Abstract
This review aimed to identify the features of coronavirus disease 2019 (COVID-19) in pediatric patients after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. According to the literature, the incidence of COVID-19 was reported to be 1–5% among children. However, the incidence of infection with the new variant of the virus is higher in children. The most common features were fever and respiratory manifestation. The milder severity and lower mortality of COVID-19 among children are related to their less contact, immature immune system, and different features of angiotensin-converting enzyme 2 (ACE2), an important receptor of the virus to invade the host cells. Several complications were observed in severe pediatric patients, such as coinfections, encephalitis, multisystem inflammatory syndrome, and multiorgan failure. The most frequent laboratory data were the procalcitonin elevation. The enhanced inflammatory factors and lymphocytopenia were less common among this population. In the CT findings, the ground-glass opacities, pulmonary consolidation, fine mesh shadow, and tiny nodules were most common. While some children were admitted to the ICU, mechanical ventilation was rarely reported. The vertical intrauterine transmission from mother to child has not been proven. The treatment mainly focuses on maintaining balance in the fluids and electrolytes, nutritional support, and oxygen therapy for this vulnerable population.
Keywords: COVID-19, SARS-CoV-2, Pediatric patients, Viral infections
INTRODUCTION
Coronavirus disease 2019 (COVID-19), an infectious disease caused by severe acute respiratory syndrome 2 (SARS-CoV2), was first reported in Wuhan, China (1). SARS-CoV, SARS-CoV-2, and the Middle East respiratory syndrome (MERS) belong to the family of human coronaviruses (HCoVs), which may cause severe illness, causing a high mortality rate (2). SARS-CoV-2 is a type of β-CoVs with large, enveloped, positive-strand RNA viruses (3). SARS-COV-2 has rapidly involved the whole world and impacted pediatric and adolescent populations (4). Recently, SARS-CoV-2 was transmitted by respiratory droplets of different sizes and through direct contact. Also, the fecal transmission of the virus should be considered (5). SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) receptor to invade the host cell (6, 7). Th virus can lead to multiorgan dysfunction by infecting other organs with higher expression of ACE2 (8).
In mild adult patients, the common symptoms of COVID-19 are fever, shortness of breath, dry cough, tiredness, chest tightness, and dyspnea, and develop severe conditions can be seen in 10–20% of them (9). In the chest computed tomography (CT) scan of severe adult cases with COVID-19, the bilateral ground-glass opacity (GGO) was prominent (10). The risk of death is higher in older people (aged more than 65 years) and patients with underlying conditions due to acute respiratory distress syndrome (ARDS) and multiorgan dysfunction (8).
Although the incidence of COVID-19 among infants and children was not prominent and the severity of disease and incidence of severe cases are lower among pediatric patients (11, 12), the UK variant of SARS-CoV-2 (B.1.1.7) can cause those under 20 years (13). For COVID-19 in pediatric patients, the median time between the onset and diagnosis of the illness was two days (0–42 days) (14), which increases the risk of virus transmission from pediatric patients to older people. Additionally, the presence of a novel pediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2 (PIMS-TS) or Kawasaki disease–like multisystem inflammatory syndrome has been reported in severe pediatric cases (15).
Due to the importance of understanding the features of COVID-19 in pediatric patients as a susceptible population, we reviewed the recent literature to identify the case definitions and management strategies of COVID-19 among infants and children.
Epidemiology of COVID-19 among children
The clinical manifestations of children may be less severe compared to the adult population. In an epidemiology study in China, 2135 pediatric patients were identified with a median age of 7 years (2–13 years) (14). In a similar study in China, out of 1391 children examined for SARS-CoV-2 infection, 171 cases (12.3%) were infected with COVID-19. One death, 21 patients with stable condition, and 149 discharged patients were recorded (16). In another epidemiologic study in the United States, 22% of COVID-19 patients were infants, children, and adolescents less than 18 years of age (17). Also, COVID-19 in neonatal cases has been demonstrated at 16 (18), 30 (19), and 36 h (20) after delivery.
