Abstract
Background
Incidence of postherpetic neuralgia (PHN) increases with age. Epidural block in patients with herpes zoster (HZ) is expected to decrease the risk of PHN. The purpose of this study was to evaluate the effectiveness of epidural block on PHN incidence in a population-based study.
Methods
This was a retrospective matched cohort study and data were sourced from the Korean National Health Insurance Service. The study cohort comprised 427 patients diagnosed with HZ who received epidural block within 30 days after a diagnosis of HZ. The matched control cohort included 427 patients without epidural block and were randomly matched to the study cohort at a 1:1 ratio based on covariates such as sociodemographic factors. The log-rank test was used to assess differences in the incidence of PHN. Cox proportional hazards regression models were used to estimate the hazard ratio (HR) for subsequent PHN, while controlling for potential comorbidities.
Results
Among the 854 sampled patients, 30 (7.03%) from the study cohort and 18 (4.22%) from the match-control developed PHN during follow-up. There were no significant differences in the incidence of PHN between the two cohorts (p=0.08). Cox proportional hazard regressions showed that the HR for PHN in patients with epidural block was 1.66 (95% confidence interval, 0.91–3.02; p=0.10).
Conclusion
Our study indicates that epidural block did not effectively prevent PHN. However, further studies are needed to determine the effect of epidural block in patients with HZ for the prevention of PHN.
Keywords: Herpes zoster, Postherpetic neuralgia, Epidural anesthesia
INTRODUCTION
Herpes zoster (HZ) is a viral disease characterized by a painful vesicular rash involving one or more adjacent dermatomes.1) HZ is caused by the reactivation of varicella-zoster virus (VZV). This virus is dormant in the cells of the dorsal root ganglia following the resolution of chickenpox.1) VZV reactivation occurs in patients with reduced cell-mediated immunity due to aging or immunosuppressive conditions.2) The affected skin area can be extremely painful, and healing of the skin and pain resolution generally take 2–3 weeks.2,3) In some patients, residual pain can persist beyond the pathological healing process, resulting in postherpetic neuralgia (PHN).3) In PHN, pain persists even after the skin lesions have healed.4)
The incidence of PHN in patients with HZ varies from 5% to more than 50%, depending on the PHN definition and study design. The degree of pain varies from mild to severe, and pain persists for >1 year in approximately 30% of patients.2) As pain becomes chronic, PHN can negatively affect patient quality of life and cause physical, occupational, and social disabilities.5) Additionally, such patients are at high risk of developing mental health problems such as anxiety, depression, and sleep disturbances.6) Since PHN is common in older adults, its incidence is expected to increase in the upcoming aging society.7)
Therefore, it is important to prevent PHN and control acute viral infections and associated pain while treating patients with acute HZ. Owing to the complex pathophysiology of PHN, various strategies have been proposed for its prevention. These include antiviral agents, vaccines, corticosteroids, anticonvulsants, and antidepressants. However, recent studies have demonstrated the limited efficacy of these strategies in preventing PHN.2)
The application of somatic neural blocks during the acute phase of HZ has also been attempted to prevent PHN.2) Some studies have suggested that neural blocks in patients with HZ can prevent PHN.8,9) However, other studies have not demonstrated the effectiveness of neural blocks in PHN.10,11) The epidural block is one of the most frequently performed neural blocks in patients with HZ. The present study assessed the effect of epidural blocks on PHN in a large cohort from the Korean national population-based dataset.
MATERIALS AND METHODS
Study Design
We conducted a population-based retrospective cohort study of patients with HZ in Korea using data sourced from the Korean National Health Insurance Service (KNHIS) between January 1, 2002, and December 31, 2015. This study evaluated whether epidural block could cause differences in the incidence of PHN in patients with HZ. The included patients were represented by the following diagnostic names: “zoster without complications,” “zoster with other complications,” “zoster ocular disease,” and “zoster with other nervous system involvement.”
