Abstract
Lophotrochozoa is one of the most species-rich but immunologically poorly explored phyla. Although lack of acquired response in a narrow sense, lophotrochozoans possess various genetic mechanisms that enhance the diversity and specificity of innate immune system. Here, we review the recent advances of comparative immunology studies in lophotrochozoans with focus on immune recognition and effector systems. Haemocytes and coelomocytes are general important yet understudied player. Comparative genomics studies suggest expansion and functional divergence of lophotrochozoan immune reorganization systems is not as “homogeneous and simple” as we thought including the large-scale expansion and molecular divergence of pattern recognition receptors (PRRs) (TLRs, RLRs, lectins, etc.) and signaling adapters (MyD88s etc.), significant domain recombination of immune receptors (RLR, NLRs, lectins, etc.), extensive somatic recombination of fibrinogenrelated proteins (FREPs) in snails. Furthermore, there are repeatedly identified molecular mechanisms that generate immune effector diversity, including high polymorphism of antimicrobial peptides and proteins (AMPs), reactive oxygen and nitrogen species (RONS) and cytokines. Finally, we argue that the next generation omics tools and the recently emerged genome editing technicism will revolutionize our understanding of innate immune system in a comparative immunology perspective.
Keywords: Lophotrochozoa, Innate immunity, Diversification, PRRs
1. Introduction
Innate immune response is a fast, effective and semi-specific immune response, which is the first line of defense against pathogens [1], [2], [3], [4]. Nevertheless, innate immunity is relatively rarely investigated in the invertebrates, and the knowledge about key molecules from this system is fragmentary. Since the emergence of omics approaches, there is a steady increase in the availability of genomic and transcriptomic data, which has enabled researchers to investigate invertebrate immune genes. Previous studies had shown that the various genetic mechanisms of innate immune genes increased the diversity and specificity of the innate response in several model invertebrates, such as down syndrome cell adhesion molecules (Dscams) and peptidoglycan recognition proteins (PGRPs) of fruit fly Drosophila melanogaster [5], [6], Sp185/333 system in sea urchin Strongylocentrotus purpuratus [7], [8], [9] and variable domain-containing chitin binding proteins (VCBPs) in amphioxus Branchiostoma floridae [10], [11], [12].
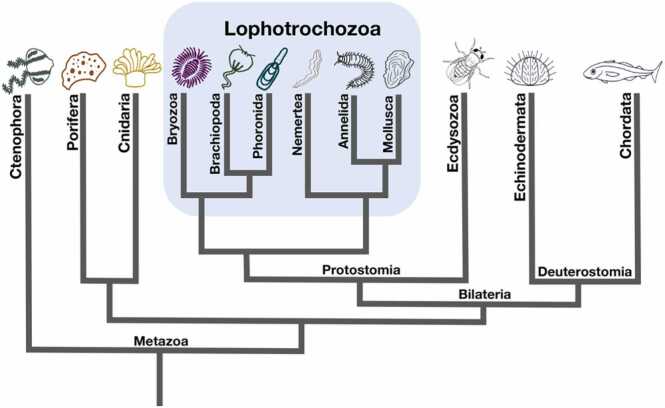
Lophotrochozoa, one of the three major clades of bilateral animals, which includes molluscs, segmented worms, and other invertebrates [13], [14] (Fig. 1). Despite lophotrochozoans lack adaptive immunity, they possess an effective and diverse innate immune system with a capacity to produce thousands of different immune proteins with an enormous range of sequence variability [15], [16]. This diverse innate immunity protects lophotrochozoans against pathogens and various environmental stressors [15], [17]. In the course of evolution, lophotrochozoans have developed some unique and novel strategies to expand the repertoire of immune responsiveness compared with vertebrates, such as the large-scale expansion and molecular divergence of pattern recognition receptors (PRRs) and signaling adapters [2], [18], [19], [20], significant domain recombination of immune receptors [2], [20], extensive somatic recombination of FREPs in snails [21], [22], [23], [24], [25], [26]. Furthermore, high polymorphism (e.g. single nucleotide polymorphisms SNPs) [27], [28], [29], [30] of immune effector molecules [31], [32], [33] are also observed in the lophotrochozoan innate immune system.
Fig. 1.
Simplified phylogenetic tree depicting the general relationships of major lophotrochozoa superphyla. The blue shaded box denote the lophotrochozoa superphyla.
This article will review recent advances of comparative immunology studies in the lophotrochozoa superphyla. The study will also provide long-needed perspectives on the evolution and function of multigene families in immune response, gaining a further understanding of innate immunity diversity in invertebrates.
2. Haemocytes and coelomocytes are key cellular players in lophotrochozoan innate immune response
Lophotrochozoans exhibit a wide range of ecological adaptations including an environment rich in pathogenic organisms and stressful factors. Thus they have evolved effective strategies, allowing them to enhance environmental adaptability. These immune responses are generally coordinated by haemocytes and coelomocytes, which acts both undertaker and supplier of in cellular and humoral immunity [34], [35], [36].
In bivalves, haemocytes have been classified into two main groups: granulocytes, which are a type of cytoplasmic cells with granules and low N/C (nuclear-to-cytoplasmic) ratio, and hyalinocytes (or agranulocytes) contain few or no granules and high N/C ratio [34], [37], [38], [39]. The two types of haemocytes have been discovered in clams, oysters, scallops, razor shells and cockles [36]. Recent studies have identified 14 granules in the clam Ruditapes philippinarum granulocytes [40] and 12 haemocyte subpopulations in the oyster Crassostrea hongkongensis by single cell RNA-seq [41]. Furthermore, a great number of hyalinocytes were also identified in various bivalves, such as mussels Perna perna [42], Mytilus chilensis [43] and Dreissena polymorpha [44]; the clams Chamelea gallina [45], Meretrix meretrix [46] and Ruditapes decussatus [47]; the scallops Argopecten irradians [48]; the oysters Crassostrea brasiliana [49], C. gigas [50], C. hongkongensis [51], C. rhizophorae [52] and so on (Table 1).
Table 1.
Immune diversification in Lophotrochozoa.
| Gene/protein/cell | Group or Species | Level of diversity | Identified diversification mechanisms | Ref. |
|---|---|---|---|---|
| TLR | Molluscs | 83 genes in Crassostrea gigas, 130 genes in C. virginica, 96 in Pinctada fucata, 103 genes in Limnoperna fortunei | Tandem duplication, lineage-specific expansion, domain shuffling, selective pressure on genetic sequence and function divergence | [18], [19], [94] |
| MyD88 | Molluscs | 10 genes in Crassostrea gigas, 19 genes in Pomacea canaliculata, 23 genes in Mizuhopecten yessoensis | Tandem duplication and lineage-specific expansion | [18], [19] |
| RLRs | Molluscs Brachiopods Annelids Nemerteans |
13 genes in Crassostrea gigas, 19 genes in Mytilus coruscus, 8 genes in Lingula anatina, 7 genes in Eisenia foetida, 6 genes in Notospermus geniculatus | Domain shuffling, tandem duplication and selective pressure on genetic sequence | [2] |
| NLRs | Molluscs Annelids |
164 genes in Pinctada fucata, 150 genes in P. imbricata, 108 genes in Capitella teleta | Lineage-specific gene duplication and domain shuffling | [20] |
| C1qDC | Molluscs | 321genes in the Crassostrea gigas, 476 genes in the C. virginica, 296 genes in the Pinctada fucata | Tandem duplication, lineage-specific expansion and SNPs | [18], [19], [27], [115] |
| FREPs | Molluscs | ≤ 45 genes per individual Biomphalaria glabrata 190 predicted FREPs in the Crassostrea gigas |
Multiple gene families, alternative splicing, somatic recombination and selective pressure on genetic sequence | [23], [24], [25], [26], [84], [140] |
| AMPs | Molluscs Annelids |
defensins, big defensins, proline-rich AMPs and bactericidal/ permeability-increasing (BPI) proteins in Crassostrea gigas, lumbricins (oligochaetes), macines (leeches), and Bri2 chondromodulin and BRICHOS-AMPs (polychaetes) in annelids | Multiple gene families, recombination events, allelic polymorphism and gene duplication, selective pressure on amino acid residues | [31], [33], [176], [177], [178], [179], [180], [181], [182], [188], [189], [193], [194] |
| IL-17 | Molluscs Annelids |
15 genes in Pinctada fucata martensii, 10 genes in Crassostrea gigas, 6 genes in Mytilus galloprovincialis, 10 genes in Mizuhopecten yessoensis, 7 genes in Capitella teleta | Many intronless IL-17 genes in mollusks may be caused by retroposition | [33], [220], [221], [222], [223], [224] |
| Immunocyte | Molluscs Annelids |
two main groups (granulocytes and hyalinocytes) of haemocytes in bivalves, 12 haemocyte subpopulations in Crassostrea hongkongensis, a great number of hyalinocytes in Perna perna, Chamelea gallina, Argopecten irradians and C. gigas, two main groups of coelomocytes (amoebocytes and eleocytes) in earth worm, four groups of coelomocytes(juvenile cells, granulocytes, vacuolated amoebocytes and spindle-shaped amoebocytes) in Arenicola marina | Multiple types of haemocytes and coelomocytes | [36], [41], [78], [79], [80], [82] |
The haemocytes of bivalves performed a primary role in phagocytosis and encapsulation. The phagocytosis of haemocytes in bivalves was firstly found in the oyster C. gigas, which was the predominant mechanism of pathogen elimination [53]. In most bivalve species, granulocytes generally exhibited higher phagocytosis activity to pathogens or foreign particles than hyalinocytes [36]. Among granulocytes, the eosinophilic granulocytes exhibited a higher phagocytosis activity than the basophilic granulocytes in oyster, clams, mussels and cockles [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]. However, only granulocytes exhibited phagocytic activity in oyster C. virginica [65], clam Tridacna crocea [66], cockles Cerastoderma edule [67], mussel M. galloprovincialis [63], mussel Pinna nobilis [68] and the pearl oyster Pinctada fucata [69]. Encapsulation of haemocytes was another cellular immune defense, which acted a crucial role in extracellularly eliminating large foreign particles. Recent studies had reported that granulocytes were involved in encapsulation in the oyster Saccostrea glomerata and C. gigas [70], [71]. Furthermore, high content of molecules and expression levels of immune-related genes including TLR, clathrin, lysozyme, defensin and IL-17 were observed in the granulocytes of the C. gigas [70]. In mussels, the defensins, mytilins and myticin C were also stored mainly in granulocytes [72]. Different molecules of MAPK and NF-κB pathways were identified from the oyster Ostrea edulis granulocytes, and many proteins of Wnt pathway were identified in hyalinocytes of Ostrea edulis [73]. A recent study had demonstrated that granulocytes were involved in immune defense by the NF-κB pathway and autophagy in oyster C. hongkongensis [41]. These findings indicated that granulocytes may be a predominant cell type in bivalve immune defense.
Annelids are coelomates with specially immunity of coelomocytes against pathogens [74]. In earthworms, coelomocytes paly a crucial role in the mediation of phagocytosis and cytotoxicity as the free-circulating immune cells [75], [76], [77]. In general, two main types of coelomocytes were discovered in earthworms (Table 1). Amoebocytes are a type of coelomocytes with large eccentric nucleus and numerous pseudopodia [78], which function in the phagocytic process. In contrast, eleocytes as a type of large cells with massive chloragosomes are derived from chloragogen tissue covering the alimentary canal [78], [79], [80], which play a role in the storage of glycogen and lipids. Post-sort phagocytosis assays revealed that amoebocytes were in favor of bacterial engulfment, which were the dominant phagocytic cell type of coelomocyte in earthworms [78]. Furthermore, Coelomic Cytolytic Factor (CCF), LPS-binding proteins and bacterial permeability increasing proteins (LBP/BPI) and Toll-like receptors (TLRs) are expressed mainly in coelomocytes of earthworms. Challenge of earthworms with Gram-positive B. subtilis increased the transcriptional expression of LBP/BPI and TLR in coelomocytes, suggested its involvement in the defense reactions [81].
In lugworm Arenicola marina, one of marine polychaetes, coelomocytes could be divided into “juvenile” cells, granulocytes, vacuolated amoebocytes and spindle-shaped amoebocytes [82] (Table 1), and the last three types of coelomocytes exhibited phagocytosis and selectivity towards Gram+ and Gram− bacteria [83]. Furthermore, immune associated molecules including complement-like factors, TLRs and various putative lectin-like proteins, C-type lectins, reactive oxygen and nitric oxide species and antimicrobial peptides had been identified in the coelomocytes with the challenge of LPS in vitro [82], revealed its immune function.
