Abstract
We present here a high-performance liquid chromatography−tandem mass spectrometry (LC-MS/MS) method for quantifying phytoestrogenic isoflavones (daidzein, equol, genistein, and O-desmethylangolensin) and lignans (enterodiol and enterolactone) in urine without the use of extraction or the preconcentration techniques inherent in existing methods. The development of this concept was made possible by use of atmospheric pressure photoionization (APPI); an ionization technique that we found to improve analyte sensitivity relative to electrospray ionization and atmospheric pressure chemical ionization for this particular group of compounds. The analytical performance of this method was equal to or exceeded that of comparable methods. Between-run coefficients of variation (CVs) across three quality control (QC) pool levels analyzed in duplicate over 20 days were 3.1–5.8% CV; within-run CVs were 2.3–6.0%. Accuracy, as determined by average spike recovery in QC pools, was generally within ±10% of being quantitative (100%). Relative limits of detection were 0.04–0.4 ng/mL urine, with absolute detection limits as low as 0.1 pg. This method was applied to the analysis of >2,500 urine specimens for the 2005–2006 Centers for Disease Control and Prevention’s National Health and Nutrition Examination Survey (NHANES). The method was capable of quantifying these compounds in 95–100% of study samples. This work is the first ever report of using APPI for the LC-MS/MS determination of these compounds in urine. It is also the first method of its kind to do so without any need for analyte extraction or preconcentration prior to analysis.
Keywords: Isoflavones, Lignans, Phytoestrogens, Urine, Atmospheric pressure photoionization, Electrospray, Mass spectrometry, ESI, APCI, APPI
Introduction
Isoflavones and lignans are two categories of secondary plant metabolites commonly encountered in the diet. Foods consisting of or derived from legumes—especially soy products—are common sources of dietary isoflavones [1], whereas fiber-rich foods such as whole grains and seeds—flaxseed in particular—are characteristic sources of lignans [2]. Once absorbed into the body, these substances or their metabolites [1, 3] can act as phytoestrogens; a class of compounds that are structurally similar to and that weakly function like natural estradiol. The dietary consumption of phytoestrogens is thought to reduce the incidence of such hormone-dependent cancers as breast [4, 5] and prostate [6, 7] cancer due to antagonistic mechanisms related to hormone receptor binding. In addition to their role as phytoestrogens, other health benefits associated with isoflavone and/or lignan consumption have been reported, including reduced severity of menopausal symptoms [8, 9], modulation of osteoporosis [10], and reduction of the risk of cardiovascular disease [11, 12].
Epidemiological interest in isoflavones and lignans has led to the development of numerous methods for measuring the biologic concentrations of these compounds in the context of pharmacokinetic studies, exposure biomonitoring, and compliance testing in clinical trials [13–15]. Of the methods developed for these purposes, those based on high-performance liquid chromatography (HPLC) separation paired with either mass spectrometry (MS) [16, 17] or tandem mass spectrometry (MS/MS) [18–23] detection have emerged as a leading class due to a favorable combination of measurement sensitivity and specificity. The current state of the art in LC-MS and LC-MS/MS methods developed for measuring isoflavones and lignans in biologic matrices generally relies upon an extraction technique, such as liquid−liquid extraction (LLE) [16–19, 22] or solid-phase extraction (SPE) [16, 20, 21, 23]. The use of SPE or LLE in these methods typically serves a dual purpose: (1) it functions as a means of extracting analytes from potentially interfering matrix components and (2) it presents a juncture at which the sample can be reconstituted in a preconcentrated form to overcome measurement sensitivity limitations and thereby enhance analyte detection. Most SPE- and LLE-based LC-MS and LC-MS/MS methods for measuring these compounds employ negative ion mode electrospray ionization (ESI) [17–22], although methods using atmospheric pressure chemical ionization (APCI) [16, 20, 23] are sometimes encountered.