Data Source
The data for this study were obtained from the KNHIS. The KNHIS is a national health insurance program established by the Korean government in 1963 that archives almost all healthcare data in a central database. The National Health Insurance Service is a compulsory healthcare plan for all Koreans, and qualified citizens are covered under this scheme through either employee or community-based plans. Because all Korean residents receive a unique identification number at birth, the medical records of any patient are not duplicated or omitted.
We used the Sample Research Database version 2 (NHIS-2020-2-116), which includes information on medical care utilization for approximately one million representative Koreans randomly selected from the total NHIS claim dataset (2002–2015). This database comprises various standard codes, including disease codes, codes for diagnostic and therapeutic procedures, medication codes, and duration of admission. The disease codes were based on the Korean Standard Classification of Diseases Eighth Revision (KCD-8), which is the Korean version of the International Classification of Diseases Tenth Revision (ICD-10). The codes for various procedures were based on those in the Korean Health Insurance Classification of Procedures in Medicine (KHICPM).
Ethics Statement
The Sample Research Database consisted of de-identified secondary data for research purposes. Hence, patient consent was not required for access to the database. This study was approved with a waiver for patient written consent from the Institutional Review Board of Konyang University Hospital, Daejeon, Korea, in February 2020 (No. KYUH 2020-02-010).
This study complied the ethical guidelines for authorship and publishing in the Annals of Geriatric Medicine and Research.12)
Study Population
We first identified patients diagnosed with HZ (KCD-8 codes: B02.2, B02.3, B02.8, B02.9) as the study cohort. The date on which the patients were first diagnosed with HZ was defined as the index date. To enhance the validity of the HZ diagnosis, this study included only cases in which antiviral agents were prescribed at the time of HZ diagnosis. We then excluded patients who had been diagnosed with PHN by excluding patients diagnosed with PHN (KCD-8 codes: G53.0) before the index date and those diagnosed with HZ and PHN at the same time on the index date. Subsequently, patients diagnosed with PHN within 30 days or >180 days were excluded, based on the index date. Among these patients, we included only those treated between 2003 and 2014, including a 1-year washout period. To exclude the effects of other procedures, we excluded patients who had undergone other interventions such as sympathetic block (KHICPM codes: LA261, LA361, LA366, and LA367), paravertebral block (KHICPM codes: LA352), and subarachnoid block (KHICPM codes: LA210), >180 days after the index date. All patients included in this study were adults (aged ≥20 years) (Fig. 1).
Fig. 1.
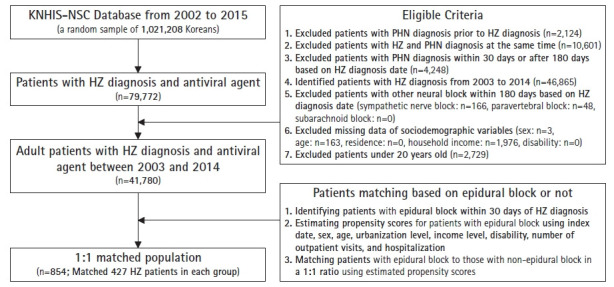
Cohort identification. This figure shows the order using inclusion and exclusion criteria to identify study cohorts. KNHIS-NSC, Korean National Health Insurance Service-National Sample Cohort; HZ, herpes zoster; PHN, postherpetic neuralgia.
Intervention and Control Cohort
A comparison cohort from KNHIS was selected to evaluate the effectiveness of epidural block on the occurrence of PHN after HZ. Patients who underwent epidural block (KHICPM codes: LA 223-227, LA321, LA322) within 30 days based on the index date were identified. We then randomly selected patients who did not undergo epidural block by propensity score matching (PSM) in a 1:1 ratio with the study cohort who underwent epidural block.
Covariates
The PSM was based on index date, sex, age (<50, 50–79, and ≥80 years), residence urbanization level (1 “most urbanized” to 3 “least urbanized”), household income level (low, middle, high), disability, number of outpatient visits, and hospitalization during the follow-up period according to the sociodemographic characteristics. These variables were considered potential confounders for PHN development.13) To minimize selection bias, we attempted PSM between the cohort groups for confounding variables. In addition, based on the findings of previous studies,13,14) we considered respiratory disease (chronic obstructive pulmonary disease, asthma, other chronic lower respiratory diseases), diabetes mellitus (DM), cancer, autoimmune disease (rheumatoid arthritis, SLE, Crohn’s disease, ulcerative colitis), and severe immunosuppressive status (human immunodeficiency virus, lymphoma, leukemia, multiple myeloma) as additional potential confounders for PHN.
