Abstract
Urease enzyme is an infectious factor that provokes the growth and colonization of virulence pathogenic bacteria in humans. To overcome the deleterious effects of bacterial infections, inhibition of urease enzyme is one of the promising approaches. The current study is designed to synthesize new 1,2-benzothiazine-N-arylacetamide derivatives 5(a-n) that can effectively provide a new drug candidate to avoid bacterial infections by urease inhibition. After structural elucidation by FT-IR, proton and carbon-13 NMR and mass spectroscopy, the synthesized compounds 5(a-n) were investigated to evaluate their inhibitory potential against urease enzyme. In vitro analysis against positive control of thiourea indicated that all the synthesized compounds have strong inhibitory strengths as compared to the reference drug. Compound 5k, being the most potent inhibitor, strongly inhibited the urease enzymes and revealed an IC50 value of 9.8 ± 0.023 µM when compared with the IC50 of thiourea (22.3 ± 0.031 µM)—a far more robust inhibitory potential. Docking studies of 5k within the urease active site revealed various significant interactions such as H-bond, π-alkyl with amino acid residues like Val744, Lys716, Ala16, Glu7452, Ala37 and Asp730.
Keywords: benzothiazine, synthesis, urease, thiourea, docking, ureolytic
1. Introduction
Enzyme inhibition remains the most attractive research domain in drug design as most of the natural physiological pathways involve a number of enzymes. Urease (EC3.5.1.5; urea amidohydrolase) enzyme having nickel in its core structure catalyses urea dissociation into ammonia and carbamate approximately a hundred trillion times faster than un-catalysed reaction [1]. The family of urease enzyme is common in a large number of prokaryotes and eukaryotes including plants and fungi [2]. Urease has become the most significant target for drug development against pathogens like Helicobacter pylori (H. pylori), a common cause of stomach-related ailments [3,4]. Production of ammonia enhances the pH, which creates an environment favourable for the progression of H. pylori in the stomach [5]. Consequently, urease activity is one of the critical stimuli for various pathogenic gastrointestinal conditions in humans and animals [6]. Thus, the inhibition of urease enzymes is conceivably good for hindering the injurious effects of urinary bacterial diseases in humans [7].
Over the past few years, different anti-urease agents have been documented due to their exceptional inhibitory potential. There are different types of effective urease inhibitors such as hydroxamic acid derivatives [8], hydrazides [9], semicarbazides [10–12], flavonoid glycoside [13], palmatine [14], epiberberine [15], Schiff bases [16], benzimidazoles [17], triazoles or oxadiazoles [18], phosphoramidates [19], boric and boronic acids [20], heavy metal ions [21], thiourea [22] and (thio)barbituric acid derivatives [23,24]. Fluoride ion as a non-competitive inhibitor is capable of inactivating the enzyme [25]. The importance of metallic ligands, hydrogen bonding and hydrophobic moieties as vital medicinal features for urease inhibitors has been explored in many literature studies [26]. Quinazolinones and their derivatives are the heterocyclic systems abundantly used in medicinal chemistry due to their functions such as anti-urease activities [27]. Nowadays, urease inhibitors have become the most vital anti-ulcer drugs that manage the deleterious effects of bacterial infections in humans and animals; however, these are accompanied by side effects and drug-resistance. Owing to a rise in the drug-resistance and toxic side effects of the available medication for urease inhibition, there is a need to prepare new, more potent but less toxic urease inhibitors for the better treatment of gastrointestinal disorders.
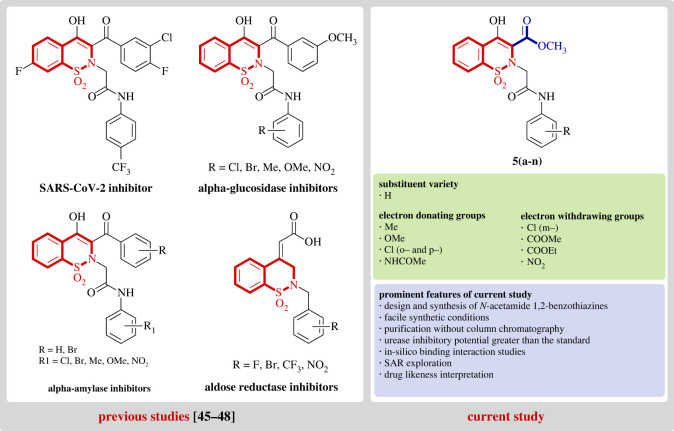
1,2-benzothiazine 1,1-dioxides are versatile pharmacophores which are widely explored for their potent biological activities and hence are considered molecules of great interest [28]. They are well-known non-steroidal anti-inflammatory drugs (NSAIDs) commercially available as oxicams [29]. Studies reveal that 1,2-benzothiazines exhibit wide pharmacological activities such as anti-inflammatory [30], calpain I inhibitors [31], anti-cancer [32], anti-microbial [33], analgesic [34], MAPK inhibitors [35], MAO inhibition [36], anti-arthritic [37], anti-oxidant [38], anti-viral [39,40], anti-diabetic [41], anti-convulsant [42] and anti-malarial [43]. Other applications include as herbicides and as dyestuff in industry [44]. Most of these compounds constitute the N-alkyl/acyl 1,2-benzothiazine framework. Similarly, 1,2-benzothiazine-N-arylacetamides have also been reported to exhibit pharmacological activities. For example, molecules containing the N-arylacetamides functional group have recently been explored as potent SARS-CoV-2 inhibitors (IC50 = 0.88 µM) [45]. Similarly, the role of related compounds has been established for inhibition of various enzymes such as aldose reductase, α-glucosidase, α-amylase and human 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) [46–48] (figure 1).
Figure 1.
Rationale of current work.
Interestingly, despite their rich biological profile benzothiazine derivatives have not been profoundly tested against urease enzyme. This inspired us to assess the urease inhibitory potential of benzothiazine derivatives. In this regard, a renowned anti-urease pharmacophore phenylacetamide was incorporated into the benzothiazine ring. Hence, continuing our previous work to explore new biologically active compounds [49–54], we herein present the synthesis and evaluation of the pharmacological potential of a series of new methyl 1,2-benzothiazine-N-arylacetamide derivatives against urease enzyme. The prepared compounds were subjected to characterization through nuclear magnetic resonance spectroscopy (proton and carbon) as well as mass spectrometry. Moreover, urease inhibition activity for the synthesized compounds was also evaluated.
2. Results and discussion
2.1. Chemistry
The schematic synthetic pathway for titled compounds is illustrated in scheme 1. N-Alkylation of sodium saccharin under anhydrous conditions gave benzisothiazole substituted ester 2 which underwent ring expansion to yield 1,2-benzothiazine framework 3 [55]. Further N-alkylation of benzothiazine bearing ester 3 was carried out by reacting it with α-chloroacetanilides 4 to achieve the desired compounds 5(a-n) in reasonable yields. All the prepared derivatives were characterized by nuclear magnetic resonance spectroscopy (proton and carbon) as well as mass spectrometry.
Scheme 1.
Synthetic route for methyl 1,2-benzothiazine-N-arylacetamides 5(a-n).
Proton NMR spectra of all the compounds contain methylene and NH protons which appeared as distinct singlets ranging between 4.34–4.56 and 9.32–10.98 ppm, respectively. The methoxy and enolic -OH protons exhibited singlet peaks at 3.91 ppm and 11.09–11.83 ppm, respectively. Aromatic protons appeared between 6.79 ppm and 8.17 ppm depending upon the extent of deshielding. In 13C NMR spectra of the synthesized derivatives, methoxy and methylene carbon atoms showed up around 53.5 ppm and 53.0 ppm respectively while carbonyl carbon appeared as the most deshielded signal at around 168.7 ppm. Signals for aromatic carbon atoms were detected between 108 ppm and 167 ppm. Similarly, in mass spectra, the observed molecular ion peaks were in accordance with the calculated molecular masses.
