Abstract
Aims:
To investigate whether TMD-related characteristics are indeed specific to TMD or whether they are also associated with other chronic overlapping pain conditions (COPCs).
Methods:
In this cross-sectional study, 22 characteristics related broadly to TMD (eg, jaw kinesiophobia, overuse behaviors, and functional limitation) were measured in 178 painful TMD cases who were also classified according to four COPCs: headache, low back pain, irritable bowel syndrome, and fibromyalgia. Differences in mean subscale scores were compared according to individual chronic pain conditions and according to number of COPCs.
Results:
Headache, low back pain, irritable bowel syndrome, and fibromyalgia were each associated (P < .05) with higher values of at least one TMD-relevant characteristic. In the multivariable analysis, TMD was independently associated with 20 of the 22 characteristics (P < .01), and other COPCs were associated variably. A critical threshold existed between the number of COPCs and TMD characteristics: all characteristics were elevated for subjects with ≥ 3 COPCs (P ≤ .01).
Conclusion:
The overlap between COPCs and characteristics typically regarded as specific to painful TMD has implications for treatment targeted at both the local TMD condition and the broader pain disorder underlying the COPC(s). In TMD patients, the overall burden of pain from COPCs may create a shift in the pain-processing systems that underlie these TMD-relevant characteristics.
Keywords: chronic overlapping pain conditions, comorbidity, pain, measurement, TMD
It is useful to distinguish between generic and condition-specific measures when studying characteristics associated with chronic pain conditions, such as temporomandibular disorder (TMD), headache, irritable bowel syndrome (IBS), low back pain (LBP), and fibromyalgia. Alternative terms for disorders with these characteristics include idiopathic pain conditions,1 chronic overlapping pain conditions (COPCs),2 central sensitivity syndromes,3 and functional pain syndromes.4 Each term is useful for certain contexts; for example, the term “idiopathic pain conditions” emphasizes that the assessment criteria selected for each disorder intentionally sidestep considerations of etiology or mechanism. To illustrate, etiology is not part of the criteria for primary headache.5 Hereafter, the collective term “chronic overlapping pain conditions” is used, consistent with the current terminology favored by the National Institutes of Health.6
Generic measures are used because they are applicable to multiple COPCs, regardless of the anatomical location and clinical characteristics of the COPC. For example, the Perceived Stress Scale (PSS) asks about an individual’s appraisal and coping with everyday stressful events, but is not tied to any particular stressor, such as a specific pain condition. Likewise, quantitative sensory testing (QST) is conducted at standardized locations whether or not they coincide with the location of the COPC—for example, heat pain thresholds are often measured on the forearm in TMD studies. Generic measures are informative because they might signify dysregulation in mechanisms that underpin multiple COPCs and might point to interventions that could be effective for pain regardless of its anatomical location.
In contrast, condition-specific measures assess attributes of a specific COPC (eg, TMD) or a system underlying that COPC (eg, the masticatory system). In some instances, condition-specific measures form part of the diagnostic criteria for the respective disorder. For example, possible hyperalgesia of the temporomandibular joints (TMJs) is evaluated with palpation when diagnosing TMD arthralgia. In other instances, condition-specific measures have treatment implications. For example, pain-free opening may be informative when jaw-stretching exercises are considered in TMD patients who have limited jaw opening.
Implicitly, the term “specific” signifies that a condition-specific measure is applicable to one pain condition but not to others. While it seems self-evident that a measure such as range of jaw opening is applicable to TMD but not to, say, LBP or headache, researchers seldom test that assumption. Certainly, other explanations are plausible—for example, if a patient with multiple COPCs has an underlying functional pain syndrome,4 it follows that an assumed condition-specific measure of one index pain condition might also be a marker of that underlying syndrome. This creates a clinical conundrum. To use the example of limited jaw opening in a TMD patient: Should jaw exercises be part of the treatment plan for TMD on the assumption that TMD is the only pain condition applicable to range of jaw motion? Or, is limited jaw opening a manifestation of a functional pain syndrome, requiring treatment targeting other COPCs that are part of that syndrome?
This paper investigates whether the putative measures germane to TMD are indeed specific to TMD or whether they are also associated with other COPCs. The other COPCs of interest were headache, IBS, LBP, and fibromyalgia. These conditions were selected because they are often cited as components of more generic dysregulation of pain processing, as described for functional pain syndromes and the related concept of central sensitization. TMD represents an excellent index pain condition for investigating the general question regarding the presumed specificity of measures developed for an index condition because TMD classification is based on multiple well-validated examination criteria. In addition, each of the TMD-specific measures have validated measurement scales that have face validity for TMD and that are seemingly not applicable to other COPCs.
Materials and Methods
Ethical Considerations
Reporting of this observational study conforms with the STROBE (Strengthening the Reporting of Observational Studies) guidelines.7 The primary data collection was from the National Institute of Dental and Craniofacial Research (NIDCR) Study Protocol 12-0520-E, conducted in the second phase of the OPPERA project. The Office of Human Research Ethics at each participating institution reviewed and approved the study.
Study Design, Setting, and Participants
This cross-sectional design used data from adults originally recruited into the first phase of OPPERA (OPPERA-1) between May 2006 and May 2013. At that time, subjects aged 18 to 44 years were selected for a community-based, case-control study of chronic TMD.
The baseline case-control study (OPPERA-1) of chronic TMD enrolled 1,008 cases with examiner-verified painful TMD and 3,258 adults with examiner-verified absence of TMD to serve as controls. All subjects were recruited at the US academic health centers located at: University at Buffalo, Buffalo, New York; University of Florida, Gainesville, Florida; University of Maryland, Baltimore, Maryland; and University of North Carolina, Chapel Hill, North Carolina. Previous papers have described the details of recruitment and baseline data collection, as well as the methods used for a subsequent prospective cohort study of the TMD-free individuals who were followed up for up to 5 years to investigate the incidence of first-onset TMD.8,9 This cross-sectional analysis reports findings from the most recent wave of data collection in OPPERA-2. Between December 2014 and May 2016, attempts were made to contact all original enrollees in the OPPERA-1 case-control study. For those who consented and attended research clinics, data were then collected using clinical examinations, QST, cardiovascular measures of autonomic function, blood samples, and self-report questionnaires. Further details of recruitment and data collection methods are provided elsewhere in this volume (see Slade et al, current issue).
Classification of COPCs
The presence or absence of five COPCs was classified as described in detail elsewhere in this volume (see Ohrbach et al, current issue), and is summarized below.
Painful TMD was classified by examiners according to the Diagnostic Criteria for TMD (DC/TMD).10 While additional diagnoses of TMD can be made using the DC/TMD, this study only classified painful TMD. In summary, to be classified as a painful TMD case, subjects had to have all four of the following characteristics: (1) history of orofacial pain in examiner-verified locations of the masseter, temporalis, submandibular, or TMJ area(s) that had occurred on 5 or more of the 30 days preceding the examination; (2) evoked pain in the same muscles and/or TMJ(s) following either palpation of those structures or jaw maneuvers; (3) reported familiarity of evoked pain, as judged by a positive response to the question: “Was the pain you felt [during palpation or jaw maneuver] familiar to the pain [or temporal headache] that you reported during the last 30 days?”; and (4) pain that was modified by jaw function, as judged by a positive response to the question: “During the last 30 days, was any of the pain modified by chewing hard food, opening the mouth, jaw habits such as clenching, or other jaw activities?”
Headache was classified using responses to a questionnaire designed for OPPERA that asked about symptoms of tension-type headache (TTH) and migraine during the preceding 12 months. Subjects who experienced more than one type of headache recorded responses separately for up to three different types of headache. Questions about TTH were from the International Classification of Headache Disorders (ICHD), third edition.5 Symptoms of migraine were based on questions used in the ID-Migraine questionnaire.11 Migraine was classified when subjects reported headache(s) on 1 or more days per month and at least two of three symptoms accompanying the headache: nausea, sensitivity to light, or being kept from everyday activities. More information is available elsewhere regarding this approach for headache classification (see Slade et al, current issue). For this analysis, headache was classified for any subject who reported symptoms consistent with TTH or migraine and who had experienced such headache(s) in the preceding 3 months. Overall, about 25% of the headache group had only TTH, while the remainder had either migraine alone or migraine in combination with TTH.
Irritable bowel syndrome (IBS) was classified using responses to four questions about abdominal pain from the Rome III diagnostic criteria.12 Subjects were classified with IBS if they met both of the following criteria: (1) abdominal pain on at least 1 day in the preceding 3 months that was not related to menstrual periods; (2) pain that was associated with at least two symptoms of bowel function (ie, pain altered by bowel movements; greater frequency of bowel movements; less frequency of bowel movements; looser stools; harder stools).
Low back pain (LBP) was classified using responses to screening questions recommended for studies of back pain prevalence.13 Subjects were classified with LBP if they reported pain that occurred in the lower back (as illustrated to the participant with a shaded manikin drawing) during the preceding 3 months that was not related to fever or menstruation and that restricted usual activities for at least 1 day.
Fibromyalgia was classified based on findings from an examination and questionnaire, consistent with the 1990 American College of Rheumatology (ACR) criteria.14 Subjects were classified with fibromyalgia when ≥ 11 of 18 body sites were tender to algometer-delivered pressure of up to 4.0 kg/cm2 and when the tenderness occurred in both the axial skeleton and in at least one set of opposing diagonal quadrants of the body. Also, fibromyalgia cases had to report a history of pain lasting for at least 1 day per month in the preceding 3 months.
Overall, these classifications represent chronic disorders. In another paper in this series (see Ohrbach et al, current issue), it was reported that pain had persisted for at least 6 months at the time of evaluation in nearly 100% of the participants for TMD, headache, and fibromyalgia each; in 88% for IBS; and in 93% for LBP.
Demographic Characteristics
The following variables were collected and coded as follows: age (in years), sex (male, female), and race/ethnicity (white, non-Hispanic, Black/African American, Hispanic, other).
Assessment of Attributes Germane to TMD
This paper focuses on the attributes likely to be considered when clinicians make a thorough assessment of patients with TMD myalgia or arthralgia. In principle, such attributes might be germane to TMD because they are a contributor to TMD (ie, as risk factors for onset, persistence, or aggravation of the condition), a consequence of TMD, or both. This study used validated measures of eight sets of attributes that are germane to TMD:
Kinesiophobia related to the jaw is defined as fear of movement of the jaw. It was measured using the Tampa Scale for Kinesiophobia for TMD (TSK-TMD) questionnaire.15 The TSK-TMD has two subscales, activity avoidance and somatic focus, each representing the degree of agreement with the items within each subscale. The TSK-TMD follows the structure of the TSK16 and equally represents a measure relevant to the fear-avoidance construct.17 The two-factor model via confirmatory factor analysis (comparative fit index [CFI] = 0.95, root mean square error of approximation [RMSEA] = 0.078) supports both subscales. Internal reliability of the subscales is 0.82 and 0.66 (Cronbach’s α), and temporal stability at 4 weeks for activity avoidance and somatic focus is 0.67 and 0.71 (intraclass correlation coefficient [ICC]), respectively.18 Jaw-related kinesiophobia was conceived as both a contributor to and consequence of TMD.
Parafunctional behaviors are defined as behaviors different from those required for, or associated with, expected jaw functional demands, such as mastication, swallowing, communication, or breathing.19 They were measured using the Oral Behaviors Checklist (OBC). The OBC presently yields a single score representing the frequency of 21 activities such as clenching, chewing gum, and holding objects between the teeth.20,21 Total score test-retest reliability at 2 weeks (ICC) is 0.88.22 It was reasoned that parafunctional behaviors could contribute to and be a consequence of TMD.
Limitations in using the jaw are defined as perceived limitation in using the jaw.23 They were measured using the Jaw Functional Limitation Scale (JFLS).24 The JFLS yields three limitation subscales: mastication, vertical jaw mobility, and verbal and emotional expression.25,26 Internal reliability of these subscales is 0.88, 0.84, and 0.92 (Cronbach's α), respectively, and temporal stability at 1 to 2 weeks is 0.87, 0.94, and 0.56 (concordance correlation coefficient [CCC] ρ), respectively. Validity, as assessed via known-groups comparison, is excellent. A total score was also computed from the three subscales when all three component scores were available (Cronbach's α = 0.87 and temporal stability = 0.87). Jaw limitation was considered primarily to be a consequence of TMD.
