Abstract
Aims:
To describe the pain characteristics of five index chronic overlapping pain conditions (COPCs) and to assess each COPC separately in order to determine whether the presence of comorbid COPCs is associated with bodily pain distribution, pain intensity, pain interference, and high-impact pain of the index COPC.
Methods:
Data were from a convenience sample of 655 US adults, of whom 388 had one or more of the five COPCs: painful temporomandibular disorders, headache, low back pain, irritable bowel syndrome, and/or fibromyalgia. Data were collected using pain location checklists and self-report questions regarding pain attributes. The contributions of the COPCs to reported pain intensity and interference were assessed using multivariable regression models.
Results/Conclusion:
Heat maps from a pain body manikin illustrated that very little of the body was pain free within these COPCs. All pain attributes were the most severe for fibromyalgia and the least severe for irritable bowel syndrome. Within each index COPC, pain intensity, pain interference, and the proportion of participants with high-impact pain increased with each additional comorbid COPC up to four or more COPCs (including the index COPC) (P < .01). High-impact pain associated with an index COPC was influenced by type and number of comorbid COPCs, largely in a gradient-specific manner.
Keywords: back pain, chronic overlapping pain conditions, comorbidity, fibromyalgia, headache, irritable bowel syndrome, measurement, pain, pain-related disability, TMD
Common practice for the assessment of pain in both clinical and research settings is to measure pain attributes—such as intensity, interference in functioning, and pain-related disability—for the primary, or index, condition of interest. An index condition could be the symptoms presented by a patient in the clinic or the primary focus in a research study; however, an index pain condition is frequently accompanied by comorbid or overlapping pain conditions, especially when the index condition is chronic.1 This pattern of comorbidity is seen for commonly occurring pain conditions, including temporomandibular disorder (TMD) myalgia or arthralgia, headache (particularly migraine or tension-type headache [TTH]), irritable bowel syndrome (IBS), low back pain (LBP), and widespread pain such as fibromyalgia.2
All five of these conditions share many similarities. For example, they lack a specific etiology in most individuals,3 and their cardinal signs of pain are typically disproportionate to physical findings.4 While there are subtypes of each pain condition defined in terms of clearly identifiable causes (for example, headache attributed to infection), these occur infrequently. Instead, the most prevalent forms of these five pain conditions are grouped using labels such as idiopathic pain disorders,4 chronic overlapping pain conditions (COPCs), central sensitivity syndromes,5 and functional pain syndromes.4 Each term has specific entailments—for example, COPCs are noted to have shared pathophysiologic mechanisms such as altered pain perception and processing, neurocognitive and behavior functions, central arousal circuits, and sleep.1,6–8 The collective term “chronic overlapping pain conditions” is used in this article, consistent with the current terminology favored by the National Institutes of Health.9
Pathophysiologic mechanisms responsible for comorbidities may therefore influence pain attributes reported for index and other pain conditions, and the clinical implication is that the pain reported for the index condition may be enhanced by the presence of comorbid COPCs through their common mechanisms. Speculation regarding an additive effect from two or more overlapping pain disorders emerged prior to the modern era of pain research.10 A synthesis of current evidence suggests that two or more overlapping pain disorders may be interchangeable in their effects on pain processing.1 However, it is equally plausible that the intensity of an index COPC is influenced differentially by overlapping conditions or that the influence is seen only above a threshold number of overlapping conditions.
The extent to which a comorbid COPC affects the reporting of pain attributes associated with an index COPC is not known, but the effect is suspected to be both additive and substantial. Such effects may offer insight into the putatively shared mechanisms underlying multiple pain conditions via the question of whether multiple COPCs are interchangeable with regard to their influence on pain processing, are simply additive, or whether particular COPCs have a specific influence. The implications for assessing pain severity, its interpretation, and the effectiveness of interventions for an index condition in the presence of other COPCs are substantial. For example, in the clinic, the immediate regional pain complaint typically becomes the singular condition for treatment; however, a singular treatment focus is rational only if the index condition and its measured pain attributes are independent of other comorbid conditions. If other pain conditions are present, a singular treatment focus may be inappropriate given that complexity. Moreover, assessing the efficacy of that singular treatment may be compromised if the markers of treatment success are influenced substantially by comorbid pain conditions.
The first aim of this study was to describe the pain characteristics of five index COPCs. For the second aim, each COPC was analyzed separately in order to determine whether the presence of one or more comorbid COPCs was associated with bodily pain distribution, pain intensity, pain interference, and/or high-impact pain of the index COPC. To test these aims, it was assumed that comorbid COPCs influence the pain attributes reported for an index COPC, and the results are reported accordingly for convenience—but, because the data are from a cross-sectional design, no specific causal pattern is implied.
Materials and Methods
Ethical Considerations
The reporting of this observational study conforms with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.11 The primary data collection was from NIDCR Study Protocol 12-050-E, conducted in the second phase of the OPPERA project (Orofacial Pain: Prospective Evaluation and Risk Assessment). The Office of Human Research Ethics at each participating institution reviewed and approved the study.
Study Design, Setting, and Participants
This cross-sectional study investigated characteristics of pain among adults who had one or more of five COPCs: TMD, headache, IBS, LBP, and fibromyalgia. They were selected from the larger sample of study participants in the OPPERA-2 study, which was comprised of both pain-free individuals and others reporting COPCs. The source population and methods of recruitment are described in detail elsewhere in this volume (see Sharma et al, current issue).
In summary, participants were recruited for OPPERA-2 between December 2014 and May 2016 at four US institutions (University at Buffalo, Buffalo, New York; University of Florida, Gainesville, Florida; University of Maryland, Baltimore, Maryland; and University of North Carolina at Chapel Hill, Chapel Hill, North Carolina). The data of those who consented and attended the research clinics were collected using clinical examinations, quantitative sensory testing, cardiovascular measures of autonomic function, blood samples, and self-report questionnaires. Of the 655 participants in OPPERA-2, 348 reported one or more COPCs and are the primary focus for this set of analyses. The sample sizes for the full set of permutations across the COPCs are available in another paper in this series (see Slade et al, current issue).
Classification of Idiopathic Pain Conditions
The presence or absence of each of the five COPCs (defined conditions based on operationalized case definitions) were determined using the best available validated criteria for each of the five conditions. Some or all of the criteria for classifying each COPC were assessed using a computer-based pain-condition questionnaire developed specifically for OPPERA-2. This questionnaire is available in the supplemental materials (Appendix 1; see all appendices in the online version of this article at www.quintpub.com/journals). The questionnaire was administered during the clinic visit (after consent and before any other study procedures) for most participants. Other information regarding the assessment instrument and its administration is available.
Each pain condition questionnaire module started with a general filter question inquiring into COPC-relevant pain during the prior 3 months as the reference period of interest. The reference periods normally used by the COPC classification sources are the prior 30 days for TMD,12 prior 12 months for headache,13 prior 3 months for IBS,14 prior 4 weeks for LBP,15 and prior 1 week for fibromyalgia.16 In OPPERA, the reference period for each COPC was operationalized as 3 months (90 days) in order to temporally align possible comorbidities among COPCs. However, the diagnostic criteria for headache also used the 12-month period specified in the International Classification of Headache Disorders (ICHD-3) for ascertainment of headache months and number of days/month. A positive response to the general pain filter question was followed by questions regarding condition-specific pain attributes (used for COPC classification), general pain questions, and additional pain attributes.
Table 1 lists the diagnostic criteria and the number of participants who reported positively to the 3-month filter question. Subthreshold cases can be computed for each COPC as the difference between those individuals positive for the filter question vs those positive for the case definition. The recognition of subthreshold cases (1) highlights the implications of the current case definition for each disorder and (2) better identifies the nature of the noncases, who are a mixture of individuals without any pain symptoms as well as those with subthreshold COPCs, for the other applied manuscripts in this series of papers that use the same case definitions.
Table 1.