3. Large-scale expansion and molecular divergence of pattern recognition receptors
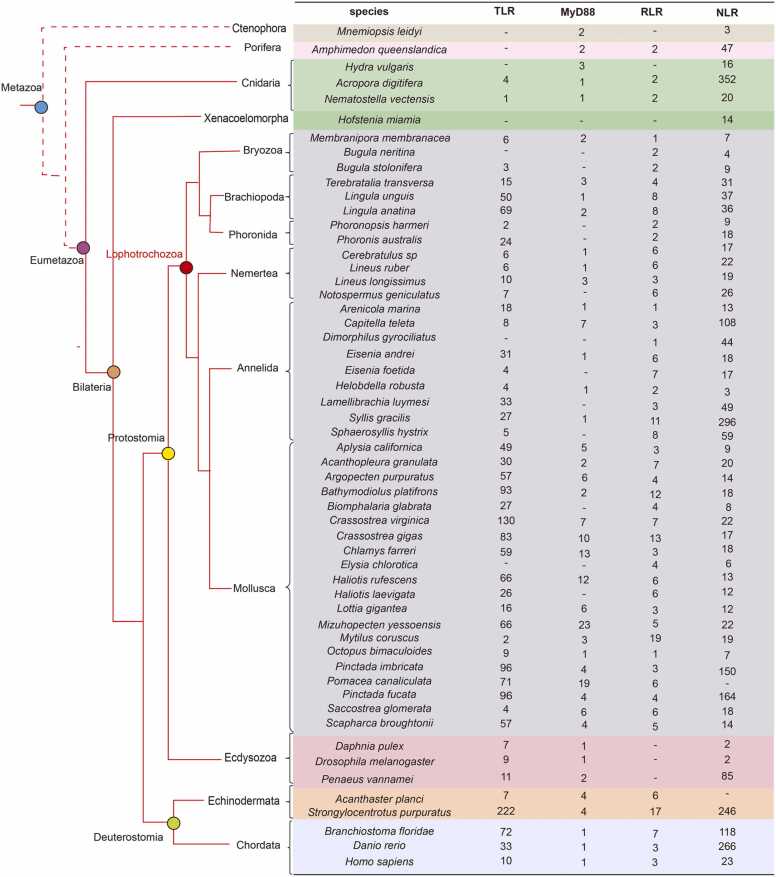
Pattern-recognition receptors as the crucial components of innate immune system, which play a key role in pathogen recognition by sensing conserved pathogen-associated molecular patterns (PAMPs), including lipopolysaccharide (LPS), lipoteichoic acids, peptidoglycan (PGNs), β-glucans and viral double-stranded RNA [84]. Upon PAMP recognition, activates the downstream pathways and stimulates a cell-mediated phagocytic response [85], [86]. Based on the sequence homology, PRRs are classified into five major groups including toll-like receptors (TLRs), retinoic acid-inducible gene I [RIG-I]-like receptors (RLRs), NACHT-leucine-rich repeat receptors (NLRs), C-type lectin receptors (CLRs) and AIM2-like receptors (ALRs) [87]. TLRs and CLRs are the extracellular or transmembrane receptors, while RLRs, NLRs and ALRs are found in the cytoplasm [88]. Significantly, TLRs and RLRs in the lophotrocozoans show a higher versatility and flexibility with large-scale expansion and molecular divergence than vertebrates at gene level (Fig. 2). Gene duplication and domain shuffling may account for the novel expansion of PRRs in lophotrochozoans.
Fig. 2.
Comparison of gene families encoding TLR, MyD88, RLR and NLR immune receptors in representative animals [2], [18], [19], [20], [74], [94], [104], [249], [250], [251], [252], [253], [254], [255], [256], [257], [258]. Data sources: http://compagen.unit.oist.jp/aurelia/datasets.html; https://www.uniprot.org/proteomes/UP000593567; https://doi.org/10.5281/zenodo.4556028; https://github.com/rannypribeiro/Regeneration_transcriptomics; http://bigd.big.ac.cn/gwh/(GWHACBE00000000); https://datadryad.org/stash/dataset/doi:10.5061/dryad.h9942.
3.1. Tandem duplication
Gene duplication is one of the primary driving forces in the evolutionary novelty of genetic systems, which generates new genes to facilitate functional divergence [89]. Tandem duplication is characterized as a structure rearrangement with serial duplication and insertion of DNA segment. Tandem gene duplication creates adjacent paralogous genes with a short distance. It frequently occurs in innate immune-related molecules [90].
3.1.1. TLR system
TLRs, as innate immune-recognition receptors with a solenoid-like LRRNT-LRR-LRRCT ectodomain for ligand recognition, play a critical role in innate immune responses by recognition of microbial pathogens [91], [92] Previous studies had revealed that individual TLRs combine with different adapters, such as MyD88, TIRAP, and TRIF, to drive a specific immune response [93].
Genomic analyses revealed high diversity of gene copy number in lophotrochozoans TLRs: Mollusca (130 in Crassostrea virginica, 96 in Pinctada fucata, 83 in Crassostrea gigas, 71 in Pomacea canaliculata), Annelida (8 in Capitella capitata, 11 in Eisenia foetida, 18 in Arenicola marina, 27 in Syllis gracilis), Bryozoa (6 in Membranipora membranacea, 3 in Bugula stolonifera), Brachiopoda (69 in Lingula anatina, 50 in Lingula unguis, 15 in Terebratalia transversa), Nemertea (10 in Lineus longissimus, 6 in Lineus ruber, 6 in Cerebratulus sp) and Phoronida (24 in Phoronis australis, 2 in Phoronopsis harmeri) [19], [94] (Fig. 2). In contrast, such phenomenon was not found in vertebrates, such as 13 in mice [18], 10 in humans [95], and 22 in chickens [96].
In the lophotrochozoans, a great expansion of TLRs is observed in parts of molluscs, such as 103 in the clam Limnoperna fortunei, 96 in the pearl oyster Pinctada fucata, 130 in the eastern oyster C. virginica, 83 in the Pacific oyster C. gigas and 66 in the scallop Mizohupecten yessoensis [18], [19]. In the oyster C. gigas, 83 TLR genes were classified into five groups: V (vertebrate-type), P (protostome-like with LRRCT [leucine-rich repeat C-terminal]-LRRNT [leucine-rich repeat N-terminal] ectodomains), sP (short protostome-like without LRRCT-LRRNT ectodomains), sPP (short protostome-like with LRRCT-LRRNT ectodomains) and Ls (LRRCT-specific ectodomains) [18]. Moreover, 57 TLRs were encoded in tandem arrays, and a cluster of eight TLRs on the Scaffold_599 was in relatively close linkage. Significantly, sP-type TLRs of C. gigas showed the most remarkable expansion, which had been diverged into three subgroups, and 8 of the 14 sP-group I-type TLRs were in tandem arrays [18]. Furthermore, 78 of 83 TLRs in an oyster were expressed, and 19 were expressed differentially against pathogen challenges, suggested its involvement in innate immune [18], and positive selection analysis suggested that the coding and promoter regions of TLR gene family in oysters were coupled with selection [18]. Furthermore, TLRs in Phoronis and Lingula were also found with lineage-specific expansion by tandem duplications [97]. These studies revealed the significant functional divergence of tightly linked TLR genes, might result in diversification and specificity of pathogen recognition.
In comparison with the three groups mentioned, no significant expansion is detected in the other groups of lophotrochozoans. Annelids are one of the important ecological groups, widespread in soil, freshwater and marine ecosystems. The first TLR of Ainnelida was identified from polychaete Capitella teleta and leech Helobdella robusta by genomic analyses [98]. In our previous study, 8 and 4 TLR genes were discovered, respectively [19]. However, the immune function of TLRs has not been characterized in two species. Meanwhile, 18 TLR genes were expressed in the coelomocytes of lugworm Arenicola marina [82]. Most TLRs in leech Hirudo medicinalis displayed a crucial role in central nervous system (CNS), especially in CNS repair [99]. In earthworm, the first TLR(EaTLR) was isolated from an oligochaete annelid Eisenia andrei, represented the single cysteine cluster TLR (sccTLR), which was closely related to the sccTLR from C. teleta [100]. Challenge with Gram-positive bacteria in earthworm increased the expression of EaTLR in coelomocytes, suggested its function in the defense reactions [100]. Recent studies had identified a novel multiple cysteine cluster TLR (mccTLR) in Eisenia andrei earthworms, which was expressed by sperm cells and suggested to participate in early embryonic development and immune response against parasites [101].
In deep-sea snail Gigantopelta aegis and Chrysomallon squamiferum, many pattern recognition receptors belonged to the TLR gene family were highly expressed in the esophageal gland [102], [103]. Other pattern recognition receptors, including peptidoglycan recognition proteins (PGRPs), fibrinogen-related proteins (FBGs), and C-type lectins (CLECs) also showed a highly expression in esophageal gland. The expansion of TLR was observed in the deep-sea tubeworm Lamellibrachia luymesi [104] and deep-sea vent/seep mussel Bathymodiolus platifrons [105]. Seven TLR4-like proteins were identified in the Lamellibrachia luymesi, suggested a potential for increased binding to LPS to enhance sensitivity to Gram-negative bacteria [104]. The TLR13 showed an expansion in Bathymodiolus platifrons, which were highly expressed in the gill [105]. Furthermore, the set of genes involved in the innate immunity recognition with TLRs, apoptosis and autophagy were highly expressed in the plume and gonad of giant tubeworm Riftia pachyptila [106]. The abundance and high expression of pattern recognition receptors may play a prominent role in bacteria recognition and acquisition and maintenance of their symbiont populations to establish the symbiosis across deep-sea chemosymbiotic host animals [104], [106].
3.1.2. C1qDC
The complement system plays a crucial role in innate immune response as a sophisticated proteolysis system. It is organized into classical, alternative and lectin activation pathways [107]. Previous studies had revealed the origin and evolution of the immune complement-like system in Lophotrochozoa. In snail Littorina littorea, a multitude of lineage-specific complement-like systems were assumed to evolve from an ancient proto-complement [108]. Subsequently, the studies showed that proto-complement of Lophotrochozoa evolved moderately in the bivalves and brachiopods, and rapidly in the annelids, cephalopods and gastropods [109]. Moreover, it suggested that C1q molecules (C1qL, C1qDC, etc.) had the potential to form complexes with other molecules (such as protostomian MASP-Related Molecules: MReM1 and MReM2, etc.) to exert proto-complement functions and compensate for the evolutionary loss of some complement molecules, suggested the high evolutionary conservative of C1q molecules [108], [109].
C1q is the first subcomponent of the C1 complex and response for activating the classical pathway through recognizing antigen-complexed immunoglobulins [110], [111], [112], [113]. Functional genomics analyses revealed that the number of C1q genes was enormously expanded in the bivalves through extensive tandem duplication. For example, the analysis of the C. virginica genome revealed that the 476 C1qDC genes were primarily located in large clusters of tandemly duplicated paralogs on chromosomes 7 and 8 [114], consistent with an expanded set of 321 C1qDC genes in the C. gigas, significantly higher than in sea urchin (4 C1qDC genes) and human (31 C1qDC genes) [18]. Furthermore, multiple transcriptomes and genome analyses had confirmed the massive expansion of C1qDC genes in bivalves, such as 296 genes in Pinctada fucata [115], 445 in mussel Modiolus philippinarum [105], 554 in oyster Saccostrea glomerata [116] and over 1200 in clam Ruditapes philipinarum [117]. Moreover, hundreds of C1qDC genes were tandemly arranged in the same scaffolds, suggested that extensive duplication drived the expansion of the mostly C1qDC family in bivalves (Table 1).
In addition, many genes such as heat shock proteins 70 in the Pacific oysters [118], [119], inhibitors of apoptosis (IAPs) in clam Mercenaria mercenaria [120] and shell matrix proteins (SMPs) in Pinctada fucata [121] also exhibited an extensive tandem duplication. These findings revealed that some innate immune genes of bivalves exhibited a great expansion within the tandem duplication for immune defense against various pathogens and environmental stressors.
3.2. Domain shuffling
Domain shuffling is a process of creating new combinations of gene functional domains, which is an essential mechanism in the evolution of multi-domain proteins [122]. Previous studies revealed that domain shuffling was involved in the evolution process of innate immune pattern recognition receptors (PRRs) such as RLRs and NLRs in lophotrochozoans, and led to new domain combination patterns and expansion of PRRs, which might hence the diversity of innate immune system.
3.2.1. RLRs
Canonical RLRs are a family of three DExD/H box-containing RNA helicases, including retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2), which function as intracellular sensors of dsRNA responsible for the induction of type I IFN [123], [124], [125]. Functional domain analysis showed that RIG-I and MDA5 contain two N-terminal caspase recruitment domains (CARDs), a C-terminal RIG-I_C-RD domain, and an intermediate DEAD/DEAH box helicase domain [125]. Functionally, RIG-I and MDA5 can interact with the CARD domain of IFN-β promoter stimulator-1 (IPS-1) to recruit TNF-receptor associated factor-3 (TRAF-3), NF-κB-activator binding kinase-1 (TBK1) and inducible IκBkinase (IKK) [126], [127], [128]. Then, these kinases phosphorylate IRF-3 and IRF-7 and activate NF-κB to induce the expression of type I IFN [129]. Due to the loss of CARD domains, LGP2 can not trigger immune responses but can inhibit MDA-5 and RIG-I antiviral action [130], [131].
According to the form of domain composition, there are 11 different types of lophotrochozoan RLRs, among which the N-terminal domain is particularly abundant, including five domains, namely Death, DED, SAM, IG and CASc domain, in addition to the typical CARD [2]. A great expansion and diversity of the RLRs was observed in several groups (Fig. 2), including Mollusca (13 RLRs in C. gigas, 7 RLRs in C.virginica, 9 RLRs in Mytilus coruscus, 12 RLRs in Bathymodiolus platifrons, and 7 RLRs in Acanthopleura granulata), Annelida (7 RLRs in Eisenia foetida, 11 RLRs in Syllis gracilis, 8 RLRs in Sphaerosyllis hystrix, 6 RLRs in Eisenia andrei), Brachiopoda (8 RLRs in Lingula anatina, 8 RLRs in Lingula uguis), Nemertea (6 RLRs in Notospermus geniculatus, 6 RLRs in Lineus ruber, 6 RLRs in Cerebratulus sp), and all the RLRs except C. virginica could be divided into at least three types [2]. Significantly, transcriptome analysis showed that most of these lophotrochozoan RLRs with specific N-terminal domains showed immune-related tissue (digestive gland, mantle, gut and haemocytes) expression patterns, suggested their potential function in mucosal and hemocyte-mediated immunity [2]. In oyster C. gigas, the expression of 11 RLRs were significantly upregulated under virus challenge, suggested a crucial role in antiviral immune recognition [2]. In comparison with molluscs, the function of RLRs have not been described in other lophotrochozoan groups.
The diversity of lophotrochozoan RLRs N-terminal domains evolved through domain shuffling [2]. Intron-exon structure analysis had revealed that three types of structures were identified, including intraexonic insertion/deletion, gain/loss of exon/intron and exonization/pseudoexonization, and the structure divergence was prevalent in all N-specific domains of RLRs, such as CASc, DED, Death, and IG domains [2]. These findings suggested that exon-intron divergence was one of the main forces to drive domain shuffling, which created the expansion and diversity of RLRs, and led to distinct functional lophotrochozoan RLRs.
