Abstract
The aims of present works were to explore the difference in pulp breakdown of ‘Fuyan’ and ‘Dongbi’ longans and its relationship with cell wall metabolism. Comparison with ‘Fuyan’ longan fruit, postharvest ‘Dongbi’ longan fruit showed lower pulp breakdown index, lower activities of PE, PG, cellulase, β-Gal, XET, and lower expression levels of their corresponding genes. In addition, higher levels of cell wall polysaccharides including ISP, CSP, cellulose and hemicellulose were exhibited in ‘Dongbi’ longan pulp. These findings implied that, the reduced activities of enzymes and the down-regulated expressions of genes-involved in cell wall disassembly were shown in ‘Dongbi’ longan pulp, which might reduce the dissolution of polysaccharides and maintain a higher structural integrity in ‘Dongbi’ longan pulp cell wall, and consequently the mitigated pulp breakdown was displayed in ‘Dongbi’ longan during storage.
Keywords: Longan fruit, Cultivars, Pulp breakdown, Cell wall-degrading enzymes, Gene expression, Cell wall polysaccharides
Graphical abstract

Highlights
-
•
Dongbi longan showed a higher level of pulp cell wall materials than Fuyan longan.
-
•
Dongbi longan had higher contents of pulp cell wall polysaccharides than Fuyan longan.
-
•
Dongbi longan had lower pulp cell wall degrading enzymes activities than Fuyan longan.
-
•
Dongbi longan had lower expression level of cell wall degrading enzymes related-genes.
-
•
Dongbi longan had better integrity of the pulp cell wall structure than Fuyan longan.
Abbreviations
- ANOVA
analysis of variance
- CSP
covalent-soluble pectin
- Cx
cellulase
- CWDEs
cell wall-disassembling enzymes
- CWM
cell wall materials
- DlPE, DlPG, DlCx, DlXET and
DlPE, DlPG, DlCx, DlXET expresses the protein and gene of PG, PE, Cx and XET in pulp of longan fruit, separately
- β-Gal
β-galactosidase
- ISP
ionic-soluble pectin
- NCBI
National Center for Biotechnology Information
- PCR
polymerase chain reaction
- PE
pectinesterase
- PG
polygalacturonase
- PVP
polyvinyl pyrrolidone
- RH
relative humidity
- WSP
water-soluble pectin
- XET
xyloglucan endotransglycosylase
1. Introduction
Longan is widely appreciated for its various health benefits, unique taste, and high nutritional value (L.J. Lin et al., 2019; Y.F. Lin et al., 2019). However, the concentrated harvest season of fresh longan is the season with high temperature from July to August, the harvested longans are easy to fruit decay, pulp breakdown and pericarp browning, and lose their commercial value after being stored for about a week (L.J. Lin et al., 2019; Lin et al., 2020; Chen et al., 2021; Lin et al., 2022). The pulp breakdown of longan is analogous to the softening of climacteric fruits, which manifests as the pulp quickly softening, juice-flow and erosion (Chen et al., 2022). This phenomenon led to the quality deterioration and the declined commodity value of harvested fresh longan (L.J. Lin et al., 2019; Chen et al., 2021, 2022). Therefore, studying the mechanism of longan pulp breakdown and looking for its control method are very important for maintaining quality of longan fruit and improving the economic benefit of longan industry.
The structural composition of the cell wall is deemed to be a crucial factor in the softening of fresh produces like plum (He et al., 2022), blueberry (Ji et al., 2021), and apricot (Cui et al., 2021). Cellulose, hemicellulose and pectin are the primary polysaccharides in plant cell walls (Y.F. Lin et al., 2019; Liu et al., 2020; Frempong et al., 2022; He et al., 2022; Huang et al., 2022; Wang et al., 2022; Yu et al., 2022). β-Galactosidase (β-Gal), cellulase (Cx), pectinesterase (PE), xyloglucan endotransglycosylase (XET), and polygalacturonase (PG) are the main enzymes causing the degradation of cell wall components (Lin et al., 2018, 2022; Chen et al., 2021; Huang et al., 2022). Ren et al. (2020) reported that cell wall polysaccharides, mainly pectin, and the enzymes (PG, Cx and PE) involving in cell wall-disassembly, were engaged in the softening process of custard apple. However, the softening of postharvest fruit could be accelerated by the disintegration of cell wall polysaccharides under certain treatments. For example, exogenous ethylene (Fan et al., 2018) or abscisic acid (Zhou et al., 2021) treatments could aggravate postharvest fruit softening by promoting cell wall metabolism. Whereas, ethanol vapor (Ji et al., 2021), 1-methylcyclopropene (Wang et al., 2020), and trisodium phosphate (Ge et al., 2020) treatment could retard the breakdown of polysaccharides in the cell wall by suppressing cell wall-degrading enzymes (CWDEs) activities, thereby delaying fruit softening.
In addition, different varieties of fresh produces present different symptoms of texture softening, resulting from different dissolution degrees of cell wall components in different varieties of fresh produces after harvest (Yoshioka et al., 2011; Burdon et al., 2021; Win et al., 2021). Wei et al. (2015) demonstrated that the difference of softening behavior between ‘Jingbaili’ and ‘Yali’ Chinese pear fruits was intimately associated with cell wall pectin fraction, CWDEs activities and the related gene expression. Our previous work showed that different cultivars of longan fruit differed in pulp breakdown and fruit storability (Zhang et al., 2013). Further study revealed that the different pulp breakdown processes of the two cultivars were related to the metabolisms of respiratory, energy, and membrane lipid (L.J. Lin et al., 2019; Lin et al., 2023). Compared to ‘Fuyan’ longan, ‘Dongbi’ longan, which less prone to aril breakdown, exhibited a weaker respiratory rate (L.J. Lin et al., 2019), and the reduced metabolisms of energy and membrane lipid (Lin et al., 2023). Importantly, part components of the cell wall are also crucial substrates for respiration. Whereas, the differences in pulp breakdown between the two longan cultivars and their relationship with cell wall metabolism remain unclear. Therefore, the difference of pulp breakdown and cell wall polysaccharides amounts, CWDEs activities and relative gene expression levels in ‘Fuyan’ and ‘Dongbi’ longans were carried out in this study, so as to provide theoretical basis for the selection of longan varieties that can be stored and transported for long-distance sale.