Classification Criteria for COPC Cases
| IPC | Initial filter question | OPPERA-2 criteria for case classification | |
|---|---|---|---|
| TMD1 | In the last 3 months, have you had any of the following symptoms? a. Pain in the face b. Pain in your jaw c. Pain in your ear d. Pain in front of your ear e. Headaches in your temples f. Pain in your temples other than headache Yes / no (Yes to any location: n = 406) |
All 5 of the following criteria must be met: 1. Examiner verification that pain or headache from history is from temporalis or that pain from history is from masseter, temporalis, submandibular, or TMJ areas 2. Pain or headache on ≥ 5 d of prior 30 d 3. Evoked pain or headache in same locations as criterion 1 following palpation or jaw maneuver 4. Evoked pain or headache in criterion 3 is familiar pain or headache experienced in prior 30 d, as stated in criterion 1 5. History of pain or headache that is modified by jaw function (n = 182) |
|
| Headache | In the last 3 months, have you had any headaches? Yes / no (Yes: n = 520) |
Either of the following criteria must be met: 1. ICHD-3 criteria A–D for any tension-type headache 2. Headache on ≥ 1 d/mo accompanied by at least 2 of 3 symptoms: nausea, sensitivity to light, or being kept from everyday activities (n = 269) |
|
| IBS | In the last 3 months (that is, the last 90 days), how often have you had discomfort or pain anywhere in your abdomen? Never / Less than one day a month / One day a month / Two to three days a month / One day a week / More than one day a week (but not every day) / Every day (Any response other than “never”: n = 324) |
All 3 of the following criteria must be met: 1. Abdominal pain or discomfort on ≥ 1 d in the preceding 3 mo 2. Pain or discomfort unrelated to menstrual periods 3. Pain or discomfort associated with ≥ 2 symptoms of bowel function: Relieved by bowel movements; greater or lesser frequency of bowel movements; looser or harder stools (n = 158) |
|
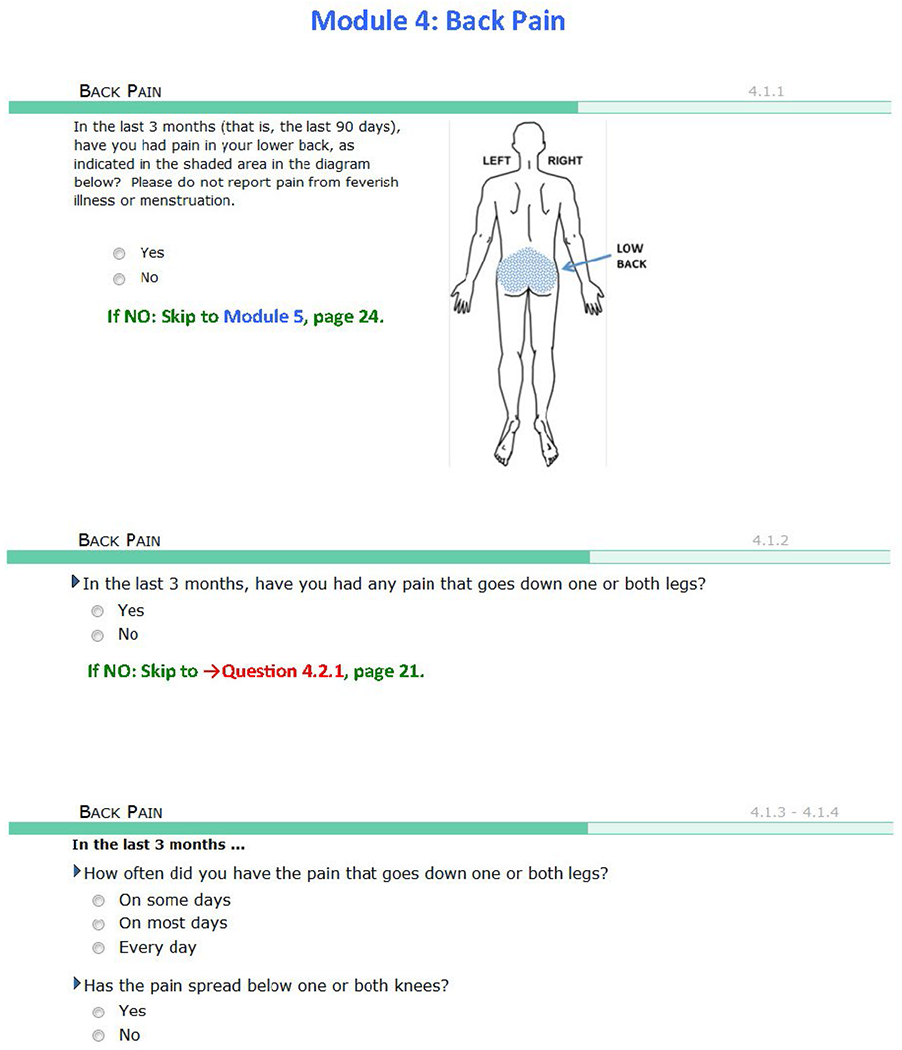
| LBP | In the last 3 months (that is, last 90 days), have you had pain in your lower back, as indicated in the shaded area in the diagram? Please do not report pain from feverish illness or menstruation. Yes / no (Yes: n = 329) |
|
All 3 of the following criteria must be met: 1. Pain occurring in lower back (see shaded manikin drawing) during the preceding 3 mo 2. Pain unrelated to fever or menstruation 3. Pain restricted usual activities for ≥ 1 d (n = 139) |
| Fibromyalgia | In the last 3 months, have you had any aches or pains anywhere in your body that have lasted for one day or longer? Yes / no (Yes: n = 397) |
All 3 of the following criteria must be met: 1. Pain for ≥ 1 d per mo in ≥ 3 mo of last 12 mo 2. Painful areas from history, in both the axial skeleton and in at least 1 set of opposing diagonal quadrants of the body 3. Evoked pain in ≥ 11 of 18 body sites in response to algometer-delivered pressure of up to 4.0 kg/cm2 at 1 kg/cm2/s (n = 52) |
|
TMD classification was based on the DC/TMD criteria for myalgia, arthralgia, or both. Positive responses to both the filter question and classification criteria were required for a given COPC (see text for procedural methods and citations). For the initial filter question, the text within parentheses refers to the number of participants who reported positive for that level of screening, and for the OPPERA-2 criteria, it refers to the unweighted number of participants meeting the respective criteria.
Examiners were trained and calibrated according to the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD)13,17 standards and the American College of Rheumatology (ACR)18 1990 standards for body palpation. Examiners were assessed for reliability annually, and classification accuracy remained at the κ = 0.9 to 1.0 level previously reported for OPPERA-1.19
Temporomandibular Disorders.
The initial filter question for TMD pain queried six face-area locations for pain over the prior 90 days. A full clinical examination according to the DC/TMD was performed, and examiners used those decision rules for a diagnosis of either masticatory muscle myalgia or temporomandibular joint (TMJ) arthralgia,12 referred to subsequently as simply “TMD” or “painful TMD.” Criterion 4 (Table 1) was met with a positive response from the participant to the question “Was the pain you felt (during palpation or jaw maneuver) familiar to the pain (or temporal headache) that you reported during the last 30 days?” as per the DC/TMD protocol.17 Criterion 5 was met with a positive response to the question “During the last 30 days, was any of the familiar pain (identified during palpation or jaw maneuver) modified by chewing hard food, opening the mouth, jaw habits such as clenching, or other jaw activities?”, which was administered during the examination in order to anchor the questions to the provoked pain. This is in contrast to the DC/TMD protocol, in which the questions are administered as a symptom checklist.
Headache.
After ascertaining the presence of any headache in the prior 12 months for the purpose of assessing any responsiveness to migraine medications (see pain condition questionnaire), the filter question for headache probed into the presence of any headache in the prior 3 months. The participant was requested to then describe up to three types of headache. For each headache description, the questionnaire contained migraine questions based on the ID-Migraine questionnaire20 and tension-type headache (TTH) questions based on the ICHD-3 beta21 and consistent with the ICHD-3.13 For this analysis, headache was classified for any participant who reported symptoms for at least one headache (among the potential maximum of three) and met the criteria for migraine or TTH, as listed in Table 1. TTH included infrequent, frequent, and chronic types. See Slade et al (current issue) for further information regarding the use of ID-Migraine in this study.
Irritable Bowel Syndrome.
The initial filter question for IBS probed into the presence of abdominal discomfort or pain over the prior 90 days. An acceptable positive response was a reported frequency of anything greater than none. IBS was classified based on Rome III diagnostic criteria,14,22 as listed in Table 1.
Low Back Pain.
The initial filter question for LBP probed into the presence of lower back pain in the prior 90 days. The location for “lower back” was anchored using an anatomical image in the pain-condition questionnaire. LBP was classified using responses to questions that consider a relatively neutral stance regarding the role of sciatica-type pain as ruling out idiopathic LBP and that were designed for epidemiologic-type research.15 Classification criteria and an anatomical image are presented in Table 1.
Fibromyalgia.
The initial filter question for fibromyalgia probed into the presence of aches or pains anywhere in the body that lasted for 1 day or longer in the prior 90 days.18 Fibromyalgia was classified based on the filter question as well as tender point examination, consistent with the 1990 ACR criteria,18 as listed in Table 1.
Assignment of Classifications
Based on the above criteria, a set of algorithms was used to assign specific classifications for each participant, ranging from 0 to 5. Of the classifications assigned to a given person, each would serve as the index COPC for a set of analyses that would examine how other COPCs might affect the primary pain attribute (see below) when associated with the index COPC. In a clinical setting, the condition that a person complains about and for which the pain attributes would be assessed is analogous to the designation of a given COPC as the index COPC throughout the analyses.
Demographic Characteristics
The following variables were collected and coded as follows: age (years), sex (male, female), and race/ethnicity (white non-Hispanic, Black/African American, Hispanic, other).
Assessment of Clinical Pain
This paper focuses on the relationship between COPCs and explanatory variables that measure clinical primary attributes of pain.
Primary Pain Attributes.
The Graded Chronic Pain Scale (GCPS)23,24 provided the general pain questions for each COPC. The GCPS is a well-established instrument using a 0 to 10 numeric rating scale (NRS) for three pain intensity items (anchored as “no pain” and “pain as bad as can be”) and three pain interference items (anchored as “no interference” and “unable to carry on any activities”) over the prior 3 months. In addition, one item requests the number of disability days due to pain over the preceding 90 days. Multiple publications utilizing the GCPS for various pain conditions, including for those in this study, have demonstrated its reliability, validity, and utility.25–27 For example, the GCPS classification exhibits excellent short-term reliability (κ = 0.87) and both convergent and discriminant validity in individuals with TMD. The component scores similarly exhibit strong psychometric characteristics.28
Characteristic pain intensity (CPI) was computed as the average current pain, average pain in the prior 3 months, and worst pain in the prior 3 months. Pain interference was computed as the average interference in daily, recreational, and work activities. These two scores were combined with disability days according to established rules,29 producing the graded chronic pain status: 0 = no pain; 1 = low pain and no more than low pain interference; 2a = high pain and no more than low pain interference; 2b = high pain and high pain interference but no pain disability days; 3 = moderate pain-related disability (based on both pain interference and pain disability days); and 4 = severe pain-related disability.30 High-impact pain was classified as present if a grade of 2b to 4 was identified for the respective COPC.
Additional Pain Attributes.
The pain condition questionnaire for each COPC contained four other pain questions: number of pain days in the last 3 months; duration of pain episodes; number of months of pain in the prior 12 months; and age of onset. Appendix 2 indicates how each of these questions was administered and scored for each COPC. These pain attributes, for which reliability and validity statistics are scarce, were used only for descriptive statistics.
Pain Manikin and Specific Locations for Face-Area Pain and Headache.
The pain-condition questionnaire included a body manikin on which participants marked any of 42 named pain locations. Separate modules in the questionnaire asked more specifically about pain locations relevant to TMD and headache.
