Abstract
Background:
Acute exacerbation events, which can develop during the natural course of chronic obstructive pulmonary disease (COPD) can lead to worsening quality of life, increased hospital costs, and higher rates of morbidity and mortality. In recent years, individuals at heightened risk of COPD exacerbations have been said to display a so-called “frequent exacerbator (FE)” phenotype, defined as having two or more exacerbation events (or ≥ 1 exacerbation with a hospitalization) within 1 year.
Materials and Methods:
We conducted a retrospective study involving 299 patients with COPD. Patients were divided into 2 groups as non-exacerbator phenotype (group-1, n=195) and FE phenotype (group-2, n=104).
Results:
FE phenotype was identified in 35.1% of patients. There were no significant differences between these two phenotypes in terms of gender, smoking status, or leukocyte count. However, FEs were found to be older (p=0.04), with more frequent detection of emphysema (p=0.02) and lower eosinophil levels (p=0.02). FEs also demonstrated worse pulmonary function parameters.
Conclusion:
COPD patients with the FE phenotype likely require a different treatment algorithm due to differing clinical features such as poorer respiratory function, lower eosinophil levels, and more frequent emphysema.
Keywords: COPD, Frequent exacerbator, Phenotype, Hospitalization, Clinical characteristic
INTRODUCTION
Acute exacerbation events, which can develop during the natural course of chronic obstructive pulmonary disease (COPD), are characterized by increases in patient-reported respiratory complaints, including cough, shortness of breath, and sputum production, necessitating further treatment or drug changes (1). These exacerbations can lead to worrisome outcomes, including decline in lung function, worsening quality of life, increased hospital costs, and higher rates of morbidity and mortality (2,3).
In recent years, individuals at heightened risk of COPD exacerbations have been said to display a so-called “frequent exacerbator (FE)” phenotype, defined as having two or more exacerbation events (or ≥ 1 exacerbation with a hospitalization) within 1 year (4). Previous studies have identified various risk factors associated with more frequent exacerbations, including chronic respiratory symptoms (i.e., persistent cough and sputum production); older age; COPD severity; emphysematous phenotype; low forced expiratory pressure in 1 second (FEV1), low partial pressure of oxygen in arterial blood (PaO2); high levels of systemic inflammation; bacterial load; comorbid extrapulmonary diseases; anxiety; depression; and the body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE) category of patients (5–7). It has been reported that the FE phenotype can be identified in 22 to 47% of patients depending on COPD severity (4).
In a previous large population study, it was reported that combination therapy with inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA) was used in 75.6% of patients with the FE phenotype compared to 46.5% of patients with the non-FE phenotype (8). Unfortunately, despite more frequent use of combination therapy, this population is still at a higher risk of frequent exacerbations, necessitating further study into the pathophysiologic mechanisms underlying this phenotype. Another previous study has reported that patients with≥300 blood eosinophils/μL are at higher risk of exacerbations and could benefit from triple therapy, including use of long-acting muscarinic antagonists, LABAs, and ICS (9). In addition, patients with the FE phenotype have been reported to have a worse prognosis (10). Finally, sputum microbiota have been examined from patients during COPD exacerbations, revealing a decrease in Bacteroidetes strains and an increase in Proteobacteria strains (11); however, the clinical relevance of this finding is not yet known.
These previous studies have highlighted the prognostic and therapeutic importance of identifying patients with the FE phenotype; therefore, the aim of this study was to identify significant clinical, laboratory, or radiological predictors of the FE phenotype in COPD patients from our clinical practice.
MATERIALS AND METHODS
Study design
We conducted a retrospective, case-control study involving 299 patients with COPD who were admitted to outpatient clinics at the Yedikule Hospital for Chest Disease and Thoracic Surgery in Istanbul, Turkey from January 2014 to January 2020. This study was approved by Yedikule Chest Disease and Chest Surgery Research and Training Hospital and was approved by the Ethics Committee of the Istanbul Research and Training Hospital (No. 2411, date 12.06.2020). Patient informed consent was not required because of the retrospective nature of the study.
Patient Selection
The hospital electronic data system was searched to identify potentially eligible participants. Patients were considered eligible for inclusion in this study if they i) had been diagnosed with COPD based on the recommendations of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (1), ii) had received COPD treatment for more than 1 year, iii) had at least a 10-pack-year smoking history, and iv) had undergone thoracic computed tomography (CT) imaging during the preceding 6 months. Patients were excluded from participation if they i) were nonsmokers, ii) had a condition that prevented completion of the COPD Assessment Test (CAT) or modified Medical Research Council (mMRC) questionnaires; iii) had malignant disease; or iv) had GOLD stage I disease.
