Abstract
Inflammatory myofibroblastic tumor (IMT) is a rare tumor with intermediate biologic potential, in which lack of understanding often poses difficulties in preoperative diagnosis and treatment. The aim of the present study was to characterize the computed tomography (CT) features of the bladder IMT. The CT images of nine pathologically confirmed bladder IMT were retrospectively reviewed. All patients underwent both unenhanced CT and contrast-enhanced CT. The diameter, location, contour, growth pattern, margin, boundary, density and enhancement pattern of the lesions were assessed. The mean Ki67 value of an irregular blood clot was 18% and that of no blood clot was 12%. A total of eight (89%) patients had one tumor and 1 (11%) patient had multiple tumors. An endophytic growth pattern was observed in 4 (44%) patients, an exophytic growth pattern in 2 (22%) patients, and a mixed growth pattern in 3 (33%) patients. The tumor manifests morphologically as either polypoid (n=5), or cauliflower-like (n=1) soft-tissue mass with a wide base in the cavity, or a limited thick-walled (n=3). The tumor margins were smooth (n=8) or lobulated (n=1), and the tumor boundaries were either clear (n=7) or ill-defined (n=2). The lesions showed either ring-shaped (n=3) or heterogeneous (n=6). The polypoid and cauliflower-like soft-tissue mass showed a symmetrical change in the center of the lesion after enhancement. The bladder IMT is mostly a single polypoid nodule in the superior wall, mostly endophytic growth, with ring-haped enhancement and symmetrical change after enhancement as its characteristic manifestations.
Keywords: bladder cancer, inflammatory myofibroblastic tumor, X-ray computed tomography, diagnosis
Introduction
Since the first report by Brunn in 1939 as a primary myofibroblastic tumor of the lung (1), an inflammatory myofibroblastic tumor (IMT) has been acknowledged as a distinctive, though rare, intermediate soft tissue tumor that commonly in the orbit and lung (2,3). Although previously believed to be proliferative lesions deriving from submucosal stroma, of low or indeterminate malignant potential (4), IMT is now considered to be different from pseudotumor for the distinct histological and molecular features, specifically characteristic cellular spindle cell proliferation alongside mutations in the anaplastic lymphoma kinase (ALK)-1 gene loci (5), and there has been an increasing incidence to be found in the pelvic cavity, head and neck, trunk, retroperitoneum, abdomen and limbs (6).
An IMT diagnosis is characterized by the lymphocytic infiltrate and spindle myoepithelial cell proliferation (7). Coffin et al (8) described the IMT with three different histological patterns, which may be present simultaneously within a single specimen. Based in fact that IMT is considered as a low malignant potential neoplasm, surgical resection is the first choice of treatment, and several studies proved that successful resolution with radiotherapy, chemotherapy, steroids, or even non-steroidal inflammatory drugs (2,4,9). According to a previous study, ~50% of tumours present with ALK overexpression, which is considered to identify the characteristics and provide the opportunity for targeted therapy (10). Although the exact incidence remains unclear, it has been reported that the recurrence rate and distant metastasis rate of IMT are low (recurrence: 2% in pulmonary, 25% in extrapulmonary sites; metastasis: less than 5%) (3).
Since the first study of a thirty-two-year-old woman of bladder IMT in 1980(11), the bladder has become one of the common sites of the tumor involving urinary system, and more than 200 cases of bladder IMT were reported mostly in the ophthalmologic and pathologic literature. Due to the lack of specificity clinical symptoms and laboratory results, a preoperative imaging-based examination is very important for determining bladder IMT and selecting appropriate ways to manage conditions of patients requiring emergency treatment (including bladder cancer and lymphoma) (6). However, to the best of our knowledge, there are seldom studies systematically analyzing the CT features of bladder IMT in the radiology literature. The purpose of the present study was to describe the characteristic CT findings of 9 patients with bladder IMT confirmed by pathology.
Materials and methods
Patients
The study was exempt from the Institutional Review Board of the first Hospital of Zhengzhou University. The written informed consent was waived by the Institutional Review Board because this is a retrospective study. Between 2011 and 2021, a review of medical records based on the pathology records and the PACS system of our institution revealed patients with bladder IMT proved on pathologic examination, and the requirement for written informed consent was waived in this retrospective study.