3.2.2. NLRs
Nod-like receptors (NLRs), as a group of intracellular PRRs, have shown a canonical role in the host’s fight against pathogens. In vertebrates, NLRs consists of N-terminal domains for signaling, a C-terminal LRR region for ligand recognition, and a central NACHT domain for oligomerization [91], [132]. However, NLRs have not been systematically described in lophotrochozoans. Recent studies had shown that no less than three NLRs were identified in the 24 molluscan genomes [20]. However, the expression of these molluscan NLRs were not induced under bacterial or viral infections, suggested the immune function of canonical NLRs might be lost in molluscs [20]. Instead, incomplete NLR (incNLR) genes (without NACHT domain) were upregulated under pathogen infection. Furthermore, the species-specific expansion of NLRs was observed in the clades Polychaeta (108 NLRs in Capitella teleta, 196 NLRs Syllis gracilis) and Pteriidae (164 NLRs in Pinctada fucata and 150 NLRs in P. imbricata), and lineage-specific gene duplication and domain shuffling might play important roles in the expansion of NLRs [20] (Fig. 2). NLRs were also discovered in leech (Hirudo medicinalis), which could be induced by Gram-positive bacteria and bacterial cell wall component muramyl dipeptide [99].
Inhibitors of apoptosis proteins (IAPs) are known to play a crucial role in suppressing apoptosis and promoting cell cycle progression [133]. The extensive domain shuffling was also found in IAPs, which had shaped IAPs architectural diversity in hard clam Mercenaria mercenaria against immune and environmental stresses [120]. Furthermore, the significantly expansion of IAPs was also observed in deep-sea vent/seep mussel Bathymodiolus platifrons (130 IAPs) [105] and deep-sea chemosymbiotic clam Archivesica marissinica (204 IAPs) [133]. The highly expression of IAPs in the gill tissue of two species could reveal increased host cell survival in response to symbionts, as had been reported in other symbiosis systems [105], [133], [134].
Furhermore, contractions also play a role in modeling the immune system. Peptidoglycan recognition proteins (PGRPs) as one of the PRRs play a role in recognition of peptidoglycan cell wall of bacteria [102]. Only three PGRPs were identified from the clam Archevesica marissinica (hosting Ca. V. marissinica without a cell wall) genome, compared with the expansion of PGRPs in the deep-sea mussel G. platifrons (hosting symbiotic bacteria with an intact cell wall) [102]. The contraction of PGRP gene family in deep-sea pliocardiine immune system might be an evolutionary adaptation to the close relationship between the host and symbiont.
4. Expansion of signaling adapters
Immune adapters consist of various protein-binding modules that link to receptors and drive the formation of signaling complexes to regulate a complicated immunity signal network. One of the most typical immune adapters is myeloid differentiation primary response gene 88 (MyD88). MyD88 is an adapter of TLRs, which plays a pivotal role in immune cell activation through TLRs [93]. In addition, MyD88 has a single death domain (Death) that interacts with the TLR-TIR domain to participate in TLR-mediated signaling pathways, activating the transcription factor NF-κB to regulate the expression of immune-related genes [135], [136].
In lophotrochozoans, the expansion of MyD88 is another novel strategy to expand the repertoire of innate immune responsiveness (Fig. 2). Recent studies had reported that an unique expansion of MyD88 in molluscans, such as 19 MyD88s in snail Pomacea canaliculata, 12 MyD88s in abalone Haliotis rufescens, 13 MyD88s in scallop Chlamys farreri, 23 MyD88s in scallop Mizuhopecten yessoensis, 10 MyD88-like genes in oyster C. gigas, 7 MyD88s in oyster C. virginica and 7 MyD88s in snail Biomphalaria glabrata [19] (Fig. 2). However, no more than 3 MyD88s were observed in other groups of lophotrochozoans, except 7 MyD88s in Annelida Capitella teleta. In C. gigas, four MyD88s possess only a TIR domain and six possess a typical Death-TIR domain combination [18]. The MyD88 of C. gigas were clustered in three or in pairs, suggested that the expansion might be attributed to multiple local tandem duplication and lineage-specific expansion. Furthermore, patterns of functional diversification resembling were observed with the MyD88-like genes, 4 MyD88s were up-regulated expressed in response to OsHV-1 virus, 7 MyD88s were up-regulated expressed in response to Vibrios, and some MyD88-like genes were sensitive to abiotic challenges, such as temperature, salinity and metal [18]. These findings indicated that the expansion of TLR and MyD88 drived the creation of complex and specific regulatory networks that mediated innate immune responses in lophotrochozoans. However, 222 TLRs had been observed in sea urchin Strongylocentrotus purpuratus, but only 4 MyD88s were identified [18], suggested that sea urchin increased the specificity of innate immune recognition, but the the number of genes coding for adapters was still conservative. It is suggested that the expansion of TLR system in sea urchins and molluscs may have evolved independently.
5. Somatic recombination
Somatic recombination is a type of DNA rearrangement by somatic cells physically cutting out small regions of DNA and then combining the DNA fragments, which occurs physiologically in the assembly of the B cell receptor and T-cell receptor genes (V(D)J recombination) [137], [138], [139]. In the immune system, somatic recombination is one of the critical mechanisms to produce diverse antibodies to defend bodies against various pathogens. The most representative somatic recombination in lophotrochozoans is fibrinogenrelated proteins (FREPs) in freshwater snail Biomphalaria glabrata.
A diverse family of fibrinogenrelated proteins (FREPs) had been identified in B. glabrata, and the IgSF1 domain of the FREP3 subfamily showed a higher diversity rate than those recorded for control genes [21], [22]. Among them, a small number of germline sequences were maintained within each subfamily of FREP genes, which retained potential sequences to account for variations in the cDNAs [23], [24], [25], [26]. It suggested that somatic recombination is present in B. glabrata which is capable of diversifying FREP genes involved in immune defense as vertebrates. Furthermore, approximately 190 predicted FREPs were also identified in C. gigas [140], [141]. The diversification may resulted from lineage-specific duplication and somatic recombination (Table 1).
6. Single nucleotide polymorphisms (SNPs)
Single nucleotide polymorphisms (SNPs), as the most frequent type of genetic variation, is a substitution of a single DNA building block (nucleotide) at a specific position in the genome, which is associated with diversity in the population, susceptibility to diseases, immune response against different pathogens and environmental stress [142], [143], [144], [145]. SNPs have been used as a genetic marker for resistance breeding in different economic aquatic species, such as oyster [146], [147], rainbow trout [148], turbot [149]and common carp [150], [151] and shrimp [152].
Meanwhile, various studies had supported that SNPs in immune-related genes of lophotrochozoans could affect the susceptibility and resistance of individuals against invading pathogens by influencing the expression of the proteins. Population diversity analysis in C. gigas had revealed that innate immune genes families, including C1qDC, CTL, and SRCR, showed higher nucleotide diversity than non-immune genes [27]. A genome-wide association study found 20,353 SNPs were mapped to the ten pairs of chromosomes in C. gigas challenged with ostreid herpesvirus (OsHV), suggested SNPs might be an effective tool to enhance oyster resistance to this problematic pathogen [28]. Yu found 12 SNPs in the coding region of serine protease inhibitors (SPIs), and the polymorphisms were associated with dermo or MSX resistance in C. virginica. Additionally, SNP of SPI in bay scallop Argopecten irradians was associated with resistance to Listonella anguillarum [29]. The analysis of haemocytes transcriptome sequencing from mussel Mytilus chilensis suggested that 20,306 polymorphisms were associated with immune-related genes, which were classified into various pathways of the immune response, such as TLR pathway, apoptosis pathway and immune-related molecular functions (molecular chaperones and antimicrobial peptides) [30].
These observations had revealed that higher nucleotide diversity of immune genes was closely associated with specific immune traits, suggested that SNP might be one of the pivotal mechanisms to promote the diversity of lophotrochozoans innate immunity, which enhanced the adaptability to the pathogen-rich environment.
7. Alternative splicing
Alternative splicing is a powerful mechanism to increase post-genomic diversity from a single coding sequence [153]. In general, genes generate limited mRNA isoforms, but several genes generate a vast repertoire of isoforms by alternative splicing, which allows gene products with different functions. Cell surface receptors involved in immune responses are most frequently associated with alternative splicing [154]. Previous studies in mammals have revealed that alternative splicing exhibited a crucial role in the activation of T-cell [155], [156], whilst insects down syndrome cell adhesion molecule (Dscam) exhibited pathogen-specific splice form expression infected with different pathogens [157], such as Drosophila Dscam create 38,016 isoforms through the alternative splicing of 95 variable exons [158]. Furthermore, alternative splicing of fibrinogen-related proteins (FREPs) is also identified in invertebrates, especially in lophotrochozoans.
FREPs are a family of fibrinogen-related domain (FReD) lectins with a unique molecular structure, consisting of a fibrinogen domain in C-terminal region and one or two N-terminal immunoglobulin superfamily (IgSF) domains [21], [22], [159], which is widely identified in vertebrates [160], [161], urochordates [162] and invertebrates [21], [25], [141], [163], [164], [165]. The gene family comprises diverse proteins, such as tenascins, tachylectins, ficolins, angiopoietins, ixoderins, and fibrinogen β and γ chains [25], [166], [167]. Previous studies in invertebrates had exhibited high levels of sequence diversity in FREPs, which were sensitive to relatively specific immunostimulation, suggested that diverse FREPs might contribute to a crucial role in the innate immune response of invertebrates. In C. gigas, approximately 190 predicted FREPs with more than 200 fibrinogen-like (FBG) domains were identified, which were highly expressed in immune-related tissues, suggested a versatile immune function [140], [141]. The high variability of FREPs from the M. galloprovincialis were up-regulate with bacterial infection or PAMPs treatment, suggested a potential capacity in recognization and elimination of pathogens [168].
Significantly, a diverse family of FREPs is found in freshwater snail B. glabrata, which are up-regulated after exposure to digenetic trematode to enhance internal defense ability. Previous studies had revealed that the IgSF1 domain of the FREP3 subfamily was highly diverse. It included three truncated forms that lack a partial exon, one complete exon, or two complete exons plus the 3’UTR [23], [24]. Additionally, five truncated cDNAs were generated by alternative splicing of full-length FREP genes [23], [24]. Collectively, the diversification of FREP kept pace with pathogen diversification, which was an effective strategy to prevent tracking by pathogens over multiple host generations to strengthen the innate immune diversity and specificity (Table 1).
8. Immune effector molecules
Lophotrochozoans have evolved high variability and diversity of immune effector molecules to help them against a wide range of pathogens and environmental stressors by killing invading microbes, reducing pathogenicity, or modulating the immune response [33]. In this review, we focus on the three classes of effector molecules, such as antimicrobial peptides and proteins (AMPs), reactive oxygen and nitrogen species (RONS) and cytokines.
8.1. Antimicrobial peptides and proteins
Antimicrobial peptides and proteins (AMPs) are evolutionarily ancient molecules that act as critical parts of the innate immune system against invading pathogens in all living organisms [169], [170], [171]. AMPs are small cationic amphipathic molecules that range in length from 15 to 200 amino acids [172]. AMPs show a broad spectrum of activity against Gram-negative and positive bacteria, viruses, fungi, yeast and protozoa [171], [173], [174], [175]. Over the past years, considerable diversity in sequence, structure and biological activity of AMPs had been observed in invertebrates.
In bivalves, there are a wide repertoire of different AMPs with immune-related functions or direct antibacterial activity [31]. In mussels, biochemical analyses have revealed a huge diversity of AMPs, suggesting a complex effector system of the anti-infectious innate immunity. On the basis of structural characteristics, no fewer than six AMP families have been identified in mussels, such as defensin, big defensin, myticin, mytilin, mytimycin and mytimacins [176]. Defensins are the most well-known and described family of AMPs, which are initially characterized and isolated from mussels [31], [177]. The expression of defensins is detected in different tissues and at different expression levels in mussels with the bacterial challenge [178]. Furthermore, Myticin C (MytC) is the most expressed AMP in adult mussels, which exhibits the highest RNA polymorphism among mussel AMPs [179]. Previous studies showed that overexpression of MytC in haemocytes could regulate the transcriptional expression of other immune-related genes, such as AMPs, lysozyme and complement proteins. The results revealed that MytC might also act as an immune system modulator with chemokine-like activities [179], [180].
Meanwhile, a diversity of AMPs is also observed in the oyster, including defensins, big defensins, proline-rich AMPs, and bactericidal/permeability-increasing (BPI) proteins [181] (Table 1). In C. gigas, sequences analysis identified 43 variants of proline-rich AMPs, 24 Cg-BPI, and 19 defensins [182]. The oyster defensins consist of the CSαβ-defensin and the big defensin AMP families, and CSαβ-containing defensins comprise a large and diverse family, where a separate gene encodes each member with the different genomic organization [181], [182], [183]. Various genetic mechanisms have promoted the defensin diversity, including gene duplication, allelic polymorphism, and recombination events [182], [184]. In addition, the high gene copy number variation (CNV) between individuals generates a variable expression of the defensins gene in oyster haemocytes. The defensins are sensitive to Gram-positive bacteria and act as inhibitors in the bacterial biosynthesis pathway [182], [184]. Furthermore, the Big defensins from oyster C. gigas and scallop Argopecten purpuratus display a characteristic mechanism of action. The N-terminal domain of Big defensins can drive bacteria-triggered peptide assembly into antimicrobial nanonets, which entraps bacteria [185], [186], [187].
In contrast, there are only three main classes of AMPs in annelids, including lumbricins (oligochaetes), macines (leeches), and Bri2 chondromodulin and BRICHOS-AMPs (polychaetes)[33]. Lumbricins as the first discovered AMPs in annelids were identified from the earthworm Lumbricus rubellus [188]. Related peptides with antibacterial activities including Hm-lumbricin [189], peptide PP-1 [190], lumbricin-PG [191], and lumbricin and lumbricin-related peptide [192] were subsequently discovered from several annelids. Macins are a group of long cationic cysteine-rich AMPs with an a-helix/b-sheet structure. There are three groups of macins in leeches, such as theromacin from Theromyzon tessulatum [189], Hmneuromacin and Hm-theromacin from H. medicinalis [193]. The Bri2 chondromodulin and prosurfactant protein C (BRICHOS-AMPs) are enriched with cysteine and only discovered in polychaetes. The arenicin 1 and 2 from polychaete Arenicola marina are the first discovered groups of the BRICHOS family [194]. Subsequently, similar AMPs, alvinellacin from hydrothermal Pompeii worm Alvinella pompejana [195], capitellacin from Capitella teleta [196], nicomicin-1 and-2 from artic polychaete Nicomache minor were identified [197]. These AMPs with membranolytic activity displayed a broad spectrum of antimicrobial activities against Gram-negative and Gram-positive bacteria [198], caused the permeabilization of microbes membrane.
