Abstract
Aims:
To investigate associations between experimental pain sensitivity and five chronic pain conditions among 655 participants in the OPPERA study.
Methods:
Quantitative sensory tests were used to measure sensitivity to three modalities of nociception: blunt pressure pain, mechanical pinprick pain, and thermal heat pain. Participants were also classified according to the presence or absence of five chronic pain conditions: temporomandibular disorders, headache, low back pain, irritable bowel syndrome, and fibromyalgia.
Results:
Univariate analyses found each modality to be significantly associated with at least one pain condition, most consistently for pressure pain sensitivity (8 of 15 instances) and least consistently for heat pain sensitivity (5 of 35 instances). Yet, multivariable analyses that evaluated the independent contributions of all five pain conditions found few significant associations (12 of 75 instances). Instead, pain sensitivity consistently varied according to the total number of pain conditions a person experienced, implying that the combination of pain conditions influences each nociceptive modality.
Conclusion:
When evaluating nociceptive sensitivity in a chronic pain patient, comorbid pain conditions should be considered, as the more salient feature underlying sensitivity is likely the number rather than the type(s) of pain conditions.
Keywords: chronic overlapping pain conditions, heat pain, pinprick pain, pressure pain, quantitative sensory testing
Many chronic pain conditions have poorly understood underlying pathophysiologies. Such conditions include temporomandibular disorders, fibromyalgia or widespread pain, low back pain (LBP), headache, and irritable bowel syndrome (IBS).1,2 These pain conditions frequently co-occur at a higher rate than would be predicted by independent occurrences despite each having distinctly different features and anatomical expressions. Moreover, factors associated with one condition are often also associated with other conditions, such as female sex, psychosocial features, and even genetic variants. This pattern of overlap implies the possibility of common pathophysiologic mechanisms and risk factors that underpin multiple chronic pain conditions.1-3 Accordingly, the collective term “chronic overlapping pain conditions” (COPCs) is used herein, consistent with the current terminology promoted by the National Institutes of Health.4
Many studies report that individuals with chronic pain conditions have enhanced sensitivity to experimental pain, which in some cases is interpreted as evidence of central sensitization.5-7 Yet, the literature is not consistent in this regard. A minority of studies have failed to find such enhanced pain sensitivity. In most of these reports, multiple types of pain assessments are conducted, and chronic pain cases show greater pain sensitivity than controls for some assessments but not for others. This has been reported for individuals with TMD,8,9 various types of chronic headache,10,11 IBS,12,13 chronic LBP,14,15 and fibromyalgia.16 It is not clear what underlying pathophysiologic processes influence experimental pain sensitivity among individuals with chronic pain, as most studies evaluate only a single chronic pain condition and use a small number of pain assessments. Three factors that likely play a role in determining the presence or magnitude of such an association are (1) the type of pain assessment, (2) the particular chronic pain condition, and (3) the coexistence of multiple pain conditions.
The purpose of the current study was to evaluate the association of experimental pain sensitivity with five selected COPCs: TMD, headache, LBP, IBS, and fibromyalgia. The associations of combined COPCs with each measure of experimental pain were further explored, with the combined COPCs as independent types of clinical pain and as a simple count of total burden of clinical pain. Specifically, the following hypotheses were tested: (1) quantitative sensory testing (QST) measures would show increased pain sensitivity across multiple COPCs; (2) some COPCs would be associated with greater pain sensitivity than other COPCs; and (3) increasing numbers of COPCs would be monotonically associated with higher levels of pain sensitivity.
Materials and Methods
This section summarizes the study methods that are explained in detail elsewhere in this volume (see Slade et al, current issue). Reporting of this observational study conforms to the STROBE guidelines.17 The primary data collection was from National Institute of Dental and Craniofacial Research (NIDCR) Study Protocol 12-052-E, conducted in the second phase of the OPPERA project (OPPERA-2; Orofacial Pain: Prospective Evaluation and Risk Assessment). This study received approval from the institutional review boards of all participating institutions.
Study Design, Setting, and Participants
The current report involved adults originally recruited into the first phase of OPPERA (OPPERA-1) between May 2006 and May 2013. At that time, subjects aged 18 to 44 years were selected for a community-based, case-control study of chronic TMD. Cases were 1,008 adults with examiner-verified painful TMD. Controls were 3,258 adults with examiner-verified absence of TMD. All subjects were recruited at US academic health centers located at: University at Buffalo, Buffalo, New York; University of Florida, Gainesville, Florida; University of Maryland, Baltimore, Maryland; and University of North Carolina, Chapel Hill, North Carolina. Previous papers have described details of recruitment and baseline data collection, as well as methods used for a subsequent prospective cohort study of the TMD-free individuals who were followed up for up to 5 years to investigate the incidence of first-onset TMD.18,19
This current cross-sectional analysis reports findings from the most recent wave of data collection in OPPERA-2. Between December 2014 and May 2016, attempts were made to contact all original enrollees from OPPERA-1. Data were then collected using clinical examinations, QST, cardiovascular measures of autonomic function, blood samples, and self-report questionnaires. Further details of recruitment and data collection methods are provided elsewhere in this volume (see Slade et al, current issue).
Classification of COPCs
The presence or absence of five COPCs was classified as described in detail elsewhere in this volume (see Ohrbach et al, current issue) and is summarized below.
TMD was classified by examiners who used the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD).20 In summary, to be classified a TMD case, subjects had to have all four of the following findings: (1) history of orofacial pain in the examiner-verified locations of the masseter, temporalis, submandibular, or temporomandibular joint (TMJ) area(s) and/or history of headache in the verified location of the temporal region that had occurred on 5 or more of the 30 days preceding the examination; (2) evoked pain in the same muscles and/or TMJ(s) following palpation of those structures or jaw maneuvers; (3) reported “familiarity” of evoked pain, as judged by a positive response to the question “Was the pain you felt [during palpation or jaw maneuver] familiar to the pain [or temporal headache] that you reported during the last 30 days?”; and (4) pain that was modified by jaw function, as judged by a positive response to the question “During the last 30 days, was any of the pain modified by chewing hard food, opening the mouth, jaw habits such as clenching, or other jaw activities?”
Headache was classified using responses to a questionnaire designed for OPPERA that asked about symptoms of tension-type headache (TTH) and migraine during the preceding 12 months. Subjects who experienced more than one type of headache recorded responses separately for up to three different types of headache. Questions about TTH were from the International Classification of Headache Disorders, third edition (ICHD-3).21 Symptoms of migraine were based on questions used in the ID-Migraine questionnaire.22 Migraine was classified when subjects reported headache(s) on 1 or more day per month and at least two of three symptoms accompanying the headache: nausea; sensitivity to light; or being kept from everyday activities. For this analysis, headache was classified for any subject who reported symptoms consistent with probable TTH, TTH, or migraine, and who had experienced such headache(s) in the preceding 3 months.
IBS was classified using responses to four questions about abdominal pain from the Rome III diagnostic criteria.23 Subjects were classified with IBS if they met both of the following criteria: (1) abdominal pain on at least 1 day in the preceding 3 months that was not related to menstrual periods; and (2) pain that was associated with at least two symptoms of bowel function (ie, pain altered by bowel movements; greater frequency of bowel movements; less frequency of bowel movements; looser stools; harder stools).
LBP was classified using responses to screening questions recommended for studies of back pain prevalence.24 Subjects were classified with LBP if they reported pain that occurred in the lower back (as indicated with a shaded manikin drawing) during the preceding 3 months that was not related to fever or menstruation and that restricted usual activities for at least 1 day.
Fibromyalgia was classified based on findings from examinations and questionnaires, consistent with the 1990 American College of Rheumatology criteria.25 Subjects were classified with fibromyalgia when ≥ 11 of 18 body sites were tender to algometer-delivered pressure of up to 4.0 kg/cm2 and when the tenderness occurred in both the axial skeleton and in at least one set of opposing diagonal quadrants of the body. Also, fibromyalgia cases had to report a history of pain lasting for at least 1 day per month in the preceding 3 months.
Assessment of Pain Sensitivity
QST was conducted in three sensory domains in the following order: pressure pain; mechanical cutaneous (pricking) pain; and heat pain.
Pressure Pain
Pressure pain thresholds (PPTs) were assessed using a commercially available pressure algometer (Somedic). Three body sites were tested, bilaterally, in the following order: (1) the center of the temporalis muscle; (2) the center of the trapezius muscle; and (3) the center of the anterior tibialis muscle. The protocol involved manual application of the algometer, with which the examiner would increase the pressure at a steady rate (30 kPa/second) until the participant indicated the first pain sensation by pressing a button. If no response was given at the point the stimulus reached 600 kPa, a value of 600 was used as the threshold value. A minimum of two trials were administered at each site, with an interstimulus interval of 2 to 3 seconds. If the values derived from those two trials were not within 20 kPa of one another, additional trials were administered until either (1) two trials were within 20 kPa of one another (not necessarily sequential), or (2) a total of five trials were administered. In the former case, the value midway between the two values was recorded as the PPT for that site; in the latter case, the median of the five values was used.
The examiners from each of the four study sites participated in a reliability exercise at an early stage of this project. PPTs were assessed in a group of 16 subjects (not part of the main study), each of whom was tested by two examiners at each body site. The overall intraclass correlation coefficient was 0.91, ranging from 0.87 to 0.94. This was considered an acceptable level of inter-examiner reliability.
Mechanical Cutaneous (Pinprick) Pain.
