Abstract
Palm sap sugar is a sweetener which is made from the sap or nectar collected from different varieties/species of palm trees. It has huge scope as an alternative sweetener in Indian market. It is a natural alternative to unhealthy cane sugar and is more beneficial for farmers as well. Some of its characteristic features are low GI value and its macro (Glucose: 0.49–86.90 g/100 ml, Fructose: 0.26–1.61, Sucrose: 5.30–27.00 g/100 ml) and micro (K: 65.28–1326.0, Na: 2.85–117.5, Mg: 0.54–31.00, Ca: 0.24–79.00 mg/100 ml) nutritional content. Palm sugar also has impact on colour, aroma and taste profile of the final product. The taste, sensory profile and nutritional attributes of palm sugar vary on the basis of its species, region of growth and climatic conditions. At present, traditional processing of palm sap leads to lower yield and higher expenses. There is huge potential in the field of development in processing techniques (Traditional processing, spray drying, membrane technology, and vacuum drying) to optimize the production of palm sugar. Palm sugar and other products from different parts of palm can be used to make a variety of other value-added products like toffees, chocolates, cola, toddy wine, candy, and palm vinegar etc. The purpose of this review paper is to summarise the composition of palm sap, distinctive qualities of the extracted sap, various production procedures, nutritional and physico-chemical properties of palm sugar, and the development of functional foods using palm sugar.
Keywords: Phytochemicals, Alternative sweetener, Tropical fruit, Health benefits
1. Introduction
Sweetness in food products has a powerful sensory appeal for people of all age groups, especially children and young adults. Along with this hedonic appeal, lesser cost and the ubiquitous availability of sweeteners that contain energy have led to a burgeoning demand for the same. Food choices are often directed by sweetness and familiarity, especially with infants and small children. As per clinical studies, the scientific basis of the sensory pleasure derived from tasting sweet foods is “the activation of pleasure-generating brain circuitry”. Research has shown that sweet taste preferences may even be expressed before birth. The ability of new-borns to differentiate between different levels of sweetness, followed by consumption of the solution that tastes the sweetest is further demonstration of the power sweet tastes have in controlling children’s behaviours. There are noticeable age-related differences as well when it comes to consumption of sugar. The taste and preference of sugar is significantly higher in younger children and adolescents than in adults. Sugar has also been reported to have pain reducing and analgesic effects. Although sweetness is largely acknowledged as a favoured hedonic attribute, a variety of additional factors such as chronic disease, pharmaceutical usage, heredity, ethnicity, race, chronic disease, addictions, and nutritional inadequacies influence sweet taste preference [1].
However, according to few studies the consumption of added free sugars is linked to a number of health risk factors (chronic diseases), including cancer, diabetes, obesity, cognitive decline and non-alcoholic fatty liver disease. Many studies have established the relation of reduced sugar intake with improved dietary quality and health. There is a healthy degree of scepticism about excessive sugar consumption, despite the fact that the available information is not clear. As a result, alternatives to cane sugar are being incorporated in diets [2].
There is an increasing public awareness about potential health impacts of added sugars in foods. This has led to a growing interest in “plant-derived, natural low-calorie or zero-calorie sweeteners” [1]. Low-calorie sweeteners like saccharine, cyclamates, aspartame and acesulfame K became popular in the 1980s. However, such synthetic sweeteners have been embroiled in controversies and conflicts as well including allegations of serious health dangers like bladder and liver toxicity, foetus malformation, carcinogenicity etc [1]. Even though investigations have shown that these sweeteners are generally harmless, there has still been a certain amount of consumer mistrust. As a result, some laws forbid the use of specific artificial sweeteners. Moreover, customers believe that naturalness is good for food as a whole. A survey on consumers has consistently shown that they have an increasing awareness of food additives and most often choose natural additives over their artificial alternatives [1,3,4]. Therefore, it may be inferred that natural sweeteners will be positively accepted by consumers as long as they have a sufficient level of hedonic appeal.
Sugar is manufactured from sap or nectar that is collected from the flowers of different types of palms, including the coconut palm, sugar palm, and nipa palm. In Asia, palm sugars are generally used as sweeteners for a very long time. Due to its natural origin, little processing, and healthiness, it is currently gaining favour on a global scale. It has lower glycemic index as compared to cane sugar. It also has nutritionally significant quantities of vitamins and minerals. Additionally, it shows antioxidant activity. It is minimally processed, unrefined and its natural forms contain dietary fibre. It also possesses antioxidant properties as 2,3- dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (DDMP) is also present. DDMP is not only known for its antioxidative properties but can also reduce the chance of colon cancer [5]. Thus, palm sap sugar can be used as an alternative to cane sugar because of its popularity and high production in South East and South Asia (Indonesia, India, Malaysia, Philippines, and Thailand etc.), leading to economic benefits. Secondly, its sugar content and aroma profile create distinct, unique characteristics, when added to food. Thirdly, its positive health effects make it a ready alternative to cane sugar [6].
It is predicted that by end of 2025, the global palm sugar market will be valued over 2,000 million USD. Its expected CAGR is around 3.4% over 2017–25 (the forecast period). The global palm sugar market is also predicted to reach 958,512 MT in terms of volume by the end of 2025 with a CAGR of 3.2%. At present, the market consists of a large number of regional and local players. Around 70–75% share of the market is held by local players. There is huge market potential in regions like Western Europe and North America because they are witnessing a rise in number of health-conscious individuals who crave for healthy and organic food alternatives. Due to the high cost of palm sugar, multinational companies hold a very small (5–10%) market share compared to local competitors. Indonesia is the largest producer of palm sugar. The annual output of India’s crude sugar is around 3,000,000 tons and around 10% of this output is derived from palms. At present, export from India is mostly to Belgium, U.K, Japan, France, Ireland, Italy, Canada, Germany, Australia, Philippines etc. The greatest opportunities of export can be in north Western Europe because the consumers in this region have high per capita income and relatively higher share of disposable income to spend on higher cost natural sugar alternatives and are willing to try out new and unique food products like palm sugar [7].
Existing literature in this field separately talk about specific palm specie(s) or specific production and processing techniques for palm sugar. Reviews also cover salient features of palm sap anthology beginning from production and processing to value addition [6,8,9]. The novelty of this review is that it collates the existing data and gives an in depth and comprehensive view of the journey of palm sugar from its sap stage to the finally marketed product. It also summarizes the sugar content of different palm varieties in Indian sub-continent and nutritional composition of the final palm sugar produced from different palm varieties. To the best of our knowledge, these aspects with regards to palm sugar have not been covered in any paper before.
This review discusses about the composition of palm sap, regulating factors, different sugar palm varieties in the Indian subcontinent and their characteristic features, the existing traditional production techniques of palm sugar, alternative technologies and innovations to optimize production, the nutritional composition and physical properties of palm sugar and health benefits related to the consumption of palm sugar. It also outlines the value-added products with palm, storage and preservation of palm sugar and future prospects of the industry in India.
2. Composition of palm sap
Nutritional composition of palm sap varies greatly depending upon factors the species, genus, geographical region of growth, tapping duration and variety. The content of water in the sap varies while the total sugar content is around 66% (Table 1). Out of the total sugars present in palm sap, sucrose is in largest amount followed by glucose, fructose, inositol and raffinose sugars in minor quantities. Sugar obtained from Borassus flabeliffer and Phoenix sylvestris sap is healthier than cane sugar because of higher calcium content (1.32 mg/100 ml) and protein content (3.63%) [10]. Several studies corroborate that sugars having higher sucrose levels are healthier. This is because sucrose has very less fattening effect when compared to starch or glucose. There is no weight gain reported even with an increase of energy consumption by 15%. Additionally, the glycemic index (GI) value of sugars rich in fructose and sucrose is low. Thus, palm sap has low GI value [11].
Table 1.
Composition of general palm sap.
| Component | Amount | Recommended daily intake (mg/day) | Reference |
|---|---|---|---|
| Ash | 1.8% | [[11], [12], [13], [14], [15], [16], [17], [18]] | |
| Moisture | 35.2% | ||
| Total Sugars | 9–18.6 g/100 ml | ||
| Glucose | 9.5%; 0.49–86.90 g/100 ml | ||
| Fructose | 4.8%; 0.26–1.61 g/100 ml | ||
| Sucrose | 37.9%; 5.30–27.00 g/100 ml | ||
| Maltose | <0.5% | ||
| Monohydrate lactose | <0.5% | ||
| Total sugars (in glucose) | 66.0% | ||
| Fat | 0.20% | ||
| Water soluble Vitamins | (mg/Kg) | ||
| C | <5 | 75–90 | |
| B1 | 0.20 | 1.1–1.2 | |
| B2 | <0.10 | 1.1–1.3 | |
| B3 | 31.7 | 14–18 | |
| B6 | <0.40 | 1.3–1.7 | |
| B9 | 0.10 | 0.4–0.6 | |
| B12 | 0.0049 | 0.00378–0.00594 | |
| Fat soluble vitamins | (mg/Kg) | ||
| A | <0.50 | 700-900 Retinol equivalent | |
| D | <5.00 | 0.01–0.02 | |
| E | <0.50 | 15–19 | |
| K | <0.001 | 0.122–0.138 | |
| Micro nutrients | mg/100 ml | ||
| Fe | 0.04–1.58 | 8.7–14.8 | |
| Zn | 0.013–0.71 | 8–11 | |
| Cu | 0.02–0.21 | 0.9–1.3 | |
| Mn | 0.009–2.23 | 1.8–2.3 | |
| Ni | 0.06 | 0.18–0.25 | |
| Macro nutrients | mg/100 ml | ||
| K | 65.28–1326.0 | 1600–2000 | |
| Na | 2.85–117.5 | <2300 | |
| Mg | 0.54–31.00 | 310–420 | |
| Ca | 0.24–79.00 | 700 |
Fresh palm sap is a hub for amino acids. The amino acids present in fresh palm sap are mainly asparagine and glutamine. These amino acids have polar side chains. Seventeen acids are present in palm sugar. Amino acids are building units of proteins and help in maintaining neutral pH. These amino acids play a prominent role in Maillard reaction, where free amino acids are released. Based on heating time and cooking temperatures, the amino acid substrates that are released may either catalyze sucrose to monosaccharides or take part in retro aldol reactions, producing C2–C5 dicarbonyl compounds. These dicarbonyl compounds react with amino acids to produce aldehydes and α-amino ketones. This is followed by chain reaction in the later stage including cyclization, dehydrations, retro aldolizations and isomerizations. The amino acids present in palm sap are tryptophan, phenylalanine, tyrosine, lysine, histidine, leucine, isoleucine, arginine, proline, aspartic acid, glutamic acid, valine, threonine, glycine, serine, and alanine. The amino acid profile of palm sap varies depending on the species. For example, the glutamic acid content of Cocos nucifera is 34.20 g/100 mL palm sap whereas Phoenix dactylifera contains only 1.18 g/100 ml concentration of glutamic acid. In general, Cocos nucifera has relatively higher amino acid content as compared to other palm saps. Phoenix dactylifera has relatively lesser amino acid content [6,11].
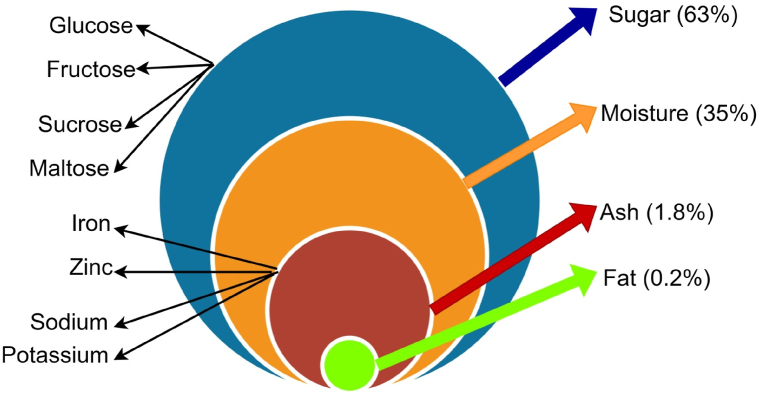
Palm sap also has a wide range of vitamins and minerals. Thirteen minerals were reported in palm sap (Fig. 1). These consist of eight microelements (P, Mg, Si, Cl, Na, S, Ca and K) and five oligoelements (Cu, Fe, Mn, Br and Zn). Vitamins contained in palm sap include vitamin C and B. For Borassus flabellifer, the amount of Vitamin C is around 13.25 mg/100 ml. Ascorbic acid has been used widely in food industries as an antioxidant agent. The maximum permissible limit for ascorbic acid is 550 ppm (for fruit products).