2.2. In vitro inhibition and SAR analysis (structure activity relationship)
The synthesized compounds 5(a-n) were evaluated for their inhibitory potential against urease while using thiourea as a positive control (IC50 value = 22.3 ± 0.031 µM). The experimental data are outlined in table 1. A general observation of all synthetic analogues was that they had varying degrees of urease inhibitory activity and were remarkably potent. The synthesized compounds showed inhibition in a range of 9.8–20.7 µM. The in vitro analysis exhibited that all the synthesized derivatives were exceptionally active and potent inhibitors of urease; even the least active compound of the series 5i (IC50 = 20.7 ± 0.25 µM) revealed better inhibitory potential than the positive control (thiourea) (22.3 ± 0.031 µM). Hence, we analysed a diversification of structural features and influence of different electron donating and withdrawing groups present in the active N-phenylacetamide-substituted benzothiazine carboxylate framework. Compound 5k was selected to be the most potent and effective inhibitor having a IC50 value of 9.8 ± 0.023 µM with a twofold stronger inhibitory efficacy than thiourea. The effective structural feature of the most active inhibitor comprised an N-phenylacetamide ring bearing a substituent of empirical ethoxy carbonyl at para-position. The unsubstituted derivative 5a exhibited better inhibitory potential against urease (IC50 = 10.1 ± 0.90 µM) as compared to urease. A notable decline in the activity was observed when the 2-carboxymethyl group was introduced in the phenylacetamide ring in 5d (IC50 = 14.1 ±0.12 µM). Consequently, substituting the acetamido group at para-position on the afore-mentioned ring in 5l, an IC50 value of 12.6 ± 0.10 µM was observed, suggesting the impact of substituents on the efficacy profile of the synthesized analogues. The presence of the halogen (chloro) group as a substituent on different positions of the phenylacetamide ring resulted in moderate inhibitory potentials of 5i (20.7 ± 0.25 µM), 5m (14 ± 0.15 µM) and 5n 13 ± 0.10 µM.
Table 1.
Urease inhibitory activity of benzothiazine derivatives 5(a-n).
| compound | substituent | urease inhibition IC50 ± s.e.m. (μM) |
|---|---|---|
| 5a | -H | 10.1 ± 0.90 |
| 5b | 2-Methoxy | 14.9 ± 1.50 |
| 5c | 2-Methyl | 17.2 ± 1.10 |
| 5d | 2-Carboxymethyl | 14.1 ± 0.12 |
| 5e | 3-Methoxy | 18.2 ± 1.69 |
| 5f | 3-Methyl | 15.5 ± 1.35 |
| 5g | 4-Methoxy | 20.4 ± 1.81 |
| 5h | 4-Methyl | 16.4 ± 1.40 |
| 5i | 4-Chloro | 20.7 ± 0.25 |
| 5j | 4-Nitro | 17.06 ± 1.45 |
| 5k | 4-Carboxyethyl | 9.8 ± 0.023 |
| 5l | 4-Acetamido | 12.6 ± 0.10 |
| 5m | 2,3-Dichloro | 14.06 ± 0.15 |
| 5n | 2,4-Dichloro | 13.06 ± 0.10 |
| thiourea | 22.3 ± 0.031 |
In parallel, the substitution of the methyl group on different positions of the phenylacetamide ring exhibited effective and comparable inhibitory potential for compounds 5c (IC50 = 17.2 ± 1.10 µM), 5f (IC50 = 15.5 ± 1.35 µM) and 5h (IC50 = 16.4 ± 1.40 µM). Furthermore, the presence of the methoxy group on different positions of phenylacetamide ring in 5b, 5e and 5g exhibited almost the same inhibitory potency as the IC50 values 14.9 ± 1.50 µM, 18.2 ± 1.69 µM and 20.4 ± 1.81 µM, respectively. Further modifications such as the entry of the nitro group (strong electron withdrawing) at para-position on phenylacetamide ring in 5j showed better inhibition (IC50 value = 17.06 ± 1.45 µM) when compared with the standard drug.
2.3. Molecular docking studies
The most potent compounds (5a, 5l and 5k) were docked within the active pocket of urease to elaborate important interactions with the amino acid residues of the receptor (PDB; 3LA4) [56]. The most potent compound 5k presented various major interactions inside the urease active pocket with amino acid residues such as Lys709, Lys716, Lys745, Ala16, Ala37, Glu742, Val744, Tyr32 and Asp730. Conventional hydrogen bonds were observed with several amino acid residues including Lys716, Lys709 and Glu742 with the oxygen atom of thiazine derivative compound. π-Alkyl interactions were also observed with the benzothiazine ring with amino acid residues such as Ala16, Ala37 and Val744 with the phenylacetamide ring of compound 5k. π-anion interactions were also exhibited in the phenylacetamide ring with Asp730, whereas Tyr32 and Lys745 were also found to interact with phenylacetamide ring and carboxylate part of the compound at distances of 4.10 Å and 3.60 Å, respectively (figure 2).
Figure 2.
Three- and two-dimensional visualization of compound 5k against urease.
The docking study of compound 5a exhibited multiple interactions with amino acids such as Lys716 (2.80 Å and 2.89 Å), Glu742 (3.24 Å) and Thr33 (3.01 Å) depicting conventional hydrogen bond, with the oxygen atoms present in the benzothiazine compound. However, some other interactions like π-donor H-bond with Tyr32 (4.16 Å), π-anion with Asp730 (3.90 Å) and π-alkyl bond with Val744 (5.36 Å) were also observed in the phenylacetamide ring of the compound 5a. Meanwhile, Ala16 (4.92 Å) and Ala37 (4.44 Å) formed π-alkyl interactions in the benzothiazine part of the compound. Moreover, Thr33 was also involved in the C–H bond with the phenyl ring via distance of 3.60 Å as shown in figure 3.
Figure 3.
Three- and two-dimensional visualization of compound 5a against urease.
Another potent inhibitor 5l was docked inside the urease depicting various interactions with amino acid residues such as Phe712, Tyr32, Val36, Thr33, Ala37, Val744 and Lys716. Among them, Lys716 and Val744 showed conventional hydrogen bonding with the benzothiazine bearing oxygen atoms with distances of 3.11 Å and 3.24 Å, respectively. Lys716 also exhibited an π-cationic interaction with the phenylacetamide part of the compound 5l via a distance of 4.64 Å. Additionally, π-sigma interactions were also observed between the amino acid residues such as Phe712 and Thr33 with the benzothiazine part of the compound. However, π-alkyl interactions were also revealed in the active moiety of the thiazine with amino acid residues Val36 and Ala37. Moreover, a C–H bond was established between carbon atom and hydroxyl group of the Tyr32 with a distance of 3.73 Å as shown in figure 4.
Figure 4.
Three- and two-dimensional visualization of compound 5l against urease.
2.4. Molecular dynamic simulations
The conformational dynamic studies of protein and compound 5k complex were explored by employing molecular dynamic simulation through normal mode analysis (NMA) which is done by iMODS. Deformability results show a low level of deformation with all residues and the Eigen value of the complex is 3.111139 × 10−5. Figure 5 explains the results of MD simulation.
Figure 5.
