Abstract
Background
Despite widespread adoption during COVID‐19, there is limited evidence supporting the quality of telemedicine care in managing patients with abnormal BMI.
Objective
To evaluate the comparability of telemedicine and in‐person (office) quality performance for abnormal body mass index (BMI kg/m2) screening and management in primary care.
Methods
This retrospective cohort study measured Healthcare Effectiveness Data and Information Set (HEDIS) quality performance for abnormal BMI screening (patients with BMIs <18.5 or >25 kg/m2 and a qualifying documented follow up plan) across an 8‐hospital integrated health system seen via primary care from 4/1/20 ‐ 9/30/21. Encounters were divided into three exposure groups: office (excluding telemedicine), telemedicine (excluding office), and blended telemedicine (office + telemedicine). Demographic stratification compared group composition. Chi squared tests determined statistical differences in quality performance (p = <0.05).
Results
Demographics of sub‐groups for the 287,387 patients (office: 222,333; telemedicine: 1,556; blended‐telemedicine: 63,489) revealed a modest female predominance, majority ages 26–70, mostly White non‐Hispanics of low health risk, and the majority BMI representation was overweight, followed closely by class 1 obesity. In both HEDIS specified and HEDIS modified performance, blended‐telemedicine performed better than office (12.56%, 95% CI 12.29%–13.01%; 11.16%, 95% CI: 10.85%–11.48%; p < 0.0001); office performed better than telemedicine (4.29%, 95% CI 2.84%–5.54%; 4.79%, 95% CI 3.99%–5.35%; p < 0.0001).
Conclusion
Quality performance was highest for blended‐telemedicine, followed by office‐only, then telemedicine‐only. Given the known cost savings, adding telemedicine as a care venue might promote value within health systems without negatively impacting HEDIS performance.
Keywords: abnormal BMI screening, HEDIS quality measures, primary care, telemedicine
1. BACKGROUND
Obesity is an increasingly prevalent disease in the United States that contributes to other costly comorbidities like heart disease, diabetes and cancer. 1 The CDC estimates that obesity affects >40% of Americans, and World‐wide, the incidence of obesity has tripled since 1975. 2 Recent literature has shown the pandemic has only exacerbated the prevalence of obesity. 4 Body mass index (BMI kg/m2) remains a reliable measure for screening abnormal weight and routine screening for abnormal BMI has widespread institutional, governmental, and medical society support. 4 , 5 , 6 , 7 , 8 , 9 Moreover, Healthcare Effectiveness Data and Information Set (HEDIS) measurement from health system medical records has shown to be representative means of evaluating the prevalence and quality of obesity care, demonstrating consistency with national data. 10
The literature supports telemedicine's capability in managing patients with abnormal BMI, where telemedicine augments delivery of comprehensive and effective care. 11 , 12 The literature also describes telemedicine's capability to increase access to care, 13 , 14 promote cost savings, 15 , 16 , 17 and achieve comparable outcomes to in‐office care. 18 , 19 , 20 Telemedicine has proven in randomized, blinded studies to directly show favorable impact weight loss efforts, and for patients with failed outpatient efforts, 12 telemedicine's ability to mitigate barriers for access to bariatric surgery care has also been demonstrated. 11 Thus, telemedicine has a promising role for positive impact by preventing the complications of patients with abnormal BMI.
But during COVID‐19, preventive care around the world was negatively impacted, 21 and much of the increased use of telemedicine in the US was not prevention focused. 22 The evidence supporting quality of telemedicine in obesity care is limited, 23 , 24 and this study adds to the quality evaluation literature by comparing performance under standardized quality performance measures during COVID‐19 to in‐office care. 25 Given the known increase in obesity prevalence during the COVID‐19 pandemic, 26 , 27 , 28 , 29 it is important to understand if the quality of telemedicine care affects the management of patients with abnormal BMI. Moreover, to understand its optimal role in preventive care moving forward, telemedicine's quality in the context of measures substantiated by national quality organizations can facilitate a standardized comparison. 6 , 30 , 31
Thus, the objective of this study was to compare the quality performance of abnormal BMI screening between telemedicine and office‐based care. The hypothesis was that these care venues would have comparable quality performance.
2. METHODS
Quality performance for abnormal BMI management was retrospectively compared in patients with graded exposure to telemedicine (office‐only, blended‐telemedicine, and telemedicine‐only) over a 1.5 years timeframe during the COVID‐19 pandemic. The Healthcare Effectiveness Data and Information Set (HEDIS) measure for abnormal BMI prevention was adapted from the National Quality Forum (NQF) 30 and Centers for Medicare and Medicaid (CMS) 11 to compare quality performance between groups seen in primary care encounters within an integrated healthcare system across >200 outpatient care sites in central Pennsylvania and northern Maryland (WellSpan Health: 20,000 employees, 8 hospitals and 2600 clinicians 32 ). SlicerDicer, a clinical data mining tool within EPIC's electronic medical record (EMR), was used to mine de‐identified patient data. The study was exempt from full board review by the WellSpan Health Institutional Review Board (1760263‐1) and STROBE guidelines for cohort studies were followed.