CT evaluation
The pelvic CT scans (Discovery CT750HD; GE Healthcare) were typically obtained after the bladder had a sensation of urine (Fig. 1) and intravenous administration of non-ionic iohexol (iopromide, 370 mg/ml, GE Medical Systems, 1.5 ml/kg, and 5 ml/s) by a dual-head pump injector (Medrad, Inc.; Bayer AG), with a section thickness of 5 mm and a pitch of 1.5.
Figure 1.
Computed tomography image of normal bladder.
Image analyses
All CT images were reviewed blindly by two abdominal radiologists with 3 and 6 years of experience at AW4.6 workstation (GE Healthcare). The following CT features of the bladder IMT were assessed: i) Diameter of the lesion, ii) location of the lesion (posterior wall; superior wall; front wall; left wall; right wall), iii) contour of the lesion (polypoid; cauliflower-like; limited thick-walled), iv) growth pattern of the lesion (endophytic; exophytic; mixed), v) margin of the lesion (smooth; lobulated), vi) boundary of the lesion (clear; ill-defined), vii) density of the lesion on non-enhancement CT scan (low density; iso-density; slightly high density), viii) presence of non-enhancement low-density area within the lesion (homogeneous; heterogeneous), ix) type of enhancement of the lesion (ring-shaped; heterogeneous) and x) degree of enhancement of the lesion (significant; moderate; mild). The lesion showed a symmetrical change in the center of the lesion after enhancement: taking the base of the lesion as the tangent position, and making the median line perpendicular to the base of the lesion, it could be observed that the lesion showed a symmetrical change. In addition, the clinical data (including age, sex and symptoms) were also recorded by the review of medical records.
Results
Patient characteristics
The nine patients (four men and five women) who were evaluated were 7-75 years old (mean age, 40 years). The most common presenting clinical symptoms were gross hematuria (9/9), followed by frequent urination (4/9) and painful urination (4/9). Relevant history included appendicitis resection (1/9) and nephrotic syndrome (1/9). Of the 9 patients, 6 (67%) had irregular blood clots in the bladder. The mean Ki67 value of the presence of an irregular blood clot in the bladder was 18% and that of no blood clot was 12% (Tables I and II).
Table I.
Summary of CT findings for nine patients with IMT of bladder.
| Patient | Sex | Age, years | Clinical Symptom(s) | Diameter (mm2) | Location | Number | Contour | Growth pattern | Margin | Boundary | Tumor Uniformity | Type of enhancement | Ki67 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | Gross hematuria, frequent urination, painful urination | 48x51 | Posterior wall | One | Polypoid | Exophytic | Lobulated | Ill-defined | Ring-shaped | Persistent | 5 |
| 2 | Female | 60 | Gross hematuria, painful urination, irregular blood clot | 33x34 | Superior wall | One | Cauliflower- like | Endophytic | Smooth | Clear | Ring-shaped | Persistent | 5 |
| 3 | Female | 35 | Gross hematuria, | 33x34 | Superior wall | One | Polypoid | Endophytic | Smooth | Clear | Ring-shaped | Persistent | 20 |
| 4 | Male | 28 | Gross hematuria, frequent urination, painful urination, irregular blood clot | 34x48 | Superior wall | One | Polypoid | Exophytic | Smooth | Clear | Ring-shaped | Persistent | 30 |
| 5 | Female | 14 | Gross hematuria, irregular blood clot | 22x19 | Superior wall | One | Polypoid | Endophytic | Smooth | Clear | Ring-shaped | Persistent | 20 |
| 6 | Male | 35 | Gross hematuria, frequent urination, irregular blood clot | 44x47 | Front wall | One | Limited thick- walled | Mixed | Smooth | Clear | Heterogeneous | Persistent | 30 |
| 7 | Female | 7 | Gross hematuria, painful urination, irregular blood clot | 29x32 | Left wall | One | Polypoid | Endophytic | Smooth | Clear | Ring-shaped | Persistent | 20 |
| 8 | Male | 75 | Gross hematuria, frequent urination, irregular blood clot | 35x48 | Posterior wall | One | Limited thick- walled | Mixed | Smooth | Clear | Heterogeneous | Persistent | 15 |
| 9 | Male | 47 | Gross hematuria | 13x21 | Superior wall | Multiple | Limited thick- walled | Mixed | Smooth | Clear | Heterogeneous | Persistent | 10 |
Table II.