The molecular dynamic simulation study of docked complex 5k with urease. Deformability (a), β-factor (b), eigen values (c), variance (d), covariance map (e) and elastic network (f). In (d), green colour indicates cumulative variances and red colour indicates individual variances, while in (f) darker grey area represents high stiff regions. In (e), covariance map represents correlated, anticorrelated or uncorrelated motions shown in red, blue or white colour, respectively.
3. Experimental set-up
3.1. Synthesis
Chemicals acquired from E. Merck, Fluka or BDH were used as received, however solvents were distilled to purify. Gallenkamp melting point apparatus was used for determination of melting points of all the synthesized compounds. For FT-IR spectra, a Thermo Nicolet IR 200 spectrometer with KBr discs was used. A Brücker Avance NMR instrument was used for 13C NMR spectra at 75 MHz and 1H NMR spectra at 300 MHz. Mass spectra were recorded using the EI mode on a JEOL 600H-1 instrument. A LECO 630–200–200 TRUSPEC CHNS microanalyzer was used for elemental analysis and the data obtained was within ±0.4% of the calculated results. Compounds 2–4 were prepared according to the literature [55,57].
General synthetic procedure for 1,2-Benzothiazine-N-arylacetamides 5(a-n)
To a solution of compound 3 (1.0 mmol) in 5 ml dry acetone was added anhydrous K2CO3 (1.5 mmol) and a solution of the corresponding 2-chloro-N-arylacetamide 4 (1.0 mmol) in 2 ml acetone drop-wise. The reaction mixture was refluxed for 6–8 h until the complete consumption of reactants as indicated by thin layer chromatography. Workup of the reaction was done by cooling, dilution with cold distilled water and acidification with cold dilute HCl (20%). The resulted precipitates were collected via filtration, washed with excess distilled water and dried at 70°C. Good to excellent isolated yields were recorded for all the products after re-crystallization with distilled methanol.
Methyl 4-hydroxy-2-(2-oxo-2-(phenylamino)ethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5a)
Yield 81%. White solid; m.p. 188–190°C. IR (KBr)cm−1: 3260 (N–H), 2951 (C–H), 1681 (C=O), 1602 (C=C), 1247 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.91 (s, 3H, OCH3), 4.46 (s, 2H, CH2), 6.98 (t, J = 7.2 Hz, 1H, ArH), 7.21 (t, J = 7.8 Hz, 2H, ArH), 7.31 (d, J = 7.5 Hz, 2H, ArH), 7.79–7.88 (m, 3H, ArH), 8.04 (dd, J = 6.3 Hz, 2.1 Hz, 1H, ArH), 9.98 (s, 1H, NH), 11.82 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.0, 53.5, 108.3, 119.4, 122.0, 123.8, 126.5, 128.2, 129.2, 132.8, 133.2, 138.3, 138.8, 158.0, 165.8, 168.7 ppm; Anal. calculated for C18H16N2O6S: C, 55.66; H, 4.15; N, 7.21; S, 8.26; Found: C, 55.70; H, 4.25; N, 7.29; S, 8.32; MS (EI+) m/z: 388.3 (M+).
Methyl 4-hydroxy-2-(2-((2-methoxyphenyl)amino)-2-oxoethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5b)
Yield 78%. Light yellow solid; m.p. 176–178°C. IR (KBr)cm−1: 3260 (N–H), 2950 (C–H), 1674 (C=O), 1605 (C=C), 1265 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.83 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.42 (s, 2H, CH2), 6.79–6.85 (m, 1H, ArH), 6.99–7.03 (m, 2H, ArH), 7.75 (d, J = 7.8 Hz, 1H, ArH), 7.83–7.90 (m, 3H, ArH), 8.02–8.05 (m, 1H, ArH), 9.32 (s, 1H, NH), 11.80 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.5, 53.8, 56.2, 108.9, 111.5, 120.7, 121.1, 122.6, 124.7, 126.7, 127.2, 128.2, 133.1, 133.3, 137.5, 149.3, 157.8, 166.1, 168.6 ppm; Anal. calculated for C19H18N2O7S: C, 54.54; H, 4.34; N, 6.70; S, 7.66; Found: C, 54.58; H, 4.36; N, 6.76; S, 7.70; MS (EI+) m/z: 418.3 (M+).
Methyl 4-hydroxy-2-(2-oxo-2-(o-tolylamino)ethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5c)
Yield 76%. White solid; m.p. 172–174°C. IR (KBr)cm−1: 3282 (N–H), 2959 (C–H), 1672 (C=O), 1609 (C=C), 1253 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 2.04 (s, 3H, CH3), 3.93 (s, 3H, OCH3), 4.45 (s, 2H, CH2), 7.00–7.09 (m, 2H, ArH), 7.13–7.16 (m, 2H, ArH), 7.80–7.86 (m, 3H, ArH), 8.00 (dd, J = 6.3 Hz, 1.2 Hz, 1H, ArH), 9.41 (s, 1H, NH), 11.09 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 18.0, 53.0, 53.5, 108.4, 122.1, 125.0, 125.8, 126.4, 126.5, 128.3, 130.7, 132.0, 132.9, 133.2, 136.0, 138.0, 158.0, 165.8, 168.7 ppm; Anal. calculated for C19H18N2O6S: C, 56.71; H, 4.51; N, 6.96; S, 7.97; Found: C, 56.75; H, 4.55; N, 7.00; S, 8.03; MS (EI+) m/z: 402.1 (M+).
Methyl 4-hydroxy-2-(2-((2-(methoxycarbonyl)phenyl)amino)-2-oxoethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5d)
Yield 80%. White solid; m.p. 178–180°C. IR (KBr)cm−1: 3247 (N–H), 2959 (C–H), 1672 (C=O), 1590 (C=C), 1252 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.84 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.34 (s, 2H, CH2), 7.19 (t, J = 7.8 Hz, 1H, ArH), 7.56 (t, J = 7.8 Hz, 1H, ArH), 7.87–7.92 (m, 4H, ArH), 8.05 (d, J = 6.3 Hz, 1H, ArH), 8.17 (d, J = 8.4 Hz, 1H, ArH), 10.98 (s, 1H, NH), 11.83 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.0, 53.4, 54.4, 109.2, 118.2, 121.3, 123.0, 124.0, 126.8, 128.0, 131.1, 133.5, 134.4, 136.8, 139.3, 157.6, 166.7, 167.5, 168.5 ppm; Anal. calculated for C20H18N2O8S: C, 53.81; H, 4.06; N, 6.27; S, 7.18; Found: C, 53.97; H, 4.22; N, 6.43; S, 7.30; MS (EI+) m/z: 446.3 (M+).
Methyl 4-hydroxy-2-(2-((3-methoxyphenyl)amino)-2-oxoethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5e)
Yield 84%. White solid; m.p. 178–180°C. IR (KBr)cm−1: 3260 (N–H), 2950 (C–H), 1681 (C=O), 1674 (C=C), 1265 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.65 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.43 (s, 2H, CH2), 6.58 (dd, J = 8.1 Hz, 1.8 Hz, 1H, ArH), 6.88–6.93 (m, 2H, ArH), 7.12 (t, J = 8.1 Hz, 1H, ArH), 7.79–7.88 (m, 3H, ArH), 8.04 (dd, J = 6.3 Hz, 2.1 Hz, 1H, ArH), 9.98 (s, 1H, NH), 11.81 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.1, 53.5, 55.4, 105.5, 108.3, 109.0, 111.8, 122.0, 126.5, 128.3, 130.0, 132.8, 133.2, 138.2, 139.9, 158.0, 159.9, 165.8, 168.7 ppm; Anal. calculated for C19H18N2O7S: C, 54.54; H, 4.34; N, 6.70; S, 7.66; Found: C, 54.62; H, 4.42; N, 6.74; S, 7.68; MS (EI+) m/z: 418.2 (M+).