Prior to 03/2021, there was very limited telemedicine utilization throughout the health system (there were only 463 total encounters for BMI screening during the entire month of 03/2020, and only 51 of these had documented follow up plans). Due to low volume of telemedicine throughout the health system during and prior to 3/1/20, The study timeframe commenced on 4/1/20 where consistent monthly numbers of telemedicine encounters were being conducted. Thus, the study timeframe was from 4/1/20 to 9/30/21 (near the WHO's official declaration of the COVID‐19 pandemic 33 ) to facilitate a reasonable comparison between exposure groups. Eligibility for inclusion was according to CMS's HEDIS denominator specification for the measure “Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow‐Up Plan”. 31 BMI (kg/m2) was classified according to the CDC's criteria. 5
Inclusion and exclusion criteria were specified by the HEDIS measure 31 and thus the primary outcome was quality performance. The inclusion criteria formed the HEDIS denominator: all patients in WellSpan Health with a BMI outside of normal range: <18.5 or >25 (kg/m2). This inclusion was contingent on patients having a documented weight within 1 year of their provider encounter (patient‐portal reported weights also qualified). Patients were excluded if they met the following criteria according to HEDIS specifications: palliative or hospice (Epic diagnosis grouper for palliative or hospice care), pregnant (ICD‐10‐CM: Z33*), Illness or physical disability (Epic diagnosis grouper for advanced illness AND ICD‐10‐CM: Z73.6), mental illness (ICD‐10‐CM: F99*), dementia or confusion (ICD‐10‐CM:G31*), and nutritional deficiency (ICD‐10‐CM: E64*). Quality performance was determined by the HEDIS numerator: percent of patients with BMI outside of normal range and a documented follow up plan. The collection of HEDIS specified follow up plans included: education or counseling on nutrition or exercise, referrals to dietitians, nutritionists, occupational or physical therapists, mental health services, behavior therapy, medical weight management, bariatric surgical management, and orders for nutritional or dietary supplements. Orders for BMI follow up plan were linked to the initial face‐to‐face encounter to ensure that the follow up plan was associated with the initial encounter type (office, telemedicine, or blended). A primary care service line filter was also applied to include only ambulatory (non‐surgical) encounters. The flow diagram (Figure 1) sequentially details the design of data sessions.
FIGURE 1.
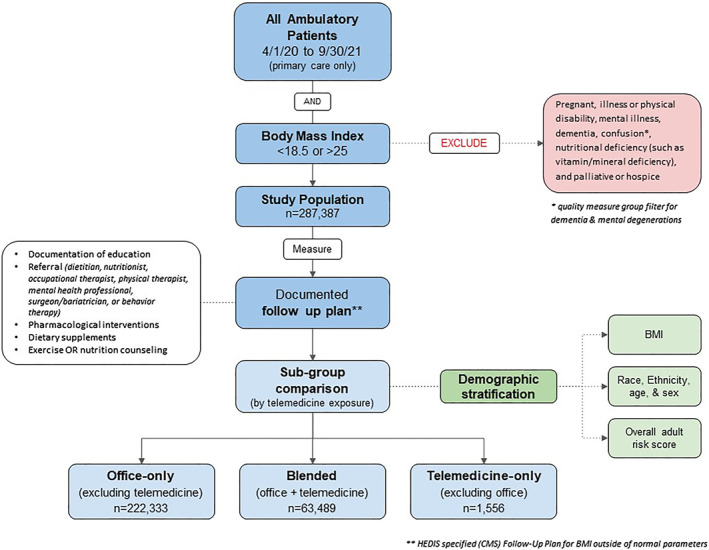
This Hierarchical data filter schema (built in EPIC SlicerDicer tool) served as inclusion and exclusion criteria according to the HEDIS measure specification for abnormal BMI screening. A primary population filter served as the inclusion criteria: all patients seen in the primary care service line (throughout the health system) during the study timeframe who had a documented weight (to populate a BMI). As indicated in the figure, patients with a disqualifying diagnosis were excluded prior to measuring the percent of patients with qualifying follow up plans (which served as the quality performance numerator). Thus, quality performance was the total number of patients meeting inclusion criteria divided by the number of those patients with a documented follow up plan. This same approach was completed for each of the sub‐group comparisons, where an additional primary data filter for encounter type (office only, blended, or telemedicine‐only) was applied to separate patients by visit type. This separation of patients by visit type also facilitated a demographic comparison between sub‐groups (Race/Ethnicity, age/sex, BMI and patient risk score).