8.2. Reactive oxygen and nitrogen species
Reactive oxygen and nitrogen species (RONS) are a group of reactive chemicals containing oxygen and nitrogen, which play an indispensable role in host resistance to microbial pathogens by causing irreversible nonspecific damage to proteins, lipids and nucleic acids [199].
Reactive oxygen species (ROS) is considered to be a variety of highly reactive chemicals containing oxygen, which is generated by a cascade of enzymatic reactions in aerobic organisms and regulated by antioxidants [199]. In various bivalve species, the production of ROS was discovered from the haemocytes of oysters [32], [200], [201], [202], [203], [204], clams[205], [206], mussels [207], [208] and scallops [209]. Due to immune function of haemocytes in host defense, ROS is considered to be involved in bivalve immune response in response to invading pathogens [210]. Furthermore, the results of transcriptome showed that the complex system of ROS was also present in the coelomocytes of the lugworm Arenicola marina with challenge of lipopolysaccharides [82].
Nitric oxide (NO) as one of the important reactive nitrogen intermediates (RNI) is generated by an oxidative reaction involved the conversion of L-arginine to L-citrulline [211]. High mounts of NO are produced by immunocytes in response to microbial and fungal invasion [211]. In molluscs, NO is considered to be an ubiquitous pathogen-killing molecule and involved in mediation of the innate immune response. Previous studies had discovered the NO and NO synthase (NOS) in the haemocytes of molluscs, such as scallop, oyster, mussels, snail and clam [212], [213], [214], [215], [216], [217]. In these molluscs, NO had been shown to play an essential role in the modulation of redox homeostasis, anti-bacterial ability, haemocytes apoptosis and phagocytosis [217], [218], [219]. Furthermore, only a single transcript of nitric oxide synthase (NOS) family was identified on coelomocytes of the lugworm Arenicola marina, and the expression of NOS was low with challenge of lipopolysaccharides [82]. However, the function of NOS had not been identified.
8.3. Cytokine
Interleukin 17 (IL-17) is an ancient pro-inflammatory cytokine, which plays an essential role in innate immunity. To date, the members of IL-17 gene family were discovered in a variety of invertebrate species. In bivalves, most current research on IL-17 mainly focused on phylogenetic analysis, related signaling pathways, and effector mechanisms in mussel M. galloprovincialis and oyster C. gigas [220], [221], [222]. IL-17 was first discovered in oyster C. gigas [223], which functioned in mucosal immunity in mussels, like in vertebrates [220]. Genomic analysis showed that there were ten IL-17 genes in C. gigas, six in M. galloprovincialis, fifteen in P. fucata martensii, and ten in scallop M. yessoensis [224] (Table 1). Some of these IL-17, such as CgIL-17–5 (C. gigas) and PmIL-17–2 (P. fucata martensii) were proved to be involved in immune response to bacterial stimulation [222], [224]. Furthermore, IL-17 had also been discovered in annelids. Seven IL-17 genes were identified in the segmented worm C. teleta by genome level prediction, but the function of IL-17 was unclear [224]. The phylogenetic analysis of IL-17 from annelids, molluscs and other invertebrates revealed that these genes were grouped together, suggested that these invertebrate IL-17 molecules shared a common ancestral gene [224].
Tumor necrosis factor α (TNFα) is a critical cytokine that consists of various transmembrane proteins with a homologous TNF domain [225]. It contributes to the proliferation, differentiation, and homeostasis of immunocytes, which is involved in the innate and adaptive immune systems [91], [225], [226]. In molluscans, the first TNFα homolog was characterized in disk abalone, which was significantly up-regulated expressed with the challenge of bacteria, viralhaemorrhagic septicemia virus (VHSV) and lipopolysaccharide (LPS), indicated its role in response to pathogenic infection or stimulation [227]. Furthermore, 18 TNF and 13 TNFR were identified in the oyster, four TNF and six TNFR members were differentially induced by bacteria, consistent with roles in immune process [18]. Among them, some TNF subgroup genes were arranged in three clusters that could be attributed to multiple local tandem duplication events. Coelomic cytolytic factor (CCF) as a cytokine was first discovered in the earthworm E. fetida and subsequently in other earthworms [228], [229], which contained a functional analog of mammalian TNFα. However, the CCF does not share gene or amino acid sequence homology with mammalian TNFα, suggesting the distinct evolutionary ancestry of invertebrate and vertebrate [230], [231], [232]. Functional analysis showed that CCF in earthworm had CCF-based cytolytic, trypanolytic activities and specific recognition of N, N’-diacetylchitobiose [229].
In addition, recent studies had shown that the diverse toxins among organisms appeared to be the defense factors of host/pathogen interactions [233], [234], [235], [236], [237]. In Biomphalaria glabrata snail, a novel and diverse toxin family was identified. Twenty-three biomphalysin genes were discovered in snail, which displayed a tissue-specific expression pattern [238]. The functional approach suggested that the diverse biomphalysins played a crucial role in recognition and elimination of pathogen through their cytolytic activity [238]. Therefore, a systematic investigation on the toxin genes is expected to help us get a new insight into innate immune response to different pathogens. Recent study in a mud-dwelling clam, Meretrix petechialis had proved the ability of synthesion, storage and secretion on the antibiotic erythromycin from specific mucus-rich cells of the clam’s mantle epithelium, revealed a new antimicrobial defense in mud-dwelling clams [239].
9. Conclusions and perspectives
Invertebrates represent roughly 90% of the planet's animal species [240]. Lophotrochozoa has evolved various effective strategies to obtain a diverse and specific innate immunity system with high adaptive capability [241], [242], [243]. Understanding lophotrochozoan innate immune responses is essential to the comprehension of innate immunity evolution and diversification.
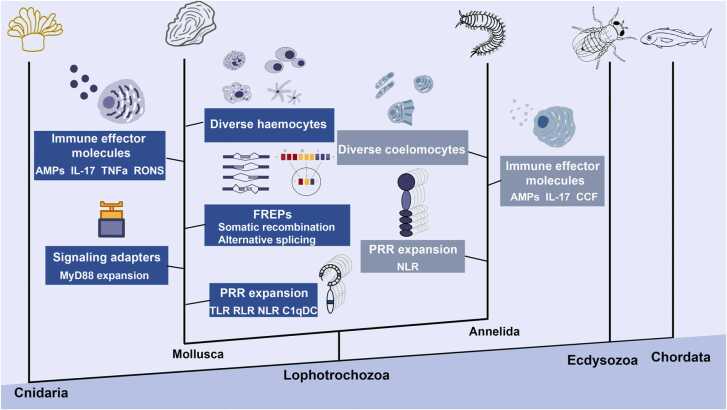
Comparative genomics studies suggest expansion and functional divergence of lophotrochozoan immune reorganization systems is not as "homogeneous and simple" as said by Pro. Eric S Loker [241], including the large-scale expansion and molecular divergence of pattern recognition receptors (PRRs) (TLRs, RLRs, lectins, etc.) and signaling adapters (MyD88s, etc.), significant domain recombination of immune receptors (RLR, NLRs, lectins, etc.), extensive somatic recombination of fibrinogenrelated proteins (FREPs) in snails (Fig. 3). These strategies keep pace with pathogen diversification to prevent tracking by pathogens over multiple host generations to help lophotrochozoans recognize and eliminate a wide range of pathogens, which promotes innate immunity diversity and specificity in lophotrochozoans.
Fig. 3.
The mechanisms of innate immunity diversity in lophotrochozoans.
Comparative immunology, derived from zoology and immunology, provides essential insights into the evolution of immunity [244], [245], [246]. Comparative immunology has made substantial advances in invertebrate immune at a cellular level and the evolutionary history of some immune-relate gene families [241], [247]. However, until recently, the signaling and regulatory networks that mediate crucial immune responses and the evolution of innate immunity are still unclear. Nowadays, next-generation sequencing (NGS) and genome editing have already shown themself to be powerful tools for a new generation of comprehensive and integrative studies in comparative immunology. For example, NGS's lower cost and effort revolutionize genomic study and provide opportunities to obtain a comprehensive view of “non-model” species. Moreover, it helps us to understand the biological relevance between gene systems and host defense and how to promote immune responses against infection. To date, the amount of genomic data for lophotrochozoans has escalated. Functional genomics, including transcriptome sequencing, single-cell transcriptome sequencing, and assay for transposase-accessible chromatin using sequencing, gives us a renewed opportunity to improve our knowledge of the genetic toolkit (core genes and gene networks) and diversity of innate immune system in lophotrochozoans. Furthermore, genome editing, such as clustered regularly interspaced palindromic repeats (CRISPR)/Cas9, makes it possible to verify the physiological function of immune-related core genes in the immune system. Recent studies in Crassostrea gigas had built a highly efficient genome editing system based on electroporation-based CRISPR/Cas9 [248]. More than ever, NGS and genome editing will be of significant interest in revealing the regulatory networks and the evolution of immune systems, which will fill the current gaps and comprehensively understand innate immune diversity in invertebrates.
CRediT authorship contribution statement
Linlin Zhang: Conceptualization, Writing – review & editing. Yongnan Li: Conceptualization, Writing – review & editing, Writing – original draft. Yu Xue: Data curation. Zhangjie Peng: Data curation.
Declaration of Competing Interest
The study funding sources are listed in the Acknowledgments. The authors have no financial/commercial conflicts of interest.
Acknowledgments
The work was supported by the National Natural Science Foundation of China 41976088 to L.Z., the Strategic Priority Research Program of the Chinese Academy of Sciences XDB42000000 to L.Z., National Key R&D Program of China 2022YFD2401400 to L.Z., tn the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao) No. 2022QNLM030004, the Postdoctoral Research Foundation of China 2020M682248 to Y.L., Key Research and Development Program of Shandong 2022LZGC015, National Key R&D Program Of China 2022YFD2400304, athe Key Development Project of Centre for Ocean Mega-Research of Science, Chinese Academy of Science COMS2019R01 to L.Z..
References
- 1.Riera Romo M., Pérez Martínez D., Castillo, Ferrer C. Innate immunity in vertebrates: an overview. Immunology. 2016;148(2):125–139. doi: 10.1111/imm.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao S., Chan J., Xu Y., Wu S., Zhang L. Divergences of the RLR gene families across lophotrochozoans: domain grafting, exon–intron structure, expression, and positive selection. Int J Mol Sci. 2022;23(7):3415. doi: 10.3390/ijms23073415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40(12):845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann J.A., Kafatos F.C., Janeway C.A., Jr, Ezekowitz R. Phylogenetic perspectives in innate immunity. Science. 1999;284(5418):1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 5.Schmucker D., Clemens J.C., Shu H., Worby C.A., Xiao J., Muda M., et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101(6):671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh J., Buckley K.M., Nair S.V., Raftos D.A., Miller C., Majeske A.J., et al. Sp185/333: a novel family of genes and proteins involved in the purple sea urchin immune response. Dev Comp Immunol. 2010;34(3):235–245. doi: 10.1016/j.dci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Smith L.C. Innate immune complexity in the purple sea urchin: diversity of the Sp185/333 system. Front Immunol. 2012;3:70. doi: 10.3389/fimmu.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith L.C., Rast J.P., Brockton V., Terwilliger D.P., Nair S.V., Buckley K.M., et al. The sea urchin immune system. Invertebr Surviv J. 2006;3(1):25–39. [Google Scholar]
- 10.Cannon J.P., Haire R.N., Litman G.W. Identification of diversified genes that contain immunoglobulin-like variable regions in a protochordate. Nat Immunol. 2002;3(12):1200–1207. doi: 10.1038/ni849. [DOI] [PubMed] [Google Scholar]
- 11.Yuan S., Tao X., Huang S., Chen S., Xu A. Comparative immune systems in animals. Annu Rev Anim Biosci. 2014;2:235–258. doi: 10.1146/annurev-animal-031412-103634. [DOI] [PubMed] [Google Scholar]
- 12.Huang S., Wang X., Yan Q., Guo L., Yuan S., Huang G., et al. The evolution and regulation of the mucosal immune complexity in the basal chordate amphioxus. J Immunol. 2011;186(4):2042–2055. doi: 10.4049/jimmunol.1001824. [DOI] [PubMed] [Google Scholar]
- 13.Halanych K. Lophotrochozoa, Diversification of; 2016.
