Abstract
Background
Pain and fatigue are highly prevalent in multiple sclerosis (MS) and are associated with adverse physical, social, and psychological outcomes. There is a critical need to identify modifiable factors that can reduce the impact of these symptoms on daily life.
Purpose
This study examined the moderating role of dispositional coping in the relationships between daily fluctuations (i.e., deviations from a person’s usual level) in pain and fatigue and same-day functional/affective outcomes.
Methods
Adults with MS (N = 102) completed a self-report measure of dispositional coping (Brief COPE), followed by 7 days of ecological momentary assessment of pain and fatigue and end-of-day diaries assessing same-day pain interference, fatigue impact, social participation, upper extremity and lower extremity functioning, depressive symptoms, and positive affect and well-being (PAWB). Multilevel models tested interactions between daily symptom fluctuations and dispositional coping (avoidant/approach) in predicting same-day outcomes.
Results
Higher approach coping mitigated the same-day association between pain and pain interference, whereas higher avoidant coping augmented this association. Daily PAWB benefits were seen for those who reported high approach coping and low avoidant coping; effects were only observed on days of low pain (for approach coping) and low fatigue (for avoidant coping). Avoidant coping was associated with worse fatigue impact, social participation, lower extremity functioning, and depressive symptoms.
Conclusions
When faced with pain and fatigue, avoidant coping is associated with increased, and approach coping with decreased, functional/affective difficulties in the daily lives of individuals with MS. Altering coping strategy use may reduce the impact of pain and fatigue.
Keywords: Multiple sclerosis, Pain, Fatigue, Coping, Ecological momentary assessment
Among individuals with multiple sclerosis, approach and avoidant coping tendencies influence the extent to which fluctuations in pain and fatigue severity are associated with functional and emotional difficulties in daily life.
Introduction
Multiple sclerosis (MS) is a chronic and progressive neurological disease that is characterized by inflammation and neurodegeneration in the brain and spinal cord [1]. MS results in a constellation of symptoms, among which pain and fatigue are highly prevalent [2, 3]. As these symptoms are associated with significant functional and affective difficulties in MS [4–6], there is a critical need to identify modifiable factors that influence the relationships between pain and fatigue and outcomes in daily life.
Dispositional coping—an individual’s habitual ways of managing stressors such as somatic symptoms [7, 8]—is one such factor that may moderate the degree to which MS symptoms affect functioning and affective health [9]. Individuals with MS, relative to the general population, report a higher tendency toward avoidant coping, such as behavioral disengagement and denial, and a lower tendency toward approach coping, such as problem-solving, planning, and seeking social support [10–15]. In MS samples, avoidant coping relates to increased disability and unemployment [16], poorer social adjustment [17], and more severe stress [18], depressive symptoms [11, 13, 19–23], and anxiety symptoms [19, 20, 24], whereas approach coping is associated with higher levels of self-rated physical health [17], social participation/satisfaction [25], and self-esteem [26]. Though fewer studies have examined coping in the context of MS symptoms, data from these investigations suggest that responding to pain and fatigue with avoidance, such as by withdrawing from activities, is associated with higher pain interference [27] and poorer work and social adjustment [28], whereas approach-oriented behaviors, including acceptance, are related to lower pain interference and depressive symptoms and higher social satisfaction and overall quality of life [29].
Despite this knowledge, important questions remain regarding the role of dispositional coping in the relationships between pain and fatigue and functional/affective outcomes in MS. It is known that individuals with MS experience significant day-to-day fluctuations in pain and fatigue severity [30, 31]. These symptom fluctuations covary with functional and affective outcomes, wherein periods of higher pain and fatigue are related to diminishments in social participation, positive affect and well-being (PAWB), and physical functioning [32, 33]. Accordingly, there is a need for self-management approaches, such as the use of coping strategies, to lessen the impact of symptom exacerbations. However, existing coping studies in MS have largely relied on cross-sectional examinations of symptoms and functional/affective responses and have utilized between-person analyses [9], leaving it unclear to what extent coping moderates within-person associations between fluctuations in symptom severity and outcomes. Moreover, there is a need for study of these phenomena in the lived environment in which people with MS experience pain and fatigue and the impacts of these symptoms. If within-person symptom-outcome relationships differ depending on dispositional coping, such data could inform the design of interventions that target coping strategy use to reduce the impact of MS-related pain and fatigue exacerbations in daily life.
We conducted a repeated-measures observational study utilizing a baseline survey of dispositional coping, ecological momentary assessment (EMA) of pain and fatigue severity, and end-of-day (EOD) diary measures of functional (i.e., pain interference, fatigue impact, ability to participate in social roles and activities [SRA], upper extremity [UE] functioning, lower extremity [LE] functioning), and affective (i.e., depressive symptoms, PAWB) outcomes. The aim of the study was to examine the moderating role of dispositional coping—specifically the use of avoidant coping and approach coping—in the association between daily changes in pain and fatigue and same-day functional and affective outcomes. In cases of nonsignificant interactions, we examined associations between dispositional coping and these outcomes. Consistent with prior between-person findings regarding coping with pain and fatigue [27–29], we hypothesized that daily pain and fatigue would be associated with significantly worse same-day functional and affective outcomes among individuals who report greater use of avoidant coping and better same-day outcomes among those who report greater use of approach coping. Given the evidence for more robust relationships between avoidant coping—as opposed to approach coping—and physical, social, and psychological functioning in MS [11, 13, 16–26], we expected avoidant coping to exhibit a stronger moderating role.
Methods
Participants
Adults with a confirmed MS diagnosis participated. Inclusion criteria were: (a) age ≥ 18 years, (b) English literacy ≥ sixth grade level, and (c) Ambulatory with or without the use of an assistive device (e.g., cane/walker). Exclusion criteria were: (a) MS exacerbation within the preceding 30 days, (b) atypical sleep/wake pattern (e.g., due to shift work), (c) diagnosis of fibromyalgia or rheumatological disease, and (d) change in disease-modifying therapy during the study.
Of 108 individuals who enrolled and completed baseline measures, six did not complete any EOD diaries, yielding an analyzed sample of 102 participants. Descriptive statistics for sociodemographic and disease characteristics are reported in Table 1. Participants were 44.68 years old on average (SD = 11.62 years; range = 23–67 years). They identified as female (69.6%) or male (30.4%). Participants were White (82.4%), Black or African American (7.8%), Asian (5.9%), Native American, American Indian, or Alaska Native (2.0%), Multiracial (1.0%), or of unspecified race (1.0%). Ethnic identities included 2.0% who identified as Hispanic or Latino/a and 98.0% who identified as not Hispanic or Latino/a. More than half had earned at least a college degree (60.7%) and were employed (57.8%). A relapsing-remitting disease course was most common (76.5%). The average MS disease duration was 9.26 years (SD = 8.25 years).
Table 1.