Outcomes
Our primary outcome measure was the difference in the incidence of PHN for 180 days from the index date between the two cohorts. By limiting the timing of PHN diagnosis from 30 to 180 days after the index date,2,15-18) we examined the direct effect of epidural blocks performed within 30 days of the index date on PHN occurrence. Our secondary outcome measure was the risk of PHN associated with potential confounding factors.
Statistical analysis
Despite being based on administrative data sources, we calculated sample sizes to ensure that the study analysis was feasible. Based on a pilot study, to detect a difference in the incidence of PHN between the groups, the estimated sample size with a type I error of 0.05 and a power of 90% was 246.
The study endpoint was the date of PHN diagnosis, end of follow-up (180 days from the index date), or death. Log-rank tests were used to assess differences in the incidence of PHN, while Kaplan–Meier curves were used to calculate the 180-day PHN incidence rates between the cohort groups. After adjusting for disease-related potential confounders (respiratory disease, DM, cancer, autoimmune disease, and severe immunosuppressive status), stratified Cox proportional hazards modeling (stratified by sex, age, residence urbanization level, household income level, disability, number of outpatient visits, hospitalization) was used to calculate hazard ratios (HR) and corresponding 95% confidence intervals (CI) for subsequent PHN. The HRs associated with potential confounding factors were confirmed using a stratified Cox proportional hazards model. SAS Enterprise Guide 6.1M1 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. Statistical significance was set at p<0.05.
RESULTS
This study identified 41,780 eligible patients with HZ diagnosis and antiviral agent use, 427 of whom underwent epidural blocks. We matched these to 427 patients without epidural blocks in a 1:1 ratio (Fig. 1).
Table 1 shows the sociodemographic characteristics and medical conditions of the individuals in the study and control cohorts. Of the 427 patients in each group, most patients were aged 50–79 years—epidural group of 328 (76.8%) and comparison group of 342 (80.1%)—and approximately 65% were female—282 (66.0%) and 274 (64.2%), respectively. Compared with the comparison group, patients with epidural blocks had more comorbidities including respiratory disease, DM, autoimmune disease, and severe immunosuppressive status (Table 1).
Table 1.
Patient characteristics
| Variable | Comparison (n=427) | Epidural block (n=427) | p-value |
|---|---|---|---|
| Sex | 0.62 | ||
| Male | 153 (35.8) | 145 (34.0) | |
| Female | 274 (64.2) | 282 (66.0) | |
| Ages (yr) | 0.51 | ||
| ≤49 | 68 (15.9) | 79 (18.5) | |
| 50–79 | 342 (80.1) | 328 (76.8) | |
| ≥80 | 17 (4.0) | 20 (4.7) | |
| Residence | 0.42 | ||
| Seoul | 60 (14.1) | 72 (16.9) | |
| Other metropolitans | 133 (31.1) | 120 (28.1) | |
| Rural and small cities | 234 (54.8) | 235 (55.0) | |
| Annual household income (million KRW) | 0.87 | ||
| ≤30.0 (low) | 86 (20.1) | 90 (21.1) | |
| 30.1–69.9 (middle) | 158 (37.0) | 151 (35.4) | |
| ≥70.0 (high) | 183 (42.9) | 186 (43.6) | |
| Disability | 0.91 | ||
| No | 378 (88.5) | 380 (89.0) | |
| Yes | 49 (11.5) | 47 (11.0) | |
| Number of outpatient visit | 0.66 | ||
| 1 time | 104 (24.4) | 98 (23.0) | |
| 2–3 times | 150 (35.1) | 143 (33.5) | |
| ≥4 times | 173 (40.5) | 186 (43.6) | |
| Hospitalization | 0.52 | ||
| No | 418 (97.9) | 414 (97.0) | |
| Yes | 9 (2.1) | 13 (3.0) | |
| RD | 0.00* | ||
| No | 171 (40.0) | 114 (26.7) | |
| Yes | 256 (60.0) | 313 (73.3) | |
| DM | 0.00* | ||
| No | 270 (63.2) | 221 (51.8) | |
| Yes | 157 (36.8) | 206 (48.2) | |
| Cancer | 0.52 | ||
| No | 381 (89.2) | 374 (87.6) | |
| Yes | 46 (10.8) | 53 (12.4) | |
| AD | 0.00* | ||
| No | 344 (80.6) | 297 (69.6) | |
| Yes | 83 (19.4) | 130 (30.4) | |
| SIS | 0.04* | ||
| No | 21 (4.9) | 9 (2.1) | |
| Yes | 406 (95.1) | 418 (97.9) |
Data area expressed as the mean or number (%).
