Abstract
Background
Obesity poses significant challenges to healthcare globally, particularly through its bi‐directional relationship with co‐morbid metabolic conditions such as type 2 diabetes and hypertension. There is also emerging evidence of an association between obesity and chronic kidney disease (CKD) which is less well characterized.
Methods
A literature search of electronic libraries was conducted to identify and present a narrative review of the interplay between obesity and CKD.
Findings
Obesity may predispose to CKD directly as it is linked to the histopathological finding of obesity‐related glomerulopathy and indirectly through its widely recognized complications such as atherosclerosis, hypertension, and type 2 diabetes. The biochemical and endocrine products of adipose tissue contribute to pathophysiological processes such as inflammation, oxidative stress, endothelial dysfunction, and proteinuria. The prevention and management of obesity may prove critical in counteracting both the development and advancement of CKD. Moreover, measures of abdominal adiposity such as waist circumference, are generally associated with worse morbidity and mortality in individuals receiving maintenance hemodialysis.
Conclusion
Obesity is a risk factor for the onset and progression of CKD and should be recognized as a potential target for a preventative public health approach to reduce CKD rates within the general population. Future research should focus on the use of glucagon‐like peptide‐1 receptor agonists and sodium–glucose cotransporter 2 inhibitors in patients with CKD and obesity due to their multi‐faceted actions on major outcomes.
Keywords: chronic kidney disease, dialysis, obesity, transplantation
Visual Abstract
Obesity poses significant challenges to healthcare globally, particularly through its bi‐directional relationship with co‐morbid metabolic conditions such as type 2 diabetes and hypertension. In addition, it increases the risk of onset and progression of CKD through direct and indirect pathways. Therefore, obesity should be recognized as a potential target for a preventative public health approach to reduce CKD rates within the general population.
1. INTRODUCTION
Obesity is an important risk factor for premature death and the development of a plethora of non‐communicable diseases such as type 2 diabetes mellitus (T2DM), heart disease, hypertension, stroke, malignancy, and chronic kidney disease (CKD). 1 , 2 CKD is a global health issue associated with poor health outcomes and quality of life, as well as serious systemic complications including cardiovascular disease (CVD), metabolic bone disorder, anemia, and acid‐base and fluid imbalance. 3 , 4 It is a complex disease, delineated by the presence of abnormalities of kidney function or structure present for more than 3 months. 5 The progressive and irreversible nature of CKD and end‐stage kidney disease (ESKD) mean that they are associated with considerable levels of morbidity, mortality and health resource expenditure 6 ; therefore, avoidable risk factors for onset and progression of CKD and ESKD should be explored. As obesity is preventable, 7 , 8 patient education and communication with regards to good lifestyle management promotion, through nutritional intake and exercise, may play a substantial role in reducing the risk of onset of both CKD and obesity.
The rising prevalence of obesity has considerable implications for CKD. 9 In England, during 2018–19, the estimated prevalence of CKD stages 3–5 in adults was 4.1%, whilst in Scotland it was 3.1%. Moreover, the incidence of patients receiving renal replacement therapy (RRT) of any form has increased over time throughout the United Kingdom. 10 A longitudinal screening study in the United Kingdom primary care setting observed that greater than 40% of people living with CKD were undiagnosed without screening, arguing the need for more robust surveillance to promptly diagnose the disease which is frequently asymptomatic in its early stages. 11 CKD is also a global concern and, in 2017, the results from a systematic review and meta‐analysis estimated the population of individuals with CKD across the planet to be 697.5 million. Meanwhile in the same year, estimated mortality from CKD was 1.2 million, constituting it as the 12th largest cause for deaths worldwide—an appreciable rise from the late twentieth century. 12
2. ETIOLOGICAL FACTORS IN OBESITY
An excessive accumulation of body fat leads to a non‐optimal health state due to a greater risk of attaining diseases, target‐organ damage 1 , 13 and a shortened life expectancy. Data from the United States showed marked race and sex differences in observed years of life lost (YLL). White men aged 20–30 years with severe obesity had a maximum of 13 YLL compared to 8 YLL for White women of the same age. However, for Black individuals, men have significantly higher YLL at 20 and women have 5 YLL. 14 In a study from Australia, higher body mass index (BMI) was associated with shorter life expectancy at any age for both men and women. The highest risk reported was for individuals in 20–39‐year age groups who had 8–10 YLL. 15 Obesity predominates in the pathogenesis of metabolic abnormalities such as hyperglycaemia, insulin resistance, dyslipidaemia and atherosclerosis. 16 , 17
Overweight and obesity manifest more commonly in individuals with a mono‐, oligo‐ or polygenic predisposition, and there are a number of genes implicated in the development of a heritable obesogenic phenotype. 18 There is no accurate estimate of the scale of the genetic contribution to heritable obesity. However, a metanalysis of 25 family studies showed a BMI heritability estimate to range from 24% to 81%. 19 Non‐syndromic, monogenic causes of obesity, though rare, are linked strongly to homozygous mutations in genes contributing to the formation of the brain‐adipose axis. These include loss of function mutations in genes which encode for components along the leptin‐melanocortin pathway such as leptin (LEP), leptin receptor (LEPR), melanocortin 4 receptor (MC4R), proconvertase 1 (PCSK1) and proopiomelanocortin (POMC), resulting in severe, early‐onset and penetrant obesity. 20 , 21 , 22 , 23 Leptin is central to alerting the brain with regards to energy intake; it is a satiety‐inducing polypeptide produced mainly by adipocytes, and functions in the regulation of food intake, energy homeostasis and body mass. 21 Oligogenic variants of obesity develop through defects in these genes when inherited in a heterozygous manner, and are characterized by greater variability in the spectrum of obesity along with more degree of dependence upon environmental factors. 22 Oligogenic obesity accounts for approximately 3% of cases of obesity in adults and children. 23
The majority of potentially heritable obesity however, is polygenic and this underpins most cases of “common” obesity. 23 Here, it is the cumulative contribution of multiple loci in synergy with epigenetic modulation and importantly, a fat‐promoting lifestyle and environment, which bring about increased adipose deposition and raised BMI. 22 For instance, the FTO gene was the first to be identified by genome‐wide association studies (GWAS) as a known and prominent contributor to polygenic obesity at a population level. SNPs on the first intron of this gene, mapped on chromosome 16, show a significant association with the onset of T2DM and increased BMI. 24 , 25 The use of GWAS technology has enabled greater pathophysiological understanding of polygenic obesity by delineating hundreds of novel polymorphisms which strongly influence BMI, although the current identities may explain a fraction of the total variance. 26 , 27 Similarly, certain genes which are implicated in monogenic obesity also display variations which influence BMI and contribute to the risk of attaining polygenic obesity. 28 , 29 , 30 , 31
A heritable link to obesity is supported strongly across the literature, 22 , 23 although the effects of environment and lifestyle factors are also crucial clinically. Increased adiposity is produced by a homeostatic imbalance between calorific input and energy expenditure resulting in weight gain due to a positive net energy balance. The prevalence of an obesogenic environment and overnutrition; coupled with lifestyle behaviors are implicated in the recent escalation of obesity globally. 32 , 33 Current United Kingdom guidelines recommend that the saturated fat content in the diet should be no greater than 11% of daily calorific input, whilst free sugars should form no more than 5%. 34 Moreover, lack of physical activity, a rise of which has developed over the past decades, has shown significant correlation with raised BMI, CVD and metabolic syndrome in studies. 35 , 36 Certain polymorphisms of the FTO gene implicated in polygenic obesity are accentuated on an environmental background of physical inactivity and overnutrition, highlighting the interaction between genetics and environment in the development of obesity. 37 , 38
3. OBESITY AND KIDNEY DISEASE: RENAL HISTOPATHOLOGICAL CHANGES AND ETIOLOGICAL FACTORS
Obesity impairs kidney function via the direct effects which adiposity exerts on the kidney, and indirectly due to systemic complications of obesity including diabetes mellitus, atherosclerosis and hypertension, Figure 1. 39 , 40 , 41 Obesity can directly injure glomeruli via hemodynamic alterations primarily due to vasodilatation of the afferent arteriole and an increase in proximal tubular salt reabsorption. These changes cause glomerular hyperfiltration and ultimately proteinuria. 42 The accelerating prevalence of obesity globally is paralleled by the increasing incidence of obesity‐related glomerulopathy (ORG); a diagnosis made upon the exclusion of clinical or histopathological evidence of other renal pathology, 43 in individuals with a BMI greater than 30. ORG is characterized by glomerulomegaly presenting alone or with secondary focal segmental glomerulosclerosis found frequently in the glomerular peri‐hilar area. 44 Biopsy studies have shown structural variations consistent with ORG, including glomerulomegaly, decreased podocyte density, and increased width of podocyte foot processes, 44 , 45 enlarged glomerular volume with a correspondingly marked decline in podocyte density, 45 , 46 increased mesangial matrix, 47 and mesangial sclerosis and glomerular basement membrane thickening. 48 The clinical features which occur secondary to these abnormalities include a gradual progression of sub‐nephrotic range proteinuria (<3.5 g/day) found alone or alongside wider renal insufficiency, 44 though the presence of overt nephrotic syndrome in the context of ORG is rare. 42
FIGURE 1.