Descriptive Statistics for Study Variables and Sociodemographic and Disease Characteristics of the Sample (N = 102)
| Variable | Mean (SD) or number (%) | Possible range | Observed range |
|---|---|---|---|
| EMA pain | 2.45 (1.73) | 0–10 | 0.00–6.73 |
| EMA fatigue | 3.48 (1.92) | 0–10 | 0.00–8.10 |
| Avoidant coping composite | 20.47 (5.23) | 12–48 | 12–33 |
| Approach coping composite | 32.93 (7.42) | 12–48 | 12–47 |
| Pain interference | 11.94 (5.78) | 6–30 | 6.00–28.00 |
| Fatigue impact | 7.86 (2.83) | 3–15 | 3.00–15.00 |
| Ability to participate in SRA | 13.94 (4.25) | 4–20 | 4.00–20.00 |
| UE functioning | 36.48 (5.52) | 8–40 | 8.00–40.00 |
| LE functioning | 33.08 (6.90) | 8–40 | 8.86–40.00 |
| Depressive symptoms | 13.29 (5.92) | 8–40 | 8.00–34.57 |
| PAWB | 35.45 (7.81) | 9–45 | 11.43–45.00 |
| Age (years) | 44.68 (11.62) | 23–67 | |
| Gender (female) | 71 (69.6%) | ||
| Race | |||
| White | 84 (82.4%) | ||
| Black or African American | 8 (7.8%) | ||
| Asian | 6 (5.9%) | ||
| Native American, American Indian, or Alaska Native | 2 (2.0%) | ||
| Multiracial | 1 (1.0%) | ||
| Not reported | 1 (1.0%) | ||
| Ethnicity | |||
| Hispanic or Latino/a | 2 (2.0%) | ||
| Not Hispanic or Latino/a | 100 (98.0%) | ||
| Education | |||
| Some high school | 1 (1.0%) | ||
| High school graduate or GED | 11 (10.8%) | ||
| Vocational or technical school | 2 (2.0%) | ||
| Some college | 26 (25.5%) | ||
| College graduate | 39 (38.2%) | ||
| Graduate school or professional school | 23 (22.5%) | ||
| Employment status | |||
| Employed full-time | 40 (39.2%) | ||
| Employed part-time | 19 (18.6%) | ||
| Unemployed | 43 (42.2%) | ||
| MS duration (years) | 9.26 (8.25) | <1–44 | |
| MS subtype | |||
| Relapsing-remitting | 78 (76.5%) | ||
| Primary progressive | 11 (10.8%) | ||
| Progressive-relapsing | 1 (1.0%) | ||
| Secondary progressive | 12 (11.8%) | ||
EMA and EOD diary variables are person-averaged.
Procedures
Data were collected from October 2014 to March 2016 from participants who enrolled in an observational, repeated-measures study incorporating baseline surveys, EMA of symptoms, EOD diaries, and accelerometry. This is a secondary analysis of these data. Findings related to symptom variability [31], covariation [34], and associations with functional and affective outcomes [32] and physical activity [33] are reported elsewhere. Participants were recruited via physician referral, printed advertisements in medical clinics and community locations, outreach at community events, electronic medical record queries, a database of previous participants, and a university-based recruitment website. Potential participants were screened by telephone. Those eligible were scheduled for an in-person baseline visit in which they provided written informed consent, completed surveys using Qualtrics, and were trained on home monitoring procedures.
On the day following the baseline visit, participants commenced 7 days of home monitoring. During this period, participants provided ratings of their pain and fatigue 5 times per day using the PRO-Diary (CamNtech, Cambridge, UK)—a compact, wrist-worn electronic diary and accelerometer with a touch-screen user interface. Ratings upon waking and at bedtime were participant-initiated, while ratings at 11 AM, 3 PM, and 7 PM were prompted via an audible alarm. Participants also completed EOD diaries via Qualtrics. Compensation was based on the number of days completed. The study was approved by the Medical Institutional Review Board of the University of Michigan.
Measures
Baseline
Sociodemographic and disease characteristics
Baseline sociodemographic and disease characteristics included age, gender, race, ethnicity, education, employment status, MS disease course, and years since MS diagnosis.
Coping
The Brief Coping Orientation to Problems Experienced (Brief COPE) [35] is a 28-item self-report scale that assesses dispositional coping. Individuals are asked to rate the frequency with which they use various coping strategies in general, without reference to a specific recall period. Items are rated on a four-point scale ranging from 1 (“I haven’t been doing this at all”) to 4 (“I’ve been doing this a lot”). Consistent with prior studies employing the Brief COPE, composite scores were generated for “avoidant coping” and “approach coping,” given their demonstrated role in pain- and fatigue-related outcomes in MS and their relevance as therapeutic targets in existing pain and fatigue management interventions, such as cognitive-behavioral therapy and acceptance and commitment therapy. The avoidant coping composite score (Cronbach’s alpha = .72) was created by summing items assessing the use of self-distraction, denial, substance use, behavioral disengagement, venting, and self-blame. The approach coping composite score (Cronbach’s alpha = .86) was created by summing items assessing the use of active coping, emotional support, instrumental support, positive reframing, planning, and acceptance. Higher scores indicate more frequent use of the respective coping strategies.
Ecological momentary assessment
EMA measures assessing pain and fatigue were based on the “right now” items from the Brief Pain Inventory [36] and Brief Fatigue Inventory [37], respectively.
Pain intensity
Participants were asked, “What is your level of pain right now?” which they rated on a scale of 0 (“no pain”) to 10 (“worst pain imaginable”).
Fatigue intensity
Participants were asked, “What is your level of fatigue right now?” which they rated on a scale of 0 (“no fatigue”) to 10 (“extremely severe fatigue”).
End-of-day diaries
EOD diaries consisted of self-report measures, adapted for daily use [38], from the Patient-Reported Outcomes Measurement Information System (PROMIS) [39] and Quality of Life in Neurological Disorders (Neuro-QoL) measurement system [40–42]. For each measure, items were summed, with total scores indicating a stronger endorsement of the respective construct.
Pain interference
The six-item PROMIS v1.0 Pain Interference Short Form 6a [43] assessed the extent (1 = “not at all” to 5 = “very much”) to which pain interfered with participants’ activities “today,” including “day to day activities,” “work around the home,” “ability to participate in social activities,” “household chores,” “things you usually do for fun,” and “enjoyment of social activities.”
Fatigue impact
Three items from the PROMIS v1.0 Fatigue item bank [44] assessed the degree (1 = “not at all” to 5 = “very much”) to which fatigue impacted participants “today,” including, “To what degree did you have to push yourself to get things done because of your fatigue?” “To what degree did your fatigue interfere with your physical functioning?” and “To what degree did your fatigue make you feel less alert?”
Ability to participate in SRA
Four items from the PROMIS v2.0 Ability to Participate in Social Roles and Activities item bank [45] assessed the frequency (1 = “always” to 5 = “never”) with which participants experienced trouble “today” doing all of the “family activities that I want to do,” “activities with friends that are really important to me,” “leisure activities with others that I want to do,” and “work that I feel I should do (include work at home).”