Methyl 4-hydroxy-2-(2-oxo-2-(m-tolylamino)ethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5f)
Yield 86%. Light brown solid; m.p. 192–194°C. IR (KBr)cm−1: 3254 (N–H), 2956 (C–H), 1670 (C=O), 1606 (C=C), 1253 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 2.19 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 4.44 (s, 2H, CH2), 6.81 (d, J = 3.6 Hz, 1H, ArH), 7.09–7.11 (m, 3H, ArH), 7.82–7.88 (m, 3H, ArH), 8.04 (dd, J = 6.3 Hz, 1.8 Hz, 1H, ArH), 9.91 (s, 1H, NH), 11.77 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 21.6, 53.0, 53.5, 108.2, 116.7, 119.9, 122.0, 124.6, 126.6, 128.2, 129.0, 132.8, 133.2, 138.2, 138.4, 138.7, 157.9, 165.7, 168.7 ppm; Anal. calculated for C19H18N2O6S: C, 56.71; H, 4.51; N, 6.96; S, 7.97; Found: C, 56.59; H, 4.47; N, 6.91; S, 7.90; MS (EI+) m/z: 402.3 (M+).
Methyl 4-hydroxy-2-(2-((4-methoxyphenyl)amino)-2-oxoethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5g)
Yield 86%. Light grey solid; m.p. 176–178°C. IR (KBr)cm−1: 3263 (N–H), 1672 (C=O), 1607 (C=C), 1249 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.66 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.42 (s, 2H, CH2), 6.79 (d, J = 9.0 Hz, 2H, ArH), 7.22 (d, J = 9.0 Hz, 2H, ArH), 7.81–7.89 (m, 3H, ArH), 8.03 (dd, J = 6.0 Hz, 2.4 Hz, 1H, ArH), 9.87 (s, 1H, NH), 11.82 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 52.9, 53.5, 55.5, 108.2, 114.3, 121.0, 122.0, 126.5, 128.3, 131.9, 132.8, 133.2, 138.2, 155.7, 158.0, 165.2, 168.7 ppm; Anal. calculated for C19H18N2O7S: C, 54.54; H, 4.34; N, 6.70; S, 7.66; Found: C, 54.50; H, 4.28; N, 6.66; S, 7.58; MS (EI+) m/z: 418.3 (M+).
Methyl 4-hydroxy-2-(2-oxo-2-(p-tolylamino)ethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5h)
Yield 82%. White solid; m.p. 182–184°C. IR (KBr)cm−1: 3254 (N–H), 2956 (C–H), 1670 (C=O), 1606 (C=C), 1253 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 2.19 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 4.44 (s, 2H, CH2), 7.01 (d, J = 8.4 Hz, 2H, ArH), 7.19 (d, J = 8.4 Hz, 2H, ArH), 7.79–7.88 (m, 3H, ArH), 8.03 (dd, J = 6.3 Hz, 2.4 Hz, 1H, ArH), 9.89 (s, 1H, NH), 11.82 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 20.9, 53.0, 53.5, 108.3, 119.4, 122.0, 126.5, 128.2, 129.5, 132.1, 132.7, 133.2, 136.3, 138.3, 158.0, 165.5, 168.7 ppm; Anal. calculated for C19H18N2O6S: C, 56.71; H, 4.51; N, 6.96; S, 7.97; Found: C, 56.83; H, 4.65; N, 7.04; S, 8.03; MS (EI+) m/z: 402.3 (M+).
Methyl 2-(2-((4-chlorophenyl)amino)-2-oxoethyl)-4-hydroxy-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5i)
Yield 80%. White solid; m.p. 148–150°C. IR (KBr)cm−1: 3253 (N–H), 3111, 2954 (C–H), 1679 (C=O), 1598 (C=C), 1250 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.90 (s, 3H, OCH3), 4.47 (s, 2H, CH2), 7.27 (dd, J = 6.9 Hz, 2.1 Hz, 2H, ArH), 7.35 (dd, J = 6.9 Hz, 2.1 Hz, 2H, ArH), 7.79–7.88 (m, 3H, ArH), 8.04 (dd, J = 6.3 Hz, 2.1 Hz, 1H, ArH), 10.16 (s, 1H, NH), 11.82 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 52.9, 53.5, 108.2, 120.9, 121.9, 126.5, 127.4, 128.2, 129.1, 132.8, 133.2, 137.8, 138.3, 158.0, 166.0, 168.7 ppm; Anal. calculated for C18H15ClN2O6S: C, 51.13; H, 3.58; N, 6.63; S, 7.58; Found: C, 51.19; H, 3.66; N, 6.71; S, 7.64; MS (EI+) m/z: 422.1 (M+).
Methyl 4-hydroxy-2-(2-((4-nitrophenyl)amino)-2-oxoethyl)-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5j)
Yield 85%. Brown solid; m.p. 192–194°C. IR (KBr)cm−1: 3349 (N–H), 3046 (C–H), 1646 (C=O), 1554 (C=C), 1286 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.91 (s, 3H, OCH3), 4.56 (s, 2H, CH2), 7.56 (d, J = 9.3 Hz, 2H, ArH), 7.81–7.91 (m, 3H, ArH), 8.06 (dd, J = 6.3 Hz, 2.1 Hz, 1H, ArH), 8.14 (d, J = 9.3 Hz, 2H, ArH), 10.63 (s, 1H, NH), 11.83 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.0, 53.5, 108.2, 119.1, 121.9, 125.5, 126.6, 128.2, 132.9, 133.3, 138.2, 142.7, 144.9, 157.9, 167.0, 168.6 ppm; Anal. calculated for C18H15N3O8S: C, 49.88; H, 3.49; N, 9.70; S, 7.40; Found: C, 49.70; H, 3.31; N, 9.68; S, 7.32; MS (EI+) m/z: 433.3 (M+).
Methyl 2-(2-((4-(ethoxycarbonyl)phenyl)amino)-2-oxoethyl)-4-hydroxy-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5k)
Yield 82%. White solid; m.p. 208–210°C. IR (KBr)cm−1: 3364 (N–H), 2956 (C–H), 1664 (C=O), 1599 (C=C), 1254 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 1.28 (t, J = 7.2 Hz, 3H, CH3), 3.90 (s, 3H, OCH3), 4.25 (q, J = 7.2 Hz, 2H, CH2), 4.51 (s, 2H, CH2), 7.46 (d, J = 9.0 Hz, 2H, ArH), 7.82–7.88 (m, 5H, ArH), 8.05 (dd, J = 6.3 Hz, 1.8 Hz, 1H, ArH), 10.36 (s, 1H, NH), 11.82 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 14.6, 53.0, 53.5, 60.9, 108.2, 118.8, 122.0, 124.8, 126.6, 128.3, 130.7, 132.9, 133.2, 138.2, 143.1, 158.0, 165.7, 166.6, 168.7 ppm; Anal. calculated for C21H20N2O8S: C, 54.78; H, 4.38; N, 6.08; S, 6.96; Found: C, 54.90; H, 4.52; N, 6.20; S, 7.10; MS (EI+) m/z: 460.3 (M+).