2. Materials and methods
2.1. Longan fruit and treatment
Fruit of two longan cultivars cv. ‘Fuyan’ and ‘Dongbi’ were obtained from a commercial orchard named Nanjin Longan Orchard in Fujian. They received the same field management such as the same kind and amount of fertilizer, fungicides and insecticides, as well as the same irrigation and pruning management. ‘Fuyan’ and ‘Dongbi’ longan were harvested about 120 and 110 days after full blossom, respectively. What's more, the values of chromaticity L*, a* and b* at harvest were 86.73 ± 1.30, −194.31 ± 5.48 and 74.97 ± 2.16 in ‘Fuyan’ longan. Those values in ‘Dongbi’ longan were 94.27 ± 0.29, −128.52 ± 10.12 and 49.89 ± 4.06, respectively. After transported to the laboratory by a refrigerated vehicle, longan fruit were selected based on without blemishes and disease, and with uniform color and shape. The selected fruit were used for following treating procedures, including sterilization, rinsing, air-drying and packing (50 fruit per polyethylene bag), afterwards the treated longans were stored at 25 ± 1 °C and 90% RH. During the storage period, 3 bags of samples (150 longan) were collected daily to determine the following metrics.
2.2. Evaluation and measurement of cell wall materials (CWM)
The methods of Y.F. Lin et al. (2019) and Nguyen et al. (2021) were applied for measuring the CWM content with some modifications. Frozen pulp samples (50 g) from 30 longan fruits were added to 80% ethanol (200 mL) and boiled for half an hour. The mixture was centrifuged (4000×g, 4 °C, 20 min), and then the sediments were washed 80% ethanol solution for three times. The residues were dissolved in 90% dimethyl sulfoxide (50 mL) for 8 h. CWM was obtained using filter under a vacuum pump, and the residues were washed with 80% acetone for three times (200 mL), and finally dried in the air oven for 3 day at 40 °C. The result was expressed with g kg−1 based on longan pulp fresh weight.
2.3. Measurement of cell wall polysaccharides
The methods of our published works (Y.F. Lin et al., 2019; Chen et al., 2021) were applied to assay polysaccharides contents in longan pulp cell wall. M-hydroxydiphenyl method was used to determine contents of pectin (WSP, CSP, ISP), additionally, the anthrone method was adopted to detect cellulose and hemicellulose contents. The units of the contents of those cell wall polysaccharides were expressed with g kg−1 based on longan pulp fresh weight.
2.4. Assessments of CWDEs activities
The methods of our published works (Y.F. Lin et al., 2019; Chen et al., 2021; Sun et al., 2022) were adopted to detect activities of CWDEs (β-Gal, PE, Cx, XET and PG). Coomassie brilliant blue was applied to determine the protein amount of enzyme solution, and bovine serum albumin was used as the standard (Bradford, 1976). U kg−1 protein was employed to express these enzyme activities.
2.5. RNA extraction
The hot borate method of Kuang et al. (2012) was employed for extracting total RNA from longan pulp tissue. The total RNA concentration and quality were measured spectrophotometrically at 260 nm. In addition, 1% agarose denaturing gel electrophoresis was used to check the integrity of RNA.
2.6. Determination of the relative gene expression
In this study, the primers were synthesized via Sangon Biotech Co., Ltd. (Shanghai, China). The full-length cDNA sequences of DlPE, DlPG, DlCx and DlXET were extracted from longans and named as DlPE1, DlPE3, DlPG3, DlPG5, DlCx1, DlCx2 and DlXET3 according to their phylogenetic characteristics (Fig. S1 and S2). The DlPE, DlPG, DlCx and DlXET genes matched to the library of longan genome sequences (Lin et al., 2017). They are DlPE1 (GenBank ID: Dlo_023498.1), DlPE3 (GenBank ID: Dlo_017851.1), DlPG3 (GenBank ID: Dlo_021895.1), DlPG5 (GenBank ID: Dlo_020054.1), DlCx1 (GenBank ID: Dlo_003232.1), DlCx2 (GenBank ID: Dlo_018095.1) and DlXET3 (GenBank ID: Dlo_011343.2).
The transcription levels of the cell wall related genes were determined by quantitative RT-PCR analysis. The RNA extracts were treated with DNase I, then the gDNA Eraser and PrimeScript ™ RT Kit was used to synthesize the first-strand cDNA from the DNA-free total RNA. Gene-specific primers (Table 1) were obtained (Primer 5.0 software) bases on sequences registered in GenBank. In addition, RT-qPCR was performed with the TB Green ™ Premix Ex Taq ™ Kit (TaKaRa, RR420A) on QuantStudio 5 Real-Time PCR system (Thermo Fisher Scientific Co. Ltd., United States). The recipe of qPCR mixture was prepared according to the method described in TB Green ™ Premix Ex Taq ™ Kit (TaKaRa, RR420A) provided by company. The qPCR conditions were set as 30 s at 95 °C, 40 cycles of 95 °C for 5 s, and then 34 s at 60 °C. The DlActin gene was set as an internal control gene for normalization. The mean threshold cycle (Ct) was used for data analyses.
Table 1.
Primers used for real time quantitative PCR analysis.
| Gene | Gene number | Forward primer (5′–3′) | Reverse primer (5′–3′) | Product (bp) |
|---|---|---|---|---|
| DlPE1 | Dlo_023498.1 | TCTCCACCACCATTTATTGC | CAACTTGAGGCAGTCATTCC | 257 |
| DlPE3 | Dlo_017851.1 | GCTCCCTCTGACTCCATCCC | ATCAAACACTGTCCCGCAAAC | 273 |
| DlPG3 | Dlo_021895.1 | GAATTGTAGCCGAGGACCCTG | GGTCCACGATGTTCCATTCCT | 287 |
| DlPG5 | Dlo_020054.1 | AAGGGACTGCTTTGTGGGC | GTGGCGATCCTGGTGTTGAG | 265 |
| DlCx1 | Dlo_003232.1 | TGCCCTCTAACCAAAGGCTCAC | ATTCAATGACACTCCAGGACAACA | 165 |
| DlCx2 | Dlo_018095.1 | CTCGCCGGAGTGGACTTGAC | CGTAGAGCTTGCCGGGAGTG | 214 |
| DlXET3 | Dlo_011343.2 | GGGAGATGGTCGTGGGAAGA | TGGTTGACCGCTCAGATTGC | 238 |
| DlActin | Dlo_013887.2 | TGGTGGTTCAACTATGTTCCCTG | ATGGACCAGACTCGTCATACTCAC | 203 |
2.7. Bioinformatics analysis
Blast and DNAMAN8 of NCBI were used to analyze the full-length sequences of the obtained genes. The cDNA sequences of the DlPEs, DlPGs, DlCxs and DlXETs were aligned with the homologous sequences of other species by NCBI Blast website (Table S1, Fig. S3). Bioinformatics analysis of DlPEs, DlPGs, DlCxs and DlXETs protein were performed using the following analytical tools: Analysis of physicochemical characterization of DlPEs, DlPGs, DlCxs and DlXETs (https://web.expasy.org/protparam/) (Table S2), predicting the subcellular localization of DlPEs, DlPGs, DlCxs and DlXETs proteins (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/) (Table S3), and drawing the phylogenetic trees based on amino acid sequences of DlPEs, DlPGs, DlCxs, DlXETs and PEs, PGs, Cxs, XETs from other plant species were established by MEGA5.0 software (Fig. S2).