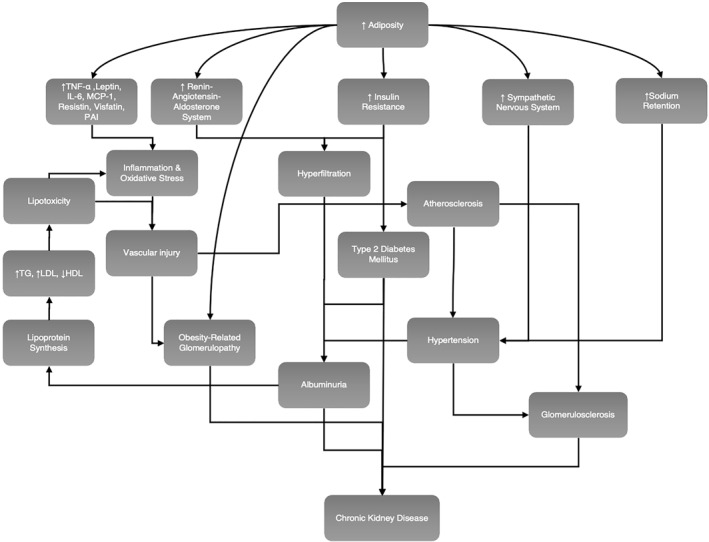
Direct and indirect mechanisms by which adiposity can perturb renal function and lead to kidney disease. HDL, high‐density lipoprotein; IL‐6, interleukin‐6; LDL, low‐density lipoprotein; MCP‐1, monocyte chemo‐attractant protein 1; PAI, plasminogen‐activator inhibitor; TG, triglycerides; TNF‐α, tumor necrosis factor alpha
Underlying mechanisms leading to ORG are thought to include a greater renal hemodynamic and metabolic demand in obesity which causes hyperfiltration, increased proximal tubular sodium reabsorption, greater glomerular tuft volume and later, glomerulosclerosis. 42 , 48 , 49 Indeed, hyperfiltration is understood to be a noteworthy contributor in the mechanism of renal damage in patients with obesity and has been observed independent of hypertension in humans. 50 Obesity can cause increase in filtration fraction (FF) that results in hemoconcentration in the postglomerular circulation and increased oncotic pressure. In a human study on selected lean patients and those with obesity, the group of patients with obesity demonstrated higher glomerular filtration rate (GFR), renal plasma flow, and FF compared to the lean group. The study showed that the increased glomerular filtration led to increased postglomerular oncotic pressure and enhancement of proximal tubular sodium reabsorption. 50 Physiological renal reaction to overcome this included compensatory renal vasodilation and increase in GFR. These processes will ultimately result in hypertension, albuminuria and more kidney damage. 50 , 51 Other processes understood to contribute to the observed glomerular hypertension and hyperfiltration within this context include insulin resistance, sympathetic nervous system over‐activity, and upregulation of the renin‐angiotensin system; this results in an elevated FF and unfavorable renal hemodynamics. 51 , 52 , 53 As such, therapeutic interventions including blockade of the renin‐angiotensin system and sodium restriction can generally have a positive influence on renal hemodynamic status. 53 , 54
The absolute risk of individuals, to develop ORG is comparatively low; indicating that an underlying renal vulnerability increases susceptibility in certain individuals. 55 Predisposing factors which increase the risk of developing ORG are generally understood to involve conditions which lower innate nephron numbers such as intrauterine growth restriction and low birth weight, impaired renal development and congenital renal abnormalities. 46 These determinants, when paired with a period of disproportionate “catch‐up” growth after infancy will cause disparity between body mass to nephron ratio, increasing the risk of glomerular hyperfiltration and hypertension throughout later life in the setting of obesity. 56 , 57 Whilst epidemiological and observational studies have reported 4%–10% of patients with obesity have proteinuria, including microalbuminuria, 42 the true incidence of ORG is unknown as guidelines and indications for renal biopsies vary globally. Consequently, the prevalence of ORG may be underestimated.
Obesity imposes a state of maladaptive persistent low‐grade inflammation, oxidative stress and cellular damage to peripheral tissues including the kidneys. 58 , 59 White adipose tissue is a complex and extremely functional endocrine organ derived from a host of cells comprising of adipocytes, endothelial cells, preadipocytes, leukocytes, macrophages, monocytes and fibroblasts. 60 Inflammatory processes are mediated by the endogenous production of nephrotoxic adipose derived cytokines and mediators, namely tumor necrosis factor, leptin, 61 interleukin 6 (IL‐6), monocyte chemo‐attractant protein 1, resistin, visfatin and plasminogen‐activator inhibitor, which cause renal injury, 41 , 62 , 63 , 64 Table 1.
TABLE 1.
Potential mechanisms to explain the effects of adipocytokines, which are cell signaling molecules secreted by adipose tissue, on renal function
| Adipocytokine | Relevance in kidney disease |
|---|---|
| Leptin |
|
| Resistin |
|
| Visfatin |
|
| TNF |
|
| IL‐6 |
|
| MCP‐1 |
|
| PAI |
|
| Adiponectin |
|
Note: This table summarizes evidence from both human studies and animal studies.
Abbreviations: CKD, chronic kidney disease; FSGS, focal segmental glomerulosclerosis; GFR, glomerular filtration rate; ICAM‐1, intercellular adhesion molecule‐1; IL‐6, interleukin 6; MCP‐1, monocyte chemo‐attractant protein 1; PAI, plasminogen‐activator inhibitor; ROS, reactive oxygen species; TGF‐β, transforming growth factor beta; TNF, tumor necrosis factor; UACR, urinary albumin/creatinine ratio; VCAM‐1, vascular cellular adhesion molecule‐1.
Moreover hypertriglyceridemia, a common abnormality noted in visceral obesity, is also implicated in the progression of kidney disease. CKD itself is also associated with dyslipidaemia, typically characterized by high levels of triglycerides, high levels of low‐density lipoprotein cholesterol, and low levels of high‐density lipoprotein cholesterol. 91 , 92 Abnormal lipid metabolism within this setting may be prompted by microalbuminuria, triggering a compensatory elevation of lipoprotein synthesis by the liver. 93 Dyslipidaemia is also an important risk factor for CVD, 94 and may contribute to renal injury by lipotoxicity, vascular injury, atherosclerosis, glomerulosclerosis, as well as inflammatory and oxidative stress mechanisms. 93 , 95
Furthermore, insulin resistance and associated hyperinsulinemia, important components of the metabolic syndrome, are pathophysiologically important in renal impairment, contributing to glomerular hyperfiltration, albuminuria, elevated vascular permeability, endothelial dysfunction, oxidative stress, and enhanced production of transforming growth factor beta and insulin like growth factor 1. 89 , 96 , 97 Beyond the systemic effects of visceral fat, researchers were interested in the effects of perivascular adipose tissue and other locally acting fat depots which secrete adipokines, cytokines and specific angiogenic factors to the vascular wall. 98 Renal sinus fat (RSF) is a perivascular fat depot located near renal arteries. Studies showed that increased RSF mass under exercise conditions aggravated microalbuminuria. 99 In a study by Wagner et al., RSF was shown to induce kidney damage when acted on by hepatokine fetuin‐A (endogenous ligand for toll‐like receptor 4). There was no measurement of albuminuria to confirm the previously mentioned study. Some studies have shown that the genetic prediction of insulin resistance is not determined by BMI. This indicates that insulin resistance might also be relevant in lean people which can result in T2DM, CVD and CKD. 100 , 101
The association between obesity and renal tubular abnormalities has not been characterized to the same extent as obesity‐induced glomerular injury. However, in a study of human renal biopsy samples in patients with non‐diabetic obesity, the changes to tubular physiology included proximal tubular epithelial cell hypertrophy accompanied with greater proximal tubular volume. 49 Similarly, histological analysis in a metabolic syndrome induced mouse model also showed signs of proximal tubular injury, thought to be instigated by increased levels of acid accumulation. 102
4. OBESITY AND KIDNEY DISEASE: CLINICAL ASSOCIATIONS
The interaction between obesity and CKD has been delineated in numerous studies. 46 , 103 , 104 , 105 A high BMI is a recognized, graded and potent risk factor for renal impairment and ESKD within the general population. 46 , 103 A large body of evidence demonstrates that in contrast to individuals with a normal BMI, individuals with overweight and obesity are at a greater risk of CKD, seen also in patients with a “metabolically‐healthy” obese phenotype. 104 Although the risk of ESKD is higher in people with metabolically healthy obesity compared to metabolically healthy people with normal weight, there is good evidence that people with metabolically healthy obesity have a lower risk compared to individuals with metabolically unhealthy obesity. 105 The risk is similar for other adverse outcomes such as cardiovascular events and all‐cause mortality. 106 Therefore, it is useful to adopt the concept of metabolically healthy obesity to encourage more people to lose weight and reduce the rate of adverse outcomes. 107 To this end, a systematic review and meta‐analysis predicted that approximately 14% and 25% of males and females respectively develop CKD as a clinical consequence of overweight or obesity in industrialized countries. 108
