Abstract
Health hazards and environmental pollution are major concerns in present world. So, it is high time to think about ecofriendly and sustainable production. In this study, pumpkin juice has been used as an ecofriendly flame retardant finish to enhance the functionality of cotton twill fabric. The pumpkin juice extracted from the fresh pumpkin without any chemicals. The cotton fabric was treated with pumpkin juice in exhaust method. The treated and untreated samples were characterized by TGA, FTIR, SEM, and EDX. The flame-retardant property of the samples were evaluated based on the LOI and vertical flame tester. The result demonstrated that the treated samples exhibited high fire-retardant properties after being finished with pumpkin juice. The LOI value of the treated samples increased to 29 from 19 after treatment. The main reason behind the increased flammability is the dehydration of pumpkin juice-treated fabric which was clarified from the TG analysis. Moreover, the FTIR, SEM, and EDX report ensured the presence of bound and unbound water molecules, different salt, and several atoms in the samples treated with pumpkin juice that enhanced the protection against the spreading of the fire and thus improved fire-retardant properties of the treated samples.
Keywords: Pumpkin juice, Cotton fabric, Fire retardant, LOI, TGA, Air permeability, Water vapour permeability
Graphical abstract
1. Introduction
Cotton fabric is a trendy bio-based clothing that has been widely used in both military and civilian areas due to its outstanding performance such as air permeability, comfortability, hydrophilicity, softness, etc. [1]. It has a worldwide monopoly in the garment industry. It has many advantages, including biocompatibility, non-molting, biodegradability, hydrophilicity, softness, and comfort. For this reason, cotton fabric is used in apparel making for both adults and kids, as outdoor, and indoor decorations such as curtains, carpets, bedding, and wallpapers [2]. Consumer demand changed over time. So, researchers in the clothing sector are forced to develop discreet smart behavior apparel to meet the requisite needs of the diversified consumer's comfortability demand. For this aspiration, various behaviors have to be introduced into clothing such as fire retardancy, disinfectants, ultraviolet security, and hydrophobicity [[4], [5], [6]].
Market research discovered that customers worldwide are keen for new clothing with functionalities like a better-quality artistic feel with healthiness and hygiene. A few examples of functionality in apparel and household textile are flame retardant, anti-microbial, water repellence, soil release, resistance to color fading, and wrinkle resistance [7,10]. Among these, flame retardant functionality in clothing is treated to be one of the crucial parameters due to its application in household furnishing, railway, hospital, aircraft, and military wear. Flame retardant clothing is also vital for employees directly engaged in the oil, gas, firefighting, welding factory, glass item, and petroleum industries [[40], [41], [42]]. Cotton fabric is offered for them due to its convenience in soft feel, comfort, and suitable moisture evaporation properties. Besides, cotton is environment-friendly, decomposable, and fabricated from sustainable sources [3,9]. Furthermore, the flame retardant properties are inserted into these clothing as a smart function to improve user well-being [16].
Due to the majority of cellulosic essence, cotton fabric is highly flammable and very hard to extinguish, causing severe health hazards and deleteriousness of textiles and wealth [8]. As cotton fabrics are worn and kept in explicit touch with human bodies, the biological security of these clothing should be contemplated with fire retardancy [11,12]. There have been many efforts made in the past using several artificial chemicals. Researchers have improved the flame retardant property of cotton fabric using different goods, those goods are available in the market but mostly fabricated products. Borax and boric acid are the simplest and most common chemical compound mixtures. Many types of techniques also have been applied to make fire retardant materials. Even, various kinds of composites have been developed for enhancing fire retardancy properties [13,48,49]. Certain flame retardant goods pose risks to the environment and human health but are regulated differently in some countries. These products are banned in some countries. Flame retardants containing silicon, nitrogen, phosphorus, and boron have become dangerous for the environment with their excellent flame retardancy [14]. Also, phosphorous-based flame retardants have been shown to work well in conjunction with nitrogenous compounds, and this synergistic effect has been observed [15].
The most effective flame retardants are phosphorus-based and nitrogenous chemicals, which have been found to act synergistically [3,47]. Cotton fabric treated with a solution of acid becomes weaker and needs to be cured before it can be used. This is expensive and time-consuming. However, antimony and halogen have not yet been seen to be very effective as flame retardants. They produce lots of environmental injuries when they're used in large quantities [3,16,38]. Although the maximum effective technique for flame retardants uses nitrogen and phosphorus, much of this type of technique is not environmentally friendly. Different types of natural elements like aloe Vera extract, bio-polishing enzymes, and cellulose-based textile finishes are getting popular in research and development because people are becoming more conscious of their health and environment [3]. To find a balance between flame retardancy and biosafety characteristics, researchers have been trying to progress with bio-based fire-retardant materials. In the last few years, researchers have been exploring polysaccharides [17,18], proteins [19], deoxyribose [20], and plant extracts [3,8,21], etc. as fire retardants from nature [22,23]. Basak et al. [3,31] proved that herbal extracts like the juice of spinach leaves could develop the fire retardancy of cotton clothing. As the consciousness of human health and hygiene is increasing, manufacturers are seeking ways to produce textile finishes using different natural products such as dyes for dyeing from nature, an enzyme for bio-finishing, and neem and aloe vera juice for anti-microbial finishing agent [3,38].