Three types of patients were identified: office only, telemedicine only, and blended telemedicine (patients with telemedicine encounters that also had office visits within the timeframe). Telemedicine included only video (Zoom) since telephone encounters in the EMR were conflated with other types of office calls (medication refills, nursing triage, billing notices etc). To prevent redundancies or duplications between these groups, separate data sessions were run for telemedicine, office, and blended encounters. Four groups were measured: (1) total patients (office + telemedicine + blended telemedicine), (2) office only (excluding telemedicine), (3) telemedicine only (excluding office), (4) blended telemedicine (office and telemedicine).
Since the BMI screening HEDIS measure examined only if a follow plan had been completed within the timeframe, there was a desire to compare the effect sequentiality between visit type and completing a follow up plan. To simulate this relationship (similar to our previous work), 25 two additional sets of statistical analysis were completed, these were termed: (1) HEDIS specified (identical measure to the NQF/CMS numerator criteria: BMI follow up plan within the study timeframe) and (2) HEDIS modified (a sequentially dependent numerator: a telemedicine or office encounter linked to a BMI follow up plan completed within 3 months after the initial encounter).
The confounding of COVID‐19 and sociodemographic sampling boas (age, race, sex, and social determinants of health—SDOH) was controlled with inclusion and exclusion criteria as specified by the HEDIS measure as above. 31 In addition, an overall adult risk score accounting for number of diagnosis, SDOH, and healthcare utilization (detailed description in the supplement of our prior work) 25 was added to the sociodemographic stratification to ensure percent composition between exposure groups. No case‐mix adjustments were made in the sample to accommodate the NQF recommendations on validity and reliability for studies completed during the COVID‐19 pandemic. 34 , 35 By using data filters for primary care service lines and face‐to‐face encounters, sampling bias was mitigated and data reliability was promoted, ensuring only outpatient primary care encounters were captured (excluded encounters for prescription refills or reviewing test results). To further address sampling bias, quality performance was compared in the pre‐study timeframe (pre‐pandemic) to provide context for the interpretation of quality performance during the study timeframe. Despite the limited amount of telemedicine throughout the health system prior to the COVID‐19 pandemic, this historical baseline served as a feasible control.
Medicals's “N‐1” Chi‐squared calculator 19 was used to detect statistical significance of proportion differences between sub‐groups. The level of significance was set at p < 0.05.
3. RESULTS
From 04/1/2020 to 09/30/21 there were 287,387 patients with provider encounters (office: 222,333; telemedicine: 1,556; blended‐telemedicine: 63,489) meeting inclusion criteria (Table 1). Across demographic comparisons for subgroups (office; telemedicine; and blended‐telemedicine), there was a modest female predominance overall and the majority of patients were adults ages 26–70. The predominant race and ethnicity were White and non‐Hispanic/Latino. The majority BMI (kg/m2) across all subgroups was overweight, followed closely by class 1 obesity. The majority of patients had a low overall risk score.
TABLE 1.
Demographic data of patients eligible for abnormal body mass index (BMI) screening
| Office (excluding telemedicine) | Blended‐telemedicine (mixed office & telemedicine) | Telemedicine (excluding office) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub‐group composition | HEDIS eligible | Completed obesity plan | Quality performance | Sub‐group composition | HEDIS eligible | Completed obesity plan | Quality performance | Sub‐group composition | HEDIS eligible | Completed obesity plan | Quality performance | ||