Summary of clinical manifestations for nine patients with IMT of bladder.
| Patient | Sex | Age, years | Immunohistochemistry | Operation | Other treatment | Follow-up time | Follow-up method | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | CK+, Vimentin+, SMA+, CD34 (Vascular+), LCA (Stove+) | Cystoscopy | 1 month | Ultrasound | No recurrence | |
| 2 | Female | 60 | Desmin (Individual+), ALK+ | Transurethral resection of bladder tumor | 1 month | Ultrasound | No recurrence | |
| 3 | Female | 35 | CK+, EMA+, ALK+, SMA (Stove+), CD30 (Minority+) | Partial cystectomy | 3 months | Bladder endoscopy | No recurrence | |
| 4 | Male | 28 | CK+, EMA+, ALK+, Vimentin+, SMA (Stove+), CD68 (Scattered+) | Partial cystectomy and pelvic lymph node dissection | 7 months | CT, ultrasound, Magnetic resonance imaging | No recurrence | |
| 5 | Female | 14 | AE1/AE3 (Weak+), CD34 (Vascular+), ALK+, SMA+, Desmin (Stove+) | Transurethral resection of bladder tumor | Bladder perfusion therapy | 2 months | Bladder endoscopy | No recurrence |
| 6 | Male | 35 | SMA (Stove+), Desmin (Stove+), ALK (Stove+) | Transurethral resection of bladder tumor plus partial cystectomy | 19 months | Bladder endoscopy, CT, ultrasound | No recurrence | |
| 7 | Female | 7 | ALK+, AE1/AE3 (Partial+), SMA (Partial+), Desmin (Partial+) | Bladder tumor excision | 2 months | Ultrasound | No recurrence | |
| 8 | Male | 75 | CK7+, CK20 (Epithelial+), SMA+, ERG (Partial+), GATA-3+ | Rod-shaped prostatic dilatation plus electrotomy of bladder neck and mouth | 1 month | Magnetic resonance imaging | No recurrence | |
| 9 | Male | 47 | AE1/AE3+, CD117 (Stove+), Vimentin+, CD99+, P53 (60%) | Laparoscopic radical cystectomy and in situ cystectomy | 4 months | CT | No recurrence |
CT, computed tomography; ALK, anaplastic lymphoma kinase-1.
CT findings
The CT findings showed that 8 (89%) patients had one tumor and 1 (11%) patient had multiple tumors. The bladder IMT size ranged from 1.3x2.1 to 4.8x5.1 cm2. Tumors occurred in the posterior wall in 2 (22%) patients, 5 (30%) patients had tumors occurred in the superior wall, 1 (11%) patient had tumors occurred in the front wall, and 1 (11%) patient had tumor occurred in the left wall (Fig. 2, Fig. 3, Fig. 4 and Fig. 5). An endophytic growth pattern (Fig. 3) was identified in 4 (44%) patients, an exophytic growth pattern (Fig. 2) was observed in 2 (22%) patients, and a mixed growth pattern (Fig. 5) was revealed in 3 (33%) patients. The tumor manifests morphologically as either polypoid (n=5), or cauliflower-like (n=1) soft-tissue mass with a wide base in the cavity, or a limited thick-walled (n=3) in the bladder. The tumor margins were smooth (n=8) or lobulated (n=1) and the tumor boundaries were either clear (n=7) or ill-defined (n=2).
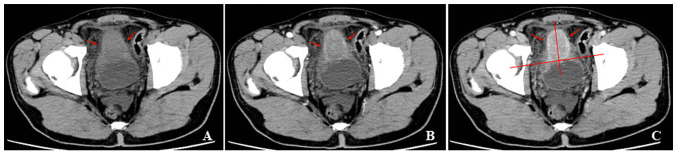
Figure 2.
A 28-year-old man with gross hematuria, frequent urination, painful urination and irregular blood clot. (A) Unenhanced CT scan of the bladder showed a polypoid soft-tissue mass with an exophytic growth pattern on the superior wall, with homogeneous density. (B and C) Contrast-enhanced CT scans revealed ring enhancement on the margin of lesion and symmetrical change in the center of the lesion. CT, computed tomography.