UE functioning
The eight-item Neuro-QoL v1.0 Upper Extremity Function Short Form [41] assessed participants’ current ability to complete UE motor tasks (1 = “unable to do” to 5 = “without any difficulty”), including “turn a key in a lock,” “brush your teeth,” “make a phone call using a touch tone key-pad,” “pick up coins from a table top,” “write with a pen or pencil,” “open and close a zipper,” “wash and dry your body,” and “shampoo your hair.”
LE functioning
The eight-item Neuro-QoL v1.0 Lower Extremity Function Short Form [41] assessed participants’ current ability to complete LE motor tasks (1 = “unable to do” to 5 = “without any difficulty”), including “get on and off the toilet,” “step up and down curbs,” “get in and out of a car,” “get out of bed into a chair,” “push open a heavy door,” “run errands and shop,” “get up off the floor from lying on your back without help,” and “go for a walk of at least 15 min.”
Depressive symptoms
The eight-item PROMIS v1.0 Emotional Distress – Depression Short Form 8a [46, 47] assessed the frequency (1 = “never” to 5 = “always”) with which participants experienced depressive symptoms “today,” including feeling “worthless,” “helpless,” “depressed,” “hopeless,” “like a failure,” “unhappy,” “that I had nothing to look forward to,” and “that nothing could cheer me up.”
PAWB
The nine-item Neuro-QoL v1.0 Positive Affect and Well-Being Short Form [48] assessed the frequency (1 = “never” to 5 = “always”) with which participants experienced PAWB “today,” including, “I had a sense of well-being,” “I felt hopeful,” “My life was satisfying,” “My life had purpose,” “My life had meaning,” “I felt cheerful,” “My life was worth living,” “I had a sense of balance in my life,” and “Many areas of my life were interesting to me.”
Data analyses
Preliminary analyses
Descriptive statistics were calculated for all variables. To reflect within-person daily fluctuations in symptoms, the EMA pain and fatigue variables were person-day centered by subtracting each participant’s average score for the week from their average score for the day; days with higher than average pain/fatigue had positive deviation values and days of lower pain/fatigue had negative deviation values. To reflect between-person variability in symptoms, the EMA pain and fatigue variables were averaged for each participant across the week. The avoidant and approach coping composites were grand-mean centered by subtracting the sample mean for each composite from each participant’s score.
Primary analyses
Multilevel models (MLMs) were used to test avoidant and approach coping as moderators of the relationships between daily changes in symptoms and functional and affective outcomes. Predictors included the person-day centered EMA pain and fatigue variables and the grand-mean centered avoidant and approach coping variables. Interaction terms were included for each combination of person-day centered EMA symptom variable and grand-mean centered coping variable. Covariates were person-averaged EMA pain and fatigue (to control for between-person variance in symptoms) and relevant sociodemographic and disease characteristics (i.e., age, gender [reference = male], MS disease course [reference = progressive types], MS disease duration).
In cases of nonsignificant interactions, MLMs were used to examine associations between avoidant and approach coping and the functional and affective outcomes. In each model, the predictors were the grand-mean centered avoidant and approach coping variables. The covariates were sociodemographic and disease characteristics (age, gender, MS disease course, MS disease duration). Critical alpha threshold was specified at p < .05. All analyses were performed using IBM SPSS Statistics v26.
Results
Preliminary Results
Rates of missing data were comparable to those in previous EMA and EOD diary studies employing chronic disease samples [49–51]. Across the sample, 84.9% of all possible EMA sessions and 87.1% of all EOD diaries were completed. Descriptive statistics for the study variables are reported in Table 1.
Avoidant and Approach Coping as Moderators of the Relationships Between Daily Symptoms and Same-Day Functional and Affective Outcomes
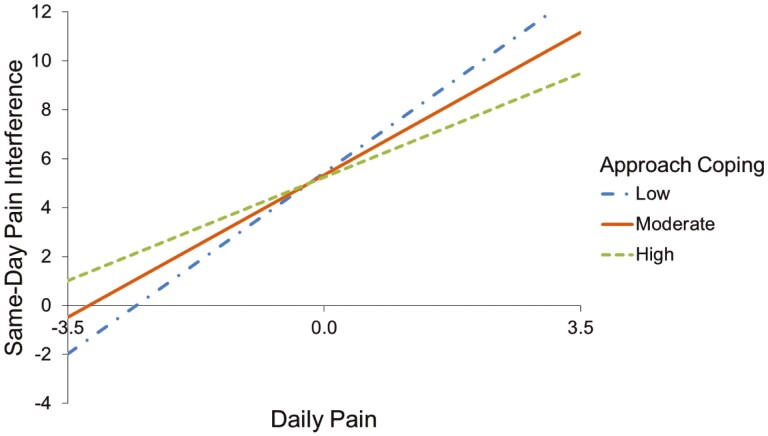
Avoidant coping and approach coping significantly moderated the association between daily pain and same-day pain interference (avoidant: B = 0.08, p = .01; approach: B = −0.06, p = .007; Table 2). Specifically, increased daily pain was related to higher same-day pain interference, and this association was augmented (greater positive association) for individuals who reported more frequent use of avoidant coping (Figure 1) but was mitigated (smaller positive association) among individuals who reported more frequent use of approach coping (Figure 2). Additionally, approach coping significantly moderated the association between daily pain and same-day PAWB (B = −0.05, p = .04). Specifically, changes in daily pain were negatively related to same-day PAWB among individuals who reported more frequent use of approach coping; in contrast, there was a positive association between daily changes in pain and same-day PAWB for individuals who reported less frequent use of approach coping (Figure 3). There were no significant interactions of daily pain with coping strategy use in models predicting same-day fatigue impact, ability to participate in SRA, UE functioning, LE functioning, and depressive symptoms (ps ≥ .05).
Table 2.