Nonspecific jaw symptoms that may represent subthreshold pain experience were evaluated in relation to the TMJ and/or masticatory muscle area using a checklist that asked about six symptoms in the preceding month: jaw stiffness, cramping, fatigue, pressure, soreness, and ache. These nonspecific symptoms might be markers of early behavioral and pathologic changes that undoubtedly represent a risk factor for TMD,27 but may also be a consequence of TMD.
Jaw mobility was measured by examiners who instructed participants to perform three jaw maneuvers: pain-free opening, maximum unassisted opening, and maximum assisted opening. Examiners then measured the vertical distance in millimeters between the maxillary and mandibular incisors.28 These measures have excellent interexaminer reliability.29 The prevailing view in the literature is that jaw mobility limitation is a consequence of TMD, although in people without the condition, painful limitation might be a marker of early changes that contribute to TMD.27
Masticatory pain was assessed by examiners who asked participants if pain was evoked by jaw mobility (described previously) and/or by palpation. Examiners also inquired whether evoked pain was familiar to the pain or the location-specific headache they had experienced in the past 1 month. For palpation pain via fingertip, a load of 1.0 kg was applied to defined sites in each of the masseter and temporalis muscles and around the lateral condylar pole of the TMJ, and a load of 0.5 kg was applied to each of the posterior mandibular and submandibular areas, the lateral TMJ pole, and the dorsal aspect of the TMJ pole. All areas were assessed bilaterally.28 Masticatory pain represents one of four criteria required for case classification; however, in people without the condition, it might be a marker of early changes that contribute to TMD.27
For mechanical status of the TMJ, a symptom questionnaire containing 14 items assessing TMJ clicking, locking, and functional TMJ pain was completed by all participants. The items were previously administered in OPPERA-1, and the results of item reduction via factor analysis were previously reported.30 That same factor model was used here for these items because it is simple, the present findings can be linked to prior published findings, and a new factor analysis in this sample did not produce any improvement in the factor model. This approach yields a 1-factor solution labeled “TMJ function by history” based on equal weighting of each of the 7 items retained by the factor model. The mechanical status of the TMJ might be a marker of anatomical and functional changes contributing to painful TMD and could represent a consequence of painful TMD.
Neck and below-the-neck pain from palpation were assessed by examiners at nine defined sites on each side of the body, as based on the 1990 ACR criteria for fibromyalgia.31 A load of up to 4 kg/cm2 was applied at a rate of 1 kg/cm2/second using a Wagner FPK 10 algometer at each site. For each palpation site, a positive or negative report of pain was recorded. Three bilateral neck sites (occiput, low cervical, and trapezius) comprised the neck scale, and six bilateral body sites (second rib, supraspinatus, lateral epicondyle, gluteal, greater trochanter, and knee) comprised the below-the-neck scale. The measure is seen here primarily as a marker for comparison to the results of associations with the measures specific to TMD.
Hereafter, three labels are used for the above measures: (1) “measures germane to TMD” for all 22 measures combined; (2) “DC/TMD–specific measures” for the 10 measures that are part of the DC/TMD examination procedures (pain-free jaw opening, maximum assisted and unassisted jaw opening, familiar and nonfamiliar masticatory pain by motion and by palpation, and TMJ function by history); and (3) “TMD-relevant measures” for the remaining 12 of the 22 measures.
Statistical Methods
Raw values of each TMD measure were used to generate descriptive statistics for cases and controls of each COPC and according to number of COPCs. All other analyses of continuous variables used z-transformed values of the measure, and the data were weighted during analysis. The goal of data transformation was to produce measures of association (eg, odds ratios [ORs], regression estimates) that could be readily compared between measures that use different scales of measurement. The goal of weighting was to adjust for the way in which study participants were selected in OPPERA-2. Weighting took into consideration the original sampling design for the OPPERA-1 case-control study (in which TMD cases were oversampled relative to their prevalence in the population) and to adjust for differential loss to follow-up of subjects between enrollment in OPPERA-1 and participation in OPPERA-2. Such weighting is important for this analysis in order to make valid estimates of association between any two variables (eg, TMD-related measures and headache) in a sample that was originally stratified according to a third variable (the presence or absence of chronic TMD in OPPERA-1).32 The analytic weights for OPPERA-2 were computed as the inverse of the sampling probability for OPPERA-1, multiplied by the inverse of the loss-to-follow-up probability between OPPERA-1 and OPPERA-2. With the exception of univariate statistics describing the distribution of explanatory variables, all means, percentages, and measures of association were calculated using generalized estimating equations with the GENMOD procedure in SAS version 9.4 (IBM), with analytic weights and robust error variance calculation.33
The analysis first assessed associations between each measure and the presence or absence of each COPC using statistical methods for case-control analysis of cross-sectional data. Adjusted ORs were estimated in separate binary logistic regression models, one for each COPC, where the main explanatory variable was the standardized (using z-score transformation) value of a single measure. The models adjusted for study site (four categories) and subjects’ demographic characteristics: age (years), gender (two categories), and race/ethnicity (five categories: white, black/African American, Asian, Hispanic, or other).
A second set of analyses examined associations with the participant’s number of COPCs. For these analyses, the TMD measures were the dependent variables, and adjusted means of the measure were plotted according to the presence of 0, 1, 2, 3, 4, or 5 COPCs. Covariates used when calculating adjusted means were study site and demographics (coded as described above).
Across models, COPCs were modeled using three approaches in order to evaluate patterns of association: (1) The number of COPCs was modeled as a categorical variable in order to evaluate potential nonlinear relationships with the explanatory variable, and pairwise comparions were tested for differences between subjects with no COPCs (the reference group) vs the other five possibilities (1, 2, 3, 4, or 5 COPCs); (2) The number of COPCs was modeled as a continuous variable in order to reveal a potential linear relationship with the dependent variable, with a test of the null hypothesis of no linear relationship (β = 0); and (3) All five COPCs were modeled as separate binary predictor variables, and parameter estimates were tested for independent associations of each pain condition with the TMD measure.
It was recognized that because painful TMD had a major influence on all measures germane to TMD, the adjusted means of the measures according to the presence of non-TMD COPCs (ie, 0 to 4 COPCs) were plotted and assessed for nonlinear and linear relationships, as described above.
Random forest modeling explored the multivariable associations of all measures with each binary COPC case classification. As described in detail in a previous OPPERA paper,34 random forest model methodology uses all potential explanatory variables (in this paper, measures germane to TMD) to create decision trees predicting the dependent variable (each pain condition). The goals were to identify individual measures germane to TMD that make the greatest contribution, statistically, to the occurrence of each pain condition and to quantify the collective accuracy of all explanatory variables in predicting each pain condition. Random forests are nonparametric statistical models that can handle interactions and nonlinear associations without the need to pre-specify the interactions or the form of the nonlinear relationships. Due to this flexibility, random forests demonstrate excellent classification performance across a broad range of tasks. For this paper, a separate random forest model was created for each pain condition, and predictor variables for each model were all 22 of the measures germane to TMD. Five steps are used to create each model34: (1) A random sample of study participants is selected with replacement; (2) A random sample of predictor variables is selected, and each one is used to partition the data and create a decision tree; (3, 4) Steps 1 and 2 are repeated 1,000 times each; and (5) The estimated probability of the dependent variable is then calculated as the average of all 1,000 probabilities.
Missing values of explanatory variables were imputed using on-the-fly imputation, which is the decision-tree analog of multiple imputation.35 Through a combination of the bootstrap aggregating and random subspace methods used in the construction of random forests, this classification performance is achieved without overfitting to the training dataset, thus maintaining good out-of-sample performance.36 Associations of individual variables in the random forest models were quantified using variable importance scores, which estimate the relative contribution of each predictor to the model’s classification of true positives and true negatives. Overall classification performance of the models was quantified with area under the receiver operating characteristic curve (AUROC) and area under the precision recall curve (AUPR). In datasets with unequal numbers of cases and controls, AUPR is a better measure of classification performance than AUROC, though no single metric can adequately capture classification performance.37 However, both measures accord equal weight to false positives and false negatives, whereas the relative importance of those errors may vary according to the pain condition. Therefore, the Brier score,38 which provides an analog to mean squared error, was also computed, as well as proportion of variance explained for the binary prediction models. Mutual information provides sensible rankings of classifiers in scenarios (such as class imbalance) that can break ties prone to occur with more commonly used measures such as precision, recall, and AUROC.39
Results
Demographic Characteristics and Descriptive Statistics
Sample counts and weighted estimates for demographic characteristics according to presence or absence of each COPC and the number of COPCs are presented in Table 1. Mean age was generally similar for both cases and controls of all COPCs and for the different numbers of COPCs. For TMD, headache, and fibromyalgia, a greater percentage of cases than controls were women, whereas for IBS and LBP, the controls were more likely women. In general, the percentage of women increased according to number of COPCs. The proportions of white and non-white individuals were generally similar for each COPC and for number of COPCs (Table 1). Appendix 1 (see all appendices in the online version of this article at www.quintpub.com/journals) presents descriptive statistics for the 22 TMD measures germane to TMD, reported separately for cases and controls of each COPC. Appendix 2 presents descriptive statistics according to number of COPCs.
Table 1.
Descriptive Univariate Analysis for Demographic Characteristics of OPPERA Study Participants
| Classification | Group | Weighted n | Mean (SE) age, y | % (SE) female | % (SE) white |
|---|---|---|---|---|---|
| TMD | Case | 108 | 33.0 (0.6) | 61.2 (3.6) | 50.7 (3.7) |
| Control | 547 | 35.4 (0.4) | 57.0 (2.3) | 52.2 (2.3) | |
| Headache | Case | 201 | 34.6 (0.5) | 71.1 (2.8) | 55.5 (3.0) |
| Control | 454 | 35.3 (0.4) | 51.7 (2.6) | 50.4 (2.6) | |
| IBS | Case | 134 | 34.6 (0.7) | 53.7 (4.0) | 60.1 (3.9) |
| Control | 521 | 35.2 (0.3) | 58.7 (2.2) | 49.8 (2.2) | |
| LBP | Case | 99 | 37.6 (0.7) | 56.7 (4.2) | 62.9 (4.1) |
| Control | 556 | 34.6 (0.3) | 57.9 (2.2) | 50.0 (2.2) | |
| Fibromyalgia | Case | 24 | 34.3 (1.1) | 77.2 (5.9) | 52.7 (7.0) |
| Control | 631 | 35.1 (0.3) | 56.9 (2.0) | 51.9 (2.0) | |
| No. of COPCs | 0 | 307 | 35.6 (0.5) | 54.0 (3.1) | 46.5 (3.1) |
| 1 | 209 | 34.4 (0.5) | 60.0 (3.7) | 55.5 (3.7) | |
| 2 | 83 | 33.6 (0.8) | 57.5 (4.8) | 58.7 (4.7) | |
| 3 | 33 | 36.5 (1.1) | 63.6 (5.8) | 68.4 (5.6) | |
| 4 | 15 | 38.7 (1.4) | 92.2 (4.7) | 39.7 (8.6) | |
| 5 | 6 | 30.9 (1.7) | 48.8 (15.1) | 52.8 (15.1) |
SE = standard error.
Univariate Associations Between Individual COPCs and Measures Germane to TMD
In the univariate analysis, each COPC was associated with most or all of the 22 measures germane to TMD, based on the nominal threshold of P < .05 for statistical significance. For painful TMD, there were 21 significant associations; for fibromyalgia, 22; for low back pain, 18; for headache, 16; and for IBS, 12. Because all measures were standardized as z scores, it is meaningful to compare the odds ratios (ORs) as measures of association with each COPC.