| Total patients | 287,387 | 77.36% | 222,333 | 27,109 | 12.19% | 22.09% | 63,489 | 15,771 | 24.84% | 0.54% | 1556 | 123 | 7.90% |
| Sex | Female | 52.28% | 116,238 | 15,986 | 13.75% | 66.72% | 42,359 | 11,237 | 26.53% | 54.31% | 845 | 85 | 10.06% |
| Age at encounter | <25 | 11.04% | 19,459 | 1759 | 9.04% | 11.51% | 7280 | 1381 | 18.97% | 13.29% | 212 | 20 | 9.43% |
| 26–45 | 29.28% | 51,607 | 6629 | 12.85% | 41.64% | 26,338 | 6651 | 25.25% | 48.21% | 769 | 65 | 8.45% | |
| 46–70 | 43.54% | 76,731 | 11,675 | 15.22% | 40.02% | 25,314 | 6952 | 27.46% | 34.29% | 547 | 31 | 5.67% | |
| >70 | 16.13% | 28,431 | 5451 | 19.17% | 6.84% | 4327 | 1249 | 28.87% | 4.20% | 67 | 7 | 10.45% | |
| Race | White | 81.50% | 182,067 | 21,936 | 12.05% | 85.29% | 55,636 | 13,062 | 23.48% | 85.64% | 1336 | 95 | 7.11% |
| Other a | 12.11% | 27,052 | 3418 | 12.63% | 8.69% | 5670 | 1719 | 30.32% | 8.59% | 134 | 16 | 11.94% | |
| Black | 5.37% | 12,006 | 1611 | 13.42% | 5.19% | 3387 | 1023 | 30.20% | 4.62% | 72 | 11 | 15.28% | |
| Asian | 1.02% | 2277 | 250 | 10.98% | 0.82% | 537 | 119 | 22.16% | 1.15% | 18 | 1 | 5.56% | |
| Ethnicity | Non‐hispanic/Latino | 85.19% | 189,401 | 23,015 | 12.15% | 89.55% | 58,249 | 13,889 | 23.84% | 89.07% | 1386 | 104 | 7.50% |
| Hispanic/Latino | 14.81% | 32,932 | 4094 | 12.43% | 10.45% | 6796 | 2005 | 29.50% | 10.93% | 170 | 19 | 11.18% | |
| BMI | <18.5 | 19.62% | 55,247 | 2985 | 5.40% | 6.65% | 6115 | 706 | 11.55% | 2.72% | 50 | 7 | 14.00% |
| 18.5–24.9 | 8.08% | 22,738 | 3618 | 15.91% | 8.41% | 7736 | 1921 | 24.83% | 4.78% | 88 | 12 | 13.64% | |
| 25–29.9 | 28.60% | 80,522 | 11,118 | 13.81% | 27.84% | 25,608 | 5853 | 22.86% | 37.17% | 684 | 43 | 6.29% | |
| 30–34.9 | 22.62% | 63,675 | 10,271 | 16.13% | 25.61% | 23,556 | 6347 | 26.94% | 27.66% | 509 | 58 | 11.39% | |
| 35–39.9 | 12.51% | 35,230 | 6517 | 18.50% | 17.30% | 15,911 | 5039 | 31.67% | 16.58% | 305 | 36 | 11.80% | |
| >40 | 8.57% | 24,139 | 5045 | 20.90% | 14.20% | 13,061 | 4900 | 37.52% | 11.09% | 204 | 30 | 14.71% | |
| Overall adult risk score b | Low (0–8) | 94.55% | 210,210 | 22,641 | 10.77% | 90.80% | 59,064 | 12,870 | 21.79% | 94.99% | 1478 | 97 | 6.56% |
| Medium (9–16) | 4.87% | 10,823 | 3881 | 35.86% | 8.34% | 5427 | 2679 | 49.36% | 4.18% | 65 | 22 | 33.85% | |
| High (>16) | 0.46% | 1013 | 540 | 53.31% | 0.77% | 500 | 327 | 65.40% | 0.39% | 6 | 2 | 33.33% | |
| No value | 0.13% | 287 | 47 | 16.38% | 0.08% | 54 | 18 | 33.33% | 0.45% | 7 | 2 | 28.57% | |
Note: Data mined during the 4/1/20 to 9/30/21 time frame and includes all HEDIS denominator eligible patients for abnormal BMI screening. Stratification by sex, age, race/ethnicity, BMI and overall risk score reveals comparable sub‐group composition between the three exposure groups.
Racial minorities include Native Alaskan or Hawiian, American Indian, other Pacific Islander, and minorities who listed “other” or not documented.
WellSpan Health scoring index that factors in (1.) Number of diagnoses (asthma, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, diabetes and hypertension) (2.) SDOH (age, depression risk, tobacco use, financial resource strain risk, food insecurity risk, transportation needs risk, physical inactivity risk, intimate partner violence risk, social isolation risk, and alcohol risk score) (3.) Healthcare utilization (recent emergency room visits or hospital admissions).
All statistical comparisons between HEDIS performance were significant with p values < 0.001 (Table 2). Blended‐telemedicine performed better than office both in HEDIS specified and HEDIS modified performance (12.56%, 11.16%); office performed better than telemedicine (4.29%, 4.79%).
TABLE 2.