Patient evaluation
All study patients were evaluated according to the recommendations of the recent GOLD guidelines, with disease severity ranging from stage II to stage IV, and from class A to D according to the new GOLD classification (1). Patients were determined to have the non-exacerbator phenotype (group-1, n = 195) if they had experienced <1 exacerbation over the preceding year without a hospitalization. Patients were determined to have the FE phenotype (group-2, n=104) if they had experienced ≥2 exacerbations or ≥1 exacerbation with a need for hospitalization during the preceding year. Patient files and data were retrospectively extracted from the hospital electronic data system, including clinical, radiological, and laboratory information, and CAT and mMRC responses, which had been completed by patients previously in the presence of a health-care worker.
Statistical analysis
Study data were analyzed with StatsDirect ver. 3.2 software (StatsDirect Ltd, Cambridge, UK). Descriptive data are presented as medians (range) or means ± standard deviation, and categorical data are presented as total numbers (percentage). Numerical variables with normal intervals were analyzed using paired t-tests, and variables with abnormal intervals were analyzed using Mann-Whitney U tests. P-values were considered statistically significant if <0.01 for the Mann-Whitney U test or <0.05 for the paired t-test. In addition, variables significant at P≤0.20 were analyzed by multivariate logistic regression models with the Wald test to determine predictive parameters for FEs. Calculated odds ratios with 95% confidence intervals and with p-values of <0.05 were considered statistically significant. Power analysis was performed to determine the minimum sample size required for this study based on a previous study by Hurst et al. (4). With consideration of this previous study’s rates, the necessary sample size in current study was determined to be a minimum of 112 people (56 in each group) for a 95% confidence interval and an 80% power.
RESULTS
In total, 299 patients with COPD were included in this study, and general characteristics as well as clinical and laboratory parameters for these patients are shown in Table 1. The study population was comprised of 265 (88.6%) men, mean age was 63.7±8.5 years, and the FE phenotype was identified in 35.1% of patients. Univariate comparisons between non-exacerbators and FEs in the total study population are summarized in Table 1. There were no significant differences between these two phenotypes in terms of gender, smoking status, or leukocyte count. However, FEs were found to be older (p=0.04), with more frequent detection of emphysema on thoracic CT imaging (p=0.02) and lower eosinophil levels (p=0.02). FEs also demonstrated worse values for several pulmonary function parameters, including FEV1, FVC, and FEV1/FVC, and reported higher CAT and mMRC scores. Finally, this group was significantly more likely to use ICS therapy than non-exacerbators (89.5 vs. 65.5%, respectively, p<0.001). As shown in Figure 1, COPD patients were also classified based on the frequency of their exacerbations and hospitalizations. Patients with Class C and D disease also had more GOLD stages III and IV disease and more COPD exacerbations and hospitalizations.
Table 1.