Results of Multilevel Models Testing the Interaction Between Daily Symptoms (Pain and Fatigue) and Coping Strategy Use (Avoidant and Approach) in Predicting Same-Day Outcomes, Controlling for Sociodemographic and Disease Characteristics
| Pain interference | Fatigue impact | Ability to participate in SRA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Random effect | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Intercept | 10.96 | 1.93 | <.0001 | 2.84 | .51 | <.0001 | 8.89 | 1.70 | <.0001 |
| AR(1) | .15 | .06 | .02 | .11 | .06 | .07 | .37 | .07 | <.0001 |
| Residual | 8.94 | .65 | <.0001 | 3.19 | .22 | <.0001 | 7.44 | .74 | <.0001 |
| Fixed effect | B | SE | p | B | SE | p | B | SE | p |
| Between-person variables | |||||||||
| Intercept | 5.35 | 2.04 | .01 | 2.90 | 1.06 | .007 | 20.02 | 1.89 | <.0001 |
| Age | .04 | .04 | .33 | .03 | .02 | .07 | −.09 | .03 | .01 |
| Gender | −1.24 | .85 | .15 | −.20 | .44 | .65 | .55 | .79 | .48 |
| MS subtype | −.16 | .93 | .86 | −.46 | .49 | .35 | 1.95 | .87 | .03 |
| MS duration | −.01 | .05 | .85 | .004 | .03 | .88 | −.02 | .05 | .69 |
| Pain * | 2.13 | .30 | <.0001 | .13 | .16 | .39 | −.16 | .28 | .57 |
| Fatigue * | .22 | .27 | .41 | 1.00 | .14 | <.0001 | −1.01 | .25 | <.001 |
| Avoidant coping | .10 | .08 | .19 | .03 | .04 | .41 | −.12 | .07 | .10 |
| Approach coping | −.01 | .05 | .81 | −.01 | .03 | .76 | .05 | .05 | .25 |
| Within-person variables | |||||||||
| Δ Pain | 1.66 | .17 | <.0001 | .22 | .10 | .04 | −.50 | .14 | .001 |
| Δ Fatigue | .53 | .15 | <.001 | .72 | .09 | <.0001 | −.32 | .12 | .008 |
| Cross-level interactions | |||||||||
| Δ Pain × avoidant coping | .08 | .03 | .01 | .01 | .02 | .68 | −.02 | .03 | .39 |
| Δ Pain × approach coping | −.06 | .02 | .007 | −.01 | .01 | .48 | .02 | .02 | .21 |
| Δ Fatigue × avoidant coping | .02 | .03 | .47 | .01 | .02 | .70 | −.002 | .02 | .94 |
| Δ Fatigue × approach coping | .01 | .02 | .52 | −.01 | .01 | .57 | −.01 | .02 | .55 |
| UE functioning | LE functioning | ||||||||
| Random effect | Est. | SE | p | Est. | SE | p | |||
| Intercept | 22.17 | 4.41 | <.0001 | 26.39 | 4.58 | <.0001 | |||
| AR(1) | .45 | .07 | <.0001 | .39 | .07 | <.0001 | |||
| Residual | 19.29 | 2.20 | <.0001 | 13.76 | 1.39 | <.0001 | |||
| Fixed effect | B | SE | p | B | SE | p | |||
| Between-person variables | |||||||||
| Intercept | 36.91 | 3.04 | <.0001 | 38.54 | 3.14 | <.0001 | |||
| Age | −.05 | .06 | .33 | −.16 | .06 | .007 | |||
| Gender | .37 | 1.26 | .77 | −.24 | 1.30 | .86 | |||
| MS subtype | 3.31 | 1.39 | .02 | 7.31 | 1.44 | <.0001 | |||
| MS duration | .07 | .07 | .32 | .01 | .08 | .90 | |||
| Pain* | −.37 | .45 | .41 | −.63 | .46 | .18 | |||
| Fatigue* | −.18 | .40 | .66 | −.67 | .42 | .11 | |||
| Avoidant coping | −.08 | .12 | .52 | −.12 | .12 | .32 | |||
| Approach coping | .07 | .07 | .32 | .07 | .08 | .36 | |||
| Within-person variables | |||||||||
| Δ Pain | −.70 | .22 | .001 | −.62 | .19 | .001 | |||
| Δ Fatigue | .13 | .18 | .49 | −.04 | .16 | .82 | |||
| Cross-level interactions | |||||||||
| Δ Pain × avoidant coping | −.02 | .04 | .57 | −.06 | .04 | .09 | |||
| Δ Pain × approach coping | −.01 | .03 | .72 | .01 | .03 | .66 | |||
| Δ Fatigue × avoidant coping | .03 | .04 | .48 | .02 | .03 | .49 | |||
| Δ Fatigue × approach coping | .01 | .02 | .81 | .004 | .02 | .86 | |||
| Depressive symptoms | PAWB | ||||||||
| Random effect | Est. | SE | p | Est. | SE | p | |||
| Intercept | 25.35 | 4.10 | <.0001 | 49.75 | 7.80 | <.0001 | |||
| AR(1) | .27 | .06 | <.0001 | .27 | .07 | <.0001 | |||
| Residual | 9.36 | .75 | <.0001 | 11.15 | .96 | <.0001 | |||
| Fixed effect | B | SE | p | B | SE | p | |||
| Between-person variables | |||||||||
| Intercept | 9.35 | 2.99 | .002 | 38.79 | 4.12 | <.0001 | |||
| Age | .01 | .05 | .91 | .01 | .08 | .90 | |||
| Gender | −1.43 | 1.24 | .25 | 1.54 | 1.70 | .37 | |||
| MS subtype | 1.67 | 1.37 | .23 | −1.93 | 1.89 | .31 | |||
| MS duration | −.04 | .07 | .62 | .04 | .10 | .67 | |||
| Pain * | .19 | .44 | .67 | −.20 | .61 | .74 | |||
| Fatigue * | .93 | .40 | .02 | −.93 | .54 | .09 | |||
| Avoidant coping | .21 | .12 | .07 | −.29 | .16 | .07 | |||
| Approach coping | −.11 | .07 | .13 | .22 | .10 | .03 | |||
| Within-person variables | |||||||||
| Δ Pain | .34 | .17 | .04 | −.10 | .19 | .60 | |||
| Δ Fatigue | .70 | .14 | <.0001 | −.84 | .16 | <.0001 | |||
| Cross-level interactions | |||||||||
| Δ Pain × avoidant coping | .03 | .03 | .31 | −.02 | .04 | .62 | |||
| Δ Pain × approach coping | −.01 | .02 | .60 | −.05 | .02 | .04 | |||
| Δ Fatigue × avoidant coping | −.002 | .03 | .94 | .06 | .03 | .049 | |||
| Δ Fatigue × approach coping | .02 | .02 | .28 | −.03 | .02 | .13 | |||
Est. covariance parameter estimate; B unstandardized beta; SE standard error. Bold values denote statistical significance at p < .05.
*Between-person term.
ΔWithin-person term.
Fig. 1.
Simple slopes depicting interaction between daily pain and use of avoidant coping in predicting same-day pain interference among individuals with MS (N = 102).
Fig. 2.
Simple slopes depicting interaction between daily pain and use of approach coping in predicting same-day pain interference among individuals with MS (N = 102).
Fig. 3.
Simple slopes depicting interaction between daily pain and use of approach coping in predicting same-day PAWB among individuals with MS (N = 102).
Avoidant coping significantly moderated the relationship between daily fatigue and same-day PAWB (B = 0.06, p = .049). That is, a daily increase in fatigue was negatively related to same-day PAWB; this association was augmented (stronger negative association) among individuals who reported less frequent use of avoidant coping (Figure 4). There were no significant interactions of daily fatigue with coping strategy use in models predicting same-day pain interference, fatigue impact, ability to participate in SRA, UE functioning, LE functioning, and depressive symptoms (ps ≥ .05).