Methyl 2-(2-((4-acetamidophenyl)amino)-2-oxoethyl)-4-hydroxy-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5l)
Yield 88%. White solid; m.p. 202–204°C. IR (KBr)cm−1 : 3402, 3277 (N–H), 3052, 2822 (C–H), 1671 (C=O), 1619 (C=C), 1252 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 1.98 (s, 3H, CH3), 3.91 (s, 3H, OCH3), 4.43 (s, 2H, CH2), 7.22 (d, J = 8.7 Hz, 2H, ArH), 7.41 (d, J = 8.7 Hz, 2H, ArH), 7.79–7.89 (m, 3H, ArH), 8.03 (dd, J = 6.3 Hz, 2.1 Hz, 1H, ArH), 9.84 (s, 1H, NH), 9.93 (s, 1H, NH), 11.81 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 24.3, 52.9, 53.5, 108.2, 119.8, 122.0, 126.5, 128.2, 132.8, 133.2, 134.0, 135.4, 138.2, 158.0, 165.4, 168.4, 168.7 ppm; Anal. calculated for C20H19N3O7S: C, 53.93; H, 4.30; N, 9.43; S, 7.20; Found: C, 54.01; H, 4.36; N, 9.53; S, 7.32; MS (EI+) m/z: 445.3 (M+).
Methyl 2-(2-((2,3-dichlorophenyl)amino)-2-oxoethyl)-4-hydroxy-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5m)
Yield 85%. White solid; m.p. 154–156°C. IR (KBr)cm−1: 3267 (N–H), 2957 (C–H), 1681 (C=O), 1573 (C=C), 1247 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.92 (s, 3H, OCH3), 4.51 (s, 2H, CH2), 7.27 (t, J = 8.1 Hz, 1H, ArH), 7.41 (dd, J = 8.1 Hz, 1.5 Hz, 1H, ArH), 7.54 (dd, J = 8.1 Hz, 1.5 Hz, 1H, ArH), 7.82–7.87 (m, 3H, ArH), 8.02 (dd, J = 6.0 Hz, 1.8 Hz, 1H, ArH), 9.79 (s, 1H, NH), 11.80 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.1, 53.6, 108.5, 122.3, 123.9, 124.7, 126.6, 127.1, 128.2, 128.6, 132.3, 133.1, 133.3, 136.6, 137.7, 157.8, 166.6, 168.6 ppm; Anal. calculated for C18H14Cl2N2O6S: C, 47.28; H, 3.09; N, 6.13; S, 7.01; Found: C, 47.32; H, 3.11; N, 6.19; S, 7.13; MS (EI+) m/z: 456.1(M+).
Methyl 2-(2-((2,4-dichlorophenyl)amino)-2-oxoethyl)-4-hydroxy-2H-benzo[e][1,2]thiazine-3-carboxylate 1,1-dioxide (5n)
Yield 82%. White solid; m.p. 200–202°C. IR (KBr)cm−1: 3364 (N–H), 2957 (C–H), 1663 (C=O), 1581 (C=C), 1246 (C–N); 1H NMR (DMSO-d6, 300 MHz) δ: 3.92 (s, 3H, OCH3), 4.50 (s, 2H, CH2), 7.21 (dd, J = 8.7 Hz, 2.4 Hz, 1H, ArH), 7.50 (d, J = 8.7 Hz, 1H, ArH), 7.69 (d, J = 2.4 Hz, 1H, ArH), 7.84–7.90 (m, 3H, ArH), 8.04 (dd, J = 6.0 Hz, 2.4 Hz, 1H, ArH), 9.72 (s, 1H, NH), 11.79 (s, 1H, OH) ppm; 13C NMR (DMSO-d6, 100 MHz) δ: 53.2, 53.6, 108.6, 122.5, 124.0, 126.1, 126.7, 128.2, 131.3, 132.0, 133.2, 133.4, 135.9, 137.6, 157.7, 166.9, 168.5 ppm; Anal. calculated for C18H14Cl2N2O6S: C, 47.28; H, 3.09; N, 6.13; S, 7.01; Found: C, 47.36; H, 3.21; N, 6.25; S, 7.13; MS (EI+) m/z: 456.1 (M+).
3.2. Urease inhibition activity assay
Compounds 5(a-n) were investigated to evaluate the inhibitory activity employing the slightly modified indophenol method [58,59]. Assay mixture containing 1 unit of urease enzyme solution and 60 µl of buffer comprising urea substrate (100 mM) were pre-incubated with synthesized compounds (10 µl of 1 mM solution) at room temperature for 10 min in 96-well plates. Inhibitory activity of all test compounds was noted by measuring NH3 production using the above-mentioned protocol. Momentarily, phenolic reagent (50 µl) and alkali reagent (70 µl) were added in each well. After half an hour, absorbance at 630 nm was determined for all, using a Bio-Tek ELx 800, Instruments, Inc. USA Elisa-plate reader. Triplicate data for all the experiments was obtained and percentage inhibitory activities were calculated using the following formula:
3.3. Docking protocol
3.3.1. Structure assortment and preparation
Using the molecular docking protocol, the jack bean urease's crystallographic structure (3LA4; PDB) was downloaded from the protein data bank library [56] and prepared for the investigation by forming the complex with urease enzyme. Before docking analysis, structures of compounds and enzyme were prepared. To protonate the structure of the enzyme within the molecular modelling software MOE, the protonate3D technique was used [60,61]. Structure energy of molecules as well as molecules of solvent was minimized using the Amber99 force field. To overcome the collapsing of binding pockets during the calculations of minimization of energy, small force was used to strengthen the backbone atoms. Consequently, the ligands as well as water molecules were removed and addition of polar hydrogens was carried out in the crystallographic structure employing MOE.
3.3.2. Compounds preparation
A ‘wash' module was applied to the three-dimensional structural coordinates of synthesized compounds (5a, 5l and 5k) for the assignment of protonation process and ionization state within the specific range of pH by MOE. Subsequently, for the investigation of docking, the energy of the chemical structure was minimized using the MMFF94x force field.
3.3.3. Docking studies
LeadIT software from BioSolveIT, GmbH Germany [62] was used for calculations and docking investigations. The LeadIT tool, receptor utility was uploaded by Load or Prepare Receptor tool along with metal ions identified as part of it. The binding site was defined within the 9.0 spacing of residues. Docking of the inhibitor was performed employing the FlexX utility from LeadIT. Depending upon the free binding energies for all the three compounds 5a, 5l and 5k, almost 50 different conformations for each complex were collected. Already defined parameters of docking were used, and the best 30 poses were selected for further analysis [63]. Poses having less binding free energy were selected as the stable and exhibited maximum affinity with the load receptor. Such three-dimensional models were fabricated using Discovery Studio Visualization v4 to evaluate the interactions between ligand–protein complex having minimum free binding energy [64].
3.3.4. Molecular dynamics simulations
These were performed by online server iMODs for the docking investigation as well as the dynamics of the complex to determine the motion of molecules. Normal Mode Analysis (NMA) was also determined through the iMODs server [65]. This tool also generates different parameters such as deformability, B-factors, eigenvalue, variance, covariance and elastic network. For these investigations, a PDB file was used to upload the input file on the iMODs server.
4. Conclusion
In summary, a series of new benzothiazine 5(a-n) derivative compounds were designed and synthesized through a multistep and facile approach, considering the significance of thiazine as a versatile category of new drugs for the treatment of ureolytic infections. Substitution of electron deficient or rich groups at different positions of the phenyl ring provided a huge opportunity for demonstrating important interactions with different amino acid residues. All the compounds 5(a-n) exhibited outstanding inhibitory potential (9.8–20.7 µM) when compared with positive control thiourea (22.3 ± 0.031), among which 5k emerged as the best candidate with strong inhibition depicting an IC50 value (9.8 ± 0.023 µM). To identify the important parameters required to increase potency, structure–activity relationship was generated. An in silico study showed the conventional hydrogen bonding, π-sigma, π-alkyl as well as π-cation interactions with different amino acid residues like Lys716, Phe712, Ala16 and Val744. Molecular dynamics simulations validated the stability and deformability of the protein with the inhibitors. Therefore, a new template based on the identified inhibitors could be developed for the treatment of ureolytic bacterial infections using the identified inhibitors.