Statistical comparison of primary care Healthcare Effectiveness Data and Information Set (HEDIS) specified and HEDIS modified quality performance
| Combined totals | Office (excluding telemedicine) | Blended telemedicine | Telemedicine (excluding office) | Office versus blended‐telemedicine | Office versus telemedicine | |
|---|---|---|---|---|---|---|
| HEDIS specified ‐ follow up plan within the study timeframe | ||||||
| Follow up plan | 43,003 | 27,109 | 15,771 | 123 | Difference 12.65% 95% CI 12.2886% to 13.0137%Chi‐squared 6198.379 p < 0.0001 | Difference 4.29% 95% CI 2.8374%–5.5364% chi‐squared 26.623 p < 0.0001 |
| BMI outside normal range | 287,378 | 222,333 | 63,489 | 1556 | ||
| Quality performance | 14.96% | 12.19% | 24.84% | 7.90% | ||
| HEDIS modified ‐ follow up plan within 3 months of provider encounter | ||||||
| Follow up plan | 25,948 | 14,648 | 11,272 | 28 | Difference: 11.16% 95% CI: 10.8470%–11.4762% chi‐squared: 7458.725 p < 0.0001 | Difference: 4.79% 95% CI3.9943%–5.3514% chi‐squared: 57.865 p < 0.0001 |
| BMI outside normal range | 287,378 | 222,333 | 63,489 | 1556 | ||
| Quality performance | 9.03% | 6.59% | 17.75% | 1.80% | ||
Note: Data represents patients seen from 4/1/20 ‐ 9/30/21 with BMI <18.5 or >25 meeting HEDIS specified criteria for abnormal BMI prevention. Comparisons reported as absolute percent differences in quality performance between exposure groups. Two comparisons: (1) office only versus blended telemedicine and (2) office only versus telemedicine only. Significant p‐values indicate true differences between quality performance in exposure groups favoring blended‐telemedicine overall.
Compared to a baseline control for abnormal BMI screening (293,070 patients), which was overwhelming office‐only encounters (99.14%), quality performance resembled study‐time frame performance, and the best performance was similarly in the blended group (Table 3).
TABLE 3.
Historical (pre‐COVID‐19) baseline Healthcare Effectiveness Data and Information Set (HEDIS) quality measure performance by health system patients from 9/1/18 to 3/31/21 (measuring the CMS HEDIS measure ‐ not the 3 months follow up)
| Office (excluding telemedicine) | Blended‐telemedicine (mixed office & telemedicine) | Telemedicine (excluding office) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sub‐group composition | HEDIS eligible | Quality performance | Sub‐group composition | HEDIS eligible | Quality performance | Sub‐group composition | HEDIS eligible | Quality performance | ||
| Pre‐COVID baseline (9/1/18‐3/31/20) | 293,070 | 99.14% | 290,554 | 11.79% | 0.85% | 2481 | 25.88% | 0.01% | 35 | 5.71% |
| Study timeframe (4/1/20‐9/30/21) | 287,387 | 77.36% | 222,333 | 12.19% | 22.09% | 63,489 | 24.84% | 0.01 | 1556 | 7.90% |
Note: A 1.5 years historical time frame (pre‐COVID‐19) revealed limited telemedicine or blended telemedicine utilization (<1% of all encounters) throughout the health system for abnormal BMI care compared to a 1.5 years study timeframe (26.6% telemedicine and blended telemedicine utilization). Quality performance is largely comparable or improved with the significant increase utilization of telemedicine during the study (pandemic) timeframe.
4. DISCUSSION
This 1.5‐year retrospective cohort study of 287,378 patients during the COVID‐19 pandemic found statistically significant differences in HEDIS quality performance for BMI screening favoring office‐only encounters over telemedicine‐only, but favoring blended‐telemedicine overall. These findings suggest that patients might achieve optimal performance in addressing and preventing complications of abnormal BMIs, and perhaps better long term management of abnormal BMI, by blending telemedicine and office encounters. Given the pandemic‐accelerated widespread utilization of telemedicine, and given the progression of modern healthcare incorporating telemedicine into standard of care, our findings provide generalizable insight on telemedicine care. Especially for overweight and class 1 obesity patients, telemedicine could significantly increase access to care and might provide increased follow up for weight management interventions. As findings reveal, those with a blend of telemedicine and office encounters had more documented plans for addressing abnormal BMIs. Thus, telemedicine likely plays an important role in increasing high‐value opportunities for intervention.
By including two versions of quality performance: a process‐type measure (CMS's retrospective HEDIS measure for BMI screening and follow‐up plan) and an outcome‐type measure (modified HEDIS measure looking at orders placed at or after the encounter), the effect of abnormal BMI intervention was simulated. This facilitated analytic granularity in the comparisons of telemedicine types (telemedicine‐only and blended‐telemedicine).
First, in the pure office and pure telemedicine comparison, there was better quality performance in the office‐only group. It seemed reasonable that the office‐only group might be more comfortable leaving their home and engaging in healthcare activities. This probably put the office‐only group at a relative advantage for higher quality performance, especially since many of the qualifying BMI preventions rely on follow up with in‐person interventions (like nutritional education, physical therapy, or behavioral health). The office‐only group was also the largest, and had the most patients of all subgroups. An ironic observation was that despite a prerequisite degree of healthcare literacy and technology proficiency, telemedicine‐only patients had the lowest rates of performance, both the HEDIS‐specified and the HEDIS‐modified comparisons (Table 2). It is uncertain why the telemedicine‐only group was lower performing. These telemedicine‐only patients may have had an inability or unwillingness to leave home, falling on the spectrum of bed ridden to simply fearful of being in public during the pandemic. It is also plausible that these patients had lower motivation for treatment, considering the comparative effort it takes to log into a telemedicine encounter versus following up with a treatment plan. But the most likely consideration is the methodological limitations of the telemedicine‐only group. Patients with even one office encounter during the time frame were excluded from the telemedicine‐only group (and included only in the blended group); an important consideration for interpretation.