With regard to the 10 DC/TMD–specific measures, 9 were associated with painful TMD, 6 with headache, 7 with IBS, 8 with LBP, and all 10 with fibromyalgia. For pain-free jaw opening, the 5 significant associations ranged from OR = 1.35 (IBS) to 3.20 (fibromyalgia). However, there were fewer significant associations for the two other measures of jaw mobility, and the associated ORs were no greater than 2.38. ORs were generally much larger for the six examiner-assessed measures of masticatory muscle pain, ranging up to 11.95 for the association between painful TMD and masticatory familiar pain. Fewer associations for TMJ function by history were significant, and the largest OR was not greater than 3.17 (for painful TMD).
As for the remaining 12 TMD-relevant measures, all 12 were associated with painful TMD, 10 with headache, 5 with IBS, 11 with LBP, and 12 with fibromyalgia. Most associations were significant for the four examiner-assessed measures of neck and body pain, ranging from OR = 1.37 to 5.61; however, the strongest association was between neck pain and fibromyalgia, at OR = 24.52. Associations between nonspecific jaw symptoms and each of the five COPCs were all significant and ranged up to OR = 8.78 for painful TMD. Similarly, the four measures of jaw functional limitation were associated mostly with painful TMD, headache, LBP, and fibromyalgia, but not with IBS. The same pattern was seen for the two measures for kinesiophobia. The largest ORs were for the associations between painful TMD and global score (OR = 3.12), JFLS opening (OR = 2.99), and somatic focus (OR = 2.46). The OBC measure of oral parafunctional behaviors was likewise significantly associated with each COPC, although the OR did not exceed 3.08. A graphical depiction as a heat map showing strength of association is presented in an orange panel in Fig 1 and shows the standardized ORs reported in Table 2 that quantify the strength of association between measures germane to TMD and each individual COPC.
Fig 1.

Beta coefficient and standard error (SE) for the linear relationship of each measure with the number of COPCs. The orange panel is a heat map depicting standardized odds ratios (SORs) (reported in Table 2) that quantify the strength of association between measures germane to TMD and each individual COPC. The blue panel is a heat map depicting TMD measure z-score differences according to each COPC (data presented in Appendix 3). For example, the top row depicts the mean pain-free jaw opening z-score difference between cases and controls for each COPC. HA = headache; FM = fibromyalgia; (r) = reverse scoring (negative z scores used for SORs represent increase in odds of being a case associated with reduction of 1 SD in the value of the variable); MM = masticatory muscle; TMQ = Temporomandibular Questionnaire; JFLS = Jaw Functional Limitation Scale; V&E = verbal and emotional expression subscale; TSK = Tampa Scale for Kinesiophobia (activity and somatic subscales); OBC = Oral Behaviors Checklist.
Table 2.
Standardized Odds Ratios (SORs) of Measures Germane to TMD with 95% Confidence Limits (CL) for Individual COPCs, Adjusted for Study Site and Demographic Characteristics
| TMD |
Headache |
|||
|---|---|---|---|---|
| Measure | SOR (95% CL) | P | SOR (95% CL) | P |
| Pain-free jaw opening (r) | 2.43 (1.73, 3.42) | < .001 | 1.38 (1.08, 1.77) | .010 |
| Maximum unassisted jaw opening (r) | 1.61 (1.16, 2.23) | .005 | 1.11 (0.86, 1.43) | .409 |
| Maximum assisted jaw opening (r) | 1.29 (0.95, 1.75) | .099 | 1.08 (0.84, 1.39) | .541 |
| MM pain: Motion | 3.01 (2.00, 4.52) | < .001 | 1.25 (0.96, 1.64) | .103 |
| MM pain: Palpation | 7.68 (5.39, 0.95) | < .001 | 1.69 (1.30, 2.18) | < .001 |
| MM pain: Motion + palpation | 8.74 (5.52, 3.85) | < .001 | 1.56 (1.20, 2.02) | .001 |
| MM familiar pain: Motion | 7.09 (3.47, 4.50) | < .001 | 1.68 (1.23, 2.31) | .001 |
| MM familiar pain: Palpation | 11.43 (6.60, 19.82) | < .001 | 1.94 (1.44, 2.63) | < .001 |
| MM familiar pain: Motion + palpation | 11.95 (7.12, 20.05) | < .001 | 1.88 (1.41, 2.51) | < .001 |
| Neck pain: Palpation | 2.74 (1.89, 3.97) | < .001 | 1.23 (0.96, 1.57) | .105 |
| Body pain: Palpation | 2.92 (1.92, 4.45) | < .001 | 1.27 (0.98, 1.65) | .072 |
| Neck familiar pain: Palpation | 4.21 (2.86, 6.20) | < .001 | 1.69 (1.30, 2.20) | <. 001 |
| Body familiar pain: Palpation | 3.82 (2.30, 6.33) | < .001 | 1.41 (1.05, 1.90) | .023 |
| Nonspecific jaw symptoms | 8.78 (5.33, 14.46) | < .001 | 2.29 (1.68, 3.13) | < .001 |
| TMJ function by history | 3.17 (2.37, 4.23) | < .001 | 1.79 (1.33, 2.42) | < .001 |
| JFLS: Chewing | 2.77 (1.83, 4.20) | < .001 | 1.65 (1.27, 2.16) | < .001 |
| JFLS : Opening | 2.99 (1.96, 4.56) | < .001 | 1.43 (1.06, 1.94) | .020 |
| JFLS: Expressiveness | 1.77 (1.29, 2.45) | < .001 | 1.22 (0.97, 1.54) | .091 |
| JFLS: Global | 3.12 (2.05, 4.76) | < .001 | 1.58 (1.18, 2.11) | .002 |
| TSK-TMD: Activity avoidance | 2.18 (1.53, 3.11) | < .001 | 1.47 (1.12, 1.92) | .005 |
| TSK-TMD: Somatic focus | 2.46 (1.79, 3.40) | < .001 | 1.44 (1.08, 1.93) | .014 |
| OBC: Total | 3.08 (2.10, 4.54) | < .001 | 1.74 (1.30, 2.33) | < .001 |
| IBS |
LBP |
Fibromyalgia |
||||
|---|---|---|---|---|---|---|
| Measure | SOR (95% CL) | P | SOR (95% CL) | P | SOR (95% CL) | P |
| Pain-free jaw opening (r) | 1.35 (1.02, 1.77) | .033 | 1.94 (1.37, 2.73) | < .001 | 3.20 (1.98, 5.16) | < .001 |
| Maximum unassisted jaw opening (r) | 1.08 (0.83, 1.41) | .564 | 1.40 (0.98, 2.01) | .062 | 2.38 (1.34, 4.25) | .003 |
| Maximum assisted jaw opening (r) | 1.02 (0.80, 1.31) | .873 | 1.27 (0.90, 1.79) | .166 | 1.67 (1.00, 2.78) | .049 |
| MM pain: Motion | 1.37 (1.07, 1.76) | .014 | 1.38 (1.08, 1.76) | .011 | 2.34 (1.58, 3.46) | < .001 |
| MM pain: Palpation | 1.93 (1.45, 2.58) | < .001 | 1.66 (1.20, 2.30) | .002 | 6.02 (2.46, 14.74) | < .001 |
| MM pain: Motion + palpation | 1.84 (1.39, 2.45) | < .001 | 1.61 (1.18, 2.21) | .003 | 5.38 (2.15, 13.48) | < .001 |
| MM familiar pain: Motion | 1.44 (1.09, 1.91) | .012 | 1.67 (1.26, 2.22) | < .001 | 2.89 (2.05, 4.08) | < .001 |
| MM familiar pain: Palpation | 1.72 (1.29, 2.29) | < .001 | 1.68 (1.23, 2.31) | .001 | 4.08 (2.53, 6.58) | < .001 |
| MM familiar pain: Motion + palpation | 1.70 (1.28, 2.26) | < .001 | 1.74 (1.28, 2.38) | < .001 | 4.21 (2.59, 6.85) | < .001 |
| Neck pain: Palpation | 1.60 (1.17, 2.19) | .003 | 1.69 (1.18, 2.43) | .004 | 24.52 (4.96, 121.14) | < .001 |
| Body pain: Palpation | 1.48 (1.09, 2.01) | .012 | 1.83 (1.26, 2.65) | .001 | 5.61 (3.50, 8.98) | < .001 |
| Neck familiar pain: Palpation | 1.37 (1.01, 1.87) | .044 | 1.62 (1.18, 2.22) | .003 | 4.05 (2.58, 6.36) | < .001 |
| Body familiar pain: Palpation | 1.33 (0.94, 1.87) | .105 | 2.16 (1.58, 2.95) | < .001 | 3.94 (2.58, 6.00) | < .001 |
| Nonspecific jaw symptoms | 1.55 (1.15, 2.10) | .004 | 1.96 (1.46, 2.62) | < .001 | 3.30 (2.23, 4.88) | < .001 |
| TMJ function by history | 1.32 (0.95, 1.82) | .097 | 2.11 (1.48, 3.01) | < .001 | 2.20 (1.35, 3.59) | .002 |
| JFLS: Chewing | 1.18 (0.92, 1.52) | .184 | 1.50 (1.14, 1.99) | .004 | 2.28 (1.70, 3.06) | < .001 |
| JFLS : Opening | 1.30 (0.99, 1.72) | .060 | 1.46 (1.09, 1.95) | .010 | 1.95 (1.39, 2.73) | < .001 |
| JFLS: Expressiveness | 1.13 (0.91, 1.39) | .266 | 1.22 (0.94, 1.58) | .126 | 1.50 (1.10, 2.04) | .010 |
| JFLS: Global | 1.25 (0.98, 1.59) | .069 | 1.49 (1.12, 1.99) | .006 | 2.06 (1.48, 2.86) | < .001 |
| TSK-TMD: Activity avoidance | 1.19 (0.91, 1.55) | .198 | 1.29 (0.97, 1.71) | .080 | 1.80 (1.20, 2.72) | .005 |
| TSK-TMD: Somatic focus | 1.21 (0.91, 1.62) | .192 | 1.43 (1.05, 1.95) | .024 | 2.00 (1.31, 3.06) | .001 |
| OBC: Total | 1.42 (1.11, 1.82) | .005 | 1.56 (1.18, 2.06) | .002 | 2.13 (1.47, 3.08) | < .001 |
(r) = reverse scoring; MM = masticatory muscle; JFLS = Jaw Functional Limitation Scale; TSK-TMD = Tampa Scale for Kinesiophobia; OBC = Oral Behaviors Checklist.
Univariate Associations Between Number of COPCs and Measures Germane to TMD
Appendix 3 summarizes associations according to number of COPCs. Virtually all 22 measures germane to TMD increased significantly according to the number of COPCs (ie, positive beta coefficients for the linear association), and in many instances, the increase was monotonic (ie, successively greater differences in the contrasts between each COPC group and the reference group with no COPCs).
Of the 10 DC/TMD–specific measures, the one notable exception was maximum assisted jaw opening, where none of the contrasts differed significantly from the group with 0 COPCs. The most pronounced positive relationships with number of COPCs were seen for the six examiner-assessed measures of masticatory muscle pain and for TMJ function by history. Each additional increase in COPC was associated with an approximate 0.4-SD increase for each of the familiar and nonfamiliar masticatory muscle pain provocation reports from palpation alone and from both palpation and motion.
Of the 12 TMD-relevant measures, the OBC measure of parafunctional behaviors increased monotonically according to the number of COPCs. Other monotonic increases were two of the four examiner-assessed measures of neck and body. Other notable associations with number of COPCs were seen for measures of jaw functional limitation, jaw mobility, and kinesiophobia (Fig 1 and Appendix 3).
Figure 2 shows plots for the linear association between the number of COPCs, modeled as a continuous predictor, and six selected measures germane to TMD. A critical threshold of ≥ 3 comorbid conditions was apparent between the number of COPCs and each outcome measure. Appendix 4 summarizes associations according to the presence of the non-TMD COPCs (that is, for a count of 0 to 4 COPCs). Virtually all 22 measures germane to TMD increased significantly according to the number of COPCs (ie, positive beta coefficients for the linear association), and, in many instances, the increase was monotonic (ie, successively greater differences in the comparisons between each COPC group and the reference group with no COPCs).
Fig 2.