Obesity is a strong risk factor for kidney disease. A cohort study with greater than 320,000 patients showed a stepwise increase in the risk of ESKD with higher BMI. The relative risk of ESKD was 1.87, 3.57, 6.12, and 7.07 for overweight (BMI 25–29 kg/m2), class I obesity (BMI 30–34.9 kg/m2), class II obesity (BMI 35–39.9 kg/m2) and extreme obesity (BMI ≥40 kg/m2) respectively. The ESKD risk was consistently observed after adjustment for sex, race, and presence or absence of diabetes and hypertension. 103 The mechanisms thought to underlie this involved those already mentioned in this review, including hypertension and diabetes which possess a causal relationship with CKD, as well as the harmful effects exerted by adiposity itself. 103 Moreover, in a population‐based Swedish study of adults between the ages of 18 and 74 years, being overweight at the age of 20 years posed a three‐fold increased risk of CKD whilst obesity in males and clinically severe obesity in females at any age was linked to a three‐to‐four‐fold increase in risk, compared to lean adults. The risk was still three‐fold in analyses of patients without underlying diabetes and hypertension, again highlighting the causative association between adiposity and CKD onset. 107
Silverwood et al. 109 also outlined a greater risk of CKD with respect to early age of onset of overweight and obesity. In this study, based upon a cohort born in the year 1946 in England, Scotland and Wales, CKD at the ages of 60–64 years was twice as likely to occur if participants were overweight when assessed at the age timepoints of 26 and 36 years. The strength of the association declined significantly as the age of first‐onset obesity increased. In addition, the study suggested prevention of elevated BMI at any age is linked to a lower burden of CKD, but more so when obesity is prevented during early adulthood. 109 Several other studies, with participants from diverse ethnic backgrounds, similarly report the association of a greater BMI conferring a larger risk of CKD onset and development. 110 , 111 , 112 , 113 , 114
In addition to BMI, anthropometric measures such as waist circumference (WC) and waist/hip ratio (WHR) positively correlate as a risk determinant of CKD. This is perhaps because visceral adiposity poses a more potent metabolic risk than fat located elsewhere and is central to pathological sequelae caused by obesity such as insulin resistance and inflammation. 16 , 115 For example, Nerpin et al. showed a direct correlation between insulin sensitivity and subsequent eGFR level irrespective of BMI in patients with normal fasting glucose and normal glucose tolerance. 116 Evans et al. found significant correlations between WHR and estimated GFR decline, as well as with an increase in urinary albumin‐creatinine ratio and uric acid levels. 117 Central adipose distribution was also detrimental in lean persons in a cohort of 7676 non‐diabetic patients. 118 In this study, participants with normal BMI and central adiposity (apple‐shaped) displayed diminished glomerular filtration, in a dose dependent manner; with increased visceral adiposity conferring increased CKD risk. This was similar in participants with overweight and obesity (pear‐shaped), though importantly, renal impairment was present irrespective of peripheral or central fat distribution in the context of obesity, in this study. 118 In non‐diabetic patients with existing CKD, normal‐weight obesity (characterized by a normal BMI (<25 kg/m2) and a large proportion of body fat mass) was associated with the worst clinical outcome with regards to cardiovascular event composites and all‐cause mortality when compared to normal‐weight lean patients and patients with overweight and obesity. Highest levels of the inflammatory cytokine IL‐6 were also noted in patients with normal‐weight obesity. 119
WC also positively associates with risk of all‐cause mortality in patients with CKD, and may prove an effective, relatively simple and time efficient tool to be used alongside BMI to assess mortality risk in population studies. 120
Obesity is also documented to hasten renal deterioration in pathologies such as IgA nephropathy, 121 and constitutes a greater level of risk for the developing nephrolithiasis. 122 Non‐alcoholic fatty liver disease (NAFLD) is another important condition interlinking obesity and CKD. 120 NAFLD is a condition characterized by the intrahepatic accumulation of fat in the form of triglycerides in people who take minimal or no alcohol. There is conflicting evidence regarding the link between NAFLD and CVD. Some studies shown NAFLD as a risk factor for CKD and CVD independent of the components of metabolic syndrome. 123 , 124 However, an exome‐wide association study identified genetic variants located at the 148Met allele in patatin‐like phospholipase domain‐containing protein 3, and Lysine at residue number 167 (167Lys) allele in transmembrane‐6 superfamily member 2 (TM6SF2) as strong factors for increased liver fat content, progression to cirrhosis, and protection from coronary artery disease. 125 The discordance of the effects of these alleles is not well understood. In a 2017 study, Musso et al. studied the impact of polymorphism on lipoprotein metabolism in 60 individuals without obesity with NAFLD and a matched cohort. They concluded that the 167Lys allele in TM6SF2 might divert cholesterol from vessels into the liver inducing steatosis and cirrhosis. 126
There are multiple putative mechanisms that interlink the pathogenesis of NAFLD and CKD including decreased adiponectin, activated protein kinase (AMPK) activity, and increased fibroblast growth factor 21 activity, increased fetuin‐A, mammalian target of rapamycin activity, and sodium–glucose cotransporter 2 (SGLT2) activity. 120 , 127 In general, an increased production of pro‐inflammatory cytokines and oxidative stress mechanisms promote the onset and progression of both NAFLD and CKD. 128 NAFLD is also shown to be a strong and independent risk factor for CVD in people with advanced CKD. 129 Despite the identification of multiple treatment targets, the only current proven management strategy for NAFLD is weight loss by lifestyle modification (diet and exercise) 130 or bariatric surgery, 131 which further strengthens the link between obesity and NAFLD.
5. WEIGHT MANAGEMENT CONSIDERATIONS IN KIDNEY DISEASE
Because surplus adiposity impacts negatively on renal health both in the general population and in those with existing CKD, 110 targeting obesity is critical in the endeavor to reduce the national and global burden of the disease, and other ailments accelerated by excessive adiposity and metabolic syndrome. Fortunately, volitional weight management fosters good health outcomes in patients with obesity and reduces mortality, 132 improves glycemic status and triglyceride levels, and better cardiometabolic outcomes are highlighted in the literature. 123 , 133 In consideration of renal health, targeted weight loss interventions improve outcomes such as microalbuminuria, proteinuria, hyperfiltration and can normalize GFR. 124 , 134 , 135 Weight loss through caloric restriction and exercise is associated with decreases in body weight and fat proportion, as well as reduced inflammation and oxidative stress in patients with moderate‐to‐severe CKD. 136 Bariatric surgery remains the most effective and enduring form of management of weight and comorbid conditions in patients with clinically severe obesity, 137 and is associated with meaningful post‐surgical benefits in patients with CKD such as stabilized eGFR and significantly slower progression to ESKD. 134 , 138 Coleman et al. reported that bariatric surgery in pre‐dialysis CKD stages 3–5 and obesity stages II and III was associated with a reduction in mortality, irrespective of whether these patients developed ESKD. 139
6. PHARMACOLOGICAL THERAPY TARGETS BOTH OBESITY AND CHRONIC KIDNEY DISEASE
In recent years, glucagon‐like peptide‐1 receptor agonists (GLP1 RA) and SGLT2 inhibitors emerged as effective glucose‐lowering agents with a good safety profile. Although they are licensed primarily to treat patients with T2DM, they proved to have other major positive outcomes including weight loss. SGLT2 inhibitors were proved to reduce the risk of ESKD, cardiovascular events, and mortality in patients with T2DM and CKD. 140 The effects of SGLT2 inhibitors were proved to go beyond the glucose‐lowering effect by showing similar outcomes in people without T2DM but with CKD and proteinuria. 141 Although SGLT2 inhibitors induce weight loss, they are not currently licensed for people with CKD and obesity with no significant proteinuria and/or T2DM. However, they work on different aspects of the metabolic syndrome and can indirectly benefit patients with obesity. The primary outcomes of several studies showed that GLP‐1 RAs reduce major cardiovascular events and all‐cause mortality in patients with T2DM. Trials also showed that they may have a role in slowing the progression of CKD in patients with T2DM as per secondary outcome data. 142 , 143 , 144 , 145 In an animal study, liraglutide, a GLP‐1 RA, restored renal metabolism by inhibiting renal lipid accumulation and rescued renal mitochondria function after inducing obesity in rats using a high fat diet. 146 These data are encouraging to consider GLP‐1 RAs in people with obesity and CKD in future clinical trials.