Fig. 1.
Palm sap composition (curated by the author).
Phenolic compounds are important because they impact the colour, nutritional, antioxidant and sensory properties of foods. Flavonoids are composed of dietary phenols (two-third) and the rest is composed of phenolic acid. Phenolic acids show antioxidant activities and numerous health advantages of phenolic acids have also been reported. Fresh sap contains around 0.33 g/L phenolic compounds [11]. The phenolic content rises during fermentation up to a peak of 1.24 g/L at 58 h, beyond which there is very little change. Phenolic compounds are also produced by activity of some microorganisms. Presence of gallic acid, protocatechucic acid, galangin, caffeic acid and p-coumaric acid has also been reported in palm sap [6,11].
3. Regulating factors for palm sap quality
To process the palm sap, first the nectar is tapped/harvested. Initially, the flowers are cut and then a bamboo container is used to collect the nectar. Natural fermentation may occur before the sugar processing, resulting in physical and microbiological changes. The fermentation process consists of three stages, firstly lactic acid fermentation, followed by alcoholic fermentation, and finally acetic acid fermentation. Thus, the different levels of fermentation impact the quality profile of the sugar produced by the farmer [6].
There is a chance of contamination of palm sap as the tapping process is done in an open environment. As a result of contamination, sugar gets consumed by microbes. This results in greater concentrations of acids. Afterwards, change in amount of amino acids and reducing sugar as well was noted. Hence, good hygiene, equipment, and sanitary facilities are very essential in maintaining palm sap quality.
The timing and duration of palm sap tapping also influences the composition of the sap. Even morning and afternoon sap have minor difference in composition. Sap tapping duration also influences physical, chemical and microbiological properties. Climatic conditions, the variety of palm tree, age of inflorescence and fertility of soil are other important factors that influence palm sap quality [6].
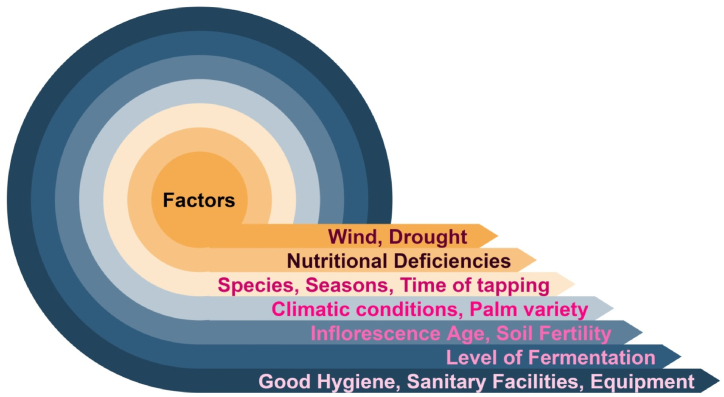
The yield of palm sap is influenced by various factors like “species, seasons, spathes of the same tree and time of tapping (morning/evening)”. The biotic and abiotic stress also heavily influences the yield of sap from palm trees. Yellowing affected trees show low or no sap production. Some abiotic factors like nutritional deficiencies, wind and drought also lead to the low yield. On the other hand, rains positively affect yield and greater yield is seen in monsoon season [10]. Fig. 2 describes the key factors affecting the palm sap quality.
Fig. 2.
Parameters for palm sap quality (curated by the author).
4. Sugar palm varieties in Indian subcontinent
In India, palm is majorly concentrated in north eastern and eastern Himalayan regions, the Andaman Nicobar forests and Western ghats. As many as thirteen genera and twenty-four species are found in Andaman Nicobar Islands. B. flabellifer, C. urens and P. sylvestris spread throughout peninsular India but the other species are limited to specific geographical locations and regions of India (Table 2). Nypa fruticans is solely found in certain mangrove species in salt marshes of Andaman Nicobar and Sundarban. Most forest palms see growth under the shade of dominant trees along streams and slopes in humid and warm conditions. Most forest palms grow in shady places under forest trees along hill slopes and streams in warm and humid environment. However, some like B. flabellifer and P. sylvestris grow under full exposure to sunlight. A typical characteristic of most palms is tall or dwarf woody trunk. Branching of aerial trunk is uncommon and is produced by damaging the terminal growing bud. This phenomenon has been reported in coconut palm (Cocos nucifera) and Palmyra palm (B. flabellifer). Regular annual flowering and fruiting is observed. But, the Corypha species shows flowering and fruiting only once in its lifespan after the attainment of complete vegetative growth (around 40 years) and then dies, leaving behind huge seed population to extend its progeny. Palms of this kind are known as monocarpic. In Caryota sp. (fishtail palm), basipetal flowering is observed, where the topmost bud turns into a huge drooping flowering axis while the other buds are placed on the successive basal older nodes of the trunk. This phenomenon is referred to as basipetal flowering. Of all Caryota sp., C. urens is the most widespread in India and C. mitis is restricted to Andaman-Nicobar islands [19].
Table 2.
Sugar palm varieties in Indian subcontinent.
| Palm variety | Geographical availability | Amount of sap produced (L/Palm/Day) | Sugar percentage | Reference |
|---|---|---|---|---|
| Areca catechu | Tropical rainforests of South, South east Asia and East Africa: India, Indonesia | 1.5–3 | [10] | |
| Arenga pinnata | Humid areas of tropical Southeast and South Asia (Papua New Guinea, Thailand, Sri lanka, Vietnam, Indonesia and India) | 3–6 | 10–20 | [10,20] |
| Arenga wightii | India | 2 | [10] | |
| Borassus aethiopum | Tropical zone from West Africa through India and Southeast Asia to New Guinea and Australia | 10 | 7–15 | [10,15] |
| Borassus flabellifer | Tropical countries of Asia (Sri Lanka, Nepal Malaysia, India, Indonesia, Vietnam, Philippines) | 6–10 | 9–17 | [10,20] |
| Caryota mitis | India, Brunei, Malaysia, Myanmar, Indonesia, Thailand, Vietnam | 1.5–3 | [10] | |
| Caryota urens | Humid areas of Southeast and South Asia (Indonesia, India, Sri Lanka, Philippines, Malaysia and Sri Lanka.) | 45 | 13–17 | [10,21] |
| Cocos nucifera | Common in tropical lands | 1.7–4.3 | 15–18 | [10,20] |
| Corypha umbraculifera | Tropical rainforest of South and Southeast Asia (Sri Lanka, Cambodia, Thailand, India and Myanmar) | 20 | [10] | |
| Corypha utan | Widely distributed in open and dry areas of Asia (Bangladesh, India, Philippines, Australia, Sri Lanka, Indonesia and Malayasia) | 45 | 17 | [10,22] |
| Nypa fruticans | Tidal areas with slow movement and soft mud for eg. Coastlines, mangrove forests and estuaries (Sri Lanka, Cambodia, Bangladesh, Nigeria, Philippines Thailand, Malaysia, Burma and Indonesia) | 1.3 | 15 | [10,20] |
| Phoenix sylvestris | Arid and desert areas of South Asia, Middle east and north Africa (Pakistan, Bangladesh, India, Iran, Arabian Peninsula) | 1.2–2.5 | 10–14 | [10,22] |
5. Processing of palm sugar
Palm sugar processing in micro and small-scale industries take place in traditional methods, while membrane technology, vacuum processing is the state-of-the-art technique, spray drying is also adapted for medium scale industries. Table 3, describes the key-feature, merits and demerits of the processing techniques available.
Table 3.
Palm sugar processing techniques.
| Processing techniques | Key feature | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Traditional processing | Long heating time (3–4 h), Dark brown sugar formed | Does not require skilling up of labour | Very dark colour, reduction in antioxidant and phenolic content, sucrose inversion, bitter taste of sugar | [20,24] |
| Spray drying | Short heating time at higher temperature | Low moisture content, convenient storage, higher phenolic content, higher antioxidant activity | Stickiness to spray dryer wall, undesirable lump formation, low yield | [20,24] |
| Membrane technology | Usage of ultrafiltration using membranes like ceramic membranes | Higher anti-oxidant activity | Chance of fouling, lesser phenolic content | [20] |
| Vacuum drying | Optimum efficiency at 40 °C for 3 h | Decreases phenolic compound and anti-oxidant loss, reduces sucrose inversion | Low efficiency, expensive | [27] |
5.1. Traditional processing
In the traditional method of palm sugar preparation, the processing is done by taking a large pan/vessel and then filtered palm sap is poured into it. The palm sap is heated and cooked by stirring and manual agitation for around 3–4 h until water evaporates and highly viscous and sticky appearance is attained. This appearance is indicative of high levels of brown sugar concentration (around 93 g/100 g). The sugar is brown in colour due to Maillard reaction [23]. This is poured into a coconut, wood or bamboo mold. The sugar cools and hardens within 1 h [20].
Disadvantages of the method include undesirable colour (dark brown). Also, antioxidant properties and phenolic content of palm sugar reduce due to long heat treatment. Since an open pan evaporator is used, the observed sucrose inversion is also greater. Also, hydroxyl methyl furfural is formed. The taste of the sugar formed is a little bitter [20,24].
5.2. Spray drying
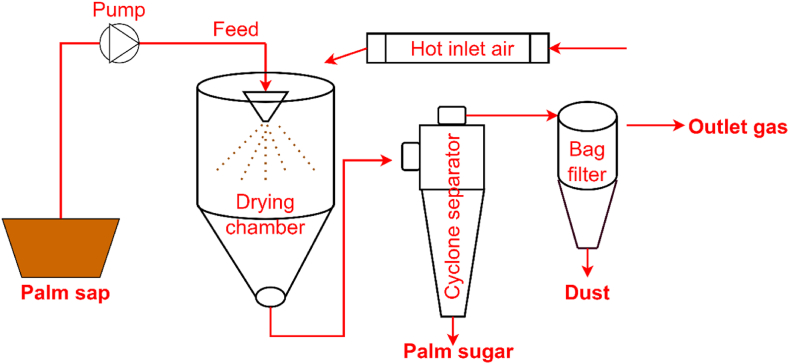
Filtered palm sap is placed in a spray dryer feed tank. The inlet and outlet temperature of the spray dryer are set. The optimum inlet and outlet temperatures are 220 °C and 85 °C respectively. It is observed that at lower inlet temperatures (140, 160, 180 and 200 °C), the sugar is prone to a sticky appearance and formation of big lumps. However, the dark colour of the sugar formed also increases with increase in temperature. The first stage is contact with the flowing air and heater. Palm sap starts flowing from the feed tank to the nozzle, where it gets atomized. It also comes in contact with heated air. Operating parameters that strongly affect palm sugar formation process are feed rate, inlet and outlet temperature, drying air temperature, colour of particles, size of particles, bulk density, nutrient and moisture content [20,24]. Fig. 3 illustrates an overview of palm sugar preparation via spray drying technology.
Fig. 3.
Spray drying technology for palm sugar preparation [25].
This method is highly effective under optimal conditions. The sugar formed by this method has lower moisture content than that formed by other drying techniques and can be more conveniently stored. The initial moisture content (wet basis) is around 1.06–2.95% and increases to 1.15–3.10% after 6 months of storage at a temperature of 30 °C. The antioxidant content is also maintained, unlike in the conventional method. Although this method uses a higher temperature than conventional heating, its phenolic content is higher than the latter. The reason behind this is short contact time between the hot air and sap droplets [20,24].
One disadvantage of this method is that the drying walls of the spray dryer become sticky due to adhesion-cohesion phenomenon. In cohesion, the particles stick to each other to form lumps. On the other hand, in adhesion, particle powders stick to the walls. Undesirable lumps are a major challenge in this process. The final yield of the product is also quite low [20,24].
5.3. Membrane technology
There are many fields in the sugar industry, where membrane technology is used. For example, juice treatment following liming (ultrafiltration), liquid thickness post evaporation is treated using ultrafiltration, molasses is treated using ultrafiltration or electrodialysis, and raw materials can be utilized by using ultrafiltration. The raw materials in case of juice are salt, protein, acid and pectin. Calcium oxide (CaO) can deposit organic acids and magnesium and ferri-hydroxides. UF membranes can remove high molecular weight compounds before the process of liming. They can decrease the usage of calcium oxide [20].