Mechanical cutaneous pain (MCP) sensitivity was assessed using a set of weighted probes, manufactured locally, matching those described by the German Neuropathic Pain Network.26 This set of probes had a flat contact area 0.2 mm in diameter and exerted forces between 8 and 512 mN. Stimuli were applied to the dorsum of digits 2 to 4. Measures included the pain threshold (MCPT), ratings of pain intensity in response to the 512-mN probe, temporal summation (TS) of pain with the same probe, and ratings of aftersensations following the TS protocol. The execution of these protocols followed that of the German Neuropathic Pain Network.26
The MCPT was determined using a standard single-staircase protocol and was followed by assessment of suprathreshold MCP sensitivity. The MCPT was derived using an adaptive staircase method, calculated as the geometric mean of five series of ascending and descending stimulus intensities. If subjects gave two “no” responses in a row using the 512-mN probe, the staircase was halted, and a value of 512 was used as the threshold value.
Participants judged the pain intensity evoked by suprathreshold stimuli, verbally reporting a number between 0 and 100 without a visual reference. Participants were instructed that “0” represented no pain, while “100” represented the most intense pain imaginable. Participants reported pain intensity after a single stimulus (applied for approximately 0.5 seconds) and then again after a series of 10 stimuli were applied at 1-second intervals. For the series of 10 stimuli, participants were asked to report an overall pain intensity for the series of stimuli. At 15 and 30 seconds after the series of 10 stimuli were administered, participants were asked to rate the pain intensity of any residual sensation at the stimulated finger, which served as the aftersensation measure. This series of suprathreshold tests was administered four times, and average values were taken for analysis. TS of MCP was calculated as the difference between the rating of the series of 10 stimuli and the rating of the single stimulus. In the course of this testing, if the participant reported “100,” the protocol was halted. The participant was then offered the option of continuing with the next series or omitting the rest of this set of tests. The participant was also instructed that they could stop testing at any time by telling the examiner to stop.
Heat Pain.
Heat pain sensitivity was assessed using a commercially available thermal stimulator (Pathway, Medoc). Stimuli were applied on the ventral forearm. Heat pain measures included threshold, tolerance, ratings of single suprathreshold stimuli, TS of heat pain, and aftersensations following the TS protocol.
Heat pain threshold (HPT) was determined using a protocol similar to that for PPT. The Medoc ATS thermode (2.56 cm2) was manually placed in contact with the skin on the right ventral forearm at a temperature of 32°C. After a few seconds, the temperature increased at a rate of 0.5°C/second until the participant pushed a button indicating they just then felt a pain sensation. The temperature of the thermode at the time of the button press was recorded as a threshold estimate. This was repeated four times, moving the thermode to a new site on the forearm each time. Following this, pain tolerance was estimated using the same protocol. The sole difference was that the participant was instructed to press the button when they could no longer tolerate the pain. This was repeated four times, moving the thermode for each trial. For both threshold and tolerance testing, a ceiling temperature was set at 52°C, which was entered as the threshold or tolerance estimate if the participant failed to press the button in a given trial.
Following heat pain tolerance testing, participants judged the pain intensity evoked by suprathreshold heat stimuli applied to the left ventral forearm, verbally reporting a number between 0 and 100. As with the MCP ratings, participants were instructed that “0” represented no pain, while “100” represented the most intense pain imaginable. Participants were told that they would receive 10 thermal stimuli in a row and would be verbally cued to report their peak pain intensity after each stimulus. Practice trials were administered to provide the participant a sense of the timing of stimulus delivery and to verify understanding of the protocol. The Medoc CHEPS thermode (5.73 cm2) was manually placed on the skin at a temperature of 38°C, and then a series of 10 temperature pulses were given at 2.4- to 2.5-second interstimulus intervals. The temperature pulses reached a peak temperature of 48°C, with a ramp rate of 20°C/second and a hold time of 750 milliseconds at the peak temperature. The participant was cued to report pain intensity when the temperature just started to decline after reaching the peak. Based on this protocol, three variables were derived: (1) single-stimulus pain rating (the response to the first trial in the series); (2) series of 10 stimuli area under the curve (AUC), (calculated as the sum of all 10 ratings in the series); and (3) TS of pain (the difference between the highest rating in the series of 10 and the first stimulus rating). At intervals of 15 and 30 seconds after the 10th thermal pulse, the participant was asked to rate the pain intensity of any lingering sensation using the same 0 to 100 scale, which served as the aftersensation measure. In the course of the testing, if the participant reported “100,” the thermode was removed, and that series of thermal stimuli was halted. The participant was also instructed that they could stop testing at any time by telling the examiner to stop.
Statistical Analyses
Raw values of each QST measure were used to generate descriptive statistics for cases and controls of each COPC and according to the number of COPCs. All other analyses of continuous variables used z-transformed values of QST measures, and the data were weighted during analysis. The goal of data transformation was to produce measures of association (eg, odds ratios [ORs], regression estimates) that could be readily compared between QST measures that use different scales of measurement. The goal of weighting was to adjust for the way in which study participants were selected in OPPERA-2. This took into consideration the original sampling design for the OPPERA-1 case-control study, where TMD cases were oversampled relative to their prevalence in the population, and adjusted for differential loss to follow-up of subjects between enrollment in OPPERA-1 and participation in OPPERA-2. Such weighting is important for this analysis in order to make valid estimates of association between any two variables (eg, QST measures and headache) in a sample that was originally stratified according to a third variable (ie, presence or absence of chronic TMD in OPPERA-1).27 The analytic weights for OPPERA-2 were computed as the inverse of the sampling probability for OPPERA-1, multiplied by the inverse of loss to follow-up probability between OPPERA-1 and OPPERA-2. Except for univariate statistics describing the distribution of explanatory variables, all means, percentages, and measures of association were calculated using generalized estimating equations (GEE) with the GENMOD procedure in SAS version 9.4, with analytic weights and robust error variance calculation.28
The analysis first assessed associations between QST variables and the presence or absence of each COPC using statistical methods for case-control analysis of cross-sectional data. For descriptive purposes, mean values of continuous variables and percentages of categorical variables were generated for cases and controls for each of the five COPCs. To quantify univariate associations, adjusted ORs were estimated in separate binary logistic regression models, one for each COPC, where the main explanatory variable was the standardized (ie, using z-score transformation) value of a single QST variable. If up to one-half of the items for any given variable were missing, the variable’s values were imputed using the expectation maximization method. However, if more than one-half of the items were missing, the observation for study participant was excluded from the model. The models adjusted for study site (four categories) and subjects’ demographic characteristics: age (measured in years), gender (two categories: male and female), and race/ethnicity (five categories: white, Black/African American, Asian, Hispanic, or other). In order to determine independent associations between individual COPCs and QST variables, all five COPCs were modeled as separate binary variables in a multivariable model to predict the dependent variable, with tests of the null hypotheses that individual COPCs did not contribute independently to the dependent variable. Such multivariable analyses allowed for determining the impact of any single pain condition on a given QST measure, independent of the impact of comorbid COPCs.
Random forest modeling explored the multivariable contributions of all QST variables to each binary COPC case classification. Missing values of explanatory variables were imputed using on-the-fly imputation, which is the decision-tree analog of multiple imputation.29 Random forests are nonparametric statistical models that can handle interactions and nonlinear associations without the need to pre-specify the interactions or the form of the nonlinearities. Due to this flexibility, random forests demonstrate excellent classification performance across a broad range of tasks. Through a combination of the bootstrap-aggregating and random-subspace methods used in the construction of random forests, they achieve this classification performance without overfitting to the training dataset, thus maintaining good out-of-sample performance.30 For the present study, random forest classification models were evaluated using their out-of-bag AUC, which is an unbiased estimate of the model’s discrimination accuracy in predicting binary COPC status. Contributions of individual variables were quantified using variable importance scores, which estimate the relative contribution of each predictor to the model’s performance.
A second set of analyses examined associations with the subjects’ number of COPCs. For descriptive purposes, unadjusted means or percentages of QST variables were estimated for subjects with 0, 1, 2, 3, 4, or 5 COPCs. For these analyses, the standardized variable was used as the dependent variable in a linear regression model where the main predictor variable was the number of COPCs, and covariates were adjusted for study site and demographics (coded as described above). The number of COPCs was modeled using three approaches to evaluate different ways in which COPCs might be associated with each QST variable: (1) the number of COPCs was modeled as a categorical variable to evaluate potential nonlinear relationships with the explanatory QST variable, and pairwise comparisons were used to test for differences between subjects with no COPCs (the reference group) vs the other five possibilities (1, 2, 3, 4, or 5 COPCs); (2) the number of COPCs was modeled as a continuous variable to reveal a potential linear relationship with the dependent variable, with a test of the null hypothesis of no linear relationship (β = 0); and (3) all five COPCs were modeled as separate binary predictor variables, and parameter estimates were used to test for independent contributions of each COPC to each QST measure.
The 0–5 count variable for number of COPCs results in somewhat sparse data in regard to the pain variables in this study for the group defined by 4 pain conditions, and definitely sparse data for the group defined by 5 pain conditions. Therefore, a 0–4 count variable was also created, collapsing groups 4 and 5—the results remain the same for the 0–3 groups, and for 4 groups, the results tend to fall between the current findings for 4 groups and for 5 groups; thus, there was no change inthe interpretation of the findings. While the findings are likely more reliable when collapsing 4 and 5 COPCs into one group, it was elected to present the results for 0–5 pain conditions in order to remain parallel with the other papers in this series. In addition, this approach allows the reader to fully appreciate the impact that smaller groups of all 5 pain conditions can have on the estimates of the variables of interest.