Relationships between number of pain conditions and measures germane to TMD in the OPPERA-2 study (n = 655 participants). (a) Pain-free opening. (b) Jaw muscle pain on palpation. (c) Neck muscle pain on palpation. (d) Nonspecific jaw symptoms. (e) Jaw Functional Limitation Scale global score. (f) Oral Behaviors Checklist. Each TMD measure was the dependent variable in separate linear regression models that used weighted estimates from generalized estimating equations with robust error variance calculation and adjustments for study site, age, gender, and race. Each plot summarizes three separate regression models for the same dependent variable. (1) The number of COPCs was modeled as a dummy predictor. Vertical axis plots estimated the mean value of the selected TMD variable (transformed to a z-score) as the dependent variable and the dummy variable for the number of COPCs (5 degrees of freedom) as a categorical predictor. The model was adjusted for study site, age, gender, and race. Whiskers signify ± 1 standard error (SE). (2) Beta (β) estimate (SE) represents the amount of change in the same dependent variable as in (1) for each increase in the number of COPCs, modeled as a continuous variable. The model was adjusted for study site, age, gender, and race. aP < .05 for the test of the null hypothesis that β = 0. (3) Each COPC was modeled as an independent binary predictor to show independent associations of each COPC. The numbers in the tables are parameter estimates for the corresponding dummy variables for the COPCs, denoted as T = temporomandibular disorder, H = headache, I = IBS, B = low back pain, and F = fibromyalgia. The model adjusted for study site, age, gender, and race. bP < .05 for the null hypothesis that the parameter estimate for the dummy variable = 0.
Multivariable Associations Between Independent COPCs and Measures Germane to TMD
Independent associations of COPCs with the variables germane to TMD are presented in Table 3 and summarized graphically in Fig 1 (blue panel). Of the 22 measures, painful TMD was independently associated with 20 variables, headache with 2, IBS with 2, LBP with 2, and fibromyalgia with 13.
Table 3.
Independent Associations of Each COPC with Standardized Mean Measures Germane to TMD Adjusted for Other COPCs, Study Site, and Demographics
| Measure | TMD | Headache | IBS | LBP | Fibromyalgia |
|---|---|---|---|---|---|
| Pain-free jaw opening (r) | 0.49 (0.14), < .01 | 0.13 (0.10), .19 | 0.03 (0.10), .76 | 0.26 (0.18), .15 | 0.75 (0.25), < .01 |
| Maximum unassisted jaw opening (r) | 0.27 (0.15), .08 | 0.01 (0.11), .91 | −0.03 (0.11), .77 | 0.23 (0.19), .21 | 0.58 (0.27), .03 |
| Maximum assisted jaw opening (r) | 0.13 (0.14), .36 | 0.03 (0.11), .77 | −0.05 (0.11), .64 | 0.21 (0.19), .26 | 0.49 (0.27), .07 |
| MM pain: Motion | 0.86 (0.17), < .01 | 0.00 (0.11), .98 | 0.08 (0.12), .47 | 0.03 (0.11), .81 | 0.40 (0.20), .05 |
| MM pain: Palpation | 1.17 (0.13), < .01 | 0.16 (0.10), .13 | 0.30 (0.13), .03 | 0.00 (0.10), 1.00 | 0.54 (0.16), < .01 |
| MM pain: Motion + palpation | 1.19 (0.11), < .01 | 0.12 (0.11), .28 | 0.28 (0.13), .03 | 0.03 (0.10), .74 | 0.41 (0.17), .01 |
| MM familiar pain: Motion | 1.08 (0.18), < .01 | 0.09 (0.09), .29 | 0.02 (0.11), .85 | 0.02 (0.10), .83 | 0.53 (0.21), .01 |
| MM familiar pain: Palpation | 1.32 (0.16), < .01 | 0.15 (0.08), .06 | 0.12 (0.11), .27 | −0.09 (0.08), .29 | 0.62 (0.23), < .01 |
| MM familiar pain: Motion + palpation | 1.35 (0.14), < .01 | 0.14 (0.08), .08 | 0.12 (0.11), .28 | −0.03 (0.09), .72 | 0.54 (0.22), .01 |
| Neck pain: Palpation | 0.48 (0.12), < .01 | 0.02 (0.11), .86 | 0.22 (0.12), .06 | 0.14 (0.15), .38 | 0.54 (0.15), < .01 |
| Body pain: Palpation | 0.58 (0.16), < .01 | 0.00 (0.09), .99 | 0.11 (0.10), .28 | 0.13 (0.14), .35 | 0.81 (0.16), < .01 |
| Neck familiar pain: Palpation | 0.97 (0.18), < .01 | 0.18 (0.10), .06 | 0.00 (0.10), .99 | 0.00 (0.11), .97 | 0.75 (0.19), < .01 |
| Body familiar pain: Palpation | 0.80 (0.20), < .01 | −0.01 (0.10), .94 | −0.04 (0.10), .70 | 0.25 (0.11), .03 | 1.05 (0.22), < .01 |
| Nonspecific jaw symptoms | 1.18 (0.12), < .01 | 0.30 (0.07), < .01 | 0.03 (0.09), .73 | 0.05 (0.08), .58 | 0.41 (0.22), .07 |
| TMJ function by history | 0.67 (0.14), < .01 | 0.18 (0.09), .05 | −0.01 (0.09), .95 | 0.31 (0.13), .02 | 0.15 (0.21), .49 |
| JFLS: Chewing | 0.71 (0.13), < .01 | 0.20 (0.10), .05 | −0.06 (0.08), .44 | 0.06 (0.10), .57 | 0.53 (0.22), .01 |
| JFLS: Opening | 0.75 (0.14), < .01 | 0.05 (0.09), .60 | 0.05 (0.09), .55 | 0.09 (0.09), .32 | 0.32 (0.24), .17 |
| JFLS: Expressiveness | 0.38 (0.11), < .01 | 0.03 (0.06), .68 | 0.00 (0.06), .98 | 0.03 (0.08), .73 | 0.34 (0.22), .13 |
| JFLS: Global | 0.72 (0.13), < .01 | 0.11 (0.08), .18 | 0.00 (0.07), 1.00 | 0.07 (0.09), .42 | 0.45 (0.23), .05 |
| TSK-TMD: Activity avoidance | 0.72 (0.20), < .01 | 0.21 (0.13), .10 | −0.01 (0.13), .95 | −0.09 (0.14), .52 | 0.20 (0.28), .48 |
| TSK-TMD: Somatic focus | 0.72 (0.14), < .01 | 0.15 (0.10), .16 | −0.03 (0.10), .75 | −0.05 (0.13), .72 | 0.38 (0.29), .20 |
| OBC: Total | 0.81 (0.19), < .01 | 0.31 (0.12), < .01 | 0.12 (0.10), .21 | 0.05 (0.11), .65 | 0.18 (0.19), .34 |
Data are reported as estimated mean difference (standard error), P. (r) = reverse scoring; MM = masticatory muscle; JFLS = Jaw Functional Limitation Scale; TSK-TMD = Tampa Scale for Kinesiophobia; OBC = Oral Behaviors Checklist.
There were significant independent associations with painful TMD for all DC/TMD–specific measures except for maximum assisted and unassisted opening. Similarly, after adjusting for the other four COPCs, fibromyalgia was also associated with 8 out of the 10 DC/TMD–specific measures, but not with maximum assisted opening or masticatory pain from motion. With regard to the other COPCs, very few to none of the DC/TMD–specific measures were significantly associated with IBS, LBP, or headache, while IBS was associated with two measures (masticatory pain on palpation and with motion), and LBP was significantly associated with only one measure (TMJ function by history). Headache was not significantly associated with any of the 10 DC/TMD–specific measures.
For the 12 TMD-relevant measures, all were significantly associated with painful TMD. Fibromyalgia was independently associated with 5 TMD-relevant measures, including neck and body pain and jaw functional limitation associated with chewing. As for the other COPCs, headache was independently associated with nonspecific jaw symptoms and oral parafunctional behaviors, LBP was associated with familiar body pain evoked by palpation, and IBS was not independently associated with any of the 12 TMD-related measures.
Results from Fig 2 show the independent associations of each COPC, modeled as binary dummy predictors, with each of the six standardized TMD measures, adjusted for study site and demographics, using regression models. In the multivariable analysis, TMD, headache, and fibromyalgia were each significantly (P < .05) associated with higher values of at least one of the six outcome measures of nonspecific jaw symptoms, jaw overuse behavior, jaw functional limitation, jaw mobility, and pain on palpation of masticatory or neck muscles. Among the COPCs, TMD was the only comorbid COPC independently associated with all six outcome measures.
Overall, the results as depicted in Fig 1 indicate high variability across the COPCs regarding the magnitude of the associations between the measures germane to TMD and the COPCs. For example, when COPCs are assessed collectively as depicted on the left panel, the positive beta coefficients suggest that all 22 measures germane to TMD increased significantly according to the number of COPCs. When each individual COPC was assessed in univariate models, the orange heat map in the middle panel depicts that a majority of the measures germane to TMD were associated, with an OR of at least 2, with each COPC. As indicated by the orange color gradient, measures of masticatory muscle pain evoked by palpation, by motion, and of nonspecific jaw symptoms had the strongest magnitudes of association with TMD and fibromyalgia each. Similarly, when the COPCs were assessed independently in the multivariable models, the blue heat map in the right panel readily depicts that, aside from TMD and fibromyalgia, COPCs were strongly associated with only a few of the TMD-relevant measures. Yet, the blue heat map does indicate a general hypothesis-relevant pattern that, besides painful TMD, the COPCs had independent associations with measures germane to TMD.
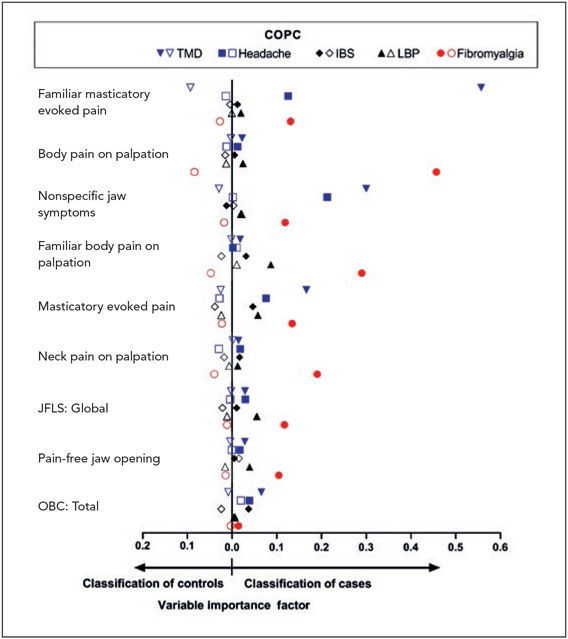
Random Forest Plots Using Measures Germane to TMD to Predict Each COPC
When all 22 TMD-specific measures were assessed for their multivariable associations with TMD using a random forest model, the most important predictors were familiar and nonfamiliar masticatory evoked pain and nonspecific jaw symptoms, as indicated by variable importance scores of 0.1 or more (plotted in Fig 3 with blue triangles). Using that same threshold, 8 TMD-specific measures differentiated fibromyalgia cases from controls, while oral parafunctional behaviors did not (Fig 3, red circles). For headache, nonspecific jaw symptoms and masticatory familiar pain were the most important predictors. In contrast, for LBP and IBS, none of the TMD-specific measures were important predictors. Variables with low importance in discriminating cases from controls included masticatory evoked familiar pain (for IBS and LBP), nonspecific jaw symptoms (IBS), familiar body pain evoked by palpation (LBP, TMD, and headache), neck pain evoked by palpation (TMD), pain-free opening (headache and IBS), and oral behaviors (headache). Indices of model fit are provided in Appendix 5.
Fig 3.