2.8. Statistical analyses
Three replicates for each experiment were conducted. The data in the figure were indicated as mean ± standard error (n = 3). SPSS 21.0 software was applied to analyze the experimental data through ANOVA, the notable discrepancies between ‘Fuyan’ and ‘Dongbi’ longans on the same day of storage were presented by * (P < 0.05) or ** (P < 0.01). The mark *, ** or *** in the value of correlation coefficient r respectively represented the correlation at the level P < 0.05, P < 0.01, or P < 0.001.
3. Results and discussion
3.1. The relationship between the difference of pulp breakdown and CWM content in ‘Fuyan’ and ‘Dongbi’ longans
The CWM is mainly composed of polysaccharides, whose massive decomposition result in the destruction of the overall structure (Chen et al., 2021). Previous works verified that electron beam radiation treatment restrained the decomposition of CWM, keeping a relatively complete structure and delaying the softening of postharvest mangoes (Nguyen et al., 2021). Therefore, reducing the degradation of CWM is an important factor to keep the structural integrity of cell wall.
Our research showed that the CWM content (Fig. 1) in ‘Fuyan’ and ‘Dongbi’ longans decreased constantly at 0–6 d. Both two longan cultivars decreased rapidly at 2–5 d, then ‘Dongbi’ longan fuit decreased slightly at the last day, while ‘Fuyan’ longans decreased sharply. Furthermore, correlation analysis indicated negative relationship between pulp breakdown index (Fig. S1) and CWM amount (Fig. 1) of ‘Fuyan’ longans (rFuyan = −0.985∗∗∗) and ‘Dongbi’ longans (rDongbi = −0.956∗∗∗). Consequently, it could draw the conclusion that pulp breakdown might be resulted from the reduction of dry weight of CWM. In addition, the CWM content in ‘Dongbi’ longans was maintained at a higher level compared with that of ‘Fuyan’ longans over the entire storage. Its content in ‘Dongbi’ longans was 32.83% higher than ‘Fuyan’ longans at the end of storage. These data illustrated that ‘Dongbi’ longan fruit could maintain better pulp integrity of longan fruit because of its high CWM content. This finding was in line with previous study on the suppression of CWM disassembly by valeric acid treatment to sustain the firmness of postharvest ‘Waizuili’ plum fruit (He et al., 2022).
Fig. 1.
Changes in contents of cell wall materials of ‘Fuyan’ and ‘Dongbi’ longan fruit stored for 6 d at 25 °C. Each value is expressed as the mean ± standard error (n = 3). *P < 0.05 and **P < 0.01 indicated the level of significant differences between ‘Fuyan’ and ‘Dongbi’ longans.
3.2. The relationship between the difference of pulp breakdown and cell wall polysaccharides compositions in ‘Fuyan’ and ‘Dongbi’ longans
Postharvest fruit softening or pulp breakdown is owing to polysaccharides degradation, which lead to the changes of cell structure and texture in fruit (Luo, 2006; He et al., 2022). Hemicelluloses, cellulose, and pectin are the major composition of cell wall polysaccharides in fresh produces (Y.F. Lin et al., 2019; Hassan et al., 2020; Ji et al., 2021; Nguyen et al., 2021). More in detail, pectin exists in the intercellular layer between adjacent cell walls and acts to stick cells together (Willats et al., 2001; Li et al., 2021). During fruit ripening, the degradation of pectin can lead to the breakdown of cellulose-hemicellulose network in cell wall, resulting to a decrease in fruit hardness, which was related to the rise of WSP content and the decline of ISP and CSP content (Duan et al., 2008; Xie et al., 2017; Chen et al., 2021). Cellulose consists of linear chains primarily made up of β-1,4-D-glucan, which constitutes the primary and secondary cell walls in plant (Broxterman and Schols, 2018). Hemicellulose is composed of xylans, xyloglucans and mannans, which are attached to cellulose microfibrils to constitute a cellulose-matrix network that maintains cell wall intensity (Y.F. Lin et al., 2019; Damasceno Junior et al., 2022). Zhou et al. (2021) elucidated that cell wall polysaccharides in blueberry fruit was enhanced by abscisic acid treatment, thereby promoted the postharvest softening of blueberry. Therefore, cell wall polysaccharides contribute greatly to fruit softening or pulp breakdown.
In this study, ISP, CSP, cellulose and hemicellulose contents (Fig. 2A-B, 2D-E) showed a decreasing trend as storage time prolonged in ‘Fuyan’ and ‘Dongbi’ longans. In contrast, the WSP content (Fig. 2C) gradually incremented. As showed in Table 2, correlation analyses indicated that the breakdown index of longan pulp (Fig. S1) showed the negative relationships with CSP content (Fig. 2A) (r1 Fuyan = −0.977∗∗∗; r1 Dongbi = −0.886∗∗, respectively), ISP content (Fig. 2B) (r2 Fuyan = −0.889∗∗; r2 Dongbi = −0.852∗∗, respectively), cellulose content (Fig. 2D) (r3 Fuyan = −0.964∗∗∗; r3 Dongbi = −0.917∗∗, respectively), and hemicellulose content (Fig. 2E) (r4 Fuyan = −0.942∗∗; r4 Dongbi = −0.840∗, respectively) in ‘Fuyan’ and ‘Dongbi’ longans. On the contrary, there was a positive relationship between pulp breakdown index (Fig. S1) and WSP amount (Fig. 2C) of ‘Fuyan’ longans (rFuyan = 0.867∗∗) and ‘Dongbi’ longans (rDongbi = 0.879∗∗). These data illustrated that there were the close correlations between the degradation of cell wall polysaccharides (ISP, CSP, cellulose and hemicellulose) and the pulp breakdown in postharvest longan.
Fig. 2.
Changes in contents of CSP (A), ISP (B), WSP (C), cellulose (D) and hemicellulose (E) in pulp of ‘Fuyan’ and ‘Dongbi’ longan fruit stored for 6 d at 25 °C. Each value is expressed as the mean ± standard error (n = 3). *P < 0.05 and **P < 0.01 indicated the level of significant differences between ‘Fuyan’ and ‘Dongbi’ longans.
Table 2.