Results
Table 1 presents sample sizes and demographic weighted estimates for each COPC. Mean age was similar for cases and controls across all COPCs, although TMD cases were slightly younger than controls, while LBP cases were slightly older than controls. No consistent pattern of age with number of COPCs emerged. A greater proportion of cases vs controls was female for TMD, headache, and fibromyalgia. Non-Hispanic white race/ethnicity was overrepresented in cases vs controls for all COPCs.
Table 1.
Sample Counts and Weighted Estimates (Standard Error) for Demographic Characteristics
| Classification | Group | Weighted no. | Mean age (SE), y | % female (SE) | % white (SE) |
|---|---|---|---|---|---|
| TMD | Case | 108 | 33.0 (0.6) | 61.2 (3.6) | 50.7 (3.7) |
| Control | 547 | 35.4 (0.4) | 57.0 (2.3) | 52.2 (2.3) | |
| Headache | Case | 201 | 34.6 (0.5) | 71.1 (2.8) | 55.5 (3.0) |
| Control | 454 | 35.3 (0.4) | 51.7 (2.6) | 50.4 (2.6) | |
| IBS | Case | 134 | 34.6 (0.7) | 53.7 (4.0) | 60.1 (3.9) |
| Control | 521 | 35.2 (0.3) | 58.7 (2.2) | 49.8 (2.2) | |
| LBP | Case | 99 | 37.6 (0.7) | 56.7 (4.2) | 62.9 (4.1) |
| Control | 556 | 34.6 (0.3) | 57.9 (2.2) | 50.0 (2.2) | |
| Fibromyalgia | Case | 24 | 34.3 (1.1) | 77.2 (5.9) | 52.7 (7.0) |
| Control | 631 | 35.1 (0.3) | 56.9 (2.0) | 51.9 (2.0) | |
| No. of COPCs | 0 | 307 | 35.6 (0.5) | 54.0 (3.1) | 46.5 (3.1) |
| 1 | 209 | 34.4 (0.5) | 60.0 (3.7) | 55.5 (3.7) | |
| 2 | 83 | 33.6 (0.8) | 57.5 (4.8) | 58.7 (4.7) | |
| 3 | 33 | 36.5 (1.1) | 63.6 (5.8) | 68.4 (5.6) | |
| 4 | 15 | 38.7 (1.4) | 92.2 (4.7) | 39.7 (8.6) | |
| 5 | 6 | 30.9 (1.7) | 48.8 (15.1) | 52.8 (15.1) |
Associations Between Individual COPCs and QST Measures
Univariate Associations.
Table 2 presents demographically adjusted ORs depicting the association of each z-transformed QST variable with the binary case classification of each COPC. (For reference, descriptive statistics for the original QST variables are presented in Appendix 1; see all appendices in the online version of this article at www.quintpub.com/journals). Lower PPTs at all body sites were associated with significantly greater odds of TMD (with the largest ORs for the temporalis site) and of fibromyalgia (most strongly for the trapezius site). Lower PPTs at the temporalis and trapezius sites were associated with significantly greater odds of IBS, while headache and LBP cases failed to show significant PPT ORs at any body site.
Table 2.
Univariate Associations of Phenotype z-Scores and Individual COPCs, Adjusted for Study Site and Demographics
| TMD |
Headache |
IBS |
LBP |
Fibromyalgia |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| QST measure | OR (95% CL) | P | OR (95% CL) | P | OR (95% CL) | P | OR (95% CL) | P | OR (95% CL) | P |
| PPT: Temporalis (r) | 3.94 (2.42, 6.41) | < .001 | 1.19 (0.94, 1.50) | .143 | 1.62 (1.14, 2.30) | .007 | 1.38 (0.99, 1.93) | .057 | 2.81 (1.31, 6.02) | .008 |
| PPT: Trapezius (r) | 1.85 (1.29, 2.66) | .001 | 1.08 (0.85, 1.37) | .534 | 1.41 (1.02, 1.96) | .039 | 1.21 (0.85, 1.71) | .286 | 4.94 (2.06, 11.80) | < .001 |
| PPT: Anterior tibialis (r) | 1.78 (1.28, 2.49) | .001 | 1.06 (0.83, 1.36) | .616 | 1.27 (0.94, 1.71) | .117 | 1.06 (0.77, 1.44) | .732 | 2.36 (1.36, 4.08) | .002 |
| MCPT (r) | 1.77 (1.25, 2.50) | .001 | 1.40 (1.12, 1.76) | .003 | 1.32 (0.99, 1.76) | .062 | 1.39 (1.00, 1.93) | .052 | 2.63 (1.18, 5.86) | .018 |
| MCP: Single stimulus (0–100) | 1.56 (1.24, 1.97) | < .001 | 1.18 (0.92, 1.50) | .196 | 1.16 (0.91, 1.47) | .233 | 1.27 (0.94, 1.72) | .124 | 1.48 (NE) | NE |
| MCP: Series of 10 stimuli (0-100) | 1.44 (1.09, 1.90) | .011 | 1.12 (0.89, 1.41) | .346 | 1.25 (0.98, 1.59) | .070 | 1.12 (0.84, 1.51) | .429 | 1.34 (NE) | NE |
| MCP: TS (−100 to 100) | 1.00 (0.73, 1.38) | .997 | 0.99 (0.78, 1.25) | .921 | 1.19 (0.94, 1.52) | .147 | 0.92 (0.68, 1.25) | .601 | 0.93 (NE) | NE |
| MCP: 15-s after-sensation (0 to 100) | 1.37 (1.07, 1.76) | .011 | 1.22 (0.96, 1.56) | .105 | 1.16 (0.93, 1.46) | .190 | 1.59 (1.20, 2.10) | .001 | 1.66 (NE) | NE |
| HPT, °C (r) | 1.24 (0.88, 1.74) | .222 | 1.22 (0.96, 1.55) | .101 | 1.36 (1.00, 1.85) | .052 | 1.03 (0.78, 1.34) | .848 | 1.49 (0.92, 2.42) | .104 |
| HP: Tolerance, °C (r) | 1.27 (0.92, 1.75) | .151 | 1.02 (0.77, 1.35) | .901 | 0.98 (0.70, 1.37) | .892 | 1.31 (0.99, 1.73) | .055 | 1.10 (0.81, 1.48) | .547 |
| HP: Single stimulus, (0 to 100) | 1.52 (1.06, 2.18) | .023 | 0.95 (0.72, 1.25) | .728 | 1.15 (0.81, 1.63) | .426 | 1.36 (0.93, 1.99) | .114 | 1.12 (0.80, 1.57) | .517 |
| HP: 10 stimuli AUC (0 to 100) | 1.68 (1.20, 2.36) | .003 | 1.08 (0.84, 1.40) | .544 | 1.19 (0.87, 1.63) | .287 | 1.34 (0.97, 1.86) | .078 | 1.34 (0.92, 1.97) | .131 |
| HP: Maximum of 10 stimuli (0 to 100) | 1.80 (1.28, 2.52) | .001 | 1.12 (0.87, 1.45) | .368 | 1.32 (0.96, 1.82) | .088 | 1.48 (1.06, 2.07) | .023 | 1.51 (0.96, 2.38) | .076 |
| HP: TS (0 to 100) | 1.17 (0.82, 1.67) | .374 | 1.03 (0.75, 1.42) | .835 | 1.08 (0.77, 1.52) | .660 | 1.00 (0.70, 1.42) | .980 | 1.41 (0.81, 2.44) | .225 |
| HP: 15-s aftersensation (0 to 100) | 1.20 (0.89, 1.61) | .233 | 1.09 (0.84, 1.41) | .515 | 1.19 (0.88, 1.60) | .266 | 1.33 (0.96, 1.84) | .090 | 1.61 (1.11, 2.34) | .012 |
Values in bold are significant (P < .05). AUC = area under the curve. OR = odds ratio; CL = confidence limit; (r) = reverse scoring (negative z scores used for standardized odds ratios represent increase in odds of being a case associated with reduction of 1 SD in the value of the variable); NE = not estimated due to failure of model convergence; PPT = pressure pain threshold; MCP(T) = mechanical cutaneous pain (threshold); HP(T) = heat pain (threshold); TS = temporal summation.
Lower MCPTs were associated with significantly greater odds of TMD, headache, and fibromyalgia, and showed nonsignificant differences in the same direction for IBS and LBP. Higher ratings for single-pinprick stimuli and the series of 10 stimuli were associated with significantly greater odds only for TMD cases, while none of the COPC groups showed significant ORs for TS of mechanical stimuli. Greater MCP aftersensation ratings were associated with significantly greater odds of TMD and LBP.
Neither HPT nor tolerance showed significant case-control ORs for any COPC. Greater ratings of heat pain stimuli were associated with significantly greater odds of TMD, while ORs for heat pain TS were not significant for any COPC group. Heat pain aftersensation ratings were associated with significantly greater odds for fibromyalgia only (Table 2). The overall pattern of all these associations is depicted in Fig 1 (orange heat map).
Fig 1.