KRW, Korean won; RD, respiratory disease; DM, diabetes mellitus; AD, autoimmune disease; SIS, severe immunosuppressive status.
p<0.05.
The 180-day PHN incidence rate in the patients included in the study was 5.62% (48/854; 95% CI, 4.14–7.45). Of the 427 patients in each group, 30 (7.03%) in the epidural group and 18 (4.22%) in the comparison group advanced to PHN. The Kaplan–Meier curve is shown in Fig. 2. The log-rank tests revealed no significant differences in the incidence rates of PHN between the two cohorts (p=0.08) (Fig. 2).
Fig. 2.
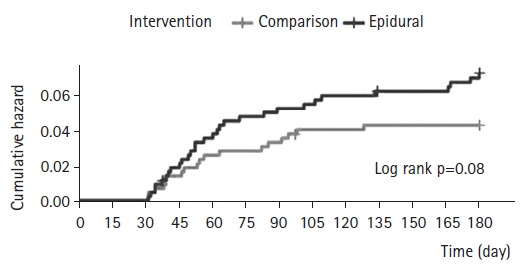
Cumulative hazard ratio of postherpetic neuralgia (PHN) in patients with and without epidural block. There was no statistically significant different between the two cohorts.
Table 2 shows the HR for PHN associated with the potential confounding factors. Females, older adults, and residents living in rural areas tended to have an increased risk of developing PHN. Additionally, respiratory and autoimmune diseases, along with a severe immunosuppressive status, increased the risk of PHN. However, the HRs associated with these factors were not significant. Meanwhile, as the number of outpatient visits increased (≥4 times), and in the case of hospitalization, the HR of PHN was significantly higher—3.55; 95% CI, 1.37–9.17; p=0.01; and 3.08; 95% CI, 1.03–9.21; p=0.04, respectively.
Table 2.
Risk of postherpetic neuralgia associated with potential confounding factors
| Confounder |
Cox-regressiona) | |
|---|---|---|
| HR (95% CI) | p-value | |
| Sex (female) | 1.67 (0.91–1.98) | 0.10 |
| Ages (yr) | ||
| ≤49 | 1.0 | |
| 50–79 | 1.52 (0.58–3.98) | 0.40 |
| ≥80 | 1.70 (0.37–7.86) | 0.50 |
| Residence | ||
| Seoul | 1.0 | |
| Other metropolitans | 1.08 (0.41–2.85) | 0.88 |
| Rural and small cities | 1.39 (0.57–3.40) | 0.47 |
| Annual household income (million KRW) | 0.87 | |
| ≤30.0 (low) | 1.0 | |
| 30.1–69.9 (middle) | 0.5 (0.21–1.17) | 0.11 |
| ≥70.0 (high) | 1.07 (0.53–2.14) | 0.85 |
| Disability (yes) | 1.15 (0.49–2.73) | 0.75 |
| Number of outpatient visit | ||
| 1 time | 1.0 | |
| 2–3 times | 1.23 (0.42–3.65) | 0.71 |
| ≥4 times | 3.55 (1.37–9.17) | 0.01* |
| Hospitalization (yes) | 3.08 (1.03–9.21) | 0.04* |
| RD (yes) | 1.11 (0.58–2.14) | 0.74 |
| DM (yes) | 0.85 (0.46–1.58) | 0.62 |
| Cancer (yes) | 0.44 (0.13–1.46) | 0.18 |
| AD (yes) | 1.16 (0.61–2.22) | 0.65 |
| SIS (yes) | 1.12 (0.15–8.39) | 0.91 |
HR, hazard ratio; CI, confidence interval; KRW, Korean won; RD, respiratory disease; DM, diabetes mellitus; AD, autoimmune disease; SIS, severe immunosuppressive status.