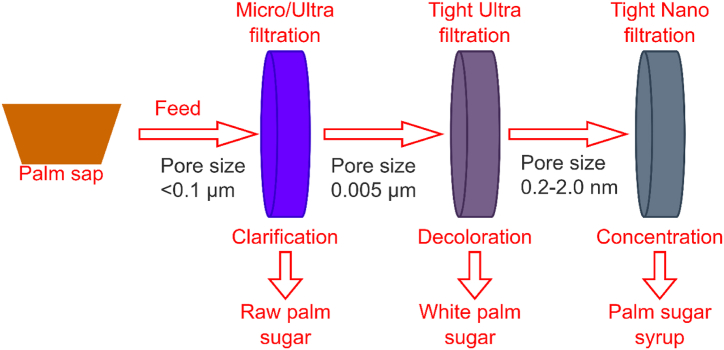
In membrane technology, a commonly used membrane is the ultrafiltration membrane. This method concentrates the solution and separates while also retaining desirable components. A membrane that is widely used in this method is the ceramic membrane. In this method, the sap undergoes microfiltration followed by ultrafiltration and finally, evaporation and crystallization [20]. Fig. 4 illustrates an overview of palm sugar preparation via membrane technology.
Fig. 4.
Membrane technology for palm sugar preparation [26].
There is a chance of fouling in this method. Raw materials may be affected which reduces the sugar content in sap syrup. There is reduction in sucrose and pectin content. The phenolic content of sugar produced by conventional method is 352.3 mg GAE/mL which is higher as compared to 328.36 mg GAE/mL of total polyphenols produced by membrane technology. The anti-oxidant activity of sugar from thermal process is 3.76 mg/mL whereas the antioxidant activity of sugar from ultrafiltration is 4.93 mg/mL. Since the antimicrobial content depends on total phenolic content, it can be inferred that antimicrobial content with membrane technology is greater than in thermal process [20].
5.4. Vacuum drying
Vacuum drying can also be used to produce palm sap sugar. This method reduces the loss of phenolic compounds and antioxidant characteristics. Increase in temperature and time are responsible for altering the physicochemical properties of vacuum-dried palm sap sugars. The optimum vacuum drying parameters is at 40 °C for 3 h. It also decreases the phenomenon of sucrose inversion. This method has low efficiency, high cost of set up and maintenance and requires skilled labour [27].
In a nutshell, there are basically four methods for making palm sap sugar. Even though the traditional method [20,23] is simple and inexpensive to use, they cannot be controlled. The best conventional method, in terms of enhanced antioxidant and phenolic content and superior storage capacity, is spray drying [20,24]. However, undesirable lump formation and stickiness to spray dryer wall should be taken care of by some post-processing techniques [28,29].
6. Proximate composition of palm sugar
The largest constituent of Borassus flabeliffer granulated sugar is sugar which makes up about 91.04–93.28% of the entire composition. It is observed that sugar content is higher when the temperature is more (100 °C) than at lesser temperatures (80 or 90 °C). The content of reducing sugar ranges from 5.55 to 6.61% (Table 4). Reducing sugar content reduces marginally when the drying time and temperature are increased. This may be because the reducing sugars take part in Maillard reaction which leads to browning of the sugar. Degradation of reducing sugar with time creates different MRPs and intermediates like melanoidins, organic acids and α-dicarbonyl compounds. The different values of reducing sugar and total sugar and the difference between the two indicate contamination with lactic acid bacteria. This is because sucrose can be converted fructose and glucose as well as to alcohols or organic acids. The mineral composition of Borassus flabeliffer includes high concentration of sodium, phosphorus, iron and potassium with potassium being the maximum in content. Palm from Palmyra palm also has significant vitamin content like riboflavin, thiamine, niacin and vitamin A [30].
Table 4.
Proximate composition of palm sugar (granulated and syrup).
| Borassus flabeliffer (granulated) | Nypa Fruticans (syrup) | Phoenix dactylifera (syrup) | Phoenix canariensis (syrup) | Arenga pinnata (granulated) | Reference | |
|---|---|---|---|---|---|---|
| Protein (g/100g) | – | 2.0–2.9 | 0.30–1.30 | _ | _ | [31,32] |
| Fat (g/100g) | – | – | - | <0.20 | 0.11 | [12,33] |
| Total sugar (g/100g) | 91.04–93.28 | 93.1–93.6 | 58.49–75.28 | 66.0 | 95.29 | [12,[30], [31], [32], [33]] |
| Reducing sugars (g/100g) | 5.55–6.61 | 10.0–19.6 | 6.65–8.10 | _ | 9.31 | [12,30,31,33] |
| Ash (g/100g) | – | 3.8–4.2 | 2.13–2.60 | 1.78 | 0.47 | [12,[31], [32], [33]] |
| Moisture (g/100g) | 2.91–5.12 | 28.9–31.5 | 20.79–39.06 | 35.3 | 4.11 | [12,[30], [31], [32], [33]] |
| 5-hydroxymethyl-furfural (HMF) (mg/100g) | 2.18–41.92 | 0.5–1.8 | _ | _ | _ | [30,31] |
| Total phenolic content (mg/100g) | 2.77–8.94 | 16.9–44.3 | 14.76–22.45 | _ | 194.3 | [[30], [31], [32], [33]] |
| Mineral | ||||||
| Ca (mg/100g) | - | 2–6 | 1.68–1.87 | 2.43 | - | [12,31,32] |
| Fe (mg/100g) | 0.188–0.205 | 1.5–2.3 | 0.55–1.11 | 1.16 | - | [12,[30], [31], [32]] |
| K (mg/100g) | 688.45–705.27 | 1026–1250 | 939.6–1149.3 | 451 | - | [12,[30], [31], [32]] |
| Na (mg/100g) | 23.10–24.50 | 34–47.4 | – | 59.7 | - | [12,30,31] |
| P (mg/100g) | _ | 52–71 | 57.2–67.1 | _ | - | [31,32] |
| Mg (mg/100g) | – | 26–34 | 26.9–30.7 | 17.4 | - | [12,31,32] |
| Si (mg/100g) | – | 21–43 | – | _ | - | [31] |
| Vitamin | ||||||
| A (mg/100g) | 1.54–1.95 | - | - | <0.05 | - | [12,30] |
| B1(mg/100g) | 0.66–1.06 | - | - | 0.02 | - | [12,34] |
| B2(mg/100g) | 0.04–0.07 | - | - | <0.01 | - | [12,34] |
| B3(mg/100g) | 1.88–2.19 | - | - | 3.17 | - | [12,34] |
| B5(mg/100g) | 0.40–0.74 | - | - | _ | - | [30] |
| B6(mg/100g) | 0.08–0.21 | - | - | <0.04 | - | [12,34] |
| Folic Acid (μg/100 g) | 2.51–3.33 | - | - | 0.001 | - | [12,34] |
| C (mg/100g) | 2.78–4.01 | - | - | <0.5 | 1.76 | [12,33,34] |
| D2(mg/100g) | 2.11–2.23 | - | - | <0.005 | - | [12,34] |
| E | 52.15–55.12 | - | - | <0.05 | - | [12,34] |
The moisture content of nipa palm syrups is in the range between 28.9 and 31.5%. The major constituent is sugar which makes up 93.1–93.6% of the entire composition. The protein levels in Nypa fruticans is 2.0–2.9% on dry weight basis. Ash content varies between 3.8 and 4.2%. The presence of fibers and lipids is negligible and at times, completely absent in the samples [31].
The most abundant component of date palm syrup is sugar and of the total sugar, reducing makes up about 10.63–11.37%. Ash content is between 2.13 and 2.16%. Potassium was the mineral with the highest presence in date palm [32].
The solid and moisture content in Phoenix canariensis syrup is 64.7% and 35.3% respectively while the ash content is 1.78%. Among the carbohydrates, sucrose, glucose and fructose are the most abundant in that order. Fats have negligible presence (less than 0.2%). Fat soluble vitamin presence is lesser than water soluble vitamin presence. The presence of fat-soluble vitamins in Phoenix canariensis is in the order: A, E, D and K respectively with D and K having a very minor presence. Niacin was the most abundant among the water-soluble vitamins. Potassium and iron have the highest concentration among macro and micro elements respectively [12].
7. Physical properties
7.1. Colour
Maillard reaction occurs during the production of palm sugar. Thus, a darker colour is formed. Caramelization and Maillard reaction result in an increase in a* value and the L* value decreases (L* a* b* colour system) because of greater heating time and temperature [6] (Table 5).
Table 5.
Colour characteristics of Palm sap/sap sugar.
| Palm sap/sap sugar | L* | a* | b* | C* | Reference |
|---|---|---|---|---|---|
| Arenga pinnatasap | 54.07–62.03 | −2.83 to −3.80 | 6.82 - 7.28 | 7.38–8.19 | [36] |
| Phoenix Dactylifera L. sap | 72.01 ± 0.07 | 0.64 ± 0.02 | 15.64 ± 0.02 | – | [37] |
| Borassus flabellifer Linn. sap | 61.49–87.53 | 1.46–3.25 | 13.10–19.31 | – | [38] |
| Granulated sugar (Arenga pinnata) | 24.6–29.0 | 1.9–6.7 | 2.8–7.3 | – | [35] |
| Coarse sugar (A. pinnata) | 48.53 | 12.8 | 33.63 | 35.98 | [39] |
| Coarse palm sugar | 37–42.1 | 17–20.9 | 60–64 | – | [3] |
L*: Lightness; a*: Redness; b*: Yellowness; C*: Chroma.
In Arenga pinnata, the production of granulated sugar is done by boiling palm sap up to a temperature of 127 ᴼC with total suspended solids more than 93%. It is followed by continuous stirring till the granules appear. In the entire process, L* values show a small increase at the start i.e., the values rise from 24.6 to 29.0. After this, the value shows a sharp rise to 42.6 with the production of granulated sugar. The a* and b* values show a different trajectory. The a* values initially increase from 1.9 to 6.7, followed by a small increase to 7.4. On the other hand, the b* value increases from 2.8 to 7.3, followed by a major increase to 13.5 [35].
7.2. Aroma and flavour
The aroma profile is alike to other thermally processed foods. In general, volatile compounds are mainly responsible for aroma and flavour. So, firstly, extraction of volatile compounds was done by using headspace solid phase micro extraction process. Many flavour compounds are formed in the course of heating as a result of Strecker degradation, Maillard reaction, and lipid oxidation. Additionally, to obtain the characteristic sweet, nutty and roasty aroma of palm sugar, the heating temperatures for extraction need to be considerably greater than 110ᴼC. After proper extraction, identification and quantification of these volatile compounds can be done by GC-MS [40].
The time-temperature curve of heating process of palm sap shows that in the first half hour, the temperature of sap drastically increases up to 76.9ᴼC. Then, the sample temperature increases up to 100 ± 5 ᴼC at the rate of 60-min heating for 2 h. The temperature then continuously increases and the final temperature of approximately 150 ᴼC at 4 h. Thus, there is a drastic increase of the sap temperature twice during the entire process. The first increase in the first 1 h of the process is partially due to water dehydration from the sap. This evaporation is important because it brings down the water activity to a moderate level, thus creating a fertile environment for inducing subsequent reactions. However, the second increment is related to formation of a volatile compound that imparts a sweet caramel and roasted nutty flavour to the final sugar product [40].
Some important volatile compounds identified during heating process for palm sugar production and their characteristic flavours are: 2- Methyl pyrazine and 5- Methyl furfural give a nutty flavour; 2,5 (6)-Dimethyl pyrazine gives a sweet, nutty and roasty flavour; 2-Ethyl pyrazine and 2-Ethyl-5-methyl pyrazine give a sweet and roasty flavour; 2,3-Dimethyl pyrazine gives a roasted nut, peanut, nutty, coffee and sweet flavour; 2-Ethyl-3,5-dimethyl pyrazine and 2,3-Diethyl-5-methyl pyrazine give an earthy and roasty flavour; 2-Furfural gives a burnt and smoky flavour; 4-Hydroxy-2,5-dimethyl-3(2H)-furanone gives a burnt sugar, strawberry, sweet, and caramel-like flavour [40]. The unique flavour of palm toddy comes from acetoin, 2-acetyl-1-pyrroline, ethylhexanoate and 3-isobutyl-2-methoxypyrazine. Acetoin gives a buttery flavour whereas 3-I’sobutyl-2-methoxypyrazine gives an earthy flavour [40].
Sc-, O- and N-heterocyclic compounds are there in palm sugar in good amount. As many as thirty-six volatiles have been reported in palm sugar. Six aromatics, four acids, two ketones, two aldehydes, five furans, fourteen pyrazines and two furanones have been identified. In separate studies, thirty and twenty-seven volatile compounds have also been reported [6].