- 14.Kocot K.M. On 20 years of Lophotrochozoa. Org Divers Evol. 2016;16(2):329–343. [Google Scholar]
- 15.Canesi L., Pruzzo C. Specificity of innate immunity in bivalves: a lesson from bacteria. Lessons Immun. 2016:79–91. [Google Scholar]
- 16.Guo X., He Y., Zhang L., Lelong C., Jouaux A. Immune and stress responses in oysters with insights on adaptation. Fish Shellfish Immunol. 2015;46(1):107–119. doi: 10.1016/j.fsi.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Seppälä O., Çetin C., Cereghetti T., Feulner P.G., Adema C.M. Examining adaptive evolution of immune activity: opportunities provided by gastropods in the age of ‘omics’. Philos Trans R Soc B. 1825;2021(376):20200158. doi: 10.1098/rstb.2020.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Li L., Guo X., Litman G.W., Dishaw L.J., Zhang G. Massive expansion and functional divergence of innate immune genes in a protostome. Sci Rep. 2015;5(1):1–11. doi: 10.1038/srep08693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., Chan J., Li Q., Zhang L. Evolutionary history of the Toll-like receptor and myd88 in molluscs. Oceanol Limnol Sin. 2021;52:4. [Google Scholar]
- 20.Zhu X., Mu K., Wan Y., Zhang L. Evolutionary history of the NLR gene families across lophotrochozoans. Gene. 2022;843 doi: 10.1016/j.gene.2022.146807. [DOI] [PubMed] [Google Scholar]
- 21.Adema C.M., Hertel L.A., Miller R.D., Loker E.S. A family of fibrinogen-related proteins that precipitates parasite-derived molecules is produced by an invertebrate after infection. Proc Natl Acad Sci. 1997;94(16):8691–8696. doi: 10.1073/pnas.94.16.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Léonard P.M., Adema C.M., Zhang S.M., Loker E.S. Structure of two FREP genes that combine IgSF and fibrinogen domains, with comments on diversity of the FREP gene family in the snail Biomphalaria glabrata. Gene. 2001;269(1–2):155–165. doi: 10.1016/s0378-1119(01)00444-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S.M., Adema C.M., Kepler T.B., Loker E.S. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305(5681):251–254. doi: 10.1126/science.1088069. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S.M., Loker E.S. The FREP gene family in the snail Biomphalaria glabrata: additional members, and evidence consistent with alternative splicing and FREP retrosequences. Dev Comp Immunol. 2003;27(3):175–187. doi: 10.1016/s0145-305x(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S.M., Léonard P.M., Adema C.M., Loker E.S. Parasite-responsive IgSF members in the snail Biomphalaria glabrata: characterization of novel genes with tandemly arranged IgSF domains and a fibrinogen domain. Immunogenetics. 2001;53(8):684–694. doi: 10.1007/s00251-001-0386-8. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S.M., Loker E.S. Representation of an immune responsive gene family encoding fibrinogen-related proteins in the freshwater mollusc Biomphalaria glabrata, an intermediate host for Schistosoma mansoni. Gene. 2004;341:255–266. doi: 10.1016/j.gene.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song K., Li Y., Huang B., Li L., Zhang G. Genetic and evolutionary patterns of innate immune genes in the Pacific oyster Crassostrea gigas. Dev Comp Immunol. 2017;77:17–22. doi: 10.1016/j.dci.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Ierrez A.P., Bean T.P., Hooper C., Stenton C.A., Sanders M.B., Paley R.K., et al. A genome-wide association study for host resistance to ostreid herpesvirus in Pacific oysters (Crassostrea gigas). G3: Genes, Genomes. Genetics. 2018;8(4):1273–1280. doi: 10.1534/g3.118.200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siva V.S., Wang L., Qiu L., Zhou Z., Liu C., Yang J., et al. Polymorphism in a serine protease inhibitor gene and its association with the resistance of bay scallop (Argopecten irradians) to Listonella anguillarum challenge. Fish Shellfish Immunol. 2016;59:1–8. doi: 10.1016/j.fsi.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Núñez Acuña G., Gallardo, Escárate C. Identification of immune-related SNPs in the transcriptome of Mytilus chilensis through high-throughput sequencing. Fish Shellfish Immunol. 2013;35(6):1899–1905. doi: 10.1016/j.fsi.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Hubert F., Noël T., Roch P. A member of the arthropod defensin family from edible Mediterranean mussels (Mytilus galloprovincialis) Eur J Biochem. 1996;240(1):302–306. doi: 10.1111/j.1432-1033.1996.0302h.x. [DOI] [PubMed] [Google Scholar]
- 32.Labreuche Y., Lambert C., Soudant P., Boulo V., Huvet A., Nicolas J.L. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes Infect. 2006;8(12–13):2715–2724. doi: 10.1016/j.micinf.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Canesi L., Auguste M., Balbi T., Prochazkova P. Soluble mediators of innate immunity in annelids and bivalve mollusks: a mini-review. Front Immunol. 2022;13:1051155. doi: 10.3389/fimmu.2022.1051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowley A., Ratclife N. Invertebrate blood cells. Parasitology. 1981;2:421. [Google Scholar]
- 35.Lau Y.T., Sussman L., Espinosa E.P., Katalay S., Allam B. Characterization of hemocytes from different body fluids of the eastern oyster Crassostrea virginica. Fish Shellfish Immunol. 2017;71:372–379. doi: 10.1016/j.fsi.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 36.De la Ballina N.R., Maresca F., Cao A., Villalba A. Bivalve haemocyte subpopulations: a review. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.826255. 826255-826255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisher W. Structure and functions of oyster hemocytes. Immun Invertebr. 1986:25–35. [Google Scholar]
- 38.Cheng T.C. A classification of molluscan hemocytes based on functional evidences. Invertebr Blood. 1984:111–146. [Google Scholar]
- 39.Auffret M. Bivalve hemocyte morphology. Disease processes in marine bivalve molluscs; 1988.
- 40.Liu J., Zhao Y. Morphological and functional characterization of clam Ruditapes philippinarum haemocytes. Fish Shellfish Immunol. 2018;82:136–146. doi: 10.1016/j.fsi.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 41.Meng J., Zhang G., Wang W.X. Functional heterogeneity of immune defenses in molluscan oysters Crassostrea hongkongensis revealed by high-throughput single-cell transcriptome. Fish Shellfish Immunol. 2022;120:202–213. doi: 10.1016/j.fsi.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Barracco M.A., Medeiros I.D., Moreira F.M. Some haemato-immunological parameters in the mussel Perna perna. Fish Shellfish Immunol. 1999;9(5):387–404. [Google Scholar]
- 43.Astuya A., Carrera C., Ulloa V., Aballay A., Núñez Acuña G., Hégaret H., et al. Saxitoxin modulates immunological parameters and gene transcription in Mytilus chilensis hemocytes. Int J Mol Sci. 2015;16(7):15235–15250. doi: 10.3390/ijms160715235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evariste L., Auffret M., Audonnet S., Geffard A., David E., Brousseau P., et al. Functional features of hemocyte subpopulations of the invasive mollusk species Dreissena polymorpha. Fish Shellfish Immunol. 2016;56:144–154. doi: 10.1016/j.fsi.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 45.Pampanin D.M., Marin M.G., Ballarin L. Morphological and cytoenzymatic characterization of haemocytes of the venus clam Chamelea gallina. Dis Aquat Org. 2002;49(3):227–234. doi: 10.3354/dao049227. [DOI] [PubMed] [Google Scholar]
- 46.Yanyan Z., Sulian R., Dexiu W., Weibo S. Structure and classification of haemocytes in the bivalve mollusc Meretrix meretrix. J Ocean Univ China. 2006;5(2):132–136. [Google Scholar]
- 47.Ladhar Chaabouni R., Ayadi W., Sahli E., Mokdad Gargouri R. Establishment of primary cell culture of Ruditapes decussatus haemocytes for metal toxicity assessment. Vitr Cell Dev Biol Anim. 2021;57(4):477–484. doi: 10.1007/s11626-021-00561-x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W., Wu X., Wang M. Morphological, structural, and functional characterization of the haemocytes of the scallop, Argopecten irradians. Aquaculture. 2006;251(1):19–32. [Google Scholar]
- 49.Queiroga F.R., Marques-Santos L.F., Hégaret H., Soudant P., Farias N.D., Schlindwein A.D., et al. Immunological responses of the mangrove oysters Crassostrea gasar naturally infected by Perkinsus sp. in the Mamanguape Estuary, Paraíba state (Northeastern, Brazil) Fish Shellfish Immunol. 2013;35(2):319–327. doi: 10.1016/j.fsi.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 50.Donaghy L., Hong H.K., Lee H.J., Jun J.C., Park Y.J., Choi K.S. Hemocyte parameters of the Pacific oyster Crassostrea gigas a year after the Hebei Spirit oil spill off the west coast of Korea. Helgol Mar Res. 2010;64(4):349–355. [Google Scholar]
- 51.Li J., Zhang Y., Mao F., Lin Y., Xiao S., Xiang Z., et al. The first morphologic and functional characterization of hemocytes in Hong Kong oyster, Crassostrea hongkongensis. Fish Shellfish Immunol. 2018;81:423–429. doi: 10.1016/j.fsi.2018.05.062. [DOI] [PubMed] [Google Scholar]
- 52.MdF Rebelo, EdS Figueiredo, Mariante R.M., Nóbrega A., de Barros C.M., Allodi S. New insights from the oyster Crassostrea rhizophorae on bivalve circulating hemocytes. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng S. Pinocytosis of proteins by oyster leucocytes. Biol Bull. 1965;129(1):95–105. [Google Scholar]
- 54.Canesi L., Gallo G., Gavioli M., Pruzzo C. Bacteria–hemocyte interactions and phagocytosis in marine bivalves. Microsc Res Tech. 2002;57(6):469–476. doi: 10.1002/jemt.10100. [DOI] [PubMed] [Google Scholar]
- 55.Anisimova A.A. Morphofunctional parameters of hemocytes in the assessment of the physiological status of bivalves. Russ J Mar Biol. 2013;39(6):381–391. [Google Scholar]
- 56.López C., Carballal M.J., Azevedo C., Villalba A. Differential phagocytic ability of the circulating haemocyte types of the carpet shell clam Ruditapes decussatus (Mollusca: Bivalvia) Dis Aquat Org. 1997;30(3):209–215. [Google Scholar]
- 57.Tame A., Ozawa G., Maruyama T., Yoshida T. Morphological and functional characterization of hemocytes from two deep-sea vesicomyid clams Phreagena okutanii and Abyssogena phaseoliformis. Fish Shellfish Immunol. 2018;74:281–294. doi: 10.1016/j.fsi.2017.12.058. [DOI] [PubMed] [Google Scholar]
- 58.Matozzo V., Rova G., Marin M.G. Haemocytes of the cockle Cerastoderma glaucum: morphological characterisation and involvement in immune responses. Fish Shellfish Immunol. 2007;23(4):732–746. doi: 10.1016/j.fsi.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Wootton E.C., Dyrynda E.A., Ratcliffe N.A. Bivalve immunity: comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua) Fish Shellfish Immunol. 2003;15(3):195–210. doi: 10.1016/s1050-4648(02)00161-4. [DOI] [PubMed] [Google Scholar]
- 60.Tame A., Yoshida T., Ohishi K., Maruyama T. Phagocytic activities of hemocytes from the deep-sea symbiotic mussels Bathymodiolus japonicus, B. platifrons, and B. septemdierum. Fish Shellfish Immunol. 2015;45(1):146–156. doi: 10.1016/j.fsi.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 61.Pipe R.K., Farley S.R., Coles J.A. The separation and characterisation of haemocytes from the mussel Mytilus edulis. Cell Tissue Res. 1997;289(3):537–545. doi: 10.1007/s004410050899. [DOI] [PubMed] [Google Scholar]
- 62.Lin T., Zhang D., Lai Q., Sun M., Quan W., Zhou K. A modified method to detect the phagocytic ability of eosinophilic and basophilic haemocytes in the oyster Crassostrea plicatula. Fish Shellfish Immunol. 2014;40(1):337–343. doi: 10.1016/j.fsi.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Carballal M.J., López C., Azevedo C., Villalba A. In vitrostudy of phagocytic ability of Mytilus galloprovincialis Lmk. haemocytes. Fish Shellfish Immunol. 1997;7(6):403–416. [Google Scholar]
- 64.Friebel B., Renwrantz L. Application of density gradient centrifugation for separation of eosinophilic and basophilic hemocytes from Mytilus edulis and characterization of both cell groups. Comp Biochem Physiol Part A Physiol. 1995;112(1):81–90. [Google Scholar]
- 65.Wikfors G.H., Alix J.H. Granular hemocytes are phagocytic, but agranular hemocytes are not, in the Eastern Oyster Crassostrea virginica. Invertebr Immun. 2014;1:15–21. [Google Scholar]
- 66.Nakayama K., Nomoto A.M., Nishijima M., Maruyama T. Morphological and functional characterization of hemocytes in the giant clamTridacna crocea. J Invertebr Pathol. 1997;69(2):105–111. doi: 10.1006/jipa.1996.4626. [DOI] [PubMed] [Google Scholar]
- 67.Russell Pinto F., Reimão R., de Sousa M. Haemocytes in Cerastoderma edule (Mollusca, Bivalvia): distinct cell types engage in different responses to sheep erythrocytes. Fish Shellfish Immunol. 1994;4(5):383–397. [Google Scholar]
- 68.Matozzo V., Pagano M., Spinelli A., Caicci F., Faggio C. Pinna nobilis: a big bivalve with big haemocytes? Fish Shellfish Immunol. 2016;55:529–534. doi: 10.1016/j.fsi.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 69.Huang J., Li S., Liu Y., Liu C., Xie L., Zhang R. Hemocytes in the extrapallial space of Pinctada fucata are involved in immunity and biomineralization. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-22961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W., Li M., Wang L., Chen H., Liu Z., Jia Z., et al. The granulocytes are the main immunocompetent hemocytes in Crassostrea gigas. Dev Comp Immunol. 2017;67:221–228. doi: 10.1016/j.dci.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 71.Aladaileh S., Nair S.V., Birch D., Raftos D.A. Sydney rock oyster (Saccostrea glomerata) hemocytes: morphology and function. J Invertebr Pathol. 2007;96(1):48–63. doi: 10.1016/j.jip.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Rey Campos M., Moreira R., Valenzuela Muñoz V., Gallardo Escárate C., Novoa B., Figueras A. High individual variability in the transcriptomic response of Mediterranean mussels to Vibrio reveals the involvement of myticins in tissue injury. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-39870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nuria R., Villalba A., Cao A. Shotgun analysis to identify differences in protein expression between granulocytes and hyalinocytes of the European flat oyster Ostrea edulis. Fish Shellfish Immunol. 2021;119:678–691. doi: 10.1016/j.fsi.2021.10.045. [DOI] [PubMed] [Google Scholar]
- 74.Hoeger U, Harris JR (Eds.). Vertebrate and invertebrate respiratory proteins, lipoproteins and other body fluid proteins (Vol. 94). Springer Nature; 2020.
