thrombolysis is an adverse prognostic marker for in-hospital and long-term survival [14, 15]. We had previously reported a similar survival to discharge rate of 70% and 89.2% for high-risk and intermediate-risk PE out of 321 acute PE patients from our institution [8]. Thus, there exists a clinical need to safely, expediently and effectively relieve pulmonary vascular obstruction, reduce right heart strain without the need for major invasive surgery and avoiding the excessive bleeding risk associated with thrombolytic use.
RV dysfunction and failure due to acute pressure overload is considered the primary cause of death in severe PE. Hence early reperfusion to reduce pressure overload, avoid decompensation and re-establish RV-PA coupling, in the most expedient, safest way, should theoretically improve survival from acute PE. In the multi-centre, prospective FLASH registry of 800 American PE patients (7.9% high-risk and 92.1% intermediate-risk) undergoing ST, mean PAP post ST decreased by 23% accompanied by a 11.2% reduction in heart rate and a normalisation of RV dilatation on echocardiography. These findings are strikingly consistent with our haemodynamic findings. The 30-day all-cause mortality was only 0.8% in the FLASH registry, which is far lower than expected based on historical data described above. Uniquely in our study, changes in TASPE, PASP and RVOT VTI in response to ST were measured and demonstrated for the first time that RV-PA coupling and RV output significantly improved post ST, which may underlie improvements in patient outcomes.
Mean procedural and device dwell times in the FLASH registry were much shorter than in our experience, which reflects the learning curve associated with ST. Despite the longer procedural times, procedural complications were uncommon, which may be attributed to 2 experienced interventional cardiologists performing ST in tandem. Ongoing randomized studies such as HI-PEITHO and PEERLESS will confirm and quantify the additive benefit of catheter-based therapies for PE compared to established medical therapy alone.
The limitations of this study include the study design of being a single-arm, all-comers registry of a relatively small sample size and lack of direct comparison with a contemporaneous control group treated more conservatively. However, the inclusion of consecutive patients allows for unique insight into the safety, feasibility of performing suction thrombectomy in any centres providing such therapies for the first time. Survival and more robust clinical endpoints would require further clarification from ongoing randomized multi-centre studies.
Conclusions
Large bore suction thrombectomy was acutely successful in all PE patients and significantly improved haemodynamics, pulmonary perfusion, reduced right ventricular overload, with minimal blood loss, low procedural complication rates and avoidance of thrombolytics. In patients who had not decompensated or developed cardio-pulmonary arrest, clinical outcomes were excellent with survival to discharge above 94%.
Acknowledgements
Preliminary data of the first 30 patients in this cohort was presented as an abstract at the 2022 AICT-AsiaPCR conference, Singapore and the abstract was awarded as the best abstract of the conference.
Author Contribution
All authors contributed to the study conception and design. Data collection and analysis were performed by Dr Pipin Kojodjojo, Lim Yinghao and Dr Ong Heng Ann. The first draft of the manuscript was written by Dr Pipin Kojodjojo and all authors commented on various versions of this manuscript. All authors read and approved the final manuscript for submission.
Funding
No funding was received for conducting this study.