Fig. 4.
Simple slopes depicting interaction between daily fatigue and use of avoidant coping in predicting same-day PAWB among individuals with MS (N = 102).
Associations Between Avoidant and Approach Coping and Functional and Affective Outcomes
Higher use of avoidant coping was associated with higher fatigue impact (B = 0.18, p = .002), reduced ability to participate in SRA (B = −0.27, p = .001), worse LE functioning (B = −0.28, p = .02), and more severe depressive symptoms (B = 0.36, p = .002), but was not associated with UE functioning (p = .19; Table 3). There were no significant associations between use of approach coping and fatigue impact, ability to participate in SRA, UE functioning, LE functioning, and depressive symptoms (ps ≥ .05).
Table 3.
Results of Multilevel Models Testing Avoidant Coping and Approach Coping as Correlates of Daily Outcomes, Controlling For Sociodemographic and Disease Characteristics
| Fatigue impact | Ability to participate in SRA | UE functioning | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Random effect | Est. | SE | p | Est. | SE | p | Est. | SE | p |
| Intercept | 6.43 | 1.06 | <.0001 | 12.90 | 2.26 | <.0001 | 22.44 | 4.41 | <.0001 |
| AR(1) | .12 | .06 | .047 | .34 | .07 | <.0001 | .45 | .07 | <.0001 |
| Residual | 3.87 | .27 | <.0001 | 7.70 | .72 | <.0001 | 19.39 | 2.17 | <.0001 |
| Fixed effect | B | SE | p | B | SE | p | B | SE | p |
| Intercept | 5.89 | 1.46 | <.001 | 16.70 | 2.11 | <.0001 | 35.63 | 2.92 | <.0001 |
| Age | .04 | .03 | .14 | −.09 | .04 | .03 | −.06 | .06 | .29 |
| Gender | −.26 | .62 | .68 | .73 | .89 | .42 | .64 | 1.24 | .61 |
| MS subtype | .17 | .69 | .80 | 1.34 | 1.00 | .18 | 3.10 | 1.38 | .03 |
| MS duration | .01 | .04 | .70 | −.03 | .05 | .57 | .07 | .08 | .34 |
| Avoidant coping | .18 | .05 | .002 | −.27 | .08 | .001 | −.14 | .11 | .19 |
| Approach coping | .02 | .04 | .68 | .03 | .05 | .55 | .07 | .07 | .38 |
| LE functioning | Depressive symptoms | ||||||||
| Random effect | Est. | SE | p | Est. | SE | p | |||
| Intercept | 29.98 | 5.07 | <.0001 | 28.70 | 4.53 | <.0001 | |||
| AR(1) | .38 | .07 | <.0001 | .22 | .06 | <.001 | |||
| Residual | 14.00 | 1.38 | <.0001 | 9.75 | .73 | <.0001 | |||
| Fixed effect | B | SE | p | B | SE | p | |||
| Intercept | 35.63 | 3.18 | <.0001 | 12.39 | 3.03 | <.0001 | |||
| Age | −.17 | .06 | .005 | .01 | .06 | .85 | |||
| Gender | .14 | 1.34 | .92 | −1.55 | 1.28 | .23 | |||
| MS subtype | 6.65 | 1.51 | <.0001 | 2.28 | 1.44 | .12 | |||
| MS duration | .005 | .08 | .95 | −.03 | .08 | .71 | |||
| Avoidant coping | −.28 | .12 | .02 | .36 | .11 | .002 | |||
| Approach coping | .04 | .08 | .60 | −.09 | .08 | .26 | |||
Est. covariance parameter estimate; B unstandardized beta; SE standard error. Bold values denote statistical significance at p < .05.
Discussion
This study examined the importance of dispositional coping in moderating associations between fluctuations in pain and fatigue and functional and affective outcomes in the daily lives of people with MS. Results showed avoidant coping strategies to be maladaptive, and approach coping strategies to be adaptive, in relation to the outcomes. Dispositional coping moderated the relationships between within-person fluctuations in symptom severity and same-day pain interference and PAWB. These findings provide important insights regarding the potential for interventions that modify coping strategy use to reduce the impact of fluctuations in pain and fatigue on functioning and quality of life in MS.
First, significant interactions emerged between daily fluctuations in pain and dispositional coping in determining same-day pain interference. Specifically, analyses showed higher daily pain to be associated with greater same-day pain interference in individuals who reported more frequent use of avoidant coping strategies and with lower same-day pain interference in individuals who reported more frequent use of approach coping strategies. There were also significant interactions between daily fluctuations in pain and fatigue and coping in determining same-day PAWB. In particular, the relationship between daily pain and PAWB varied with frequency of approach coping, and the relationship between daily fatigue and PAWB varied with frequency of avoidant coping. Although both approach coping and avoidant coping moderated the association between symptoms and PAWB as we expected, the nature of this moderating effect was not as hypothesized. We expected to find that high approach coping and low avoidant coping would work to buffer the negative effects of increased pain and fatigue on PAWB. Instead, the findings indicate that high approach coping and low avoidant coping are related to higher PAWB only when symptoms are quite low. The beneficial effects of a positive coping profile wane in the context of increasing pain and fatigue. These findings can be interpreted according to the Dynamic Model of Affect (DMA), which purports that under conditions of low stress (e.g., days of lower pain/fatigue severity), negative affect and positive affect are relatively independent, reflecting high affective complexity [52, 53]. According to the DMA, at higher levels of stress (e.g., days of higher pain/fatigue severity), negative affect and positive affect show a strong inverse relationship, wherein negative affect predominates and positive affect is restrained. Although changes in coping strategy use were not assessed here, the results suggest that, due to this shrinking of affective complexity under conditions of high stress, altering coping strategy use may be a less effective approach for maintaining PAWB on days of particularly high pain/fatigue severity. Other types of coping responses, such as chronic pain acceptance, have been shown to relate to higher and sustained levels of positive affect [49], and may be a useful adjunct to avoidant and approach coping as studied here.
Although significant interactions were not observed when assessing other outcomes of interest, additional analyses revealed avoidant coping to be associated with higher fatigue impact, reduced ability to participate in SRA, poorer LE functioning, and more severe depressive symptoms. Prior research has likewise demonstrated significant relationships between coping in MS and diverse outcomes spanning the physical, social, and psychological domains, with more robust relationships observed for avoidant, as opposed to approach, coping [11, 13, 16–26]. The present findings are a significant contribution to this literature given the study’s in vivo, repeated measurement of symptoms and outcomes—qualities that foster measurement reliability and ecological validity [54], thus adding confidence in the relevance of dispositional coping for functioning and affective health in this population.