Correlation analysis of breakdown index in pulp of ‘Fuyan’ and ‘Dongbi’ longans.
| Breakdown index | CWM | CSP | ISP | WSP | Cellulose | Hemicellulose | PE | PG | Cx | β-Gal | XET | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Fuyan’ longans | ||||||||||||
| Breakdown index | 1 | |||||||||||
| CWM | −0.985 | 1 | ||||||||||
| CSP | −0.977 | – | 1 | |||||||||
| ISP | −0.889 | – | – | 1 | ||||||||
| WSP | 0.867 | – | −0.933 | −0.818 | 1 | |||||||
| Cellulose | −0.964 | – | – | – | – | 1 | ||||||
| Hemicellulose | −0.942 | – | – | – | – | – | 1 | |||||
| PE | 0.936 | – | −0.965 | −0.901 | 0.774 | – | – | 1 | ||||
| PG | 0.926 | – | −0.972 | −0.901 | 0.864 | – | – | – | 1 | |||
| Cx | 0.946 | – | – | – | – | −0.972 | −0.964 | – | – | 1 | ||
| β-Gal | 0.905 | – | −0.965 | −0.906 | 0.913 | – | – | – | – | – | 1 | |
| XET | 0.883 | – | – | – | – | −0.914 | −0.905 | – | – | – | – | 1 |
| ‘Dongbi’ longans | ||||||||||||
| Breakdown index | 1 | |||||||||||
| CWM | −0.956 | 1 | ||||||||||
| CSP | −0.886 | – | 1 | |||||||||
| ISP | −0.852 | – | – | 1 | ||||||||
| WSP | 0.879 | – | −0.986 | −0.950 | 1 | |||||||
| Cellulose | −0.917 | – | – | – | – | 1 | ||||||
| Hemicellulose | −0.840 | – | – | – | – | – | 1 | |||||
| PE | 0.953 | – | −0.859 | −0.839 | 0.808 | – | – | 1 | ||||
| PG | 0.934 | – | −0.949 | −0.930 | 0.941 | – | – | – | 1 | |||
| Cx | 0.880 | – | – | – | – | −0.974 | −0.972 | – | – | 1 | ||
| β-Gal | 0.921 | – | −0.961 | −0.939 | 0.957 | – | – | – | – | – | 1 | |
| XET | 0.977 | – | – | – | – | −0.859 | −0.777 | – | – | – | – | 1 |
Note: “-” means the correlation analysis is not done. CWM, cell wall materials; CSP, covalent-soluble pectin; ISP, ionic-soluble pectin; WSP, water-soluble pectin; PE, pectinesterase; PG, polygalacturonase; Cx, cellulase; β-Gal, β-galactosidase; XET, xyloglucan endotransglycosylase.
Moreover, WSP content of ‘Fuyan’ longans and ‘Dongbi’ longans were negatively associated with CSP content (rFuyan = −0.933∗∗; rDongbi = −0.986∗∗∗, respectively) and ISP content (rFuyan = −0.818∗; rDongbi = −0.950∗∗, respectively, Table 2) at 0–5 d. WSP level increased significantly at 0–5 d, while CSP and ISP levels decreased, indicating a gradual dissolution of water-insoluble pectin into WSP, which could cause a continuous lysis of the cell wall. In addition, ‘Dongbi’ longans had lower WSP content and higher amounts of ISP, CSP, cellulose and hemicellulose compared with that of ‘Fuyan’ longans over the entire storage. These findings implied that the cell wall polysaccharides of ‘Dongbi’ longan pulp were less susceptible to decomposition under the same storage conditions, which could keep an integrity of pulp cell walls and delay the occurrence of pulp breakdown in ‘Dongbi’ longan.
3.3. The relationship between the difference of pulp breakdown and the activities of CWDEs in ‘Fuyan’ and ‘Dongbi’ longans
CWDEs promote softening development in fresh fruit owing to the depolymerization of cell wall substances, causing the ultrastructural changes in cell walls (Ren et al., 2020). Therefore, CWDEs containing β-Gal, PE, Cx, XET and PG have a significant impact on the decomposition and softening development of cell wall polysaccharides (Lin et al., 2018; Y.F. Lin et al., 2019; Lin et al., 2020). First of all, PE and PG regulate the enzymatic hydrolysis of pectin polysaccharides (Lin et al., 2018). The role of PE is to promote the conversion of pectin ester acid into pectin acid, to increase the solubility of pectin in water, and to provide necessary conditions for the catalytic effect of PG (Rugkong et al., 2010; Lin et al., 2018, 2020). PG hydrolyzes the 1, 4-2-D-galactoside bonds of pectic acid, disintegrates the structure of the cell wall and finally results in a loss of fruit hardness (Nunes et al., 2008; Y.F. Lin et al., 2019). Cx is mainly involved in cellulose and hemicellulose disintegration, which can destroy the pectin-cellulose-hemicellulose network (Chen et al., 2017). β-Gal also has an important effect on pectin disassembly (Lin et al., 2018), which contributes to fruit softening by removing galactose residues from cell wall polymers (Wei et al., 2010). XET depolymerizes the xyloglucan chains connected with cellulose microfibers, causing irreversible rupture of the xyloglucan chains (Li et al., 2009; Y.F. Lin et al., 2019). Ji et al. (2021) showed that ethanol vapor treatment could reduce CWDEs related activities to prevent decomposition of cell wall components, thereby delaying blueberry softening. A similar work was found in jujube that trisodium phosphate treatment could delay fruit softening by inhibiting CWDEs (PE, PG, cellulase, β-Gal) activities (Ge et al., 2020).
The current study showed that PE, PG and β-gal activities in ‘Fuyan’ and ‘Dongbi’ longans enhanced at 0–6 d (Fig. 3A, B, 3D). Correlation analyses indicated that there was a negative relationship between CSP content (Fig. 2A) and the activities of PE (Fig. 3A) (r1 Fuyan = −0.965∗∗∗; r1 Dongbi = −0.859∗∗, respectively, Table 2), PG (Fig. 3B) (r2 Fuyan = −0.972∗∗∗; r2 Dongbi = −0.949∗∗, respectively, Table 2) and β-gal (Fig. 3D) (r3 Fuyan = −0.965∗∗∗; r3 Dongbi = −0.961∗∗∗, respectively, Table 2) in ‘Fuyan’ and ‘Dongbi’ longans. The relationships between content of ISP (Fig. 2B) with activities of PE, PG and β-Gal (Fig. 3A, B, 3D) were similar to that of CSP content, but the correlation coefficients were different (Table 2). Nevertheless, the PE, β-Gal and PG activities were positively associated to WSP content, with rFuyan and rDongbi values of 0.774, 0.864, 0.913 and 0.808, 0.941, 0.957, separately. Additionally, there was a positive relationship between pulp breakdown index (Fig. S1) and activities of PE (Fig. 3A) (r1 Fuyan = 0.936∗∗; r1 Dongbi = 0.953∗∗∗, respectively, Table 2), PG (Fig. 3B) (r2 Fuyan = 0.926∗∗; r2 Dongbi = 0.934∗∗, respectively, Table 2) and β-gal (Fig. 3D) (r3 Fuyan = 0.905∗∗; r3 Dongbi = 0.921∗∗, respectively, Table 2) in ‘Fuyan’ and ‘Dongbi’ longans. The above results indicated that the higher activities of PE, β-Gal and PG help promote the degradation of CSP to WSP, and thus stimulate the breakdown in ‘Fuyan’ and ‘Dongbi’ longan pulp.