Multivariable stratified Cox regression analysis.
p<0.05
Table 3 presents the unadjusted and adjusted HRs for PHN for the cohorts. After adjusting for respiratory disease, DM, cancer, autoimmune disease, and severe immunosuppressive status, stratified Cox proportional hazard regressions showed that the HR for PHN diagnosis within the 180-day period for patients with epidural blocks was 1.66 (95% CI, 1.91–3.02; p=0.10) that of comparison patients (Table 3).
Table 3.
Incidence and risk of postherpetic neuralgia associated with epidural block
| Cox-regressiona) |
||||
|---|---|---|---|---|
| Unadjusted | p-value | Adjusted | p-value | |
| Comparison (n=427) | 1.00 | |||
| Epidural block (n=427) | 1.69 (0.94–3.03) | 0.08 | 1.66 (0.91–3.02) | 0.10 |
Values are presented as hazard ration (95% confidence interval).
Stratified Cox proportional hazard regressions (stratified on sex, age, urbanization level of residence, household income level, disability, number of outpatient visits, hospitalization) with adjusting comorbidities (respiratory disease, diabetes mellitus, cancer, autoimmune disease, severe immunosuppressive status).
DISCUSSION
This population-based matched case-control study explored the effectiveness of epidural blocks for PHN prevention in the Korean population. We observed that the epidural block group did not show a decreased incidence of PHN compared to that in the control group. Epidural blocks have been used for decades to treat HZ-associated pain and prevent PHN, and their positive effects on preventing PHN have been reported in several studies.15,19-22) A recent systematic review recommended epidural blocks to prevent PHN in patients with HZ and showed that continuous or repeated epidural blocks significantly reduced the incidence of PHN.2) However, the conclusion of a review conducted by the International Association for the Study of Pain Neuropathic Pain Special Interest Group (NeuPSIG) disagreed with this conclusion.23) This may be due to the lack of high-quality randomized controlled studies on the effects of epidural blocks on PHN. In addition, several studies have failed to demonstrate the effectiveness of epidural blocks in preventing PHN.5,7,24)
This discrepancy in the findings is probably due to the absence of a consensus on PHN definition. Although PHN has been defined as persistent pain after the healing of an HZ rash,20) no clinical cutoff points for its diagnosis have been established. Generally, PHN is defined as pain persisting for >3 months2,15-18) after the diagnosis of HZ; however, the clinical cutoff points for PHN diagnosis vary between 1 and 6 months.11,25,26) The absence of a single, uniform cutoff point could have affected the study results. In addition, the definition of pain in the diagnosis of PHN remains controversial. The criteria for pain intensity for diagnosis differs between studies. In each study, based on pain scales (numerical rating scale or visual analog scale), PHN was defined differently: higher than 10/100,2,13,16-18,27) 25/100,28) and 30/100.2) Furthermore, PHN has a wide spectrum of symptoms including spontaneous pain, paroxysmal pain, allodynia, hyperalgesia, and abnormal sensations.20) Therefore, the reported results may have varied depending on which of these symptoms were included in the PHN definitions.