The behavioral inhibition (BIS)-behavioral approach system (BAS) model [55, 56] provides a useful context for understanding the results. The model holds that the BIS is a neurophysiological mechanism that is activated by environmental or internal cues that an individual associates with a potential for punishment. BIS activation drives avoidance-related behaviors, emotions, and cognitions that aim to reduce the possibility of punishment. In contrast, the BAS is activated by cues that signal a potential for reward. BAS activation facilitates approach-related responses that aim to increase the possibility of reinforcement. Importantly, the degree to which a cue activates or inhibits each system varies from individual to individual. The present study’s results suggest that, when faced with pain exacerbation, individuals with a tendency toward BIS-related responses (e.g., withdrawal from activity) are likely to experience more pain interference in their day-to-day life. The reverse appears true for those with a tendency toward higher BAS activation—these individuals likely continue to engage in valued activities despite pain, thus reducing pain-related interference. Sánchez-Rodríguez et al [57] and Turner et al [58] similarly showed BIS sensitivity to be associated with greater pain interference in chronic pain samples, whereas BAS sensitivity was not correlated with pain interference and was a relatively weaker correlate of other functional and affective outcomes. The present study’s findings of more frequent and stronger associations between avoidant, as opposed to approach, coping and outcomes are thus consistent with a growing body of research showing that BIS is more important than BAS to pain-related outcomes across multiple clinical populations [57, 58].
Together, the results demonstrate the importance of assessing in clinical practice how individuals with MS cope with their pain and fatigue. Those individuals who report more frequent use of avoidant strategies and less frequent use of approach strategies to cope with pain and fatigue endorse more functional and affective difficulties and may benefit from follow-up care. Cognitive-behavioral therapy [59–61], mindfulness-based cognitive therapy [62–64], and fatigue/self-management programs [65–67] have demonstrated efficacy in improving pain- and fatigue-related outcomes in people with MS. Given accumulating evidence for the particular importance of BIS phenomena in determining functional/affective outcomes, targeting BIS sensitivity may improve treatment outcomes. Future research should examine whether individual differences in BIS/BAS sensitivity moderate treatment effects and whether tailoring interventions based on individual differences in BIS/BAS sensitivity improves outcomes.
Our findings suggest that interventions that aim to decrease avoidant coping and increase approach coping may be particularly beneficial for mitigating the impact of pain exacerbations on pain interference. However, in the case of PAWB, results showed that the benefits of a positive coping profile likely wane during high pain/fatigue, suggesting that modifying coping strategy use during pain/fatigue exacerbations may not substantially impact PAWB. Though dispositional coping appeared to buffer the association between pain/fatigue and PAWB at low symptom levels, this phenomenon is likely not unique to individuals with MS, as previous research utilizing nonclinical samples with no/low symptom levels has shown similar associations wherein avoidant and approach coping strategies differentially moderate the relationship between stress and PAWB [68–71]. Thus, the results suggest a need for future research to identify other pain and fatigue management strategies that lessen the impact of symptom exacerbations on this outcome.
Study Limitations
It is not possible from these data to infer causal relationships between symptoms and outcomes. There is a lack of published data defining clinically meaningful differences in scores on the daily adaptations of PROMIS and Neuro-QoL measures used here, thus limiting interpretation of the magnitude of the observed moderation effects. However, given the propensity for effects observed in psychological studies to cumulate over time within a given individual [72], it is reasonable to expect that the associations observed here may underestimate the magnitude of the functional and affective impacts of coping over longer periods. Given their prevalence and impact on MS, pain and fatigue were the focus of the analyses, though examining coping in relation to other MS symptoms is also warranted. Coping strategy use was examined as a dispositional construct and was assessed only at baseline. Future research should aim to refine momentary measures of coping strategy use that can be incorporated into EMA studies to assess situational change in coping strategy implementation. This would allow for examining dynamic changes in coping strategy use in relation to fluctuations in pain and fatigue intensity, and to determine whether individuals vary their strategy use in response to different symptoms. Consistent with many previous studies employing the Brief COPE, and supported by evidence for the importance of the approach-avoidance dichotomy for MS-related outcomes, we utilized coping composite scores in the present investigation. This additionally permitted limiting Type I error. Future studies might add to the present findings by examining the moderating role of individual coping strategies (e.g., acceptance, behavioral disengagement) in symptom-outcome relationships. The participants were predominantly White, most had a modest symptom burden, and all were ambulatory. Whether these findings are generalizable to individuals from more diverse backgrounds and with more severe disease merits further study. Additionally, future investigations should examine whether coping tendencies, and the impact of coping strategy use, differ depending on MS disease characteristics (e.g., disease duration, disability) to inform the tailoring of interventions based on individual differences.
Conclusions
The results suggest that fluctuations in pain and fatigue severity interact with dispositional coping to determine same-day functional and affective outcomes. Interventions that modify coping strategy use may help individuals with MS reduce the impact of pain and fatigue exacerbations on pain interference and PAWB in daily life.
Acknowledgments
This research was supported by the National Institute of Nursing Research of the National Institutes of Health under award number R03NR014515; PI: Kratz. Dr. Valentine was supported during manuscript preparation by the Mentor-Based Postdoctoral Fellowship Program in Rehabilitation Research from the National Multiple Sclerosis Society (MB-1706-27943; PI: Kratz). Dr. Kuzu was supported during manuscript preparation by a postdoctoral fellowship award funded by the University of Michigan’s Advanced Rehabilitation Research Training Program in Community Living and Participation from the National Institute on Disability, Independent Living, and Rehabilitation Research, Administration for Community Living (90ARCP0003). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Multiple Sclerosis Society, or the National Institute on Disability, Independent Living, and Rehabilitation Research. The manuscript submitted does not contain information about medical device(s).
Contributor Information
Thomas R Valentine, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, MI, USA.
Duygu Kuzu, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, MI, USA.
Anna L Kratz, Department of Physical Medicine and Rehabilitation, University of Michigan, Ann Arbor, MI, USA.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions T.V.: Conceptualization, Methodology, Formal Analysis, Writing—Original Draft, Writing—Review, and Editing, Visualization. D.K.: Conceptualization, Methodology, Writing—Review, and Editing. A.K.: Conceptualization, Methodology, Resources, Data Curation, Writing—Review, and Editing, Supervision, Project Administration, Funding Acquisition.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Open Science This study was not formally registered. The analysis plan was not formally pre-registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author. Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author. Materials used to conduct the study are not publicly available.