7.3. Crystal behaviour
The factors that influence the crystallization of palm sugar are: reducing sugar, processing conditions, acidity, storage conditions and formulation. Crystallization affects the hardness of palm sugar. Hardness enables a material to resist deformation when penetration or compression is applied to it. Greater intermolecular bonding leads to greater hardness. The textural properties of palm sugar include stickiness and hardness. Stickiness is the phenomenon of a material adhering to a surface. This depends on temperature, water and food ingredients. In low moisture food, water-solid interaction is the prime reason of stickiness. Hardness and stickiness of palm sugar range from 30.83 to 69.00 N and 0.11–0.33 N respectively. The main sugar in palm sugar is sucrose. Sucrose gets hydrolysed to glucose and fructose during heating under acidic conditions. Glucose, reducing sugar and fructose can retard sugar crystallization and lead to low hardness of the final product [41].
When heated, the crystalline state of matter changes to the liquid state in a process known as melting. The melting point of a substance is lowered by the presence of impurities. The melting curve of various sugars showed that coarse sucrose had a sharper and symmetric peak with a smaller width value, in contrast to coarse palm sugar, which generally had a flat, broader, and asymmetric peak with larger width values. This is due to the fact that coarse sucrose had a high degree of purity and a melting temperature that was equivalent to sucrose tested by others, ranging from 185 to 190 °C [42,43]. Thermal behaviour of these sugars were studied by using differential scanning colorimeter having a refrigerated cooling system [3].
Scanning electron microscopy or SEM can also be used to examine the microstructure of various sugars. The existence of layers in the palm sap sugar revealed by SEM and caused the sugar crystals to clump together and form agglomerates. The relatively high moisture level and the presence of hygroscopic substances including fructose, glucose, and amorphous sugar may have contributed to this [3]. Another study [24], claimed that the rectangular shape of palm sap sugar is quite similar to the morphology of commercial jaggery. Instead of powder, they displayed a sticky, clumped result. Caramelization is most likely to blame for the agglomeration. According to reports, during caramelization hot sucrose is exposed to air which causes oxidation of carbohydrates, leading to browning. The operating temperature of the spray drier also has a significant effect on shape and morphology of palm sugar [44].
The XRD patterns demonstrate that the palm sap sugar produced by the spray dryer was primarily sucrose since they matched the XRD patterns of the pure sucrose described in the literature. The crystalline sucrose produced by the spray drier rather than the amorphous one was indicated by the sucrose XRD pattern. Crystalline sucrose results from quite high temperature operations (140–220 °C). Most likely, the low temperature and high moisture content of the sugar produced resulted in the low crystallinity of sucrose [24]. Temperature, sugar type, and supersaturation are a few of the parameters that have a significant impact on sugar crystallization. Typically, palm sap or syrup is heated until a crystalline structure forms in order to create palm sugar cake. The crystallinity of palm sugar cake is influenced by the characteristics of the palm sap, such as the amount of reducing sugar present and overall pH. When pH is lowered during palm sugar cake preparation, quantity of reducing sugar increases. Most sugars, in particular non-reducing sugars, can be made to crystallise. However, because anomers and ring isomers are present in the solution, making the reducing sugars intrinsically "impure," it can be challenging to produce some reducing sugars in crystalline form. Thus, the presence of reducing sugars could prevent the crystallization of non-reducing sugar. Ten samples of palm sugar cake were examined, and it was found that their crystallinity varied from 73.40% to 78.56% [41].
The level of Arenga pinnata sugar (AS) adulteration with coconut sugar was determined using Fourier transform near-infrared (FT-NIR) and infrared (FT-IR) spectroscopy. The absorbance spectra of the resultant mixes were measured by FT-NIR and FT-IR when different quantities of coconut sugar (CS) were added to AS, ranging from 0 to 100%. In comparison to the FT-NIR spectra, the FT-IR spectra produced greater determination coefficients and lower error values overall. The outcomes support the possible application of FT-IR spectroscopy for CS adulteration in AS detection [39]. It was observed that the peak absorption of functional groups in palm sugar from a 220 °C spray dryer temperature was comparable to that of commercial jaggery by using FT-IR [24].
8. Bioactive composition of palm sugar
8.1. Phenolic compounds
Antioxidants are molecules (exogenous or endogenous) that diminish oxidative stresses and their results. Phenolic compounds are the most abundant antioxidants which can be found in fruits and plants. An aromatic ring is present in phenol in hydroxylated form. This is what imparts redox properties to phenolic compounds. The phenolic compounds benefit overall health of humans and do not show any harmful side effects.
Phenolic components are present in the palm sap itself and the content increases during heating because bound polyphenols are released in this case. It has been observed from different studies that phenolic content is highest at around 80ᴼC. But when the temperature is increased to 90 or 100ᴼC, the total phenolic content reduces because of its destruction during the heating. Phenolic content and antioxidant activity are closely related. In relation to the phenomenon of free radical scavenging, mechanisms affect antioxidation potential of the palm sugars. It was reported that DPPH % was highest for (100ᴼC, 90 min) and lowest for (80ᴼC, 60 min). The reason for the former having a higher DPPH activity is because of more caramelization and Maillard reaction products. Also, samples prepared at greater temperatures and for longer periods of time show greater FRAP activity. However, Palm sugar syrup shows greater amount of phenolic content than palm sugar powder and both show higher amount of phenolic content than refined cane sugar [30].
The acid predominantly present in the palm varieties is p-hydroxybenzoic acid. Additionally, ferulic acid and tiny amounts of p-coumaric acid are also present. Gallic, vanillic, syringic and catechuic acids are some other phenolics that are derivatives of benzoic acid and have been found in palm varieties. Their aldehydic forms like vanillin (present in coconut); syringaldehyde, protocatechuic aldehyde and p-hydroxybenzoic aldehyde (present in leaves and some other tissues) have been detected as well. Ferulic, sinapic, chlorogenic and caffeic acids are present in mesocarp of several varieties. In E. guineensis, apart from Vanillic, p-Coumaric p-Hydroxybenzoic, Protocatechuic, Gallic, Syringic, Caffeic, and ferulic acids, caffeoyl shikimic acid isomers constitute the major component of phenolic compounds [[45], [46], [47], [48], [49], [50]]. These isomers have also been found in ripened fruits and seeds of fruits in P. dactylifera and has also been detected in flowers of palm. Many of these phenolic acids and their derivatives show antifungal and antibacterial properties apart from antioxidant activities [[51], [52], [53], [54], [55], [56], [57], [58], [59]]. Another bioactive compound, resveratrol and its methoxy analogs also show antioxidant properties and studies have indicated preventive effects on cancer as well. Fruits of E. oleracea [[60], [61], [62], [63], [64]] O. bataua and E. edulis [[65], [66], [67]] have shown the presence of this compound. An unrecognized stilbene has been isolated from the fruit of H. thebaica [68]. Derivatives of cinnamic acid and p-hydroxybenzoic acid have been detected in several types of palms (Table 6).
Table 6.
Benzoic acids, cinnamic acids and anthocyanins in palms.
| Species | Plant Part | Benzoic acids | Cinnamic acids | Anthocyanins | References |
|---|---|---|---|---|---|
| Areca catechu L. | fruit | p-Hydroxybenzoic | p-Coumaric, Ferulic | – | [45,70] |
| Nypa fruticans | fruit | p-Hydroxybenzoic, Protocatechuic, Gallic | Chlorogenic | – | [71] |
| Borassus flabellifer | fruit | p-Hydroxybenzoic | p-Coumaric, Ferulic | – | [45] |
| Euterpe edulis | fruit | p-Hydroxybenzoic, Protocatechuic, Vanillic, Gallic, Syringic | p-Coumaric, Caffeic, Ferulic, Sinapic, Chlorogenic, Stilbene | Cyanidin-3-glucoside, Cyanidin-3-rutinoside | [[65], [66], [67]] |
| Butia capitata | fruit | _ | Chlorogenic | – | [72] |
| Butia odorata Barb. Rodr | fruit | p-Hydroxybenzoic, Vanillic, Gallic | p-Coumaric, Caffeic, Ferulic, Chlorogenic | Cyanidin-3-glucoside, Cyanidin-3-rutinoside | [69] |
| Bactris setosa Mart. | fruit | p-Hydroxybenzoic | Stilbene | – | [73] |
| Euterpe oleracea | pulp | p-Hydroxybenzoic, Protocatechuic, Vanillic, Gallic, Syringic | p-Coumaric, Caffeic, Ferulic, Chlorogenic | Cyanidin-3-glucoside, Cyanidin-3-rutinoside | [[60], [61], [62], [63], [64]] |
| Hyphaene thebaica | fruit | _ | Caffeic, Chlorogenic, Stilbene | – | [68] |
| Mauritia flexulosa L. f. | fruit | Protocatechuic | p-Coumaric, Caffeic, Ferulic, Chlorogenic | Cyanidin-3-glucoside | [74,75] |
| Mauritia flexulosa L. f. | leaves | _ | Caffeic, Chlorogenic | – | [75,76] |
| Phoenix dactylifera L. | date fruit | p-Hydroxybenzoic, Protocatechuic, Vanillic, Gallic, Syringic | p-Coumaric, Caffeic, Ferulic, Sinapic | Cyanidin-3-glucoside | [[51], [52], [53], [54], [55], [56], [57], [58], [59]] |
| Oenocarpus distichus Mart. | Bacaba-de-leque fruit | p-Hydroxybenzoic, Vanillic, Syringic | p-Coumaric, Caffeic, Ferulic, Sinapic, Chlorogenic | – | [77] |
| Oenocarpus distichus Mart. | Patawa fruit | Syringic | Caffeic, Chlorogenic, stilbene | Cyanidin-3-rutinoside | [78] |
| Cocos nucifera L. | Husk fibre | p-Hydroxybenzoic, Vanillic, Gallic, Syringic | Ferulic, Chlorogenic | – | [[79], [80], [81], [82]] |
| Cocos nucifera L. | oil | – | p-Coumaric, Caffeic, Ferulic | – | [83] |
| Elaeis guineensis Jacq | fruit | p-Hydroxybenzoic, Protocatechuic, Vanillic, Gallic, Syringic | p-Coumaric, Caffeic, Ferulic | Cyanidin-3-glucoside | [[45], [46], [47], [48], [49], [50]] |
8.2. Pigments
The fruit, E. precatoria is rich in anthocyanin and studies have shown that anthocyanin can prevent Alzheimer. Cyanidin-3-O-rutinoside and cyanidin-3-O-glucoside are mainly responsible for pigmentation in fruits of palms. Peonidin-3-O-glucoside, peonidin-3-O-rutinoside and pelargonidin-3-O-rutinoside are present in the fruits of E. edulis [[65], [66], [67]]. Similarly, fruit of Butia odorata [69] and pulp of Euterpe oleracea are rich in anthocyanin mainly Cyanidin-3-glucoside and Cyanidin-3-rutinoside [[60], [61], [62], [63], [64]].
8.3. Flavones, flavonols and flavanols
Flavonoids have polyphenolic structures. They are actually a part of plant secondary metabolites. Flavonoids have fifteen carbon skeletons where two aromatic rings are linked by a 3-carbon chain, creating a heterocyclic oxygenated ring. Flavonoids are classified into flavones and flavan-3-ols. In flavan-3-ols, C3 position has a hydroxyl group and in flavones, the position between C3 and C2 is unsaturated and C4 has a carbonyl group. They have positive health effects like antioxidant effects. They have protective impact like prevention of diseases like Alzheimer’s disease, cancer, atherosclerosis etc. Palm sugars have high flavonoid content. Palm sugar syrup has higher flavonoid component as compared to palm sugar powders [84]. The most commonly prevalent components of palm leaf extracts are tricin and flavon-C-glycosides. Tricin has anti-viral, immunomodulatory, antioxidant, anti-inflammatory, anti-tubercular, anti-cancer and anti-ulcerogenic nutraceutical properties. The major constituent of several species of Attalea genus are free tricin, tricin-7-glycosides, flavone C-glycosides and tricin-5-glycosides. B. capitata leaves contain luteolin 7-O-glucoside and tricin 7-O-rutinoside.
The characteristic feature of flavonols are O-glycosidic linkages. Free isorhamnetin and quercetin occurs more commonly in cocosoid palm leaves, as compared to the rest of palmae family. Quercetin, along with its derivatives, has been garnering attention because of its antiviral, anti-carcinogenic, anti-inflammatory and antioxidant effects [85]. The different flavones flavonols detected in various palms are listed in Table 7.