The pathophysiology of PHN remains unclear.21) In patients with PHN, damage to the sensory nerve, dorsal root ganglion, and dorsal horn of the spinal cord has been reported.29) These injuries can result in pain, allodynia, and hyperalgesia. This damage may be attributed to two pathophysiological mechanisms: deafferentation and sensitization. Deafferentation is the interruption or destruction of afferent connections of nerve cells. Reactivation of VZV in the dorsal root ganglion can lead to inflammation, resulting in sequential edema, increased intrafascicular pressure, and neural destruction.30) Sensitization is an abnormal state of responsiveness or increased gain in the nociceptive system. Peripheral nociceptors can induce ongoing discharge after acute tissue injury, which subsequently affects the neurons of the dorsal horn ganglion, leading to hyperexcitability and hypersensitivity.7) Epidural blocks are expected to be effective in PHN by preventing deafferentation and sensitization. Epidural blocks using local anesthetics (with or without steroids) reduce inflammation and prevent profound sympathetic stimulation. These effects may prevent a decrease in intraneural blood flow and subsequent ischemic neuronal damage.3,9) In addition, the analgesic effects of epidural blocks can prevent central sensitization by stopping the continuous accumulation of nociceptive inputs.15)
However, the use of epidural blocks in our study did not demonstrate any significant differences in the preventive effects on PHN. These results may be attributed to the absence of standardized guidelines for epidural block in patients with HZ, such as the timing of intervention and frequency, number, duration, and types of local anesthetics with or without steroids, as previously reported.31) It is well recognized that, for the best efficacy, HZ treatment should be started as soon as possible. Many researchers have suggested that an epidural block appears to exhibit efficacy if performed within 10–15 days of HZ diagnosis.20) Once reactivated, VZV damage can extend centrally to the dorsal horn of the spinal cord, with central lesions generally appearing in 9–12 days.20) Therefore, if possible, an epidural block should be performed within 2 weeks of HZ diagnosis. In clinical practice, epidural blocks are not routinely applied to HZ treatment and are considered only in patients at high risk for PHN or in the absence of a response to other HZ treatments. Hence, epidural blocks are often delayed in HZ treatment. In our study, 66.5% (n=284) of patients underwent epidural block within 14 days of HZ diagnosis, whereas 33.5% (n=143) of patients were administered the block after 14 days. Epidural blocks conducted later (15–30 days after HZ diagnosis) may have negatively affected PHN prevention.
The technique employed for the epidural block may have affected the results of this study. Previous reports have documented that continuous epidural catheters or repeated single-shot techniques reduce the incidence of PHN.2) In comparison, a single-shot epidural block may be insufficient to prevent the accumulation of continuous nociceptive input. Therefore, continuous or repeated epidural blocks are necessary to effectively prevent PHN.2) However, continuous epidural blocks requiring a catheter are limited in clinical practice because of the risk of infection and the probability of hospitalization. In this study, 98.2% (n=397) of patients underwent epidural blocks more than twice; hence, it is unlikely that the number of epidural blocks affected our results. Meanwhile, the rate of continuous epidural block was 7.3% (n=30). Although the proportion of continuous epidural blocks was low, both continuous and repeated single-shot epidural blocks are expected to be effective in preventing PHN. Therefore, it seems that this wouldn't have had a decisive effect on our study results.
Among the potential confounders related to PHN in this study, the age-related risk of PHN did not increase significantly. However, previous studies reported an increased risk of PHN with age.13,14) This discrepancy may be attributed to the fact that our study included a matched cohort. The study findings cannot be generalized as the patients were matched based on epidural block. Thus, the demographic characteristics of the study population may have differed. Meanwhile, as the number of outpatient visits increased, as well as the cases of hospitalization, the risk of PHN was significantly higher in our study. Patients with severe symptoms such as pain likely visited the hospital more frequently, many of whom progressed to PHN. Similarly, considering that the epidural group showed more comorbidities such as respiratory disease, DM, autoimmune disease, and severe immunosuppressive status, due to the severe symptoms of HZ, epidural block may have been more likely to be performed.
The limitation of this study was its retrospective nature based on the National Sample Cohort of the KNHIS. The KNHIS database does not contain important information such as the location, intensity, and quality of pain or the start time of antiviral agents after symptom onset, which are covered in the medical records. Therefore, although the use of antiviral agents is generally recommended within 72 hours of HZ onset,32) we could not ascertain the time interval between symptom onset and the administration of antiviral agents. Moreover, the effectiveness of antiviral agents in preventing PHN has not yet been confirmed.33) In addition, a recent systematic review and meta-analysis showed that the presence of prodromal pain, severe acute pain, and severe rash in patients with HZ increased the risk of PHN.14) However, we could not adjust for these potential confounders in this study. Finally, a selection bias may have affected the study outcomes. As patients with severe zoster-associated pain are more likely to receive intensive treatment such as epidural blocks, the baseline pain severity may have differed between the control and epidural block groups.