References
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG.. Multiple sclerosis. N Engl J Med. 2000; 343(13):938–952. [DOI] [PubMed] [Google Scholar]
- 2. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta-analysis. Pain. 2013; 154(5):632–642. [DOI] [PubMed] [Google Scholar]
- 3. Hadjimichael O, Vollmer T, Oleen-Burkey M; North American Research Committee on Multiple Sclerosis. Fatigue characteristics in multiple sclerosis: The North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes. 2008; 6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Day MA, Ehde DM, Ward LC, et al. An empirical investigation of a biopsychosocial model of pain in multiple sclerosis. Clin J Pain. 2016; 32(2):155–163. [DOI] [PubMed] [Google Scholar]
- 5. Salter A, Fox RJ, Tyry T, Cutter G, Marrie RA.. The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Mult Scler Relat Disord. 2019; 31:165–172. [DOI] [PubMed] [Google Scholar]
- 6. Veldhuijzen van Zanten J, Douglas MR, Ntoumanis N.. Fatigue and fluctuations in physical and psychological wellbeing in people with multiple sclerosis: A longitudinal study. Mult Scler Relat Disord. 2021; 47:102602. [DOI] [PubMed] [Google Scholar]
- 7. Lazarus RS, Folkman S.. Stress, appraisal, and coping. New York, NY: Springer, 1984. [Google Scholar]
- 8. Carver CS, Scheier MF, Weintraub JK.. Assessing coping strategies: A theoretically based approach. J Pers Soc Psychol. 1989; 56:267–283. [DOI] [PubMed] [Google Scholar]
- 9. Kar MK, Whitehead L, Smith CM.. Characteristics and correlates of coping with multiple sclerosis: A systematic review. Disabil Rehabil. 2019; 41:250–264. [DOI] [PubMed] [Google Scholar]
- 10. McCabe MP, McKern S, McDonald E.. Coping and psychological adjustment among people with multiple sclerosis. J Psychosom Res. 2004; 56:355–361. [DOI] [PubMed] [Google Scholar]
- 11. Goretti B, Portaccio E, Zipoli V, et al. Coping strategies, psychological variables and their relationship with quality of life in multiple sclerosis. Neurol Sci. 2009; 30:15–20. [DOI] [PubMed] [Google Scholar]
- 12. Goretti B, Portaccio E, Zipoli V, et al. Impact of cognitive impairment on coping strategies in multiple sclerosis. Clin Neurol Neurosurg. 2010; 112:127–130. [DOI] [PubMed] [Google Scholar]
- 13. Lode K, Bru E, Klevan G, Myhr KM, Nyland H, Larsen JP.. Depressive symptoms and coping in newly diagnosed patients with multiple sclerosis. Mult Scler. 2009; 15:638–643. [DOI] [PubMed] [Google Scholar]
- 14. McCabe M, Battista JD.. Role of health, relationships, work and coping on adjustment among people with multiple sclerosis: A longitudinal investigation. Psychol Health Med. 2004; 9:431–439. [Google Scholar]
- 15. McCabe MP, Stokes M, McDonald E.. Changes in quality of life and coping among people with multiple sclerosis over a 2 year period. Psychol Health Med. 2009; 14:86–96. [DOI] [PubMed] [Google Scholar]
- 16. Holland DP, Schlüter DK, Young CA, et al. Use of coping strategies in multiple sclerosis: Association with demographic and disease-related characteristics. Mult Scler Relat Disord. 2019; 27:214–222. [DOI] [PubMed] [Google Scholar]
- 17. Pakenham KI, Stewart CA, Rogers A.. The role of coping in adjustment to multiple sclerosis-related adaptive demands. Psychol Health Med. 1997; 2:197–211. [Google Scholar]
- 18. Grech LB, Kiropoulos LA, Kirby KM, Butler E, Paine M, Hester R.. Target coping strategies for interventions aimed at maximizing psychosocial adjustment in people with multiple sclerosis. Int J MS Care. 2018; 20:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan-Kristanto S, Kiropoulos LA.. Resilience, self-efficacy, coping styles and depressive and anxiety symptoms in those newly diagnosed with multiple sclerosis. Psychol Health Med. 2015; 20:635–645. [DOI] [PubMed] [Google Scholar]
- 20. Farran N, Ammar D, Darwish H.. Quality of life and coping strategies in Lebanese Multiple Sclerosis patients: A pilot study. Mult Scler Relat Disord. 2016; 6:21–27. [DOI] [PubMed] [Google Scholar]
- 21. Arnett PA, Higginson CI, Voss WD, Randolph JJ, Grandey AA.. Relationship between coping, cognitive dysfunction and depression in multiple sclerosis. Clin Neuropsychol. 2002; 16:341–355. [DOI] [PubMed] [Google Scholar]
- 22. Lynch SG, Kroencke DC, Denney DR.. The relationship between disability and depression in multiple sclerosis: The role of uncertainty, coping, and hope. Mult Scler. 2001; 7:411–416. [DOI] [PubMed] [Google Scholar]
- 23. Mohr DC, Goodkin DE, Gatto N, Van der Wende J.. Depression, coping and level of neurological impairment in multiple sclerosis. Mult Scler. 1997; 3:254–258. [DOI] [PubMed] [Google Scholar]
- 24. Pakenham KI. Investigation of the coping antecedents to positive outcomes and distress in multiple sclerosis (MS). Psychol Health. 2006; 21:633–649. [Google Scholar]
- 25. Vanotti S, Cabral N, Eizaguirre MB, et al. Coping strategies: Seeking personalized care in relapsing-remitting multiple sclerosis. A patient reported measure–coping responses inventory. Mult Scler J Exp Transl Clin. 2021; 7:2055217320987588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Brien MT. Multiple sclerosis: The relationship among self-esteem, social support, and coping behavior. Appl Nurs Res. 1993;6: 54–63. [DOI] [PubMed] [Google Scholar]
- 27. Harrison AM, Silber E, McCracken LM, Moss-Morris R.. Beyond a physical symptom: The importance of psychosocial factors in multiple sclerosis pain. Eur J Neurol. 2015; 22:1443–1452. [DOI] [PubMed] [Google Scholar]
- 28. Skerrett TN, Moss-Morris R.. Fatigue and social impairment in multiple sclerosis: The role of patients’ cognitive and behavioral responses to their symptoms. J Psychosom Res. 2006; 61:587–593. [DOI] [PubMed] [Google Scholar]
- 29. Kratz AL, Hirsh AT, Ehde DM, Jensen MP.. Acceptance of pain in neurological disorders: Associations with functioning and psychosocial well-being. Rehabil Psychol. 2013; 58:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powell DJH, Liossi C, Schlotz W, Moss-Morris R.. Tracking daily fatigue fluctuations in multiple sclerosis: Ecological momentary assessment provides unique insights. J Behav Med. 2017; 40:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kratz AL, Murphy SL, Braley TJ.. Ecological momentary assessment of pain, fatigue, depressive, and cognitive symptoms reveals significant daily variability in multiple sclerosis. Arch Phys Med Rehabil. 2017; 98:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kratz AL, Braley TJ, Foxen-Craft E, Scott E, Murphy J, Murphy SL.. How do pain, fatigue, depressive, and cognitive symptoms relate to well-being and social and physical functioning in the daily lives of individuals with multiple sclerosis? Arch Phys Med Rehabil. 2017; 98:2160–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kratz AL, Fritz NE, Braley TJ, Scott EL, Foxen-Craft E, Murphy SL.. Daily temporal associations between physical activity and symptoms in multiple sclerosis. Ann Behav Med. 2018; 53:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kratz AL, Murphy SL, Braley TJ.. Pain, fatigue, and cognitive symptoms are temporally associated within but not across days in multiple sclerosis. Arch Phys Med Rehabil. 2017; 98:2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carver CS. You want to measure coping but your protocol’s too long: Consider the brief COPE. Int J Behav Med. 1997; 4:92–100. [DOI] [PubMed] [Google Scholar]