Fig. 3.
Changes in activities of PE (A), PG (B), Cx (C), β-Gal (D) and XET(E) in pulp of ‘Fuyan’ and ‘Dongbi’ longan fruit stored for 6 d at 25 °C. Each value is expressed as the mean ± standard error (n = 3). *P < 0.05 and **P < 0.01 indicated the level of significant differences between ‘Fuyan’ and ‘Dongbi’ longans.
In addition, it showed that the activities of Cx (Fig. 3C) and XET (Fig. 3E) of ‘Fuyan’ and ‘Dongbi’ longans also gradually raised with the prolonged storage day. Correlation analyses indicated that there was a negative relationship between Cx activity (Fig. 3C) with the contents of cellulose (Fig. 2D) (rFuyan = −0.972∗∗∗; r Dongbi = −0.974∗∗∗, respectively, Table 2) and hemicelluloses (Fig. 2F) (rFuyan = −0.964∗∗∗; rDongbi = −0.972∗∗∗, respectively, Table 2). But the Cx activity displayed the positive correlations with the index of pulp breakdown (r3 Fuyan = 0.946∗∗; r3 Dongbi = 0.880∗∗, respectively, Table 2) in ‘Fuyan’ and ‘Dongbi’ longans. The relationships between XET activity (Fig. 3E) with cellulose (Fig. 2D) and hemicelluloses contents (Fig. 2F) were similar to that of Cx activity (Fig. 3C), but with rFuyan and rDongbi values of −0.914, −0.905 and −0.859, −0.777, separately. These results suggested that Cx and XET activities were involved in the decompositions of hemicellulose and cellulose, which led to the breakdown of longan pulp.
Additionally, the β-Gal, PE, Cx, XET and PG activities of ‘Dongbi’ longans were maintained at lower levels compared with that of ‘Fuyan’ longans over the entire storage (Fig. 3). The above results demonstrated that ‘Dongbi’ longans had lower cell wall degrading related enzymes that could slow down the decomposition of cell wall material including pectic substances, cellulose and hemicellulose, thus maintain a higher CWM content. Therefore, ‘Dongbi’ longans maintained a better pulp cell wall structure, so as to delay the occurrence of pulp breakdown.
3.4. The relationship between the difference of pulp breakdown and the expressions of CWDEs related genes in ‘Fuyan’ and ‘Dongbi’ longans
Different levels of genes expression can affect the change of cell wall related enzyme activities, so as to influence the occurrence of pulp breakdown (Nguyen et al., 2021; Zhou et al., 2021). The transcriptional levels of the DlPEs and DlPGs are related to PE and PG activities, respectively, thus affect the cell wall decomposition in fruits (Fan et al., 2018; Win et al., 2021). In addition, Cx and XET activities are contributed greatly to the decomposition of hemicelluloses and cellulose, so their related genes may also play an important part in pulp breakdown (Shi et al., 2019; Lin et al., 2020). Some reports demonstrated that the close relationship between cell wall-degrading enzymes related genes and softening in fresh produces like apple (Win et al., 2021), cherimoya (Li et al., 2009) and mango (Nguyen et al., 2021) fruit.
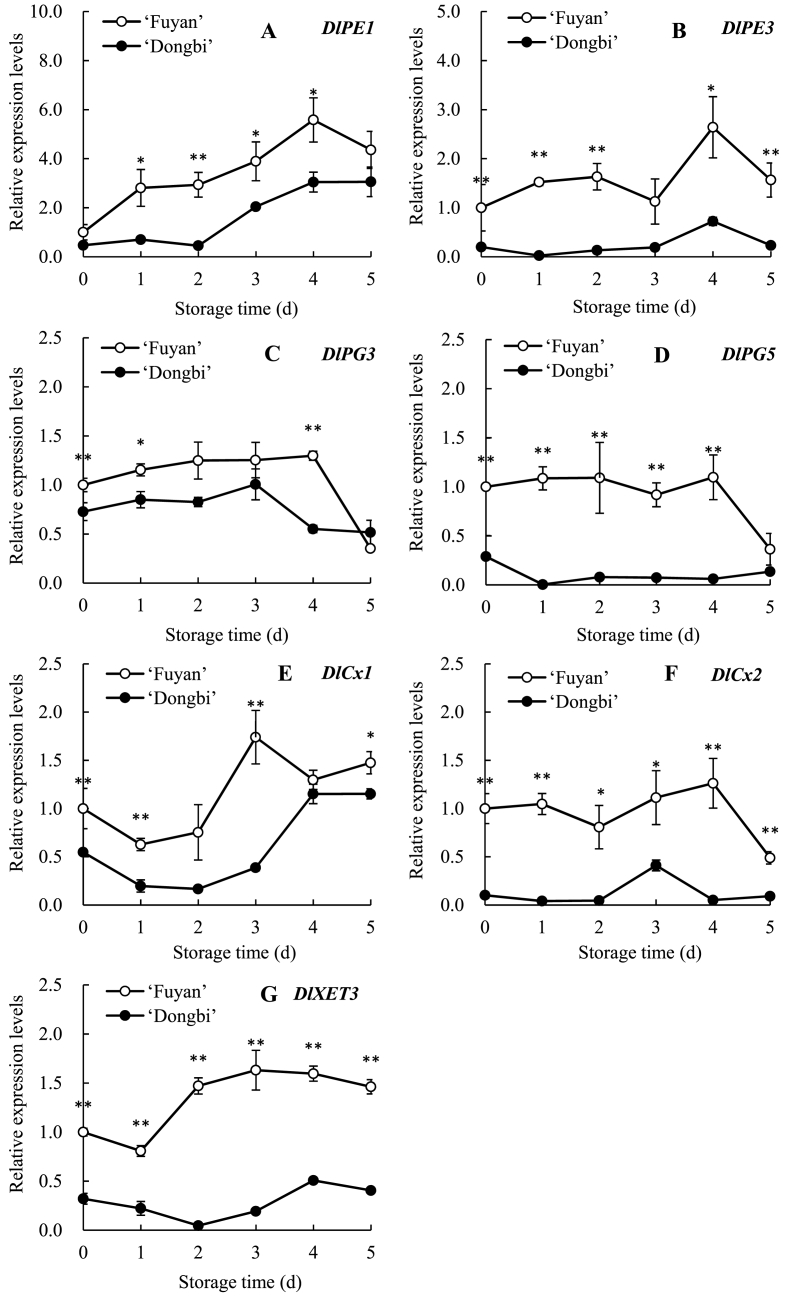
In our experiments, the expression characteristics of cell wall enzymes related genes were studied by Real-time-PCR. The results showed that the degree of expression varies for different genes. For the relative expression level of DlPE1, DlPE3, DlPG3, DlPG5, DlCx1, DlCx2 and DlXET3 in ‘Fuyan’ longans, they rose in the early stage of storage, peaked at 3 or 4 days, and then declined. The decrease of gene expression at the later stage of storage might be due to the RNA degradation with the prolongation of storage time and the acceleration of pulp breakdown. However, these change in ‘Dongbi’ longans was more gradual. Additionally, among them, the relative expression level of DlPE1, DlPE3, DlPG3 and DlXET3 (Fig. 4A–C, 4G) in pulp of ‘Fuyan’ and ‘Dongbi’ longans displayed a similar pattern of variation with the PE activity (Fig. 3A), PG activity (Fig. 3B) and XET activity (Fig. 3E) at 0–4 d. Therefore, it could be inferred that these genes were closely related to their corresponding enzyme activities. However, DlPG5 (Fig. 4D) in the two groups demonstrated different expression patterns during 0–4 d, with an overall increase in DlPG5 expression in ‘Fuyan’ longan, but an overall decrease in ‘Dongbi’ longan. These inconsistent patterns may be attributed to different cultivars longan fruits. In addition, the variation of Cx activity (Fig. 3C) was not tightly accorded with the transcription level of DlCx1 and DlCx2 (Fig. 4E and F), i.e., the activities of Cx increased over the entire storage, while DlCx1 and DlCx2 expression showed a rise only at 2–4 d. These results showed that Cx genes might play a primary role in the middle stage storage and the activities of Cx might be regulated by post-translational mechanisms.