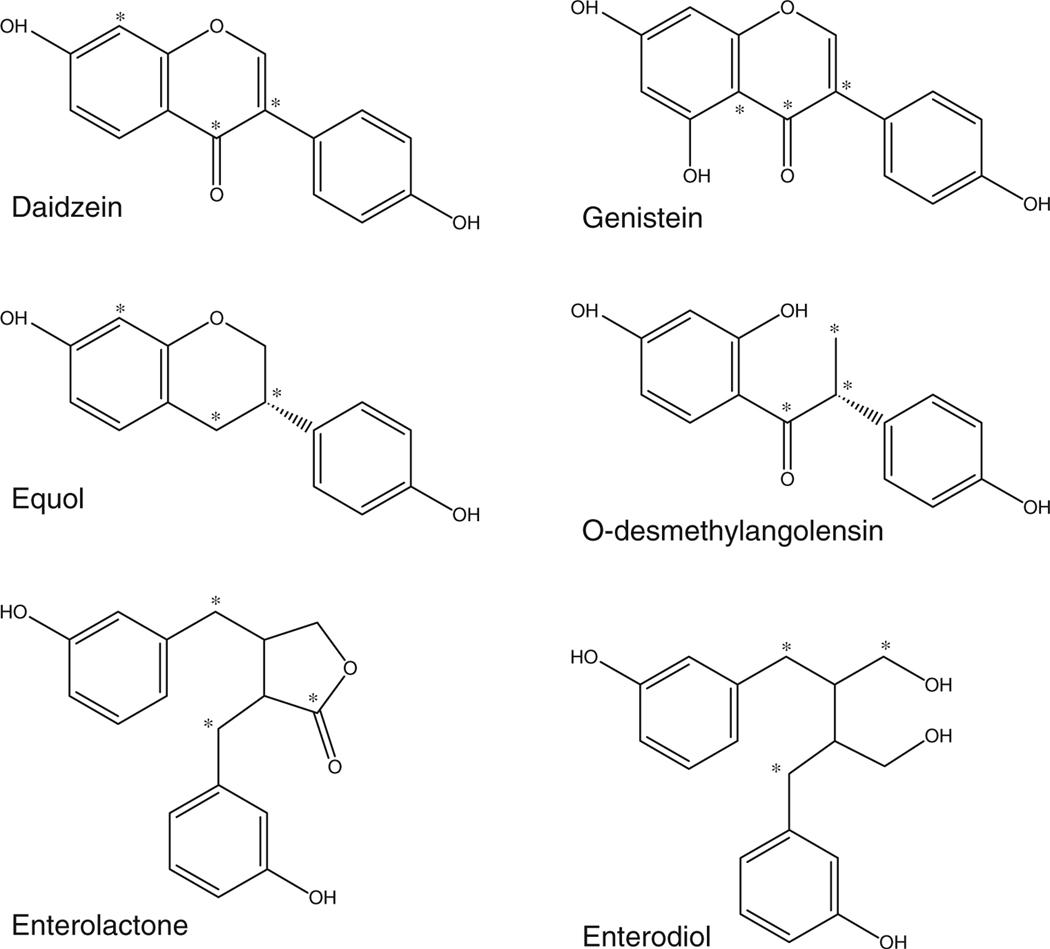
Urinary isoflavone and lignan concentrations can serve as biomarkers of dietary intake [24]. The urinary concentrations of several phytoestrogenic isoflavones (daidzein, genistein, equol, O-desmethylangolensin) and lignans (enterolactone and enterodiol; Fig. 1) have been measured since 1999 as part of the National Health and Nutrition Examination Survey (NHANES), a program of continuous studies conducted by the U.S. Centers for Disease Control and Prevention and designed to assess the health and nutritional status of adults and children in the USA [25]. Our laboratory has considerable experience in performing these measurements for NHANES by using SPE-based LC-MS/MS, first using negative ion mode APCI [23, 26] and later negative ion mode ESI [20]. Our development of a negative ion mode ESI method for measuring urinary isoflavones and lignans was driven by preliminary experiments that showed improved measurement sensitivity for certain analytes. We subsequently performed a thorough comparison of our ESI and APCI methods and confirmed the presence of substantial measurement sensitivity improvements when ESI was used. The case was particularly compelling for equol, whereby using ESI resulted in a tenfold sensitivity improvement that improved our ability to quantify this analyte in NHANES samples from 81% to 98% [20].
Fig. 1.
Chemical structures of the urinary isoflavones (daidzein, genistein, equol, O-desmethylangolensin) and lignans (enterolactone, enterodiol). Positions of the 13C atoms in the corresponding internal standard are denoted by an asterisk
Building on our experience with ESI vs. APCI, we sought to explore the use of other ionization techniques as a means of further improving measurement sensitivity and selectivity in the hopes of eliminating the need for analyte extraction and preconcentration entirely. Atmospheric pressure photoionization (APPI) is an ionization technique in which UV radiation is used to cause the formation of molecular radical ions in the gas phase [27, 28]. To overcome the low statistical probability of direct analyte ionization, the process introduces an excess of an easily ionizable substance (i.e., dopant) into the APPI source to initiate a series of direct or solvent/oxygen-mediated proton transfer, charge exchange, electron capture, and/or substitution reactions that eventually ionize the analyte [27]. Although originally intended as an ionization source for compounds not amenable to either ESI or APCI, APPI has been applied for a wide variety of compounds with reports of enhanced selectivity, expanded linear dynamic ranges, and reduced ion suppression [28]. On the basis of the chemical structures of isoflavones and lignans, we hypothesized that APPI held the potential to facilitate developing an LC-MS/MS method for urinary isoflavones and lignans with improved analyte selectivity and sensitivity and that these enhancements would permit the development of a sample preparation protocol that eliminates the need for SPE or LLE.