Pumpkin (Cucurbita maxima), a member of the Cucurbitaceae family, is famous in Bangladesh. It is rich in carbohydrates and, vitamins A, C, and minerals. It is grown up in Bangladesh in an area of around 140,000 ha, and the regular yield is 20–25 metric tons per hectare [28]. Pumpkin is produced plenty amount in Bangladesh pasture land. It takes a low cost to be produced. It is also very cheap at the market as it is used only for vegetable purposes. Its value can be increased if it can be used for various purposes and exported [24,25]. Pumpkin is an excellent origin of functional food, composed of minerals, vitamins, and dietary fibers. Pumpkin is rich in carotenoids, vitamins, minerals, and dietary fibers, like spinach [24]. If it is possible to add the value of pumpkin by using it for different purposes, pumpkin production will be increased and can be exported [26]. Pumpkin contains silicate, phosphorus, mineral salts, and minerals which may be employed to enhance flame retardant characteristics of different non-cellulosic and cellulosic materials. This research attempted to improve cotton clothing's flame retardant properties using pumpkin extract, as pumpkin juice consists of magnesium, sodium, iron, silicate, proteinous nitrogen, and metallic constituents [1,24,27].
The twill fabric has an excellent thermal insulation capacity. It is a widely used fabric for firefighters and the military though it is not fire-retardant. Due to the interlacement, the strength of the twill fabrics is high; this has increased the popularity of these fabrics among the defense person [29,30,43,44]. Alongside the strength, adding fire retardant properties to the twill fabric would greatly increase the demand for the twill fabric for defense. This research will add value to the cheap and available vegetable pumpkin and increase the demand for twill fabric.
The research aimed to assess the effect of pumpkin juice to increase the flame retardant properties of cotton twill fabric in terms of LOI, ignition time, burning time, and thermal gravimetry.
2. Materials and methods
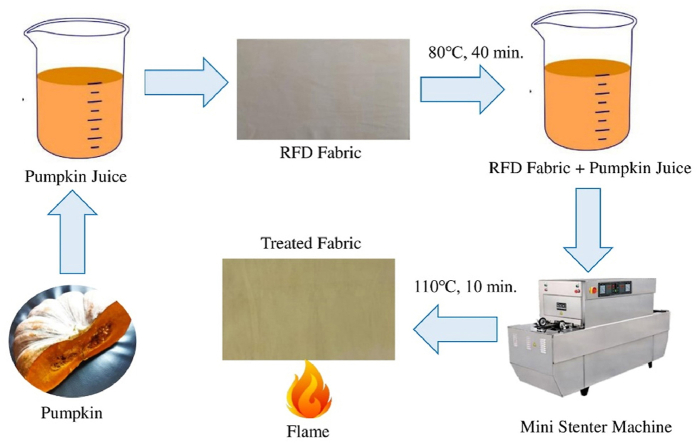
100% cotton 3/1 LH twill RFD fabric, specification 128 × 60/20 × 16, 245 GSM, was collected from Thermax Yarn Dyed Fabrics Ltd., Bangladesh, for this study. The pumpkin used for the analysis was procured from the local market. Pumpkin with a concentration of 500 g/L is first made into juice using a blender and filtered using a nylon strainer six times. Deionized water was used for the making of the juice. The pH of the pumpkin extract is 6.3 which was naturally obtained. RFD fabric was then impregnated in pumpkin juice at 80 °C temperature for 40 min. After the bath drain, the sample is rinsed for 10 min at 50 °C. The treated sample was then dried at 110 °C temperature for 10 min in a mini stenter machine. The recipe was optimized based on the burning time of the samples. Treated samples look a bit yellowish.
2.1. Testing
All treated and control samples were conditioned using a desiccator for 48 h at 65% RH and 27 °C before starting testing.
2.2. Determination of add-on%
The gravimetric principle was used to determine the add-on percentage of the treated sample following the oven dry weight principle; weight is taken of the sample before and after treatment, and was express the results in percentage of the weight in terms of before treatment weight.
| [1] |
Where M1 and M2 are the weight of the sample before treatment and after treatment, respectively. The testified results are an average of ten tests in each case.
2.3. Flammability assessment
The burning behavior of the samples both before and after treatment was evaluated by different usual methods. IS 13501 test method was used for testing limiting oxygen index (LOI) analysis. Ignition time and burning time were measured as per the testing procedures of ASTM D6413.
2.4. Washing durability
The fire-retardant property of the treated samples was evaluated against two wash cycles in laundry wash. The treated sample was washed using available detergent 1 g/L at 40 °C for 40 min and another sample for 60 min. The samples were then rinsed in the normal water for 5 min, then it was dried at 100 °C for 5 min. Then it was conditioned in a desiccator for 24 h before starting the flammability test.
2.5. Thermogravimetry (TG) analysis
Thermogravimetry (TG) is used to measure the regular weight loss of a sample concerning time and temperature variation. It similarly specifies the outcome of flame retardant on the pyrolysis of the polymeric residues. The TG analysis of both control and treated fabrics have been recognized using a thermogravimetric analyzer (TGA 4000, PerkinElmer, CT 06484, USA).
2.6. Fourier transform infrared spectroscopy (FTIR) analysis
IR Spirit FTIR spectrophotometer with ATR (QATR-S) analyzer machine, Shimadzu has been used to evaluate the FTIR curves of the control and the treated fabrics in this research.
2.7. Scanning electron microscope (SEM) and energy dispersive X-ray fluorescence spectrometer (EDX-FS) analysis
The surface analysis of the control and treated samples have been analyzed by Field Emission Scanning Electron Microscope (FE-SEM), ZEISS SIGMA, Germany. To determine the type and content of the elements comprising the sample was carried out by Shimadzu EDX-FS, model EDX-8000, Shimadzu, Japan.