Demographic, laboratory and clinical characteristics of patients with the non-exacerbator or frequent exacerbator phenotypes
| Baseline | Group-1 (non FE) | Group-2 (FE) | p | |
|---|---|---|---|---|
| Number of patients | 299 | 194 | 105 | |
| Gender (male), n (%) | 265 (88.6%) | 172 (88.7) | 93 (88.6) | 0.98 |
| Age, years, mean (SD) | 63.7 (8.5) | 63.0 (8.5) | 65.1 (8.3) | 0.04 |
| History of smoking, pack-years (SD) | 48.2 (25.6) | 47.7 (25.7) | 49.1 (25.5) | 0.66 |
| Coexisting radiological factors | ||||
| Emphysema, n (%) | 147 (49.1) | 86 (44.1) | 61 (58.1) | 0.02 |
| Biomass exposure, n (%) | 130 (43.4) | 90 (47.1) | 40 (38.1) | 0.13 |
| Bronchiectasis, n (%) | 90 (30.1) | 52 (26.8) | 38 (36.2) | 0.09 |
| Interstitial lung disease, n (%) | 10 (3.3) | 4 (2.1) | 6 (5.7) | 0.09 |
| Spirometry | ||||
| FEV1 (L), mean (SD) | 1.32 (0.5) | 1.48 (0.5) | 1.03 (0.4) | <0.001 |
| FEV1 (%), mean (SD) | 46.1 (16.2) | 50.5 (14.7) | 38.0 (15.7) | <0.001 |
| FVC (L), mean (SD) | 2.19 (0.7) | 2.37 (0.7) | 1.87 (0.6) | <0.001 |
| FVC (%), mean (SD) | 59.5 (17.2) | 63.5 (15.9) | 52.2 (17.2) | <0.001 |
| FEV1/FVC (%), mean (SD) | 55.6 (9.7) | 57.4 (8.9) | 52.3 (10.2) | <0.001 |
| COPD stage, classic | ||||
| GOLD II, n (%) | 123 (41.1) | 100 (51.5) | 23 (21.9) | |
| GOLD III, n (%) | 121 (40.5) | 79 (40.7) | 42 (40.0) | |
| GOLD IV, n (%) | 55 (18.4) | 15 (7.7) | 40 (38.1) | |
| COPD stage, new | ||||
| Group A, n (%) | 131 (43.8) | 131 (67.5) | - | |
| Group B, n (%) | 63 (21.1) | 63 (32.5) | - | |
| Group C, n (%) | 30 (10.0) | - | 30 (29.6) | |
| Group D, n (%) | 75 (25.1) | - | 75 (71.4) | |
| Symptom questionnaires | ||||
| CAT score (point), median (SD) | 11.2 (8.6) | 8.5 (6.5) | 10.2 (9.6) | <0.001 |
| mMRC score (point), mean (SD) | 1.5 (1.0) | 1.26 (0.8) | 2.17 (1.1) | <0.001 |
| Cell Blood Count | ||||
| Leukocyte, 109/L (SD) | 8.7 (1.7) | 8.7 (1.8) | 8.8 (1.6) | 0.60 |
| Neutrophil, 109/L (SD) | 5.2 (1.4) | 5.1 (1.4) | 5.4 (1.4) | 0.09 |
| Eosinophil, 109/L (SD) | 235.1 (181.9) | 247.8 (204.3) | 211.6 (128.6) | 0.06 |
| Eosinophil, median, % (SD) | 2.7 (2.0) | 2.88 (2.2) | 2.37 (1.5) | 0.02 |
| Platelet, 109/L (SD) | 273.8 (76.1) | 277.5 (75.0) | 266.9 (78.2) | 0.25 |
| PNR, mean (SD) | 56.5 (23.6) | 58.2 (24.4) | 53.3 (21.7) | 0.13 |
| Clinical Condition, last 12 M | ||||
| Hospitalization, mean (SD) | 0.17 (0.5) | 0.04 (0.1) | 0.42 (0.8) | <0.001 |
| COPD exacerbation, mean (SD) | 1.71 (3.2) | 0.27 (0.4) | 4.34 (4.2) | <0.001 |
| Treatment | ||||
| Use of only LABA and LAMA, n (%) | 78 (26.1) | 67 (34.5) | 11 (10.5) | <0.001 |
| Use of LABA and LAMA plus ICS, n (%) | 221 (73.9) | 127 (65.5) | 94 (89.5) | |
Data are presented as mean ± standard deviation. CAT, Chronic Obstructive Pulmonary Disease (COPD) Assessment Test; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; FEV1, forced expiratory volume in 1 s; FE, frequent exacerbator; FVC, forced vital capacity; ICS, inhaled corticosteroid; L, liter; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; M, month; mMRC, modified Medical Research Council; PNR, platelet-to-neutrophil ratio
Figure 1.