- 75.Bilej M., Procházková P., Silerová M., Josková R. Earthworm immunity. Adv Exp Med Biol. 2010;708:66–79. doi: 10.1007/978-1-4419-8059-5_4. [DOI] [PubMed] [Google Scholar]
- 76.Cooper E.L., Kauschke E., Cossarizza A. Digging for innate immunity since Darwin and Metchnikoff. Bioessays. 2002;24(4):319–333. doi: 10.1002/bies.10077. [DOI] [PubMed] [Google Scholar]
- 77.Cooper E.L., Kauschke E., Cossarizza A. Phylogenetic perspectives on the vertebrate immune system. 2001. Cossarizza A.: annelid humoral immunity: cell lysis in earthworms; pp. 169–183. [DOI] [PubMed] [Google Scholar]
- 78.Engelmann P., Hayashi Y., Bodó K., Ernszt D., Somogyi I., Steib A., et al. Phenotypic and functional characterization of earthworm coelomocyte subsets: linking light scatter-based cell typing and imaging of the sorted populations. Dev Comp Immunol. 2016;65:41–52. doi: 10.1016/j.dci.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 79.Homa J., Zorska A., Wesolowski D., Chadzinska M. Dermal exposure to immunostimulants induces changes in activity and proliferation of coelomocytes of Eisenia andrei. J Comp Physiol B. 2013;183(3):313–322. doi: 10.1007/s00360-012-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cholewa J., Feeney G.P., O'Reilly M., Stürzenbaum S.R., Morgan A.J., Płytycz B. Autofluorescence in eleocytes of some earthworm species. Folia Histochem Cytobiol. 2006;44(1):65–71. [PubMed] [Google Scholar]
- 81.Prochazkova P., Roubalova R., Dvorak J., Navarro Pacheco N.I., Bilej M. Pattern recognition receptors in annelids. Dev Comp Immunol. 2020;102 doi: 10.1016/j.dci.2019.103493. [DOI] [PubMed] [Google Scholar]
- 82.Stanovova M.V., Gazizova G.R., Gorbushin A.M. Transcriptomic profiling of immune-associated molecules in the coelomocytes of lugworm Arenicola marina (Linnaeus, 1758) J Exp Zool B Mol Dev Evol. 2023;340(1):34–55. doi: 10.1002/jez.b.23135. [DOI] [PubMed] [Google Scholar]
- 83.Fitzgerald S.W., Ratcliffe N.A. Evidence for the presence of subpopulations of Arenicola marina coelomocytes identified by their selective response towards Gram+ve and Gram-ve bacteria. Dev Comp Immunol. 1982;6(1):23–34. doi: 10.1016/0145-305x(82)90004-0. (Winter) [DOI] [PubMed] [Google Scholar]
- 84.Ghosh J., Lun C.M., Majeske A.J., Sacchi S., Schrankel C.S., Smith L.C. Invertebrate immune diversity. Dev Comp Immunol. 2011;35(9):959–974. doi: 10.1016/j.dci.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 85.Grabacka M., Pierzchalska M., Płonka P.M., Pierzchalski P. The role of PPAR alpha in the modulation of innate immunity. Int J Mol Sci. 2021;22(19):10545. doi: 10.3390/ijms221910545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang W., Song X., Wang L., Song L. Pathogen-derived carbohydrate recognition in molluscs immune defense. Int J Mol Sci. 2018;19(3):721. doi: 10.3390/ijms19030721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magadum S., Banerjee U., Murugan P., Gangapur D., Ravikesavan R. Gene duplication as a major force in evolution. J Genet. 2013;92(1):155–161. doi: 10.1007/s12041-013-0212-8. [DOI] [PubMed] [Google Scholar]
- 90.Oren M., Barela Hudgell M.A., D’Allura B., Agronin J., Gross A., Podini D., et al. Short tandem repeats, segmental duplications, gene deletion, and genomic instability in a rapidly diversified immune gene family. BMC Genom. 2016;17(1):1–19. doi: 10.1186/s12864-016-3241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang S., Yuan S., Guo L., Yu Y., Li J., Wu T., et al. Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Res. 2008;18(7):1112–1126. doi: 10.1101/gr.069674.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 93.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 94.Orús-Alcalde A., Lu T.M., Børve A., Hejnol A. The evolution of the metazoan Toll receptor family and its expression during protostome development. BMC Ecol Evol. 2021;21(1):208. doi: 10.1186/s12862-021-01927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roach J.C., Glusman G., Rowen L., Kaur A., Purcell M.K., Smith K.D., et al. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA. 2005;102(27):9577–9582. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Werling D., Jann O.C., Offord V., Glass E.J., Coffey T.J. Variation matters: TLR structure and species-specific pathogen recognition. Trends Immunol. 2009;30(3):124–130. doi: 10.1016/j.it.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Luo Y.J., Kanda M., Koyanagi R., Hisata K., Akiyama T., Sakamoto H., et al. Nemertean and phoronid genomes reveal lophotrochozoan evolution and the origin of bilaterian heads. Nat Ecol Evol. 2018;2(1):141–151. doi: 10.1038/s41559-017-0389-y. [DOI] [PubMed] [Google Scholar]
- 98.Davidson C.R., Best N.M., Francis J.W., Cooper E.L., Wood T.C. Toll-like receptor genes (TLRs) from Capitella capitata and Helobdella robusta (Annelida). Dev Comp Immunol. 2008;32(6):608–612. doi: 10.1016/j.dci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Cuvillier-Hot V., Boidin-Wichlacz C., Slomianny C., Salzet M., Tasiemski A. Characterization and immune function of two intracellular sensors, HmTLR1 and HmNLR, in the injured CNS of an invertebrate. Dev Comp Immunol. 2011;35(2):214–226. doi: 10.1016/j.dci.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 100.Škanta F., Roubalová R., Dvořák J., Procházková P., Bilej M. Molecular cloning and expression of TLR in the Eisenia andrei earthworm. Dev Comp Immunol. 2013;41(4):694–702. doi: 10.1016/j.dci.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 101.Prochazkova P., Roubalova R., Skanta F., Dvorak J., Pacheco N.I.N., Kolarik M., et al. Developmental and immune role of a novel multiple cysteine cluster TLR from Eisenia andrei earthworms. Front Immunol. 2019;10:1277. doi: 10.3389/fimmu.2019.01277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lan Y., Sun J., Chen C., Sun Y., Zhou Y., Yang Y., et al. Hologenome analysis reveals dual symbiosis in the deep-sea hydrothermal vent snail Gigantopelta aegis. Nat Commun. 2021;12(1):1165. doi: 10.1038/s41467-021-21450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun J., Chen C., Miyamoto N., Li R., Sigwart J.D., Xu T., et al. The scaly-foot snail genome and implications for the origins of biomineralised armour. Nat Commun. 2020;11(1):1657. doi: 10.1038/s41467-020-15522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y., Tassia M.G., Waits D.S., Bogantes V.E., David K.T., Halanych K.M. Genomic adaptations to chemosymbiosis in the deep-sea seep-dwelling tubeworm Lamellibrachia luymesi. BMC Biol. 2019;17(1):91. doi: 10.1186/s12915-019-0713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun J., Zhang Y., Xu T., Zhang Y., Mu H., Zhang Y., et al. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes. Nat Ecol Evol. 2017;1(5):1–7. doi: 10.1038/s41559-017-0121. [DOI] [PubMed] [Google Scholar]
- 106.de Oliveira A.L., Mitchell J., Girguis P., Bright M. Novel insights on obligate symbiont lifestyle and adaptation to chemosynthetic environment as revealed by the giant tubeworm genome. Mol Biol Evol. 2022;39(1):msab347. doi: 10.1093/molbev/msab347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun J., Wang L., Song L. The primitive complement system in molluscs. Dev Comp Immunol. 2022;139 doi: 10.1016/j.dci.2022.104565. [DOI] [PubMed] [Google Scholar]
- 108.Gorbushin A.M. Immune repertoire in the transcriptome of Littorina littorea reveals new trends in lophotrochozoan proto-complement evolution. Dev Comp Immunol. 2018;84:250–263. doi: 10.1016/j.dci.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 109.Gorbushin A.M. Derivatives of the lectin complement pathway in Lophotrochozoa. Dev Comp Immunol. 2019;94:35–58. doi: 10.1016/j.dci.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 110.Kishore U., Reid K.B. C1q: structure, function, and receptors. Immunopharmacology. 2000;49(1–2):159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 111.Matsushita M., Matsushita A., Endo Y., Nakata M., Kojima N., Mizuochi T., et al. Origin of the classical complement pathway: lamprey orthologue of mammalian C1q acts as a lectin. Proc Natl Acad Sci. 2004;101(27):10127–10131. doi: 10.1073/pnas.0402180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fouët G., Bally I., Chouquet A., Reiser J.B., Thielens N.M., Gaboriaud C., et al. Molecular basis of complement C1q collagen-like region interaction with the immunoglobulin-like receptor LAIR-1. Int J Mol Sci. 2021;22(10):5125. doi: 10.3390/ijms22105125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mortensen S.A., Sander B., Jensen R.K., Pedersen J.S., Golas M.M., Jensenius J.C., et al. Structure and activation of C1, the complex initiating the classical pathway of the complement cascade. Proc Natl Acad Sci. 2017;114(5):986–991. doi: 10.1073/pnas.1616998114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gerdol M., Greco S., Pallavicini A. Extensive tandem duplication events drive the expansion of the C1q-domain-containing gene family in bivalves. Mar Drugs. 2019;17(10):583. doi: 10.3390/md17100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takeuchi T., Koyanagi R., Gyoja F., Kanda M., Hisata K., Fujie M., et al. Bivalve-specific gene expansion in the pearl oyster genome: implications of adaptation to a sessile lifestyle. Zool Lett. 2016;2(1):1–13. doi: 10.1186/s40851-016-0039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Powell D., Subramanian S., Suwansa Ard S., Zhao M., O’Connor W., Raftos D., et al. The genome of the oyster Saccostrea offers insight into the environmental resilience of bivalves. DNA Res. 2018;25(6):655–665. doi: 10.1093/dnares/dsy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mun S., Kim Y.-J., Markkandan K., Shin W., Oh S., Woo J., et al. The whole-genome and transcriptome of the Manila clam (Ruditapes philippinarum) Genome Biol Evol. 2017;9(6):1487–1498. doi: 10.1093/gbe/evx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang G., Fang X., Guo X., Li L., Luo R., Xu F., et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490(7418):49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L., Li L., Zhu Y., Zhang G., Guo X. Transcriptome analysis reveals a rich gene set related to innate immunity in the Eastern oyster (Crassostrea virginica) Mar Biotechnol. 2014;16(1):17–33. doi: 10.1007/s10126-013-9526-z. [DOI] [PubMed] [Google Scholar]
- 120.Song H., Guo X., Sun L., Wang Q., Han F., Wang H., et al. The hard clam genome reveals massive expansion and diversification of inhibitors of apoptosis in Bivalvia. BMC Biol. 2021;19(1):1–20. doi: 10.1186/s12915-020-00943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miyamoto H., Endo H., Hashimoto N., Isowa Y., Kinoshita S., Kotaki T., et al. The diversity of shell matrix proteins: genome-wide investigation of the pearl oyster, Pinctada fucata. Zool Sci. 2013;30(10):801–816. doi: 10.2108/zsj.30.801. [DOI] [PubMed] [Google Scholar]
- 122.Babushok D., Ostertag E., Kazazian H. Current topics in genome evolution: molecular mechanisms of new gene formation. Cell Mol Life Sci. 2007;64(5):542–554. doi: 10.1007/s00018-006-6453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loo Y.M., Gale Jr M. Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoneyama M., Fujita T. RNA recognition and signal transduction by RIG‐I‐like receptors. Immunol Rev. 2009;227(1):54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 125.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Immunol Rev. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 126.Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., et al. IPS-1, an adaptor triggering RIG-I-and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6(10):981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 127.Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437(7062):1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 128.Seth R.B., Sun L., Ea C.K., Chen Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122(5):669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 129.Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 130.Bamming D., Horvath C.M. Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem. 2009;284(15):9700–9712. doi: 10.1074/jbc.M807365200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., et al. LGP2 is a positive regulator of RIG-I–and MDA5-mediated antiviral responses. Proc Natl Acad Sci. 2010;107(4):1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kanneganti T.D., Lamkanfi M. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27(4):549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 133.Ip J.C.H., Xu T., Sun J., Li R.S., Chen C., Lan Y., et al. Host–endosymbiont genome integration in a deep-sea chemosymbiotic clam. Mol Biol Evol. 2021;38(2):502–518. doi: 10.1093/molbev/msaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kremer N., Charif D., Henri H., Gavory F., Wincker P., Mavingui P., et al. Influence of Wolbachiaon host gene expression in an obligatory symbiosis. BMC Microbiol. 2012;12(1):1–16. doi: 10.1186/1471-2180-12-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Medzhitov R., Preston Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2(2):253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 136.Chen H., Jiang Z. The essential adaptors of innate immune signaling. Protein Cell. 2013;4(1):27–39. doi: 10.1007/s13238-012-2063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alt F.W., Oltz E.M., Young F., Gorman J., Taccioli G., Chen J.Z. VDJ recombination. Immunol Today. 1992;13(8):306–314. doi: 10.1016/0167-5699(92)90043-7. [DOI] [PubMed] [Google Scholar]
- 138.Dudley D.D., Chaudhuri J., Bassing C.H., Alt F.W. Mechanism and control of V (D) J recombination versus class switch recombination: similarities and differences. Adv Immunol. 2005;86:43–112. doi: 10.1016/S0065-2776(04)86002-4. [DOI] [PubMed] [Google Scholar]
- 139.Gellert M. Molecular analysis of V (D) J recombination. Annu Rev Genet. 1992;26:425–446. doi: 10.1146/annurev.ge.26.120192.002233. [DOI] [PubMed] [Google Scholar]