Second, in pure office and mixed office‐telemedicine comparison, there was better quality performance in the blended‐telemedicine group. Until the blended‐telemedicine comparison was observed, it was assumed that the significantly better performance by office‐only patients was due to better patient‐provider interactions in a live format. However, as the literature has supported, a combination of care venues might be the best way to mitigate blind spots in telemedicine care (i.e., non‐verbal communication and physical exam) while expanding the opportunity for trust‐building interactions beyond traditional office‐based care. 36 This was evident in the study where the blended‐telemedicine group was the highest performing—doubling the office‐only HEDIS‐specified performance and nearly tripling the HEDIS‐modified quality performance (Table 2). The results of the blended‐telemedicine group inferred sequential nuance for telemedicine and office visits in abnormal BMI prevention. Although generalizability may depend on the population, in person care might be fitting for initial engagement of patients with abnormal BMIs, especially in target sub‐groups—overweight and class 1 obesity, non‐Hispanic, White patients ages 26–70 (Table 1). But thereafter, telemedicine might augment the patient‐provider engagement capacity needed to foster an environment of trust and facilitate increased opportunity for preventive interventions. This cooperative, feed‐forward effect of blended‐telemedicine might best explain increased quality performance.
These findings have important practice implications for clinicians managing patients with abnormal BMI. The non‐detrimental effect of telemedicine on HEDIS performance supports telemedicine as a potentially appropriate care space. As the healthcare industry continues toward stronger embrace of value based care, the clinical clout of HEDIS performance measures not only bolsters the reimbursement argument for telemedicine, but more importantly, supports the quality of telemedicine in abnormal BMI care moving forward.
The study had several limitations. First, there were sampling limitations. There was a very small telemedicine‐only exposure group. Unfortunately, the best method for selecting out telemedicine was to exclude all other types of visits. This severely limited the telemedicine‐only group since any office visit in the timeframe would preclude inclusion in the group. Nonetheless, at least this provided a high level of reassurance that the telemedicine‐only group included patients who were seen only via telemedicine. Similarly, the sample size was limited by EMR accuracy which affects HEDIS specified inclusion criteria (denominator). Especially for patients with telemedicine, it is unclear if more patients were uncaptured due to inaccurate encounter designation in the EMR. Especially with the evolving policies and rapid workflow changes with the pandemic, there may be unintended sampling bias which exceeded bias control strategies (inclusion/exclusion criteria and encounter type stratification).
Second, the HEDIS‐modified quality performance has a limited reach. This is due to the high volume follow up plans that qualify in the HEDIS measure and the high variability of time frame completion. For example, appetite suppressing medication could be ordered at the visit, but it may take a patient a month or two until he gets into nutritional education classes or behavioral therapy. This makes it difficult to reliably distinguish completion of intervention (outcome measure) versus ordering of that intervention (process measure). The better take away from the comparison of HEDIS‐modified versus HEDIS‐specified is the consistency of quality performance between care venue types.
Third, insurance type comparison was unable to be completed in our demographic analysis. Payer types were unable to be accurately separated out to stratify by BMI. Thus, speculations on the relationship between insurance type and telemedicine utilization cannot be made.
Lastly, the number of visits between groups were unable to be controlled. Given the limitations of SlicerDicer, a patient data model, primarily filtering by number of patients (not by number of visits) had to be used in order to build the HEDIS measure. This unfortunately precluded the ability to compare the percent of exposure for a more graded association. The ability to compare for example, a 25% blend versus a 75% blend of telemedicine exposure, would add value to future studies.
5. CONCLUSION
Quality performance for abnormal BMI screening was higher in the office‐only group compared to the telemedicine‐only group, but the blended telemedicine group performed best overall. Given the likely progression toward blended telemedicine‐office management in post‐pandemic medicine, and given the known cost savings benefits of telemedicine, adding telemedicine as a care venue might promote value within health systems without negatively impacting HEDIS performance. Future research could incorporate randomization to establish non‐inferiority of blended care, and analyzing individual follow‐up plans would provide more nuanced insight of how telemedicine could be used most effectively in BMI screening.