Multivariable associations of TMD measures with pain conditions in OPPERA-2 (n = 655 participants). Random forest modeling explored the multivariable associations of all TMD measures with each binary COPC case classification, with study site, age, gender, and race also included as covariates. Associations of individual variables in the random forest models were quantified using variable importance scores, which estimate the relative association of each predictor to the model’s classification of true positives and true negatives. Other measures germane to TMD were included in the models but are excluded from the figure due to negligible variable importance scores. The threshold for exclusion from the figure was set to 0.0004 in order to ensure a clear, concise plot. A variable importance score < 0.0004 means that in the presence of all of the other measures included in the random forest model, these TMD measures improved the misclassification error rate by less than 0.04 percentage points. Filled symbols = COPC cases; open symbols = controls; JFLS = Jaw Functional Limitation Scale; OBC = Oral Behaviors Checklist.
Discussion
This study investigated 22 measures germane to TMD, including measures from the DC/TMD examination procedures as well as other TMD-relevant measures because they represent domains with high face validity: jaw function, pain in masticatory tissues, nonspecific jaw symptoms, jaw kinesiophobia, and oral behaviors. As expected, virtually all were significantly associated with the odds of having painful TMD, with ORs signifying moderate to strong effect estimates. Unexpectedly, there were significant associations with the other four COPCs, although the effect estimates were less pronounced. Furthermore, in multivariable models that adjusted for the effect of painful TMD and the other COPCs, fibromyalgia was associated independently with most of the 22 measures germane to TMD. The multivariable models also revealed independent associations of headache, IBS, and LBP with a few of the 22 TMD measures. When the measures were separated into the two sets of DC/TMD–specific and TMD-relevant measures, both univariate and multivariable analyses showed that most measures from both sets were associated with painful TMD and fibromyalgia. When the other COPCs were considered agnostically as a count of the total number of COPCs, there was a positive monotonic association with each of the 22 TMD measures. After excluding painful TMD, the association between the other 1 to 4 COPCs and the 22 TMD measures showed a similar linear association, as when painful TMD was included in the count of COPCs. In summary, most of the 22 TMD measures displayed patterns of association with other COPCs. Moreover, the analyses of each index COPC and of the number of COPCs (with and without painful TMD) indicate that the 22 TMD measures are not as condition-specific, as often assumed irrespective of whether they are separated into DC/TMD–specific and TMD-relevant measures.
Findings from this study reject the assumption that attributes germane to TMD are elevated only in painful TMD cases relative to non-TMD subjects. Nevertheless, the magnitude of association was higher in those with painful TMD. The multivariable analyses highlight that, independent from the associations demonstrated by painful TMD, the association of fibromyalgia with measures germane to TMD was nearly twice the magnitude of that for painful TMD alone for 4 of the 22 measures germane to TMD: pain-free opening (painful TMD = 0.49; fibromyalgia = 0.75), maximum unassisted opening (painful TMD = 0.27; fibromyalgia = 0.58), maximum assisted opening (painful TMD = 0.13; fibromyalgia = 0.49), and body pain on palpation (painful TMD = 0.58; fibromyalgia = 0.81). Not only are the 22 measures germane to TMD not specific to a painful TMD diagnosis, but these 22 TMD measures may represent markers of a functional pain syndrome beyond their seeming relevance to only painful TMD.
The implications of these findings are of clinical relevance when diagnosing and classifying painful TMD. For painful TMD characteristics that are used as criteria in validated diagnostic systems, the implications are troubling. For example, palpation-evoked pain in masticatory muscles represents one criterion for classifying TMD myalgia in the DC/TMD system, yet the current findings reveal that fibromyalgia and IBS are both associated with the extent of palpation-evoked masticatory muscle pain, independently of TMD itself. While palpation-evoked pain in masticatory muscles is generally assumed to be TMD-specific, this finding is especially troubling because it suggests that the DC/TMD’s criterion for masticatory pain from clinical provocation could be fulfilled in the presence of fibromyalgia or IBS, and not necessarily by TMD. However, any such concern is mitigated by the fact that palpation-evoked masticatory muscle pain is a necessary, though not sufficient, criterion for classification of painful TMD. Specifically, the DC/TMD criteria also require that the evoked pain be familiar to previously experienced painful TMD symptoms and that the pain be changed by jaw function. Furthermore, the masticatory muscle pain criterion can be fulfilled by pain evoked either by palpation or by jaw maneuvers. Nonetheless, the observed associations of fibromyalgia and IBS with palpation-evoked masticatory muscle pain draw attention to the importance of a thorough examination of all regional pain systems when diagnosing painful TMD. Indeed, the current results endorse the value of a rigorous and holistic examination when classifying painful TMD. Equally important, the results refute the premise that diagnostic devices measuring characteristics of painful TMD (eg, joint vibration analysis, precision jaw tracking) might be sufficient for classifying painful TMD. Indeed, the current findings may be part of the reason that diagnostic devices perform poorly in classifying clinical painful TMD.40,41
Another noteworthy finding concerns masticatory muscle pain evoked by palpation. Compared to pain evoked by motion, the magnitude of association of masticatory muscle pain evoked by palpation with each COPC and the number of COPCs was stronger. Similar results were found by Ohrbach et al42 with chronic TMD cases compared to TMD controls, but not for predicting first-onset TMD.27 Pain evoked by palpation vs motion vis-à-vis other COPCs raises a question as to why stronger associations of palpation scores are seen with TMD and other COPCs than with pain evoked by motion. Weaker associations with mobility may either reflect the absence of structural damage (no variance to be explained; that is, muscles are normal from a structural perspective, and so pain evoked by movement is less likely) or reflect increased adaptability of the jaw despite pain; that is, motivation to function (to eat and talk). An implication is that the impact of motivation to function with the system must not be underestimated. Alternatively, pain evoked by motion may be a region-specific measure, whereas pain evoked by palpation may be a more global measure of hyperalgesia; that is, an increased response to a suprathreshold stimulus that normally evokes pain and is not limited to the specific tissue. It is also possible that pain from palpation is much more local, focused, and nociceptive than pain from motion, which may be modulated by controlled movement once an aversive signal is felt.
In addition, and most notably, the extent of overlapping pain conditions is not only associated with pain and function, but extends its influence beyond the boundaries of physical symptoms: COPCs also influence attitudes, beliefs, and behaviors ostensibly specific to painful TMD or the masticatory system. The diagnostic and behavioral overlap between headache and painful TMD is considerable,43-45 and among comorbid pain conditions, headache has the strongest association with chronic painful TMD (OR = 8.8).42 It seems self-evident that headache should reliably and substantially influence measures germane to TMD, including the DC/TMD–specific measures; however, among COPCs, headache exerted the least influence on any of the measures germane to TMD, despite 75% of this group having some type of migraine headache, which has a strong relationship with TMD itself.46 However, given these results, as well as the concurrent findings from Slade et al (current issue) that show 49% of headache cases occurred in isolation (that is, with no overlap with the other pain conditions), it appears that headache and painful TMD appear to be more independent despite being proximal pain conditions. It may be that a simple headache-TMD relationship is transcended by painful TMD being part of a functional pain condition (see Slade et al, current issue), consistent with the strong associations between each measure germane to TMD and fibromyalgia, which is currently regarded as the exemplar for functional pain disorders and was associated with 16 of the 22 measures germane to TMD. While these findings may not be a surprise given that 75% of patients with fibromyalgia will have TMD, only 18% of patients with TMD will have fibromyalgia,47 such that the number of associations with fibromyalgia is surprising. This may help explain the difference between the 75% when considering fibromyalgia vs 18% when considering TMD.
The 22 TMD measures represent a range of domains: physical examination measures, limitations, beliefs, and behaviors, each germane to the character of TMD as a localized condition. Yet, 21 of these 22 measures were associated with at least one other COPC in addition to TMD. The findings regarding pain-free jaw opening are particularly noteworthy because it is measured reliably29 and is generally considered to be a relatively pure measure of jaw functional status. Because pain-free jaw opening, measured as “open as wide as you can without any pain,” is considered to be a pain-dependent measure and because fear of inducing pain may lead the patient to not open as far as they could without pain, any factors that affect pain processing (eg, pain-related fear, anxiety)48 would be expected to affect a pain-free opening measure. Yet, the overlap of other COPCs with the jaw mobility measures signifies the ubiquity of pain processing and the impact of the multiple central nervous system influences on potentially all aspects of pain measures. The association of fibromyalgia, for example, with the measured extent of simple jaw opening (estimated as β = 0.75) highlights that pain-free extent of opening only partially reflects jaw status. For example, in an experimental trial of intraoral appliances for TMD myofascial pain, the presence of fibromyalgia significantly affected treatment response to the appliance, with the conclusion that comorbidities matter.49 These findings suggest an additional interpretation: Measured outcomes of TMD pain and function may have been equally affected by the presence of fibromyalgia, raising the question of whether the comorbid condition (fibromyalgia) affected the outcome, the measurement of the outcome (for, say, chewing), or both. Both effects would occur in the same direction.
Among other noteworthy findings is the measure of nonspecific jaw symptoms. Univariate results reveal associations of all COPCs with nonspecific jaw symptoms, random forest plots show that nonspecific jaw symptoms predict painful TMD and headache cases from their respective controls, and multivariable analyses confirm independent influences of painful TMD and headache on this measure. The above findings on nonspecific jaw symptoms parallel findings that pain conditions and their overlaps were associated with greater somatic symptom burden (ie, the Pennebaker Inventory of Limbic Languidness [PILL] and Symptom Checklist (SCL)-90 Somatization Scale), as concurrently shown in Fillingim et al (current issue). Consequently, nonspecific jaw symptoms, a regional measure, appear to respond in the same way as measures not tied to any single part of the body. This suggests that nonspecific jaw symptoms tap into a more global level of pain processing, as well as dimensions beyond pain.
As in all cross-sectional studies, the current study was unable to determine whether TMD-specific characteristics were present before or after the onset of COPCs. While prior OPPERA publications have shown associations between TMD measures and first-onset TMD,27,42 in this study, the TMD measures are a reflection of underlying pain processing/burden, which in turn could affect the threshold by which an individual will meet the diagnostic criteria for a given disorder. However, future studies still need to explain the natural history and evolution of TMD symptoms in individuals with multiple overlapping pain conditions. Furthermore, external validity of these findings is compromised, as the individuals recruited for the study were a convenience sample that may not be representative of the general population. There was limited statistical power for comparing individuals with fibromyalgia or with five COPCs due to the small sample sizes in these respective groups.
Conclusions
In this study, COPCs were associated with many attributes generally assumed to be germane to only painful TMD. Yet, both univariate and multivariable analyses demonstrated that measures of pain-free opening, masticatory muscle palpation, and nonspecific jaw symptoms were the TMD measures most strongly associated with COPCs other than painful TMD. The implications of these findings are considerable for making a “simple localized” clinical pain diagnosis vs a “complex multisystem” pain diagnosis. Painful TMD is seldom an isolated condition, and these findings further highlight the importance of considering assessment of other chronic pain conditions, which will help better understand the functional syndromic nature of painful TMD. Management of painful TMD should be approached from an integrated pain-processing model, which in turn determines selection of specific interventions and treatments for specific pain conditions. Thus, these findings reinforce the value of a rigorous and holistic clinical examination. If any of the other pain conditions were present, the patient could then benefit from specific interventions for the overall pain disorder as well as for painful TMD, which would be well-reflected in the measures germane to TMD.
Acknowledgments
This work was supported by National Institutes of Health grants U01DE017018 (NIDCR) and UL1TR001427 (NCATS). The OPPERA program also acknowledges resources provided for this project by the participating institutions: University at Buffalo; University of Florida; University of Maryland; and University of North Carolina. Dr Fillingim has equity ownership in Algynomics Inc. The authors have nothing else to disclose.
Appendices
Appendix 1.