2.8. Strength properties analysis
The tensile strength of both the samples were evaluated by a James Heal tester, USA as per ASTM D5034-09 method. The tear strength properties of both the fabrics sample were evaluated by James Heal tester, USA as per ISO 13937-1 method.
2.9. Permeability properties analysis
Air permeability tests of the woven sample were tested according to EN ISO 9237:1995. The permeability of the air was measured using a GT-N44 Air Permeability Tester with a head area of 20 cm2. On the other hand, Water vapor permeability is measured using a reference fabric in grams per square meter per 24 h (g/m2/day). Each dish of the tester is filled up with distilled water with a 10 mm gap between the water surface and the fabric. Samples are kept on each dish to keep the fabric level. The adhesive is applied to the rim of the dish and the specimen, which is 96 mm in diameter, is sensibly positioned on top. After a time, interval, the samples are weighed and noted against time. The water vapor permeability is calculated by the following relationship.
| [2] |
Where: M = loss in mass (g), t = time between weighing (h), A = internal area of the dish (m2).
3. Results and discussion
3.1. Mechanism of flame retardant property
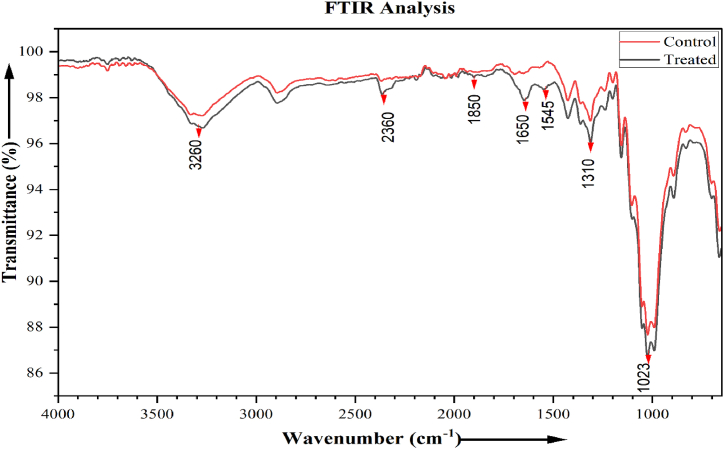
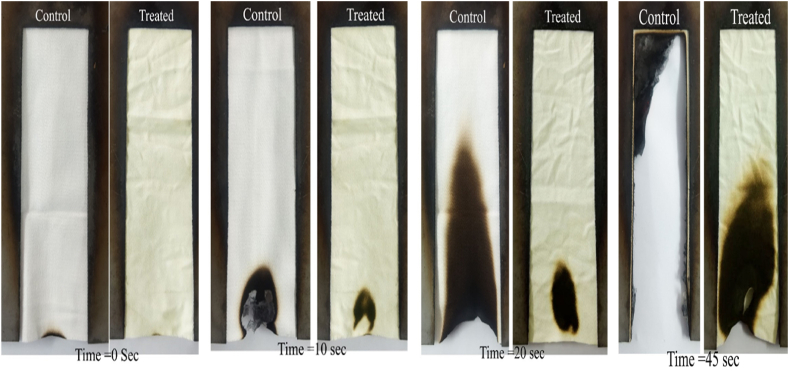
Naturally, cotton fabric is pure cellulose, so it has a low LOI value (LOI:19) which does not show any retardancy from flame. The sample treated with pumpkin juice ensures a good add-on (2.24%). This indicates that the cotton sample becomes flame retardant only because of the treatment of pumpkin juice and its chemical composition, which is inserted into the sample. Development of the fire-retardant properties due to the treatment with pumpkin juice may be credited to mineral salts and elements present in the pumpkin juice. Metal elements exist in the treated sample in the form of salts, such as potassium silicate, magnesium chloride, calcium chloride, etc. The presence of these elements and molecules is also detected in FTIR analysis (as shown in Fig. 3) and the elemental analysis (EDX-ray). Different descriptions prove that mineral salts present in the pumpkin juice retarded the thermal progress of the treated sample, and developed a dehydration process with an increasing amount of char formation, as seen in the burning image of Fig. 1 [46]. It is known that chloride and silicate-containing molecules are flame retardants in nature [3].
Fig. 3.
FTIR analysis of control and treated fabric.
Fig. 1.
Comparison in burning behavior of control and treated fabric at the different time intervals.
Unbound water molecule present in the treated sample with pumpkin juice, as seen in the FTIR image (as shown in Fig. 3), helps the sample to be flame retardant. Unbound water helps lower the rise of temperature by gripping the heat of evaporation. The moisture peak has been seen in the image of FTIR and TGA curves (as shown in Fig. 2, Fig. 3). So, the flame retardancy effect in the treated cotton sample might have developed for the existence of metal salts, different elements, and bound and unbound water fragments. These have supported the development of additional char and flammable gases like CO2 and H2O [46]. These gases might become weak the flammable gases such as levoglucosan and pyroglucosan, which are produced in the time of cellulose pyrolysis [36,45]. The total amount of organic material (cellulose) as per Table 3 has reduced in the treated sample, which might help to form char, which may lower the production of flammable gases in the cellulose pyrolysis process, as seen in the TGA curve.
Fig. 2.
TGA analysis graph of control and treated fabric.
Table 3.