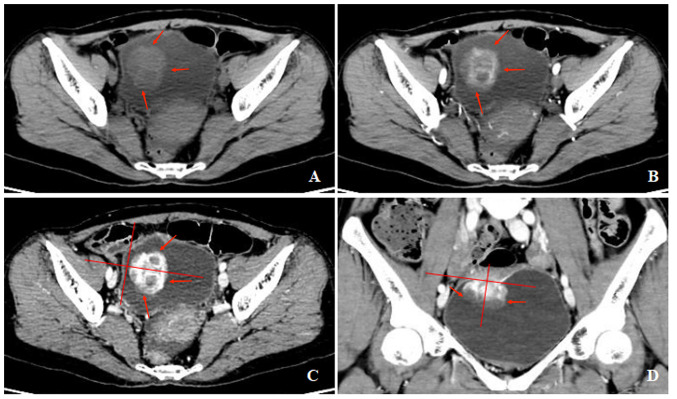
Figure 3.
A 60-year-old woman with gross hematuria, painful urination, irregular blood clot. (A) Unenhanced CT scan of bladder showed a cauliflower-like soft-tissue mass with an endophytic growth pattern on the superior wall, with homogeneous density. (B-D) Contrast-enhanced CT scans revealed heterogeneous enhancement of the lesion and symmetrical change in the center of the lesion. CT, computed tomography.
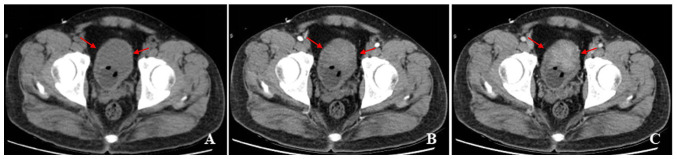
Figure 4.
A 35-year-old man with gross hematuria, frequent urination, irregular blood clot. (A) Unenhanced CT scan of bladder showed a limited thick-walled with a mixed growth pattern on the front wall, with homogeneous density. (B and C) Contrast-enhanced CT scans revealed heterogeneous enhancement of the lesion.
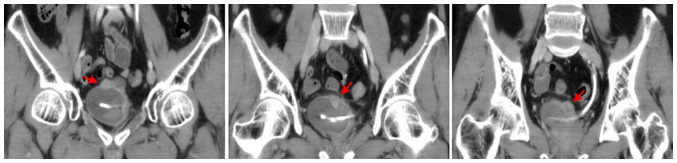
Figure 5.
A 47-year-old man with gross hematuria. Coronal computed tomography scan of bladder shows multiple polypoid soft-tissue mass on the superior wall.
The unenhanced CT examination of the lesions revealed either low density (n=4), iso-density (n=3), or slightly high density (n=1), and density was either homogeneous (n=3) or heterogeneous (n=6). The enhanced CT examination of the lesions showed either ring-shaped (n=3) or heterogeneous (n=6), and the degree of enhancement was either significant (n=6), or moderate (n=3). The enhancement pattern was persistent (n=9). In addition, all polypoid and cauliflower-like soft-tissue masses showed a symmetrical change in the center of the lesion after enhancement on the CT image.
Discussion
IMT is a rare tumor with intermediate biological potential. It is characterized by irregular proliferation of spindle cells in a mucoid to the collagenous stroma, inflammatory infiltration mainly composed of plasma cells and lymphocytes, and occasional mixing of eosinophils and neutrophils (12). Since the first description of IMT in 1939, the understanding of its biological and clinical characteristics has undergone significant changes (9). Based on morphological and histopathological features, IMT used to be described as fibrous histiocytoma, plasma cell granuloma, inflammatory pseudotumor, inflammatory fibroid polyp, fibroxanthoma, xanthoma and xanthogranuloma (9). At present, in the fourth edition of the World Health Organization Classification of Soft Tissue and Bone Tumors, IMT is classified as an intermediate tumor lesion (invasive, occasionally metastatic) (10). Microscopic examination showed that IMT is a tumor with myxoid/vascular type, compact spindle cell type, and hypocellular fibrous type, which could easily lead to misdiagnosis, including inflammatory malignant fibrous histiocytoma, leiomyosarcoma, gastrointestinal stromal tumor rich in inflammatory cells and solitary fibrous tumors (13). The diagnosis of IMT can be consolidated by immunohistochemical analysis (13). IMT tumor cells characteristically stain positive for ALK-1 in 87.5% cases, smooth muscle actin (SMA) in 90% cases, pan-cytokeratin focally in >50% cases, and desmin in ~50% cases (14). In addition, a young woman diagnosed as having IMT of the urinary bladder showed the expression of CD10 in a recently published study (14). The pathogenesis of the tumor remains unknown, and it may be related to infections, immunosuppression, chemotherapy, radiotherapy, local trauma and autoimmune disorders (15). In addition, several diseases, including Crohn's disease, congenital neutropenia, gastrointestinal stromal tumor, and pregnancy have been related to the development of IMT (16). Previous studies have reported the correlation between ALK-1 gene expression and local recurrence, distant metastasis, and overall prognosis of IMT patients (17-20). Moreover, in certain studies (8,21,22), the bladder IMT was associated with the Epstein-Barr virus, bacteria such as Campylobacter Equi, the human herpes virus HHV8, Campylobacter jejuni, Escherichia coli, trauma, radio- and steroid therapy. Previous studies reported that a history of inflammation in the bladder, previous instrumentation, radiation, or a history of surgery was a potential cause of IMT (6). One patient in the present study had a history of appendicitis resection, and one patient had a history of nephrotic syndrome; however, it could not be confirmed whether the surgery was considered to be a causative factor of IMT, because the lesion occurred in the other parts of the pelvis.