Unadjusted, Unweighted Estimates for Measures Germane to TMD for Cases of the Five COPCs and Controls
| TMD |
Headache |
|||
|---|---|---|---|---|
| Measure | Case | Control | Case | Control |
| Pain-free jaw opening | 35.52 (0.84), 182 | 45.13 (0.39), 472 | 39.32 (0.67), 269 | 44.64 (0.46), 385 |
| Maximum unassisted jaw opening | 47.33 (0.67), 182 | 50.73 (0.33), 472 | 48.51 (0.50), 269 | 50.68 (0.39), 385 |
| Maximum assisted jaw opening | 51.38 (0.61), 182 | 53.25 (0.34), 471 | 51.79 (0.46), 269 | 53.39 (0.39), 384 |
| MM pain: Motion | 3.11 (0.14), 182 | 1.00 (0.06), 473 | 2.19 (0.12), 270 | 1.16 (0.08), 385 |
| MM pain: Palpation | 6.04 (0.15), 182 | 1.92 (0.11), 473 | 4.20 (0.18), 270 | 2.27 (0.13), 385 |
| MM pain: Motion + palpation | 6.40 (0.12), 182 | 2.43 (0.11), 473 | 4.60 (0.18), 270 | 2.78 (0.13), 385 |
| MM familiar pain: Motion | 2.87 (0.14), 182 | 0.30 (0.04), 473 | 1.77 (0.13), 270 | 0.48 (0.06), 385 |
| MM familiar pain: Palpation | 5.16 (0.18), 182 | 0.61 (0.06), 473 | 3.22 (0.19), 270 | 0.93 (0.10), 385 |
| MM familiar pain: Motion + palpation | 5.55 (0.16), 182 | 0.73 (0.07), 473 | 3.49 (0.19), 270 | 1.07 (0.10), 385 |
| Neck pain: Palpation | 5.02 (0.10), 182 | 3.60 (0.09), 473 | 4.36 (0.11), 270 | 3.74 (0.10), 385 |
| Body pain: Palpation | 6.80 (0.26), 182 | 3.37 (0.15), 473 | 5.30 (0.24), 270 | 3.63 (0.18), 385 |
| Neck familiar pain: Palpation | 3.38 (0.17), 182 | 0.83 (0.07), 473 | 2.41 (0.14), 270 | 0.92 (0.09), 385 |
| Body familiar pain: Palpation | 3.90 (0.27), 182 | 0.72 (0.07), 473 | 2.49 (0.20), 270 | 0.98 (0.10), 385 |
| Nonspecific jaw symptoms | 3.51 (0.13), 178 | 0.59 (0.05), 446 | 2.42 (0.13), 262 | 0.70 (0.07), 362 |
| TMJ function by history | 2.89 (0.14), 166 | 0.82 (0.06), 427 | 2.09 (0.12), 251 | 0.89 (0.07), 342 |
| JFLS: Chewing | 1.83 (0.13), 178 | 0.47 (0.05), 454 | 1.29 (0.10), 265 | 0.54 (0.06), 367 |
| JFLS: Opening | 1.89 (0.16), 178 | 0.33 (0.05), 454 | 1.23 (0.12), 265 | 0.43 (0.06), 367 |
| JFLS: Expressiveness | 0.72 (0.10), 178 | 0.17 (0.04), 454 | 0.48 (0.07), 265 | 0.21 (0.05), 367 |
| JFLS: Global | 1.48 (0.11), 178 | 0.32 (0.04), 454 | 1.00 (0.08), 265 | 0.39 (0.05), 367 |
| TSK-TMD: Activity avoidance | 13.14 (0.36), 178 | 9.18 (0.15), 466 | 11.61 (0.29), 268 | 9.32 (0.18), 376 |
| TSK-TMD: Somatic focus | 9.19 (0.27), 178 | 5.98 (0.10), 466 | 7.92 (0.22), 268 | 6.11 (0.12), 376 |
| OBC: Total | 31.88 (0.82), 180 | 19.74 (0.46), 457 | 27.60 (0.71), 267 | 19.96 (0.54), 370 |
| IBS |
LBP |
Fibromyalgia |
||||
|---|---|---|---|---|---|---|
| Measure | Case | Control | Case | Control | Case | Control |
| Pain-free jaw opening | 39.85 (0.88), 157 | 43.27 (0.44), 497 | 38.40 (1.00), 139 | 43.55 (0.42), 515 | 30.08 (1.55), 52 | 43.52 (0.38), 602 |
| Maximum unassisted jaw opening | 49.22 (0.68), 157 | 49.96 (0.35), 497 | 48.20 (0.73), 139 | 50.21 (0.34), 515 | 45.23 (1.33), 52 | 50.18 (0.31), 602 |
| Maximum assisted jaw opening | 52.64 (0.61), 157 | 52.76 (0.34), 496 | 51.66 (0.65), 139 | 53.02 (0.34), 514 | 49.63 (1.24), 52 | 53.00 (0.30), 601 |
| MM pain: Motion | 2.09 (0.15), 158 | 1.42 (0.08), 497 | 2.45 (0.18), 139 | 1.35 (0.07), 516 | 3.63 (0.33), 52 | 1.41 (0.07), 603 |
| MM pain: Palpation | 4.28 (0.23), 158 | 2.68 (0.13), 497 | 4.62 (0.25), 139 | 2.65 (0.12), 516 | 6.79 (0.27), 52 | 2.74 (0.11), 603 |
| MM pain: Motion + palpation | 4.70 (0.22), 158 | 3.16 (0.13), 497 | 4.98 (0.24), 139 | 3.14 (0.12), 516 | 6.88 (0.26), 52 | 3.24 (0.11), 603 |
| MM familiar pain: Motion | 1.54 (0.15),158 | 0.85 (0.07), 497 | 1.99 (0.19), 139 | 0.75 (0.06), 516 | 3.46 (0.32), 52 | 0.80 (0.06), 603 |
| MM familiar pain: Palpation | 2.95 (0.24), 158 | 1.54 (0.11), 497 | 3.33 (0.26), 139 | 1.48 (0.11), 516 | 5.71 (0.37), 52 | 1.55 (0.10), 603 |
| MM familiar pain: Motion + palpation | 3.21 (0.24), 158 | 1.71 (0.12), 497 | 3.62 (0.27), 139 | 1.65 (0.11), 516 | 5.98 (0.34), 52 | 1.73 (0.10), 603 |
| Neck pain: Palpation | 4.52 (0.13), 158 | 3.82 (0.09), 497 | 4.54 (0.15), 139 | 3.84 (0.08), 516 | 5.81 (0.08), 52 | 3.84 (0.08), 603 |
| Body pain: Palpation | 5.27 (0.30), 158 | 4.02 (0.16), 497 | 5.76 (0.33), 139 | 3.93 (0.16), 516 | 9.10 (0.33), 52 | 3.91 (0.14), 603 |
| Neck familiar pain: Palpation | 2.26 (0.19), 158 | 1.31 (0.09), 497 | 2.59 (0.20), 139 | 1.26 (0.09), 516 | 4.37 (0.29), 52 | 1.30 (0.08), 603 |
| Body familiar pain: Palpation | 2.48 (0.26), 158 | 1.33 (0.11), 497 | 3.17 (0.29), 139 | 1.18 (0.10), 516 | 6.08 (0.49), 52 | 1.22 (0.09), 603 |
| Nonspecific jaw symptoms | 2.00 (0.16) ,152 | 1.24 (0.08), 472 | 2.36 (0.19), 134 | 1.17 (0.08), 490 | 3.90 (0.26), 52 | 1.20 (0.07), 572 |
| TMJ function by history | 1.85 (0.15), 140 | 1.26 (0.08), 453 | 2.05 (0.17), 126 | 1.22 (0.07), 467 | 2.92 (0.27), 50 | 1.26 (0.07), 543 |
| JFLS: Chewing | 1.04 (0.12), 152 | 0.80 (0.06), 480 | 1.50 (0.15), 134 | 0.68 (0.06), 498 | 2.24 (0.23), 52 | 0.73 (0.05), 580 |
| JFLS: Opening | 1.12 (0.15), 152 | 0.66 (0.07), 480 | 1.43 (0.18), 134 | 0.59 (0.06), 498 | 2.26 (0.32), 52 | 0.63 (0.06), 580 |
| JFLS: Expressiveness | 0.42 (0.09), 152 | 0.29 (0.04), 480 | 0.64 (0.11), 134 | 0.24 (0.04), 498 | 0.88 (0.19), 52 | 0.27 (0.04), 580 |
| JFLS: Global | 0.86 (0.11), 152 | 0.58 (0.05), 480 | 1.19 (0.13), 134 | 0.50 (0.05), 498 | 1.79 (0.21), 52 | 0.55 (0.04), 580 |
| TSK-TMD: Activity avoidance | 11.03 (0.35), 156 | 10.03 (0.18), 488 | 11.71 (0.42), 137 | 9.89 (0.17), 507 | 13.94 (0.70), 52 | 9.95 (0.16), 592 |
| TSK-TMD: Somatic focus | 7.56 (0.28), 156 | 6.65 (0.13), 488 | 8.00 (0.31), 137 | 6.56 (0.12), 507 | 9.35 (0.50), 52 | 6.65 (0.12), 592 |
| OBC: Total | 26.64 (0.95), 156 | 22.04 (0.51), 481 | 27.06 (1.10), 136 | 22.11 (0.49), 501 | 31.84 (1.69), 52 | 22.40 (0.46), 585 |
Data are reported as mean (standard error), number of participants. MM = masticatory muscle; JFLS = Jaw Functional Limitation Scale; TSK-TMD = Tampa Scale for Kinesiophobia; OBC = Oral Behaviors Checklist.
Appendix 2.
Unadjusted, Unweighted Estimates According to Number of COPCs
| No. of COPCs | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Pain-free jaw opening | 45.85 (0.52), 252 | 44.24 (0.67), 178 | 40.09 (0.91), 108 | 37.32 (1.41), 71 | 31.85 (1.81), 33 | 25.17 (3.02), 12 |
| Maximum unassisted jaw opening | 51.00 (0.47), 252 | 50.36 (0.55), 178 | 49.05 (0.73), 108 | 48.24 (1.06), 71 | 45.48 (1.46), 33 | 43.42 (3.73), 12 |
| Maximum assisted jaw opening | 53.43 (0.50), 251 | 53.12 (0.52), 178 | 52.38 (0.67), 108 | 52.13 (0.97), 71 | 48.61 (1.42), 33 | 50.33 (2.96), 12 |
| MM pain: Motion | 0.85 (0.08), 252 | 1.29 (0.12), 178 | 2.00 (0.15), 109 | 2.85 (0.24), 71 | 3.79 (0.37), 33 | 4.08 (0.69), 12 |
| MM pain: Palpation | 1.60 (0.14), 252 | 2.40 (0.20), 178 | 4.30 (0.26), 109 | 5.44 (0.28), 71 | 6.97 (0.24), 33 | 7.67 (0.22), 12 |
| MM pain: Motion + palpation | 2.13 (0.15), 252 | 2.94 (0.20), 178 | 4.65 (0.25), 109 | 5.92 (0.26), 71 | 7.09 (0.21), 33 | 7.75 (0.18), 12 |
| MM familiar pain: Motion | 0.17 (0.05), 252 | 0.57 (0.10), 178 | 1.59 (0.16), 109 | 2.54 (0.24), 71 | 3.58 (0.38), 33 | 4.00 (0.70), 12 |
| MM familiar pain: Palpation | 0.33 (0.06), 252 | 1.22 (0.15), 178 | 3.03 (0.27), 109 | 4.49 (0.32), 71 | 5.85 (0.40), 33 | 7.17 (0.37), 12 |
| MM familiar pain: Motion + palpation | 0.42 (0.07), 252 | 1.35 (0.16), 178 | 3.39 (0.27), 109 | 4.97 (0.31), 71 | 6.06 (0.38), 33 | 7.25 (0.33), 12 |
| Neck pain: Palpation | 3.41 (0.12), 252 | 3.82 (0.14), 178 | 4.49 (0.16), 109 | 4.76 (0.17), 71 | 5.45 (0.16), 33 | 5.75 (0.18), 12 |
| Body pain: Palpation | 3.06 (0.21), 252 | 3.56 (0.25), 178 | 5.74 (0.35), 109 | 5.97 (0.46), 71 | 7.85 (0.52), 33 | 9.67 (0.58), 12 |
| Neck familiar pain: palpation | 0.53 (0.08), 252 | 1.11 (0.13), 178 | 2.45 (0.22), 109 | 3.08 (0.28), 71 | 3.85 (0.33), 33 | 5.25 (0.30), 12 |
| Body familiar pain: palpation | 0.48 (0.08), 252 | 0.92 (0.12), 178 | 2.61 (0.30), 109 | 3.18 (0.43), 71 | 4.76 (0.59), 33 | 8.17 (0.89), 12 |
| Nonspecific jaw symptoms | 0.36 (0.06), 232 | 0.92 (0.10), 174 | 2.42 (0.18), 106 | 3.00 (0.25), 68 | 4.09 (0.33), 32 | 4.58 (0.38), 12 |
| TMJ function by history | 0.63 (0.07), 225 | 1.04 (0.11), 164 | 2.33 (0.18), 97 | 2.53 (0.23), 65 | 2.89 (0.36), 30 | 3.43 (0.54), 12 |
| JFLS: Chewing | 0.42 (0.07), 238 | 0.64 (0.09), 174 | 0.96 (0.13), 108 | 1.66 (0.21), 69 | 2.51 (0.30), 31 | 2.81 (0.53) n = 12 |
| JFLS: Opening | 0.31 (0.07), 238 | 0.43 (0.08), 174 | 0.86 (0.14), 108 | 1.94 (0.26), 69 | 2.23 (0.40), 31 | 3.38 (0.76), 12 |
| JFLS: Expressiveness | 0.22 (0.07), 238 | 0.15 (0.04), 174 | 0.26 (0.06), 108 | 0.59 (0.16), 69 | 1.10 (0.29), 31 | 1.80 (0.54), 12 |
| JFLS: Global | 0.32 (0.06), 238 | 0.40 (0.06), 174 | 0.70 (0.10), 108 | 1.39 (0.18), 69 | 1.95 (0.28), 31 | 2.66 (0.47), 12 |
| TSK-TMD: Activity avoidance | 8.92 (0.20), 246 | 9.57 (0.28), 175 | 11.50 (0.42), 109 | 11.93 (0.53), 70 | 14.97 (0.93), 32 | 14.92 (1.42), 12 |
| TSK-TMD: Somatic focus | 5.89 (0.13), 246 | 6.18 (0.18), 175 | 7.62 (0.31), 109 | 8.76 (0.45), 70 | 9.59 (0.64), 32 | 11.83 (1.05), 12 |
| OBC: Total | 17.97 (0.60), 238 | 22.00 (0.80), 177 | 27.34 (0.96), 108 | 30.79 (1.40), 70 | 33.09 (2.06), 32 | 34.95 (4.26), 12 |
Data are reported as mean (standard error), number of participants. MM = masticatory muscle; TSK-TMD = Tampa Scale for Kinesiophobia; OBC = Oral Behaviors Checklist.