7. OBESITY AND DIALYSIS
In patients receiving maintenance hemodialysis [maintenance hemodialysis (MHD)], abdominal adiposity was linked to inflammatory processes and protein energy wasting, and is highlighted as a risk factor for mortality. 147 In a Korean study of 18,699 participants all receiving MHD; whilst a higher BMI associated negatively with mortality; increased WC in fact conferred a higher risk of mortality. 148 Moreover, Wang et al. 149 observed that lean body mass, which accounts for the proportion of adipose‐free body mass, associated negatively with mortality risk. The proportion of lean body mass can remain greatly unaccounted for in measures of BMI as a person with a large proportion of skeletal muscle can be classified in the overweight or obese categories of BMI. 117 With this in mind, it is important to reference that sarcopenia, which describes a loss in skeletal muscle mass and strength, is a common comorbidity within the elderly MHD population and is associated with considerable levels of frailty, protein energy wasting and disability. Sarcopenic obesity can also occur whereby sarcopenia can co‐exist with obesity, and this is associated with a lower quality of life, greater risk of frailty, mortality and inflammation in ESKD and in patients in receipt of MHD. 150 , 151
With regards to patients receiving peritoneal dialysis (PD), previous reports have shown that obesity is associated with worse treatment outcomes, in relation to treatment survival rate, frailty and risk of mortality. 152 , 153 A significantly greater risk of peritonitis has also been noted in some, though not all studies. 154 , 155 Due to raised intra‐abdominal pressure, a higher incidence of peritoneal leaks is also observed in the population of patients receiving PD who have obesity. 156 Severe obesity often may pose a contraindication to being treated with PD. 157 , 158 Obi et al. 159 demonstrated that patients with a higher BMI were transferred more quickly to receive hemodialysis, were at a greater risk of peritonitis‐related complications and had quicker deterioration in residual renal function. However, in patients with severe obesity (BMI >35 kg/m2), the survival was analogous to matched MHD patients. 159
8. OBESITY AND KIDNEY TRANSPLANTATION
Obesity is common both amongst recipients of and donors for kidney transplantation 156 and it contraindicates transplantation as a feasible treatment option in many patients. 160 Nevertheless, renal transplantation is the preferred treatment for ESKD and is associated with better longevity and quality of life in contrast to dialysis. 161 Indeed, studies have observed a several‐fold greater survival advantage with transplantation in ESKD patients with obesity, more so than those who remained on the waiting list and receiving dialysis. 162 , 163 Nevertheless, obesity is classified as a risk factor, because of a range of surgical difficulties namely prolonged operative time, delayed wound healing, higher risk of venous‐thromboembolism, peripheral nerve injury and incidence of cardiac events. 164 , 165 , 166 Many studies have shown that obesity at the time of transplantation correlates independently with delayed wound healing, wound complication, graft loss and greater duration of hospitalization; along with a larger burden of readmissions 1‐year post‐transplant, increased CVD, diabetes mellitus risk and premature patient death in studies across the literature. 161 , 167 , 168 , 169 In contrast, there are studies that have observed similar outcomes post‐kidney transplant, 163 , 170 , 171 in patients with or without obesity; suggesting that renal transplantation may be conducted safely within this subpopulation. For example, an analysis from the United Kingdom transplant registry noted that the survival advantage of kidney transplantation in ESKD was greater across all BMI bands. 163 Marks et al. observed comparable results with regards to patient survival and graft survival in patients with clinically severe obesity in comparison to those without obesity 3 years post‐transplant, although the patients with clinically severe obesity displayed longer in‐hospital stays, and higher rates of readmission and major wound complications. 172
Whether weight management should be advocated in patients with obesity prior to receiving renal transplantation requires further research; some studies have reported that substantial loss in body weight before transplantation is seen to pose either no observable benefit, 173 or might in fact increase the risk of adverse events such as lengthened hospitalization and graft loss. 174 Greater mortality risk is also detected in some studies, potentially as reductions in BMI may be associated with processes such as protein malnutrition, muscle mass loss and systemic inflammation. 174 , 175 Therefore, volitional weight loss efforts may require robust monitoring, with consideration of unique patient adjustments in those awaiting renal transplantation. 173 Bariatric surgery however, prior to renal transplantation, is observed to be a safe, feasible and beneficial procedure, 176 , 177 which improves surgical access and kidney transplantation in patients with clinically severe obesity.
9. CLINICAL IMPLICATIONS AND FUTURE RESEARCH
Obesity is a tangible risk factor for the development and progression of CKD. 105 The prevalence of kidney disease amongst patients with overweight or obesity varies amongst studies, although in a systematic review and meta‐analysis it was reported that approximately 24% and 34% of kidney disease cases amongst American males and females, respectively, were related to overweight and obese. 108
The rising prevalence of CKD is paralleled by an increase in the aging population, as well as the number of individuals with obesity, type 2 diabetes and hypertension; resulting in a greater population of individuals who are potentially susceptible to CKD. 178 With a considerable escalation in the incidence of CKD and associated risk of ESKD there will be a greater number of people requiring RRT 179 over the coming decades, and this should influence changes at the population level aimed at reducing the initiation and progression of CKD. Modifiable risk factors including overweight or obesity should be targeted for prevention implementation strategies such as healthy eating, physical activity and improved energy balance to counteract the onset and worsening of kidney disease. In a systematic review of trials studying lifestyle and behavioral modifications in adults with CKD, 180 approximately 70% of studies demonstrated significant improvement in measured physiological metrics such as GFR, blood pressure, albumin excretion and body composition with the implementation of lifestyle change. Commonly utilized interventions and behavioral techniques included patient education, enablement, social support and feedback and structure, whilst education was the basis for the most promising intervention in terms of achieving physiological improvement. 180 The need for larger population and epidemiological‐based studies to assess the efficacy of lifestyle‐based approaches in managing CKD, and the subsequent scope of implementing standardized approaches to lifestyle modification, is recognized.
The postulated mechanisms by which overweight and obesity may contribute toward the development and progression of CKD require further research, although there is an appreciation that adiposity both directly and indirectly affects renal function. The metabolic complications of obesity exert pathophysiological changes in renal performance, including glomerular dysfunction. 108 Excessive adipose tissue in individuals with obesity may be associated with an adverse hormonal and cytokine profile, ectopic lipid accumulation and altered renal hemodynamics which result in nephrotoxicity and latent changes such as ORG, causing proteinuria and a spectrum of wider renal dysfunction. 44 However, a significant proportion of patients with overweight, obesity and/or components of the metabolic syndrome will not develop CKD, 42 and the reasons for this differentiation of risk are unclear. Future insight should build on genetic, epigenetic and phenotypic understanding of why certain individuals may be more inclined to develop renal impairment secondary to these metabolic conditions, and to explore other potential mechanisms of kidney injury.
In patients with CKD and obesity, WC possesses a greater correlation with adverse renal outcomes and mortality compared to overall BMI. This may be linked, at least in part, to the higher risk associated with visceral fat in terms of metabolic outcomes and future cardiovascular events, in comparison to peripheral or gluteo‐femoral adiposity. 181 Ongoing clinical studies and routine patient care may benefit from the incorporation of WC measurements into daily practice. 117
The main strategies utilized to manage obesity in patients with CKD are lifestyle modification and bariatric surgery. Bariatric surgery remains effective in treating clinically severe obesity, type 2 diabetes and hypertension, although its therapeutic effects extend beyond weight reduction. Indeed, studies have shown that bariatric surgery may be associated with decreased risk of CKD onset 182 and progression to ESKD 183 in at‐risk individuals, and the surgical risk appears only slightly greater in comparison to the general bariatric surgery population. 183 Benefits of bariatric surgery before renal transplantation are also recorded in the literature. 177 However, assessment is required to carefully and judiciously weigh up the benefits of bariatric surgery in patients with clinically severe obesity and CKD, compared to potential risks and thus, rigorous patient selection is necessary to optimize outcomes. 184 There is also a need to focus research on the benefits of bariatric surgery in lower risk groups who are currently not eligible for it.
Furthermore, future research should explore the benefits of pharmaco‐therapeutic means to treat obesity, either alone, or in synergy with surgery. SGLT2 inhibitors and GLP‐RAs may be beneficial in this cohort of patients due to their multi‐faceted actions in improving several outcomes such as weight loss, glycemic control, progression of CKD, cardiovascular events and all‐cause mortality. 140 Recent contemporary advances in the treatment of obesity and diabetes has also guided the development of co‐agonistic approaches to drug therapy such as GLP‐1/glucagon, glucose‐dependent insulinotropic polypeptide (GIP)–GLP1, amylin–calcitonin dual agonists, and GIP–GLP1–glucagon tri‐agonists. 185 Ongoing studies should determine the efficacious properties of these drugs in a population of patients with overweight/obesity and CKD, so as to explore the potential of narrowing the therapeutic gap between pharmacotherapy and surgical treatment of obesity in this cohort.
AUTHOR CONTRIBUTIONS
Saira Nawaz wrote the first draft of the manuscript. Saif Al‐Chalabi and Saira Nawaz wrote the final version of the manuscript. Rajkumar Chinnadurai, Philip Evans, Philip A. Kalra, Akheel A. Syed, and Smeeta Sinha revised the manuscript and made corrections. All authors approved the final manuscript.
CONFLICT OF INTEREST
Smeeta Sinha reports receiving research funding from Amgen, Ethicon, and AstraZeneca and receiving honoraria from Astra Zeneca, Napp Pharmaceuticals, Vifor Pharma, Novartis, Bayer, and Sanofi‐Genzyme. Philip A. Kalra reports grants from Vifor and receiving Honoria from Astra Zeneca, Astellas, Pharmacosmos, Boehringer Ingelheim, Napp, and Vifor. The other authors have nothing to disclose.
ACKNOWLEDGMENTS
No funding was obtained for this study.