In conclusion, in our study, the clinical outcome of PHN incidence in patients with HZ did not differ significantly between those with and without epidural block. This is the first population-based cohort study to investigate the preventive effects of epidural block on PHN. Additional studies are required to evaluate the effect of epidural blocks on PHN incidence. In addition, a consensus-based definition of PHN and standardized guidelines for epidural block in patients with HZ must be established.
Footnotes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
This study was supported by the Myunggok Medical Research Institute
AUTHOR CONTRIBUTIONS
Conceptualization, CBI, JYK, JYH; Data curation, JYH, IK, MC; Funding acquisition, CBI, JYK; Investigation, CBI, JYH, IK; Methodology, CBI, JYK, JYH, IK, MC; Project administration, IK, MC; Supervision, CBI, JYK, JYH; Writing-original draft, CBI, JYK, JYH; Writing-review & editing, CBI, JYK, JYH.
REFERENCES
- 1.Arvin AM. Varicella-zoster virus. Clin Microbiol Rev. 1996;9:361–81. doi: 10.1128/cmr.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HJ, Ahn HS, Lee JY, Choi SS, Cheong YS, Kwon K, et al. Effects of applying nerve blocks to prevent postherpetic neuralgia in patients with acute herpes zoster: a systematic review and meta-analysis. Korean J Pain. 2017;30:3–17. doi: 10.3344/kjp.2017.30.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makharita MY, Amr YM, El-Bayoumy Y. Single paravertebral injection for acute thoracic herpes zoster: a randomized controlled trial. Pain Pract. 2015;15:229–35. doi: 10.1111/papr.12179. [DOI] [PubMed] [Google Scholar]
- 4.Wen B, Wang Y, Zhang C, Xu W, Fu Z. Efficacy of different interventions for the treatment of postherpetic neuralgia: a Bayesian network meta-analysis. J Int Med Res. 2020;48:300060520977416. doi: 10.1177/0300060520977416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Wijck AJ, Opstelten W, Moons KG, van Essen GA, Stolker RJ, Kalkman CJ, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. 2006;367:219–24. doi: 10.1016/S0140-6736(06)68032-X. [DOI] [PubMed] [Google Scholar]
- 6.Tang Y, Wang M, Zheng T, Xiao Y, Wang S, Han F, et al. Structural and functional brain abnormalities in postherpetic neuralgia: a systematic review of neuroimaging studies. Brain Res. 2021;1752:147219. doi: 10.1016/j.brainres.2020.147219. [DOI] [PubMed] [Google Scholar]
- 7.Opstelten W, van Wijck AJ, Stolker RJ. Interventions to prevent postherpetic neuralgia: cutaneous and percutaneous techniques. Pain. 2004;107:202–6. doi: 10.1016/j.pain.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Tajima K, Iseki M, Inada E, Miyazaki T. [The effects of early nerve blocks for prevention of postherpetic neuralgia and analysis of prognostic factors] Masui. 2009;58:153–9. [PubMed] [Google Scholar]
- 9.Winnie AP, Hartwell PW. Relationship between time of treatment of acute herpes zoster with sympathetic blockade and prevention of post-herpetic neuralgia: clinical support for a new theory of the mechanism by which sympathetic blockade provides therapeutic benefit. Reg Anesth. 1993;18:277–82. [PubMed] [Google Scholar]
- 10.Yanagida H, Suwa K, Corssen G. No prophylactic effect of early sympathetic blockade on postherpetic neuralgia. Anesthesiology. 1987;66:73–6. doi: 10.1097/00000542-198701000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Riopelle JM, Naraghi M, Grush KP. Chronic neuralgia incidence following local anesthetic therapy for herpes zoster. Arch Dermatol. 1984;120:747–50. [PubMed] [Google Scholar]