The atomic percentage of control and treated fabric using EDX.
| Elements | Control | Treated |
|---|---|---|
| C | 46.10% | 33.50% |
| O | 53.90% | 57.20% |
| P | 0 | 2.09% |
| K | 0 | 1.97% |
| Ca | 0 | 1.56% |
| Mg | 0 | 1.26% |
| S | 0 | 1.14% |
| Si | 0 | 0.74% |
| Al | 0 | 0.21% |
| Fe | 0 | 0.17% |
| Mn | 0 | 0.07% |
| Cu | 0 | 0.05% |
| Cr | 0 | 0.05% |
| 100.00% |
3.2. Flammability testing
RFD (Ready For Dye) cotton twill fabrics were finished with pumpkin juice which was discussed in the section on materials and methods. The flammability test result and LOI test of both samples (control and treated) are declared in Table 1. The LOI express the flammability condition of the sample. The minimum quantity of oxygen in the oxygen-nitrogen mixture mandatory for ignition is defined as limiting oxygen index. Fibers taking a value LOI of 21 or below ignite, and it is burned rapidly in the open atmosphere. Sample with LOI value above 21 ignites but burns slowly. When value of LOI equal to or more than 26, is considered the material as a flame retardant material [3,31]. Both samples’ LOI values are shown in Table 1. As described previously, cotton having pure cellulose, catch flame easily and shows less LOI value 19. Before the application of pumpkin juice, the sample was scoured and bleached. After applying pumpkin juice to the cotton sample, the LOI value increased significantly, which is very higher than that of the control sample.
Table 1.
Flammability testing.
| Flammability parameters | Control | Treated | Increase/Decrease % |
|---|---|---|---|
| Add-on (%) | – | 2.24 | |
| LOI | 19 | 29 | 52.63 |
| Ignition time (second) | 10 | 12 | 20.00 |
| Burning Time (second) | 43.5 | 67.7 | 55.63 |
| Vertical flammability occurring of flashing over the surface | Yes | No | |
| Observed burning rate (mm/min) warp way | 175.17 | 112.56 | −35.74 |
In vertical flammability measurement, It is seen that the total burning time increased to 67.7s from 43.5s in the treated sample. It is also seen in Table 1 that the burning rate was reduced from 175.17 mm/min to 112.56 mm/min in the treated sample. As sample was burned completely, char length observation was ignored, though it was an important parameter.
3.3. Wash durability
Treated sample was washed with soap solution, then the sample was tested to determine how long fire-retardancy would last. After washing, the treated fabric had different burning behavior. This might have happened as a result of the components and mineral salts in pumpkin juice washing off the fabric's surface during washing. The burning time value of the washed cloth fell from 67.7S to 50.28S after 40 min wash, and to 49.03S after 60 min of washing, as shown in Table 2, but it remained higher than the value for the control fabric.
Table 2.
Wash durability of samples.
| Time (Second) | Control | Before wash | 40 min wash | 60 min wash |
|---|---|---|---|---|
| Ignition time | 10 | 12 | 10 | 10 |
| Burning time | 43.5 | 67.7 | 50.28 | 49.03 |
3.4. TGA analysis
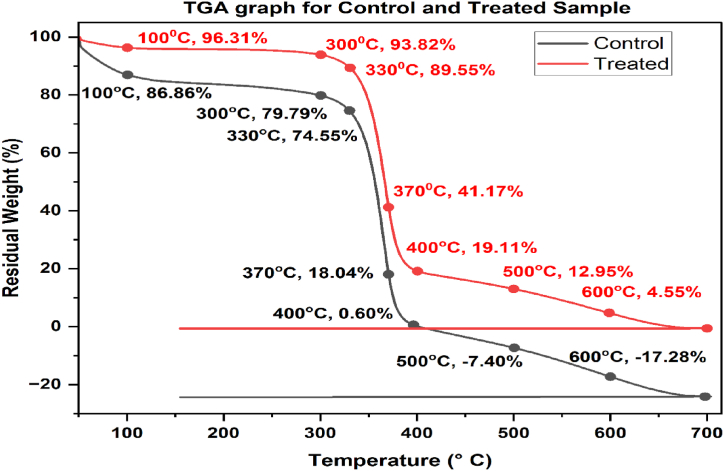
To measure the effects of heat degradation on the fabrics, the TGA analysis method was used. The TGA curves of the control and treated samples are shown in Fig. 2. TGA is performed in a nitrogen (N2) atmosphere at a heating rate of 10 °C/min. Three stages were seen in the TG curves of the control and treated samples, with the first stage occurring at a temperature below 100 °C. Here, the polymer's mass loss was primarily responsible for absorbed moisture. The primary pyrolysis stage took place at temperatures around 300 °C. When mass loss has begun to occur quickly, cellulose pyrolysis products may be produced at this stage.
The TG curve indicates that rapid breakdown begins at 300 °C; treated fabric loses only approximately 7% of its mass, which is significantly less than control fabric cause control fabric loses 20% of its mass. At 400 °C, the treated fabric lost 79% of its mass, whereas the control fabric lost all of its weight and started to make char. Weight loss of treated fabric is slower than the control fabric. It might be because of inorganic salt in the treated fabric. The control sample started to lose its mass (pyrolysis) from 100 °C gradually, but the treated fabric started pyrolysis at 300 °C, which is 200 °C below the degradation temperature of the treated sample. Hence, it is assumed that pumpkin juice treatment widens the transformation region and conjointly reduces the mass loss within the transformation. Additionally, it reduces the ignition temperature of the treated sample with the weakening of inflammable volatiles by the growing of non-oxidizable CO2 gas and H2O compound at relatively low temperatures. It may be determined from the TG curve that within the third stage, the treated material started char formation at a lower temperature. Also, char residue remained higher compared to the control fabric at a higher temperature. Char formation was started at around 300 °C, which is prior about 20–30 °C to the control cotton fabric. Thus, compared to the Control fabric, char residue remaining at 400 °C will increase from 19% in the treated sample. It shows that pumpkin juice positively impacted earlier dehydration and char formation. The pumpkin juice-treated sample might retain a lot of mass at higher temperatures. Within the treated samples, the reduction in burnable gas production and increased char formation have assisted the treated sample to be flame retardant. Therefore, it's that pumpkin juice will improve the treated fabric's general resistance to fire retardant and conjointly reduce the weight loss rate of cellulose.