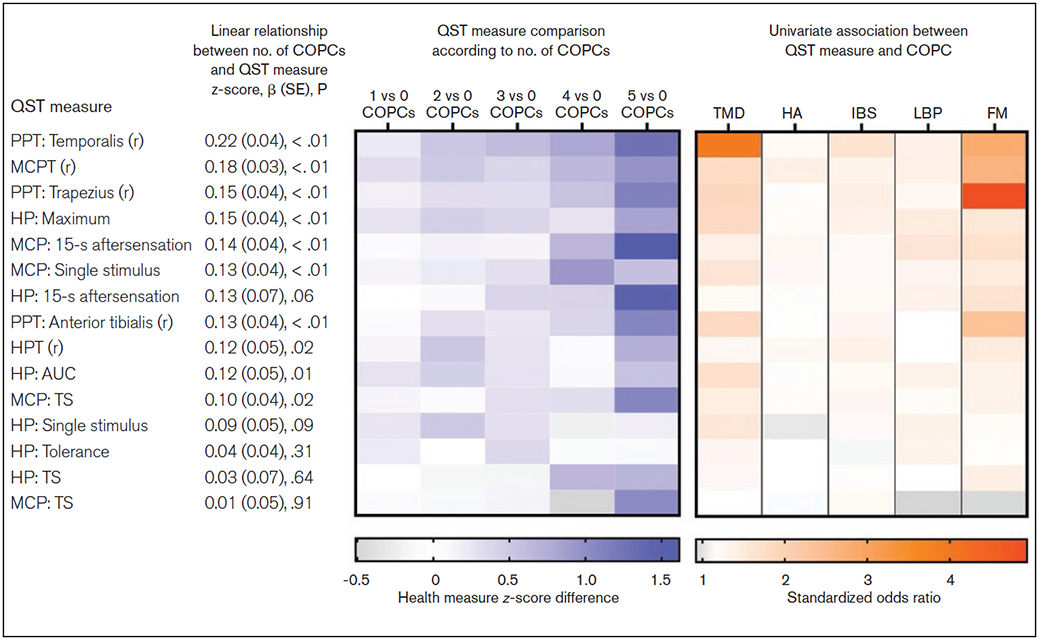
Summary of univariate measures of individual QST variables. The blue panel is a heat map depicting QST measure z-score differences according to number of COPCs based on the data presented in Table 4; for example, the first cell in the top row depicts the mean temporalis PPT z-score difference between groups with 1 COPC vs 0 COPCs. Rows are ordered in descending strength of association, as determined by beta coefficients, reported in Table 4. The orange panel is a heat map depicting standardized odds ratios (reported in Table 2) that quantify the strength of association between QST measures and each individual COPC. (r) = reverse scoring; OR = odds ratio; PPT = pressure pain threshold; MCP(T) = mechanical cutaneous pain (threshold); AUC = area under the curve; HP = heat pain; TS = temporal summation.
Considering the separate COPCs, the majority of QST measures were significantly associated with TMD, while only four were significantly associated with fibromyalgia; and only one or two were associated with headache, IBS, and LBP. The same pattern was seen based on the magnitude of ORs: values of 1.5 or more were common for TMD and fibromyalgia, but not for the other COPCs. Indeed, for headache, ORs were below 1.2 for 12 of the 15 QST measures.
Multivariable Associations.
The multivariable regression analysis revealed the extent of independent contributions of individual COPCs to each of the QST measures, separate from potential contributions of other COPCs (Table 3). In general, none of the individual COPCs had significant independent associations with any of the pinprick or thermal pain measures; the exceptions were TMD—which was independently associated with all five PPT measures, two pinprick measures, and two HP measures—and fibromyalgia, which was independently associated with the two noncranial body PPT measures. Two other exceptions were noted: Fibromyalgia was independently associated with one heat pain measure, and headache was independently associated with one pinprick pain measure. No other QST measures showed significant independent associations with any other COPC.
Table 3.
Independent Contribution of Each COPC to Standardized Mean QST Measures, Adjusted for Study Site and Demographics
| QST measure | TMD | Headache | IBS | LBP | Fibromyalgia |
|---|---|---|---|---|---|
| PPT: Temporalis (r) | 0.68 (0.11), < .01 | −0.02 (0.09), .84 | 0.24 (0.12), .05 | 0.01 (0.14), .97 | 0.08 (0.15), .61 |
| PPT: Trapezius (r) | 0.30 (0.13), .02 | −0.08 (0.10), .40 | 0.19 (0.12), .10 | −0.08 (0.13), .55 | 0.56 (0.15), < .01 |
| PPT: Anterior tibialis (r) | 0.38 (0.14), < .01 | −0.07 (0.10), .50 | 0.13 (0.11), .26 | −0.14 (0.12), .25 | 0.46 (0.19), .02 |
| MCPT (r) | 0.31 (0.13), .02 | 0.23 (0.11), .03 | 0.10 (0.12), .40 | −0.01 (0.16), .97 | 0.35 (0.18), .05 |
| MCP: Single stimulus (0 to 100) | 0.39 (0.15), < .01 | 0.05 (0.12), .66 | 0.01 (0.11), .94 | −0.02 (0.16), .91 | 0.05 (0.26), .85 |
| MCP: Series of 10 stimuli (0 to 100) | 0.26 (0.14), .06 | 0.01 (0.11), .90 | 0.11 (0.10), .28 | −0.04 (0.14), .77 | 0.04 (0.25), .87 |
| MCP: TS (−100 to 100) | −0.02 (0.16), .91 | −0.04 (0.11), .73 | 0.17 (0.11), .13 | −0.04 (0.15), .76 | 0.01 (0.26), .97 |
| MCP: 15-s aftersensation (0 to 100) | 0.10 (0.10), .33 | 0.12 (0.10), .22 | −0.03 (0.09), .76 | 0.21 (0.15), .15 | 0.53 (0.28), .06 |
| HPT, °C (r) | 0.08 (0.17), .62 | 0.12 (0.11), .26 | 0.23 (0.16), .14 | −0.11 (0.13), .43 | 0.32 (0.28), .26 |
| HP: Tolerance, °C (r) | 0.20 (0.15), .20 | −0.07 (0.12), .56 | −0.08 (0.14), .55 | 0.17 (0.11), .13 | −0.24 (0.16), .13 |
| HP: Single stimulus (0 to 100) | 0.42 (0.18), .02 | −0.15 (0.13), .25 | 0.01 (0.17), .97 | 0.08 (0.19), .69 | −0.24 (0.19), .19 |
| HP: 10 stimuli AUC (0 to 100) | 0.41 (0.16), .01 | −0.05 (0.13), .69 | 0.03 (0.15), .85 | 0.10 (0.15), .50 | −0.14 (0.18), .42 |
| HP: Maximum of 10 stimuli (0 to 100) | 0.39 (0.15), < .01 | −0.04 (0.13), .72 | 0.11 (0.14), .43 | 0.17 (0.13), .21 | −0.13 (0.15), .41 |
| HP: TS (0 to 100) | 0.10 (0.18), .60 | 0.00 (0.16), .99 | 0.06 (0.16), .72 | 0.05 (0.18), .77 | 0.21 (0.26), .43 |
| HP: 15-s aftersensation (0 to 100) | 0.06 (0.17), .73 | 0.05 (0.15), .74 | 0.06 (0.15), .68 | 0.15 (0.24), .54 | 0.69 (0.30), .02 |
Data are reported as estimated mean difference (standard error), P value. Values in bold are significant (P < .05). PPT = pressure pain threshold; (r) = reverse scoring; MCP(T) = mechanical cutaneous pain (threshold); HP(T) = heat pain (threshold); TS = temporal summation; AUC = area under the curve.
To evaluate which QST measures best differentiated cases from controls, random forest algorithms simultaneously assessed all associations and interactions of the full set of QST measures with individual COPCs, quantifying the relative discriminative ability with a variable importance score (Fig 2). TMD cases and controls were best differentiated by temporalis PPT and less so for PPT derived from noncranial sites. In contrast, fibromyalgia cases and controls were best differentiated by temporalis and trapezius PPTs, and less so for anterior tibialis PPT. Fibromyalgia cases were also modestly differentiated from controls by MCP threshold and aftersensation measures. The data underlying Fig 2 are reported in Appendix 2.
Fig 2.
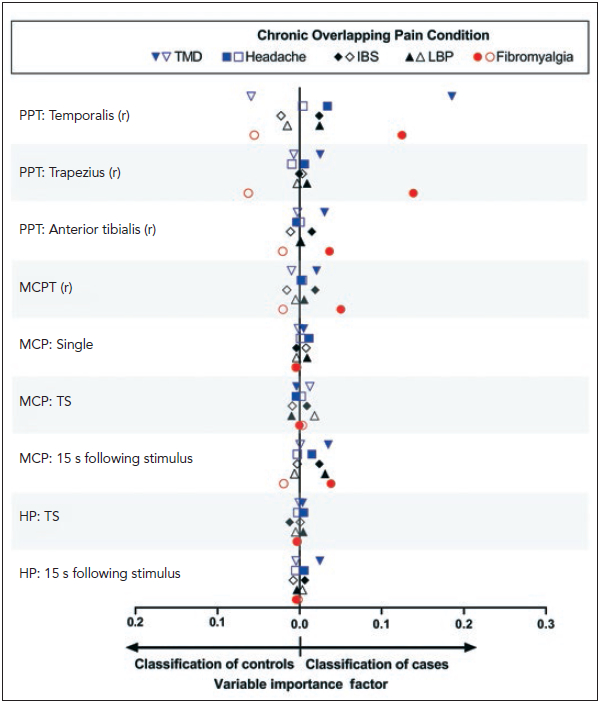
Random forest modeling was used to explore multivariable contributions of all QST measures to each binary COPC case classification, with study site, age, gender, and race included as covariates. Contributions of individual variables in the random forest models were quantified using variable importance scores, which estimate the relative contribution of each predictor to the model’s classification of true positives and true negatives. Other QST measures were included in the models but are not plotted because their variable importance factors did not exceed 0.0004. Filled symbols = COPC cases; open symbols = controls. PPT = pressure pain threshold; MCP(T) = mechanical cutaneous pain (threshold); TS = temporal summation; HP = heat pain.