There have been certain case reports of bladder IMT, but the shortcomings are obvious. As this tumor is a rare tumor, it is easy to be misdiagnosed and missed. At present, there is a lack of literature studies on the systematic analysis of imaging manifestations of bladder IMT, leading to a lack of understanding among clinicians. Therefore, the clinical imaging data of 9 patients were systematically analyzed and combined with previous literature studies, some new insights were found, aiming to improve our understanding of bladder IMT.
The bladder IMT is uncommon accounting for less than 1% of all bladder tumors (23). The distribution of 182 IMT of the bladder was 51.7% in the female patients, and 48.3% in the male patients, with a mean age of 38.9±16.6 years, according to Teoh et al (24). In a previous study by Harik et al (25), the age of onset of bladder IMT ranged from 7 to 77 years (average 47 years), and the incidence rate of male patients was 3.2 times that of female patients. According to a previous study (6), the average age of onset of bladder IMT was 53 years old, and the incidence rate of male patients was slightly higher than that of female patients. In the current study, the age of patients ranged from 7 to 75 years (average, 40 years), and the proportion of women preferring men to women was 4:5. Due to the limited number of cases, this demographic difference in the study may be caused by selection bias.
The most common clinical symptoms of patients with bladder IMT are anemia and gross hematuria, accompanied by pain occasionally during urination (6,26). In addition, there was one case report by Harik et al (25), which had no clinical symptoms. Due to the rarity of this tumor, it is not always possible to predict the presence of IMT preoperatively based solely on clinical and radiological findings (24).
CT is a common examination method for bladder space-occupying lesions, and it has been reported in the literature on the detection, diagnosis and differentiation of bladder lesions as well as the judgment of curative effect. Since bladder IMT is a rare tumor, if some typical imaging diagnostic information can be obtained before treatment, it will be helpful to develop treatment methods and improve the prognosis of patients. Therefore, it is very necessary to systematically analyze the imaging findings of bladder IMT.
Due to its rarity, the CT-based analysis of the bladder IMT remains largely unknown. Lack of understanding often brings difficulties to the preoperative diagnosis and treatment of such tumors. Therefore, it was decided to systematically review the bladder IMT and focus on image-based analysis. Usually, the tumor is identified as a polypoid or cauliflower-like soft-tissue mass with a broad base on the CT image. Occasionally, narrow-base polypoid lesions, nodules, or limited thick-walled bladders have been reported (6,27,28). In the present study, tumors of 5 patients (30%) were located in the superior wall, and 2 (22%) patients' tumors were located in the posterior wall, which was more similar to the distribution reported by Liang et al (6). In addition, it was found that 8 of the included patients (89%) had well-defined boundaries, and in the aforementioned study (6), more than five-six of patients also had a well-defined tumor in the bladder. Thus, well-defined tumors were considered to be a feature of the bladder IMT on CT imaging. In the present study, there was a patient with a tumor invasion in the perivesical soft tissue of the bladder. According to the results of the histopathological examination, it was confirmed that it is caused by inflammatory changes. Liang et al (6) proposed that the relationship between the number of lesion and biological behavior needed further investigation. In the current study, there were eight patients with one lesion, and one patient with multiple lesions. The present data showed that there was no significant relationship between one lesion and bladder IMT. Liang et al (6) stated that endophytic tumors are more common in bladder IMT. In addition, there have been previous studies that early-stage bladder IMT can appear as a limited thick-walled bladder (27,28). Similarly, the present series of studies revealed that endophytic tumors were more common than exophytic tumors; 44% of the cases were endophytic, 11% exophytic and 22% mixed growth pattern.