Statistical Methods
Unless stated otherwise, data were weighted during analysis to adjust for the selection process of study participants in OPPERA-2. As described elsewhere in this volume (see Slade et al, current issue), weights were computed as the inverse of the sampling probability for the original OPPERA-1 case-control study, multiplied by the inverse of probability of cohort retention between OPPERA-1 and OPPERA-2. Such weighting is important in order for this analysis to make valid estimates of association between any two COPCs in a sample that was originally stratified according to a third variable31 (ie, the presence or absence of chronic TMD in OPPERA-1). For weighted estimates, the means, percentages, and measures of association were calculated using generalized estimating equations with the GENMOD procedure in SAS v. 9.4, with analytic weights and robust error variance calculation.
An anatomical heat map depicting the weighted proportions of participants reporting pain on the body manikin was created for each COPC. The proportion of participants reporting pain at each anatomical point on the manikin was converted to the natural log scale in order to broaden the display of proportions across the lower third and to compress the display of proportions across the upper two-thirds of the distribution, thereby better delineating the body locations least likely to be reported as painful by participants. Coordinates mapped the location of the anatomical points on the manikin checklist to a human silhouette figure, and the measured log values were assigned to the corresponding mapped points on the figure. Values for regions outside of those specific mapped points were determined utilizing multilevel B-splines, which provided bivariate smoothing as implemented in the MBR R package. The first 60 values of the tim. colors palette were used for coloration, and a tuning parameter was used to adjust the color transitions. The scale reports the original, not logarithmic, values as proportions. Because the more detailed questions about location relevant to TMD and headache could not be visualized on the heat map, these proportions were tabulated based on separate questions in their respective modules.
In order to evaluate potential thresholds in the additive effects of overlapping pain regardless of the COPC(s) involved, the primary pain attributes were plotted according to the number of comorbid COPCs experienced by those subjects. With regard to pain variables, the 0- to 5-count variable for number of pain conditions resulted in somewhat sparse data for the group defined by 4 pain conditions, and definitely sparse data for the group defined by 5 pain conditions. Therefore, a 0- to 4-count variable, collapsing groups 4 and 5, was also created. Since the results remain the same for 0 to 3 groups and the results in the 4 group tend to fall between the current findings for 4 and 5 groups, there was no change in the interpretation of the findings. While the findings are probably more reliable for the maximal group when collapsing 4 and 5 COPCs into one group, it was elected to present the results for 0 to 5 pain conditions in order to remain parallel with the other papers in this series. In addition, this approach allows the reader to fully appreciate the impact that smaller groups of all 5 pain conditions—which will be true for any such study—can have on the estimates of the variables of interest.
In order to investigate variations according to the type of comorbid COPC experienced (if any), linear regression models were created separately for each group of subjects with an index COPC. The dependent variables were the pain characteristics for that COPC (eg, pain intensity due to TMD in the model for subjects with TMD). The model covariates were four dummy variables, one for each possible comorbid COPC, to signify the presence or absence of that comorbid COPC. In these models, the intercept represented the estimated mean value for the dependent variable for subjects with the index COPC alone (ie, when all four dummy variables had a value of 0). In total, there were 15 regression models: 3 dependent variables (excluding high-impact pain) assessed for each of the 5 COPCs.
Results
Demographics
Participants with LBP were older, while the participants in the other four COPC groups were of similar ages (Table 2). A greater proportion of women than men had TMD, headache, and fibromyalgia, while approximately equal proportions of men and women reported IBS and LBP. White individuals accounted for approximately 50% of TMD and fibromyalgia, while the proportions of white individuals for the other three COPCs were higher, up to 63%.
Table 2.
Descriptive Univariate Statistics of OPPERA Study Participants: Demographic Variables for Each COPC
| COPC classification |
|||||
|---|---|---|---|---|---|
| Characteristic | TMD | Headache | IBS | LBP | Fibromyalgia |
| Unweighted n | 182 | 269 | 158 | 139 | 52 |
|
| |||||
| Weighted n | 108 | 201 | 134 | 99 | 24 |
|
| |||||
| Mean (SE) age, y | 33.0 (0.6) | 34.6 (0.5) | 34.6 (0.7) | 37.6 (0.7) | 34.3 (1.1) |
|
| |||||
| % female (SE) | 61.2 (3.6) | 71.1 (2.8) | 53.7 (4.0) | 56.7 (4.2) | 77.2 (5.9) |
|
| |||||
| % white (SE) | 50.7 (3.7) | 55.5 (3.0) | 60.1 (3.9) | 62.9 (4.1) | 52.7 (7.0) |
SE = standard error.
Pain Attributes
Table 3 displays the weighted means of the primary pain attributes for each of the five COPCs. The highest means for all attributes were reported for LBP and fibromyalgia. For pain intensity, IBS was the lowest, while TMD and headache were similar. For pain interference, TMD and IBS were the lowest. For the number of days inactive, headache and IBS were the lowest. The pattern for proportion of participants with high-impact pain generally followed that of pain intensity. Appendix 3 lists the unweighted descriptive statistics, including means and percentiles, for the same variables; some of the variables are normally distributed while others are skewed. The weighted and unweighted means were generally similar for all variables, but the medians differed from the means for about one-third of the variables.
Table 3.
Descriptive Statistics for Each Pain Characteristic for Each Index Pain Condition
| Index COPC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain characteristic | TMD (n = 107) | Headache (n = 201) | IBS (n = 134) | LBP (n = 99) | Fibromyalgia (n = 24) | |||||
| Pain intensity | 45.8 | (1.4) | 46.4 | (1.1) | 32.2 | (1.2) | 52.0 | (1.6) | 58.1 | (2.0) |
|
| ||||||||||
| Pain interference | 27.6 | (1.9) | 32.9 | (1.6) | 18.8 | (2.0) | 49.0 | (2.3) | 38.0 | (3.8) |
|
| ||||||||||
| No. of days missed due to pain | 8.8 | (1.6) | 4.1 | (0.6) | 4.4 | (1.1) | 20.5 | (2.6) | 12.5 | (3.2) |
|
| ||||||||||
| % with high-impact pain | 32.5 | (3.5) | 34.9 | (2.9) | 13.5 | (2.7) | 57.8 | (4.2) | 56.0 | (6.9) |
Data are reported as mean (standard error [SE]). Statistics are based on weighted number of participants.
The distributions of the additional pain attributes are listed for each of the five COPCs in Appendix 4. The episode frequency attributes (number of pain days/month and number of months with pain) were variably skewed. The conditions with the highest median numbers of days/month with pain were: TMD10; headache6; IBS3; LBP; and fibromyalgia,24 paralleling the pattern for number of days inactive. The median number of months of pain in the prior 12 months was 12 for all COPCs except for IBS, which was 6. The age and chronicity variables were more normally distributed. The mean age when each of the COPCs was first experienced ranged from about 18 to 28 years: TMD = 20.6; headache = 17.6; IBS = 23.9; LBP = 28.4; and fibromyalgia = 20.9. The percentages within each COPC of those with pain that had persisted for at least 6 months at the time of evaluation were nearly 100% for TMD, headache, and fibromyalgia; 87.7% for IBS; and 92.8% for LBP. Chronicity ranged up to 38 years.
Pain Locations
The heat maps of the manikin-based pain sites, indicating pain of at least 1-day duration in the prior 3 months, differed notably for participants with any COPC from participants who did not meet the criteria for any of the identified COPCs in this study (Fig 1). For those participants without an identified COPC, very little pain was reported throughout the body, with the major exceptions being at the lower back and upper back (about 20%) and the head (about 30%). Of note is that facial pain and abdominal pain occured in about 2% of the non-COPC group. Notable findings from among the COPCs were the following: the extent of neck and shoulder pain in those with TMD but not with headache, IBS, or LBP; a similar proportion of upper and lower limb pain in those with TMD, headache, and IBS; LBP in about 30% of those with TMD, headache, and IBS; equally high proportions (40% or more) of head pain in each of the COPCs; and lower proportions (5% to 15%) of pain in the arms, mid-chest, thighs, and feet in those with fibromyalgia.
Fig 1.
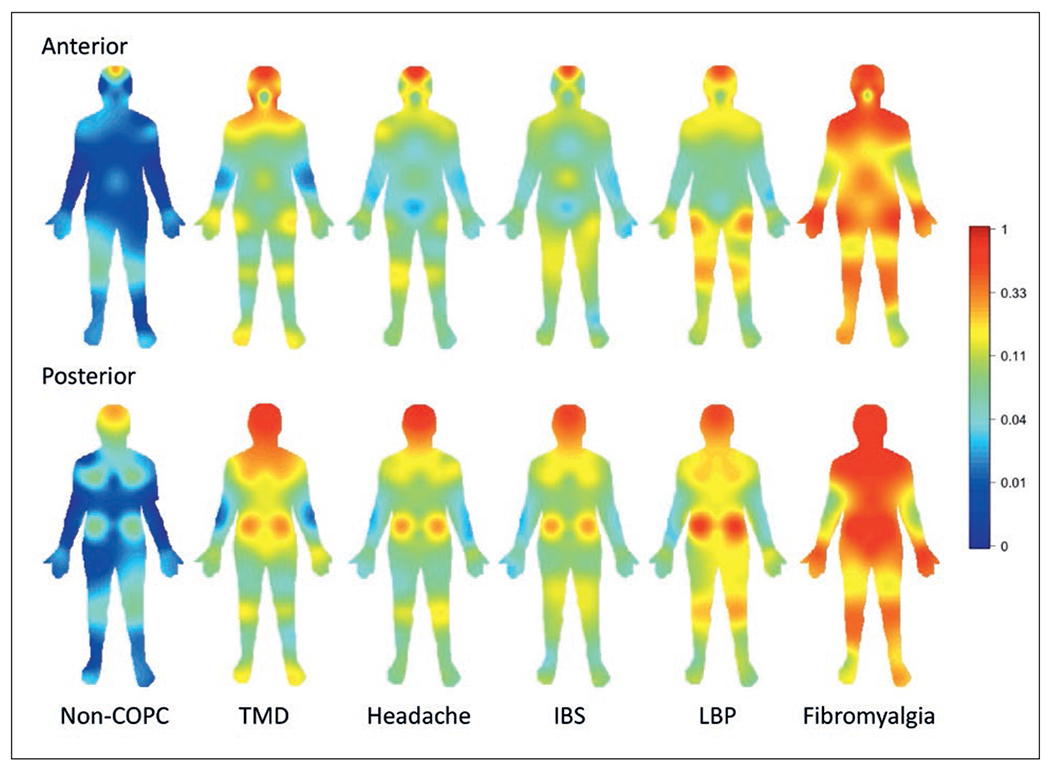
Heat map of manikin-based pain sites for pain lasting 1 day or longer within the prior 3 months for each index COPC. The color spectrum refers to the proportion of individuals with pain reported at the given site on the manikin. The skewed scaling of the color spectrum allows for the detection of body areas reported as painful by relatively few participants, with red color saturation starting at areas reported by approximately one-third of the participants. Non-COPC = individuals not meeting criteria for any of the five listed COPCs.