Fig. 4.
Changes in relative expression levels of DlPE1 (A), DlPE3 (B), DlPG3 (C), DlPG5 (D), DlCx1 (E), DlCx2 (F) and DlXET3 (G) genes transcripts in pulp of two cultivars longan fruit stored for 6 d at 25 °C. Each value is expressed as the mean ± standard error (n = 3). *P < 0.05 and **P < 0.01 indicated the level of significant differences between ‘Fuyan’ and ‘Dongbi’ longans.
More importantly, compared two different varieties longans, ‘Dongbi’ longans displayed the lower expression levels of DlPE1, DlPE3, DlPG3, DlPG5, DlCx1, DlCx2 and DlXET3 (Fig. 4A–G), the lower PE, PG, Cx, XET activities (Fig. 3, Fig. 4E) and a lower pulp breakdown index (Fig. S1), but the higher values of ISP, CSP, cellulose and hemicellulose (Fig. 2A and B, 2D-E). These results indicated that the mitigated pulp breakdown in ‘Dongbi’ longan was due to the lower expression levels of CWDEs related-genes, which leading to the lower CWDEs activities and reducing the decompositions of cell wall polysaccharides, and thus maintaining a better structure of cell walls and mitigating the pulp breakdown of ‘Dongbi’ longan.
Based on the above data, the possible mechanisms for the different pulp breakdown performances of ‘Fuyan’ and ‘Dongbi’ longan from the perspective of cell wall metabolism are shown in Fig. 5. Therefore, ‘Dongbi’ longan can be selected for long-distance sales and longer storage. For ‘Fuyan’ longan, which is prone to pulp breakdown, it can be sold locally or short distance. Alternatively, we can use the treatments such as acidic electrolyzed water (Sun et al., 2022) or chitosan (Y.F. Lin et al., 2019) to delay the breakdown of cell wall structures to maintain better quality of longan.
Fig. 5.
The possible mechanisms of the different pulp breakdown performances in ‘Fuyan’ and ‘Dongbi’ longan from the perspective of cell wall metabolism.
4. Conclusions
It could be concluded from this study that, compared two longan cultivars cv. ‘Fuyan’ and ‘Dongbi’, ‘Dongbi’ longans had the lower activities of CWDEs (PE, PG, Cx, β-Gal and XET) and lower expression levels CWDEs related-genes (DlPE1, DlPE3, DlPG3, DlPG5, DlCx1, DlCx2 and DlXET3). All these actions reduced the decompositions of CWM and cell wall polysaccharide (ISP, CSP, cellulose and hemicellulose), thereby, maintaining a better structure of cell walls and slowing down fruit softening and pulp breakdown. These findings not only expose the difference of pulp breakdown between ‘Fuyan’ and ‘Dongbi’ longans and its relationship to cell wall metabolism, but also provide a theoretical basis for selecting longan varieties with a slow pulp breakdown and a durable storage.
CRediT authorship contribution statement
Lijuan Lin: Investigation, Formal analysis, Data curation, Writing – original draft. Yazhen Chen: Writing – original draft. Hetong Lin: Conceptualization, Supervision, Writing – review & editing, Project administration. Yixiong Lin: Project administration, Investigation. Zhongqi Fan: Investigation. Hui Wang: Investigation. Wangjin Lu: Supervision. Jianye Chen: Supervision. Yihui Chen: Investigation. Yifen Lin: Conceptualization, Supervision, Writing – review & editing, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant numbers U21A20275, 31701653, and 32102047), the Natural Science Foundation of Fujian Province (Grant numbers 2020J01560, and 2021J01996), and the Science and Technology Innovation Foundation at Fujian Agriculture and Forestry University of China (Grant numbers KFb22078XA, KFb22079XA, and CXZX2020113A).
Handling Editor: Professor A.G. Marangoni
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100496.
Contributor Information
Hetong Lin, Email: hetonglin@163.com.