While the incidence of severe disease in adults was 18.5% in a report by the CDC in China (21), many pediatric patients were asymptomatic with mild or moderate disease (90%). Accordingly, 5.8% of pediatric patients exerted severe symptoms (e.g., central cyanosis, dyspnea, and oxygen saturation<92%) and critical illness (e.g., ARDS, shock, abnormal coagulation, encephalopathy, heart failure, acute renal failure, and multiorgan failure). These alterations were attributed to the severe outcomes mainly among cases of less than one-year-old and one patient who died. Frequency of severe disease in pediatric patients was calculated for each age group; 10.6% for <1, 7.3% for 1–5, 4.2% for 6–10, 4.1% for 11–15, and 3.0% for >15 year-old groups (14). The incidence of diagnosed children with COVID-19 was 1–5% of cases with milder conditions and better prognoses (22). Pneumonia was observed in about half of children with mild or asymptomatic (28%) disease (23). The incidence of disease in patients aged <18 years was 1.7%, and infants <1 year were 15% of pediatric COVID-19 patients (11). Hospitalization rates were lower in pediatric patients (5.7–20%), and 0.58–2.0% of cases were admitted to the intensive care unit (ICU) (14, 16). COVID-19 was more common in infant patients aged < 1 year and those with underlying conditions (11). Therefore, children with asymptomatic or mild infections are silent carriers of the virus and contribute to the rapid amplification and transmission of SARS-CoV-2 in the community, especially among elderly adults. Subsequently, providing social and public health policies to limit the interaction of this age group is necessary (24).
Several studies have reported a slightly higher rate of infection in boys than girls (14, 16). Additionally, the case-fatality rate among men was higher than in women (21). The mechanisms underlying to make the potential difference in COVID-19 severity or incidence between males and females have not been detected. In all age groups, particularly in patients aged less than one year, males were predominant infected sex group, suggesting that susceptibility to infection by sex is affected by biological factors (11). Hospitalization of infants and young children following viral respiratory tract infections, e.g., influenza virus (IV) and respiratory syncytial virus (RSV), is frequent and due to the immaturity of the respiratory tract and immune system in this age group (25). Nevertheless, the lower hospitalization rate among children with COVID-19 has created a puzzle for scientists, epidemiologists, and clinicians.
COVID-19 among pediatric patients: clinical, laboratory, and radiological findings
According to the literature, the typical clinical manifestations of COVID-19 are fever, cough, sore throat, sneezing, fatigue, myalgia, and rarely wheezing among children (14). Other symptoms are diarrhea, rhinorrhea, and vomiting—the oxygen saturation < 92% is observed in 2.3% of patients. During the hospital admission, tachycardia (42.1%) and tachypnoea (28.7%) were recorded (16). Vomiting is more prominent in pediatric patients (6.5% [2/31]-66.7% [4/6]) (26). Data from pediatric patients in the US showed the following symptoms: fever (73%), cough (54%), and shortness of breath (13%) (11). Also, in a systematic review of the features of COVID-19 in 7780 pediatric patients, the most frequent symptoms were fever (59·1%) and cough (55·9%), and 19·3% were asymptomatic. Abnormal lung radiography and chest CT findings include patchy lesions (21·0%) and GGO (32·9%), respectively. Also, 152/233 cases were observed as immunocompromised children (respiratory/cardiac disease) with underlying medical conditions. In 5.6% of cases, coinfections were reported. The laboratory findings confined the presence of abnormal factors, such as creatine kinase, D-dimer, procalcitonin, and interleukin (IL)-6. The multisystem inflammatory syndrome was seen in 0.14%, and the incidence of death was 0·09% (27). According to World Health Organization, the most frequent clinical finding in children with severe COVID-19 was pneumonia (53%). Also, fever, dry cough, or both, and shortness of breath were the most frequent symptoms (28). However, 93% of adults aged 18 to 64 had these symptoms in a similar period (16). In a study on pediatric