Associations Between Number of COPCs and QST Measures
When combinations of COPCs were considered as a count of the total number of COPCs experienced, they were positively associated with greater pain sensitivity for at least one measure in each of the three nociceptive modalities (beta estimates and tests for linear association in Table 4). Specifically, there was a significant linear association for 10 of the 15 QST variables, including all PPT measures, most MCP measures except for TS, and three heat pain measures. Furthermore, when significant linear associations were observed, there was usually a threshold effect, as indicated by pairwise comparisons in QST measures between the reference group (ie, no COPCs) and groups with successively greater numbers of COPCs (estimated mean differences for pairwise comparisons in Table 4). For example, relative to subjects with no COPCs, significant differences in most PPT measures were seen for subjects with two COPCs, but not for subjects with one. The thresholds for heat or pinprick pain tended to be observed at higher numbers of COPCs. (For reference, descriptive statistics for the original QST variables are presented in Appendix 3.)
Table 4.
Estimates of Linear Association and Pairwise Comparisons Between Extent of COPC Overlap and Quantitative Sensory Testing Phenotype z-Score, Adjusted for Site and Demographics
| Linear association |
Est (SE), P |
|||||
|---|---|---|---|---|---|---|
| QST measure | β (SE), P | 1 vs 0 COPCs | 2 vs 0 COPCs | 3 vs 0 COPCs | 4 vs 0 COPCs | 5 vs 0 COPCs |
| PPT: Temporalis (r) | 0.22 (0.04), < .01 | 0.21 (0.12), .08 | 0.51 (0.14), < .01 | 0.61 (0.15), < .01 | 0.80 (0.21), < .01 | 1.27 (0.28), < .01 |
| PPT: Trapezius (r) | 0.15 (0.04), < .01 | 0.17 (0.11), .11 | 0.31 (0.12), .01 | 0.31 (0.19), .09 | 0.54 (0.22), .01 | 1.13 (0.27), < .01 |
| PPT: Anterior tibialis (r) | 0.13 (0.04), < .01 | 0.06 (0.11), .60 | 0.30 (0.13), .01 | 0.24 (0.15), .10 | 0.39 (0.26), .14 | 1.07 (0.33), < .01 |
| MCPT (r) | 0.18 (0.03), < .01 | 0.31 (0.12), .01 | 0.49 (0.14), < .01 | 0.38 (0.14), < .01 | 0.63 (0.16), < .01 | 0.97 (0.18), < .01 |
| MCP: Single stimulus (0 to 100) | 0.13 (0.04), < .01 | 0.13 (0.12), .27 | 0.20 (0.15), .18 | 0.29 (0.15), .05 | 0.92 (0.42), .03 | 0.56 (0.24), .01 |
| MCP: Series of 10 stimuli (0 to 100) | 0.10 (0.04) , .02 | 0.13 (0.12), .27 | 0.06 (0.15), .68 | 0.29 (0.16), .07 | 0.31 (0.26), .22 | 1.07 (0.30), < .01 |
| MCP: TS (−100 to 100) | 0.01 (0.05), .91 | 0.05 (0.13), .66 | −0.12 (0.13), .35 | 0.14 (0.16), .40 | −0.51 (0.19), < .01 | 1.04 (0.48), .03 |
| MCP: 15-s aftersensation (0 to 100) | 0.14 (0.04), < .01 | 0.07 (0.10), .46 | 0.16 (0.11), .14 | 0.14 (0.13), .30 | 0.66 (0.46), .14 | 1.62 (0.38), < .01 |
| HPT, °C (r) | 0.12 (0.05), .02 | 0.12 (0.12), .30 | 0.52 (0.16), < .01 | 0.26 (0.18), .13 | 0.07 (0.35), .84 | 0.72 (0.47), .12 |
| HP: Tolerance, °C (r) | 0.04 (0.04), .31 | 0.19 (0.12), .09 | 0.01 (0.11), .92 | 0.36 (0.15), .01 | −0.07 (0.25), .77 | 0.09 (0.20), .63 |
| HP: Single stimulus (0 to 100) | 0.09 (0.05), .09 | 0.24 (0.14), .07 | 0.49 (0.17), < .01 | 0.28 (0.19), .12 | −0.22 (0.36), .55 | 0.18 (0.22), .40 |
| HP: 10 stimuli AUC (0 to 100) | 0.12 (0.05), .01 | 0.27 (0.14), .05 | 0.44 (0.16), < .01 | 0.27 (0.16), .09 | 0.09 (0.27), .73 | 0.55 (0.17), < .01 |
| HP: Maximum of 10 stimuli (0 to 100) | 0.15 (0.04), < .01 | 0.26 (0.14), .05 | 0.44 (0.16), < .01 | 0.39 (0.16), .01 | 0.27 (0.20), .17 | 0.84 (0.21), < .01 |
| HP: TS (0 to 100) | 0.03 (0.07), .64 | −0.04 (0.17), .81 | −0.16 (0.17), .33 | −0.17 (0.35), .62 | 0.65 (0.39), .09 | 0.68 (0.37), .06 |
| HP: 15-s aftersensation (0 to 100) | 0.13 (0.07), .06 | −0.06 (0.18), .76 | 0.09 (0.16), .58 | 0.39 (0.24), .10 | 0.41 (0.56), .46 | 1.49 (0.42), < .01 |
Values in bold are significant (P < .05). Est = estimated mean difference; SE = standard error; PPT = pressure pain threshold; (r) = reverse scoring; MCP(T) = mechanical cutaneous pain threshold; HP(T) = heat pain (threshold); TS = temporal summation; AUC = area under the curve.
Discussion
In this community-based study, enhanced sensitivity to at least 1 of the 15 QST measures of experimental pain was associated with greater odds of at least 1 of the 5 COPCs assessed. However, positive associations were not the norm: pressure pain sensitivity was the modality most likely to be associated with several COPCs, while TMD and fibromyalgia were the clinical conditions most likely to be associated with various QST measures. Furthermore, few of the COPCs contributed independently to any of the QST measures when all 5 COPCs were evaluated in multivariable models. Instead, experimental pain sensitivity varied more consistently according to the total number of COPCs, usually in a nonlinear manner, with a threshold of at least 2 or 3 COPCs required to observe a significant increase in sensitivity.
Pain Sensitivity Associations with Individual COPCs
TMD cases showed the greatest extent of enhanced pain sensitivity, having the greatest number of significant associations with QST measures—10 of 15 QST measures in the univariate analysis, and 8 of 15 in the multivariable analyses. Fibromyalgia cases showed the next greatest extent of enhanced pain sensitivity, with 5 of 11 QST measures being significant in univariate analyses and 3 of 15 in the multivariable analysis. The other COPCs only showed one (headache, LBP) or two (IBS) significant QST associations in the univariate analysis, and one (headache, IBS) or no (LBP) significant associations in the multivariable analysis. For both TMD and fibromyalgia, significant case-control differences were most consistent for PPT (P < .05 for all 3 PPT measures for both COPCs) and least consistent for heat pain measures (P < .05 for 3/7 for TMD, and 1/7 for fibromyalgia). The MCP measures were intermediate for TMD (P < .05 for 4/5) and inconclusive for fibromyalgia.
The larger and more consistent association of PPT with this group of COPCs could reflect the greater relevance of deep muscle vs cutaneous hyperalgesia in chronic pain conditions. Indeed, the strongest effects found were for the association of PPT with TMD and fibromyalgia—two COPCs with major myalgic features. One would very much expect this result from testing of symptomatic regions on these patients; however, significant pain sensitivity associations are found for testing on nonsymptomatic sites, particularly for TMD cases, indicating that something other than local factors are in play. The fact that pain sensitivity is not found to be globally enhanced in these patient populations implies the relevance of a complex set of factors, potentially beyond a generalized central sensitization.
Consistent with this perspective, the literature on enhanced pain sensitivity among chronic pain patients is mixed. While the largest study of this kind found TMD cases to be significantly more sensitive to many of the same QST measures used in the current study,31 other studies found either no case-control differences8,32,33 or mixed results.9,34,35 The literature generally reports that fibromyalgia patients have greater pain sensitivity than controls, particularly for PPT and HPT. Regarding PPT, the present results coincide with the majority of studies reporting significantly lower values for fibromyalgia patients vs controls when testing on the forearm,36 trapezius,37,38 and cranial sites.39 In contrast, Hermans et al16 found no group difference in PPT tested on the trapezius. Regarding HPT, the present results do not coincide with five studies that found lower HPTs for fibromyalgia patients when tested on the forearm40-43 or fingertip.36
IBS patients have been most frequently tested for heat pain sensitivity. Among reports of testing on the forearm, two found significantly lower HPTs for IBS patients,44,45 and one found no group difference.12 Studies of suprathreshold heat pain sensitivity on the forearm, including heat pain tolerance, report greater pain sensitivity of IBS patients.44-46 However, tests at other body sites provided mixed results.13,47-49 These results, finding no group differences for any heat pain or MCP measure, do not support the thesis of enhanced cutaneous sensitivity for IBS patients.