Table 7.
Flavones, flavonols and flavanols present in palm leaves and fruits.
| Palm variety | Flavones | Flavonols | Flavan-3-ols | References |
|---|---|---|---|---|
| Leaves | ||||
| B. capitata | tricin 7-O-rutinoside, luteolin 7-O-glucoside | Isorhamnetin-3-O-rutinoside, quercetin-3-O-rutinoside, kaempferol-3-O-rutinoside | [94] | |
| M. flexuosa | apigenin 6-C-arabinoside, luteolin 8-C-glucoside, apigenin 6-C-arabinoside-8-C-glucoside, tricin 7-O-rutinoside, luteolin 6-C-glucoside | Rutin, nicotiflorin | [75,76] | |
| Phoenix loureiroi | tricin 7-neohesperidoside, apigenin, tricin 7-O-glucoside, isoorientin, luteolin 7-neohesperidoside, orientin 7-O-glucoside, 6-C-glucoside | Quercetin-3-glucoside | [95] | |
| Phoenix canariensis | tricin 7-neohesperidoside, flavone C-glycosides, tricin 7-glycosides, luteolin 7-rutinoside | [84] | ||
| Fruits | ||||
| Areca catechu L. | Catechins | [45,70] | ||
| Butia odorata | hesperitin | Catechins, Procyanidins | [69] | |
| P. dactylifera | luteolin, apigenin, diosmetin 7-O-arabinosyl apioside, diosmetin 7-O-apioside | Quercetin-3-O-rhamnoside, quercetin, rutin | Catechins, Procyanidins | [[51], [52], [53], [54], [55], [56], [57], [58], [59]] |
| M. flexulosa | Chrysoeriol 8-C-glucoside, apigenin 8-C-glucoside, | [74,75] | ||
| E. oleracea | isovitexin, apigenin 6,8-C-hexoside, luteolin 7-O-glucoside, orientin, apigenin 6-C-pentoside-8-C-hexoside, isoorientin, 5,4’-dihydroxy-7,3’,5’-trimethoxyflavone, luteolin 7,3’-dimethyl ether | [[60], [61], [62], [63], [64]] | ||
| H. thebaica | luteolin O-rutinoside, chrysoeriol O-rutinoside | Narcissoside, isoquercitrin | [68] | |
| E. edulis | 6-O-Methylapigenin | Catechins | [[65], [66], [67]] | |
Dihydroflavonoids have also been reported in palm species. The trunk and leaves of M. flexuosa show the presence of naringenin [86]. Naringenin, along with dihydrokaempferol and dihydrotricin are present in the stems of Calamus formosanus. Becc. The fruits of E. oleracea show the presence of eriodictyol, dihydroquercetin, aromadendrin 3-O-glucoside, aromadendrin and taxifolin deoxyhexose. Taxifolin and aromadendrin have also been found in the pulp of E. edulis. Recently, pinocembrin has been detected in the fruits of B. odorata [84].
Procyanidins and flavan-3-ols are present in different parts of different palm species [87]. Flavan-3-ols like epicatechin and catechin show a positive effect on coronary heart health [88]. Epigallocatechin gallate shows ant-inflammatory, antioxidative and antiproliferative effects [89]. E. guineensis leaf extract contains catechin, epigallocatechin, epicatechin, epicatechin gallate and epigallocatechin gallate [47]. Catechin-based procyanidins with varying degrees of polymerization are present in A. catechu nut. The antioxidant activity is higher when the polymerization degree is greater [90]. The fruits of P. dactylifera contain procyanidin oligomers whereas the seeds contain procyanidin dimers and trimers. The seeds also contain epicatechin and catechin [91]. Presence of these flavan-3-ols is similarly observed in the seed of S. coronata and husk fiber of coconut [84].
Pinoresinol, lariciresionol, dihydroconiferyl alcohol and syringaresinol are lignan precursors, present in the fruits of E. oleracea [92]. Salcolin A and calquiquelignan A were detected in the Calamus formosanus stems [93]. The complete chemical profile of many lignans found in palms has not been established yet [84].
8.4. Volatiles
Thirty-eight volatile compounds have been identified in palm sugar. The volatile compounds present in palm sugar are further subdivided into pyrazines, ketones, aldehydes, carboxylic acids and furan derivatives. Palm sugars having a higher volatile content have roasty and nutty taste. Also, these volatiles impart sweet aroma to the palm sugar. Furaneol has a kind of caramel like flavour. Thus, the sweet caramel like qualities of palm sugar may be due to these volatiles [96]. Palm sugars with higher pyrazine contents are shown to have increased roasty and nutty flavour but have reduced burnt and caramel like flavours. Thus, every volatile compound can possibly and distinctly reduce, enhance or cover the sensory qualities of food products like palm sugars [34].
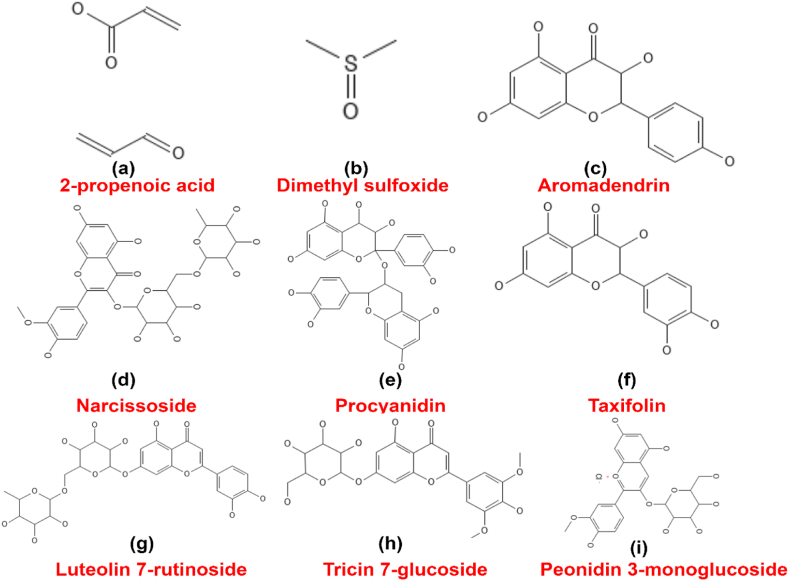
The volatile components that are present in greater quantity in palm sugar syrup include 2,3-dihydro-3,5-dihydroxy-6-methyl-4 H-pyran-4-one, R-(R’,R’)-2,3-butanediol, 2-propenoic acid, S-(R, R’)-2,3-butanediol, benzoic acid and dimethyl sulfoxide. On the other hand, the volatile components that are present in lower amounts include 3-methyl-1,2-cyclopentanedione, 4,5-dihydro-2-methyl-3(2H)-furanone and 5-methyl-2-pyrazinylmethanol. S-(R, R’)-2,3-butanediol is believed to have a role in imparting the typical flavour associated with palm syrup [34,96]. Nine kinds of acids are present in palm syrup with 2-propenoic acid having the highest concentration among them all in both ultrafiltration and thermal processes [34,96]. Though acid content tended to show a reduction at greater temperatures, several volatile components were retained which do not impart any aroma. On the other hand, the 12 types of ketone compounds identified increased in content when the temperature was raised [34]. Ketone compounds impart different flavours and odours to the palm syrup e.g., 2,3-dihydro-3,5-dihydroxy-6-methyl-4 H-pyran-4-one produces caramel, maple type, sweet odour. The volatile compounds that contain sulphur show anti-cancer and antioxidant characteristics but also produce an untoward odour so excessive amounts are undesirable [97,98]. The number of volatiles was higher with thermal process as compared with ultrafiltration process [34]. Fig. 5 represents the important bioactive components present in palm sap.
Fig. 5.
Potential bioactive compounds present in palm sap, a) 2-propenoic acid; b) Dimethyl sulfoxide; c) Aromadendrin; d) Narcissoside; e) Procyanidin; f) Taxifolin; g) Luteolin 7-rutinoside; h) Tricin 7-glucoside; i) Peonidin 3-monoglucoside (curated by the authors themselves).
9. Value added product with palm
9.1. Palm sugar
Palm sugar is a popular alternative sweetener in countries like Indonesia, India, Philippines and Thailand. It is traditionally produced from palm sap locally. Philippines and Indonesia are leaders in palm sugar production globally [6]. Palm sugar not only adds sweetness but it also impacts the colour, texture and flavour of the food product [99]. Palm sugar has rich nutritional profile consisting of amino acids, antioxidants, dietary fibres and vitamins [100]. Thus, from a health perspective, palm sugar has a wide array of nutritional benefits.
9.2. Toddy
Microorganisms naturally present in palm sap initiate fermentation of the sap into a white milky substance, commonly known as toddy or palm wine. Palm wine has alcohol content of around 4–4.5% [101]. It is a popular drink in tropical areas of South America, Africa and Asia. The most popular toddy drinks in India are made from coconut palm, silver date palm and palmyra palm [102]. Palm wines display a wide diversity in tastes and sensory profile because of influence of species and geographical location [103]. Toddy also shows some antibacterial and nutraceutical properties.
9.3. Palm jaggery
Palm jaggery is colloquially known as palm gur. Jaggery is produced from unfermented sap. It has an intense and earthy taste, very similar to that of chocolate. In the beginning, earthen pots are treated with calcium hydroxide and palm sap is collected here. The sap then undergoes sedimentation and is filtered after that. Next, the sap is shifted to a furnace where boiling takes place at a temperature of 110 °C. This leads to the formation of a thick sticky fluid that is kept in moulds to solidify [104]. Palm jaggery is expensive because of its mineral rich nutritional composition and therapeutic effects. Additionally, it is deficient in sodium and rich in potassium, ascorbic acid and vitamin B12. This composition leads to reduction in possibility of health ailments like oedema, anaemia and hypertension [105].
9.4. Treacle
To prepare a treacle, the sap is first concentrated by heating at a temperature below 107 °C up to approximately one-sixth of its volume. A viscous syrup is subsequently formed which is dark and thick in appearance. A temperature more than 107 °C will lead to darkening and caramelization of the sugar. Treacle is an excellent alternative to sugar [106].
9.5. Palm toffee
The ingredients to make palm toffee are palm fruit pulp, glucose, skimmed milk powder, sugar, starch and refined wheat flour. The aforementioned ingredients are mixed thoroughly by continuously stirring for 40 min. After heating is completed, the mixture is spread over a tray. The tray should be made of aluminium and butter or oil must be spread over it. After 24 h, toffees are cut into desirable shapes and sizes and packaged accordingly [104].
9.6. Palm sap vinegar
Palm vinegar has several applications as food preservatives, additives and medicinal agent [107]. It is widely popular in countries of pacific islands and Asia. For the production of palm vinegar, alcoholic and acetic acid fermentations are done successively. The produced acetic acid gives characteristic odour and flavour to the vinegar. Palm vinegar has also been associated with hypoglycaemic effect [10,108].
9.7. Palm tamarind candy
To make palm tamarind candy, heating of palm syrup is done for 2 h. This creates syrup with consistency, akin to that of honey. The syrup is pored over mud pots and seedless, shelled, dry and ripe tamarind fruits are added to the neera. The pots are clamped strongly with clothes and preserved in a dry and cool place for 130–180 days. Crystallization of sugar takes place in the seams of tamarind to transform the fruits into tasty candies [104].
9.8. Palm cola
The ingredients to make palm cola are palm sugar, citric acid, food colour and cola concentrate. Addition of palm sugar to milk is followed by heating at 110–115 °C for purification. After the solution is cooled, cola essence is added at the rate of 250 ml/1000 bottles [106,109].