- 12.Noh JH, Jung HW, Ga H, Lim JY. Ethical guidelines for publishing in the Annals of Geriatric Medicine and Research. Ann Geriatr Med Res. 2022;26:1–3. doi: 10.4235/agmr.22.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Mansfield K, et al. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: a cohort study. Neurology. 2016;87:94–102. doi: 10.1212/WNL.0000000000002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157:30–54. doi: 10.1097/j.pain.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YN, Kim DW, Kim ED. Efficacy of continuous epidural block in acute herpes zoster: incidence and predictive factors of postherpetic neuralgia, a retrospective single-center study. Medicine (Baltimore) 2016;95:e4577. doi: 10.1097/MD.0000000000004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dworkin RH, Portenoy RK. Proposed classification of herpes zoster pain. Lancet. 1994;343:1648. doi: 10.1016/s0140-6736(94)93106-2. [DOI] [PubMed] [Google Scholar]
- 17.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 19.Perkins HM, Hanlon PR. Epidural injection of local anesthetic and steroids for relief of pain secondary to herpes zoster. Arch Surg. 1978;113:253–4. doi: 10.1001/archsurg.1978.01370150025003. [DOI] [PubMed] [Google Scholar]
- 20.Pasqualucci A, Pasqualucci V, Galla F, De Angelis V, Marzocchi V, Colussi R, et al. Prevention of post-herpetic neuralgia: acyclovir and prednisolone versus epidural local anesthetic and methylprednisolone. Acta Anaesthesiol Scand. 2000;44:910–8. doi: 10.1034/j.1399-6576.2000.440803.x. [DOI] [PubMed] [Google Scholar]
- 21.Seo YG, Kim SH, Choi SS, Lee MK, Lee CH, Kim JE. Effectiveness of continuous epidural analgesia on acute herpes zoster and postherpetic neuralgia: a retrospective study. Medicine (Baltimore) 2018;97:e9837. doi: 10.1097/MD.0000000000009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar V, Krone K, Mathieu A. Neuraxial and sympathetic blocks in herpes zoster and postherpetic neuralgia: an appraisal of current evidence. Reg Anesth Pain Med. 2004;29:454–61. doi: 10.1016/j.rapm.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Dworkin RH, O'Connor AB, Kent J, Mackey SC, Raja SN, Stacey BR, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154:2249–61. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutgers MJ, Dirksen R. The prevention of postherpetic neuralgia: a retrospective view of patients treated in the acute phase of herpes zoster. Br J Clin Pract. 1988;42:412–4. [PubMed] [Google Scholar]
- 25.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaglstein WH, Katz R, Brown JA. The effects of early corticosteroid therapy on the skin eruption and pain of herpes zoster. JAMA. 1970;211:1681–3. [PubMed] [Google Scholar]
- 27.Lee YB, Park JT, Han JW, Yoon KB. The efficacy of epidural blockade on acute herpes zoster. Korean J Pain. 1999;12:183–7. [Google Scholar]
- 28.Lee IH, Kim BS, Lee SC, Jo DH. The effect of stellate ganglion block on the pain of acute stage and the prevention of postherpetic neuralgin in the treatment of senile herpes zoster patients. Korean J Dermatol. 1999;37:571–9. [Google Scholar]
- 29.Smith FP. Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol. 1978;10:50–3. [PubMed] [Google Scholar]
- 30.Lundborg G. Structure and function of the intraneural microvessels as related to trauma, edema formation, and nerve function. J Bone Joint Surg Am. 1975;57:938–48. [PubMed] [Google Scholar]
- 31.Johnson RW, Rice AS. Clinical practice: postherpetic neuralgia. N Engl J Med. 2014;371:1526–33. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 32.Cho SJ, Kim SW, Lee SA, Kim JH. Ultrasoud guided transverse abdomins plane block for postherptic neuralgia. Ann Geriatr Med Res. 2016;20:163–5. [Google Scholar]
- 33.Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;(2):CD006866. doi: 10.1002/14651858.CD006866.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]