Although blood clot is a non-specific sign of any bladder tumor and can be observed in other diseases such as hemorrhagic cystitis, the present study aimed to analyze the relationship between blood clots in the bladder and Ki67 value of the bladder IMT. Of the 9 patients, 6 (67%) had irregular blood clots in the bladder and 9 (100%) had gross hematuria. Therefore, the presence of irregular blood clots in the bladder was considered to be helpful in the diagnosis of bladder IMT (6). The mean Ki67 value of an irregular blood clot of the bladder was 18 and that of no blood clot was 12. It was found that a large Ki67 value appeared to be related to the irregular blood clot.
To expound the relationship between Ki67 value and the prognosis of patients with bladder IMT is of great value for improving the prognosis of patients. However, due to the limited sample size included in the present study and the time of follow-up, the prognosis assessment was limited. This is a limitation to the current study. As a result, sample size will be further accumulated to analyze the relationship between Ki67 value and the prognosis of patients with bladder IMT in future studies.
Although it is not a pathological diagnosis, it has been reported that ring enhancement is the most significant feature of bladder IMT, because more tumor cells gather at the edge of the lesion, accompanied by inflammatory cell infiltration and a relatively rich blood supply (6). Similarly, all 9 lesions in the present study showed homogeneous or heterogeneous enhancement, and six of the nine lesions also showed ring enhancement on CT imaging. It is interesting to note that all polypoid and cauliflower-like soft-tissue mass exhibited a symmetrical change in the center of the lesion after enhancement on the CT image, which had not been reported before. It is considered that symmetrical enhancement may be the clue of the bladder IMT diagnosis. In the present study, all 9 lesions were examined with CT-enhanced examination, intense enhancement at arterial phase scanning, followed by progressive enhancement at intravenous phase scanning. The possible reason is the presence of a contrast agent in the interstitial space in the center of the lesion (6).
The bladder IMT is often difficult to diagnose correctly before surgery, leading to unnecessary radical bladder resection. The analysis may be due to: i) The clinical symptoms and signs are not characteristic, ii) Clinicians and radiologists have no or insufficient knowledge of it and iii) Preoperative cystoscopy is often due to insufficient materials, resulting in a low rate of diagnosis.
Surgical treatment of the bladder IMT is preferred, and the appropriate surgical method should be determined after a comprehensive examination and full evaluation before surgery. Based on the presence of inflammatory cell infiltration in the pathological tissue of the bladder IMT, anti-infection therapy can play a role in reducing the tumor, so perioperative anti-inflammatory measures should be strengthened. The selection of surgical methods should be comprehensively evaluated according to the size of the tumor and the extent of bladder invasion. Although transurethral resection of bladder tumor has the advantages of less trauma and faster recovery, a multicenter study (24) showed that the rate of second surgery after surgery was 21%, markedly higher than the rate of 2% in patients who underwent partial cystectomy. Total cystotomy should be carefully selected considering the patient's condition, tumor size and invasion degree. Chemotherapy after bladder IMT is controversial. Among the 9 patients admitted to our hospital, 1 patient with preoperative biopsy tendency to bladder malignant tumor received bladder perfusion therapy, and the remaining 8 patients did not receive bladder perfusion therapy after the operation. It remains to be verified whether bladder perfusion could reduce the recurrence rate. It has been reported that prednisone, COX-2 inhibitors and other anti-inflammatory drugs have a favorable effect on bladder IMT in children before surgery (29).
The prognosis of bladder IMT was favorable. The nine patients in the current group were reviewed after surgery for bladder endoscopy, CT, ultrasound and magnetic resonance examination. The follow-up time was 1-19 months. No recurrence or distant metastasis was observed in all patients. It has been reported that the recurrence rate and distant metastasis rate of IMT are 2-25% (3). Although most bladder IMTs are benign tumors and still have malignant potential, it is recommended that patients should be reexamined by cystoscopy every 3 months after surgery for 2 years. Then, ultrasound and CT have performed annually.