9.9. Chocolate products
Investigations were done on milk chocolate's capacity to withstand heat upon incorporation of fat and palm sap sugar [110]. The durability of chocolate against storage temperature was assessed using the hardness of the chocolate as a measure. Three levels of fat content—30%, 32%, and 34%—as well as five levels of palm sap sugar—0%, 25%, 50%, 75%, and 100%—were employed in this investigation. The findings demonstrated that milk chocolate's hardness was considerably influenced by fat level, the amount of palm sap sugar, and their interaction. The amount of moisture in chocolate caused its hardness to rise as the proportion of palm sap sugar did. Conversely, the increase in fat content lowered the hardness of milk chocolate at the same level of palm sap sugar percentage [110]. Another study looked at the effects of drying time and the ratio of palm sugar to sucrose (P:S) on the properties of cocoa drink powder produced by a continuous-type steam jet agglomerator. The outcome shown that as the proportion of palm sugar and drying time increased, so did the solubility, mass, and tapped density. However, as the percentage of palm sugar grew, the lightness tended to be equivalent and the moisture content after drying dropped [111]. Dark chocolate products with a unique flavour or aroma may be created by replacing the sucrose in the chocolate with palm sap-based sugar [112]. Additionally, sugar-sweetened chocolate made from palm sap is better for diabetics as it contains additional minerals, vitamins, antioxidative and anti-carcinogenic substances compared to ordinary chocolate. High amounts of pyrazine-based chemicals and the presence of 2,3-dihydro-3,5-dihydroxy-6-methyl-4(H)-pyran-4-one (DDMP), which was absent from sucrose-sweetened dark chocolate, were both distinguishing characteristics of the olfactory profile of sugar-sweetened chocolate made from palm sap [4].
10. Storage and preservation of palm sugar
To maintain and preserve the quality of a food product, it is vital to keep in check the decay and decline of sensory and nutritional aspects of stored food. This is to be done by extending shelf life of food products and reducing non enzymatic browning. For example, nonenzymatic browning reactions are heavily influenced by processing techniques used for coconut palm sugar syrup. Adequate steps need to be taken at both the production, as well storage stage. It has been reported that microbes which cause deterioration of jaggery blocks grow the fastest at 30 °C and moisture content of around 10%. For storage, the ideal moisture content is 7–8% and relative humidity 40–45%. During rainy season, crystallinity, colour and sweetness of blocked sugar remain unaffected if it is kept in air tight containers. Sugar blocks with greater moisture content are more prone to deterioration. An alkathene film can be used to wrap sugar blocks. A plastic pouch can be used to cover the film and a paper box used for final packaging. The above steps can ensure quality is retained better for long periods of time [27].
To extend the shelf life of fresh palm sap, sterilization or pasteurization may be done. But for such thermally processed palm sap, there is a chance of murkiness developing, while being in storage. Interaction between proteins and polyphenolic compounds creates insoluble particles that get dispersed throughout the sap. Formation of brown pigments also contribute to cloudiness. So, gelatin, polyvinylpolypyrrolidone, chitosan and bentonite may be added to enhance quality. Similar clarifying agents can be used for palm syrup as well but the biggest challenge concerning palm syrup is the development of crystals. This can happen within one or two days. Ensuring high viscosity or adding sugars like corn syrup can inhibit crystallization. Also, appropriately formulating the product can kinetically inhibit crystal formation. Significant inhibition of nucleation is necessary to avoid undesirable crystal formation [113].
Heating increases viscosity and decreases water activity. Cubic and powdered sugars are produced by evaporation sap till a water activity lower than 0.60 (for powdered sugar) is attained. The hygroscopic nature of palm sugars requires dry and cool storage conditions, as well as appropriate packaging like food grade moisture absorbers. Maltodextrin and other bulking agents may also be added [113].
11. Conclusion and future perspective
Palm sugar is a natural and healthy alternative to the conventional cane sugar as well as artificial sweeteners. It has a wide variety of functional and nutraceutical properties (5-hydroxymethyl-furfural (HMF): 2.18–41.92 mg/100 g, Total phenolic content: 16.9–44.3 mg/100 g, Vit A: 1.54–1.95 mg/100 g, Vit B: 1.88–2.19 mg/100 g, Vit C: 2.78–4.01 mg/100 g, Folic Acid: 2.51–3.33 μg/100 g, Vit D: 2.11–2.23 mg/100 g, Vit E: 52.15–55.12 mg/100 g). Processing of palm sugar which is done minimally helps in retention of different phytonutrients. There are a range of bioactive components like polyphenols, antioxidants, flavonoids and volatile compounds present in palm sugar, which are both beneficial to health and impart characteristic sensory attributes as well.
In India and other countries, the production and processing of palm sugar is still localized and small scale (3 lakh tonnes). Moreover, commercial scale production of palm sap incurs huge expenses. More modern technologies need to be developed to enable large scale production of palm sugar to meet national and international market standards.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Contributor Information
Tanmay Sarkar, Email: tanmays468@gmail.com.
Runu Chakraborty, Email: crunu@hotmail.com.
References
- 1.Drewnowski A., Mennella J.A., Johnson S.L., Bellisle F. Sweetness and food preference. J. Nutr. 2012;142:1142–1148. doi: 10.3945/jn.111.149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rippe J.M., Angelopoulos T.J. Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients. 2016;8 doi: 10.3390/nu8110697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saputro A.D., Van de Walle D., Dewettinck K. Physicochemical properties of coarse palm sap sugars as natural alternative sweetener. Food Biosci. 2020;38 doi: 10.1016/j.fbio.2020.100780. [DOI] [Google Scholar]
- 4.Saputro A.D., Van De Walle D., Philip R., Amoafo M., Claudia M., Nathalie D., Van Durme J., Dewettinck K. Quality attributes of dark chocolates formulated with palm sap - based sugar as nutritious and natural alternative sweetener. Eur. Food Res. Technol. 2017;243:177–191. doi: 10.1007/s00217-016-2734-9. [DOI] [Google Scholar]
- 5.Saputro A.D., Van de Walle D., Kadivar S., Bin Sintang M.D., Van der Meeren P., Dewettinck K. Investigating the rheological, microstructural and textural properties of chocolates sweetened with palm sap-based sugar by partial replacement. Eur. Food Res. Technol. 2017;243:1729–1738. doi: 10.1007/s00217-017-2877-3. [DOI] [Google Scholar]
- 6.Saputro A.D., Van de Walle D., Dewettinck K. Palm sap sugar: a review. Sugar Tech. 2019;21:862–867. doi: 10.1007/s12355-019-00743-8. [DOI] [Google Scholar]
- 7.Kearney J. Food consumption trends and drivers. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2010;365:2793–2807. doi: 10.1098/rstb.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naknean P., Meenune M. Impact of clarification of palm sap and processing method on the quality of palm sugar syrup (Borassus flabellifer Linn.) Sugar Tech. 2015;17:195–203. [Google Scholar]
- 9.Ben Thabet I., Attia H., Besbes S., Deroanne C., Francis F., Drira N.-E., Blecker C. Physicochemical and functional properties of typical tunisian drink: date palm sap (Phoenix dactylifera L.) Food Biophys. 2007;2:76–82. [Google Scholar]
- 10.Sarma C., Mummaleti G., Sivanandham V., Kalakandan S., Rawson A., Anandharaj A. Anthology of palm sap: the global status, nutritional composition, health benefits & value added products. Trends Food Sci. Technol. 2022;119:530–549. doi: 10.1016/j.tifs.2021.12.002. [DOI] [Google Scholar]
- 11.Hebbar K.B., Pandiselvam R., Manikantan M.R., Arivalagan M., Beegum S., Chowdappa P. Palm sap—quality profiles, fermentation chemistry, and preservation methods. Sugar Tech. 2018;20:621–634. doi: 10.1007/s12355-018-0597-z. [DOI] [Google Scholar]
- 12.Luis G., Rubio C., Gutiérrez A.J., Hernández C., González-Weller D., Revert C., Castilla A., Abreu P., Hardisson A. Miel de palma; composición nutricional de un edulcorante natural. Nutr. Hosp. 2012;27:548–552. doi: 10.3305/nh.2012.27.2.5586. [DOI] [PubMed] [Google Scholar]
- 13.Barh D., Mazumdar B.C. Comparative nutritive values of palm saps before and after their partial fermentation and effective use of wild date (Phoenix sylvestris Roxb.) sap in treatment of anemia. Res. J. Med. Med. Sci. 2008;3:173–176. [Google Scholar]
- 14.Victor I., Orsat V. Characterization of Arenga pinnata (palm) sugar. Sugar Tech. 2017;20 doi: 10.1007/s12355-017-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zongo O., Tapsoba F., Leray F., Bideaux C., Guillouet S., Traoré Y., Savadogo A. Nutritional, biochemical and microbiological composition of Borassus aethiopum Mart. sap in Burkina Faso. J. Food Sci. Technol. 2020;57:495–504. doi: 10.1007/s13197-019-04078-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranasinghe P., Premakumara G.A.S., Wijayarathna C.D., Ratnasooriya W.D. Antioxidant activity of Caryota urens L. (Kithul) sap. Trop. Agric. Res. 2015;24:117. doi: 10.4038/tar.v24i2.7997. [DOI] [Google Scholar]
- 17.Ikegwu T., Iwouno J. Effect of preservation methods of oil palm (elaeis guineensis) sap and wine on the mineral and vitamin compositions for reproductive health. Food Sci. Qual. Manag. 2015;43:14–20. [Google Scholar]
- 18.Saithong P., Nitipan S., Permpool J. Optimization of vinegar production from nipa (Nypa fruticans wurmb.) sap using surface culture fermentation process. Appl. Food Biotechnol. 2019;6:193–200. doi: 10.22037/afb.v6i3.24653. [DOI] [Google Scholar]
- 19.Kulkarni A.R., Mulani R.M. Indigenous palms of India. Curr. Sci. 2004;86:1598–1603. http://www.jstor.org/stable/24108009 [Google Scholar]
- 20.Kurniawan T., Jayanudin J., Kustiningsih I., Adha Firdaus M. Palm sap sources, characteristics, and utilization in Indonesia. J. Food Nutr. Res. 2018;6:590–596. doi: 10.12691/jfnr-6-9-8. [DOI] [Google Scholar]
- 21.Kapilan R. Optimization of the usage of commercial lime for the inhibition of fermentation of sweet sugary saps of Borassus flabellifer and Caryota urens. Int. J. Adv. Res. Biol.Sci. 2015;2:12–20. [Google Scholar]
- 22.Dalibard C. Overall view on the tradition of tapping palm trees and prospects for animal production. Livest. Res. Rural Dev. 1999;11 [Google Scholar]
- 23.Ho C.W., Aida W.M., Maskat M.Y., Osman H. Effect of thermal processing of palm sap on the physico-chemical composition of traditional palm sugar. Pakistan J. Biol. Sci. 2008;11:989–995. doi: 10.3923/pjbs.2008.989.995. [DOI] [PubMed] [Google Scholar]
- 24.Jayanudin J., Kurniawan T., Kustiningsih I. Phenolic analysis and characterization of palm sugar (Arenga pinnata) produced by the spray dryer, orient. J. Chem. 2019;35:150–156. doi: 10.13005/ojc/350116. [DOI] [Google Scholar]