Several studies have evaluated experimental pain sensitivity in both acute and chronic LBP patients. Most of these studies evaluated pain sensitivity at symptomatic sites on the back, which was not one of the test sites in the present study. Of those studies evaluating pain sensitivity on body sites comparable to this study, one found lower PPTs on the tibialis of chronic LBP patients,50 and one found no case-control difference in PPTs on the forearm of acute LBP patients.14 Regarding HP sensitivity, most “off-site” testing has been done on the forearm. One study found significant case-control differences for HPT in acute LBP patients,51 while two studies found no case-control differences in either acute or chronic LBP patients.14,15 Two studies evaluated TS of heat pain on the forearm. One found a significant case-control difference,52 while the other found no difference.15 Overall, both positive and negative results have been reported from studies that use assessments similar to those of the present study, which found no significant case-control differences in PPT at any body site that was tested or for HPT or heat pain TS on the forearm.
A recent systematic review and meta-analysis examined QST-based pain sensitivity differences between migraineurs and nonmigraine controls.11 Considering head and neck pain sensitivity, the meta-analysis identified significantly lower PPTs for migraineurs, but no case-control differences in measures of heat pain or MCP. The few studies that evaluated pain sensitivity at other body sites found no group differences in any of these measures. The present results contradict portions of that meta-analysis in that no significant case-control differences in PPT were found at any body site tested, except for a significant difference in MCPT tested on the hand. However, the present headache sample was not limited to migraine, which could explain some of the discrepant findings.
Pain Sensitivity Associations with Multiple COPCs
Despite the finding that individual COPCs are only modestly associated with experimental pain sensitivity (if at all), combinations of COPCs more consistently demonstrated associations between the number of COPCs an individual experienced and increased pain sensitivity. A significant linear association emerged for 10 of 15 QST measures. This suggests that even when individual COPCs do not show a significant independent association with a given QST measure, the combination of small effects can lead to a significant effect overall. The strongest example of this is reflected in HPT, in which none of the COPCs were found to provide independent contributions to the measure, yet a significant linear trend was found showing declining HPT with an increasing number of COPCs (Fig 3).
Fig 3.
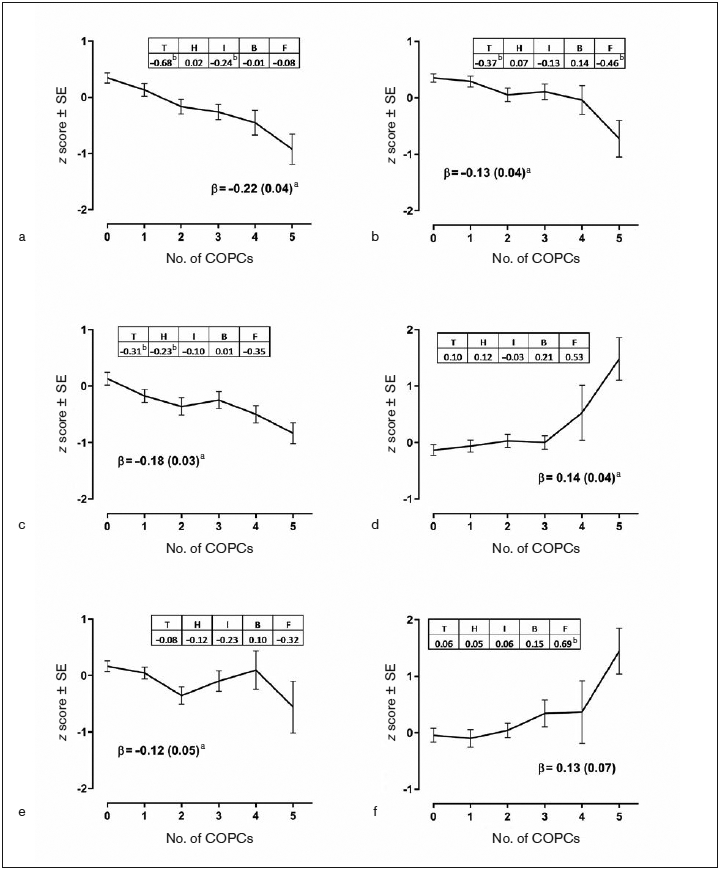
Impact of individual COPCs and number of COPCs on selected QST measures. (a) Pressure pain threshold (PPT) temporalis. (b) PPT anterior tibialis. (c) Mechanical cutaneous pain (MCP) threshold. (d) MCP aftersensation (15 seconds). (e) Heat pain (HP) threshold. (f) HP aftersensation (15 seconds). Each QST measure was the dependent variable in separate linear regression models that used weighted estimates from generalized estimating equations with robust error variance calculation and with adjustment for study site, age, gender, and race. Each of the six panels summarizes results from three linear regression analyses. (1) Plotted values are adjusted means of the z-transformed QST measure ± standard error (SE) from models in which the number of COPCs was the categorical predictor variable. (2) Beta (β) estimate (SE) represents the amount of change in the dependent variable associated with a unit increase in number of COPCs, modeled as a continuous variable. aP < .05 for the null hypothesis that β = 0. (3) Each COPC was modeled as a separate binary predictor variable in a multivariable linear regression model to show independent contributions of COPCs. Tabulated numbers are parameter estimates for COPCs, denoted as T = temporomandibular disorder, H = headache, I = IBS, B = low back pain, and F = fibromyalgia. bP < .05 for the null hypothesis that parameter estimate for the dummy variable equals 0.
One study addressing this question evaluated QST-based pain sensitivity among groups of TMD patients, migraineurs, and TMD + migraine patients, reporting that only the combined patient group had significantly lower HPTs than the control group when tested on the forearm.32 These results failed to show HPTs to be significantly associated with any individual COPC, but exhibited a significant association with the number of COPCs, thus generally supporting the results of the Chaves et al study (Fig 3).32 Additionally, another study evaluated the influence of comorbid conditions (painful and otherwise) on TMD pain and found a significant positive association between the number of comorbidities and both TMD pain intensity and pain duration.53 This result suggests that clinical pain, not just experimental pain, may be enhanced with multiple comorbidities.
Study Limitations
These findings should be interpreted in light of several study limitations. First, this is a cross-sectional study, which prohibits causal inferences regarding the association of pain sensitivity measures and COPCs. Second, the convenience sample recruited for this study may not be representative of the general population. Third, while the sample was relatively large, the number of individuals with fibromyalgia and the number experiencing five COPCs were small, which limited the statistical power for comparisons involving these groups. Accordingly, greater emphasis was placed on ORs than P values when interpreting associations for fibromyalgia, in particular when comparing them with the ORs for other COPCs seen for the same QST measure.
Conclusions
Acute pain sensitivity was enhanced for subjects experiencing some, but not all, of the five COPCs, although not uniformly. PPT and MCPT were most likely to exhibit significant associations and generally had high ORs across COPCs, while the other MCP and HP measures showed weaker and less consistent effects. For most QST measures, an increasing number of COPCs was usually associated with greater pain sensitivity, even when individual COPCs had only weak associations with the QST measure. In nearly all instances, the additive effect was seen only at a threshold of at least two or three COPCs. Thus, the presence of multiple COPCs, more so than the type of COPC, appears to signify enhanced nociceptive processing. The potential clinical significance of these results is in recognizing that the presence of multiple chronic pain conditions is likely to influence the clinical presentation and potential treatment success of any single one of them. Furthermore, the additive effects of multiple COPCs suggest that accounting for the number of comorbidities is an important factor in a patient’s overall pain sensitivity.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Dental and Craniofacial Research (U01-DE017018). Drs Fillingim and Maixner have equity ownership in Algynomics, Inc. The authors have nothing else to disclose.
Appendices
Appendix 1.