Locations of reported facial pain are listed in Appendix 5. Among all participants (including those without a COPC), headache in the temple region was the most common of the facial pain locations. Overwhelmingly, participants with TMD reported pain in the jaw and headache in the temple, and pain in the temple other than headache was reported at the lowest level among the six locations. Participants with headache, IBS, and LBP also reported temple headache as the most common among the six facial pain locations. Except for the jaw region, participants with fibromyalgia reported pain at each location at a greater percentage than participants with TMD. Consistent with the above findings, participants with no COPC or one COPC reported headache in the temple region at a much higher percentage compared to all other locations. As COPCs increased from two to five, the relative percentage of pain reported in other locations increased, but headache in the temple remained the maximum, with pain in the jaw a close second.
Locations of reported headache are listed in Appendix 6. Among all participants (including those without a COPC), temple location was the most frequent, with forehead and behind the eyes or inside the head a close second. For participants with TMD or headache, temple and behind the eyes or inside the head were the most frequent, and the frequency at the other three locations was virtually the same for both groups. For IBS and LBP, the temple and forehead were the most frequent locations. Participants with fibromyalgia reported headache most frequently in the temple, forehead, and behind the eyes or inside the head. Consistent with the above findings, participants without a COPC reported headache most frequently in the temple and behind the eyes or inside the head, whereas those with one COPC reported headache most frequently only in the temple. For participants with two to five COPCs, the most frequent locations were the temple, forehead, and behind the eyes or inside the head.
Effects of Overlapping Pain Conditions on Primary Pain Attributes
The hypothesis tests regarding the contribution of COPCs to a pain attribute of the index COPC are shown using 15 models (3 pain attributes and 5 index COPCs [Table 4]). Using Model 1 as an example, the reported pain intensity for TMD was 38.9 when no other COPC was present. Headache and IBS, when present, did not significantly contribute to the reported TMD pain intensity, whereas the reported intensity would be 59.9 if both LBP and fibromyalgia were also present (that is, the sum of the significant associations from all COPCs plus that reported for TMD: 38.9 + 11.2 + 9.8 = 59.9). The pain intensity of TMD was influenced by LBP and fibromyalgia, and, similarly, the pain intensity of fibromyalgia was influenced by TMD and LBP; however, the pain intensity of LBP was influenced only by fibromyalgia. The pain intensities reported for headache and IBS were not influenced by any other COPCs. Overall, the pain intensity reported for each index COPC was most strongly (and significantly) associated with the respective index COPC compared to the contribution from the comorbid COPCs.
Table 4.
Results of 15 Regression Models
| Index pain condition of subjects in model |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | TMD (n = 107) | Headache (n = 201) | IBS (n = 134) | LBP (n = 99) | Fibromyalgia (n = 24) | |||||
| Predictor variables | β | (SE) | β | (SE) | β | (SE) | β | (SE) | β | (SE) |
| Pain intensity | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||
| Intercept | 38.9 | (4.1) | 43.4 | (1.9) | 26.6 | (2.7) | 48.9 | (3.7) | 39.7 | (6.4) |
| Comorbid COPC | ||||||||||
| TMD (0, 1) | N/A | 6.6 | 3.9 | (3.1) | (2.9) | 1.4 | (3.9) | 15.9 | (5.6) | |
| Headache (0, 1) | 3.0 | (4.9) | N/A | 5.8 | (3.1) | −4.5 | (4.0) | −6.3 | (4.4) | |
| IBS (0, 1) | −0.2 | (4.1) | −2.3 | (3.2) | N/A | 4.7 | (4.3) | −0.7 | (3.9) | |
| LBP (0, 1) | 11.2 | (5.2) | 4.0 | (4.0) | 4.6 | (2.9) | N/A | 17.3 | (3.5) | |
| Fibromyalgia (0, 1) | 9.8 | (4.3) | 8.8 | (5.7) | 11.5 | (6.2) | 18.1 | (4.7) | N/A | |
|
| ||||||||||
| Pain interference | Model 6 | Model 7 | Model 8 | Model 9 | Model 10 | |||||
| Intercept | 17.4 | (5.1) | 29.1 | (3.4) | 5.7 | (3.3) | 40.6 | (4.3) | 21.7 | (8.1) |
| Comorbid COPC | ||||||||||
| TMD (0, 1) | N/A | 4.8 | (5.1) | 9.4 | (5.9) | 12.7 | (6.8) | −16.7 | (6.9) | |
| Headache (0, 1) | 4.0 | (5.7) | N/A | 15.4 | (5.0) | −5.8 | (6.1) | 19.8 | (5.7) | |
| IBS (0, 1) | 1.9 | (5.8) | 0.4 | (4.7) | N/A | 13.3 | (7.0) | −6.6 | (6.8) | |
| LBP (0, 1) | 26.2 | (7.3) | 8.5 | (5.2) | 11.7 | (5.6) | N/A | 31.1 | (5.9) | |
| Fibromyalgia (0, 1) | −6.3 | (7.1) | 5.1 | (7.8) | 4.2 | (14.6) | 8.7 | (9.6) | N/A | |
|
| ||||||||||
| No. of days missed due to pain | Model 11 | Model 12 | Model 13 | Model 14 | Model 15 | |||||
| Intercept | −0.3 | (3.0) | 1.7 | (0.5) | −1.8 | (1.8) | 13.9 | (5.9) | 2.7 | (6.3) |
| Comorbid COPC | ||||||||||
| TMD (0, 1) | N/A | 3.1 | (1.4) | 6.5 | (4.1) | 0.7 | (8.3) | 3.1 | (6.4) | |
| Headache (0, 1) | 5.5 | (3.7) | N/A | 5.8 | (3.0) | −1.7 | (7.2) | −12.9 | (11.1) | |
| IBS (0, 1) | 0.4 | (5.4) | 1.8 | (2.4) | N/A | 13.4 | (8.3) | −10.1 | (8.3) | |
| LBP (0, 1) | 16.6 | (7.1) | 2.7 | (1.8) | 7.2 | (4.8) | N/A | 32.6 | (13.2) | |
| Fibromyalgia (0, 1) | 3.2 | (8.4) | 4.2 | (3.2) | −3.9 | (8.6) | 12.1 | (12.0) | N/A | |
The intercept represents the estimated mean (β) and standard error (SE) of the dependent variable (primary pain characteristic) for subjects with no comorbid COPCs (ie, those having the index COPC alone). Other estimates represent the mean (SE) change in the dependent variable when associated with the presence of the designated comorbid COPC. Estimates of comorbid effects are not applicable (N/A) for the index COPC because that effect is represented by the intercept.
Unique contributions to each primary pain characteristic (dependent variable) from each COPC (predictor variable) are reported as mean (β) and standard error (SE) within each index COPC, as indicated by the column headings. Each comorbid COPC was entered as a dummy predictor variable (0 = no, 1 = yes). Bold values signify P < .05 for the null hypothesis that β = 0.
The model was weighted using generalized estimating equation multivariable linear regression. Significant β coefficients from a comorbid COPC can be considered to represent a percent increase (or decrease) in the pain reported (intercept) for the index COPC; for example, in Model 1, LBP and fibromyalgia each contribute another 25% (11.2 and 9.8, respectively) beyond what TMD itself contributes (38.9) for the pain intensity reported for TMD.
However, that simple and expected relationship observed for pain intensity did not hold for pain interference or for number of days missed due to pain. Pain interference in TMD was also influenced by LBP, whereas pain interference in headache and LBP were not influenced by any other COPCs. Pain interference for IBS was influenced by both headache and LBP, but was not linked directly to IBS alone, and in fibromyalgia, it was influenced by all COPCs except for IBS. From the perspective of COPCs that contribute to other COPCs, neither IBS nor fibromyalgia contributed to the pain interference reported for other COPCs.
The number of days missed due to pain for TMD, LBP, and fibromyalgia each was influenced only by LBP, whereas TMD exerted a greater influence on the number of days missed due to headache than did headache on its own. The number of days missed for IBS was not influenced by any of the COPCs. From the perspective of COPCs that contribute to others, neither IBS nor fibromyalgia contributed to the number of days missed due to pain reported for other COPCs, following the same pattern as for pain interference.
Effects of Number of COPCs on Primary Pain Attributes
For each index COPC, the effect of the number of COPCs was tested using pairwise comparisons of each additional COPC to the index condition alone for each of the three primary pain attributes (Figs 2a to 2c). Pain intensity overall increased with each additional COPC, such that for each of the 5, the presence of 3 or 4 additional COPCs (for a total of 4 or 5, as graphed) significantly increased the pain intensity of the index COPC alone. Pain interference for each index COPC similarly overall increased with each additional COPC, but only up to a total of 4, after which the pain interference decreased for each of the index COPCs. For TMD, headache, and fibromyalgia, the effect of 1 additional COPC beyond the index condition alone (2 COPCs, as graphed) on pain interference was less than the impact of the index condition alone. The number of missed activity days rose substantially for several of the COPCs when 3 comorbid COPCs were present with the index condition (4 COPCs, as graphed); otherwise, the effect of additional COPCs on the number of days missed for each index COPC was modest across the number of COPCs.
Fig 2.