Acknowledgements
The authors are thankful to PCSIR laboratories complex, Lahore for providing research facilities.
Contributor Information
Maliha Uroos, Email: malihauroos.chem@pu.edu.pk.
Muhammad Zia-ur-Rehman, Email: rehman_pcsir@hotmail.com.
Data accessibility
The data presented in this study are available in electronic supplementary material which include NMR and mass spectra of all the synthesized compounds, SeeSAR visual drug design and ADMET Properties of the synthesized compounds [66].
Authors' contributions
S.H.: investigation, writing—original draft; S.Z.: methodology, software, supervision, validation; M.U.: supervision; M.Z.R.: conceptualization, methodology, project administration, resources; R.M.: data curation, formal analysis, writing—review and editing; H.R.: investigation; Q.S.: formal analysis; S.H.I.A.: formal analysis, resources.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Liu Q, et al. 2018. Arylamino containing hydroxamic acids as potent urease inhibitors for the treatment of Helicobacter pylori infection. Eur. J. Med. Chem. 156, 126-136. ( 10.1016/j.ejmech.2018.06.065) [DOI] [PubMed] [Google Scholar]
- 2.Follmer C. 2010. Ureases as a target for the treatment of gastric and urinary infections. J. Clin. Pathol. 63, 424-430. ( 10.1136/jcp.2009.072595) [DOI] [PubMed] [Google Scholar]
- 3.Zaib S, Younas MT, Zaraei S-O, Khan I, Anbar HS, El-Gamal MI. 2022. Discovery of urease inhibitory effect of sulfamate derivatives: biological and computational studies. Bioorg. Chem. 119, 105545. ( 10.1016/j.bioorg.2021.105545) [DOI] [PubMed] [Google Scholar]
- 4.Karita M, Tsuda M, Nakazawa T. 1995. Essential role of urease in vitro and in vivo Helicobacter pylori colonization study using a wild-type and isogenic urease mutant strain. J. Clin. Gastroenterol. 21, S160-S163. [PubMed] [Google Scholar]
- 5.Mobley H, Island MD, Hausinger RP. 1995. Molecular biology of microbial ureases. Microbiol. Rev. 59, 451-480. ( 10.1128/mr.59.3.451-480.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pervez H, Khan N, Iqbal J, Zaib S, Yaqub M, Tahir MN, Naseer MM. 2018. Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors. Heterocycl. Commun. 24, 51-58. ( 10.1515/hc-2017-0148) [DOI] [Google Scholar]
- 7.Kosikowska P, Berlicki L. 2011. Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin. Ther. Pat. 21, 945-957. ( 10.1517/13543776.2011.574615) [DOI] [PubMed] [Google Scholar]
- 8.Odake S, Morikawa T, Tsuchiya M, Imamura L, Kobashi K. 1994. Inhibition of Helicobacter pylori Urease Activity by Hydroxamic Acid Derivatives.. Biol. Pharm. Bull. 17, 1329-1332. ( 10.1248/bpb.17.1329) [DOI] [PubMed] [Google Scholar]
- 9.Betkas H, Ceylan S, Demisbas N, Alpay-Karaoglu S, Sokmen BB. 2013. Antimicrobial and antiurease activities of newly synthesized morpholine derivatives containing an azole nucleus. Med. Chem. Res. 22, 3629-3639. ( 10.1007/s00044-012-0318-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali B, Khan KM, Hussain S, Hussain S, Ashraf M, Riaz M, Wadood A, Perveen S. 2018. Synthetic nicotinic/isonicotinic thiosemicarbazides: In vitro urease inhibitory activities and molecular docking studies. Bioorg. Chem. 79, 34-45. ( 10.1016/j.bioorg.2018.04.004) [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T, Kawase M, Tani S. 2003. Urease inhibitory activity of simple α,β-unsaturated ketones. Life Sci. 73, 2985-2990. ( 10.1016/S0024-3205(03)00708-2) [DOI] [PubMed] [Google Scholar]
- 12.Pervez H, Chohan ZH, Ramzan M, Nasim FU, Khan KM. 2009. Synthesis and biological evaluation of some new N4-substituted isatin-3-thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 24, 437-446. ( 10.1080/14756360802188420) [DOI] [PubMed] [Google Scholar]
- 13.Babu TMC, Rajesh SS, Bhaskar BV, Devi S, Rammohan A, Sivaraman T, Rajendra W. 2017. Molecular docking, molecular dynamics simulation, biological evaluation and 2D QSAR analysis of flavonoids from Syzygium alternifolium as potent anti-Helicobacter pylori agents. RSC Adv. 7, 18 277-18 292. ( 10.1039/C6RA27872H) [DOI] [Google Scholar]
- 14.Zhou JT, et al. 2017. Inhibition of Helicobacter pylori and its associated urease by palmatine: investigation on the potential mechanism. PLoS one 12, e0168944. ( 10.1371/journal.pone.0168944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L, et al. 2017. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. Eur. J. Pharm. Sci. 110, 77-86. ( 10.1016/j.ejps.2017.02.004) [DOI] [PubMed] [Google Scholar]
- 16.Aslam MAS, Mahmood SU, Shahid M, Saeed A, Iqbal J. 2011. Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur. J. Med. Chem. 46, 5473-5479. ( 10.1016/j.ejmech.2011.09.009) [DOI] [PubMed] [Google Scholar]
- 17.Arshad T, et al. 2017. 5-Bromo-2-aryl benzimidazole derivatives as non-cytotoxic potential dual inhibitors of α -glucosidase and urease enzymes. Bioorg. Chem. 72, 21-31. ( 10.1016/j.bioorg.2017.03.007) [DOI] [PubMed] [Google Scholar]
- 18.Menteşe E, Bektaş H, Sokmen BB, Emirik M, Çakır D, Kahveci B. 2017. Synthesis and molecular docking study of some 5,6-dichloro-2-cyclopropyl-1 H -benzimidazole derivatives bearing triazole, oxadiazole, and imine functionalities as potent inhibitors of urease. Bioorg. Med. Chem. Lett. 27, 3014-3018. ( 10.1016/j.bmcl.2017.05.019) [DOI] [PubMed] [Google Scholar]
- 19.Dixon NE, Gazzola C, Watters JJ, Blakeley RL, Zerner B. 1975. Inhibition of jack bean urease (EC 3.5.1.5) by acetohydroxamic acid and by phosphoramidate. Equivalent weight for urease. J. Am. Chem. Soc. 97, 4130-4131. ( 10.1021/ja00847a044) [DOI] [PubMed] [Google Scholar]
- 20.Breitenbach JM, Hausinger RP. 1988. Proteus mirabilis urease. Partial purification and inhibition by boric acid and boronic acids. Biochem. J. 250, 917-920. ( 10.1042/bj2500917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krajewska B. 2008. Mono- (Ag, Hg) and di- (Cu, Hg) valent metal ions effects on the activity of jack bean urease. Probing the modes of metal binding to the enzyme. J. Enzyme Inhib. Med. Chem. 23, 535-542. ( 10.1080/14756360701743051) [DOI] [PubMed] [Google Scholar]
- 22.Bano B, Khan KM, Lodhi A, Salar U, Begum F, Ali M, Taha M, Perveen S. 2018. Synthesis, in vitro urease inhibitory activity, and molecular docking studies of thiourea and urea derivatives. Bioorg. Chem. 80, 129-144. ( 10.1016/j.bioorg.2018.06.007) [DOI] [PubMed] [Google Scholar]