- 140.Huang B., Zhang L., Li L., Tang X., Zhang G. Highly diverse fibrinogen-related proteins in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2015;43(2):485–490. doi: 10.1016/j.fsi.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 141.Zhang L., Li L., Zhang G. Sequence variability of fibrinogen-related proteins (FREPs) in Crassostrea gigas. Chin Sci Bull. 2012;57(25):3312–3319. [Google Scholar]
- 142.Hawn T.R., Verbon A., Lettinga K.D., Zhao L.P., Li S.S., Laws R.J., et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003;198(10):1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Z., Cordes J. DNA marker technologies and their applications in aquaculture genetics. Aquaculture. 2004;238(1–4):1–37. [Google Scholar]
- 144.Gao L., Du X., Su H., Gao X., Li Y., Bao X., et al. The polymorphisms of chemokine gene in channel catfish (Ictalurus punctatus) and the associations with susceptibility/resistance to Edwardsiella ictaluri. HOAJ Biol. 2013;2:3. [Google Scholar]
- 145.Li L., Zhao J., Wang L., Qiu L., Zhang H., Dong C., et al. The polymorphism of lysozyme gene in Zhikong scallop (Chlamys farreri) and its association with susceptibility/resistance to Listonella anguillarum. Fish Shellfish Immunol. 2009;27(2):136–142. doi: 10.1016/j.fsi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 146.Zhang L., Guo X. Development and validation of single nucleotide polymorphism markers in the eastern oyster Crassostrea virginica Gmelin by mining ESTs and resequencing. Aquaculture. 2010;302(1–2):124–129. [Google Scholar]
- 147.Yu H., He Y., Wang X., Zhang Q., Bao Z., Guo X. Polymorphism in a serine protease inhibitor gene and its association with disease resistance in the eastern oyster (Crassostrea virginica Gmelin) Fish Shellfish Immunol. 2011;30(3):757–762. doi: 10.1016/j.fsi.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 148.Kongchum P., Rexroad C., Hallerman E., David L., Palti Y. Single nucleotide polymorphism identification, genetic mapping and tissue expression of the rainbow trout TLR9 gene. Anim Genet. 2009;40(6):1001. doi: 10.1111/j.1365-2052.2009.01924.x. [DOI] [PubMed] [Google Scholar]
- 149.Vera M., Álvarez Dios J.A., Millán A., Pardo B.G., Bouza C., Hermida M., et al. Validation of single nucleotide polymorphism (SNP) markers from an immune Expressed Sequence Tag (EST) turbot, Scophthalmus maximus, database. Aquaculture. 2011;313(1–4):31–41. [Google Scholar]
- 150.Kongchum P., Palti Y., Hallerman E.M., Hulata G., David L. SNP discovery and development of genetic markers for mapping innate immune response genes in common carp (Cyprinus carpio) Fish Shellfish Immunol. 2010;29(2):356–361. doi: 10.1016/j.fsi.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 151.Xu J., Ji P., Zhao Z., Zhang Y., Feng J., Wang J., et al. Genome-wide SNP discovery from transcriptome of four common carp strains. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.KGutZenger K.R., Khatkar M.S., Jones D.B., Khalilisamani N., Jerry D.R., Raadsma H.W. Genomic selection in aquaculture: application, limitations and opportunities with special reference to marine shrimp and pearl oysters. Front Genet. 2019;9:693. doi: 10.3389/fgene.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Litman G.W., Dishaw L.J., Cannon J.P., Haire R.N., Rast J.P. Alternative mechanisms of immune receptor diversity. Curr Opin Immunol. 2007;19(5):526–534. doi: 10.1016/j.coi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Modrek B., Resch A., Grasso C., Lee C. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 2001;29(13):2850–2859. doi: 10.1093/nar/29.13.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lynch K.W. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4(12):931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 156.Lynch K.W. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4(12):931–940. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- 157.Dong Y., Taylor H.E., Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006;4(7) doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Watson F.L., Püttmann Holgado R., Thomas F., Lamar D.L., Hughes M., Kondo M., et al. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309(5742):1874–1878. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- 159.Stout B., Adema C., Zhang S., Loker E. Biology of FREPs: diversified lectins with fibrinogen-related domains from the freshwater snail Biomphalaria glabrata. Anim Lectins. 2009:471–491. [Google Scholar]
- 160.Matsushita M., Endo Y., Taira S., Sato Y., Fujita T., Ichikawa N., et al. A novel human serum lectin with collagen-and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271(5):2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 161.Doolittle R.F. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci. 1992;1(12):1563–1577. doi: 10.1002/pro.5560011204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kenjo A., Takahashi M., Matsushita M., Endo Y., Nakata M., Mizuochi T., et al. Cloning and characterization of novel ficolins from the solitary ascidian, Halocynthia roretzi. J Biol Chem. 2001;276(23):19959–19965. doi: 10.1074/jbc.M011723200. [DOI] [PubMed] [Google Scholar]
- 163.Yang C., Wang L., Zhang H., Wang L., Huang M., Sun Z., et al. A new fibrinogen-related protein from Argopecten irradians (AiFREP-2) with broad recognition spectrum and bacteria agglutination activity. Fish Shellfish Immunol. 2014;38(1):221–229. doi: 10.1016/j.fsi.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 164.Liberti A., Natarajan O., Atkinson C.G.F., Dishaw L.J. Secreted immunoglobulin domain effector molecules of invertebrates and management of gut microbial ecology. Immunogenetics. 2022;74(1):99–109. doi: 10.1007/s00251-021-01237-2. [DOI] [PubMed] [Google Scholar]
- 165.Xu X., Doolittle R.F. Presence of a vertebrate fibrinogen-like sequence in an echinoderm. Proc Natl Acad Sci. 1990;87(6):2097–2101. doi: 10.1073/pnas.87.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gorbushin A., Panchin Y., Iakovleva N. In search of the origin of FREPs: characterization of Aplysia californica fibrinogen-related proteins. Dev Comp Immunol. 2010;34(4):465–473. doi: 10.1016/j.dci.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 167.Lu J., Le Y. Ficolins and the fibrinogen-like domain. Immunobiology. 1998;199(2):190–199. doi: 10.1016/S0171-2985(98)80026-0. [DOI] [PubMed] [Google Scholar]
- 168.Romero A., Dios S., Poisa Beiro L., Costa M.M., Posada D., Figueras A., et al. Individual sequence variability and functional activities of fibrinogen-related proteins (FREPs) in the Mediterranean mussel (Mytilus galloprovincialis) suggest ancient and complex immune recognition models in invertebrates. Dev Comp Immunol. 2011;35(3):334–344. doi: 10.1016/j.dci.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 169.RHancock R.E., Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8(9):402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 170.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 171.Tassanakajon A., Amparyup P., Somboonwiwat K., Supungul P. Cationic antimicrobial peptides in penaeid shrimp. Mar Biotechnol. 2011;13(4):639–657. doi: 10.1007/s10126-011-9381-8. [DOI] [PubMed] [Google Scholar]
- 172.Bulet P., Stöcklin R., Menin L. Anti‐microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198(1):169–184. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- 173.Brown K.L., Hancock R.E. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006;18(1):24–30. doi: 10.1016/j.coi.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 174.Hancock R.E., Brown K.L., Mookherjee N. Host defence peptides from invertebrates–emerging antimicrobial strategies. Immunobiology. 2006;211(4):315–322. doi: 10.1016/j.imbio.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 175.Yang D., Biragyn A., Kwak L.W., Oppenheim J.J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23(6):291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 176.Mitta G., Vandenbulcke F., Roch P. Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Lett. 2000;486(3):185–190. doi: 10.1016/s0014-5793(00)02192-x. [DOI] [PubMed] [Google Scholar]
- 177.Charlet M., Chernysh S., Philippe H., Hetru C., Hoffmann J.A., Bulet P. Innate immunity: isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J Biol Chem. 1996;271(36):21808–21813. doi: 10.1074/jbc.271.36.21808. [DOI] [PubMed] [Google Scholar]
- 178.Rosa R.D., Santini A., Fievet J., Bulet P., Destoumieux-Garzón D., Bachere E. Big defensins, a diverse family of antimicrobial peptides that follows different patterns of expression in hemocytes of the oyster Crassostrea gigas. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Balseiro P., Falcó A., Romero A., Dios S., Martínez-López A., Figueras A., et al. Mytilus galloprovincialis myticin C: a chemotactic molecule with antiviral activity and immunoregulatory properties. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Figueras A., Moreira R., Sendra M., Novoa B. Genomics and immunity of the Mediterranean mussel Mytilus galloprovincialis in a changing environment. Fish Shellfish Immunol. 2019;90:440–445. doi: 10.1016/j.fsi.2019.04.064. [DOI] [PubMed] [Google Scholar]
- 181.Bachère E., Rosa R.D., Schmitt P., Poirier A.C., Merou N., Charrière G.M., et al. The new insights into the oyster antimicrobial defense: cellular, molecular and genetic view. Fish Shellfish Immunol. 2015;46(1):50–64. doi: 10.1016/j.fsi.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 182.Schmitt P., Gueguen Y., Desmarais E., Bachère E., De Lorgeril J. Molecular diversity of antimicrobial effectors in the oyster Crassostrea gigas. BMC Evolut Biol. 2010;10(1):1–12. doi: 10.1186/1471-2148-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schmitt P., Rosa R.D., Destoumieux Garzón D. An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes. Biochim Biophys Acta Biomembr. 2016;1858(5):958–970. doi: 10.1016/j.bbamem.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 184.Schmitt P., Santini A., Vergnes A., Degremont L., de Lorgeril J. Sequence polymorphism and expression variability of Crassostrea gigas immune related genes discriminate two oyster lines contrasted in term of resistance to summer mortalities. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0075900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Loth K., Vergnes A., Barreto C., Voisin S.N., Meudal H., Da Silva J., et al. The ancestral N-terminal domain of big defensins drives bacterially triggered assembly into antimicrobial nanonets. mBio. 2019;10(5) doi: 10.1128/mBio.01821-19. e01821-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ouellette A.J., Selsted M.E. Immunology. HD6 defensin nanonets. Science. 2012;337(6093):420–421. doi: 10.1126/science.1225906. [DOI] [PubMed] [Google Scholar]
- 187.Stambuk F., Ojeda C., Machado Matos G., Rosa R.D., Mercado L., Schmitt P. Big defensin from the scallop Argopecten purpuratus ApBD1 is an antimicrobial peptide which entraps bacteria through nanonets formation. Fish Shellfish Immunol. 2021;119:456–461. doi: 10.1016/j.fsi.2021.10.037. [DOI] [PubMed] [Google Scholar]
- 188.Cho J.H., Park C.B., Yoon Y.G., Kim S.C. Lumbricin I, a novel proline-rich antimicrobial peptide from the earthworm: purification, cDNA cloning and molecular characterization. Biochim Biophys Acta. 1998;1408(1):67–76. doi: 10.1016/s0925-4439(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 189.Schikorski D., Cuvillier-Hot V., Leippe M., Boidin-Wichlacz C., Slomianny C., Macagno E., et al. Microbial challenge promotes the regenerative process of the injured central nervous system of the medicinal leech by inducing the synthesis of antimicrobial peptides in neurons and microglia. J Immunol. 2008;181(2):1083–1095. doi: 10.4049/jimmunol.181.2.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang X., Wang X., Zhang Y., Qu X., Yang S. An antimicrobial peptide of the earthworm Pheretima tschiliensis: cDNA cloning, expression and immunolocalization. Biotechnol Lett. 2003;25(16):1317–1323. doi: 10.1023/a:1024999206117. [DOI] [PubMed] [Google Scholar]
- 191.Li W., Li S., Zhong J., Zhu Z., Liu J., Wang W. A novel antimicrobial peptide from skin secretions of the earthworm, Pheretima guillelmi (Michaelsen) Peptides. 2011;32(6):1146–1150. doi: 10.1016/j.peptides.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 192.Bodó K., Boros Á., Rumpler É., Molnár L., Böröcz K., Németh P., et al. Identification of novel lumbricin homologues in Eisenia andrei earthworms. Dev Comp Immunol. 2019;90:41–46. doi: 10.1016/j.dci.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 193.Tasiemski A., Vandenbulcke F., Mitta G., Lemoine J., Lefebvre C., Sautière P.E., et al. Molecular characterization of two novel antibacterial peptides inducible upon bacterial challenge in an annelid, the leech Theromyzon tessulatum. J Biol Chem. 2004;279(30):30973–30982. doi: 10.1074/jbc.M312156200. [DOI] [PubMed] [Google Scholar]
- 194.Ovchinnikova T.V., Aleshina G.M., Balandin S.V., Krasnosdembskaya A.D., Markelov M.L., Frolova E.I., et al. Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett. 2004;577(1–2):209–214. doi: 10.1016/j.febslet.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 195.Tasiemski A., Jung S., Boidin-Wichlacz C., Jollivet D., Cuvillier-Hot V., Pradillon F., et al. Characterization and function of the first antibiotic isolated from a vent organism: the extremophile metazoan Alvinella pompejana. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0095737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Safronova V.N., Panteleev P.V., Sukhanov S.V., Toropygin I.Y., Bolosov I.A., Ovchinnikova T.V. Mechanism of action and therapeutic potential of the β-hairpin antimicrobial peptide capitellacin from the marine polychaeta Capitella teleta. Mar Drugs. 2022;20(3):167. doi: 10.3390/md20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Panteleev P.V., Tsarev A.V., Bolosov I.A., Paramonov A.S., Marggraf M.B., Sychev S.V., et al. Novel antimicrobial peptides from the arctic polychaeta Nicomache minor provide new molecular insight into biological role of the BRICHOS domain. Mar Drugs. 2018;16(11):401. doi: 10.3390/md16110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Edwards I.A., Elliott A.G., Kavanagh A.M., Zuegg J., Blaskovich M.A., Cooper M.A. Contribution of amphipathicity and hydrophobicity to the antimicrobial activity and cytotoxicity of β-hairpin peptides. ACS Infect Dis. 2016;2(6):442–450. doi: 10.1021/acsinfecdis.6b00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Al-Shehri S.S. Reactive oxygen and nitrogen species and innate immune response. Biochimie. 2021;181:52–64. doi: 10.1016/j.biochi.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 200.Larson K.G., Roberson B.S., Hetrick F.M. American oyster Crassostrea virginica. Dis Aquat Org. 1989;6:131–136. [Google Scholar]