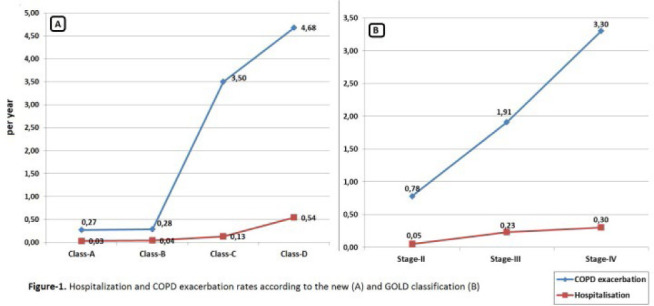
Hospitalization and chronic obstructive pulmonary disease (COPD) exacerbation rates based on the (A) new Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) classification system and (B) GOLD 2007 classification
Table 2 demonstrates univariate comparisons between non-exacerbators and FEs in the subgroup of patients with GOLD stage III or IV disease. In this subgroup analysis, age (p=0.42) and emphysema (p=0.37) did not significantly differ between non-exacerbators and FEs; however, FEs again displayed lower eosinophil levels (p=0.001), lower pulmonary function parameters (p<0.001), and higher symptom scores (p<0.001) than non-exacerbators. ICS treatment was significantly high among FEs, with 90.2% (p=0.02) of these patients using ICS, as shown in Figure 2. Multivariate analysis of data from all study participants revealed three significant predictors for FE, including the mMRC score, ICS use, and FEV1% predicted (OR: 1.88, p<0.001; OR: 2.47, p=0.02; OR: 0.97, p<0.001, respectively) (Table 3). For the subgroup of patients with GOLD stage III or IV disease, mMRC score and FEV1% predicted (OR: 0.93, p=0.001; OR: 1.96, p<0.001) were found to be significant predictors.
Table 2.
Clinical characteristics of patients with Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) stage III or IV disease and the frequent exacerbator phenotype
| Parameters |
Group-1 (non FE)
n=94 |
Group-2 (FE)
n= 82 |
P |
|---|---|---|---|
| Age, years | 64.1 ± 7.8 | 64.7 ± 8.5 | 0.42 |
| Gender (Male), n (%) | 89 (94.7) | 76 (92.7) | 0.59 |
| History of smoking, pack-years (SD) | 48.9 ± 25.8 | 51.4 ± 26.2 | 0.88 |
| Coexisting radiological factors | |||
| Emphysema, n (%) | 51 (54.2) | 50 (61.0) | 0.37 |
| Biomass exposure, n (%) | 36 (38.2) | 33 (40.2) | 0.79 |
| Bronchiectasis, n (%) | 32 (34.0) | 31 (37.8) | 0.60 |
| Interstitial lung disease, n (%) | 2 (2.1) | 6 (7.3) | 0.10 |
| Complete Hemogram | |||
| Leukocyte, 109/L | 8.8 ± 1.7 | 8.9 ± 1.6 | 0.82 |
| Neutrophil, 109/L | 5.4 ± 1.4 | 5.7 ± 1.4 | 0.18 |
| Eosinophil, 109/L | 241.4 ± 181.0 | 202.8 ± 126.5 | 0.001 |
| Eosinophil, median, % | 2.79 ± 1.8 | 2.23 ± 1.5 | 0.03 |
| Platelet, 109/L | 278.6 ± 64.5 | 263.5 ± 76.7 | 0.23 |
| PNR | 55.4 ± 21.8 | 50.6 ± 20.3 | 0.19 |
| Respiratory function | |||
| FEV1, L | 1.10 ± 0.2 | 0.88 ± 0.2 | <0.001 |
| FEV1, % | 37.7 ± 7.7 | 31.2 ± 9.3 | <0.001 |
| FVC, L | 1.96 ± 0.5 | 1.71 ± 0.5 | 0.001 |
| FVC, % | 52.5 ± 12.7 | 46.3 ± 13.5 | 0.002 |
| FEV1/FVC, % | 52.4 ± 9.1 | 50.2 ± 10.1 | 0.13 |
| COPD stage, classic | |||
| GOLD-III, n (%) | 79 (84.1) | 42 (51.3) | |
| GOLD-IV, n (%) | 15 (15.9) | 40 (48.7) | |
| COPD stage, new | |||
| Group-A, n (%) | 54 (57.4) | - | |
| Group-B, n (%) | 40 (42.6) | - | |
| Group-C, n (%) | - | 19 (23.2) | |
| Group-D, n (%) | - | 63 (76.8) | |
| Clinicalevaluation questionnaire | |||
| CAT score | 10.1 ± 6.6 | 17.3 ± 9.6 | <0.001 |
| mMRC score | 1.46 ± 0.9 | 2.34 ± 1.1 | <0.001 |
| Exacerbations, Previous 12 M | |||
| Hospitalization | 0.05 ± 0.2 | 0.50 ± 0.9 | <0.001 |
| COPD exacerbation | 0.35 ± 0.5 | 4.65 ± 4.6 | <0.001 |
| Treatment | |||
| Use of only LABA + LAMA, n (%) | 21 (22.4) | 8 (9.8) | 0.02 |
| Use of LABA and LAMA plus ICS, n (%) | 73 (77.6) | 74 (90.2) | |
Data are presented as mean ± standard deviation. CAT, Chronic Obstructive Pulmonary Disease (COPD) Assessment Test; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; FEV1, forced expiratory volume in 1 s; FE, frequent exacerbator; FVC, forced vital capacity; ICS, inhaled corticosteroid; L, liter; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; M, month; mMRC, modified Medical Research Council; PNR, platelet-to-neutrophil ratio
Figure 2.