3.5. FTIR analysis
FTIR analysis of the control and treated sample are shown in Fig. 2, and it is not found any important variation between the curves. The treated curve showed a wide band of water in the near region of 3300 cm−1. It demonstrates that the treated fabric has more bound and unbound water molecules, which show fire-retardant properties. The absorption bands at 2360 cm−1 in the FTIR spectrum of the treated are sharp and clear, which is missing in the control's spectrum. These bands link to symmetrical stretching vibrations of O–H bonds in C–OH and CH2–OH groups characteristic for the polysaccharide structure of β-CDx and in adsorbed Н2О molecules and confirm the use of the pumpkin during the finishing [32]. The carbonyl, C O−NHR band is observed at 1650 cm−1 at the treated fabric but is absent in the control sample [33]. The newly observed peaks at 1850 cm−1 wavelength was mainly due to the presence of Fe-in the treated sample [34]. Spectra at 1545 cm−1 prove the presence of a salt of copper in the treated sample [35]. It is assumed that more amount of bound and unbound water molecules and different mineral salt presence helps the treated fabric to show fire retardant properties.
3.6. SEM and EDX analysis
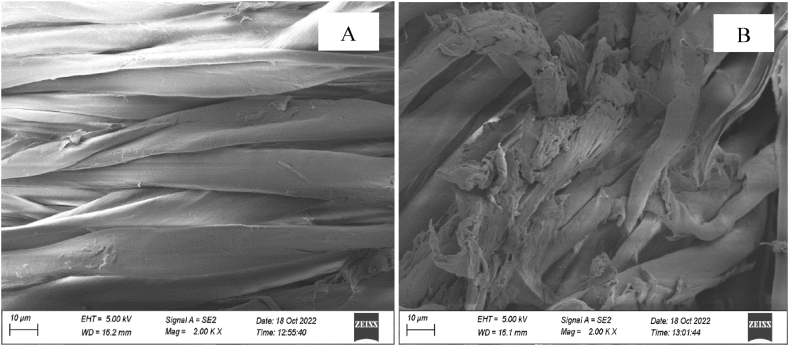
Fig. 4 shows the image of SEM of the both control and treated sample. The control sample is seen very clean, and uniform. However, a thin layer of pumpkin juice could be readily visible after pumpkin juice treatment. Pumpkin juice is un-uniformly and very tiny distributed over the entire surface. It is seen that treated fabric is ruptured, which indicates that the fabric will lose its strength; the tensile strength report (as shown in Table 4) shows that treated fabric loses its strength after treatment. It may be due to the effect of salt absorbed from pumpkin juice.
Fig. 4.
SEM images of control fabric and treated fabric (A. Control fabric; B. Treated fabric).
Table 4.
Strength and elongation report of control and treated sample.
| Cotton Fabric | Tensile Strength |
Tear Strength(N) |
Elongation% |
|||
|---|---|---|---|---|---|---|
| Warp | Weft | Warp | Weft | Warp | Weft | |
| Control | 494.15 | 285.91 | 10.37 | 17.34 | 14.51 | 15.49 |
| Treated | 379.09 | 244.65 | 23.67 | 17.39 | 18.42 | 16.25 |
EDX-FS analysis data is informed in Table 3 of both the control and the treated fabric. Only the carbon and oxygen atoms are seen in the control sample as the analyzer could not detect hydrogen atoms. But, in the treated sample, numerous atoms are visible in a tiny amount. These correspond to phosphorus, potassium, calcium, sodium, magnesium, sulfur, silicon, aluminum, and iron. Phosphorus helps to prevent fire flame from spreading [39]. The Phosphorus percentage in the treated cotton fabric is higher than other atoms. It is seen treated carbon amount in the treated sample was reduced by about 27.33%. Nevertheless, the Oxygen proportion of the treated sample increased by 6.12%. All elements are present in the pure pumpkin except silicone. Silicone may have come from sample handling because it was present in silicate (SiO2 or Na2SiO3) in the treated fabric.
3.7. Strength properties analysis
Table 4 displays the tensile and tear strength of the control and treated fabric samples. The application of pumpkin juice was found to affect the tensile strength, tear strength, and elongation percentage. In most cases of application of conventional and commercial flame retardant finishes, a significant loss of tensile strength occurred [37]. Another assumption is that elements penetrate inside the fibers and react with the hydroxyl groups inside the macromolecule so that it either produces cellulosate or links to the molecules through the pulling forces and increases the amorphous region, which leads to a decrease in tensile strength. As shown in Table 4, there is a bit decrease in tensile strength. There is a very slight increase in tear strength, which might be due to the metal salts on the surface of the cotton fabric. Surface metals might increase stiffness as well as tear strength [23]. On the other hand, no noticeable significant increase in elongation percentage is found.