Nawaz S, Chinnadurai R, Al‐Chalabi S, et al. Obesity and chronic kidney disease: a current review. Obes Sci Pract. 2023;9(2):61‐74. 10.1002/osp4.629
REFERENCES
- 1. Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol. 2017;2(7):e17. 10.1097/ij9.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Angelantonio E, Di Angelantonio E, Bhupathiraju SN, et al. Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776‐786. 10.1016/s0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu JL, Kalantar‐Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25(9):2088‐2096. 10.1681/asn.2013070754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bello AK, Alrukhaimi M, Ashuntantang GE, et al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl. 2017;7(2):122‐129. 10.1016/j.kisu.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levey, AS , Coresh J, Bolton K, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1‐S266. [PubMed] [Google Scholar]
- 6. Kent S, Schlackow I, Lozano‐Kühne J, et al. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate‐to‐severe kidney disease? BMC Nephrol. 2015;16(1):65. 10.1186/s12882-015-0054-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organisation . Obesity and overweight; 2020. https://www.who.int/en/news‐room/fact‐sheets/detail/obesity‐and‐overweight
- 8. De Lorenzo A, Romano L, Di Renzo L, Di Lorenzo N, Cenname G, Gualtieri P. Obesity: a preventable, treatable, but relapsing disease. Nutrition. 2020;71:110615. 10.1016/j.nut.2019.110615 [DOI] [PubMed] [Google Scholar]
- 9. Whaley‐Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92(2):313‐323. 10.1016/j.kint.2016.12.034 [DOI] [PubMed] [Google Scholar]
- 10. Public Health Information for Scotland . Kidney disease: UK and international data/comparisons; 2020. https://www.scotpho.org.uk/health%2Dwellbeing%2Dand%2Ddisease/kidney%2Ddisease/data/uk%2Dand%2Dinternational%2Ddata%2Dcomparisons/%23%3A%7E%3Atext%3DThe%20prevalence%20of%20CKD%20%28stage%2C%2C%20Scotland%2C%20Northern%20Ireland
- 11. Hirst JA, Hill N, O'Callaghan CA, et al. Prevalence of chronic kidney disease in the community using data from OxRen: a UK population‐based cohort study. Br J Gen Pract. 2020;70(693):e285‐e293. 10.3399/bjgp20x708245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8(1):200. 10.1186/1471-2458-8-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187‐193. 10.1001/jama.289.2.187 [DOI] [PubMed] [Google Scholar]
- 15. Lung T, Jan S, Tan EJ, Killedar A, Hayes A. Impact of overweight, obesity and severe obesity on life expectancy of Australian adults. Int J Obes. 2019;43(4):782‐789. 10.1038/s41366-018-0210-2 [DOI] [PubMed] [Google Scholar]
- 16. Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist‐hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254(6):555‐563. 10.1111/j.1365-2796.2003.01229.x [DOI] [PubMed] [Google Scholar]
- 17. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9(1):88. 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khera AV, Chaffin M, Wade KH, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587‐596.e9. 10.1016/j.cell.2019.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elks C, Den Hoed M, Zhao JH, et al. Variability in the heritability of body mass index: a systematic review and meta‐regression. Front Endocrinol. 2012;3. 10.3389/fendo.2012.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thaker VV. Genetic and epigenetic causes of obesity. Adolesc Med State Art Rev. 2017;28(2):379‐405. [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Y, Rui L. Leptin signaling and leptin resistance. Front Med. 2013;7(2):207‐222. 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rohde K, Keller M, la Cour Poulsen L, Blüher M, Kovacs P, Böttcher Y. Genetics and epigenetics in obesity. Metabolism. 2019;92:37‐50. 10.1016/j.metabol.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 23. Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. 2016;9(3):158‐173. 10.1159/000445061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science (New York, NY). 2007;316(5826):889‐894. 10.1126/science.1141634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spoto B, Mattace‐Raso F, Sijbrands E, et al. The fat‐mass and obesity‐associated gene (FTO) predicts mortality in chronic kidney disease of various severity. Nephrol Dial Transplant. 2012;27(suppl 4):iv58‐iv62. 10.1093/ndt/gfs550 [DOI] [PubMed] [Google Scholar]
- 26. Yengo L, Sidorenko J, Kemper KE, et al. Meta‐analysis of genome‐wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641‐3649. 10.1093/hmg/ddy271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nead KT, Li A, Wehner MR, et al. Contribution of common non‐synonymous variants in PCSK1 to body mass index variation and risk of obesity: a systematic review and meta‐analysis with evidence from up to 331 175 individuals. Hum Mol Genet. 2015;24(12):3582‐3594. 10.1093/hmg/ddv097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choquet H, Kasberger J, Hamidovic A, Jorgenson E. Contribution of common PCSK1 genetic variants to obesity in 8, 359 subjects from multi‐ethnic American population. PLoS One. 2013;8(2):e57857. 10.1371/journal.pone.0057857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benzinou M, Creemers JW, Choquet H, et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008;40(8):943‐945. 10.1038/ng.177 [DOI] [PubMed] [Google Scholar]
- 31. Vázquez‐Moreno M, Locia‐Morales D, Valladares‐Salgado A, et al. The MC4R p.Ile269Asn mutation confers a high risk for type 2 diabetes in the Mexican population via obesity dependent and independent effects. Sci Rep. 2021;11(1):3097. 10.1038/s41598-021-82728-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mark AL. Dietary therapy for obesity is a failure and pharmacotherapy is the future: a point of view. Clin Exp Pharmacol Physiol. 2006;33(9):857‐862. 10.1111/j.1440-1681.2006.04454.x [DOI] [PubMed] [Google Scholar]
- 33. Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280(5368):1371‐1374. 10.1126/science.280.5368.1371 [DOI] [PubMed] [Google Scholar]
- 34. National Health Service England . Statistics on Obesity, Physical Activity and Diet; 2020. https://digital.nhs.uk/data‐and‐information/publications/statistical/statistics‐on‐obesity‐physical‐activity‐and‐diet/england‐2020/part‐6‐diet‐copy
- 35. Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian diabetes, obesity and lifestyle study (AusDiab). Diabetes Care. 2008;31(2):369‐371. 10.2337/dc07-1795 [DOI] [PubMed] [Google Scholar]
- 36. Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK, Rauramaa R, Lakka TA. Low levels of leisure‐time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25(9):1612‐1618. 10.2337/diacare.25.9.1612 [DOI] [PubMed] [Google Scholar]
- 37. Ahmad T, Lee IM, Paré G, et al. Lifestyle interaction with fat mass and obesity‐associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care. 2011;34(3):675‐680. 10.2337/dc10-0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andreasen CH, Stender‐Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57(1):95‐101. 10.2337/db07-0910 [DOI] [PubMed] [Google Scholar]
- 39. Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41(3):625‐633. 10.1161/01.hyp.0000052314.95497.78 [DOI] [PubMed] [Google Scholar]
- 40. Hall JE, Henegar JR, Dwyer TM, et al. Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11(1):41‐54. 10.1053/j.arrt.2003.10.007 [DOI] [PubMed] [Google Scholar]
- 41. Ellington AA, Malik AR, Klee GG, et al. Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension. 2007;50(4):708‐714. 10.1161/hypertensionaha.107.095257 [DOI] [PubMed] [Google Scholar]
- 42. D'Agati VD, Chagnac A, de Vries AP, et al. Obesity‐related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453‐471. 10.1038/nrneph.2016.75 [DOI] [PubMed] [Google Scholar]
- 43. Okabayashi Y, Tsuboi N, Sasaki T, et al. Glomerulopathy associated with moderate obesity. Kidney Int Rep. 2016;1(4):250‐255. 10.1016/j.ekir.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang S, Cao C, Deng T, Zhou Z. Obesity‐related glomerulopathy: a latent change in obesity requiring more attention. Kidney Blood Press Res. 2020;45(4):510‐522. 10.1159/000507784 [DOI] [PubMed] [Google Scholar]
- 45. Chen H‐M, Liu Z‐H, Zeng C‐H, Li S‐J, Wang Q‐W, Li L‐S. Podocyte lesions in patients with obesity‐related glomerulopathy. Am J Kidney Dis. 2006;48(5):772‐779. 10.1053/j.ajkd.2006.07.025 [DOI] [PubMed] [Google Scholar]
- 46. Pommer W. Preventive nephrology: the role of obesity in different stages of chronic kidney disease. Kidney Dis. 2018;4(4):199‐204. 10.1159/000490247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Donnell MP, Kasiske BL, Cleary MP, Keane WF. Effects of genetic obesity on renal structure and function in the Zucker rat. II. Micropuncture studies. J Lab Clin Med. 1985;106(5):605‐610. [PubMed] [Google Scholar]
- 48. Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD. Obesity‐related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498‐1509. 10.1046/j.1523-1755.2001.0590041498.x [DOI] [PubMed] [Google Scholar]
- 49. Tobar A, Ori Y, Benchetrit S, et al. Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria. PLoS One. 2013;8(9):e75547. 10.1371/journal.pone.0075547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chagnac A, Herman M, Zingerman B, et al. Obesity‐induced glomerular hyperfiltration: its involvement in the pathogenesis of tubular sodium reabsorption. Nephrol Dial Transplant. 2008;23(12):3946‐3952. 10.1093/ndt/gfn379 [DOI] [PubMed] [Google Scholar]