- 201.Hégaret H., Wikfors G.H., Soudant P. Flow cytometric analysis of haemocytes from eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation: II. Haemocyte functions: aggregation, viability, phagocytosis, and respiratory burst. J Exp Mar Biol Ecol. 2003;293(2):249–265. [Google Scholar]
- 202.Lambert C., Soudant P., Choquet G., Paillard C. Measurement of Crassostrea gigas hemocyte oxidative metabolism by flow cytometry and the inhibiting capacity of pathogenic vibrios. Fish Shellfish Immunol. 2003;15(3):225–240. doi: 10.1016/s1050-4648(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 203.Goedken M., De Guise S. Flow cytometry as a tool to quantify oyster defence mechanisms. Fish Shellfish Immunol. 2004;16(4):539–552. doi: 10.1016/j.fsi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 204.Donaghy L., Kim B.K., Hong H.K., Park H.S., Choi K.S. Flow cytometry studies on the populations and immune parameters of the hemocytes of the Suminoe oyster, Crassostrea ariakensis. Fish Shellfish Immunol. 2009;27(2):296–301. doi: 10.1016/j.fsi.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 205.Cima F., Matozzo V., Marin M.G., Ballarin L. Haemocytes of the clam Tapes philippinarum (Adams & Reeve, 1850): morphofunctional characterisation. Fish Shellfish Immunol. 2000;10(8):677–693. doi: 10.1006/fsim.2000.0282. [DOI] [PubMed] [Google Scholar]
- 206.Buggé D.M., Hégaret H., Wikfors G.H., Allam B. Oxidative burst in hard clam (Mercenaria mercenaria) haemocytes. Fish Shellfish Immunol. 2007;23(1):188–196. doi: 10.1016/j.fsi.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 207.Pipe R.K. Generation of reactive oxygen metabolites by the haemocytes of the mussel Mytilus edulis. Dev Comp Immunol. 1992;16(2–3):111–122. doi: 10.1016/0145-305x(92)90012-2. [DOI] [PubMed] [Google Scholar]
- 208.Ordás M.C., Novoa B., Figueras A. Modulation of the chemiluminescence response of Mediterranean mussel (Mytilus galloprovincialis) haemocytes. Fish Shellfish Immunol. 2000;10(7):611–622. doi: 10.1006/fsim.2000.0276. [DOI] [PubMed] [Google Scholar]
- 209.Chen M.Y., Yang H.S., Delaporte M., Zhao S.J., Xing K. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J Exp Mar Biol Ecol. 2007;345(1):52–60. [Google Scholar]
- 210.Donaghy L., Lambert C., Choi K.S., Choi K.S., Soudant P. Hemocytes of the carpet shell clam (Ruditapes decussatus) and the Manila clam (Ruditapes philippinarum): current knowledge and future prospects. Aquaculture. 2009;297(1–4):10–24. [Google Scholar]
- 211.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 212.Arumugam M., Romestand B., Torreilles J., Roch P. In vitro production of superoxide and nitric oxide (as nitrite and nitrate) by Mytilus galloprovincialis haemocytes upon incubation with PMA or laminarin or during yeast phagocytosis. Eur J Cell Biol. 2000;79(7):513–519. doi: 10.1078/0171-9335-00068. [DOI] [PubMed] [Google Scholar]
- 213.Tafalla C., Gómez-León J., Novoa B., Figueras A. Nitric oxide production by carpet shell clam (Ruditapes decussatus) hemocytes. Dev Comp Immunol. 2003;27(3):197–205. doi: 10.1016/s0145-305x(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 214.Serfozo Z., Veréb Z., Roszer T., Kemenes G., Elekes K. Development of the nitric oxide/cGMP system in the embryonic and juvenile pond snail, Lymnaea stagnalis L. A comparative in situ hybridization, histochemical and immunohistochemical study. J Neurocytol. 2002;31(2):131–147. doi: 10.1023/a:1023945522690. [DOI] [PubMed] [Google Scholar]
- 215.Nieto-Fernandez F.E., Alcide K., Rialas C. Heavy metals and neuroimmunomodulation in Mytilus edulis. Acta Biol Hung. 2000;51(2–4):325–329. [PubMed] [Google Scholar]
- 216.Novas A., Barcia R., Ramos-Martínez J.I. After the Prestige oil spill modifications in NO production and other parameters related to the immune response were detected in hemocytes of Mytilus galloprovincialis. Aquat Toxicol. 2007;85(4):285–290. doi: 10.1016/j.aquatox.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 217.Jiang Q., Zhou Z., Wang L.L., Shi X., Wang J., Yue F., et al. The immunomodulation of inducible nitric oxide in scallop Chlamys farreri. Fish Shellfish Immunol. 2013;34(1):100–108. doi: 10.1016/j.fsi.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 218.Tafalla C., Novoa B., Figueras A. Production of nitric oxide by mussel (Mytilus galloprovincialis) hemocytes and effect of exogenous nitric oxide on phagocytic functions. Comp Biochem Physiol B Biochem Mol Biol. 2002;132(2):423–431. doi: 10.1016/s1096-4959(02)00050-7. [DOI] [PubMed] [Google Scholar]
- 219.Franchini A., Fontanili P., Ottaviani E. Invertebrate immunocytes: relationship between phagocytosis and nitric oxide production. Comp Biochem Physiol Part B Biochem Mol Biol. 1995;110(2):403–407. [Google Scholar]
- 220.Saco A., Rey-Campos M., Rosani U., Novoa B., Figueras A. The evolution and diversity of interleukin-17 highlight an expansion in marine invertebrates and its conserved role in mucosal immunity. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.692997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cao W., Wang W., Fan S., Li J., Li Q., Wu S., et al. The receptor CgIL-17R1 expressed in granulocytes mediates the CgIL-17 induced haemocytes proliferation in Crassostrea gigas. Dev Comp Immunol. 2022;131 doi: 10.1016/j.dci.2022.104376. [DOI] [PubMed] [Google Scholar]
- 222.Lv X., Sun J., Li Y., Yang W., Wang L., Leng J., et al. CgIL17-5 regulates the mRNA expressions of immune effectors through inducing the phosphorylation of CgMAPKs and the nuclear translocation of CgRel and CgAP-1 in the Pacific oyster Crassostrea gigas. Dev Comp Immunol. 2022;127 doi: 10.1016/j.dci.2021.104263. [DOI] [PubMed] [Google Scholar]
- 223.Roberts S., Gueguen Y., de Lorgeril J., Goetz F. Rapid accumulation of an interleukin 17 homolog transcript in Crassostrea gigas hemocytes following bacterial exposure. Dev Comp Immunol. 2008;32(9):1099–1104. doi: 10.1016/j.dci.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 224.Cao Y., Yang S., Feng C., Zhan W., Zheng Z., Wang Q., et al. Evolution and function analysis of interleukin-17 gene from Pinctada fucata martensii. Fish Shellfish Immunol. 2019;88:102–110. doi: 10.1016/j.fsi.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 225.Chu W.M. Tumor necrosis factor. Cancer Lett. 2013;328(2):222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 227.De Zoysa M., Jung S., Lee J. First molluscan TNF-alpha homologue of the TNF superfamily in disk abalone: molecular characterization and expression analysis. Fish Shellfish Immunol. 2009;26(4):625–631. doi: 10.1016/j.fsi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 228.Beschin A., Bilej M., Torreele E., De Baetselier P. On the existence of cytokines in invertebrates. Cell Mol Life Sci. 2001;58(5–6):801–814. doi: 10.1007/PL00000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Silerová M., Procházková P., Josková R., Josens G., Beschin A., De Baetselier P., et al. Comparative study of the CCF-like pattern recognition protein in different Lumbricid species. Dev Comp Immunol. 2006;30(9):765–771. doi: 10.1016/j.dci.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 230.Beschin A., Bilej M., Brys L., Torreele E., Lucas R., Magez S., et al. Convergent evolution of cytokines. Nature. 1999;400(6745):627–628. doi: 10.1038/23164. [DOI] [PubMed] [Google Scholar]
- 231.Beschin A., Bilej M., Magez S., Lucas R., De, Baetselier P. Functional convergence of invertebrate and vertebrate cytokine-like molecules based on a similar lectin-like activity. Prog Mol Subcell Biol. 2004;34:145–163. doi: 10.1007/978-3-642-18670-7_6. [DOI] [PubMed] [Google Scholar]
- 232.Bilej M., Josková R., Van den Bergh R., Procházková P., Šilerová M., Ameloot P., et al. An invertebrate TNF functional analogue activates macrophages via lectin–saccharide interaction with ion channels. Int Immunol. 2006;18(12):1663–1670. doi: 10.1093/intimm/dxl100. [DOI] [PubMed] [Google Scholar]
- 233.Beceiro A., Tomás M., Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26(2):185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dal Peraro M., van der Goot F.G. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol. 2016;14(2):77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 235.Parisi M.G., Parrinello D., Stabili L., Cammarata M. Cnidarian immunity and the repertoire of defense mechanisms in anthozoans. Biology. 2020;9(9):283. doi: 10.3390/biology9090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang Y. Why do we study animal toxins? Dongwuxue Yanjiu. 2015;36(4):183–222. doi: 10.13918/j.issn.2095-8137.2015.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Podobnik M., Anderluh G. Pore-forming toxins in Cnidaria. Semin Cell Dev Biol. 2017;72:133–141. doi: 10.1016/j.semcdb.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 238.Pinaud S., Tetreau G., Poteaux P., Galinier R., Chaparro C., Lassalle D., et al. New insights into biomphalysin gene family diversification in the vector snail Biomphalaria glabrata. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.635131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yue X., Zhang S., Wang H., Yu J., Peng Q., McFall-Ngai M., et al. The mud-dwelling clam Meretrix petechialis secretes endogenously synthesized erythromycin. Proc Natl Acad Sci USA. 2022;119(49) doi: 10.1073/pnas.2214150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dheilly N.M., Adema C., Raftos D.A., Gourbal B., Grunau C., Du Pasquier L. No more non-model species: the promise of next generation sequencing for comparative immunology. Dev Comp Immunol. 2014;45(1):56–66. doi: 10.1016/j.dci.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Loker E.S., Adema C.M., Zhang S.M., Kepler T.B. Invertebrate immune systems--not homogeneous, not simple, not well understood. Immunol Rev. 2004;198:10–24. doi: 10.1111/j.0105-2896.2004.0117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Venier P., Varotto L., Rosani U., Millino C., Celegato B., Bernante F., et al. Insights into the innate immunity of the Mediterranean mussel Mytilus galloprovincialis. BMC Genom. 2011;12:69. doi: 10.1186/1471-2164-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang L., Song X., Song L. The oyster immunity. Dev Comp Immunol. 2018;80:99–118. doi: 10.1016/j.dci.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 244.Cooper EL (Ed.). Advances in comparative immunology. Springer; 2018.
- 245.Cooper E.L. Comparative immunology. Curr Pharm Des. 2003;9(2):119–131. doi: 10.2174/1381612033392297. [DOI] [PubMed] [Google Scholar]
- 246.Schultz J.H., Adema C.M. Comparative immunogenomics of molluscs. Dev Comp Immunol. 2017;75:3–15. doi: 10.1016/j.dci.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hoffmann J.A., Reichhart J.M. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3(2):121–126. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 248.Chan J., Zhang W., Xu Y., Xue Y., Zhang L. Electroporation-based CRISPR/Cas9 mosaic mutagenesis of b-tubulin in the cultured oyster. Front Mollusk Breed Genet Improv. 2022;9 [Google Scholar]
- 249.Kamesh N., Aradhyam G.K., ManojN The repertoire of G protein-coupled receptors in the sea squirt Ciona intestinalis. BMC Evol Biol. 2008;8:129. doi: 10.1186/1471-2148-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gilmore T.D., Wolenski F.S. NF-κB: where did it come from and why? Immunol Rev. 2012;246(1):14–35. doi: 10.1111/j.1600-065X.2012.01096.x. [DOI] [PubMed] [Google Scholar]
- 251.Jiao Y., Gu Z., Luo S., Deng Y. Evolutionary and functional analysis of MyD88 genes in pearl oyster Pinctada fucata martensii. Fish Shellfish Immunol. 2020;99:322–330. doi: 10.1016/j.fsi.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 252.Zhao Q.P., Gao Q., Zhang Y., Li Y.W., Huang W.L., Tang C.L., et al. Identification of Toll-like receptor family members in Oncomelania hupensis and their role in defense against Schistosoma japonicum. Acta Trop. 2018;181:69–78. doi: 10.1016/j.actatropica.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 253.Nam B.H., Jung M., Subramaniyam S., Yoo S.I., Markkandan K., Moon J.Y., et al. Transcriptome analysis revealed changes of multiple genes involved in Haliotis discus hannai innate immunity during Vibrio parahemolyticus infection. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0153474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Buckley K.M., Rast J.P. Diversity of animal immune receptors and the origins of recognition complexity in the deuterostomes. Dev Comp Immunol. 2015;49(1):179–189. doi: 10.1016/j.dci.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 255.McTaggart S.J., Conlon C., Colbourne J.K., Blaxter M.L., Little T.J. The components of the Daphnia pulex immune system as revealed by complete genome sequencing. BMC Genom. 2009;10:1–19. doi: 10.1186/1471-2164-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Gerdol M., Venier P., Edomi P., Pallavicini A. Diversity and evolution of TIR-domain-containing proteins in bivalves and Metazoa: new insights from comparative genomics. Dev Comp Immunol. 2017;70:145–164. doi: 10.1016/j.dci.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 257.Bryant C.E., Monie T.P. Mice, men and the relatives: cross-species studies underpin innate immunity. Open Biol. 2012;2(4) doi: 10.1098/rsob.120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li Y.F., Liu Y.Z., Chen Y.W., Chen K., Batista F.M., Cardoso J.C.R., et al. Two toll-like receptors identified in the mantle of Mytilus coruscus are abundant in haemocytes. Fish Shellfish Immunol. 2019;90:134–140. doi: 10.1016/j.fsi.2019.05.001. [DOI] [PubMed] [Google Scholar]