Yifen Lin, Email: yifenlin@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broxterman S.E., Schols H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018;192:263–272. doi: 10.1016/j.carbpol.2018.03.070. [DOI] [PubMed] [Google Scholar]
- Burdon J., Martin P., Ireland H., Schaffer R., McAtee P., Boldingh H., Nardozza S. Transcriptomic analysis reveals differences in fruit maturation between two kiwifruit cultivars. Sci. Hortic. 2021;286 doi: 10.1016/j.scienta.2021.110207. [DOI] [Google Scholar]
- Chen Y.H., Hung Y.C., Chen M.Y., Lin H.T. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT--Food Sci. Technol. 2017;84:650–657. doi: 10.1016/j.lwt.2017.06.011. [DOI] [Google Scholar]
- Chen Y.Z., Yu J., Lin H.T., Zheng Y., Fan Z.Q., Wang H., Chen Y.H., Lin Y.F. Phomopsis longanae Chi-induced longan pulp breakdown and softening in relation to cell wall polysaccharides disassembly. Postharvest Biol. Technol. 2022;186 doi: 10.1016/j.postharvbio.2022.111837. [DOI] [Google Scholar]
- Chen Y.Z., Zhang S., Lin H.T., Lu W.J., Chen Y.H., Lin Y.F., Fan Z.Q. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chem. 2021;351 doi: 10.1016/j.foodchem.2021.129294. [DOI] [PubMed] [Google Scholar]
- Cui K.B., Yang L.L., Shu C., Liu J., Zhu Z.J., Yang Z.Q., Zhu X., Jiang W.B. Near freezing temperature storage alleviates cell wall polysaccharide degradation and softening of apricot (Prunus armeniaca L.) fruit after simulated transport vibration. Sci. Hortic. 2021;288 doi: 10.1016/j.scienta.2021.110296. [DOI] [Google Scholar]
- Damasceno Junior C.V., Godoy S., Gonela A., Scapim C.A., Grandis A., Santos W.D., Mangolin C.A., Buckeridge M.S., Machado M.F.P.S. Biochemical composition of the pericarp cell wall of popcorn inbred lines with different popping expansion. Curr. Res. Food Sci. 2022;5:102–106. doi: 10.1016/j.crfs.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.W., Cheng G.P., Yang E., Yi C., Ruenroengklin N., Lu W.J., Luo Y.B., Jiang Y.M. Modification of pectin polysaccharides during ripening of postharvest banana fruit. Food Chem. 2008;111:144–149. doi: 10.1016/j.foodchem.2008.03.049. [DOI] [Google Scholar]
- Fan X.G., Shu C., Zhao K., Wang X.M., Cao J.K., Jiang W.B. Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci. Hortic. 2018;232:63–70. doi: 10.1016/j.scienta.2017.12.061. [DOI] [Google Scholar]
- Frempong K.E.B., Chen Y., Liang L.L., Lin X.Y. Effect of calcium chloride and 1-methylcyclopropene combined treatment on pectin degradation and textural changes of Eureka lemon during postharvest storage. Curr. Res. Food Sci. 2022;5:1412–1421. doi: 10.1016/j.crfs.2022.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y.H., Wu Y.F., Zhang J.H., Li C.Y., Xue W.J., Zhang S.Y., Lv J.Y. Trisodium phosphate delays softening of jujube fruit by inhibiting cell wall-degrading enzyme activities during ambient storage. Sci. Hortic. 2020;262 doi: 10.1016/j.scienta.2019.109059. [DOI] [Google Scholar]
- Hassan S.S., Williams G.A., Jaiswal A.K. Computational modelling approach for the optimization of apple juice clarification using immobilized pectinase and xylanase enzymes. Curr. Res. Food Sci. 2020;3:243–255. doi: 10.1016/j.crfs.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M.Y., Wu Y.F., Wang Y., Hong M., Li T.T., Deng T.J., Jiang Y.M. Valeric acid suppresses cell wall polysaccharides disassembly to maintain fruit firmness of harvested ‘Waizuili’ plum (Prunus salicina Lindl) Sci. Hortic. 2022;291 doi: 10.1016/j.scienta.2021.110608. [DOI] [Google Scholar]
- Huang X.J., Ai C., Yao H.Y.Y., Zhao C.G., Xiang C.H., Hong T., Xiao J.B. Guideline for the extraction, isolation, purification, and structural characterization of polysaccharides from natural resources. eFood. 2022;3(6):e37. doi: 10.1002/efd2.37. [DOI] [Google Scholar]
- Ji Y., Hu W.Z., Liao J., Xiu Z.L., Jiang A.L., Guan Y.G., Yang X.Z., Feng K. Ethanol vapor delays softening of postharvest blueberry by retarding cell wall degradation during cold storage and shelf life. Postharvest Biol. Technol. 2021;177 doi: 10.1016/j.postharvbio.2021.111538. [DOI] [Google Scholar]
- Kuang J.F., Chen J.Y., Luo M., Wu K.Q., Sun W., Jiang Y.M., Lu W.J. Histone deacetylase HD2 interacts with ERF1 and is involved in longan fruit senescence. J. Exp. Bot. 2012;63:441–454. doi: 10.1093/jxb/err290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.R., Wei B.S., Wang J.L., Jiang Y.M., Xie J.H., Chen J.Y. 1-MCP delayed softening and affected expression of XET and EXP genes in harvested cherimoya fruit. Postharvest Biol. Technol. 2009;52:254–259. doi: 10.1016/j.postharvbio.2008.12.009. [DOI] [Google Scholar]
- Li L., Gao X.L., Liu J.G., Chitrakar B., Wang B., Wang Y.C. Hawthorn pectin: extraction, function and utilization. Curr. Res. Food Sci. 2021;4:429–435. doi: 10.1016/j.crfs.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.J., Lin Y.X., Lin H.T., Lin M.S., Ritenour M.A., Chen Y.H., Wang H., Hung Y.C., Lin Y.F. Comparison between 'Fuyan' and 'Dongbi' longans in aril breakdown and respiration metabolism. Postharvest Biol. Technol. 2019;153:176–182. doi: 10.1016/j.postharvbio.2019.04.008. [DOI] [Google Scholar]
- Lin Y.F., Lin L.J., Lin Y.X., Lin M.S., Ritenour M.A., Lin H.T. Comparison between two cultivars of longan fruit cv. ‘Dongbi’ and ‘Fuyan’ in the metabolisms of lipid and energy and its relation to pulp breakdown. Food Chem. 2023;398 doi: 10.1016/j.foodchem.2022.133885. [DOI] [PubMed] [Google Scholar]
- Lin Y.F., Lin Y.X., Lin H.T., Lin M.S., Li H., Yuan F., Chen Y.H. Effects of paper containing 1-MCP postharvest treatment on the disassembly of cell wall polysaccharides and softening in Younai plum fruit during storage. Food Chem. 2018;264:1–8. doi: 10.1016/j.foodchem.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Lin Y.F., Lin Y.Z., Lin Y.X., Lin M.S., Chen Y.H., Wang H., Lin H.T. A novel chitosan alleviates pulp breakdown of harvested longan fruit by suppressing disassembly of cell wall polysaccharides. Carbohydr. Polym. 2019;217:126–134. doi: 10.1016/j.carbpol.2019.04.053. [DOI] [PubMed] [Google Scholar]