Unadjusted, Unweighted Estimates According to COPC
| TMD |
Headache |
|||
|---|---|---|---|---|
| QST measure | Case | Control | Case | Control |
| PPT: Temporalis | 132.36 (3.80), 177 | 190.05 (3.32), 465 | 160.15 (4.44), 264 | 183.92 (3.56), 378 |
| PPT: Trapezius | 243.51 (9.34), 177 | 332.60 (6.86), 465 | 291.62 (9.13), 264 | 319.50 (7.48), 378 |
| PPT: Anterior tibialis | 335.85 (10.84), 177 | 427.30 (6.89), 465 | 379.95 (9.37), 264 | 417.55 (7.80), 378 |
| MCPT | 185.84 (10.33), 176 | 266.54 (7.61), 470 | 216.55 (9.39), 265 | 264.03 (8.46), 381 |
| MCP: Single stimulus (0 to 100) | 24.62 (1.72), 169 | 17.35 (0.86), 456 | 20.47 (1.23), 255 | 18.52 (1.03), 370 |
| MCP: Series of 10 stimuli (0 to 100) | 45.30 (2.13), 169 | 39.32 (1.31), 456 | 42.07 (1.71), 255 | 40.15 (1.48), 370 |
| MCP: TS (−100 to 100) | 20.68 (1.30), 169 | 21.97 (0.87), 456 | 21.60 (1.09), 255 | 21.64 (0.96), 370 |
| MCP: 15-s aftersensation (0 to 100) | 12.99 (1.21), 169 | 8.13 (0.62), 455 | 10.82 (0.92), 255 | 8.50 (0.71), 369 |
| HPT, °C | 41.49 (0.27), 147 | 41.96 (0.17),418 | 41.61 (0.22), 231 | 41.99 (0.19), 334 |
| HP: Tolerance, °C | 45.70 (0.21), 147 | 45.95 (0.14), 418 | 45.88 (0.17), 231 | 45.88 (0.16), 334 |
| HP: Single stimulus (0 to 100) | 49.98 (2.61), 142 | 44.36 (1.57), 395 | 45.08 (2.09), 220 | 46.38 (1.76), 317 |
| HP: 10 stimuli AUC (0 to 100) | 619.65 (20.29), 142 | 541.16 (14.24), 395 | 568.34 (18.07), 220 | 557.45 (15.70), 317 |
| HP: Maximum of 10 stimuli (0 to 100) | 77.35 (2.14), 142 | 68.27 (1.60), 395 | 71.72 (2.01), 220 | 69.94 (1.74), 317 |
| HP: TS (0 to 100) | 25.85 (2.13), 142 | 20.81 (1.38), 395 | 23.55 (1.92), 220 | 21.17 (1.45), 317 |
| HP: 15-s aftersensation (0 to 100) | 17.73 (1.70), 138 | 9.84 (0.80), 378 | 13.65 (1.31), 211 | 10.77 (0.90), 305 |
| IBS |
LBP |
Fibromyalgia |
||||
|---|---|---|---|---|---|---|
| QST measure | Case | Control | Case | Control | Case | Control |
| PPT: Temporalis | 153.07 (5.01), 155 | 180.85 (3.30), 487 | 159.48 (5.92), 139 | 178.19 (3.18), 503 | 125.59 (6.58), 52 | 178.42 (2.94), 590 |
| PPT: Trapezius | 272.18 (10.79), 155 | 319.45 (6.77), 487 | 280.03 (12.32), 139 | 315.77 (6.55), 503 | 195.67 (12.45), 52 | 317.94 (6.06), 590 |
| PPT: Anterior tibialis | 367.59 (12.62), 155 | 413.07 (6.80), 487 | 376.92 (13.01), 139 | 409.05 (6.78), 503 | 299.41 (19.54), 52 | 411.14 (6.20), 590 |
| MCPT | 211.48 (12.58), 156 | 255.08 (7.32), 490 | 228.49 (13.57), 139 | 248.96 (7.20), 507 | 139.17 (17.28), 51 | 253.59 (6.62), 595 |
| MCP: Single stimulus (0 to 100) | 21.41 (1.62), 148 | 18.66 (0.90), 477 | 20.85 (2.00), 129 | 18.91 (0.85), 496 | 25.83 (3.51), 47 | 18.78 (0.80), 578 |
| MCP: Series of 10 stimuli (0 to 100) | 45.29 (2.24), 148 | 39.59 (1.29), 477 | 42.41 (2.59), 129 | 40.55 (1.24), 496 | 46.10 (4.13), 47 | 40.52 (1.16), 578 |
| MCP: TS (−100 to 100) | 23.88 (1.43), 148 | 20.92 (0.84), 477 | 21.56 (1.71), 129 | 21.64 (0.80), 496 | 20.27 (2.61), 47 | 21.73 (0.75), 578 |
| MCP: 15-s aftersensation (0 to 100) | 12.41 (1.31), 148 | 8.53 (0.61), 476 | 13.69 (1.67), 129 | 8.34 (0.55), 495 | 17.11 (2.85), 47 | 8.82 (0.56), 577 |
| HPT, °C | 41.12 (0.30), 130 | 42.05 (0.16), 435 | 41.56 (0.32), 118 | 41.91 (0.16), 447 | 41.26 (0.52), 39 | 41.88 (0.15), 526 |
| HP: Tolerance, °C | 45.72 (0.24), 130 | 45.93 (0.13), 435 | 45.46 (0.25), 118 | 45.99 (0.13), 447 | 45.81 (0.47), 39 | 45.89 (0.12), 526 |
| HP: Single stimulus (0 to 100) | 48.69 (2.85), 123 | 45.00 (1.53), 414 | 48.92 (3.06), 110 | 45.05 (1.50), 427 | 46.64 (4.66), 37 | 45.79 (1.41), 500 |
| HP: 10 stimuli AUC (0 to 100) | 597.75 (23.31), 123 | 551.26 (13.70), 414 | 616.95 (25.38), 110 | 547.73 (13.33), 427 | 593.76 (41.29), 37 | 559.56 (12.36), 500 |
| HP: Maximum of 10 stimuli (0 to 100) | 76.29 (2.47), 123 | 69.00 (1.53), 414 | 78.56 (2.66), 110 | 68.64 (1.49), 427 | 75.22 (4.59), 37 | 70.33 (1.37), 500 |
| HP: TS (0 to 100) | 24.11 (2.73), 123 | 21.56 (1.27), 414 | 25.89 (3.01), 110 | 21.18 (1.24), 427 | 26.01 (4.48), 37 | 21.86 (1.20), 500 |
| HP: 15-s aftersensation (0 to 100) | 16.22 (1.84), 119 | 10.67 (0.81), 397 | 14.86 (1.96), 105 | 11.21 (0.81), 411 | 20.67 (3.69), 36 | 11.30 (0.76), 480 |
Appendix 2.
Summary Measures of Model Fit for Random Forest Models
| Metric | TMD | Headache | IBS | LBP | Fibromyalgia |
|---|---|---|---|---|---|
| Observed % of cases | 0.278 | 0.412 | 0.241 | 0.212 | 0.079 |
| Area under precision-recall curve | 0.556 | 0.491 | 0.267 | 0.306 | 0.221 |
| Area under receiver operator characteristic curve | 0.774 | 0.591 | 0.548 | 0.617 | 0.765 |
| Brier score | 0.178 | 0.240 | 0.220 | 0.202 | 0.141 |
| Mutual information index | 0.069 | 0.008 | 0.000 | 0.010 | 0.020 |
| Proportion of variance explained | 0.154 | 0.049 | 0.047 | 0.051 | 0.151 |
| Maximum variable importance factor: Predicting cases | 0.198 | 0.059 | 0.035 | 0.051 | 0.138 |
| Maximum variable importance factor: Predicting controls | 0.012 | 0.010 | 0.008 | 0.018 | 0.008 |
| Maximum variable importance factor: All | 0.009 | 0.009 | 0.002 | 0.004 | 0.003 |
Appendix 3.
Unadjusted, Unweighted Estimates According to Number of COPCs
| No. of COPCs |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| PPT: Temporalis | 192.04 (4.43), 247 | 186.88 (5.53), 174 | 157.16 (6.81), 107 | 136.91 (6.22), 69 | 129.86 (8.27), 33 | 108.31 (11.40), 12 |
| PPT: Trapezius | 334.26 (9.27), 247 | 328.69 (11.27), 174 | 291.75 (14.37), 107 | 254.36 (16.26), 69 | 214.66 (15.96), 33 | 179.44 (17.62), 12 |
| PPT: Anterior tibialis | 425.32 (9.41), 247 | 434.00 (11.42), 174 | 376.74 (14.26), 107 | 341.13 (17.76), 69 | 323.27 (24.02), 33 | 254.42 (37.27), 12 |
| MCPT | 281.56 (10.28), 250 | 249.39 (12.37), 176 | 202.67 (14.91), 105 | 218.19 (17.62), 71 | 175.81 (24.53), 32 | 108.54 (22.80), 12 |
| MCP: Single stimulus (0 to 100) | 17.19 (1.18), 244 | 19.14 (1.53), 171 | 19.88 (1.83), 105 | 22.45 (2.56), 64 | 23.52 (4.68), 30 | 34.05 (7.00), 11 |
| MCP: Series of 10 stimuli (0 to 100) | 38.65 (1.83), 244 | 40.63 (2.06), 171 | 42.31 (2.69), 105 | 44.45 (3.51), 64 | 41.47 (5.44), 30 | 61.43 (6.47), 11 |
| MCP: Temporal summation (−100 to 100) | 21.46 (1.20), 244 | 21.49 (1.32), 171 | 22.44 (1.81), 105 | 22.00 (2.29), 64 | 17.95 (2.85), 30 | 27.39 (5.40), 11 |
| MCP: 15-s aftersensation (0 to 100) | 7.14 (0.76), 243 | 8.71 (1.08), 171 | 11.08 (1.28), 105 | 12.10 (2.06), 64 | 15.00 (3.71), 30 | 25.91 (5.72), 11 |
| HPT, °C | 42.06 (0.23), 223 | 42.11 (0.26), 158 | 41.36 (0.35), 90 | 41.63 (0.46), 58 | 41.05 (0.64), 27 | 39.91 (1.09), 9 |
| HP: Tolerance, °C | 45.91 (0.20), 223 | 45.96 (0.22), 158 | 46.10 (0.24), 90 | 45.77 (0.31), 58 | 45.14 (0.57), 27 | 44.66 (1.35), 9 |
| HP: Single stimulus (0 to 100) | 44.31 (2.16), 214 | 44.66 (2.47), 148 | 49.15 (3.36), 85 | 48.57 (4.55), 55 | 48.39 (6.62), 26 | 46.67 (7.07), 9 |
| HP: 10 stimuli AUC (0 to 100) | 532.48 (19.75), 214 | 545.71 (22.24), 148 | 626.11 (26.40), 85 | 583.25 (36.63), 55 | 604.54 (56.42), 26 | 668.22 (42.93), 9 |
| HP: Maximum of 10 stimuli (0 to 100) | 66.57 (2.22), 214 | 69.51 (2.50), 148 | 77.27 (2.87), 85 | 73.64 (3.86), 55 | 76.65 (5.85), 26 | 89.44 (3.32), 9 |
| HP: TS (0 to 100) | 20.23 (1.69), 214 | 21.07 (2.27), 148 | 26.12 (3.09), 85 | 21.45 (4.00), 55 | 26.72 (5.23), 26 | 38.89 (10.46), 9 |
| HP: 15-s aftersensation (0 to 100) | 9.51 (1.08), 204 | 10.30 (1.35), 143 | 14.51 (1.61), 83 | 15.09 (2.91), 53 | 19.33 (5.24), 24 | 31.67 (7.50), 9 |
Data are reported as mean (standard error), number of unweighted participants. PPT = pressure pain threshold; MCP(T) = mechanical cutaneous pain threshold; HP(T) = heat pain (threshold); TS = temporal summation; AUC = area under the curve.