In conclusion, the bladder IMT had certain characteristic CT findings that differ from other bladder lesions. The present study of cases suggested that there may be a certain relationship between Ki67 value and radiology feathers of the bladder IMT, but the number of cases is limited, and further study is needed for in-depth analysis.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the Outstanding Youth Project in Henan for Young and Middle-aged Health and Health Technology Innovation (grant no. YXKC2020053).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
PL primarily wrote the manuscript. JG and PL critically reviewed the paper and revised it. JG and PL confirm the authenticity of all the raw data. PL and XR performed the database search and literature review. PL, BZ and XR analyzed the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was exempt from the Institutional Review Board of the first Hospital of Zhengzhou University (Zhengzhou, China). Written informed consent was waived by the Institutional Review Board since this is a retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Brunn H. Two interesting benign lung tumours of contradictory histopathology: Remarks on the necessity for maintaining the chest tumour registry. J Thorac Surg. 1939;9:119–131. [Google Scholar]
- 2.Song D, Jiao W, Gao Z, Liu N, Zhang S, Zong Y, Fang Z, Fan Y. Inflammatory myofibroblastic tumor of urinary bladder with severe hematuria: A Case report and literature review. Medicine (Baltimore) 2019;98(e13987) doi: 10.1097/MD.0000000000013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B, Li S, Fang X, Xu H, Yu J, Liu L, Wei Q. Inflammatory myofibroblastic tumor of the urinary system on computed tomography at a high-volume institution in China. Urol Int. 2020;104:960–967. doi: 10.1159/000506779. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, He B, Tu X, Bao Y, Yang L, Zhuo H, Wei Q. Minimally invasive surgery for inflammatory myofibroblastic tumor of the urinary bladder: Three case reports. Medicine (Baltimore) 2018;97(e13474) doi: 10.1097/MD.0000000000013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cresser S, Nzenza T, Tripathy S, Hall R. Case series: Inflammatory myofibroblastic bladder tumor in regional Australia. Int J Surg Case Rep. 2021;82(105898) doi: 10.1016/j.ijscr.2021.105898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Zhou X, Xu S, Lin S. CT manifestations of inflammatory myofibroblastic tumors (Inflammatory Pseudotumors) of the urinary system. AJR Am J Roentgenol. 2016;206:1149–1155. doi: 10.2214/AJR.15.14494. [DOI] [PubMed] [Google Scholar]
- 7.Gass J, Beaghler M, Kwon M. Inflammatory myofibroblastic tumor of the urinary bladder: A case report. J Endourol Case Rep. 2019;5:31–33. doi: 10.1089/cren.2018.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Saleem MI, Ben-Hamida MA, Barrett AM, Bunn SK, Huntley L, Wood KM, Yelbuz TM. Lower abdominal inflammatory myofibroblastic tumor-an unusual presentation-a case report and brief literature review. Eur J Pediatr. 2007;166:679–683. doi: 10.1007/s00431-006-0305-y. [DOI] [PubMed] [Google Scholar]
- 10.Zeng X, Huang H, Li J, Peng J, Zhang J. The clinical and radiological characteristics of inflammatory myofibroblastic tumor occurring at unusual sites. Biomed Res Int. 2018;2018(5679634) doi: 10.1155/2018/5679634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth JA. Reactive pseudosarcomatous response in urinary bladder. Urology. 1980;16:635–637. doi: 10.1016/0090-4295(80)90578-6. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Sharma S, Mittal A, Barwad A, Rastogi S. Recurrent infantile inflammatory myofibroblastic tumor of mesentery-Case report and review of imaging findings. Radiol Case Rep. 2020;16:504–510. doi: 10.1016/j.radcr.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerier E, Beal EW, Dillhoff ME. Inflammatory myofibroblastic tumour: An unusual presentation including small bowel obstruction and palpable abdominal mass. BMJ Case Rep. 2018;2018(bcr2018224549) doi: 10.1136/bcr-2018-224549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patne SC, Katiyar R, Chaudhary D, Trivedi S. Dysuria and fever in a young woman diagnosed as having inflammatory myofibroblastic tumour of the urinary bladder. BMJ Case Rep. 2016;2016(bcr2015214059) doi: 10.1136/bcr-2015-214059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YH, Zhong DJ, Tang J, Han JJ, Yu JD, Wang J, Wan YL. Inflammatory myofibroblastic tumor of the liver following renal transplantation. Ren Fail. 2012;34:789–791. doi: 10.3109/0886022X.2012.673446. [DOI] [PubMed] [Google Scholar]