patients with COVID-19 aged under 16 years in China, the main symptoms were fever (41.5%) and cough (48.5%), and 1.8% of cases were admitted to the ICU (16). In another study, cough (48.5%), pharyngeal erythema (46.2%), and fever (41.5%) were common, respectively. Furthermore, 15.8% of patients were asymptomatic. Radiologic features of pneumonia without any symptoms of infection were recorded in 12 patients, and three needed ICU admission and mechanical ventilation. Laboratory finding showed six patients (3.5%) with lymphopenia and CT abnormalities, including GGO (32.7%), local (18.7%), and bilateral (12.3%) patchy shadowing and interstitial abnormalities (1.2%). Also, one death due to multiorgan failure was reported (16). In an investigation of clinical and CT characteristics of 20 pediatric COVID-19 patients, fever (60%) and cough (65%) were the most common symptoms. The higher levels of procalcitonin (80%) were essential laboratory findings. Unilateral pulmonary lesions (30%), bilateral pulmonary lesions (50%), no abnormality on chest CT (20%) as well as GGOs (60%), pulmonary consolidation (50%), fine mesh shadow (20%), and tiny nodules (15%) were reported. Coinfection was recorded in 40% of patients (29). Laboratory findings of 12 studies (66 pediatric patients) have confirmed the average leucocyte count of 69.2%. Moreover, neutrophilia (4.6%) and neutropenia (6.0%) were rare compared to adults. Lymphocytopenia was confirmed in 3.0% of children. A high concentration of C-reactive protein and procalcitonin (correlated with the severity of illness) were observed in 13.6% and 10.6% of patients, respectively. The elevated level of inflammatory factors was rare, but levels of IL-6 were high in very sick infants (30). In a small pediatric cohort of ten- to six-year-old COVID-19 patients, similar but modest CT lung abnormalities (patchy GGOs) were recorded (31). In the Regina Margherita Children’s Hospital (Italy), the evaluation of COVID-19 features in eight children (two severe, two moderate, and four mild cases) showed that two cases required noninvasive oxygen therapy (no mechanical ventilation). The lung ultrasound of these patients revealed five patients with confluent B-lines and two with sub-pleural consolidations. In seven out of eight children, these data were confirmed by the radiologic findings. Despite a normal radiography finding, an interstitial B-lines pattern was observed in the remaining cases. Also, follow-up examinations of a severe case presented reduced B-lines bilateral pattern one day before radiographic and clinical improvement (32). All features of COVID-19 in pediatric patients are summarized in figure 1.
Figure 1.
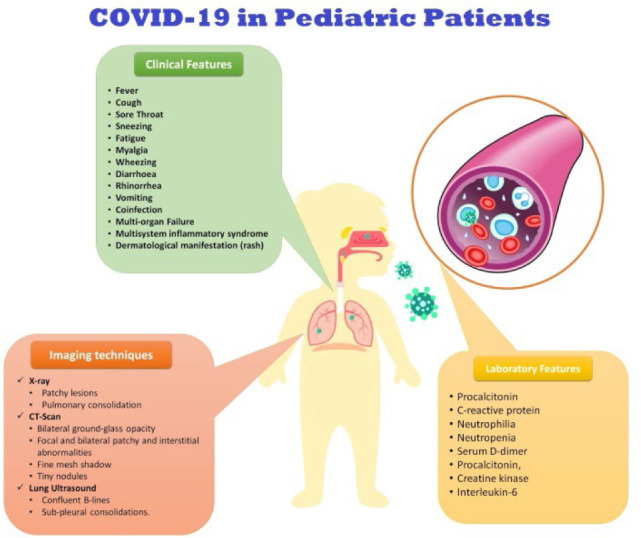
The summery of COVID-19 features among pediateric patiens
Secondary complications of COVID-19 among pediatric patients
Rare evidence has confirmed severe complications in pediatric patients with COVID-19. However, severe respiratory infections (e.g., IV) were associated with some complications in children, including respiratory failure (33), neurologic abnormalities (34), myositis, myoglobinuria, acute renal failure (35), myocarditis (36), and gastrointestinal symptoms (i.e., diarrhea, nausea, or vomiting) (36). A case study reported a six-month-old infant with SASR-CoV-2 infection with PIMS-TS. In this case, fever and minimal respiratory symptoms were accompanied by acute vasculitis, leading to heart disease (37). Additionally, a cluster of eight children with hyperinflammatory shock was reported in the UK. Disease characteristics were similar to those observed in toxic shock syndrome, known as Kawasaki disease shock syndrome, atypical Kawasaki disease, or PIMS-TS. Four out of eight cases were from a family with COVID-19 exposure (38). Also, fever, rash from macular to maculopapular or morbilliform (dermatological manifestations), and PIMS-TS were recorded in a 3-year-old girl with COVID-19 (39). Primary viral pneumonia can be accompanied by a secondary bacterial infection (Staphylococcus aureus) (35, 40). Wheezing illness caused by viral infections in infants may increase the risk of asthma in their later life with unclear mechanisms (41). Wheezing in the first years of life following severe RSV resulted in allergic asthma in later childhood and early adulthood (42).