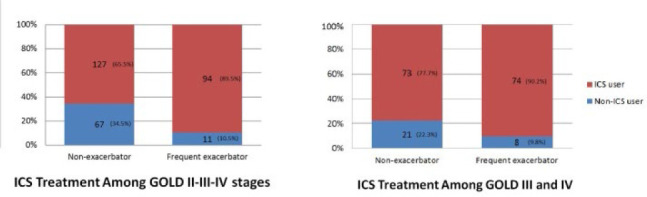
Frequency of inhaled corticosteroid treatment based on phenotype in patients with (A) Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) stage II, III, or IV disease and (B) GOLD stage III or IV disease
Table 3.
Multivariate models for prediction of the frequent exacerbator phenotype
|
Multivariate analysis in GOLD Stage II-III, and IV patients
| |||||||
|---|---|---|---|---|---|---|---|
| Wald | OR | 95% CI | Coeff.β | SE | P | ||
| Lower | Upper | ||||||
| Age | 1.70 | 1.02 | 0.98 | 1.05 | 0.02 | 0.17 | 0.19 |
| Emphysema | 0.12 | 1.10 | 0.63 | 1.94 | 0.01 | 0.28 | 0.73 |
| Eosinophil % | 0.56 | 0.94 | 0.81 | 1.10 | −0.06 | 0.76 | 0.45 |
| FEV1% predicted | 11.3 | 0.97 | 0.95 | 0.99 | −0.03 | 0.01 | <0.001 |
| ICS User | 5.49 | 2.47 | 1.16 | 5.27 | 0.90 | 0.38 | 0.02 |
| mMRC | 18.6 | 1.88 | 1.41 | 2.51 | 0.63 | 0.14 | <0.001 |
| Constant | 3.18 | −2.25 | 1.26 | 0.07 | |||
| Multivariate analysis in GOLD Stage III and IV patients | |||||||
| Age | 0.71 | 1.02 | 0.97 | 1.06 | 0.02 | 0.02 | 0.39 |
| Emphysema | 0.97 | 0.89 | 0.44 | 1.81 | −0.11 | 0.36 | 0.75 |
| Eosinophil % | 1.13 | 0.89 | 0.73 | 1.10 | −0.11 | 0.10 | 0.28 |
| FEV1% predicted | 9.80 | 0.93 | 0.90 | 0.97 | −0.07 | 0.02 | 0.001 |
| ICS User | 1.16 | 1.71 | 0.64 | 4.53 | 0.53 | 0.50 | 0.28 |
| mMRC | 14.4 | 1.96 | 1.38 | 2.77 | 0.67 | 0.18 | <0.001 |
| Constant | 0.05 | −0.37 | 1.58 | 0.81 | |||
CI, confidence interval; SE, Standard error
DISCUSSION
The present study demonstrated that the mMRC score, ICS use, and FEV1% predicted were significant predictors of frequent exacerbations, findings that are consistent with previous studies to date. To our knowledge; however, this is the first study to evaluate the characteristics of FEs in Turkey. In recent years, there has been extensive debate regarding differences in COPD phenotypes worldwide. At the same time, the concept of an FE phenotype has been introduced, accepted, and defined as the occurrence of two or more exacerbations per year. Since COPD rates have been shown to vary between different communities, we can assume that the prevalence of the FE phenotype also varies worldwide.
Similar to our study, Capozzolo et al. (5) also found that a high mMRC score was associated with the FE phenotype. In that study, 183/345 (53.0%) patients with COPD demonstrated this phenotype, with the mMRC score having an OR of 1.45 (95% CI, 1.12–1.91, p<0.01). In the present study, we identified a higher OR of 1.88 (95% CI, 1.41–2.51, p<0.001) for the mMRC score. Similarly, Wan et al. (12) reported an OR of 1.50 (95% CI, 1.15–1.97, p=0.003) for the mMRC score, although some mMRC values were missing from their data. In another related study, Le Rouzic et al. (13) reported an OR of 4.53 (95% CI, 1.63–14.8, p=0.006) for mMRC scores of 3–4, with that study developing a new scoring system for FE called ESOD (exacerbation history, chronic sputum production, GOLD stage of obstruction, and mMRC dyspnea stage), which included the mMRC score.