- 25.Trang T., Thi Van Anh L., Dang N., Nguyen D., Nhan N., Tran T.T., Nguyen Q., Bach L.G., Pham D. Microencapsulation of essential oils by spray-drying and influencing factors. J. Food Qual. 2021;2021:1–15. doi: 10.1155/2021/5525879. [DOI] [Google Scholar]
- 26.Zhang H., Luo J., Liu L., Chen X., Wan Y. Green production of sugar by membrane technology: how far is it from industrialization? Green Chem. Eng. 2021;2:27–43. doi: 10.1016/j.gce.2020.11.006. [DOI] [Google Scholar]
- 27.Tuseef Asghar Muhammad, Yusof Yus Aniza, Mokhtar Mohd Noriznan, Effendy Yaacob Mohammad, Mohd Ghazali Hasanah, Sin Chang Lee. A review of nutritional facts, production, availability and future aspects of coconut palm sugar. J. Nutr. Food Sci. 2021;715 doi: 10.1088/1755-1315/715/1/012016. [DOI] [Google Scholar]
- 28.Muzaffar K., Nayik G., Kumar P. Stickiness problem associated with spray drying of sugar and acid rich foods: a mini review. Food Nutr. Sci. 2015;s12 doi: 10.4172/2155-9600.S12-003. [DOI] [Google Scholar]
- 29.Sobulska M., Zbicinski I. Advances in spray drying of sugar-rich products. Dry. Technol. 2021;39:1774–1799. doi: 10.1080/07373937.2020.1832513. [DOI] [Google Scholar]
- 30.Le D.H.T., Lu W.C., Li P.H. Sustainable processes and chemical characterization of natural food additives: palmyra palm (Borassus flabellifer Linn.) granulated sugar. Sustain. Times. 2020;12 doi: 10.3390/su12072650. [DOI] [Google Scholar]
- 31.Saengkrajang W., Chaijan M., Panpipat W. Physicochemical properties and nutritional compositions of nipa palm (Nypa fruticans Wurmb) syrup. NFS J. 2021;23:58–65. doi: 10.1016/j.nfs.2021.04.004. [DOI] [Google Scholar]
- 32.Ben Thabet I., Besbes S., Masmoudi M., Attia H., Deroanne C., Blecker C. Compositional, physical, antioxidant and sensory characteristics of novel syrup from date palm (Phoenix dactylifera L.) Food Sci. Technol. Int. 2009;15:583–590. doi: 10.1177/1082013209353079. [DOI] [Google Scholar]
- 33.Choong C.C., Anzian A., Che Wan Sapawi C.W.N.S., Meor Hussin A.S. Characterization of sugar from Arenga pinnata and saccharum officinarum sugars. Int. Food Res. J. 2016;23:1642–1652. [Google Scholar]
- 34.Le D.H.T., Chiu C.S., Chan Y.J., Wang C.C.R., Liang Z.C., Hsieh C.W., Lu W.C., Mulio A.T., Wang Y.J., Li P.H. Bioactive and physicochemical characteristics of natural food: palmyra palm (borassus flabellifer linn.) syrup. Biology. 2021;10:1–15. doi: 10.3390/biology10101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Victor I. Colour changes during the processing of Arenga pinnata (palm) sap into sugar. J. Food Sci. Technol. 2018;55:3845–3849. doi: 10.1007/s13197-018-3314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Victor I., Wikarsa L., Orsat V. Identification of changes in the freshness of palm (Arenga pinnata) sap. Sugar Tech. 2022 doi: 10.1007/s12355-022-01181-9. [DOI] [Google Scholar]
- 37.Ben Thabet I., Besbes S., Attia H., Deroanne C., Francis F., Drira N.-E., Blecker C. Physicochemical characteristics of date sap “lagmi” from deglet nour palm (Phoenix dactylifera L.) Int. J. Food Prop. 2009;12:659–670. doi: 10.1080/10942910801993528. [DOI] [Google Scholar]
- 38.Naknean P., Meenune M., Roudaut G. Characterization of palm sap harvested in Songkhla province, southern Thailand. Int. Food Res. J. 2010;17:977–986. [Google Scholar]
- 39.Masithoh R.E., Roosmayanti F., Rismiwandira K., Pahlawan M.F.R. Detection of palm sugar adulteration by fourier transform near-infrared (FT-NIR) and fourier transform infrared (FT-IR) spectroscopy. Sugar Tech. 2022;24:920–929. doi: 10.1007/s12355-021-01058-3. [DOI] [Google Scholar]
- 40.Ho C.W., Aida W.M.W., Maskat M.Y., Osman H. Changes in volatile compounds of palm sap (Arenga pinnata) during the heating process for production of palm sugar. Food Chem. 2007;102:1156–1162. doi: 10.1016/j.foodchem.2006.07.004. [DOI] [Google Scholar]
- 41.Naknean P. 2010. Factors Affecting Browning and Crystallisation of Palm Sugar Syrup and Palm Sugar Cake, Prince of Songkla University. [Google Scholar]
- 42.Hurtta M., Pitkänen I., Knuutinen J. Melting behaviour of d-sucrose, d-glucose and d-fructose. Carbohydr. Res. 2004;339:2267–2273. doi: 10.1016/j.carres.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Roos Y. Melting and glass transitions of low molecular weight carbohydrates. Carbohydr. Res. 1993;238:39–48. doi: 10.1016/0008-6215(93)87004-C. [DOI] [Google Scholar]
- 44.Morrow E.A., Terban M.W., Thomas L.C., Gray D.L., Bowman M.J., Billinge S.J.L., Schmidt S.J. Effect of amorphization method on the physicochemical properties of amorphous sucrose. J. Food Eng. 2019;243:125–141. doi: 10.1016/j.jfoodeng.2018.08.036. [DOI] [Google Scholar]
- 45.Chakraborty M., Das K., Dey G., Mitra A. Unusually high quantity of 4-hydroxybenzoic acid accumulation in cell wall of palm mesocarps. Biochem. Syst. Ecol. - Biochem SYST ECOL. 2006;34:509–513. doi: 10.1016/j.bse.2005.11.011. [DOI] [Google Scholar]
- 46.Hazir M.H.M., Shariff A.R.M., Amiruddin M.D. Determination of oil palm fresh fruit bunch ripeness—based on flavonoids and anthocyanin content. Ind. Crop. Prod. 2012;36:466–475. [Google Scholar]
- 47.Jaffri J.M., Mohamed S., Rohimi N., Ahmad I.N., Noordin M.M., Manap Y.A. Antihypertensive and cardiovascular effects of catechin-rich oil palm (Elaeis guineensis) leaf extract in nitric oxide-deficient rats. J. Med. Food. 2011;14:775–783. doi: 10.1089/jmf.2010.1170. [DOI] [PubMed] [Google Scholar]
- 48.Kawamura F., Saary N.S., Hashim R., Sulaiman O., Hashida K., Otsuka Y., Nakamura M., Ohara S. Subcritical water extraction of low-molecular-weight phenolic compounds from oil palm biomass. Jpn. Agric. Res. Q. 2014;48:355–362. doi: 10.6090/jarq.48.355. [DOI] [Google Scholar]
- 49.Neo Y.P., Ariffin A., Tan C.P., Tan Y.A. Phenolic acid analysis and antioxidant activity assessment of oil palm (E. guineensis) fruit extracts. Food Chem. 2010;122:353–359. [Google Scholar]
- 50.Oskoueian E., Abdullah N., Zulkifli I., Ebrahimi M., Karimi E., Goh Y.M., Oskoueian A., Shakeri M. Cytoprotective effect of palm kernel cake phenolics against aflatoxin B1-induced cell damage and its underlying mechanism of action. BMC Compl. Alternat. Med. 2015;15:392. doi: 10.1186/s12906-015-0921-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abu-Reidah I.M., Gil-Izquierdo Á., Medina S., Ferreres F. Phenolic composition profiling of different edible parts and by-products of date palm (Phoenix dactylifera L.) by using HPLC-DAD-ESI/MS(n) Food Res. Int. 2017;100:494–500. doi: 10.1016/j.foodres.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Al-Farsi M., Alasalvar C., Morris A., Baron M., Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005;53:7592–7599. doi: 10.1021/jf050579q. [DOI] [PubMed] [Google Scholar]
- 53.Benmeddour Z., Mehinagic E., Le Meurlay D., Louaileche H. Phenolic composition and antioxidant capacities of ten Algerian date (Phoenix dactylifera L.) cultivars: a comparative study. J. Funct. Foods. 2013;5:346–354. doi: 10.1016/j.jff.2012.11.005. [DOI] [Google Scholar]
- 54.Hong Y.J., Tomas-Barberan F.A., Kader A.A., Mitchell A.E. The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera) J. Agric. Food Chem. 2006;54:2405–2411. doi: 10.1021/jf0581776. [DOI] [PubMed] [Google Scholar]
- 55.Kchaou W., Abbès F., Ben Mansour R., Blecker C., Attia H., Besbes S. Phenolic profile, antibacterial and cytotoxic properties of second grade date extract from Tunisian cultivars (Phoenix dactylifera L.) Food Chem. 2016;194:1048–1055. doi: 10.1016/j.foodchem.2015.08.120. [DOI] [PubMed] [Google Scholar]
- 56.Mansouri A., Embarek G., Kokkalou E., Kefalas P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera) Food Chem. 2005;89:411–420. doi: 10.1016/j.foodchem.2004.02.051. [DOI] [Google Scholar]
- 57.Michael H.N., Salib J.Y., Eskander E.F. Bioactivity of diosmetin glycosides isolated from the epicarp of date fruits, Phoenix dactylifera, on the biochemical profile of alloxan diabetic male rats. Phytother Res. 2013;27:699–704. doi: 10.1002/ptr.4777. [DOI] [PubMed] [Google Scholar]
- 58.Mrabet A., Jiménez-Araujo A., Fernández-Bolaños J., Rubio-Senent F., Lama-Muñoz A., Sindic M., Rodríguez-Gutiérrez G. Antioxidant phenolic extracts obtained from secondary Tunisian date varieties (Phoenix dactylifera L.) by hydrothermal treatments. Food Chem. 2016;196:917–924. doi: 10.1016/j.foodchem.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 59.Regnault-Roger C., Hadidane R., Biard J.-F., Boukef K. High performance liquid and thin-layer chromatographic determination of phenolic acids in palm (Phoenix dactilifera) products. Food Chem. 1987;25:61–71. doi: 10.1016/0308-8146(87)90054-9. [DOI] [Google Scholar]
- 60.Carvalho A.V., Ferreira Ferreira da Silveira T., Mattietto R. de A., Padilha de Oliveira M. do S., Godoy H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2017;97:1467–1474. doi: 10.1002/jsfa.7886. [DOI] [PubMed] [Google Scholar]
- 61.Dias A.L.S., Rozet E., Larondelle Y., Hubert P., Rogez H., Quetin-Leclercq J. Development and validation of an UHPLC-LTQ-Orbitrap MS method for non-anthocyanin flavonoids quantification in Euterpe oleracea juice. Anal. Bioanal. Chem. 2013;405:9235–9249. doi: 10.1007/s00216-013-7325-z. [DOI] [PubMed] [Google Scholar]
- 62.Gordon A., Cruz A.P.G., Cabral L.M.C., de Freitas S.C., Taxi C.M.A.D., Donangelo C.M., de Andrade Mattietto R., Friedrich M., da Matta V.M., Marx F. Chemical characterization and evaluation of antioxidant properties of açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012;133:256–263. doi: 10.1016/j.foodchem.2011.11.150. [DOI] [PubMed] [Google Scholar]
- 63.Mulabagal V., Calderón A.I. Liquid chromatography/mass spectrometry based fingerprinting analysis and mass profiling of Euterpe oleracea (açaí) dietary supplement raw materials. Food Chem. 2012;134:1156–1164. doi: 10.1016/j.foodchem.2012.02.123. [DOI] [PubMed] [Google Scholar]
- 64.Schauss A.G., Wu X., Prior R.L., Ou B., Patel D., Huang D., Kababick J.P. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae mart. (acai) J. Agric. Food Chem. 2006;54:8598–8603. doi: 10.1021/jf060976g. [DOI] [PubMed] [Google Scholar]
- 65.Bicudo M.O.P., Ribani R.H., Beta T. Anthocyanins, phenolic acids and antioxidant properties of Juçara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Foods Hum. Nutr. 2014;69:142–147. doi: 10.1007/s11130-014-0406-0. [DOI] [PubMed] [Google Scholar]
- 66.Da Silva Campelo Borges G., Gracieli Kunradi Vieira F., Copetti C., Valdemiro Gonzaga L., Zambiazi R.C., Mancini Filho J., Fett R. Chemical characterization, bioactive compounds, and antioxidant capacity of jussara (Euterpe edulis) fruit from the Atlantic Forest in southern Brazil. Food Res. Int. 2011;44:2128–2133. doi: 10.1016/j.foodres.2010.12.006. [DOI] [Google Scholar]
- 67.Schulz M., Borges G. da S.C., Gonzaga L.V., Seraglio S.K.T., Olivo I.S., Azevedo M.S., Nehring P., de Gois J.S., de Almeida T.S., Vitali L., Spudeit D.A., Micke G.A., Borges D.L.G., Fett R. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res. Int. 2015;77:125–131. doi: 10.1016/j.foodres.2015.08.006. [DOI] [Google Scholar]
- 68.Farag M.A., Paré P.W. Phytochemical analysis and anti-inflammatory potential of Hyphaene thebaica L. fruit. J. Food Sci. 2013;78:C1503–C1508. doi: 10.1111/1750-3841.12253. [DOI] [PubMed] [Google Scholar]