3.8. Permeability properties
There is no change in the air permeability of the fabric due to the pumpkin juice treatment (as shown in Table 5). It is a good sign that after finishing with pumpkin juice, the fabric can be used for the wearer's purpose, and it will not cause any problem with the comfort of the wearer in warm conditions. In the case of pumpkin juice treatment, there is also no significant change in moisture content and moisture regain percentage. No coating on the fabric surface affects water resistance, which is proven as per the water-resistant test. Due to treatment with pumpkin juice, treated fabric lost some ability to pass water vapor. The treated fabric has lower water vapor permeability than the control fabric; it may have the effect of different salt upon the fabric surface as per the SEM image.
Table 5.
Permeability properties of the control and treated sample.
| Cotton Fabric | Air Permeability (L/m2/s) | Water Vapor Permeability (g/m2/d) | MC% | MR% | Water Resistance |
|---|---|---|---|---|---|
| Control | 108.71 | 1354.92 | 5.4 | 5.71 | 0 |
| Treated | 108.11 | 821.18 | 6.1 | 6.5 | 0 |
4. Conclusion
In this work, it is seen that flame retardancy properties have been increased in the cotton fabric after treatment with pumpkin juice. LOI value increased to 29 from 19 after treatment with pumpkin juice which is very significant as total burning time increased by 27S due to the increase of LOI. This longer time will increase more chance to escape from the danger zone and extinguish the fire which will save lives. As there is no use of chemicals, it is environmentally friendly. Enhancement of fire-retardant properties of treated fabric might be developed for the presence of metal salts, silicate, and water molecules (bound and inbound). Thermogravimetry (TG) analysis report shows the dehydration of pumpkin juice-treated fabric. The pumpkin juice application procedure is straightforward and cost-effective as there are no other chemicals. Pumpkin juice as flame retardant juice could be used for home furnishing, military dresses, firefighter dress, and different types of mobile furnishing like fairs, festivals, stalls, and shops which is for a limited period but has a high risk for fire as there is a huge usage of textile material as fancy material. A higher LOI value means a higher time for ignition will help the treated material like kitchen cloth, agro textile, and military dress to be safer from danger. Pumpkin is a cheap and available vegetable in Bangladesh the air, so it will be an eco-friend vegetable source. Applying pumpkin juice for functional properties on cotton will increase the value of the available vegetables.
Author contribution statement
Abdullah Al Rakib Shikder: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Md. Abdullah Al Mamun: Conceived and designed the experiments.
Tarikul Islam: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Md. Humayun Kabir Khan: Analyzed and interpreted the data; Wrote the paper.
Mohammad Zia Uddin: Performed the experiments Analyzed and interpreted the data.
Funding statement
The authors received financial support from the Research Cell, Mawlana Bhashani Science and Technology University, Tangail, Bangladesh under the FY 2022-2023 to complete this research work (Grant number: ST23-4).
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
References
- 1.Liu Y., Wang Q.-Q., Jiang Z.-M., Zhang C.-J., Li Z.-F., Chen H.-Q., Zhu P. Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J. Anal. Appl. Pyrolysis. 2018;135:289–298. doi: 10.1016/j.jaap.2018.08.024. [DOI] [Google Scholar]
- 2.Gao W.-W., Zhang G.-X., Zhang F.-X. Enhancement of flame retardancy of cotton fabrics by grafting a novel organic phosphorous-based flame retardant. Cellulose. 2015;22:2787–2796. doi: 10.1007/s10570-015-0641-z. [DOI] [Google Scholar]
- 3.Basak S., Samanta K.K., Chattopadhyay S.K. Fire retardant property of cotton fabric treated with herbal extract. J. Text. Inst. 2015;106:1338–1347. doi: 10.1080/00405000.2014.995456. [DOI] [Google Scholar]
- 4.Attia N.F., el Ebissy A.A., Hassan M.A. Novel synthesis and characterization of conductive and flame retardant textile fabrics. Polym. Adv. Technol. 2015;26:1551–1557. doi: 10.1002/pat.3580. [DOI] [Google Scholar]
- 5.Shaheen T.I., El-Naggar M.E., Abdelgawad A.M., Hebeish A. Durable antibacterial and UV protections of in situ synthesized zinc oxide nanoparticles onto cotton fabrics. Int. J. Biol. Macromol. 2016;83:426–432. doi: 10.1016/j.ijbiomac.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Yu D., Xu L., Hu Y., Li Y., Wang W. Durable antibacterial finishing of cotton fabric based on thiol–epoxy click chemistry. RSC Adv. 2017;7:18838–18843. doi: 10.1039/c6ra28803k. [DOI] [Google Scholar]
- 7.Gulrajani M.L., Deepti G. 2011. Emerging Techniques for Functional Finishing of Textiles.http://nopr.niscpr.res.in/handle/123456789/13233 [Google Scholar]