Several neurological complications were observed in pediatric patients with the IV infection, including seizures, encephalopathy, encephalitis, Guillain-acute myelopathy, Reye’s syndrome, Barré syndrome, hemiplegia, ataxia, stroke, quadriparesis, and transverse myelitis (43). Although the recovery of neurological complications was generally observed, some secondary consequences, e.g., residual seizures, cognitive deficits related to necrotizing encephalitis, basal ganglia involvement, and cerebral volume loss, were found in these patients (35, 36). In neuroimaging of four children with COVID-19 admitted to the ICU, signal alterations in the corpus callosum were recorded (44). A 26-day-old male infant with SARS-CoV-2 infection and fever was reported with some neurological manifestations, including upward rolling of the eyes, generalized hypertonia after awakening, and generalized hypertonia and facial cyanosis during sleep. In electroencephalography (EEG) findings, a continuous background pattern with sleep/wake cycles in the absence of clinical and electrical seizures was observed. The fever was associated with the severity of neurologic manifestations (45). Recently, an 11-year-old child with encephalitis associated with COVID-19 was reported. While a CT scan did not prove the disease, the frontal intermittent delta activity was recorded by an EEG (46).
On the other hand, secondary complications of quarantined and closed schools in healthy or recovered children should be considered. Going into quarantine and social isolation may affect the mental and physical health of children. These alterations can result in irregular sleeping patterns, reduced healthy diets, and lack of physical activity, resulting in numerous comorbidities, such as obesity and cardiorespiratory diseases (47). The quarantine and isolation of children after health-related disasters (not related to SARS-CoV-2) were associated with an elevated risk of post-traumatic stress disorder (PTSD) (48). Several complications have been recorded for pediatric patients with SARS-CoV-2 infection that should be considered by clinical staff.
Multisystem Inflammatory Syndrome in Pediatric Patients with COVID-19
First described in Japan, Kawasaki disease is a vasculitis affecting medium-sized arteries with the highest incidence in under five-year-old pediatrics (49). In several studies, PIMS-TS was frequent among critically ill pediatrics with COVID-19 (50, 51). The main manifestations of PIMS-TS are shocked, cardiac dysfunction, myocarditis, left ventricular dysfunction, coronary artery dilation or aneurysm, and acute kidney injury (51, 52). Lab data of COVID-19 patients with PIMS-TS showed elevated levels of inflammatory/cardiac markers (e.g., troponin I, C-reactive protein, ferritin, lactate dehydrogenase, D-dimer, fibrinogen, interleukin-6, and N-terminal [NT]-prohormone BNP [NT-proBNP]). Hyperkalemia, hyponatremia, azotemia, neutrophilia, and lymphopenia were also recorded (51–55). The most crucial point was admitting most patients with PIMS-TS to the ICU (52). Cardiac MRI of children with PIMS-TS showed diffuse myocardial edema on T2-STIR sequences and native-T1 mapping, with no evidence of late gadolinium enhancement suggestive of replacement fibrosis or focal necrosis (56). Postmortem autopsy findings of COVID-19 patients with PIMS-TS proved endocarditis, myocarditis, and pericarditis with infiltration of inflammatory cells (such as CD68+ macrophages, CD45+ lymphocytes, neutrophils, and eosinophils). These histopathological alterations were associated with cardiomyocyte necrosis. Electron microscopy evaluations showed spherical viral particles in the extracellular sites and within multiple cell types, e.g., cardiomyocytes, capillary endothelial cells, endocardium endothelial cells, fibroblasts, macrophages, and neutrophils. Histopathological examinations of autopsied lung specimens showed microthrombi within pulmonary arterioles, mild pneumocyte hyperplasia, and patchy exudative alterations in alveolar spaces. Some alterations were observed in other organs, including microthrombi within glomerular capillaries and shock-induced acute tubular necrosis in the kidney tissues, centrilobular necrosis in liver tissue, and microglial reactivity in the brain tissue (55). These results confirmed the viral presence in the myocardial tissues of COVID-19 patients with PIMS-TS.