- 23.Khan KM, et al. 2014. Synthesis and structure–activity relationship of thiobarbituric acid derivatives as potent inhibitors of urease. Bioorg. Med. Chem. 22, 4119-4123. ( 10.1016/j.bmc.2014.05.057) [DOI] [PubMed] [Google Scholar]
- 24.Rauf A, Ahmed F, Qureshi A, Khan A, Qadir M, Choudhary M, Chohan Z, Youssoufid M, Haddad TB. 2011. Synthesis and urease inhibition studies of barbituric and thiobarbituric acid derived sulphonamides. J. Chin. Chem. Soc. 58, 528-537. ( 10.1002/jccs.201190017) [DOI] [Google Scholar]
- 25.Prakash O, Upadhyay LSB. 2004. Inhibition of water melon (Citrullus vulgaris) urease by fluoride. J. Plant Biochem. Biotechnol. 13, 61-64. ( 10.1007/BF03263193) [DOI] [Google Scholar]
- 26.Milo S, et al. 2021. A small-molecular inhibitor against Proteus mirabilis urease to treat catheter-associated urinary tract infections. Sci. Rep. 11, 3726. ( 10.1038/s41598-021-83257-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohrabi M, et al. 2022. Design and synthesis of novel nitrothiazolacetamide conjugated to different thioquinazolinone derivatives as anti-urease agents. Sci. Rep. 12, 2003. ( 10.1038/s41598-022-05736-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gouda MA, Hussein BHM, Sherif YE. 2017. Synthesis and medicinal importance of oxicams and their analogues. Synth. Commun. 47, 1709-1736. ( 10.1080/00397911.2017.1350983) [DOI] [Google Scholar]
- 29.Xu S, Rouzer CA, Marnett LJ. 2014. Oxicams, a class of nonsteroidal anti-inflammatory drugs and beyond. IUBMB Life 66, 803-811. ( 10.1002/iub.1334) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczęśniak-Sięga BM, Wiatrak B, Czyżnikowska Ż, Janczak J, Wiglusz RJ, Maniewska J. 2021. Synthesis and biological evaluation as well as in silico studies of arylpiperazine-1, 2-benzothiazine derivatives as novel anti-inflammatory agents. Bioorg. Chem. 106, 104476. ( 10.1016/j.bioorg.2020.104476) [DOI] [PubMed] [Google Scholar]
- 31.Bihovsky R, Tao M, Mallamo JP, Wells GJ. 2004. 1,2-Benzothiazine 1,1-dioxide α-ketoamide analogues as potent calpain I inhibitors. Bioorg. Med. Chem. Lett. 14, 1035-1038. ( 10.1016/j.bmcl.2003.11.037) [DOI] [PubMed] [Google Scholar]
- 32.Navarro-Ruiz E, Alvarez-Alvarez C, Pena MA, Torrado-Salmeron C, Dahma Z, de la Torre-Iglesias PM. 2022. Multiparticulate systems of meloxicam for colonic administration in cancer or autoimmune diseases. Pharmaceutics 14, 1504. ( 10.3390/pharmaceutics14071504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudek-Wicher RK, Szczęsniak-Sięga BM, Wiglusz RJ, Janczak J, Bartoszewicz M, Junka AF. 2020. Evaluation of 1,2-benzothiazine 1,1-dioxide derivatives in vitro activity towards clinical-relevant microorganisms and fibroblasts. Molecules 25, 3503. ( 10.3390/molecules25153503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczęsniak-Sięga BM, Mogilski S, Wiglusz RJ, Janczak J, Maniewska J, Malinka W, Filipek B. 2019. Synthesis and pharmacological evaluation of novel arylpiperazine oxicams derivatives as potent analgesics without ulcerogenicity. Bioorg. Med. Chem. 27, 1619-1628. ( 10.1016/j.bmc.2019.03.007) [DOI] [PubMed] [Google Scholar]
- 35.Bartolini D, Bührmann M, Barreca ML, Manfroni G, Cecchetti V, Rauh D, Galli F. 2019. Co-crystal structure determination and cellular evaluation of 1,4-dihydropyrazolo[4,3-c] [1,2] benzothiazine 5,5-dioxide p38α MAPK inhibitors. Biochem. Biophys. Res. Commun. 511, 579-586. ( 10.1016/j.bbrc.2019.02.063) [DOI] [PubMed] [Google Scholar]
- 36.Ahmad S, et al. 2019. Synthesis, X-ray crystal and monoamine oxidase inhibitory activity of 4,6-dihydrobenzo[c]pyrano[2,3-e][1,2]thiazine 5,5-dioxides: In vitro studies and docking analysis. Eur. J. Pharm. Sci. 131, 9-22. ( 10.1016/j.ejps.2019.02.007) [DOI] [PubMed] [Google Scholar]
- 37.Shabbir A, Shahzad M, Ali A, Zia-ur-Rehman M. 2014. Anti-arthritic activity of N′-[(2,4-dihydroxyphenyl)methylidene]-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-1(4H)-yl)acetohydrazide. Eur. J. Pharmacol. 738, 263-272. ( 10.1016/j.ejphar.2014.05.045) [DOI] [PubMed] [Google Scholar]
- 38.Zia-ur-Rehman M, Choudary JA, Elsegood MRJ, Siddiqui HL, Khan KM. 2009. A facile synthesis of novel biologically active 4-hydroxy-N′-(benzylidene)-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxides. Eur. J. Med. Chem. 44, 1311-1316. ( 10.1016/j.ejmech.2008.08.002) [DOI] [PubMed] [Google Scholar]
- 39.Ahmad M, et al. 2015. Molecular docking and antiviral activity of N-substituted benzyl/phenyl-2-(3,4-dimethyl-5,5-dioxidopyrazolo[4,3-c][1,2]benzothiazin-2(4H)-yl)acetamides. Bioorg. Med. Chem. Lett. 25, 1348-1351. ( 10.1016/j.bmcl.2015.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imani A, Soleymani S, Vahabpour R, Hajimahdi Z, Zarghi A. 2021. Simultaneous estimation of protein and nucleic acid using derivative spectrophotometric method. Iran. J. Pharm. Res. 20, 1-4. ( 10.18579/jopcr/v20i3.ms21041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taj S, Ahmad M, Ashfaq UA. 2022. Exploring of novel 4-hydroxy-2H-benzo[e][1,2]thiazine-3-carbohydrazide 1,1-dioxide derivative as a dual inhibitor of α-glucosidase and α-amylase: molecular docking, biochemical, enzyme kinetic and in-vivo mouse model study. Int. J. Biol. Macromol. 207, 507-521. ( 10.1016/j.ijbiomac.2022.03.023) [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T, Yajima N, Tanitame A, Kiyoshi T, Miura Y. 2015. Discovery of benzothiazine derivatives as novel, orally-active anti-epileptic drug candidates with broad anticonvulsant effect. Bioorg. Med. Chem. Lett. 25, 4518-4521. ( 10.1016/j.bmcl.2015.08.071) [DOI] [PubMed] [Google Scholar]
- 43.Barazarte A, Lobo G, Gamboa N, Rodrigues JR, Capparelli MV, Alvarez-Larena A, Lopez SE, Charris JE. 2009. Synthesis and antimalarial activity of pyrazolo and pyrimido benzothiazine dioxide derivatives. Eur. J. Med. Chem. 44, 1303-1310. ( 10.1016/j.ejmech.2008.08.005) [DOI] [PubMed] [Google Scholar]
- 44.Zhao HH, Kong CH, Xu XH. 2019. Herbicidal efficacy and ecological safety of an allelochemical-based benzothiazine derivative. Pest. Manag. Sci. 75, 2690-2697. ( 10.1002/ps.5377) [DOI] [PubMed] [Google Scholar]