Though it is widely known that FEV1 and COPD exacerbations are associated with one another, this relationship is likely nonlinear. Previous studies have reported that approximately 40% of patients with severe COPD do not present with frequent exacerbations (14,15); however, the study conducted by Le Rouzic et al. (13) showed that FEs had a significantly lower FEV1 than non-frequent exacerbators, with the FEV1 found to be a significant predictor and included in their ESOD scoring system. The present study found similar results, with significantly lower FEV1 values in FEs than in nonexacerbators (1.03±0.4 vs. 1.48 L±0.5, p< 0.001).
In contrast, some recent studies have shown the FEV1 is not associated with the risk of frequent exacerbations. For example, in a study conducted by Capozzolo et al. (5), the predicted inspiratory capacity (IC)% predicted, total lung capacity (TLC) % predicted, residual volume/TLC %, six-minute walking test, BODE index, and mMRC score were shown to be independent factors predicting FE; however, FEV1 was not. This discrepancy may have resulted from differences in patient selection, since Capozzolo et al.’s (5) study enrolled only GOLD stage III and IV patients. On the other hand, the present study included COPD patients with GOLD stages II, III, and IV disease and demonstrated that, in addition to FEV1, emphysema, mMRC score, and ICS use also played important roles in the FE phenotype.
It is also important to examine the accompanying lung abnormalities detected on thoracic CT imaging of patients in this study, including bronchiectasis, emphysema, interstitial lung diseases, and findings of biomass exposure. Among the 35.1% of FEs in our total study population, 58.1% displayed emphysema on imaging, which was a significantly higher proportion than in nonexacerbators. Subsequently, we performed a subgroup analysis including only GOLD III and GOLD IV patients and found no difference in the presence of emphysema between the FE and non-exacerbator phenotypes in this subgroup (54.2 vs. 61.0%, p=0.37). We interpreted this finding as demonstrating that accompanying emphysema may increase the rates of exacerbations in these patients, particularly during the early stages of COPD.
Similarly, a study by Oh et al. (7) demonstrated that a higher emphysema index was independently associated with the FE phenotype. That study included 380 patients with all GOLD disease stages and 77 patients with the FE phenotype (20.3%), which was a lower proportion than in our study. Although a significant relationship between emphysema and FE has not been widely accepted to date, there are an increasing number of studies focused on understanding this relationship. Furthermore, it has been suggested that FEs with emphysema may be more likely to have coexistent chronic bronchitis (6). Studies have also reported that emphysema is related to the ratio of IC/TLC rather than the FEV1 or the presence of dyspnea (16). In addition, the FE phenotype has been found to be an independent predictor of depressive symptoms in COPD patients (17).
In addition to depression, the relationships between accompanying diseases and COPD exacerbations have been the subject of significant research. In particular, coexisting cardiovascular diseases (18), gastrointestinal diseases (19), gastroesophageal reflux disease (4), history of tuberculosis (20) or post-tuberculosis pneumofibrosis (19), bacterial colonization of the lower respiratory tract (21), and bronchiectasis (22) have been reported to increase the frequency of exacerbations and/or the risk of severe exacerbations. In this study; however, we found no significant differences in the rates of bronchiectasis, interstitial lung disease, or biomass exposure findings between phenotypes.
Although no biomarkers to identify the FE phenotype are currently available, a previous small study has reported that there may be a negative correlation (RS=−0.53; p=0.02) with the soluble receptor for advanced glycation end-products (sRAGE) in patients with the FE phenotype (23). In addition, a recent study evaluating microRNAs (miRs) in patients with COPD reported that miR-23a may be a useful biomarker for detecting the FE phenotype, with a number of potential biomarkers, including miR-23a, miR-25, miR-145, and miR-224, significantly down regulated during the development and progression of COPD (24).