- 8.Basak S., Samanta K.K., Saxena S., Chattopadhyay S.K., Narkar R., Mahangade R., Hadge G.B. Flame resistant cellulosic substrate using banana pseudostem sap. Pol. J. Chem. Technol. 2015;17:123–133. doi: 10.1515/pjct-2015-0018. [DOI] [Google Scholar]
- 9.Wang W., Guo J., Liu X., Li H., Sun J., Gu X., Wang J., Zhang S., Li W. Constructing eco-friendly flame retardant coating on cotton fabrics by layer-by-layer self-assembly. Cellulose. 2020;27:5377–5389. [Google Scholar]
- 10.Chowdhury N., Faysal G.M., Al A., Shikder R., Moula A.T.M.G. 6th Int. Conf. Eng. Res. Innov. Educ., Sylhet, Bangladesh. 2021. A study on sustainable fashion supply chain of zara; p. 1. 1. [Google Scholar]
- 11.Gomes G., Ward P., Lorenzo A., Hoffman K., Stapleton H.M. Characterizing flame retardant applications and potential human exposure in backpacking tents. Environ. Sci. Technol. 2016;50:5338–5345. doi: 10.1021/acs.est.6b00923. [DOI] [PubMed] [Google Scholar]
- 12.Gong M., Weschler C.J., Zhang Y. Impact of clothing on dermal exposure to phthalates: observations and insights from sampling both skin and clothing. Environ. Sci. Technol. 2016;50:4350–4357. doi: 10.1021/acs.est.6b00113. [DOI] [PubMed] [Google Scholar]
- 13.Becenen N., Eyi G. Investigation of the flammability properties of a cotton and elastane blend denim fabric in the presence of boric acid, borax, and nano-SiO2. J. Text. Inst. 2021;112:1080–1092. doi: 10.1080/00405000.2020.1800974. [DOI] [Google Scholar]
- 14.Costes L., Laoutid F., Brohez S., Dubois P. Bio-based flame retardants: when nature meets fire protection. Mater. Sci. Eng. R Rep. 2017;117:1–25. doi: 10.1016/j.mser.2017.04.001. [DOI] [Google Scholar]
- 15.Salmeia K.A., Gaan S., Malucelli G. Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polymers. 2016;8:319. doi: 10.3390/polym8090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsayed E.M., Attia N.F., Alshehri L.A. Innovative flame retardant and antibacterial fabrics coating based on inorganic nanotubes. ChemistrySelect. 2020;5:2961–2965. doi: 10.1002/slct.201904182. [DOI] [Google Scholar]
- 17.Šimkovic I. Flame retarded composite panels from sugar beet residues. J. Therm. Anal. Calorim. 2012;109:1445–1455. doi: 10.1007/s10973-011-1879-9. [DOI] [Google Scholar]
- 18.Passauer L. Thermal characterization of ammonium starch phosphate carbamates for potential applications as bio-based flame-retardants. Carbohydr. Polym. 2019;211:69–74. doi: 10.1016/j.carbpol.2019.01.100. [DOI] [PubMed] [Google Scholar]
- 19.Bosco F., Carletto R.A., Alongi J., Marmo L., di Blasio A., Malucelli G. Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr. Polym. 2013;94:372–377. doi: 10.1016/j.carbpol.2012.12.075. [DOI] [PubMed] [Google Scholar]
- 20.Ortelli S., Malucelli G., Blosi M., Zanoni I., Costa A.L. NanoTiO2@ DNA complex: a novel eco, durable, fire retardant design strategy for cotton textiles. J. Colloid Interface Sci. 2019;546:174–183. doi: 10.1016/j.jcis.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 21.Li P., Wang B., Xu Y.-J., Jiang Z., Dong C., Liu Y., Zhu P. Ecofriendly flame-retardant cotton fabrics: preparation, flame retardancy, thermal degradation properties, and mechanism. ACS Sustain. Chem. Eng. 2019;7:19246–19256. doi: 10.1021/acssuschemeng.9b05523. [DOI] [Google Scholar]
- 22.Alongi J., Bosco F., Carosio F., di Blasio A., Malucelli G. 2014. A New Era for Flame Retardant Materials? [DOI] [Google Scholar]
- 23.Basak S., Wazed Ali S. Wastage pomegranate rind extracts (PRE): a one step green solution for bioactive and naturally dyed cotton substrate with special emphasis on its fire protection efficacy. Cellulose. 2019;26:3601–3623. doi: 10.1007/s10570-019-02327-x. [DOI] [Google Scholar]
- 24.Dhiman A.K., Sharma K.D., Attri S. Functional constituents and processing of pumpkin: a review. J. Food Sci. Technol. 2009;46:411. https://www.cabdirect.org/cabdirect/abstract/20093275557 [Google Scholar]
- 25.Khatun M., Rashid M.A., Miah M.A.M., Khandoker S., Islam M.T. Profitability of sandbar cropping method of pumpkin cultivation in char land areas of northern Bangladesh. Bangladesh J. Agric. Res. 2017;42:647–663. doi: 10.3329/bjar.v42i4.35792. [DOI] [Google Scholar]
- 26.Sharmin S., Mitra S., Rashid M. Production, yield and area growth of major winter vegetables of Bangladesh. J. Bangladesh Agric. Univ. 2018;16:492–502. https://ssrn.com/abstract=3308331 [Google Scholar]
- 27.Yadav M., Jain S., Tomar R., Prasad G., Yadav H. Medicinal and biological potential of pumpkin: an updated review. Nutr. Res. Rev. 2010;23:184–190. doi: 10.1017/S0954422410000107. [DOI] [PubMed] [Google Scholar]
- 28.Hossain S.M.M., Rahman A.K.M.M. Banglapedia, National Encyclopedia of Bangladesh. 2021. Gourd.https://en.banglapedia.org/index.php/Gourd. Accessed 18 Jun 2021 [Google Scholar]