- 16.Chen CB, Chou CT, Hsueh C, Lee KW, Chen YL. Hepatic inflammatory pseudotumor mimicking hepatocellular carcinoma. J Chin Med Assoc. 2013;76:299–301. doi: 10.1016/j.jcma.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Choi AH, Bohn OL, Beddow TD, McHenry CR. Inflammatory myofibroblastic tumor of the small bowel mesentery: An unusual cause of abdominal pain and uveitis. J Gastrointest Surg. 2011;15:584–588. doi: 10.1007/s11605-010-1408-3. [DOI] [PubMed] [Google Scholar]
- 18.Tsuzuki T, Magi-Galluzzi C, Epstein JI. ALK-1 expression in inflammatory myofibroblastic tumor of the urinary bladder. Am J Surg Pathol. 2004;28:1609–1614. doi: 10.1097/00000478-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Telugu RB, Prabhu AJ, Kalappurayil NB, Mathai J, Gnanamuthu BR, Manipadam MT. Clinicopathological study of 18 cases of inflammatory myofibroblastic tumors with reference to ALK-1 Expression: 5-Year experience in a tertiary care center. J Pathol Transl Med. 2021;51:255–263. doi: 10.4132/jptm.2017.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagnik VD. ALK 1 negative inflammatory myofibroblastic tumor of the ileum: A rare cause of ileocecal intussusception. Surg J (N Y) 2020;6:e101–e104. doi: 10.1055/s-0040-1710531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrosz Z, Ryś J, Paleń P, Właszczuk P, Ciepiela M. Inflammatory myofibroblastic tumor of the bladder-an unexpected case coexisting with an ovarian teratoma. Diagn Pathol. 2014;9(138) doi: 10.1186/1746-1596-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coffin CM, Humphrey PA, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor: A clinical and pathological survey. Semin Diagn Pathol. 1998;15:85–101. [PubMed] [Google Scholar]
- 23.Rotenberry C, Dowd K, Russell D, DeRiese W, Teeple S, Cammack T. Robot-assisted partial cystectomy for treatment of inflammatory myofibroblastic tumor of the bladder. Urol Case Rep. 2017;11:25–27. doi: 10.1016/j.eucr.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teoh JY, Chan NH, Cheung HY, Hou SS, Ng CF. Inflammatory myofibroblastic tumors of the urinary bladder: A systematic review. Urology. 2014;84:503–508. doi: 10.1016/j.urology.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Harik LR, Merino C, Coindre JM, Amin MB, Pedeutour F, Weiss SW. Pseudosarcomatous myofibroblastic proliferations of the bladder: A clinicopathologic study of 42 cases. Am J Surg Pathol. 2006;30:787–794. doi: 10.1097/01.pas.0000208903.46354.6f. [DOI] [PubMed] [Google Scholar]
- 26.Cheng L, Foster SR, MacLennan GT, Lopez-Beltran A, Zhang S, Montironi R. Inflammatory myofibroblastic tumors of the genitourinary tract-single entity or continuum? J Urol. 2008;180:1235–1240. doi: 10.1016/j.juro.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Wong-You-Cheong JJ, Woodward PJ, Manning MA, Sesterhenn IA. From the Archives of the AFIP: Neoplasms of the urinary bladder: Radiologic-pathologic correlation. Radiographics. 2006;26:553–580. doi: 10.1148/rg.262055172. [DOI] [PubMed] [Google Scholar]
- 28.Vikram R, Sandler CM, Ng CS. Imaging and staging of transitional cell carcinoma: Part 1, lower urinary tract. AJR Am J Roentgenol. 2009;192:1481–1487. doi: 10.2214/AJR.08.1318. [DOI] [PubMed] [Google Scholar]
- 29.Berger A, Kim C, Hagstrom N, Ferrer F. Successful preoperative treatment of pediatric bladder inflammatory myofibroblastic tumor with anti-inflammatory therapy. Urology. 2007;70:372.e13–5. doi: 10.1016/j.urology.2007.04.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.