Early identification of patients with the FE phenotype is of paramount importance in the prevention and reduction of future exacerbations. Since various bacterial (54.7%) and viral (48.4%) infections have been reported to accompany COPD exacerbations (25), vaccination of these patients will likely play an important role in successful management. According to national data in Turkey, the rate of vaccination for influenza was only 22.0% for people over 65 years of age (26). Furthermore, in COPD patients, the rates of vaccination for influenza and pneumococcus were only 37.9% and 13.3%, respectively (27). These rates suggest that vaccination rates in COPD patients are too low. Since patients with the FE phenotype are likely to experience two or more exacerbations per year, it is clear that higher vaccination rates need to be achieved.
There were a few unavoidable limitations to this study that need to be mentioned. First, this study did not allow for the drawing of definitive conclusions because of its retrospective and cross-sectional design. Second, this study was conducted over a 12-month follow-up period, and any necessary switching of patients between the non-FE <-> FE phenotype could not be identified after this period. However, several significant variables were identified in this study, which should certainly be confirmed in future prospective, long-term, large-scale, and randomized-controlled studies.
New studies are on the horizon for the diagnosis and treatment of patients with the FE phenotype. With the present-day increase in the use of biological drugs, a beneficial effect can be expected with use of this drug class in patients with the FE phenotype. In fact, two ongoing studies are evaluating the use of benralizumab (NCT04053634) and mepolizumab (NCT04133909) in this patient population. A synopsis of ongoing clinical studies in patients with the FE COPD phenotype is presented in Table 4.
Table 4.
Ongoing clinical trials of patients displaying the frequent exacerbator phenotype of chronic obstructive pulmonary disease
| Study | Estimated completion date | N | Trial number |
|---|---|---|---|
| Phenotypes of COPD | 04/2021 | 50 | NCT03432026 |
| The London COPD Exacerbation Cohort | 04/2021 | 300 | NCT02755974 |
| Mepolizumab as Add-on Treatment in Participants With COPD Characterized by Frequent Exacerbations and Eosinophil Level (MATINEE) | 01/2023 | 800 | NCT04133909 |
| Efficacy and Safety of Benralizumab in Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COPD) with a History of Frequent Exacerbations | 11/2023 | 868 | NCT04053634 |
CONCLUSION
COPD patients with the FE phenotype likely require a different treatment algorithm due to differing clinical features such as poorer respiratory function, lower eosinophil levels, and more frequent emphysema. A better understanding of different phenotypic/endotypic properties and identification of a significant biomarker in patients with these significant predictors will likely have important clinical implications for preventing future exacerbations in this population.
Acknowledgment
The study protocol was approved by the local ethics committee in Istanbul/Turkey (No. 2411, date: 12.06.2020). The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Footnotes
Disclosure statement
The authors report no conflicts of interest in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf (Accessed on Januar 30, 2021)
- 2.Labaki WW, Martinez FJ. Time to Understand the Infrequency of the Frequent Exacerbator Phenotype in COPD. Chest 2018;153(5):1087–8. [DOI] [PubMed] [Google Scholar]
- 3.Jinjuvadia C, Jinjuvadia R, Mandapakala C, Durairajan N, Liangpunsakul S, Soubani AO. Trends in Outcomes, Financial Burden, and Mortality for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in the United States from 2002 to 2010. COPD 2017;14(1):72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363(12):1128–38. [DOI] [PubMed] [Google Scholar]
- 5.Capozzolo A, Carratù P, Dragonieri S, Falcone VA, Quaranta VN, Liotino V, et al. Clinical and Functional Lung Parameters Associated With Frequent Exacerbator Phenotype in Subjects With Severe COPD. Respir Care 2017;62(5):572–8. [DOI] [PubMed] [Google Scholar]
- 6.Miravitlles M, Calle M, Soler-Cataluña JJ. Clinical phenotypes of COPD: identification, definition and implications for guidelines. Arch Bronconeumol 2012;48(3):86–98. [DOI] [PubMed] [Google Scholar]