- 51. Bosma RJ, Krikken JA, Homan van der Heide JJ, de Jong PE, Navis GJ. Obesity and renal hemodynamics. Contrib Nephrol. 2006;151:184‐202. [DOI] [PubMed] [Google Scholar]
- 52. Rossitto G, Maiolino G, Lerco S, et al. High sodium intake, glomerular hyperfiltration, and protein catabolism in patients with essential hypertension. Cardiovasc Res. 2021;117(5):1372‐1381. 10.1093/cvr/cvaa205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kwakernaak AJ, Tent H, Rook M, Krikken JA, Navis G. Renal hemodynamics in overweight and obesity: pathogenetic factors and targets for intervention. Expert Rev Endocrinol Metab. 2007;2(4):539‐552. 10.1586/17446651.2.4.539 [DOI] [PubMed] [Google Scholar]
- 54. Humalda JK, Navis G. Dietary sodium restriction: a neglected therapeutic opportunity in chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(6):533‐540. 10.1097/mnh.0000000000000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tsuboi N, Utsunomiya Y, Hosoya T. Obesity‐related glomerulopathy and the nephron complement. Nephrol Dial Transplant. 2013;28(suppl_4):iv108‐iv113. 10.1093/ndt/gft258 [DOI] [PubMed] [Google Scholar]
- 56. Wahba IM, Mak RH. Obesity and obesity‐initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(3):550‐562. 10.2215/cjn.04071206 [DOI] [PubMed] [Google Scholar]
- 57. Vaag AA, Grunnet LG, Arora GP, Brøns C. The thrifty phenotype hypothesis revisited. Diabetologia. 2012;55(8):2085‐2088. 10.1007/s00125-012-2589-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rüster C, Wolf G. Adipokines promote chronic kidney disease. Nephrol Dial Transplant. 2013;28(suppl 4):iv8‐iv14. 10.1093/ndt/gft191 [DOI] [PubMed] [Google Scholar]
- 59. Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47(1):42‐50. 10.1053/j.ajkd.2005.09.029 [DOI] [PubMed] [Google Scholar]
- 60. Sweiss N, Sharma K. Adiponectin effects on the kidney. Best Pract Res Clin Endocrinol Metab. 2014;28(1):71‐79. 10.1016/j.beem.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mao S, Fang L, Liu F, Jiang S, Wu L, Zhang J. Leptin and chronic kidney diseases. J Recept Signal Transduct Res. 2018;38(2):89‐94. 10.1080/10799893.2018.1431278 [DOI] [PubMed] [Google Scholar]
- 62. Tang J, Yan H, Zhuang S. Inflammation and oxidative stress in obesity‐related glomerulopathy. Int J Nephrol. 2012;2012:608397. 10.1155/2012/608397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Witko‐Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161(5):2524‐2532. [PubMed] [Google Scholar]
- 64. Yilmaz MI, Saglam M, Carrero JJ, et al. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant. 2008;23(3):959‐965. 10.1093/ndt/gfm727 [DOI] [PubMed] [Google Scholar]
- 65. Shankar A, Syamala S, Xiao J, Muntner P. Relationship between plasma leptin level and chronic kidney disease. Int J Nephrol. 2012;2012:269532. 10.1155/2012/269532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol. 2006;151:175‐183. [DOI] [PubMed] [Google Scholar]
- 67. Han DC, Isono M, Chen S, et al. Leptin stimulates type I collagen production in db/db mesangial cells: glucose uptake and TGF‐beta type II receptor expression. Kidney Int. 2001;59(4):1315‐1323. 10.1046/j.1523-1755.2001.0590041315.x [DOI] [PubMed] [Google Scholar]
- 68. Marouga A, Dalamaga M, Kastania AN, et al. Correlates of serum resistin in elderly, non‐diabetic patients with chronic kidney disease. Clin Lab. 2013;59(9–10):1121‐1128. 10.7754/clin.lab.2012.121112 [DOI] [PubMed] [Google Scholar]
- 69. Kawamura R, Doi Y, Osawa H, et al. Circulating resistin is increased with decreasing renal function in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant. 2010;25(10):3236‐3240. 10.1093/ndt/gfq155 [DOI] [PubMed] [Google Scholar]
- 70. Kawanami D, Maemura K, Takeda N, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine‐endothelial cell interactions. Biochem Biophys Res Commun. 2004;314(2):415‐419. 10.1016/j.bbrc.2003.12.104 [DOI] [PubMed] [Google Scholar]
- 71. Zhang H, Li X, Kan Y, Yang F, Hou Y, Du Y. Analysis of the correlation between serum resistin and the variability of erythropoietin responsiveness in patients with chronic kidney disease. Exp Ther Med. 2015;10(5):1925‐1930. 10.3892/etm.2015.2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem. 2008;15(18):1851‐1862. 10.2174/092986708785133004 [DOI] [PubMed] [Google Scholar]
- 73. Filippatos TD, Tsimihodimos V, Derdemezis CS, et al. Increased plasma visfatin concentration is a marker of an atherogenic metabolic profile. Nutr Metab Cardiovasc Dis. 2013;23(4):330‐336. 10.1016/j.numecd.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 74. Axelsson J, Witasp A, Carrero JJ, et al. Circulating levels of visfatin/pre‐B‐cell colony‐enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKD. Am J Kidney Dis. 2007;49(2):237‐244. 10.1053/j.ajkd.2006.11.021 [DOI] [PubMed] [Google Scholar]
- 75. Mu J, Feng B, Ye Z, et al. Visfatin is related to lipid dysregulation, endothelial dysfunction and atherosclerosis in patients with chronic kidney disease. J Nephrol. 2011;24(2):177‐184. 10.5301/jn.2010.3488 [DOI] [PubMed] [Google Scholar]
- 76. Upadhyay A, Larson MG, Guo C‐Y, et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol Dial Transplant. 2011;26(3):920‐926. 10.1093/ndt/gfq471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee BT, Ahmed FA, Hamm LL, et al. Association of C‐reactive protein, tumor necrosis factor‐alpha, and interleukin‐6 with chronic kidney disease. BMC Nephrol. 2015;16(1):77. 10.1186/s12882-015-0068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Amdur RL, Feldman HI, Gupta J, et al. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11(9):1546‐1556. 10.2215/cjn.13121215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sewter CP, Digby JE, Blows F, Prins J, O'Rahilly S. Regulation of tumour necrosis factor‐alpha release from human adipose tissue in vitro. J Endocrinol. 1999;163(1):33‐38. 10.1677/joe.0.1630033 [DOI] [PubMed] [Google Scholar]
- 80. Magno AL, Herat LY, Carnagarin R, Schlaich MP, Matthews VB. Current knowledge of IL‐6 cytokine family members in acute and chronic kidney disease. Biomedicines. 2019;7(1):19. 10.3390/biomedicines7010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Egli‐Spichtig D, Imenez Silva PH, Glaudemans B, et al. Tumor necrosis factor stimulates fibroblast growth factor 23 levels in chronic kidney disease and non‐renal inflammation. Kidney Int. 2019;96(4):890‐905. 10.1016/j.kint.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 82. Durlacher‐Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh‐Many T. Interleukin‐6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94(2):315‐325. 10.1016/j.kint.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 83. Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein‐1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282‐2289. 10.1210/jc.2004-1696 [DOI] [PubMed] [Google Scholar]
- 84. Gregg LP, Tio MC, Li X, Adams‐Huet B, de Lemos JA, Hedayati SS. Association of monocyte chemoattractant protein‐1 with death and atherosclerotic events in chronic kidney disease. Am J Nephrol. 2018;47(6):395‐405. 10.1159/000488806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Eddy AA, Fogo AB. Plasminogen activator inhibitor‐1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2006;17(11):2999‐3012. 10.1681/asn.2006050503 [DOI] [PubMed] [Google Scholar]
- 86. Kaji H. Adipose tissue‐derived plasminogen activator inhibitor‐1 function and regulation. Compr Physiol. 2016;6(4):1873‐1896. [DOI] [PubMed] [Google Scholar]
- 87. Hamano K, Iwano M, Akai Y, et al. Expression of glomerular plasminogen activator inhibitor type 1 in glomerulonephritis. Am J Kidney Dis. 2002;39(4):695‐705. 10.1053/ajkd.2002.31986 [DOI] [PubMed] [Google Scholar]
- 88. Markaki A, Psylinakis E, Spyridaki A. Adiponectin and end‐stage renal disease. Hormones (Athens). 2016;15(3):345‐354. 10.14310/horm.2002.1698 [DOI] [PubMed] [Google Scholar]
- 89. Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118(5):1645‐1656. 10.1172/jci32691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yaturu S, Reddy RD, Rains J, Jain SK. Plasma and urine levels of resistin and adiponectin in chronic kidney disease. Cytokine. 2007;37(1):1‐5. 10.1016/j.cyto.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 91. Tsuruya K, Yoshida H, Nagata M, et al. Association of the triglycerides to high‐density lipoprotein cholesterol ratio with the risk of chronic kidney disease: analysis in a large Japanese population. Atherosclerosis. 2014;233(1):260‐267. 10.1016/j.atherosclerosis.2013.12.037 [DOI] [PubMed] [Google Scholar]
- 92. Nam KH, Chang TI, Joo YS, et al. Association between serum high‐density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW‐CKD. J Am Heart Assoc. 2019;8(6):e011162. 10.1161/jaha.118.011162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. 2010;19(4):393‐402. 10.1097/mnh.0b013e32833aa4ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pol T, Held C, Westerbergh J, et al. Dyslipidemia and risk of cardiovascular events in patients with atrial fibrillation treated with oral anticoagulation therapy: insights from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. J Am Heart Assoc. 2018;7(3):e007444. 10.1161/jaha.117.007444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5(12):713‐721. 10.1038/nrneph.2009.184 [DOI] [PubMed] [Google Scholar]