- Lin Y.L., Min J.M., Lai R.L., Wu Z.Y., Chen Y.K., Yu L.L., Cheng C.Z., Jin Y.C., Tian Q.L., Liu Q.F., Liu W.H., Zhang C.G., Lin L.X., Hu Y., Zhang D.M., Thu M., Zhang Z.H., Liu S.C., Zhong C.S., Fang X.D., Wang J., Yang H.M., Varshney R.K., Yin Y., Lai Z.X. Genome-wide sequencing of longan (Dimocarpus longan Lour.) provides insights into molecular basis of its polyphenol-rich characteristics. Giga Sci. 2017;6:1–14. doi: 10.1093/gigascience/gix023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.X., Lin H.T., Wang H., Lin M.S., Chen Y.H., Fan Z.Q., Lin Y.F. Effects of hydrogen peroxide treatment on pulp breakdown, softening, and cell wall polysaccharide metabolism in fresh longan fruit. Carbohydr. Polym. 2020;242 doi: 10.1016/j.carbpol.2020.116427. [DOI] [PubMed] [Google Scholar]
- Lin Y.Z., Lin H.T., Lin M.S., Zheng Y., Chen Y.Z., Wang H., Fan Z.Q., Chen Y.H., Lin Y.F. DNP and ATP modulate the developments of pulp softening and breakdown in Phomopsis longanae Chi-infected fresh longan through regulating the cell wall polysaccharides metabolism. Food Chem. 2022;397 doi: 10.1016/j.foodchem.2022.133837. [DOI] [PubMed] [Google Scholar]
- Liu H., Xie M.Y., Nie S.P. Recent trends and applications of polysaccharides for microencapsulation of probiotics. Food Front. 2020;1:45–59. doi: 10.1002/fft2.11. [DOI] [Google Scholar]
- Luo Z.S. Extending shelf-life of persimmon (Diospyros kaki L.) fruit by hot air treatment. Eur. Food Res. Technol. 2006;222:149–154. doi: 10.1007/s00217-005-0156-1. [DOI] [Google Scholar]
- Nguyen T.T., Kato M., Ma G., Zhang L.C., Uthairatanakij A., Srilaong V., Laohakunjit N., Jitareerat P. Electron beam radiation delayed the disassembly of cell wall polysaccharides in harvested mangoes. Postharvest Biol. Technol. 2021;178 doi: 10.1016/j.postharvbio.2021.111544. [DOI] [Google Scholar]
- Nunes C., Saraiva J.A., Coimbra M.A. Effect of candying on cell wall polysaccharides of plums (Prunus domestica L.) and influence of cell wall enzymes. Food Chem. 2008;111:538–548. doi: 10.1016/j.foodchem.2008.04.016. [DOI] [Google Scholar]
- Ren Y.Y., Su P.P., Wang X.X., Zhu Z.Y. Degradation of cell wall polysaccharides and change of related enzyme activities with fruit softening in Annona squamosa during storage. Postharvest Biol. Technol. 2020;166 doi: 10.1016/j.postharvbio.2020.111203. [DOI] [Google Scholar]
- Rugkong A., Rose J.K.C., Lee S.J., Giovannoni J.J., O'Neill M.A., Watkins C.B. Cell wall metabolism in cold-stored tomato fruit. Postharvest Biol. Technol. 2010;57:106–113. doi: 10.1016/j.postharvbio.2010.03.004. [DOI] [Google Scholar]
- Shi Z.J., Yang H.Y., Jiao J.Y., Wang F., Lu Y.Y., Deng J. Effects of graft copolymer of chitosan and salicylic acid on reducing rot of postharvest fruit and retarding cell wall degradation in grapefruit during storage. Food Chem. 2019;283:92–100. doi: 10.1016/j.foodchem.2018.12.078. [DOI] [PubMed] [Google Scholar]
- Sun J.Z., Chen H.B., Xie H.L., Li M.L., Chen Y.H., Hung Y.C., Lin H.T. Acidic electrolyzed water treatment retards softening and retains cell wall polysaccharides in pulp of postharvest fresh longans and its possible mechanism. Food Chem. X. 2022;13 doi: 10.1016/j.fochx.2022.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chen Y.H., Lin H.T., Lin M.S., Chen Y.H., Lin Y.F. 1-Methylcyclopropene containing-papers suppress the disassembly of cell wall polysaccharides in Anxi persimmon fruit during storage. Int. J. Biol. Macromol. 2020;151:723–729. doi: 10.1016/j.ijbiomac.2020.02.146. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Yin J.Y., Hu J.L., Nie S.P., Xie M.Y. Gastroprotective polysaccharide from natural sources: review on structure, mechanism, and structure–activity relationship. Food Front. 2022;3(4):560–591. doi: 10.1002/fft2.172. [DOI] [Google Scholar]
- Wei J.M., Ma F.W., Shi S.G., Qi X.D., Zhu X.Q., Yuan J.W. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010;6:147–154. doi: 10.1016/j.postharvbio.2009.12.003. [DOI] [Google Scholar]
- Wei J.M., Qi X.D., Cheng Y.D., Guan J.F. Difference in activity and gene expression of pectin-degrading enzymes during softening process in two cultivars of Chinese pear fruit. Sci. Hortic. 2015;197:434–440. doi: 10.1016/j.scienta.2015.10.002. [DOI] [Google Scholar]
- Willats W.G.T., McCartney L., Mackie W., Knox J.P. Pectin: cell biology and prospects for functional analysis. Plant Mol. Biol. 2001;47:9–27. doi: 10.1023/A:1010662911148. [DOI] [PubMed] [Google Scholar]
- Win N.M., Yoo J.G., Naing A.H., Kwon J.G., Kang I.K. 1 Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021;180 doi: 10.1016/j.postharvbio.2021.111599. [DOI] [Google Scholar]
- Xie F., Yuan S.Z., Pan H.X., Wang R., Cao J.K., Jiang W.B. Effect of yeast mannan treatments on ripening progress and modification of cell wall polysaccharides in tomato fruit. Food Chem. 2017;218:509–517. doi: 10.1016/j.foodchem.2016.09.086. [DOI] [PubMed] [Google Scholar]
- Yoshioka H., Hayama H., Tatsuki M., Nakamura Y. Cell wall modifications during softening in melting type peach “Akatsuki” and non-melting type peach “Mochizuki”. Postharvest Biol. Technol. 2011;60:100–110. doi: 10.1016/j.postharvbio.2010.12.013. [DOI] [Google Scholar]
- Yu Q., Chen W., Zhong J., Huang D., Shi W.T., Chen H.Y., Yan C.Y. Purification, structural characterization, and bioactivities of a polysaccharide from Coreopsis tinctoria. Food Front. 2022;3:736–748. doi: 10.1002/fft2.145. [DOI] [Google Scholar]
- Zhang J.N., Weng H.L., Lin Z.Q., Lin Y.F., Chen Y.H., Lin H.T. Comparison of fruit storability and quality changes between harvested ‘Fuyan’ and ‘Dongbi’ longan fruit. Chin. J. Trop. Crops. 2013;34(5):989–994. [Google Scholar]
- Zhou Q., Zhang F., Ji S.J., Dai H.Y., Zhou X., Wei B.D., Cheng S.C., Wang A.D. Abscisic acid accelerates postharvest blueberry fruit softening by promoting cell wall metabolism. Sci. Hortic. 2021;288 doi: 10.1016/j.scienta.2021.110325. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.