- 69.Beskow G.T., Hoffmann J.F., Teixeira A.M., Fachinello J.C., Chaves F.C., Rombaldi C.V. Bioactive and yield potential of jelly palms (Butia odorata Barb. Rodr.) Food Chem. 2015;172:699–704. doi: 10.1016/j.foodchem.2014.09.111. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto R., Muraoka T. The catechin in the fruit of areca catechu L. Bull. Agric. Chem. Soc. Jpn. 1932;8:149–151. doi: 10.1080/03758397.1932.10857013. [DOI] [Google Scholar]
- 71.Prasad N., Yang B., Kong K.W., Khoo H.E., Sun J., Azlan A., Ismail A., Bin Romli Z. Phytochemicals and antioxidant capacity from Nypa fruticans wurmb. Fruit, evidence-based complement. Alternat. Med. 2013 doi: 10.1155/2013/154606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Genovese M.I., Da Silva Pinto M., De Souza Schmidt Gonçalves A.E., Lajolo F.M. Bioactive compounds and antioxidant capacity of exotic fruits and commercial frozen pulps from Brazil. Food Sci. Technol. Int. 2008;14:207–214. doi: 10.1177/1082013208092151. [DOI] [Google Scholar]
- 73.Boeing J.S., Ribeiro D., Chisté R.C., Visentainer J.V., Costa V.M., Freitas M., Fernandes E. Chemical characterization and protective effect of the Bactris setosa Mart. fruit against oxidative/nitrosative stress. Food Chem. 2017;220:427–437. doi: 10.1016/j.foodchem.2016.09.188. [DOI] [PubMed] [Google Scholar]
- 74.Bataglion G.A., da Silva F.M.A., Eberlin M.N., Koolen H.H.F. Simultaneous quantification of phenolic compounds in buriti fruit (Mauritia flexuosa L.f.) by ultra-high performance liquid chromatography coupled to tandem mass spectrometry. Food Res. Int. 2014;66:396–400. doi: 10.1016/j.foodres.2014.09.035. [DOI] [Google Scholar]
- 75.Koolen H.H.F., da Silva F.M.A., Gozzo F.C., de Souza A.Q.L., de Souza A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC–ESI-MS/MS. Food Res. Int. 2013;51:467–473. doi: 10.1016/j.foodres.2013.01.039. [DOI] [Google Scholar]
- 76.de Oliveira D.M., Siqueira E.P., Nunes Y.R.F., Cota B.B. Flavonoids from leaves of Mauritia flexuosa. Rev. Bras. Farmacogn. 2013;23:614–620. doi: 10.1590/S0102-695X2013005000061. [DOI] [Google Scholar]
- 77.Brabo de Sousa S.H., de Andrade Mattietto R., Campos Chisté R., Carvalho A.V. Phenolic compounds are highly correlated to the antioxidant capacity of genotypes of Oenocarpus distichus Mart. fruits. Food Res. Int. 2018;108:405–412. doi: 10.1016/j.foodres.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 78.Rezaire A., Robinson J.-C., Bereau D., Verbaere A., Sommerer N., Khan M.K., Durand P., Prost E., Fils-Lycaon B. Amazonian palm Oenocarpus bataua (“patawa”): chemical and biological antioxidant activity--phytochemical composition. Food Chem. 2014;149:62–70. doi: 10.1016/j.foodchem.2013.10.077. [DOI] [PubMed] [Google Scholar]
- 79.Dey G., Sachan A., Ghosh S., Mitra A. Detection of major phenolic acids from dried mesocarpic husk of mature coconut by thin layer chromatography. Ind. Crop. Prod. 2003;18:171–176. doi: 10.1016/S0926-6690(03)00056-6. [DOI] [Google Scholar]
- 80.Esquenazi D., Wigg M.D., Miranda M.M.F.S., Rodrigues H.M., Tostes J.B.F., Rozental S., da Silva A.J.R., Alviano C.S. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res. Microbiol. 2002;153:647–652. doi: 10.1016/s0923-2508(02)01377-3. [DOI] [PubMed] [Google Scholar]
- 81.dos M.B., Oliveira S., Valentim I.B., de Vasconcelos C.C., Omena C.M.B., Bechara E.J.H., da Costa J.G., Freitas M. de L., Sant’Ana A.E.G., Goulart M.O.F. Cocos nucifera Linn. (Palmae) husk fiber ethanolic extract: antioxidant capacity and electrochemical investigation. Comb. Chem. High Throughput Screen. 2013;16:121–129. doi: 10.2174/1386207311316020006. [DOI] [PubMed] [Google Scholar]
- 82.Valadez-Carmona L., Cortez-García R.M., Plazola-Jacinto C.P., Necoechea-Mondragón H., Ortiz-Moreno A. Effect of microwave drying and oven drying on the water activity, color, phenolic compounds content and antioxidant activity of coconut husk (Cocos nucifera L.) J. Food Sci. Technol. 2016;53:3495–3501. doi: 10.1007/s13197-016-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seneviratne K.N., Sudarshana Dissanayake D.M. Variation of phenolic content in coconut oil extracted by two conventional methods. Int. J. Food Sci. Technol. 2008;43:597–602. doi: 10.1111/j.1365-2621.2006.01493.x. [DOI] [Google Scholar]
- 84.Agostini-costa T.S. Bioactive compounds and health bene fi ts of some palm species traditionally used in Africa and the Americas – a review. J. Ethnopharmacol. 2018;224:202–229. doi: 10.1016/j.jep.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 85.Ablajan K., Abliz Z., Shang X., He J., Zhang R., Shi J. Structural characterization of flavonol 3, 7‐di‐O‐glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006;41:352–360. doi: 10.1002/jms.995. [DOI] [PubMed] [Google Scholar]
- 86.de Oliveira D.M., de Oliveira D.B.C., Nunes Y.R.F., de Almeida Alves T.M., Kohlhoff M., Andrade A.A., Cota B.B. Natural occurring phenolic derivatives from mauritia flexuosa (buriti) stems and their potential antibacterial activity against methicillin‐resistant Staphylococcus aureus (MRSA) Chem. Biodivers. 2022;19 doi: 10.1002/cbdv.202100788. [DOI] [PubMed] [Google Scholar]
- 87.Oracz J., Nebesny E., Żyżelewicz D. Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. Eur. Food Res. Technol. 2015;241:663–681. [Google Scholar]
- 88.Wiseman S.A., Balentine D.A., Frei B. Antioxidants in tea. Crit. Rev. Food Sci. Nutr. 1997;37:705–718. doi: 10.1080/10408399709527798. [DOI] [PubMed] [Google Scholar]
- 89.Liu P.-L., Liu J.-T., Kuo H.-F., Chong I.-W., Hsieh C.-C. Epigallocatechin gallate attenuates proliferation and oxidative stress in human vascular smooth muscle cells induced by interleukin-1 via heme oxygenase-1. Mediat. Inflamm. 2014:2014. doi: 10.1155/2014/523684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang P.-L., Chi C.-W., Liu T.-Y. Effects of Areca catechu L. containing procyanidins on cyclooxygenase-2 expression in vitro and in vivo. Food Chem. Toxicol. 2010;48:306–313. doi: 10.1016/j.fct.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 91.Habib H.M., Platat C., Meudec E., Cheynier V., Ibrahim W.H. Polyphenolic compounds in date fruit seed (Phoenix dactylifera): characterisation and quantification by using UPLC‐DAD‐ESI‐MS. J. Sci. Food Agric. 2014;94:1084–1089. doi: 10.1002/jsfa.6387. [DOI] [PubMed] [Google Scholar]
- 92.Pacheco-Palencia L.A., Duncan C.E., Talcott S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009;115:1199–1205. [Google Scholar]
- 93.Chang C.-L., Wang G.-J., Zhang L.-J., Tsai W.-J., Chen R.-Y., Wu Y.-C., Kuo Y.-H. Cardiovascular protective flavonolignans and flavonoids from Calamus quiquesetinervius. Phytochemistry. 2010;71:271–279. doi: 10.1016/j.phytochem.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 94.Ammar N.M., Hefnawy M.S., Al-Okbi S.Y., Mohamed D.A., El-Sayed N.K., El-Anssary A.A., Mabry T. Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt. Asian Pac. J. Trop. Biomed. 2014;4:456–462. doi: 10.12980/APJTB.4.2014C1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Y.-P., Chen T.-Y., Tseng H.-W., Lee M.-H., Chen S.-T. Neural cell protective compounds isolated from Phoenix hanceana var. formosana. Phytochemistry. 2009;70:1173–1181. doi: 10.1016/j.phytochem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 96.Ho C.W., Aida W.M.W., Maskat M.Y., Osman H. Changes in volatile compounds of palm sap (Arenga pinnata) during the heating process for production of palm sugar. Food Chem. 2007;102:1156–1162. doi: 10.1016/j.foodchem.2006.07.004. [DOI] [Google Scholar]
- 97.Miękus N., Marszałek K., Podlacha M., Iqbal A., Puchalski C., Świergiel A.H. Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules. 2020;25:3804. doi: 10.3390/molecules25173804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ashraf Z., Hamidi-Esfahani Z. Date and date processing: a review. Food Rev. Int. 2011;27:101–133. [Google Scholar]
- 99.Jose N. Neera- A potential natural health drink. Biomed. J. Sci. Tech. Res. 2018;11:8595–8597. doi: 10.26717/bjstr.2018.11.002114. [DOI] [Google Scholar]
- 100.Swamy G.M.S. Coconut Neera production and processing in Karnataka. Indian Coconut J. 2013:31–33. [Google Scholar]
- 101.Lasekan O., Abbas K.A. Flavour chemistry of palm toddy and palm juice: a review. Trends Food Sci. Technol. 2010;21:494–501. doi: 10.1016/j.tifs.2010.07.007. [DOI] [Google Scholar]
- 102.Chandrasekhar K.B., Sreevani S., Seshapani P., Pramodhakumari J. 2012. A Review on Palm Wine. [Google Scholar]
- 103.Karamoko D., Théodore Deni N., Aboya Moroh J.-L., Jean-Paul Bouatenin K.M., Dje K.M. Biochemical and microbial properties of palm wine: effect of tapping length and varietal differences. Food Nutr. Sci. 2016;7:763–771. doi: 10.4236/fns.2016.79077. [DOI] [Google Scholar]
- 104.Chaurasiya A.K., Chakraborty I., Saha J. Value addition of Palmyra palm and studies on the storage life. J. Food Sci. Technol. 2014;51:768–773. doi: 10.1007/s13197-011-0561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vengaiah P.C., Murthy G.N., Sattiraju M., Maheswarappa H.P. Value added food products from palmyrah palm (borassus flabellifer L) J. Nutr. Heal. Sci. 2017;4:1–3. doi: 10.15744/2393-9060.4.105. [DOI] [Google Scholar]
- 106.Upadhyaya A., Sonawane S.K. Palmyrah palm and its products (neera, jaggery and candy)—a review on chemistry and technology. Appl. Food Res. 2023;3 doi: 10.1016/j.afres.2022.100256. [DOI] [Google Scholar]
- 107.Onuorah S., Lina J., Obika I. Production of vinegar from oil-palm wine using acetobacter aceti isolated from rotten banana fruits. Univers. J. Biomed. Eng. 2016;4:1–5. doi: 10.13189/ujbe.2016.040101. [DOI] [Google Scholar]
- 108.Yusoff N.A., Yam M.F., Beh H.K., Abdul Razak K.N., Widyawati T., Mahmud R., Ahmad M., Asmawi M.Z. Antidiabetic and antioxidant activities of Nypa fruticans Wurmb. vinegar sample from Malaysia. Asian Pac. J. Tropical Med. 2015;8:595–605. doi: 10.1016/j.apjtm.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 109.Dyaningrum E.F., Lutfiyah R.A., Diasti D.R., Karyadi J.N.W., Saputro A.D. Physical characteristics of instanised Cocoa drink sweetened with Palm Sap Sugar: a preliminary study. IOP Conf. Ser. Earth Environ. Sci. 2019 IOP Publishing. [Google Scholar]
- 110.H E., R Nafingah A.D.S., Kurniasari J., Cahyani A. Investigating the impact of Palm Sap Sugar proportion and fat content on heat stability of Milk Chocolate. IOP Conf. Ser. Earth Environ. Sci. 2019 doi: 10.1088/1755-1315/355/1/012043. [DOI] [Google Scholar]
- 111.B Karyadi N., Setiadi J.N.W.P.A., Saputro A.D., Nurkholisa Z., Hardiyanto Y.F. Physical characteristic of instanised chocolate beverage powder produced with palm sugar and sucrose as sweeteners. IOP Conf. Ser. Earth Environ. Sci. 2021 doi: 10.1088/1755-1315/653/1/012038. [DOI] [Google Scholar]
- 112.Ibrahim S.F., Dalek N.S.E.M., Raffie Q.A.F.M., Ain M.R.F. Quantification of physicochemical and microstructure properties of dark chocolate incorporated with palm sugar and dates as alternative sweetener, Mater. Today Proc. 2020;31:366–371. [Google Scholar]
- 113.Srikaeo K., Sangkhiaw J., Likittrakulwong W. Productions and functional properties of palm sugars. Walailak J. Sci. Technol. 2019;16:897–907. doi: 10.48048/wjst.2019.5323. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.