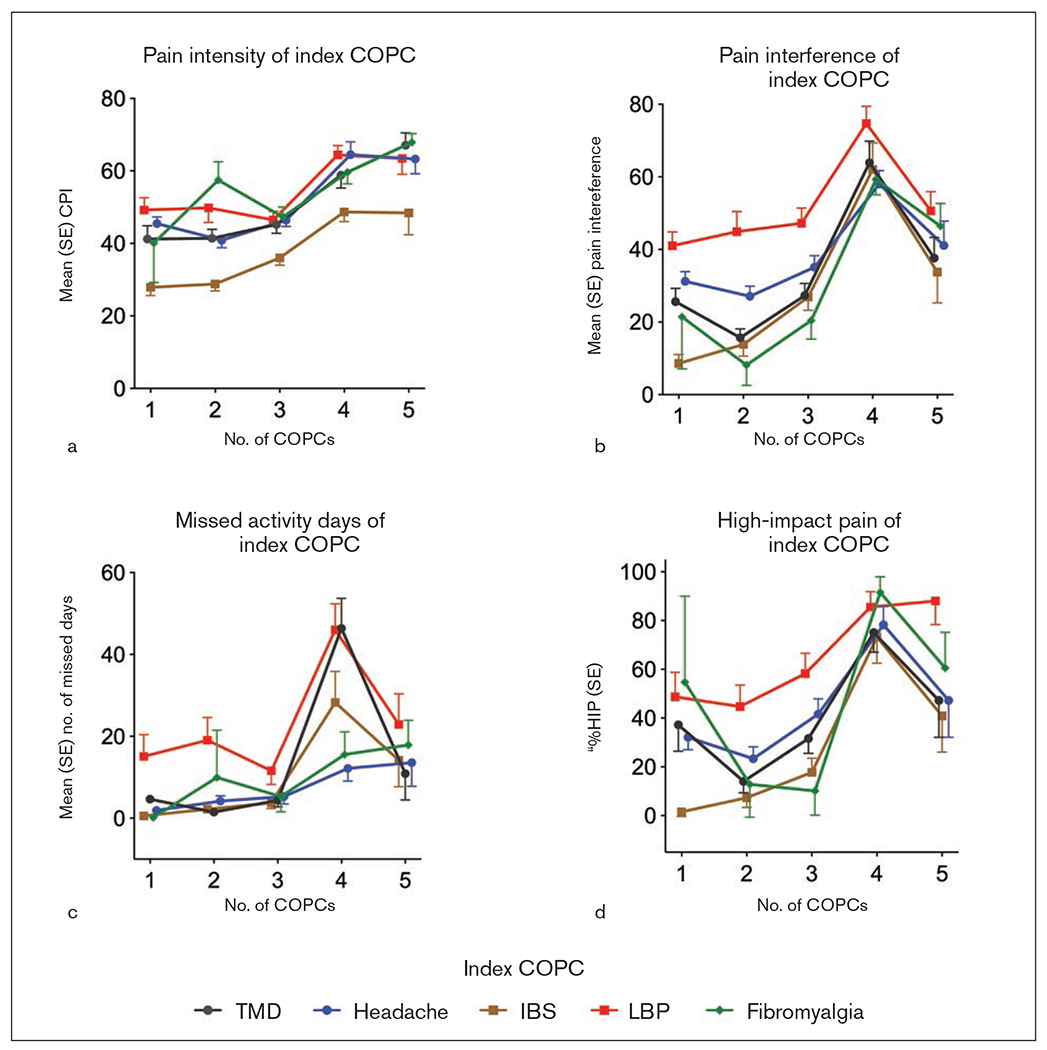
Pain characteristics for each index COPC. The index condition alone is represented by one COPC. Core measures from the Graded Chronic Pain Scale (GCPS) are measured as mean (standard error). The values of each measure are plotted for each index COPC according to the number of comorbid COPCs. Sample sizes (n) are based on weighted analyses: TMD = 107; headache = 201; IBS = 134; LBP = 99; fibromyalgia = 24. (a) Characteristic Pain Intensity (CPI). (b) Pain interference of index COPC. (c) Number of work days missed due to pain. (d) Percent (standard error) of individuals within each COPC reporting high-impact pain (%HIP), as based on Grades 2b and above from the GCPS.
Computation using the three primary pain attributes led to the graded pain classification, which was dichotomized into high (for high-impact pain) and low. The percentage of participants within each COPC reporting high-impact pain is plotted in Fig 2d according to the number of COPCs. Overall, the trend in this descriptive plot was upward from 1 to 4 COPCs; at 4 COPCs, the confidence limits (not shown) for 4 of the 5 COPCs mostly did not overlap with the confidence limits for the index condition alone, indicating a significant trend with increasing number of comorbid COPCs. Consistent with the raw data for both pain interference and missed activity days, this trend decreased at 5 COPCs.
Discussion
The overall findings of the present study demonstrate that, for each COPC, the pain intensity reported for the given COPC was strongly linked to that COPC. Among people with TMD, LBP, or fibromyalgia, the presence of an overlapping pain condition contributed substantially to the reported pain intensity of the index condition, with augmentation of up to 25% for TMD pain and 40% for LBP and fibromyalgia each (Table 4). In contrast, the reported pain intensities of headache and IBS were relatively independent of other COPCs. Collectively, these findings suggest that the intensity of musculoskeletal pains may be mutually additive, while the intensity of other pains, such as headache32–34 or IBS,35 may be processed separately. This finding is particularly important for headache, which, in this sample, included many participants with only TTH. Many TTH cases probably had an overlap with headache secondary to TMD,13 and TTH is thereby presumably similar to the COPCs that are more musculoskeletal in nature.36 The overlap between TMD and headache37–39 continues to exhibit unexpected complexities, as also shown for measures that are specific to TMD (see Sharma et al, current issue). Finally, for each index COPC, the pain intensity steadily increased with the number of comorbid COPCs, suggesting that while the COPCs exert specific effects on specific COPCs, the COPCs also exert additive influences on pain processing.
In contrast to pain intensity, the pain attribute of interference behaved differently: The interferences reported for TMD, IBS, and fibromyalgia were clearly more strongly influenced by other COPCs, whereas the interferences reported for headache and LBP were strongly linked to the respective COPC. Here, the simple dichotomy of musculoskeletal vs other pains falls apart, suggesting that pain intensity and pain interference may not be processed in parallel40,41 and that other aspects of the COPCs, such as effects on concentration (notable from headache) or mobility (notable from LBP), are more related to reported interference. In addition, when individuals have multiple COPCs, they may be less able to independently estimate the unique interference associated with one COPC vs another. In contrast to the effects of perhaps more global aspects, such as concentration and mobility, on overall functional capacity, chewing—as the most common impact from TMD pain—is a functional limitation rather than a form of disablement,42 and it appears that the overall impact of TMD as measured by interference is relatively low when TMD occurs by itself.
Compared to the contributions to pain intensity from each index COPC, the contribution from other COPCs to the magnitude of pain interference was higher: Other COPCs augmented the interference score of an index COPC by up to 150%. Unlike the more or less reciprocal pattern observed for pain intensity, the pattern for pain interference was more idiosyncratic than that reported for the index COPC. While TMD, headache, and LBP affected the reported interference in other COPCs, IBS and fibromyalgia did not do so, again indicating that the simple musculoskeletal pain additivity vs other pain (ie, headache, IBS) separateness for pain intensity may not hold for pain interference. This also suggests that, when two or more COPCs are present, the reported global impact of interference represents a complex mixture of the different ways any interference might be related to a given COPC. Finally, pain interference steadily increased with the number of COPCs, but only up to four, decreasing at five (which is addressed later). The reported number of work days missed due to pain behaved similarly to pain interference, with the exception that the report of days missed due to LBP was not influenced by other COPCs. High-impact pain, a function of interference and days missed, paralleled the pattern of pain intensity across the COPCs and steadily increased with the number of COPCs, again up to four.
For each of the primary pain attributes, it seems plausible that the role of each individual COPC in contributing to the reported attribute for the index COPC may also be a function of the steady increase in reported pain attributes with each additional COPC (up to four) within that individual. However, both the number of COPCs and the binary indicator variables for each COPC cannot be fully entered into a single model due to aliasing, as both types of variables represent the same information. However, the unique patterns displayed by the individual COPCs and their associations with the index COPC also suggest, as noted elsewhere, that specific comorbid COPCs may matter in terms of how pain is experienced from a given index condition. Investigating whether the contribution of a comorbid COPC to the reported attribute of the index COPC is also influenced by overall burden, expressed as number of COPCs, would be clinically sensible, but disentanglement of the aliasing is beyond the present scope.
The cross-COPC identification of face pain and headache locations may address a persistent problem in TMD research, which is how to frame the question regarding pain location (face, jaw, ear?). For TMD, the present data point to pain in the jaw and to headache pain in the temples (20% higher than reported by those with headache) as essential locations for pain assessment, followed by the face, in front of the ear, and in the ear—in short, a broad inquiry into possible TMD pain locations remains important. Yet, a higher percentage of those with fibromyalgia reported pain in nearly all of these locations compared to those with TMD. Simply identifying pain in these regions is not sufficient for surveys of TMD pain; rather, other queries must be added, such as pain aggravated by function.13 Similarly, those with TMD reported pain of an equal proportion to those with headache at nearly all of the headache locations, consistent with other studies regarding TMD and headache overlap.38 Other pairs of comparisons will surely be as interesting, but perhaps lead to the same conclusion: location alone is not enough to assume the presence of a regional pain disorder.