Declarations
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in a priori approval by the institutional Human Research Committee. (National Healthcare Group Domain Specific Review Board Approval number 2016/00750)
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/15/2023
A Correction to this paper has been published: 10.1007/s11239-023-02810-0
References
- 1.Martin KA, Molsberry R, Cuttica MJ, Desai KR, Schimmel DR, Khan SS. Time Trends in Pulmonary Embolism Mortality Rates in the United States, 1999 to 2018. J Am Heart Assoc. 2020;9:e016784. doi: 10.1161/JAHA.120.016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keller K, Tesche C, Gerhold-Ay A, Nickels S, Klok FA, Rappold L, Hasenfuss G, Dellas C, Konstantinides SV, Lankeit M. Quality of life and functional limitations after pulmonary embolism and its prognostic relevance. J Thromb Haemost. 2019;17:1923–1934. doi: 10.1111/jth.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galie N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV, Investigators P. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 4.Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, Tebbe U, Horstkotte J, Muller R, Blessing E, Greif M, Lange P, Hoffmann RT, Werth S, Barmeyer A, Hartel D, Grunwald H, Empen K, Baumgartner I. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129:479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Meyer G. The 2019 ESC Guidelines on the diagnosis and management of Acute Pulmonary Embolism. Eur Heart J. 2019;40:3453–3455. doi: 10.1093/eurheartj/ehz726. [DOI] [PubMed] [Google Scholar]
- 6.Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, Savani C, Patel N, Arora S, Deshmukh A, Bhatt P, Alfonso C, Cohen M, Tafur A, Elder M, Mohamed T, Attaran R, Schreiber T, Grines C, Badheka AO. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2015;86:1219–1227. doi: 10.1002/ccd.26108. [DOI] [PubMed] [Google Scholar]
- 7.Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, Khandhar S, Amin R, Weinberg M, Engelhardt T, Hunter M, Holmes D, Hoots G, Hamdalla H, Maholic RL, Lilly SM, Ouriel K, Rosenfield K, Investigators FA, Prospective Single-Arm, Multicenter Trial of Catheter-Directed Mechanical Thrombectomy for Intermediate-Risk Acute Pulmonary Embolism: the FLARE Study. JACC Cardiovasc Interv. 2019;12:859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Jen WY, Kristanto W, Teo L, Phua J, Yip HS, MacLaren G, Teoh K, Sim TB, Loh J, Ong CC, Chee YL, Kojodjojo P. Assessing the impact of a Pulmonary Embolism Response Team and Treatment Protocol on Patients presenting with Acute Pulmonary Embolism. Heart Lung Circ. 2019 doi: 10.1016/j.hlc.2019.02.190. [DOI] [PubMed] [Google Scholar]
- 9.Lyhne MD, Kabrhel C, Giordano N, Andersen A, Nielsen-Kudsk JE, Zheng H, Dudzinski DM. The echocardiographic ratio tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure predicts short-term adverse outcomes in acute pulmonary embolism. Eur Heart J Cardiovasc Imaging. 2021;22:285–294. doi: 10.1093/ehjci/jeaa243. [DOI] [PubMed] [Google Scholar]
- 10.Brailovsky Y, Lakhter V, Weinberg I, Porcaro K, Haines J, Morris S, Masic D, Mancl E, Bashir R, Alkhouli M, Rosenfield K, Mathew V, Lopez J, Bechara CF, Joyce C, Fareed J, Darki A. Right thromular outflow Doppler predicts low Cardiac Index in Intermediate Risk Pulmonary Embolism. Clin Appl Thromb Hemost. 2019;25:1076029619886062. doi: 10.1177/1076029619886062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, Just H. Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart. 1997;77:346–349. doi: 10.1136/hrt.77.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 13.Casazza F, Becattini C, Bongarzoni A, Cuccia C, Roncon L, Favretto G, Zonzin P, Pignataro L, Agnelli G. Clinical features and short term outcomes of patients with acute pulmonary embolism. The italian Pulmonary Embolism Registry (IPER) Thromb Res. 2012;130:847–852. doi: 10.1016/j.thromres.2012.08.292. [DOI] [PubMed] [Google Scholar]
- 14.Meneveau N, Ming LP, Seronde MF, Mersin N, Schiele F, Caulfield F, Bernard Y, Bassand JP. In-hospital and long-term outcome after sub-massive and massive pulmonary embolism submitted to thrombolytic therapy. Eur Heart J. 2003;24:1447–1454. doi: 10.1016/s0195-668x(03)00307-5. [DOI] [PubMed] [Google Scholar]
- 15.Toma C, Jaber WA, Weinberg MD, Bunte MC, Khandhar S, Stegman B, Gondi S, Chambers J, Amin R, Leung DA, Kado H, Brown MA, Sarosi MG, Bhat AP, Castle J, Savin M, Siskin G, Rosenberg M, Fanola C, Horowitz JM, Pollak JS. Acute outcomes for the full US cohort of the FLASH mechanical thrombectomy registry in pulmonary embolism. EuroIntervention. 2022 doi: 10.4244/EIJ-D-22-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]