Lower vulnerability of pediatric population to COVID-19: Causes
As mentioned before, children are less susceptible to SARS-CoV-2 infection, and the severity and mortality rate is lower in this population (57). A wide range of infectious diseases has less mortality and severity in children. No mortality was reported in children or adolescents <24 years with COVID-19 (58). During the 2009 H1N1 pandemic, the higher mortality and morbidity rate in severe pneumonia was related to patients aged 5–59 years (59). Also, the incidence of infection with paralytic polio and rubella was less frequent in infants and young children (58).
The critical question is why clinical symptoms of COVID-19 pediatrics were milder than those in adults. However, mechanisms underlying the milder severity of disease in pediatrics and their resistance to COVID-19 remain unknown. Some exposure and host factors may describe this question. Here, we categorized some reasons:
Exposure: Well-cared conditions at home for children provided by parents and fewer outdoor activities may decrease the risk of contracting the virus (14). Furthermore, the lower incidence of infection among pediatrics at the beginning of a pandemic cannot indicate that pediatric cases were less susceptible to COVID-19, as infection with SARS-CoV-2 was reported in infants (60).
Immune system: Children have specific mechanisms that regulate the interaction between the immune system and respiratory machinery, contributing to milder disease (23). The higher risk of exposure to respiratory infections (e.g., RSV) in the winter may increase antibodies against viruses in children. The immune system in children is still immature and undergoes substantial alterations from birth to adulthood. Therefore, this age group's immune system responses to pathogens might differ (61). In addition, fewer underlying abnormalities, healthier respiratory tracts due to a lesser chance of exposure to cigarette smoke or air pollution, and more active innate immune responses in children can lead to milder disease in this population (58). Additionally, immune system differences in the thymus activity and cross-reactive immunity to common cold CoVs are other reasons that protect children against SARS-CoV-2 infection (62).
ACE-2: One potential reason is that the difference in the distribution, maturation, and action of the ACE2 receptor is age-related (63). Children are less sensitive to SARSCoV-2 because of lower maturity and action of ACE2 (64). ACE2 expression decreases with age in mice models (65). ACE2 protects the lung against pathologic conditions, such as SARS, H5N1 virus, RSV, sepsis, and acid aspiration (66). ACE2 regulates the renin-angiotensin system via cleaving angiotensin (Ang)-II to Ang-1–7. A balanced ACE2/Ang 1–7 axis leads to protection from lung diseases. Therefore, ACE2 deficiency is associated with a rising viral respiratory infection and lung failure (57).
Management of pediatric patients with COVID-19
Many clinical and laboratory tests should be carried out to detect COVID-19 infection in children because of several differential diagnoses, including RSV, IV, parainfluenza virus, adenovirus, rhinovirus, hMPV, etc. (67). In a systemic review, the potential interventions of COVID-19 were presented for adults. In addition to nutritional support and electrolyte barnacle maintenance, treatment with thymosin alpha-1, interferons (IFNs), intravenous gamma globulin, thymopentin, levamisole, cyclosporine A, and traditional Chinese medicine were recommended for the management of oxidative stress and inflammation. Notably, it was shown that treatment with intravenous gamma globulin led to venous thromboembolism in one out of three cases during the SARS-CoV epidemic (68). In another study, COVID-19 in children was treated with aerosolized IFNα in all cases, Kaletra (lopinavir/ritonavir) syrup (twice/day for 14 days) in 14 cases (39%), and supportive oxygen therapy in six cases (17%) (28). It is suggested to use antiviral treatment for pediatric patients (67, 69). Recently, antiviral and anti-inflammatory medications have been mentioned for managing pediatric patients (70). However, it has been revealed that antiviral therapy for COVID-19 is necessary for those with critical conditions or severe diseases (71). Furthermore, anticoagulants have been recorded for hospitalized pediatric patients (72). A list of medicines, including steroids, intravenous immunoglobulin (IVIg), antiplatelet medication, and anticoagulants, was used to manage COVID-19 cases with PIMS-TS (51, 52).
In the literature, supportive approaches (e.g., oxygen supply and administration of antibiotics for secondary bacterial infectious disease) were also mentioned (30). A retrospective study confirmed that the risk of lower respiratory tract infection with CoVs is higher in immunocompromised children compared to nonimmunocompromised cases (73).