Earlier, the question was posed as to whether it is rational to measure pain intensity of only the index condition for a clinical trial if other COPCs are present. The complementary question is whether such condition-specific measures adequately inform regarding the progress of a given patient in treatment. These results suggest that a clear additive process occurs regarding reported pain intensity for TMD, LBP, and fibromyalgia, and a similar but more complex pattern exists for the report of pain interference. It seems that side-stepping other pain conditions, if only for simple pragmatic reasons, in order to focus on the condition of interest is counterproductive for understanding or for development of better treatment of that condition of interest. In this light, the additivity of COPCs for pain intensity, which was essentially proposed decades ago,43 can be extended to include additivity for both pain interference and high-impact pain. Similarly, whether greater magnitude of a pain characteristic in a comorbid COPC leads to greater magnitude of that same characteristic in the index COPC is an important extension for both clinical trials and clinical management. Based on the present findings, which focus only on the presence of the COPC, it is plausible that flare-ups of persistent comorbid back pain, for example, could result in additional effects on any of the primary pain characteristics reported for TMD as the index condition.
Moreover, it is reported separately that not only pain characteristics, but also both examination findings of the masticatory system and other variables germane to the condition of painful TMD, are influenced by the presence of other COPCs (Sharma et al, current issue). A standard characteristic of chronic pain conditions is that the reported pain is disproportionate to physical findings. Yet, the postulated—as well as the known—shared mechanisms common to many, if not all, of these COPCs may lead to a different interpretation regarding that characteristic. If the reported pain intensity for an index condition is substantially influenced by other COPCs—for example, TMD pain that could be 50% higher if both LBP and fibromyalgia are also present—then it is certain that any physical examination findings (for example, mobility, degree of hyperalgesia from provocation testing) will be disproportionate to the reported pain intensity. This is not to suggest, however, that shared mechanisms may be more important than local findings for fully understanding a given COPC—rather, both levels of information are necessary, and joint consideration may differentiate our understanding of causation behind, say, two COPCs.
As a COPC assessed for its influence on pain attributes reported for the other four index conditions, fibromyalgia warrants special consideration when interpreting these results. One general caveat is that there were 55 subjects with fibromyalgia, as classified using the 1990 ACR criteria, and in the statistical analysis, they represented only 24 weighted cases. This creates imprecise estimates with wide confidence intervals, probably contributing to anomalous findings, such as the negative parameter estimates in Table 4. Another feature of fibromyalgia is that its classification uses some criteria that may overlap with other COPCs. For example, the presence of axial pain, as required for the 1990 fibromyalgia classification, could be due to LBP. To that extent, the multivariable models in Table 4 do not truly reflect an “independent” contribution of fibromyalgia to, say, the intensity of TMD pain after accounting for LBP. Yet, in other study findings reported in the same issue of this journal, it was found that fibromyalgia was associated with pain and other features that are traditionally viewed as symptoms characteristic of TMD (eg, limited jaw opening) (see Sharma et al, current issue). This suggests that fibromyalgia might represent a marker for altered pain-processing systems underlying TMD-relevant characteristics. Likewise, in the current paper, it is conceivable that the presence of fibromyalgia signals not axial pain, but rather a systemic alteration in pain processing44 that is relevant when measuring the intensity and duration of pain attributed to other COPCs. If this is true, then the potential overlap in classification with LBP may be irrelevant, a hypothesis worth considering for future investigations of different permutations of the COPCs studied here.
The limitations of the present study should be considered. While prevalence and assumed overlap dictated the selection of COPCs used here, the presence of other COPCs was not formally identified, which could exclude the potential importance of other comorbid COPCs. Other COPCs would need to be studied in order to discover whether they have unique idiosyncratic, as well as additive, effects. Another limitation is that, for these analyses, other COPCs were identified as formally defined, and the presence of subclinical pain conditions (as identified by a positive response to the screener question but negative for the pain condition criteria) was not accounted for, but the heat maps suggest that these five COPCs identify a substantial proportion of individuals with persistent pain. A general limitation is the known bias in the assessment of days missed from work due to pain; the structure of this question in the GCPS was largely aimed at back pain as a condition with high social costs.45 But for those with other pains, such as headache or TMD, a general coping pattern often leads individuals to continue to work, but not effectively. This reduction in productivity (presenteeism) may be more accurate than days missed, so high-pain impact may consequently be underestimated at present. The epidemiologic criteria for LBP requires at least 1 disability day; consequently, no one classified as LBP reported having no disability days, creating a potential bias for back pain disability days vs other conditions. However, inspection of the distribution of disability days for LBP compared to, for example, painful TMD, did not disclose any substantial differences in the bottom 25th percentile. It is clear, however, that potential circularity is of concern in this type of research question.
Finally, the sample size of the fibromyalgia group is relatively small and probably affects both the mean and error estimates; consequently, overinterpretation of results in specific areas is cautioned against. As noted earlier for fibromyalgia alone, the small sample size of the fibromyalgia index condition group by extension contributes to the even smaller sample size for those with five COPCs (unweighted n = 12; weighted n = 6). Consequently, the findings for the five COPCs in Fig 2 should be considered with caution. This may be the case for high-impact pain at five COPCs; alternatively, it is plausible that the impact of pain processing on functional interference could also plateau at four COPCs, after which additional pain disorders do not further contribute. The present findings point to an important hypothesis for further evaluation.
Conclusions
The present findings highlight the unique effects, as well as the additive effects, that COPCs exert on an index COPC. Moreover, the findings support the role of shared pathophysiologic mechanisms coupled with local factors associated with an index COPC. TMD pain and headache are very similar in terms of measured attributes (more severe than IBS and less severe than LBP or fibromyalgia), yet they behave very differently in combination with other COPCs. Very few parts of the body are pain free in diagnostic groups based on these COPCs, consistent with the overall severity of these COPCs as measured with any of the selected pain attributes. High-impact pain occurs in about one-third of those with TMD pain or headache and in over half of those with LBP or fibromyalgia. Clinical trials, as well as care for individual patients, should carefully consider the potential importance that comorbid conditions have on measured pain attributes of the condition of interest.
Acknowledgments
This work was supported by National Institutes of Health grants U01DE017018 (NIDCR) and UL1TR001427 (NCATS). The OPPERA program also acknowledges resources provided for this project by the participating institutions: University at Buffalo; University of Florida; University of Maryland; and University of North Carolina at Chapel Hill. Dr Fillingim has equity ownership in Algynomics Inc The authors have nothing else to disclose.
Appendices
Appendix 1.












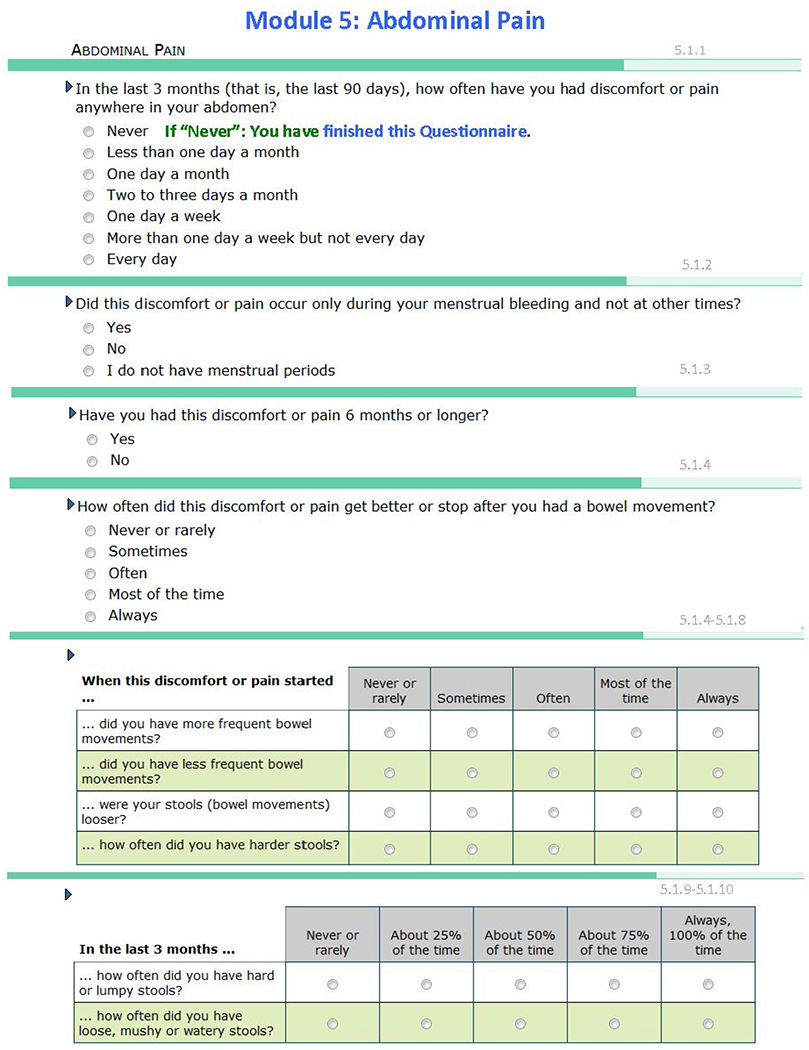


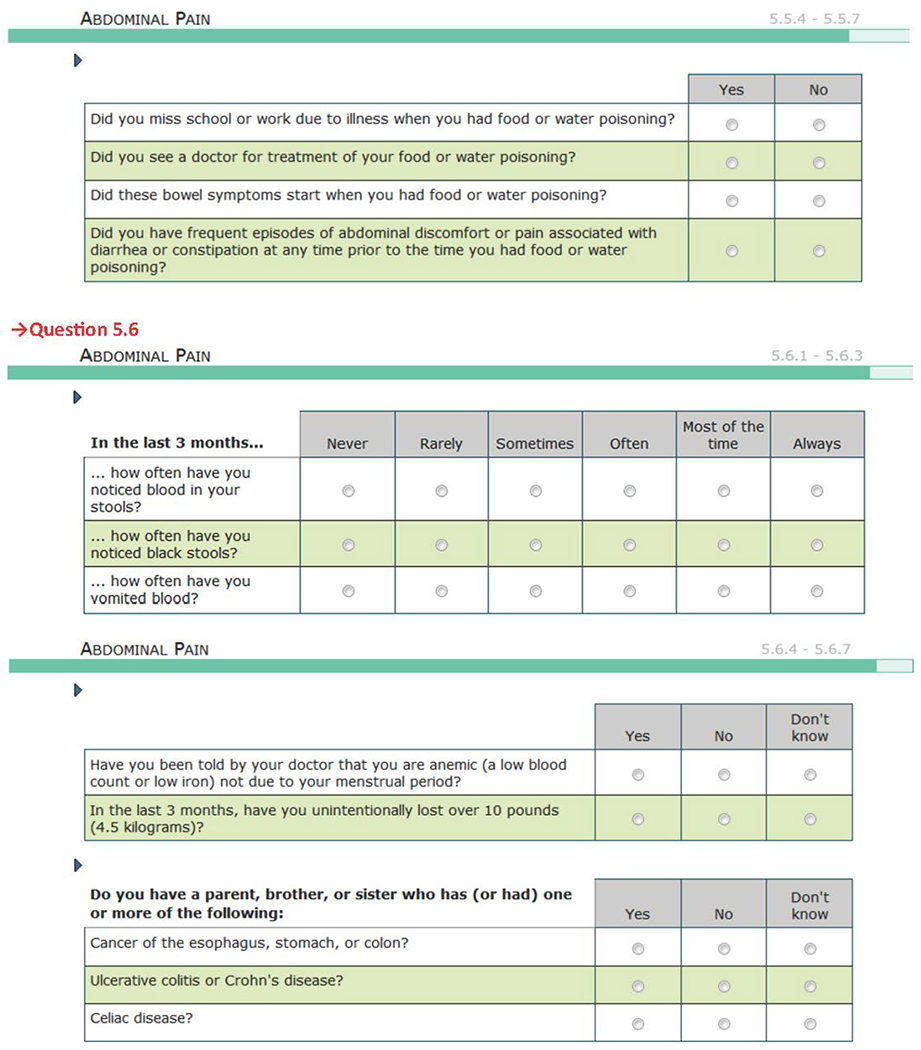
Questionnaire Used in the Study.