- 29.Tseghai G.B., Berhe B.T., Wakjira Y.T. Producing fire retardant cotton fabric using chicken eggshell. J. Textil. Sci. Eng. 2019;9 doi: 10.4172/2165-8064.1000396. [DOI] [Google Scholar]
- 30.Misnon M.I., Islam M.M., Epaarachchi J.A., Chen H., Goda K., Khan M.T.I. Flammability characteristics of chemical treated woven hemp fabric reinforced vinyl ester composites. Sci. Techn. Mater. 2018;30:174–188. [Google Scholar]
- 31.Saleh S.M., Mohammed H.A. Flame resistance of cotton fabrics and their natural dyeing using plant waste of banana pseudostem (BPS), Egypt. J. Chem. 2020;63:3355–3366. doi: 10.21608/EJCHEM.2020.27948.2599. [DOI] [Google Scholar]
- 32.Gaffney J.S., Marley N.A., Jones D.E. Fourier transform infrared (FTIR) spectroscopy. Character. Mater. 2002:1–33. doi: 10.1002/0471266965.com107.pub2. [DOI] [Google Scholar]
- 33.Osman Z., Arof A.K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochim. Acta. 2003;48:993–999. doi: 10.1016/s0013-4686(02)00812-5. [DOI] [Google Scholar]
- 34.Spoto G., Zecchina A., Berlier G., Bordiga S., Clerici M.G., Basini L. FTIR and UV–Vis characterization of Fe-Silicalite. J. Mol. Catal. Chem. 2000;158:107–114. doi: 10.1016/s1381-1169(00)00049-2. [DOI] [Google Scholar]
- 35.Galarneau A., di Renzo F., Fajula F., Vedrine J. Elsevier; Montpellier, France: 2001. Zeolites and Mesoporous Materials at the Dawn of the 21st Century: Proceedings of the 13th International Zeolite Conference. 8-13 July 2001. [DOI] [Google Scholar]
- 36.Jia P., Ma Y., Feng G., Hu L., Zhou Y. High-value utilization of forest resources: dehydroabietic acid as a chemical platform for producing non-toxic and environment-friendly polymer materials. J. Clean. Prod. 2019;227:662–674. doi: 10.1016/j.jclepro.2019.04.220. [DOI] [Google Scholar]
- 37.Banerjee S.K., Day A., Ray P.K. Fireproofing jute. Textil. Res. J. 1986;56:338–339. doi: 10.1177/004051758605600510. [DOI] [Google Scholar]
- 38.Basak S., Samanta K.K., Chattopadhyay S.K., Das S., Narkar R., Dsouza C., Shaikh A.H. 2014. Flame Retardant and Antimicrobial Ligno-Cellulosic Fabric Using Sodium Metasilicate Nonahydrate.http://nopr.niscpr.res.in/handle/123456789/29344 [Google Scholar]
- 39.Repon M.R., Siddiquee N.A., Jalil M.A., Mikucioniene D., Karim M.R., Islam T. Flame retardancy enhancement of jute fabric using chemical treatment. Tekstilec. 2021;64:70–80. doi: 10.14502/Tekstilec2021.64.70-80. [DOI] [Google Scholar]
- 40.Zhang Q., Ma L., Xue T., Tian J., Fan W., Liu T. Flame-retardant and thermal-protective polyimide-hydroxyapatite aerogel fiber-based composite textile for firefighting clothing. Compos. B Eng. 2023;248 doi: 10.1016/j.compositesb.2022.110377. [DOI] [Google Scholar]
- 41.Banks A.P.W., Wang X., Engelsman M., He C., Osorio A.F., Mueller J.F. Assessing decontamination and laundering processes for the removal of polycyclic aromatic hydrocarbons and flame retardants from firefighting uniforms. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110616. [DOI] [PubMed] [Google Scholar]
- 42.Haase J. Handbook of Fire Resistant Textiles. Elsevier; 2013. Flame resistant clothing standards and regulations; pp. 364–414. [DOI] [Google Scholar]
- 43.Chang S., Condon B., Graves E., Uchimiya M., Fortier C., Easson M., Wakelyn P. Flame retardant properties of triazine phosphonates derivative with cotton fabric. Fibers Polym. 2011;12(3):334–339. doi: 10.1002/pat.3029. [DOI] [Google Scholar]
- 44.Nguyen T.M.D., Chang S., Condon B., Uchimiya M., Graves E., Smith J., Wakelyn P. Synthesis and characterization of a novel phosphorus–nitrogen‐containing flame retardant and its application for textile. Polym. Adv. Technol. 2012;23(7):1036–1044. doi: 10.1002/pat.2008. [DOI] [Google Scholar]
- 45.Tan W., Ren Y., Xiao M., Guo Y., Liu Y., Zhang J., Liu X. Enhancing the flame retardancy of lyocell fabric finished with an efficient, halogen-free flame retardant. RSC Adv. 2021;11(55):34926–34937. doi: 10.1039/D1RA06573D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horrocks A.R., Kandola B.K., Davies P.J., Zhang S., Padbury S.A. Developments in flame retardant textiles. Review. 2005;88(1):3–12. doi: 10.1016/j.polymdegradstab.2003.10.024. [DOI] [Google Scholar]
- 47.Kader A.H.A., Dacrory S., Khattab T.A., et al. Hydrophobic and flame-retardant foam based on cellulose. J. Polym. Environ. 2022;30:2366–2377. doi: 10.1007/s10924-021-02355-4. [DOI] [Google Scholar]
- 48.Aldalbahi A., El-Naggar M.E., Khattab T.A., Hossain M. Preparation of flame-retardant, hydrophobic, ultraviolet protective, and luminescent transparent wood. Luminescence. 2021;36:1922–1932. doi: 10.1002/bio.4126. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed E., Maamoun D., Hassan T.M., Khattab T.A. Development of functional glow-in-the-dark photoluminescence linen fabrics with ultraviolet sensing and shielding. Luminescence. 2022;37:1376–1386. doi: 10.1002/bio.4310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.