- 45.Shin YS, et al. 2021. Discovery of cyclic sulfonamide derivatives as potent inhibitors of SARS-CoV-2. Bioorg. Med. Chem. Lett. 31, 127667. ( 10.1016/j.bmcl.2020.127667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saddique FA, Ahmad M, Ashfaq UA, Muddassar M, Sultan S, Zaki MEA. 2022. Identification of cyclic sulfonamides with an N-arylacetamide group as α-glucosidase and α-amylase inhibitors: biological evaluation and molecular modeling. Pharmaceuticals 15, 106. ( 10.3390/ph15010106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saddique FA, et al. 2021. Synthesis and α-glucosidase inhibition activity of 2-[3-(Benzoyl/4-bromobenzoyl)-4-hydroxy-1,1-dioxido-2H-benzo[e][1,2]thiazin-2-yl]-N-arylacetamides: an in silico and biochemical approach. Molecules 26, 3043. ( 10.3390/molecules26103043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SH, et al. 2011. Identification of cyclicsulfonamide derivatives with an acetamide group as 11.BETA.-hydroxysteroid dehydrogenase 1 inhibitors. Chem. Pharm. Bull. 59, 46-52. ( 10.1248/cpb.59.46) [DOI] [PubMed] [Google Scholar]
- 49.Zaheer M, Zia-ur-Rehman M, Munir R, Jamil N, Ishtiaq S, Saleem RSZ, Elsegood MRJ. 2021. (Benzylideneamino) triazole–thione derivatives of flurbiprofen: an efficient microwave-assisted synthesis and in vivo analgesic potential. ACS omega 6, 31 348-31 357. ( 10.1021/acsomega.1c05222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munir R, Zia-ur-Rehman M, Murtaza S, Zaib S, Javid N, Awan SJ, Iftikhar K, Athar MM, Khan I. 2021. Microwave-Assisted Synthesis of (Piperidin-1-yl)quinolin-3-yl)methylene)hydrazinecarbothioamides as potent inhibitors of cholinesterases: a biochemical and in silico approach. Molecules 26, 656. ( 10.3390/molecules26030656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Javid N, Jalil S, Munir R, Zia-ur-Rehman M, Sahar A, Arshad S, Iqbal J. 2023. 2,1-Benzothiazine – (quinolin/thiophen)yl hydrazone frameworks as new monoamine oxidase inhibitory agents; synthesis, in vitro and in silico investigation. RSC Adv. 13, 1701-1710. ( 10.1039/D2RA07045F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shabbir A, Shahzad M, Ali A, Zia-ur-Rehman M. 2016. Discovery of new benzothiazine derivative as modulator of pro- and anti-inflammatory cytokines in rheumatoid arthritis. Inflammation 39, 1918-1929. ( 10.1007/s10753-016-0427-y) [DOI] [PubMed] [Google Scholar]
- 53.Batool M, et al. 2018. Molecular docking, computational, and antithrombotic studies of novel 1,3,4-oxadiazole derivatives. Int. J. Mol. Sci. 19, 3606. ( 10.3390/ijms19113606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mustafa G, Angeli A, Zia-ur-Rehman M, Akbar N, Ishtiaq S, Supuran CT. 2019. An efficient method for the synthesis of novel derivatives 4-{5-[4-(4-amino-5-mercapto-4H-[1,2,4]triazol-3-yl)-phenyl]-3-trifluoromethyl-pyrazol-1-yl}-benzenesulfonamide and their anti-inflammatory potential. Bioorg. Chem. 91, 103110. ( 10.1016/j.bioorg.2019.103110) [DOI] [PubMed] [Google Scholar]
- 55.Zia-ur-Rehman M, Choudary JA, Ahmad S. 2005. An efficient synthesis of 2-alkyl-4-hydroxy-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxides. Bull. Korean Chem. Soc. 26, 1771-1775. ( 10.5012/bkcs.2005.26.11.1771) [DOI] [Google Scholar]
- 56.Balasubramanian A, Ponnuraj K. 2010. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J. Mol. Biol. 400, 274-283. ( 10.1016/j.jmb.2010.05.009) [DOI] [PubMed] [Google Scholar]
- 57.Xia S, et al. 2013. Synthesis of N-azaaryl anilines: an efficient protocol via smiles rearrangement. Bull. Korean Chem. Soc. 34, 394-398. ( 10.5012/bkcs.2013.34.2.394) [DOI] [Google Scholar]
- 58.Weatherburn MW. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39, 971-974. ( 10.1021/ac60252a045) [DOI] [Google Scholar]
- 59.Rauf MK, Yaseen S, Badshah A, Zaib S, Arshad R, Tahir MN, Iqbal J. 2015. Synthesis, characterization and urease inhibition, in vitro anticancer and antileishmanial studies of Ni(II) complexes with N,N,N′-trisubstituted thioureas. J. Biol. Inorg. Chem. 20, 541-554. ( 10.1007/s00775-015-1239-5) [DOI] [PubMed] [Google Scholar]
- 60.Labute P. 2007. Protonate 3D, Available online: Chemical Computing Group, (accessed on 20th September 2021), http://www.chemcomp.com/journal/proton.htm.
- 61.Chemical Computing Group's Molecular Operating Environment (MOE) MOE, 0201. 2019. Available online: http://www.chemcomp.com/MOEMolecular_Operating_Environment.htm (accessed on 20th September 2021).
- 62.LeadIT Version 2.3.2. 2017. Available online:, BioSolveIT GmbH, Sankt Augustin, Germany. (accessed on 20th September 2021). See www.biosolveit.de/LeadIT.
- 63.Schneider N, Lange G, Hindle S, Klein R, Rarey M. 2013. A consistent description of HYdrogen bond and DEhydration energies in protein–ligand complexes: methods behind the HYDE scoring function. J. Comput. Aided Mol. Des. 27, 15-29. ( 10.1007/s10822-012-9626-2) [DOI] [PubMed] [Google Scholar]
- 64.BIOVIA Discovery Studio Client v19.1.0.18287. 2019. Accelrys discovery studio. San Diego, CA: Accelrys Software Inc. [Google Scholar]
- 65.Zahra U, Zaib S, Saeed A, Rehman MU, Shabir G, Khan I. 2022. New acetylphenol-based acyl thioureas broaden the scope of drug candidates for urease inhibition: synthesis, in vitro screening and in silico analysis. Int. J. Biol. Macromol. 198, 157-167. ( 10.1016/j.ijbiomac.2021.12.064) [DOI] [PubMed] [Google Scholar]
- 66.Hina S, Zaib S, Uroos M, Zia-ur-Rehman M, Munir R, Riaz H, Syed Q, Abidi SHI. 2023. N-arylacetamide derivatives of 1,2-benzothiazine-3-carboxylate as potential drug candidates for urease inhibition. Figshare. ( 10.6084/m9.figshare.c.6492761) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hina S, Zaib S, Uroos M, Zia-ur-Rehman M, Munir R, Riaz H, Syed Q, Abidi SHI. 2023. N-arylacetamide derivatives of 1,2-benzothiazine-3-carboxylate as potential drug candidates for urease inhibition. Figshare. ( 10.6084/m9.figshare.c.6492761) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data presented in this study are available in electronic supplementary material which include NMR and mass spectra of all the synthesized compounds, SeeSAR visual drug design and ADMET Properties of the synthesized compounds [66].