Appendix 2.
Response Options and Scoring Rules for Additional Pain Attributes
| Attribute | TMD | Headachea | IBS | LBP | Fibromyalgia |
|---|---|---|---|---|---|
| No. of pain days in last 3 mo (max = 90) | ✓ | ✓b | ✓ | ✓ | ✓ |
| Episode duration (< 30 min, 30 min – < 2 h, 2 h–7 d, > 7 d) | “Average”c | “Typical”c,d | “Average”c | “Average”c | “Shortest” and “longest”c,e |
| No. of pain mo, past 12 mo | ✓ | ✓f | ✓ | ✓ | ✓ |
| Onset age, y | ✓ | ✓g | ✓ | ✓ | ✓ |
The value of each attribute for up to three headache types is included.
Add maximum (+ 50% of value of each remaining headache), up to maximum of 30; multiply by 3.
Qualifier in quotation marks refers to the specific language in the pain-condition questionnaire regarding a usual episode for the respective pain condition to use as a basis for determining episode duration.
Convert values (< 30 min, 30 min– < 2 h, 2 h– < 4 h, 4 h–72 h, > 3 d–7 d, > 7 d; DNK) to ordinal coding (1–6, with DNK [do not know] = 4) for each headache. Compute average of the ordinal coding across headaches and round up to next integer. Re-coded integers: 1 = 1; 2 = 2; 3, 4, 5 = 3; 6 = 4.
Convert values of these two related questions. For shortest: convert (< 30 min, 30 min– < 2 h, 2 h– < 4 h, 4 h–< 8 h, ≥ 8 h) to coding (1–5) and re-code integers: 1 = 1; 2 = 2; 3, 4, 5 = 3. For longest: convert (< 30 min, 30 min– < 2 h, 2 h–7 d, > 7 d) to coding (1, 2, 3.5, 4, 5). Then, compute average of the two codes for shortest and longest and round to nearest integer, with final coding (1–4).
Add maximum (+ 50% of value of each remaining headache), up to maximum of 12.
Use minimum value of the available estimates (headaches 1–3).
Appendix 3.
Unweighted Descriptive Statistics for Primary Pain Attributes for Each COPC
| TMDa | Headachea | IBS | LBP | Fibromyalgia | |
|---|---|---|---|---|---|
|
|
|||||
| Attribute | (No. of participants),b mean (5th, 25th, 50th, 75th, 95th percentile) | ||||
| Pain intensity | (180), 44.5 | (269), 46.4 | (158), 34.5 | (139), 53.9 | (52), 59.2 |
| (17, 30, 43, 57, 77) | (17, 33, 47, 60, 77) | (10, 20, 33, 43, 67) | (23, 40, 50, 70, 90) | (30, 48, 60, 70, 83) | |
|
| |||||
| Pain interference | (180), 23.0 | (269), 33.2 | (158), 22.0 | (139), 48.7 | (52), 43.6 |
| (0, 0, 13, 37, 80) | (0, 10, 27, 50, 83) | (0, 0, 10, 40, 80) | (10, 27, 50, 70, 100) | (0, 23, 47, 67, 87) | |
|
| |||||
| No. of days missed due to pain | (180), 8.1 | (269), 6.5 | (158), 5.4 | (139), 22.3 | (52), 19.2 |
| (0, 0, 0, 5, 69) | (0, 0, 2, 5, 30) | (0, 0, 0, 3, 30) | (1, 3, 5, 30, 90) | (0, 2, 8, 25, 90) | |
| (No. of participants),b % |
|||||
| Patients with high-impact pain | (180), 27.2 | (269), 36.4 | (158), 19.6 | (139), 59.0 | (52), 67.3 |
Two TMD participants and one headache participant had missing responses on the primary pain characteristic variables, with subsequent n = 180 and n = 269, respectively.
Unweighted number of participants for each COPC; means, quantiles, and percentages are unweighted estimates.
Appendix 4.
Unweighted Descriptive Statistics for Additional Pain Attributes for Each COPC
| TMDa | Headachea | IBSa | LBPa | Fibromyalgia | |
|---|---|---|---|---|---|
|
|
|||||
| Attribute | (No. of participants),b mean (5th, 25th, 50th, 75th, 95th percentile) | ||||
| No. of d with pain/mo | (178), 14.3 | (269), 9.5 | (157), 6.5 | (139), 14.4 | (52), 19.4 |
| (1, 3, 10, 27, 30) | (2, 3, 6, 13, 30) | (0, 1, 3, 10, 30) | (1, 2, 13, 30, 30) | (1, 8, 24, 30, 30) | |
|
| |||||
| No. of mo with pain/y | (180), 9.2 | (269), 9.3 | (138), 6.5 | (138), 8.4 | (52), 11.4 |
| (2, 6, 12, 12, 12) | (2, 7, 12, 12, 12) | (1, 3, 6, 12, 12) | (1, 4, 12, 12, 12) | (5, 12, 12, 12, 12) | |
|
| |||||
| Age of first pain onset, y | (179), 20.6 | (269), 17.6 | (156), 23.9 | (139), 28.4 | (52), 20.9 |
| (10, 15, 19, 25, 39) | (8, 12, 16, 20, 35) | (5, 14, 23, 35, 46) | (14, 21, 28, 35, 46) | (6, 14, 20, 27, 37) | |
|
| |||||
| No. of y since pain began | (179), 15.3 | (269), 19.6 | (156), 13.1 | (139), 11.3 | (52), 16.4 |
| (2, 9, 14, 21, 35) | (5, 13, 19, 27, 37) | (0, 3, 11, 21, 35) | (0, 4, 9, 17, 28) | (2, 10, 15, 21, 38) | |
| (No. of participants),b % |
|||||
| Pain ≥ 6 mo | (180), 98.9 | (269), 98.9 | (138), 87.7 | (138), 92.8 | (52), 100.0 |
Missing responses varied across the additional pain characteristic variables within each COPC, as follows: 2–4 TMD participants, with subsequent n = 178–180; 1 headache participant, with n = 269; 1–20 IBS participants, with n = 138–157; and 1 LBP participant, with n = 138–139.
Unweighted number of participants for each COPC; means, quantiles, and percentages are unweighted estimates.
Appendix 5.
Facial Pain Locations for Each COPC and by Number of COPCs During the Prior 3 Months
| COPC classification |
||||||
|---|---|---|---|---|---|---|
| Location | TMD (n = 108) | Headache (n = 201) | IBS (n = 134) | LBP (n = 99) | Fibromyalgia (n = 24) | |
| Pain in face | 44.2 | 34.5 | 23.5 | 29.9 | 69.2 | |
|
| ||||||
| Pain in jaw | 85.5 | 42.0 | 34.1 | 41.9 | 73.8 | |
|
| ||||||
| Pain in ear | 37.2 | 20.4 | 18.2 | 25.3 | 49.9 | |
|
| ||||||
| Pain in front of ear | 39.5 | 18.0 | 13.9 | 16.2 | 67.9 | |
|
| ||||||
| Headache in temples | 82.6 | 63.9 | 58.6 | 69.7 | 86.0 | |
|
| ||||||
| Pain in temples other than headache | 23.6 | 13.3 | 5.9 | 13.9 | 43.5 | |
| No. of COPCs |
||||||
| Location | 0 (n = 307) | 1 (n = 209) | 2 (n = 83) | 3 (n = 33) | 4 (n = 15) | 5 (n = 6) |
|
| ||||||
| Pain in face | 2.6 | 17.9 | 34.2 | 41.0 | 48.5 | 100.0 |
|
| ||||||
| Pain in jaw | 9.5 | 25.5 | 60.0 | 51.9 | 76.8 | 100.0 |
|
| ||||||
| Pain in ear | 3.6 | 12.2 | 27.5 | 31.3 | 46.4 | 38.9 |
|
| ||||||
| Pain in front of ear | 2.0 | 3.3 | 27.3 | 27.6 | 41.1 | 83.9 |
|
| ||||||
| Headache in temples | 29.1 | 51.6 | 63.3 | 84.0 | 96.4 | 100.0 |
|
| ||||||
| Pain in temples other than headache | 5.8 | 5.0 | 15.7 | 16.7 | 29.6 | 43.3 |
Data are presented as percent reporting pain in that location. Facial pain locations within COPC or number of COPCs are not mutually exclusive (percentages may add up to more than 100). Defined locations of face pain are based on self-report in the TMD section of the pain-condition questionnaire. Group sizes are based on weighted n.