- 96. Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. 2006;26(3):232‐244. 10.1159/000093632 [DOI] [PubMed] [Google Scholar]
- 97. De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2013;28(1):29‐36. 10.1093/ndt/gfs290 [DOI] [PubMed] [Google Scholar]
- 98. Siegel‐Axel DI, Häring HU. Perivascular adipose tissue: an unique fat compartment relevant for the cardiometabolic syndrome. Rev Endocr Metab Disord. 2016;17(1):51‐60. 10.1007/s11154-016-9346-3 [DOI] [PubMed] [Google Scholar]
- 99. Wagner R, Machann J, Lehmann R, et al. Exercise‐induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia. 2012;55(7):2054‐2058. 10.1007/s00125-012-2551-z [DOI] [PubMed] [Google Scholar]
- 100. Lotta LA, Gulati P, Day FR, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49(1):17‐26. 10.1038/ng.3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Scott RA, Fall T, Pasko D, et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independent of obesity. Diabetes. 2014;63(12):4378‐4387. 10.2337/db14-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Eguchi K, Izumi Y, Nakayama Y, et al. Insufficiency of urinary acid excretion of overweight or obese patients with chronic kidney disease and its involvement with renal tubular injury. Nephrology (Carlton). 2019;24(11):1131‐1141. 10.1111/nep.13553 [DOI] [PubMed] [Google Scholar]
- 103. Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end‐stage renal disease. Ann Intern Med. 2006;144(1):21‐28. 10.7326/0003-4819-144-9-200605020-00022 [DOI] [PubMed] [Google Scholar]
- 104. Chang Y, Ryu S, Choi Y, et al. Metabolically healthy obesity and development of chronic kidney disease. Ann Intern Med. 2016;164(5):305‐312. 10.7326/l16-0405 [DOI] [PubMed] [Google Scholar]
- 105. Zhang J, Jiang H, Chen J. Combined effect of body mass index and metabolic status on the risk of prevalent and incident chronic kidney disease: a systematic review and meta‐analysis. Oncotarget. 2017;8(22):35619‐35629. 10.18632/oncotarget.10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stefan N, Häring HU, Schulze MB. Metabolically healthy obesity: the low‐hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018;6(3):249‐258. 10.1016/s2213-8587(17)30292-9 [DOI] [PubMed] [Google Scholar]
- 107. Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695‐1702. 10.1681/asn.2005060638 [DOI] [PubMed] [Google Scholar]
- 108. Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta‐analysis. Kidney Int. 2008;73(1):19‐33. 10.1038/sj.ki.5002586 [DOI] [PubMed] [Google Scholar]
- 109. Silverwood RJ, Pierce M, Thomas C, et al. Association between younger age when first overweight and increased risk for CKD. J Am Soc Nephrol. 2013;24(5):813‐821. 10.1681/asn.2012070675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kim YJ, Hwang SD, Oh TJ, et al. Association between obesity and chronic kidney disease, defined by both glomerular filtration rate and albuminuria, in Korean adults. Metab Syndr Relat Disord. 2017;15(8):416‐422. 10.1089/met.2017.0053 [DOI] [PubMed] [Google Scholar]
- 111. Chang Y, Ryu S, Choi Y, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med. 2016;164(5):305‐312. 10.7326/m15-1323 [DOI] [PubMed] [Google Scholar]
- 112. Yarnoff BO, Hoerger TJ, Shrestha SS, et al. Modeling the impact of obesity on the lifetime risk of chronic kidney disease in the United States using updated estimates of GFR progression from the CRIC study. PLoS One. 2018;13(10):e0205530. 10.1371/journal.pone.0205530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non‐diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract. 2009;113(1):c16‐c23. 10.1159/000228071 [DOI] [PubMed] [Google Scholar]
- 114. Stengel B, Tarver–Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003;14(4):479‐487. 10.1097/01.ede.0000071413.55296.c4 [DOI] [PubMed] [Google Scholar]
- 115. Mittal B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J Med Res. 2019;149(5):571‐573. 10.4103/ijmr.ijmr_1910_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nerpin E, Risérus U, Ingelsson E, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community‐based cohort. Diabetes Care. 2008;31(8):1550‐1555. 10.2337/dc08-0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Evans PD, McIntyre NJ, Fluck RJ, McIntyre CW, Taal MW. Anthropomorphic measurements that include central fat distribution are more closely related with key risk factors than BMI in CKD stage 3. PLoS One. 2012;7(4):e34699. 10.1371/journal.pone.0034699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pinto‐Sietsma S‐J, Navis G, Janssen WMT, de Zeeuw D, Gans ROB, de Jong PE. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41(4):733‐741. 10.1016/s0272-6386(03)00020-9 [DOI] [PubMed] [Google Scholar]
- 119. Lin T‐Y, Lim P‐S, Hung S‐C. Normal‐weight obesity and clinical outcomes in nondiabetic chronic kidney disease patients: a cohort study. Am J Clin Nutr. 2018;107(4):664‐672. 10.1093/ajcn/nqy006 [DOI] [PubMed] [Google Scholar]
- 120. Targher G, Byrne CD. Non‐alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol. 2017;13(5):297‐310. 10.1038/nrneph.2017.16 [DOI] [PubMed] [Google Scholar]
- 121. Bonnet F, Deprele C, Sassolas A, et al. Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis. 2001;37(4):720‐727. 10.1016/s0272-6386(01)80120-7 [DOI] [PubMed] [Google Scholar]
- 122. Carbone A, Al Salhi Y, Tasca A, et al. Obesity and kidney stone disease: a systematic review. Minerva Urol Nefrol. 2018;70(4):393‐400. 10.23736/s0393-2249.18.03113-2 [DOI] [PubMed] [Google Scholar]
- 123. Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6(2):187‐194. 10.1007/s13679-017-0262-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25(4):1173‐1183. 10.1093/ndt/gfp640 [DOI] [PubMed] [Google Scholar]
- 125. Stefan N, Häring HU, Cusi K. Non‐alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313‐324. 10.1016/s2213-8587(18)30154-2 [DOI] [PubMed] [Google Scholar]
- 126. Musso G, Cipolla U, Cassader M, et al. TM6SF2 rs58542926 variant affects postprandial lipoprotein metabolism and glucose homeostasis in NAFLD. J Lipid Res. 2017;58(6):1221‐1229. 10.1194/jlr.m075028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin‐A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21(3):406‐412. 10.1681/asn.2009080820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Marcuccilli M, Chonchol M. NAFLD and chronic kidney disease. Int J Mol Sci. 2016;17(4):562. 10.3390/ijms17040562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chinnadurai R, Ritchie J, Green D, Kalra PA. Non‐alcoholic fatty liver disease and clinical outcomes in chronic kidney disease. Nephrol Dial Transplant. 2019;34(3):449‐457. 10.1093/ndt/gfx381 [DOI] [PubMed] [Google Scholar]
- 130. Baran B, Akyüz F. Non‐alcoholic fatty liver disease: what has changed in the treatment since the beginning? World J Gastroenterol. 2014;20(39):14219‐14229. 10.3748/wjg.v20.i39.14219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lee Y, Doumouras AG, Yu J, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2019;17(6):1040‐1060.e11. 10.1016/j.cgh.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 132. Willis EA, Huang W‐Y, Saint‐Maurice PF, et al. Increased frequency of intentional weight loss associated with reduced mortality: a prospective cohort analysis. BMC Med. 2020;18(1):248. 10.1186/s12916-020-01716-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 2016;23(4):591‐601. 10.1016/j.cmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Imam TH, Fischer H, Jing B, et al. Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis. 2017;69(3):380‐388. 10.1053/j.ajkd.2016.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. MacLaughlin HL, Hall WL, Patel AG, et al. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3‐4 CKD: a randomized controlled pilot study. Am J Kidney Dis. 2014;64(4):660‐663. 10.1053/j.ajkd.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 136. Ikizler TA, Robinson‐Cohen C, Ellis C, et al. Metabolic effects of diet and exercise in patients with moderate to severe CKD: a randomized clinical trial. J Am Soc Nephrol. 2018;29(1):250‐259. 10.1681/asn.2017010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hauner H, Nitschmann S. Life expectancy after bariatric surgery: Swedish Obese Subjects study. Internist (Berl). 2021;62(2):212‐214. 10.1007/s00108-021-00947-9 [DOI] [PubMed] [Google Scholar]
- 138. Liakopoulos V, Franzén S, Svensson A‐M, et al. Renal and cardiovascular outcomes after weight loss from gastric bypass surgery in type 2 diabetes: cardiorenal risk reductions exceed atherosclerotic benefits. Diabetes Care. 2020;43(6):1276‐1284. 10.2337/dc19-1703 [DOI] [PubMed] [Google Scholar]
- 139. Coleman KJ, Shu YH, Fischer H, et al. Bariatric surgery and risk of death in persons with chronic kidney disease. Ann Surg. 2021. 10.1097/SLA.0000000000004851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. 10.1056/nejmoa1811744 [DOI] [PubMed] [Google Scholar]
- 141. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436‐1446. 10.1056/nejmoa2024816 [DOI] [PubMed] [Google Scholar]