CONCLUSION
In summary, this review proposed that the severity of COVID-19 among infants and children was lower than in adults due to differences in immune system responses, ACE2 expression patterns, and lower risk of contact with COVID-19 patients. An asymptomatic or mild form of the disease leads to a higher risk of SARS-COV-2 transmission in the community. Therefore, staying at home, social distancing, and respiratory hygiene have been suggested for all age groups to reduce the spread of viral infection. On the other hand, severe pediatric patients may present several secondary complications, including multisystem inflammatory syndrome and multiorgan failures (e.g., cardiovascular disease, renal failure, neurological disease, etc.). Moreover, monitoring the pediatric patients with underlying conditions should be considered.
REFERENCES
- 1.Boccia S, Ricciardi W, Ioannidis JPA. What Other Countries Can Learn From Italy During the COVID-19 Pandemic. JAMA Intern Med 2020;180(7):927–8. [DOI] [PubMed] [Google Scholar]
- 2.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2(2):100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neerukonda SN, Katneni U. A Review on SARS-CoV-2 Virology, Pathophysiology, Animal Models, and Anti-Viral Interventions. Pathogens 2020;9(6):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020;368(6493):860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nouri-Vaskeh M, Alizadeh L. Fecal transmission in COVID-19: A potential shedding route. J Med Virol 2020;92(10):1731–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367(6485):1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46(4):586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J Mol Histol 2020;51(6):613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020;382(8):727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y, Wang X, Yang P, Zhang S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol Cardiothorac Imaging 2022(2):e200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC COVID-19 Response Team . Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69(12):343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr 2020;109(6):1082–3. [DOI] [PubMed] [Google Scholar]
- 13.Report 42 - Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. link: https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-42-sars-cov-2-variant/.
- 14.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 15.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395(10239):1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in Children. N Engl J Med 2020;382(17):1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Türkoğlu A, Gül M, Yuksel HK, Alabalik U, Ülger BV, Uslukaya O, et al. Effect of intraperitoneal curcumin instillation on postoperative peritoneal adhesions. Med Princ Pract 2015;24(2):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID-19 during Pregnancy and Possible Vertical Transmission. Am J Perinatol 2020;37(8):861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Wang C, Gao C. Neonatal coronavirus expert confirmed at 30 hours of birth: vertical transmission from mother to infant. 2020.
- 20.Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, et al. A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin Infect Dis 2020;71(15):853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41(2):145–51. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109(6):1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20(6):689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelvin AA, Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis 2020;20(6):633–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 2010;23(1):74–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther 2020;51(9):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: importance in Parkinson's disease. Ann Neurol 2004;55(6):766–73. [DOI] [PubMed] [Google Scholar]
- 28.Organization WH . Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 27 March 2020. World Health Organization; 2020. [Google Scholar]
- 29.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 2020;55(5):1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med 2020;58(7):1135–8. [DOI] [PubMed] [Google Scholar]
- 31.Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol 2020;50(6):796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denina M, Scolfaro C, Silvestro E, Pruccoli G, Mignone F, Zoppo M, et al. Lung Ultrasound in Children With COVID-19. Pediatrics 2020;146(1):e20201157. [DOI] [PubMed] [Google Scholar]
- 33.Mistry RD, Fischer JB, Prasad PA, Coffin SE, Alpern ER. Severe complications in influenza-like illnesses. Pediatrics 2014;134(3):e684–90. [DOI] [PubMed] [Google Scholar]
- 34.Mak GCK, Kwan MY, Mok CKP, Lo JYC, Peiris M, Leung CW. Influenza A(H5N1) Virus Infection in a Child With Encephalitis Complicated by Obstructive Hydrocephalus. Clin Infect Dis 2018;66(1):136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr S. Seasonal and pandemic influenza: an overview with pediatric focus. Adv Pediatr 2012;59(1):75–93. [DOI] [PubMed] [Google Scholar]
- 36.Punpanich W, Chotpitayasunondh T. A review on the clinical spectrum and natural history of human influenza. Int J Infect Dis 2012;16(10):e714–23. [DOI] [PubMed] [Google Scholar]