Appendix 3.
Estimates of Linear Association and Pairwise Comparisons Between Extent of COPC Overlap and z Scores of Measures Germane to TMD Adjusted for Study Site and Demographic Characteristics
| Linear association |
Est (SE), P |
|||||
|---|---|---|---|---|---|---|
| Measure | β (SE), P | 1 vs 0 COPCs |
2 vs 0 COPCs |
3 vs 0 COPCs |
4 vs 0 COPCs |
5 vs 0 COPCs |
| Pain-free jaw opening (r) | 0.26 (0.04), < .01 | 0.17 (0.10), .08 | 0.52 (0.11), < .01 | 0.52 (0.22), .01 | 0.89 (0.22), < .01 | 2.35 (0.38), < .01 |
| Maximum unassisted jaw opening (r) | 0.14 (0.05), < .01 | 0.18 (0.12), .12 | 0.24 (0.12), .04 | 0.16 (0.20), .41 | 0.47 (0.28), .08 | 1.53 (0.63), .01 |
| Maximum assisted jaw opening (r) | 0.09 (0.05), .06 | 0.15 (0.12), .21 | 0.15 (0.12), .21 | −0.00 (0.19), .99 | 0.48 (0.28), .08 | 1.13 (0.70), .10 |
| MM pain: Motion | 0.22 (0.04), < .01 | 0.24 (0.11), .03 | 0.43 (0.12), < .01 | 0.55 (0.17), < .01 | 0.69 (0.32), .03 | 1.79 (0.31), < .01 |
| MM pain: Palpation | 0.40 (0.03), < .01 | 0.43 (0.11), < .01 | 0.86 (0.13), < .01 | 1.03 (0.17), < .01 | 1.70 (0.17), < .01 | 2.17 (0.10), < .01 |
| MM pain: Motion + palpation | 0.38 (0.03), < .01 | 0.43 (0.12), < .01 | 0.79 (0.14), < .01 | 1.03 (0.19), < .01 | 1.59 (0.17), < .01 | 2.00 (0.09), < .01 |
| MM familiar pain: Motion | 0.29 (0.04), < .01 | 0.22 (0.09), .01 | 0.60 (0.12), < .01 | 0.76 (0.21), < .01 | 1.01 (0.35), < .01 | 2.08 (0.40), < .01 |
| MM familiar pain: Palpation | 0.38 (0.04), < .01 | 0.32 (0.09), < .01 | 0.78 (0.13), < .01 | 1.12 (0.18), < .01 | 1.39 (0.38), < .01 | 2.29 (0.24), < .01 |
| MM familiar pain: Motion + palpation | 0.39 (0.04), < .01 | 0.29 (0.10), < .01 | 0.81 (0.13), < .01 | 1.19 (0.19), < .01 | 1.36 (0.37), < .01 | 2.16 (0.28), < .01 |
| Neck pain: Palpation | 0.22 (0.04), < .01 | 0.40 (0.12), < .01 | 0.51 (0.13), < .01 | 0.53 (0.17), < .01 | 0.78 (0.20), < .01 | 1.32 (0.15), < .01 |
| Body pain: Palpation | 0.24 (0.05), < .01 | 0.26 (0.10), < .01 | 0.62 (0.13), < .01 | 0.48 (0.19), .01 | 0.76 (0.44), .08 | 1.75 (0.25), < .01 |
| Neck familiar pain: Palpation | 0.31 (0.04) , < .01 | 0.19 (0.10), .04 | 0.66 (0.14), < .01 | 0.82 (0.18), < .01 | 1.00 (0.34), < .01 | 2.18 (0.16), < .01 |
| Body familiar pain: Palpation | 0.30 (0.05), < .01 | 0.15 (0.09), .08 | 0.55 (0.15), < .01 | 0.67 (0.19), < .01 | 1.11 (0.42), < .01 | 2.55 (0.25), < .01 |
| Nonspecific jaw symptoms | 0.39 (0.04), < .01 | 0.21 (0.06), < .01 | 0.96 (0.14), < .01 | 1.03 (0.17), < .01 | 1.20 (0.28), < .01 | 2.53 (0.21), < .01 |
| TMJ function by history | 0.27 (0.04), < .01 | 0.06 (0.09), .53 | 0.72 (0.14), < .01 | 0.70 (0.14), < .01 | 0.74 (0.19), < .01 | 1.78 (0.30), < .01 |
| JFLS: Chewing | 0.27 (0.04), < .01 | 0.11 (0.10), .23 | 0.36 (0.11), < .01 | 0.65 (0.17), < .01 | 1.34 (0.32), < .01 | 2.20 (0.31), < .01 |
| JFLS: Opening | 0.25 (0.04), < .01 | 0.07 (0.08), .37 | 0.30 (0.11), < .01 | 0.87 (0.22), < .01 | 0.60 (0.27), .02 | 2.49 (0.29), < .01 |
| JFLS: Expressiveness | 0.13 (0.04), < .01 | −0.01 (0.07), 0.87 | 0.09 (0.10), .34 | 0.26 (0.15), .07 | 0.45 (0.20), .02 | 1.84 (0.40), < .01 |
| JFLS: Global | 0.26 (0.04), < .01 | 0.07 (0.08), .35 | 0.30 (0.10), < .01 | 0.72 (0.18), < .01 | 0.93 (0.23), < .01 | 2.50 (0.21), < .01 |
| TSK-TMD: Activity avoidance | 0.24 (0.04), < .01 | 0.28 (0.12), .01 | 0.44 (0.14), < .01 | 0.62 (0.16), < .01 | 0.75 (0.32), .02 | 1.83 (0.44), < .01 |
| TSK-TMD: Somatic focus | 0.24 (0.05), < .01 | 0.13 (0.09), .14 | 0.50 (0.13), < .01 | 0.62 (0.15), < .01 | 0.50 (0.32), .11 | 2.34 (0.47), < .01 |
| OBC: Total | 0.31 (0.04), < .01 | 0.27 (0.11), .01 | 0.67 (0.14), < .01 | 0.87 (0.19), < .01 | 1.24 (0.17), < .01 | 1.66 (0.39), < .01 |
Est = estimated mean difference; SE = standard error; (r) = reverse scoring; MM = masticatory muscle; JFLS = Jaw Functional Limitation Scale; TSK-TMD = Tampa Scale for Kinesiophobia; OBC = Oral Behaviors Checklist.
Appendix 4.
Estimates of Linear Association and Pairwise Comparisons Between Extent of Non-TMD COPC and z Scores of Measures Germane to TMD Adjusted for Study Site and Demographic Characteristics
| Linear association |
Est (SE), P |
||||
|---|---|---|---|---|---|
| Measure | β (SE), P | 1 vs 0 COPCs |
2 vs 0 COPCs |
3 vs 0 COPCs |
4 vs 0 COPCs |
| Pain-free jaw opening (r) | 0.26 (0.05), < .01 | 0.24 (0.10), .01 | 0.42 (0.13), < .01 | 0.43 (0.19), .02 | 1.88 (0.43), < .01 |
| Maximum unassisted jaw opening (r) | 0.13 (0.06), .05 | 0.18 (0.11), .11 | 0.09 (0.14), .52 | −0.02 (0.17), .88 | 1.47 (0.45), < .01 |
| Maximum assisted jaw opening (r) | 0.09 (0.07), .15 | 0.16 (0.12), .16 | 0.00 (0.13), .97 | −0.06 (0.18), .73 | 1.27 (0.49), < .01 |
| MM pain: Motion | 0.17 (0.05), < .01 | 0.13 (0.11), .23 | 0.27 (0.14), .04 | 0.30 (0.25), .23 | 1.25 (0.33), < .01 |
| MM pain: Palpation | 0.36 (0.05), < .01 | 0.38 (0.11), < .01 | 0.58 (0.14), < .01 | 0.91 (0.26), < .01 | 2.04 (0.08), < .01 |
| MM pain: Motion + palpation | 0.33 (0.05), < .01 | 0.34 (0.12), < .01 | 0.57 (0.14), < .01 | 0.77 (0.27), < .01 | 1.89 (0.08), < .01 |
| MM familiar pain: Motion | 0.23 (0.05), < .01 | 0.15 (0.09), .07 | 0.36 (0.14), .01 | 0.58 (0.27), .03 | 1.52 (0.36), < .01 |
| MM familiar pain: Palpation | 0.31 (0.05), < .01 | 0.24 (0.09), < .01 | 0.54 (0.13), < .01 | 0.64 (0.26), .01 | 2.14 (0.18), < .01 |
| MM familiar pain: Motion + palpation | 0.31 (0.05), < .01 | 0.22 (0.09), .01 | 0.60 (0.14), < .01 | 0.61 (0.26), .01 | 2.02 (0.21), < .01 |
| Neck pain: Palpation | 0.22 (0.05), < .01 | 0.34 (0.11), < .01 | 0.41 (0.14), < .01 | 0.34 (0.21, .09 | 1.24 (0.11), < .01 |
| Body pain: Palpation | 0.23 (0.06), < .01 | 0.23 (0.09), .01 | 0.49 (0.12), < .01 | 0.20 (0.32), .52 | 1.64 (0.18), < .01 |
| Neck familiar pain: Palpation | 0.26 (0.05), < .01 | 0.17 (0.09), .07 | 0.45 (0.13), < .01 | 0.60 (0.27), .02 | 1.77 (0.27), < .01 |
| Body familiar pain: Palpation | 0.27 (0.06), < .01 | 0.15 (0.09), .10 | 0.39 (0.13), < .01 | 0.52 (0.29), .07 | 2.26 (0.25), < .01 |
| Nonspecific jaw symptoms | 0.34 (0.05) , < .01 | 0.29 (0.08), < .01 | 0.66 (0.12), < .01 | 0.86 (0.23), < .01 | 1.73 (0.55), < .01 |
| TMJ function by history | 0.26 (0.04), < .01 | 0.12 (0.10), .22 | 0.58 (0.10), < .01 | 0.67 (0.17), < .01 | 1.28 (0.39), .01 |
| JFLS: Chewing | 0.24 (0.05), < .01 | 0.08 (0.09), .35 | 0.34 (0.12), < .01 | 0.78 (0.28), < .01 | 1.58 (0.47), < .01 |
| JFLS: Opening | 0.21 (0.06), < .01 | 0.02 (0.08), .80 | 0.39 (0.14), < .01 | 0.35 (0.20), .07 | 1.63 (0.58), < .01 |
| JFLS: Expressiveness | 0.11 (0.05), .03 | −0.03 (0.07), .63 | 0.07 (0.09), .39 | 0.18 (0.14), .20 | 1.28 (0.46), < .01 |
| JFLS: Global | 0.22 (0.05), < .01 | 0.03 (0.08), .68 | 0.33 (0.11), < .01 | 0.52 (0.19), < .01 | 1.72 (0.53), < .01 |
| TSK-TMD: Activity avoidance | 0.21 (0.05), < .01 | 0.19 (0.12), .09 | 0.38 (0.12), < .01 | 0.40 (0.23), .07 | 1.30 (0.47), < .01 |
| TSK-TMD: Somatic focus | 0.21 (0.06), < .01 | 0.17 (0.09), .06 | 0.39 (0.12), < .01 | 0.32 (0.21), .12 | 1.48 (0.70), .03 |
| OBC: Total | 0.30 (0.05), < .01 | 0.30 (0.11), < .01 | 0.60 (0.14), < .01 | 0.78 (0.23), < .01 | 1.40 (0.30), < .01 |
Est = estimated mean difference; SE = standard error; (r) = reverse scoring; MM = masticatory muscle; TSK-TMD = Tampa Scale for Kinesiophobia; OBC = Oral Behaviors Checklist.