Contributor Information
Joel D. Greenspan, Department of Neural and Pain Sciences, Brotman Facial Pain Clinic, Center to Advance Chronic Pain Research, School of Dentistry, University of Maryland, Baltimore, Maryland, USA.
Gary D. Slade, Division of Pediatric and Public Health, Adams School of Dentistry, Department of Dental Ecology, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina, USA.
Nuvan Rathnayaka, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina, USA.
Roger B. Fillingim, Department of Community Dentistry and Behavioral Science, Pain Research and Intervention Center of Excellence, College of Dentistry, University of Florida, Gainesville, Florida, USA.
Richard Ohrbach, Department of Oral Diagnostic Services, School of Dental Medicine, University at Buffalo, Buffalo, New York, USA.
William Maixner, Center for Translational Pain Medicine, Duke University, Durham, North Carolina, USA.
References
- 1.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol 2013;9:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states—Maybe it is all in their head. Best Pract Res Clin Rheumatol 2011;25:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH Working Group. Chronic Overlapping Pain Conditions. Summary of NIH Work Group Meeting to Develop Case Definition & Common Data Elements. National Institutes of Health, 2015. [Google Scholar]
- 5.Arendt-Nielsen L, Morlion B, Perrot S, et al. Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018;22:216–241. [DOI] [PubMed] [Google Scholar]
- 6.Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci 2018;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011;152(3 suppl):s2–s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oono Y, Wang K, Baad-Hansen L, et al. Conditioned pain modulation in temporomandibular disorders (TMD) pain patients. Exp Brain Res 2014;232:3111–3119. [DOI] [PubMed] [Google Scholar]
- 9.Quartana PJ, Finan PH, Smith MT. Evidence for sustained mechanical pain sensitization in women with chronic temporomandibular disorder vs healthy female participants. J Pain 2015;16:1127–1135. [DOI] [PubMed] [Google Scholar]
- 10.Beese LC, Putzer D, Osada N, Evers S, Marziniak M. Contact heat evoked potentials and habituation measured interictally in migraineurs. J Headache Pain 2015;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahman-Averbuch H, Shefi T, Schneider VJ 2nd, et al. Quantitative sensory testing in patients with migraine: A systematic review and meta-analysis. Pain 2018;159:1202–1223. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett ME, Han CJ, Cain KC, et al. Relationships of abdominal pain, reports to visceral and temperature pain sensitivity, conditioned pain modulation, and heart rate variability in irritable bowel syndrome. Neurogastroenterol Motil 2016;28:1094–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Q, Fillingim RB, Riley JL 3rd, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain 2010;148:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starkweather AR, Ramesh D, Lyon DE, et al. Acute low back pain: Differential somatosensory function and gene expression compared with healthy no-pain controls. Clin J Pain 2016;32:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hübscher M, Moloney N, Rebbeck T, Traeger A, Refshauge KM. Contributions of mood, pain catastrophizing, and cold hyperalgesia in acute and chronic low back pain: A comparison with pain-free controls. Clin J Pain 2014;30:886–893. [DOI] [PubMed] [Google Scholar]
- 16.Hermans L, Nijs J, Calders P, et al. Influence of morphine and naloxone on pain modulation in rheumatoid arthritis, chronic fatigue syndrome/fibromyalgia, and controls: A double-blind, randomized, placebo-controlled, cross-over study. Pain Pract 2018;18:418–430. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 18.Bair E, Brownstein NC, Ohrbach R, et al. Study protocol, sample characteristics, and loss to follow-up: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T2–T19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain 2011;12(11 suppl):T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 22.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology 2003;61:375–382. [DOI] [PubMed] [Google Scholar]
- 23.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 24.Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95–103. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 26.Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur J Pain 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- 27.Richardson DB, Rzehak P, Klenk J, Weiland SK. Analyses of case-control data for additional outcomes. Epidemiology 2007;18:441–445. [DOI] [PubMed] [Google Scholar]
- 28.Monsees GM, Tamimi RM, Kraft P. Genome-wide association scans for secondary traits using case-control samples. Genet Epidemiol 2009;33:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang F, Ishwaran H. Random forest missing data algorithms. Stat Anal Data Min 2017;10:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Delgado M, Cernadas E, Barro S, Amorim D. Do we need hundreds of classifiers to solve real world classification problems? J Machine Learning Res 2014;15:3133–3181. [Google Scholar]
- 31.Greenspan JD, Slade GD, Bair E, et al. Pain sensitivity risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case control study. J Pain 2011;12(11 suppl):T61–T74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaves TC, Dach F, Florencio LL, et al. Concomitant migraine and temporomandibular disorders are associated with higher heat pain hyperalgesia and cephalic cutaneous allodynia. Clin J Pain 2016;32:882–888. [DOI] [PubMed] [Google Scholar]
- 33.Garrett PH, Sarlani E, Grace EG, Greenspan JD. Chronic temporomandibular disorders are not necessarily associated with a compromised endogenous analgesic system. J Orofac Pain 2013;27:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho GF, Chaves TC, Florencio LL, Dach F, Bigal ME, Bevilaqua-Grossi D. Reduced thermal threshold in patients with temporomandibular disorders. J Oral Rehabil 2016;43:401–408. [DOI] [PubMed] [Google Scholar]
- 35.Kothari SF, Baad-Hansen L, Oono Y, Svensson P. Somatosensory assessment and conditioned pain modulation in temporomandibular disorders pain patients. Pain 2015;156:2545–2555. [DOI] [PubMed] [Google Scholar]
- 36.Martínez-Jauand M, Sitges C, Rodriguez V, et al. Pain sensitivity in fibromyalgia is associated with catechol-O-methyltransferase (COMT) gene. Eur J Pain 2013;17:16–27. [DOI] [PubMed] [Google Scholar]
- 37.Ickmans K, Meeus M, De Kooning M, Lambrecht L, Pattyn N, Nijs J. Associations between cognitive performance and pain in chronic fatigue ayndrome: Comorbidity with fibromyalgia does matter. Pain Physician 2015;18:E841–E852. [PubMed] [Google Scholar]
- 38.Schreiber KL, Loggia ML, Kim J, Cahalan CM, Napadow V, Edwards RR. Painful after-sensations in fibromyalgia are linked to catastrophizing and differences in brain response in the medial temporal lobe. J Pain 2017;18:855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Silva LA, Kazyiama HH, Teixeira MJ, de Siqueira SR. Quantitative sensory testing in fibromyalgia and hemisensory syndrome: Comparison with controls. Rheumatol Int 2013;33:2009–2017. [DOI] [PubMed] [Google Scholar]
- 40.Chalaye P, Lafrenaye S, Goffaux P, Marchand S. The role of cardiovascular activity in fibromyalgia and conditioned pain modulation. Pain 2014;155:1064–1069. [DOI] [PubMed] [Google Scholar]
- 41.Crettaz B, Marziniak M, Willeke P, et al. Stress-induced allodynia—Evidence of increased pain sensitivity in healthy humans and patients with chronic pain after experimentally induced psychosocial stress. PLoS One 2013;8:e69460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desmeules J, Chabert J, Rebsamen M, et al. Central pain sensitization, COMT Val158Met polymorphism, and emotional factors in fibromyalgia. J Pain 2014;15:129–135. [DOI] [PubMed] [Google Scholar]
- 43.Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain 2016;157:1704–1710. [DOI] [PubMed] [Google Scholar]
- 44.Piché M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010;148:49–58. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Q, Fillingim RB, Riley JL 3rd, Verne GN. Thermal hypersensitivity in a subset of irritable bowel syndrome patients. World J Gastroenterol 2009;15:3254–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues AC, Nicholas Verne G, Schmidt S, Mauderli AP. Hypersensitivity to cutaneous thermal nociceptive stimuli in irritable bowel syndrome. Pain 2005;115:5–11. [DOI] [PubMed] [Google Scholar]
- 47.Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain 2010;26:104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verne GN, Price DD, Callam CS, Zhang B, Peck J, Zhou Q. Viscerosomatic facilitation in a subset of IBS patients, an effect mediated by N-methyl-D-aspartate receptors. J Pain 2012;13:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong F, Rodrigues AC, King CD, et al. Relationships between irritable bowel syndrome pain, skin temperature indices of autonomic dysregulation, and sensitivity to thermal cutaneous stimulation. Pain Res Treat 2010;2010:949027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrêa JB, Costa LO, de Oliveira NT, Sluka KA, Liebano RE. Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: A case-control study. Exp Brain Res 2015;233:2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klyne DM, Moseley GL, Sterling M, Barbe MF, Hodges PW. Individual variation in pain sensitivity and conditioned pain modulation in acute low back pain: Effect of stimulus type, sleep, psychological and lifestyle factors. J Pain 2018;19:942.e1.e18 [DOI] [PubMed] [Google Scholar]
- 52.Owens MA, Bulls HW, Trost Z, et al. An examination of pain catastrophizing and endogenous pain modulatory processes in adults with chronic low back pain. Pain Med 2016;17:1452–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahan H, Shir Y, Velly A, Allison P. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J Headache Pain 2015;16:528. [DOI] [PMC free article] [PubMed] [Google Scholar]