AUTHOR CONTRIBUTIONS
Derek Baughman and Yalda Jabbarpour conceived the conceptual framework and analyzed the data. Derek Baughman and Kathryn Baughman built data sessions, completed the statistical analysis and designed all figures and tables. Derek Baughman and Kathryn Baughman drafted the manuscript under the support of Abdul Waheed and Yalda Jabbarpour. Abdul Waheed, Derek Baughman, Kathryn Baughman, and Yalda Jabbarpour made ICMJE authorship contributions and are accountable for all aspects of the work. Steve Strom provided expert consultation on SlicerDicer data mining. Brian Pollak, MD (WellSpan Online Primary Care) was a key clinical telemedicine advisor. The Emig research center at WellSpan York Hospital advised on statistical analysis.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
There was no financial or material support received for this project. There are no author disclosures.
Baughman D, Baughman K, Jabbarpour Y, Waheed A. Comparable quality performance between telemedicine and office‐based care for abnormal BMI screening and management. Obes Sci Pract. 2023;9(2):87‐94. 10.1002/osp4.625
REFERENCES
- 1. CDC . Obesity Is a Common, Serious, and Costly Disease. Centers for Disease Control and Prevention; 2021. Published September 30. Accessed November 17, 2021. https://www.cdc.gov/obesity/data/adult.html [Google Scholar]
- 2. Obesity and overweight. Obesity and Overweight. Accessed September 27, 2021. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight [Google Scholar]
- 3. Lange SJ, Kompaniyets L, Freedman DS, et al. Longitudinal trends in body mass index before and during the COVID‐19 pandemic among persons aged 2–19 Years — United States, 2018–2020. MMWR Morb Mortal Wkly Rep. 2021(37):70‐1283. 10.15585/mmwr.mm7037a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Screening for and Management of Obesity. Screening for and Management of Obesity. Accessed November 17, 2021. https://www.ahrq.gov/ncepcr/tools/healthier‐pregnancy/fact‐sheets/obesity.html [Google Scholar]
- 5.CDC. All about Adult BMI. Centers for Disease Control and Prevention. Published 2021. Accessed November 17, 2021. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html [Google Scholar]
- 6. Bmi Assessment A. NCQA. Accessed November 17, 2021. https://www.ncqa.org/hedis/measures/adult‐bmi‐assessment/ [Google Scholar]
- 7. Obesity. Obesity. Accessed November 17, 2021. https://www.who.int/westernpacific/health‐topics/obesity [Google Scholar]
- 8. Final Recommendation Statement. Weight Loss to Prevent Obesity‐Related Morbidity and Mortality in Adults: Behavioral Interventions | United States Preventive Services Taskforce. Accessed November 17, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/obesity‐in‐adults‐interventions [Google Scholar]
- 9. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement | Adolescent Medicine | JAMA | JAMA Network. Accessed November 17, 2021. https://jamanetwork.com/journals/jama/fullarticle/2632511 [Google Scholar]
- 10. Arterburn DE, Alexander GL, Calvi J, et al. Body mass index measurement and obesity prevalence in ten U.S. health plans. Clin Med Res. 2010;8(3‐4):126‐130. 10.3121/cmr.2010.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chao GF, Ehlers AP, Telem DA. Improving obesity treatment through telemedicine: increasing access to bariatric surgery. Surg Obes Relat Dis. 2021;17(1):9‐11. 10.1016/j.soard.2020.09.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Application of telemedicine in obesity management ‐ ScienceDirect. Accessed November 17, 2021. https://www.sciencedirect.com/science/article/abs/pii/S2212764X17300419
- 13. Barbosa W, Zhou K, Waddell E, Myers T, Dorsey ER. Improving access to care: telemedicine across medical domains. Annu Rev Publ Health. 2021;42(1):463‐481. 10.1146/annurev-publhealth-090519-093711 [DOI] [PubMed] [Google Scholar]
- 14. Latifi R, Parsikia A, Boci A, Doarn CR, Merrell RC. Increased access to care through telemedicine in Albania: an analysis of 2, 724 patients. Telemed J e Health. 2020;26(2):164‐175. 10.1089/tmj.2018.0338 [DOI] [PubMed] [Google Scholar]
- 15. Atmojo JT, Sudaryanto WT, Widiyanto A, Ernawati E, Arradini D. Telemedicine, cost effectiveness, and patients satisfaction: a systematic review. Journal of Health Policy and Management. 2020;5(2):103‐107. 10.26911/thejhpm.2020.05.02.02 [DOI] [Google Scholar]