Appendix 5.
Summary Measures of Model Fit for Random Forest Models
| Model fit for prediction of: | |||||
|---|---|---|---|---|---|
| Metric | TMD | Headache | IBS | LBP | Fibromyalgia |
| Observed cases, % | 0.278 | 0.412 | 0.241 | 0.212 | 0.079 |
| Area under precision-recall curve | 0.883 | 0.690 | 0.290 | 0.426 | 0.438 |
| Area under receiver operator characteristic curve | 0.957 | 0.743 | 0.598 | 0.745 | 0.928 |
| Brier score | 0.078 | 0.201 | 0.219 | 0.179 | 0.093 |
| Mutual information index | 0.302 | 0.064 | 0.008 | 0.042 | 0.084 |
| Proportion of variance explained | 0.642 | 0.204 | 0.083 | 0.152 | 0.405 |
| Maximum variable importance factor: Predicting cases | 0.557 | 0.213 | 0.048 | 0.087 | 0.457 |
| Maximum variable importance factor: Predicting controls | 0.002 | 0.046 | 0.021 | 0.027 | 0.001 |
| Maximum variable importance factor: All | 0.032 | 0.033 | 0.005 | 0.010 | 0.001 |
Contributor Information
Sonia Sharma, Department of Oral Diagnostic Sciences University at Buffalo School of Dental Medicine Buffalo, New York, USA; Department of Orofacial Pain and Jaw Function Faculty of Odontology, Malmö University, Malmö, Sweden.
Gary D. Slade, Division of Pediatric and Public Health Adams School of Dentistry Department of Dental Ecology Department of Epidemiology Gillings School of Global Public Health University of North Carolina Chapel Hill, North Carolina, USA.
Roger B. Fillingim, Department of Community Dentistry & Behavioral Science College of Dentistry University of Florida Gainesville, Florida, USA.
Joel D. Greenspan, Department of Neural and Pain Sciences Brotman Facial Pain Clinic Center to Advance Chronic Pain Research School of Dentistry University of Maryland Baltimore, Maryland, USA.
Nuvan Rathnayaka, Department of Epidemiology Gillings School of Global Public Health University of North Carolina Chapel Hill, North Carolina, USA.
Richard Ohrbach, Department of Oral Diagnostic Services School of Dental Medicine University at Buffalo Buffalo, New York, USA.
References
- 1.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders—Pathways of vulnerability. Pain 2006;123:226–230. [DOI] [PubMed] [Google Scholar]
- 2.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain 2016;17(9 suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin Arthritis Rheum 2007;36:339–356. [DOI] [PubMed] [Google Scholar]
- 4.Functional pain disorders: Time for a paradigm shift. In: Mayer EA, Bushnell MC (eds). Functional Pain Syndromes: Presentation and Pathophysiology. Seattle: IASP, 2009:531–565. [Google Scholar]
- 5.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, ed 3. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 6.NIH Working Group. Chronic Overlapping Pain Conditions: Summary of NIH Work Group Meeting to Develop Case Definition & Common Data Elements. https://www.iprcc.nih.gov/sites/default/files/Veasley-COPC.pdf. National Institutes of Health, 2015. Accessed May 6, 2020. [Google Scholar]
- 7.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 8.Bair E, Brownstein NC, Ohrbach R, et al. Study protocol, sample characteristics, and loss to follow-up: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T2–T19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slade GD, Bair E, By K, et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain 2011;12(11 suppl):T12–T26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 2014;28:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: The ID Migraine validation study. Neurology 2003;61:375–382. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 13.Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95–103. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 15.Visscher CM, Ohrbach R, van Wijk AJ, Wilkosz M, Naeije M. The Tampa Scale for Kinesiophobia for Temporomandibular Disorders (TSK-TMD). Pain 2010;150:492–500. [DOI] [PubMed] [Google Scholar]
- 16.Miller RP, Kori SH, Todd DD. The Tampa Scale: A measure of kinesiophobia. Clin J Pain 1991;7:51–52. [Google Scholar]
- 17.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000;85:317–332. [DOI] [PubMed] [Google Scholar]
- 18.Visscher CM, Ohrbach R, van Wijk AJ, Wilkosz M, Naeije M. The Tampa Scale for Kinesiophobia for Temporomandibular Disorders (TSK-TMD). Pain 2010;150:492–500. [DOI] [PubMed] [Google Scholar]
- 19.Ohrbach R, Markiewicz MR, McCall WD Jr. Waking-state oral parafunctional behaviors: Specificity and validity as assessed by electromyography. Eur J Oral Sci 2008;116:438–444. [DOI] [PubMed] [Google Scholar]
- 20.Markiewicz MR, Ohrbach R, McCall WD Jr. Oral behaviors checklist: Reliability of performance in targeted waking-state behaviors. J Orofac Pain 2006;20:306–316. [PubMed] [Google Scholar]
- 21.Ohrbach R. Assessment and further development of RDC/TMD Axis II biobehavioural instruments: A research programme progress report. J Oral Rehabil 2010;37:784–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan SE, Ohrbach R. Self-report of waking-state oral parafunctional behaviors in the natural environment. J Oral Facial Pain Headache 2016;30:107–119. [DOI] [PubMed] [Google Scholar]
- 23.Ohrbach R Disability assessment in temporomandibular disorders and masticatory system rehabilitation. J Oral Rehabil 2010;37:452–480. [DOI] [PubMed] [Google Scholar]
- 24.Ohrbach R, Larsson P, List T. The Jaw Functional Limitation Scale: Development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain 2008;22:219–230. [PubMed] [Google Scholar]
- 25.Ohrbach R, Granger C, List T, Dworkin S. Preliminary development and validation of the Jaw Functional Limitation Scale. Community Dent Oral Epidemiol 2008;36:228–236. [DOI] [PubMed] [Google Scholar]
- 26.Ohrbach R, Larsson P, List T. The Jaw Functional Limitation Scale: Development, reliability, and validity of 8-item and 20-item versions. J Orofac Pain 2008;22:219–230. [PubMed] [Google Scholar]
- 27.Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first-onset TMD: The OPPERA prospective cohort study. J Pain 2013;1(12 suppl):T33–T50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohrbach R, Gonzalez Y, List T, Michelotti A, Schiffman E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) Clinical Examination Protocol. http://www.rdc-tmdinternational.org/.INfORM, 2014. Accessed May 7, 2020. [Google Scholar]
- 29.Dworkin SF, LeResche L, DeRouen T, Von Korff M. Assessing clinical signs of temporomandibular disorders: Reliability of clinical examiners. J Prosthet Dent 1990;63:574–579. [DOI] [PubMed] [Google Scholar]
- 30.Ohrbach R, Bair E, Fillingim RB, et al. Clinical orofacial characteristics associated with risk of first-onset TMD: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T33–T50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–172. [DOI] [PubMed] [Google Scholar]
- 32.Richardson DB, Rzehak P, Klenk J, Weiland SK. Analyses of case-control data for additional outcomes. Epidemiology 2007;18:441–445. [DOI] [PubMed] [Google Scholar]
- 33.Monsees GM, Tamimi RM, Kraft P. Genome-wide association scans for secondary traits using case-control samples. Genet Epidemiol 2009;33:717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bair E, Ohrbach R, Fillingim RB, et al. Multivariable modeling of phenotypic risk factors for first-onset TMD: The OPPERA prospective cohort study. J Pain 2013;14(12 suppl):T102–T115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang F, Ishwaran H. Random forest missing data algorithms. Stat Anal Data Min 2017;10:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Delgado M, Cernadas E, Barro S, Amorim D. Do we need hundreds of classifiers to solve real world classification problems? J Machine Learning Res 2014;15:3133–3181. [Google Scholar]
- 37.Lever J, Krzywinski M, Altman N. Points of significance: Classification evaluation. Nature Methods 2016;13:603–604. [Google Scholar]
- 38.Rufibach K. Use of Brier score to assess binary predictions. J Clin Epidemiol 2010;63:938–939. [DOI] [PubMed] [Google Scholar]
- 39.Wallach H. Evaluation metrics for hard classifiers. Cambridge: Cavendish Laboratory, University of Cambridge, 2006. [Google Scholar]
- 40.Mohl ND. Reliability and validity of diagnostic modalities for temporomandibular disorders. Adv Dent Res 1993;7:113–119. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Crow HC, McCall WD Jr, Gonzalez YM. Systematic review of reliability and diagnostic validity of joint vibration analysis for diagnosis of temporomandibular disorders. J Orofac Pain 2013;27:51–60. [DOI] [PubMed] [Google Scholar]
- 42.Ohrbach R, Fillingim RB, Mulkey F, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: Descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12(11 suppl):T27–T45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glaros AG, Urban D, Locke J. Headache and temporomandibular disorders: Evidence for diagnostic and behavioural overlap. Cephalalgia 2007;27:542–549. [DOI] [PubMed] [Google Scholar]
- 44.Svensson P. Muscle pain in the head: Overlap between temporomandibular disorders and tension-type headaches. Curr Opin Neurol 2007;20:320–325. [DOI] [PubMed] [Google Scholar]
- 45.Von Korff M, Dworkin SF, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain 1988;32:173–183. [DOI] [PubMed] [Google Scholar]
- 46.Tchivileva IE, Ohrbach R, Fillingim RB, Greenspan JD, Maixner W, Slade GD. Temporal change in headache and its contribution to the risk of developing first-onset temporomandibular disorder in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study. Pain 2017;158:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plesh O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: Prevalence and symptom severity. J Rheumatol 1996;23:1948–1952. [PubMed] [Google Scholar]
- 48.Greenwald JD, Shafritz KM. An integrative neuroscience framework for the treatment of chronic pain: From cellular alterations to behavior. Front Integr Neurosci 2018;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raphael KG, Marbach JJ. Widespread pain and the effectiveness of oral splints in myofascial face pain. J Am Dent Assoc 2001;132:305–316. [DOI] [PubMed] [Google Scholar]