Appendix 6.
Headache Locations for Each COPC and by Number of COPCs During the Prior 3 Months
| COPC classification |
||||||
|---|---|---|---|---|---|---|
| Location | TMD (n = 108) | Headache (n = 201) | IBS (n = 134) | LBP (n = 99) | Fibromyalgia (n = 24) | |
|
| ||||||
| Temple | 72.4 | 67.0 | 53.6 | 65.0 | 65.4 | |
|
| ||||||
| Forehead | 57.0 | 57.8 | 56.4 | 68.9 | 70.0 | |
|
| ||||||
| Top of head | 23.1 | 27.8 | 22.1 | 20.3 | 38.6 | |
|
| ||||||
| Back of head | 40.4 | 45.4 | 22.9 | 21.6 | 46.9 | |
|
| ||||||
| Behind eyes or inside head | 67.9 | 66.8 | 41.9 | 50.0 | 77.5 | |
| No. of COPCs |
||||||
| Location | 0 (n = 307) | 1 (n = 209) | 2 (n = 83) | 3 (n = 33) | 4 (n = 15) | 5 (n = 6) |
|
| ||||||
| Temple | 30.6 | 56.0 | 53.1 | 83.5 | 94.3 | 60.6 |
|
| ||||||
| Forehead | 24.2 | 38.3 | 63.2 | 77.9 | 98.2 | 51.5 |
|
| ||||||
| Top of head | 4.9 | 18.4 | 19.7 | 29.2 | 49.6 | 29.9 |
|
| ||||||
| Back of head | 8.5 | 26.1 | 42.1 | 30.6 | 48.8 | 44.5 |
|
| ||||||
| Behind eyes or inside head | 27.1 | 40.7 | 64.6 | 80.0 | 55.8 | 85. |
Data are presented as percent reporting pain in that location. Headache locations within COPC or number of COPCs are not mutually exclusive (percentages may add up to more than 100). Defined locations of headache are based on self-report in the headache section of the pain condition questionnaire. The headache section allowed reporting of up to three headache types over the prior 3 months, and the locations represent all locations reported by each subject across the multiple headache types (ie, each reported location is only counted once for a given subject). Group sizes are based on weighted n.
Contributor Information
Richard Ohrbach, Department of Oral Diagnostic Sciences University at Buffalo School of Dental Medicine Buffalo, New York, USA.
Sonia Sharma, Department of Oral Diagnostic Sciences University at Buffalo School of Dental Medicine Buffalo, New York, USA; Department of Orofacial Pain and Jaw Function Faculty of Odontology Malmö University Malmö, Sweden.
Roger B. Fillingim, Department of Community Dentistry and Behavioral Science, Pain Research and Intervention Center of Excellence College of Dentistry University of Florida Gainesville, Florida, USA.
Joel D. Greenspan, Department of Neural and Pain Sciences Brotman Facial Pain Clinic, Center to Advance Chronic Pain Research, School of Dentistry, University of Maryland, Baltimore, Maryland, USA.
Jonathan D. Rosen, Department of Biostatistics University of North Carolina Chapel Hill, North Carolina.
Gary D. Slade, Division of Pediatric and Public Health Adams School of Dentistry Department of Dental Ecology Department of Epidemiology Gillings School of Global Public Health University of North Carolina Chapel Hill, North Carolina, USA.
References
- 1.Functional pain disorders: Time for a paradigm shift. In: Mayer EA, Bushnell MC (eds). Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP, 2009:531–565. [Google Scholar]
- 2.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal VR, McBeth J, Zakrzewska JM, Lunt M, Macfarlane GJ. The epidemiology of chronic syndromes that are frequently unexplained: Do they have common associated factors? Int J Epidemiol 2006;35:468–476. [DOI] [PubMed] [Google Scholar]
- 4.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders—Pathways of vulnerability. Pain 2006;123:226–230. [DOI] [PubMed] [Google Scholar]
- 5.Yunus MB. Editorial review: An update on central sensitivity syndromes and the issues of nosology and psychobiology. Curr Rheumatol Rev 2015;11:70–85. [DOI] [PubMed] [Google Scholar]
- 6.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med 2000;160: 221–227. [DOI] [PubMed] [Google Scholar]
- 7.Gatchel RJ. Comorbidity of chronic pain and mental health disorders: The biopsychosocial perspective. Am Psychol 2004;59: 795–805. [DOI] [PubMed] [Google Scholar]
- 8.Slade GD, Ohrbach R, Greenspan JD, et al. Painful temporomandibular disorder: Decade of discovery from OPPERA studies. J Dent Res 2016;95:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NIH Working Group. Chronic Overlapping Pain Conditions. Summary of NIH Work Group Meeting to Develop Case Definition & Common Data Elements. https://www.iprcc.nih.gov/sites/default/files/Veasley-COPC.pdf. Accessed 23 April, 2020.
- 10.Livingston WK. Pain and Suffering. Seattle: IASP, 1998. [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, ed 3. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis 2006;15:237–241. [PubMed] [Google Scholar]
- 15.Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95–103. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–1122. [DOI] [PubMed] [Google Scholar]
- 17.Ohrbach R, Gonzalez Y, List T, Michelotti A, Schiffman E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) Clinical Examination Protocol. http://www.rdc-tmdinter-national.org/. Accessed 23 April, 2020.
- 18.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 19.Bair E, Brownstein NC, Ohrbach R, et al. Study protocol, sample characteristics and loss-to-follow-up: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T2–T19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology 2003;61:375–382. [DOI] [PubMed] [Google Scholar]
- 21.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, ed 3 (beta version). Cephalalgia 2013;33: 629–808. [DOI] [PubMed] [Google Scholar]
- 22.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 23.Von Korff M Epidemiologic and survey methods: Chronic pain assessment. In: Turk DC, Melzack R (eds). Handbook of Pain Assessment. New York: Guilford Press, 1992:389–406. [Google Scholar]
- 24.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133–149. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin SF, Sherman JJ, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of RDC/TMD Axis II scales: Depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain 2002;16:207–220. [PubMed] [Google Scholar]
- 26.Smith BH, Penny KI, Purves AM, et al. The Chronic Pain Grade questionnaire: Validation and reliability in postal research. Pain 1997;71:141–147. [DOI] [PubMed] [Google Scholar]
- 27.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine (Phila Pa 1976) 2000;25:3140–3151. [DOI] [PubMed] [Google Scholar]
- 28.Ohrbach R, Turner JA, Sherman JJ, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. IV: Evaluation of Psychometric Properties of the Axis II Measure. J Orofac Pain 2010;24:48–62. [PMC free article] [PubMed] [Google Scholar]
- 29.Von Korff M Assessment of chronic pain in epidemiological and health services research: Empirical bases and new directions. In: Turk DC, Melzack R (eds). Handbook of Pain Assessment. New York: Guilford, 2011:455–473. [Google Scholar]
- 30.Dworkin SF, Huggins KH, Wilson L, et al. A randomized clinical trial using Research Diagnostic Criteria for Temporomandibular Disorders-Axis II to target clinic cases for a tailored self-care TMD treatment program. J Orofac Pain 2002;16:48–63. [PubMed] [Google Scholar]
- 31.Richardson DB, Rzehak P, Klenk J, Weiland SK. Analyses of case-control data for additional outcomes. Epidemiology 2007;18:441–445. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz MA. Basic mechanisms in vascular headache. Neurol Clin 1990;8:801–815. [PubMed] [Google Scholar]
- 33.Nicolodi M, Sicuteri R, Coppola G, Greco E, Pietrini U, Sicuteri F. Visceral pain threshold is deeply lowered far from the head in migraine. Headache 1994;34:12–19. [DOI] [PubMed] [Google Scholar]
- 34.Gerwin RD. Myofascial and visceral pain syndromes: Visceral-somatic pain representations. J Musculoskelet Pain 2002;10:165–175. [Google Scholar]
- 35.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci 2008;28:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen K, Tuxen C, Olesen J. Pericranial muscle tenderness and pressure-pain threshold in the temporal region during common migraine. Pain 1988;35:65–70. [DOI] [PubMed] [Google Scholar]
- 37.Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia 2008;28:832–841. [DOI] [PubMed] [Google Scholar]
- 38.Svensson P Muscle pain in the head: Overlap between temporomandibular disorders and tension-type headache. Curr Opin Neurol 2007;20:320–325. [DOI] [PubMed] [Google Scholar]
- 39.Glaros AG, Urban D, Locke J. Headache and temporomandibular disorders: Evidence for diagnostic and behavioural overlap. Cephalalgia 2007;27:542–549. [DOI] [PubMed] [Google Scholar]
- 40.Fillingim RB, Slade GD, Greenspan JD, et al. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: Findings from the OPPERA study. Pain 2018;159:24032413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dionne CE, Von Korff M, Koepsell TD, Deyo RA, Barlow WE, Checkoway H. A comparison of pain, functional limitations, and work status indices as outcome measures in back pain research. Spine (Phila Pa 1976) 1999;24:2339–2345. [DOI] [PubMed] [Google Scholar]
- 42.Ohrbach R. Disability assessment in temporomandibular disorders and masticatory system rehabilitation. J Oral Rehabil 2010;37:452–480. [DOI] [PubMed] [Google Scholar]
- 43.Rudy TE, Turk DC, Kubinski JA, Zaki HS. Differential treatment responses of TMD patients as a function of psychological characteristics. Pain 1995;61:103–112. [DOI] [PubMed] [Google Scholar]
- 44.Harper DE, Schrepf A, Clauw DJ. Pain mechanisms and centralized pain in temporomandibular disorders. Journal of Dental Research 2016;95:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Von Korff M, Dworkin SF, LeResche L, Kruger A. An epidemiologic comparison of pain complaints. Pain 1988;32:173–183. [DOI] [PubMed] [Google Scholar]