- 16. Valentino LA, Skinner MW, Pipe SW. The role of telemedicine in the delivery of health care in the COVID‐19 pandemic. Haemophilia. 2020;26(5). 10.1111/hae.14044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rozycki SW, Marvin KM, Davis KL, Ambrosio AA. Telemedicine proof of concept and cost savings during underway naval operations. Telemed J e Health. 2021;27(5):503‐507. 10.1089/tmj.2020.0181 [DOI] [PubMed] [Google Scholar]
- 18. Ullah W, Pathan SK, Panchal A, et al. Cost‐effectiveness and diagnostic accuracy of telemedicine in macular disease and diabetic retinopathy. Medicine (Baltim). 2020;99(25):e20306. 10.1097/MD.0000000000020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baughman DJ, Waheed A, Khan MN, Nicholson JM. Enhancing value‐based care with a walk‐in clinic: a primary care provider intervention to decrease low acuity emergency department overutilization. Cureus. 2021;13(2):e13284. 10.7759/cureus.13284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman DI, Rajan B, Seidmann A. A randomized trial of telemedicine for migraine management. Cephalalgia. 2019;39(12):1577‐1585. 10.1177/0333102419868250 [DOI] [PubMed] [Google Scholar]
- 21. deOliveira MM, Fuller TL, Gabaglia CR, et al. Repercussions of the COVID‐19 pandemic on preventive health services in Brazil. Prev Med. 2022;155:106914. 10.1016/j.ypmed.2021.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cortez C, Mansour O, Qato DM, Stafford RS, Alexander GC. Changes in short‐term, long‐term, and preventive care delivery in US office‐based and telemedicine visits during the COVID‐19 pandemic. JAMA Health Forum. 2021;2(7):e211529. 10.1001/jamahealthforum.2021.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitley A, Yahia N. Efficacy of clinic‐based telehealth vs. Face‐to‐Face interventions for obesity treatment in children and adolescents in the United States and Canada: a systematic review. Child Obes. 2021;17(5):299‐310. 10.1089/chi.2020.0347 [DOI] [PubMed] [Google Scholar]
- 24. Ferrara A, Hedderson MM, Brown SD, et al. A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): a randomised, parallel‐group, controlled trial. Lancet Diabetes & Endocrinol. 2020;8(6):490‐500. 10.1016/S2213-8587(20)30107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baughman D, Zain A, Waheed A. Patient Adherence to hemoglobin A1c testing recommendations in telemedicine and in‐office cohorts during COVID‐19. JAMA Netw Open. 2021;4(9):e2127779. 10.1001/jamanetworkopen.2021.27779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woolford SJ, Sidell M, Li X, et al. Changes in body mass index among children and adolescents during the COVID‐19 pandemic. JAMA. 2021;326(14):1434‐1436. 10.1001/jama.2021.15036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senthilingam M. Covid‐19 has made the obesity epidemic worse, but failed to ignite enough action. BMJ. 2021;372:n411. 10.1136/bmj.n41 [DOI] [PubMed] [Google Scholar]
- 28. Noguchi Y. Obesity Rates Rise during Pandemic, Fueled by Stress, Job Loss, Sedentary Lifestyle. NPR. Published September 29, 2021. Accessed November 17, 2021. https://www.npr.org/sections/health‐shots/2021/09/29/1041515129/obesity‐rates‐rise‐during‐pandemic‐fueled‐by‐stress‐job‐loss‐sedentary‐lifestyle [Google Scholar]
- 29. Almandoz JP, Xie L, Schellinger JN, et al. Telehealth utilization among multi‐ethnic patients with obesity during the COVID‐19 pandemic. J Telemed Telecare. Published online 2021: 1357633X21998211. 10.1177/1357633X21998211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. NQF . Obesity Measures ‐ Description. Accessed April 20, 2021. https://www.qualityforum.org/ProjectDescription.aspx?projectID=90313 [Google Scholar]
- 31. Quality ID #128 (NQF 0421): Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow‐Up Plan. :11.
- 32. Location Search ‐ WellSpan Health. Location Search ‐ WellSpan Health. Accessed December 8, 2021. https://www.wellspan.org/offices‐locations/location‐search/ [Google Scholar]
- 33. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020;91(1):157‐160. 10.23750/abm.v91i1.9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. NQF . Measuring Performance. Accessed March 20, 2021. https://www.qualityforum.org/Measuring_Performance/Measuring_Performance.aspx [Google Scholar]
- 35. NQF Quality Positioning System TM. NQF: Quality Positioning System TM. Accessed March 20, 2021. https://www.qualityforum.org/QPS/QPSTool.aspx?m=850&e=1#qpsPageState=%7B%22TabType%22%3A1 [Google Scholar]
- 36. Record JD, Ziegelstein RC, Christmas C, Rand CS, Hanyok LA. Delivering personalized care at a distance: how telemedicine can foster getting to know the patient as a person. J Personalized Med. 2021;11(2):137. 10.3390/jpm11020137 [DOI] [PMC free article] [PubMed] [Google Scholar]