- 36. Cleeland CS, Ryan KM.. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994; 23:129–138. [PubMed] [Google Scholar]
- 37. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999; 85:1186–1196. [DOI] [PubMed] [Google Scholar]
- 38. Schneider S, Choi SW, Junghaenel DU, Schwartz JE, Stone AA.. Psychometric characteristics of daily diaries for the Patient-Reported Outcomes Measurement Information System (PROMIS®): A preliminary investigation. Qual Life Res. 2013; 22:1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010; 63:1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cella D, Lai J-S, Nowinski CJ, et al. Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012; 78:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gershon RC, Lai JS, Bode R, et al. Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Qual Life Res. 2012; 21:475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cella D, Nowinski C, Peterman A, et al. The neurology quality-of-life measurement initiative. Arch Phys Med Rehabil. 2011; 92:S28–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010; 150:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lai J-S, Cella D, Choi S, et al. How item banks and their application can influence measurement practice in rehabilitation medicine: A PROMIS Fatigue item bank example. Arch Phys Med Rehabil. 2011; 92:S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hahn EA, DeWalt DA, Bode RK, et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014; 33:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, anxiety, and anger. Assessment. 2011; 18:263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC, Lawrence SM.. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS) in a three-month observational study. J Psychiatr Res. 2014; 56:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salsman JM, Victorson D, Choi SW, et al. Development and validation of the positive affect and well-being scale for the neurology quality of life (Neuro-QOL) measurement system. Qual Life Res. 2013; 22:2569–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kratz AL, Davis MC, Zautra AJ.. Pain acceptance moderates the relation between pain and negative affect in female osteoarthritis and fibromyalgia patients. Ann Behav Med. 2007; 33:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kratz AL, Ehde DM, Bombardier CH, Kalpakjian CZ, Hanks RA.. Pain acceptance decouples the momentary associations between pain, pain interference, and physical activity in the daily lives of people with chronic pain and spinal cord injury. J Pain. 2017; 18:319–331. [DOI] [PubMed] [Google Scholar]
- 51. Kratz AL, Whibley D, Kim S, Sliwinski M, Clauw D, Williams DA.. Fibrofog in daily life: An examination of ambulatory subjective and objective cognitive function in fibromyalgia. Arthritis Care Res (Hoboken). 2020; 72:1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zautra AJ, Potter PT, Reich JW.. The independence of affects is context-dependent: An integrative model of the relationship between positive and negative affect. Annu Rev Gerontol Geriatr. 1997; 17:75–103. [Google Scholar]
- 53. Reich JW, Zautra AJ, Davis M.. Dimensions of affect relationships: Models and their integrative implications. Rev Gen Psychol. 2003; 7(1):66–83. [Google Scholar]
- 54. Iida M, Shrout PE, Laurenceau J-P, Bolger N: Using diary methods in psychological research. In: APA Handbook of Research Methods in Psychology, Vol 1: Foundations, Planning, Measures, and Psychometrics. Washington, DC: American Psychological Association, 2012:.277–305. [Google Scholar]
- 55. Jensen MP, Ehde DM, Day MA.. The behavioral activation and inhibition systems: Implications for understanding and treating chronic pain. J Pain. 2016; 17(5):529.e1–529.e18. [DOI] [PubMed] [Google Scholar]
- 56. Gray JA. Brain systems that mediate both emotion and cognition. Cogn Emot. 1990; 4(3):269–288. [Google Scholar]
- 57. Sánchez-Rodríguez E, Racine M, Castarlenas E, et al. Behavioral activation and inhibition systems: Further evaluation of a BIS-BAS model of chronic pain. Pain Med. 2021; 22(4):848–860. [DOI] [PubMed] [Google Scholar]
- 58. Turner AP, Jensen MP, Day MA, Williams RM.. Behavioral activation and behavioral inhibition: An examination of function in chronic pain. Rehabil Psychol. 2021; 66(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gromisch ES, Kerns RD, Czlapinski R, et al. Cognitive behavioral therapy for the management of multiple sclerosis–related pain. Int J MS Care. 2020; 22(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E.. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008; 70(2):205–213. [DOI] [PubMed] [Google Scholar]
- 61. Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L.. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012; 50(6):415–421. [DOI] [PubMed] [Google Scholar]
- 62. Mills N, Allen J.. Mindfulness of movement as a coping strategy in multiple sclerosis. A pilot study. Gen Hosp Psychiatry. 2000; 22(6):425–431. [DOI] [PubMed] [Google Scholar]
- 63. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: A randomized trial. Neurology. 2010; 75(13):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoogerwerf AEW, Bol Y, Lobbestael J, Hupperts R, van Heugten CM.. Mindfulness-based cognitive therapy for severely fatigued multiple sclerosis patients: A waiting list controlled study. J Rehabil Med. 2017; 49:497–504. [DOI] [PubMed] [Google Scholar]
- 65. Finlayson M, Preissner K, Cho C, Plow M.. Randomized trial of a teleconference-delivered fatigue management program for people with multiple sclerosis. Mult Scler. 2011; 17(9):1130–1140. [DOI] [PubMed] [Google Scholar]
- 66. Thomas S, Thomas PW, Kersten P, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013; 84(1):1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barlow J, Turner A, Edwards R, Gilchrist M.. A randomised controlled trial of lay-led self-management for people with multiple sclerosis. Patient Educ Couns. 2009; 77(1):81–89. [DOI] [PubMed] [Google Scholar]
- 68. Koeske GF, Kirk SA, Koeske RD.. Coping with job stress: Which strategies work best? J Occup Organ Psychol. 1993; 66(4):319–335. [Google Scholar]
- 69. Chao RC-L. Managing stress and maintaining well-being: Social support, problem-focused coping, and avoidant coping. J Couns Dev. 2011; 89(3):338–348. [Google Scholar]
- 70. Cheng T, Mauno S, Lee C.. The buffering effect of coping strategies in the relationship between job insecurity and employee well-being. J Ind Econ. 2014; 35(1):71–94. [Google Scholar]
- 71. Thomsen T, Fritz V, Mößle R, Greve W.. The impact of accommodative coping on well-being in childhood and adolescence: Longitudinal findings. Int J Behav Dev. 2015; 39(5):467–476. [Google Scholar]
- 72. Funder DC, Ozer DJ.. Evaluating effect size in psychological research: Sense and nonsense. Adv Methods Pract Psychol Sci. 2019; 2(2):156–168. [Google Scholar]