- 37.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp Pediatr 2020;10(6):537–40. [DOI] [PubMed] [Google Scholar]
- 38.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395(10237):1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yozgat CY, Uzuner S, Bursal Duramaz B, Yozgat Y, Erenberk U, Iscan A, et al. Dermatological manifestation of pediatrics multisystem inflammatory syndrome associated with COVID-19 in a 3-year-old girl. Dermatol Ther 2020;33(4):e13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox TG, Christenson JC. Influenza and parainfluenza viral infections in children. Pediatr Rev 2014;35(6):217–27; quiz 228. [DOI] [PubMed] [Google Scholar]
- 41.Jat KR, Kabra SK. Wheezing in children with viral infection & its long-term effects. Indian J Med Res 2017;145(2):161–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010;65(12):1045–52. [DOI] [PubMed] [Google Scholar]
- 43.Vega K, Wee S, Hernandez DA. Viral Influenza Infection and Complications: A Pediatric-focused Review. Pediatric Emergency Medicine Reports 2018;23(9). [Google Scholar]
- 44.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol 2020;77(11):1440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chacón-Aguilar R, Osorio-Cámara JM, Sanjurjo-Jimenez I, González-González C, López-Carnero J, Pérez-Moneo B. COVID-19: síndrome febril y clínica neurológica en neonato [COVID-19: Fever syndrome and neurological symptoms in a neonate]. An Pediatr (Engl Ed) 2020;92(6):373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis Associated with COVID-19 Infection in an 11-Year-Old Child. Pediatr Neurol 2020;109:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G, Zhang Y, Zhao J, Zhang J, Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet 2020;395(10228):945–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprang G, Silman M. Posttraumatic stress disorder in parents and youth after health-related disasters. Disaster Med Public Health Prep 2013;7(1):105–10. [DOI] [PubMed] [Google Scholar]
- 49.Sharma D, Singh S. Kawasaki disease - A common childhood vasculitis. Indian J Rheumatol 2015;10:S78–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, et al. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol 2020;41(7):1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain S, Sen S, Lakshmivenkateshiah S, Bobhate P, Venkatesh S, Udani S, et al. Multisystem Inflammatory Syndrome in Children With COVID-19 in Mumbai, India. Indian Pediatr 2020;57(11):1015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020;69(32):1074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, et al. Paediatric Inflammatory Multisystem Syndrome: Temporally Associated with SARS-CoV-2 (PIMS-TS): Cardiac Features, Management and Short-Term Outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol 2020;41(7):1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, et al. COVID-19-Associated Pediatric Multisystem Inflammatory Syndrome. J Pediatric Infect Dis Soc 2020;9(3):407–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health 2020;4(10):790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, et al. Cardiac MRI in Children with Multisystem Inflammatory Syndrome Associated with COVID-19. Radiology 2020;297(3):E283–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fani M, Teimoori A, Ghafari S. Comparison of the COVID-2019 (SARS-CoV-2) pathogenesis with SARS-CoV and MERS-CoV infections. Future Virology 2020;15(5):317–23. [Google Scholar]
- 58.Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect 2020;53(3):371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009;361(7):674–9. [DOI] [PubMed] [Google Scholar]
- 60.Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA 2020;323(13):1313–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falahi S, Abdoli A, Kenarkoohi A. Claims and reasons about mild COVID-19 in children. New Microbes New Infect 2021;41:100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciaglia E, Vecchione C, Puca AA. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front Pediatr 2020;8:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367(6483):1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 2006;78(19):2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep 2016;6:19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr 2020;16(3):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol 2020;92(5):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Zhu LQ. Pharmaceutical care recommendations for antiviral treatments in children with coronavirus disease 2019. World J Pediatr 2020;16(3):271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Zhu LQ. Pharmaceutical care recommendations for antiviral treatments in children with coronavirus disease 2019. World J Pediatr 2020;16(3):271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter Initial Guidance on Use of Antivirals for Children With Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc 2020;9(6):701–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loi M, Branchford B, Kim J, Self C, Nuss R. COVID-19 anticoagulation recommendations in children. Pediatr Blood Cancer 2020;67(9):e28485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and Outcomes of Coronavirus Infection in Children: The Role of Viral Factors and an Immunocompromised State. J Pediatric Infect Dis Soc 2019;8(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