- 142. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. 10.1056/nejmoa1607141 [DOI] [PubMed] [Google Scholar]
- 143. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. 10.1056/nejmoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394(10193):121‐130. [DOI] [PubMed] [Google Scholar]
- 145. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605‐617. 10.1016/s2213-8587(18)30104-9 [DOI] [PubMed] [Google Scholar]
- 146. Wang C, Li L, Liu S, et al. GLP‐1 receptor agonist ameliorates obesity‐induced chronic kidney injury via restoring renal metabolism homeostasis. PLoS One. 2018;13(3):e0193473. 10.1371/journal.pone.0193473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Cordeiro AC, Qureshi AR, Stenvinkel P, et al. Abdominal fat deposition is associated with increased inflammation, protein–energy wasting and worse outcome in patients undergoing haemodialysis. Nephrol Dial Transplant. 2010;25(2):562‐568. 10.1093/ndt/gfp492 [DOI] [PubMed] [Google Scholar]
- 148. Kim CS, Han K‐D, Choi HS, Bae EH, Ma SK, Kim SW. Association of body mass index and waist circumference with all‐cause mortality in hemodialysis patients. J Clin Med. 2020;9(5):1289. 10.3390/jcm9051289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang J, Streja E, Rhee CM, et al. Lean body mass and survival in hemodialysis patients and the roles of race and ethnicity. J Ren Nutr. 2016;26(1):26‐37. 10.1053/j.jrn.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Saitoh M, Ogawa M, Kondo H, et al. Sarcopenic obesity and its association with frailty and protein‐energy wasting in hemodialysis patients: preliminary data from a single center in Japan. Ren Replace Ther. 2019;5(1):46. 10.1186/s41100-019-0240-9 [DOI] [Google Scholar]
- 151. Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end‐stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86(3):633‐638. 10.1093/ajcn/86.3.633 [DOI] [PubMed] [Google Scholar]
- 152. McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14(11):2894‐2901. 10.1097/01.asn.0000091587.55159.5f [DOI] [PubMed] [Google Scholar]
- 153. Chan GC‐K, Ng JKC, Chow K‐M, et al. Interaction between central obesity and frailty on the clinical outcome of peritoneal dialysis patients. PLoS One. 2020;15(10):e0241242. 10.1371/journal.pone.0241242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jegatheesan D, Johnson DW, Cho Y, et al. The relationship between body mass index and organism‐specific peritonitis. Perit Dial Int. 2018;38(3):206‐214. 10.3747/pdi.2017.00188 [DOI] [PubMed] [Google Scholar]
- 155. McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int. 2004;24(4):340‐346. 10.1177/089686080402400408 [DOI] [PubMed] [Google Scholar]
- 156. Glicklich D, Mustafa MR. Obesity in kidney transplantation: impact on transplant candidates, recipients, and donors. Cardiol Rev. 2019;27(2):63‐72. 10.1097/crd.0000000000000216 [DOI] [PubMed] [Google Scholar]
- 157. Nolph KD, Sorkin M, Rubin J, et al. Continuous ambulatory peritoneal dialysis: three‐year experience at one center. Ann Intern Med. 1980;92(5):609‐613. 10.7326/0003-4819-92-5-609 [DOI] [PubMed] [Google Scholar]
- 158. Thomson NM, Atkins RC, Humphery TJ, Agar JM, Scott DF. Continuous ambulatory peritoneal dialysis (CAPD): an established treatment for endstage renal failure. Aust N Z J Med. 1983;13(5):489‐496. 10.1111/j.1445-5994.1983.tb02700.x [DOI] [PubMed] [Google Scholar]
- 159. Obi Y, Streja E, Mehrotra R, et al. Impact of obesity on modality longevity, residual kidney function, peritonitis, and survival among incident peritoneal dialysis patients. Am J Kidney Dis. 2018;71(6):802‐813. 10.1053/j.ajkd.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Segev DL, Simpkins CE, Thompson RE, Locke JE, Warren DS, Montgomery RA. Obesity impacts access to kidney transplantation. J Am Soc Nephrol. 2008;19(2):349‐355. 10.1681/asn.2007050610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Erturk T, Berber I, Cakir U. Effect of obesity on clinical outcomes of kidney transplant patients. Transplant Proc. 2019;51(4):1093‐1095. 10.1016/j.transproceed.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 162. Glanton CW, Kao T‐C, Cruess D, Agodoa LYC, Abbott KC. Impact of renal transplantation on survival in end‐stage renal disease patients with elevated body mass index. Kidney Int. 2003;63(2):647‐653. 10.1046/j.1523-1755.2003.00761.x [DOI] [PubMed] [Google Scholar]
- 163. Krishnan N, Higgins R, Short A, et al. Kidney transplantation significantly improves patient and graft survival irrespective of BMI: a cohort study. Am J Transplant. 2015;15(9):2378‐2386. 10.1111/ajt.13363 [DOI] [PubMed] [Google Scholar]
- 164. Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31(3):556‐560. Discussion 61. 10.1007/s00268-006-0305-0 [DOI] [PubMed] [Google Scholar]
- 165. Sloan M, Sheth N, Lee G‐C. Is obesity associated with increased risk of deep vein thrombosis or pulmonary embolism after hip and knee arthroplasty? A large database study. Clin Orthop Relat Res. 2019;477(3):523‐532. 10.1097/corr.0000000000000615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ri M, Aikou S, Seto Y. Obesity as a surgical risk factor. Ann Gastroenterol Surg. 2017;2(1):13‐21. 10.1002/ags3.12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Singh D, Lawen J, Alkhudair W. Does pretransplant obesity affect the outcome in kidney transplant recipients? Transplant Proc. 2005;37(2):717‐720. 10.1016/j.transproceed.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 168. Aziz F, Ramadorai A, Parajuli S, et al. Obesity: an independent predictor of morbidity and graft loss after kidney transplantation. Am J Nephrol. 2020;51(8):615‐623. 10.1159/000509105 [DOI] [PubMed] [Google Scholar]
- 169. Foucher Y, Lorent M, Albano L, et al. Renal transplantation outcomes in obese patients: a French cohort‐based study. BMC Nephrol. 2021;22(1):79. 10.1186/s12882-021-02278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Howard RJ, Thai VB, Patton PR, et al. Obesity does not portend a bad outcome for kidney transplant recipients. Transplantation. 2002;73(1):53‐55. 10.1097/00007890-200201150-00009 [DOI] [PubMed] [Google Scholar]
- 171. Pieloch D, Dombrovskiy V, Osband AJ, Lebowitz J, Laskow DA. Morbid obesity is not an independent predictor of graft failure or patient mortality after kidney transplantation. J Ren Nutr. 2014;24(1):50‐57. 10.1053/j.jrn.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 172. Marks WH, Florence LS, Chapman PH, Precht AF, Perkinson DT. Morbid obesity is not a contraindication to kidney transplantation. Am J Surg. 2004;187(5):635‐638. 10.1016/j.amjsurg.2004.01.015 [DOI] [PubMed] [Google Scholar]
- 173. Schold JD, Srinivas TR, Guerra G, et al. A “weight‐listing” paradox for candidates of renal transplantation? Am J Transplant. 2007;7(3):550‐559. 10.1111/j.1600-6143.2006.01629.x [DOI] [PubMed] [Google Scholar]
- 174. Harhay MN, Ranganna K, Boyle SM, et al. Association between weight loss before deceased donor kidney transplantation and posttransplantation outcomes. Am J Kidney Dis. 2019;74(3):361‐372. 10.1053/j.ajkd.2019.03.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Heng AE, Aniort J, Pereira B, Fervenza F, Boirie Y, Prieto M. Renal transplant in obese patients and impact of weight loss before surgery on surgical and medical outcomes: a single‐center cohort study. Exp Clin Transplant. 2019;17(5):604‐612. [DOI] [PubMed] [Google Scholar]
- 176. Jamal MH, Corcelles R, Daigle CR, et al. Safety and effectiveness of bariatric surgery in dialysis patients and kidney transplantation candidates. Surg Obes Relat Dis. 2015;11(2):419‐423. 10.1016/j.soard.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 177. Yemini R, Nesher E, Carmeli I, et al. Bariatric surgery is efficacious and improves access to transplantation for morbidly obese renal transplant candidates. Obes Surg. 2019;29(8):2373‐2380. 10.1007/s11695-019-03925-1 [DOI] [PubMed] [Google Scholar]
- 178. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3‐15. [DOI] [PubMed] [Google Scholar]
- 179. McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30(1):127‐135. 10.1681/asn.2018050531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Evangelidis N, Craig J, Bauman A, Manera K, Saglimbene V, Tong A. Lifestyle behaviour change for preventing the progression of chronic kidney disease: a systematic review. BMJ Open. 2019;9(10):e031625. 10.1136/bmjopen-2019-031625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11(1):11‐18. 10.1111/j.1467-789x.2009.00623.x [DOI] [PubMed] [Google Scholar]
- 182. Friedman AN, Wahed AS, Wang J, et al. Effect of bariatric surgery on CKD risk. J Am Soc Nephrol. 2018;29(4):1289‐1300. 10.1681/asn.2017060707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Chang AR, Grams ME, Navaneethan SD. Bariatric surgery and kidney‐related outcomes. Kidney Int Rep. 2017;2(2):261‐270. 10.1016/j.ekir.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Docherty NG, le Roux CW. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat Rev Nephrol. 2020;16(12):709‐720. 10.1038/s41581-020-0323-4 [DOI] [PubMed] [Google Scholar]
- 185. Müller TD, Blüher M, Tschöp MH, DiMarchi RD. Anti‐obesity drug discovery: advances and challenges. Nat Rev Drug Discov. 2022;21(3):201‐223. 10.1038/s41573-021-00337-8 [DOI] [PMC free article] [PubMed] [Google Scholar]