- 7.Oh YM, Sheen SS, Park JH, Jin UR, Yoo JW, Seo JB, et al. Emphysematous phenotype is an independent predictor for frequent exacerbation of COPD. Int J Tuberc Lung Dis 2014;18(12):1407–14. [DOI] [PubMed] [Google Scholar]
- 8.McGarvey L, Lee AJ, Roberts J, Gruffydd-Jones K, McKnight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med 2015;109(2):228–37. [DOI] [PubMed] [Google Scholar]
- 9.Chapman KR, Hurst JR, Frent SM, Larbig M, Fogel R, Guerin T, et al. Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am J Respir Crit Care Med. 2018;198(3):329–39. [DOI] [PubMed] [Google Scholar]
- 10.Tomioka R, Kawayama T, Suetomo M, Kinoshita T, Tokunaga Y, Imaoka H, et al. “Frequent exacerbator” is a phenotype of poor prognosis in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2016;11:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tangedal S, Nielsen R, Aanerud M, Persson LJ, Wiker HG, Bakke PS, et al. Sputum microbiota and inflammation at stable state and during exacerbations in a cohort of chronic obstructive pulmonary disease (COPD) patients. PLoS One 2019;14(9):e0222449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan ES, DeMeo DL, Hersh CP, Shapiro SD, Rosiello RA, Sama SR, et al. Clinical predictors of frequent exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). Respir Med 2011;105(4):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Rouzic O, Roche N, Cortot AB, Tillie-Leblond I, Masure F, Perez T, et al. Defining the “Frequent Exacerbator” Phenotype in COPD: A Hypothesis-Free Approach. Chest 2018;153(5):1106–15. [DOI] [PubMed] [Google Scholar]
- 14.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157(5 Pt 1):1418–22. [DOI] [PubMed] [Google Scholar]
- 15.Dewan NA, Rafique S, Kanwar B, Satpathy H, Ryschon K, Tillotson GS, et al. Acute exacerbation of COPD: factors associated with poor treatment outcome. Chest 2000;117(3):662–71. [DOI] [PubMed] [Google Scholar]
- 16.Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005;171(6):591–7. [DOI] [PubMed] [Google Scholar]
- 17.Tse HN, Tseng CZ, Wong KY, Ng LY, Lai TL, Yee KS. Frequent Exacerbator: The Phenotype at Risk of Depressive Symptoms in Geriatric COPD Patients. Lung 2016;194(4):665–73. [DOI] [PubMed] [Google Scholar]
- 18.Niewoehner DE, Lokhnygina Y, Rice K, Kuschner WG, Sharafkhaneh A, Sarosi GA, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest 2007;131(1):20–8. [DOI] [PubMed] [Google Scholar]
- 19.Krachunov I, Kyuchukov N, Ivanova Z, Yanev NA, Hristova PA, Pavlov P, et al. Stability of Frequent Exacerbator Phenotype in Patients with Chronic Obstructive Pulmonary Disease. Folia Med (Plovdiv) 2018;60(4):536–45. [DOI] [PubMed] [Google Scholar]
- 20.Yakar HI, Gunen H, Pehlivan E, Aydogan S. The role of tuberculosis in COPD. Int J Chron Obstruct Pulmon Dis 2017;12:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finney LJ, Ritchie A, Pollard E, Johnston SL, Mallia P. Lower airway colonization and inflammatory response in COPD: a focus on Haemophilus influenzae. Int J Chron Obstruct Pulmon Dis. 2014;9:1119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamatawong T, Onnipa J, Suwatanapongched T. Relationship between the presence of bronchiectasis and acute exacerbation in Thai COPD patients. Int J Chron Obstruct Pulmon Dis 2018;13:761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miłkowska-Dymanowska J, Białas AJ, Szewczyk K, Kurmanowska Z, Górski P, Piotrowski WJ. The usefulness of soluble receptor for advanced glycation end-products in the identification of COPD frequent exacerbator phenotype. Int J Chron Obstruct Pulmon Dis 2018;13:3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Qu J, Xue W, He L, Wang J, Xi X, et al. Bioinformatics-based identification of potential microRNA biomarkers in frequent and non-frequent exacerbators of COPD. Int J Chron Obstruct Pulmon Dis 2018;13:1217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walters JA, Tang JN, Poole P, Wood-Baker R. Pneumococcal vaccines for preventing pneumonia in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;1(1):CD001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozlu T, Bulbul Y, Aydin D, Tatar D, Kuyucu T, Erboy F, et al. Immunization status in chronic obstructive pulmonary disease: A multicenter study from Turkey. Ann Thorac Med 2019;14(1):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arpinar Yigitbas B, Satici C, Tanrıverdi E, Gündüz C. Influenza vaccination frequency and associated factors among elderly population, a descriptive study. Turkish Journal of Geriatrics 2018; 4: 490–7. [Google Scholar]