In this work, we present the development, validation, and analytical performance of a negative ion mode APPI-based LC-MS/MS method for measuring selected isoflavones and lignans in urine. We demonstrate its application to population biomonitoring. We provide a direct performance comparison of our new method to our existing SPE-based ESI method on the same instrument, and we also present the adaptation of our new APPI method to a second instrument. To the best of our knowledge, our method represents two significant firsts in isoflavone and lignan measurement science: (1) it is the first ever report of using APPI for the LC-MS/MS determination of these compounds in urine and (2) is the first LC-MS/MS method for urinary phytoestrogens to feature sample preparation without any need for analyte extraction or preconcentration.
Materials and methods
Specimens, standards, and reagents
Reverse osmosis deionized water, HPLC grade solvents, and high-purity reagents were used throughout. Individual standard stock solutions of daidzein, equol, genistein (Indofine, Hillsborough, NJ, USA), enterodiol, enterolactone (Sigma-Aldrich, St. Louis, MO, USA), and O-desmethylangolensin (laboratory of Dr. Nigel Botting, University of St. Andrews, St. Andrews, Scotland) were prepared by dissolving 3–5 mg of each solid material in 0.2 mL of dimethylsulfoxide and diluting with ethanol to a final concentration of 87–193 μg/mL. Nine mixed working standards containing all six analytes were prepared in 50:50 ethanol/water and stored in 100 μL aliquots at −80 °C, from which calibrators were prepared daily. The nine calibrators ranged in concentration from 0.3 to 3,300 ng/mL, depending on the analyte (Table 1). 13C3-daidzein, 13C3-O-desmethylangolensin, 13C3-equol, 13C3-enterodiol, 13C3-enterolactone, and 13C3-genistein (laboratory of Dr. Nigel Botting, University of St. Andrews) were used for internal standardization of the analyte signals studied. Individual stock solutions of each internal standard were prepared by dissolving 1 mg of each solid material in ethanol and diluting to a final concentration of 40–60 μg/mL. A mixed internal standard solution was prepared in water from single-compound stock solutions and stored in 1 mL aliquots at −80 °C. The internal standard concentrations in this solution were from 400 to 1,200 ng/mL, depending on the compound. A 60-mg/mL solution of β-glucuronidase/sulfatase from Helix pomatia, type H-1 (Sigma), dissolved in water was prepared daily and used to enzymatically deconjugate the analytes of glucuronide and sulfate moieties during sample preparation. A deconjugation internal standard solution containing 24 μg/mL 4-methylumbelliferyl glucuronide (Sigma) and 20 μg/mL 4-methylumbelliferyl sulfate (Sigma) in ethanol for monitoring the extent of the deconjugation reaction was prepared and stored in 1 mL aliquots at −80 °C. Urine samples used for generating quality control (QC) pools were collected from anonymous donors in accordance with applicable IRB protocols and stored at −80 °C prior to use. Synthetic urine was prepared according to an established protocol [29].
Table 1.
LC-MS/MS analysis parameters
| Analyte | Retention time (min) | Calibration range (ng/mL) | ISa conc. (ng/mL) | MS/MS transitions (m/z) |
Dwell time (ms) | Mass spectrometer potentials (V) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| API 4000 |
API 5000 |
||||||||||||
| Type | Mol. Ion | Prod. Ion | DP | CE | CXP | DP | CE | CXP | |||||
|
| |||||||||||||
| Daidzein | 1.3 | 0.4–1600 | 7.0 | Q | 253 | 117 | 20 | −47 | −44 | −7 | −114 | −44 | −13 |
| C | 253 | 104 | 20 | −47 | −65 | −7 | −114 | −65 | −13 | ||||
| IS | 256 | 118 | 20 | −65 | −43 | −7 | −135 | −47 | −15 | ||||
| O-desmethylangolensin | 2.7 | 0.2–300 | 1.0 | Q | 257 | 136 | 20 | −53 | −32 | −8 | −102 | −32 | −14 |
| C | 257 | 80 | 20 | −53 | −49 | −9 | −102 | −47 | −9 | ||||
| IS | 260 | 137 | 20 | −50 | −32 | −9 | −80 | −32 | −16 | ||||
| Equol | 1.9 | 0.06–100 | 9.6 | Q | 241 | 119 | 20 | −26 | −32 | −10 | −97 | −31 | −13 |
| C | 241 | 93 | 20 | −26 | −42 | −6 | −97 | −42 | −12 | ||||
| IS | 244 | 122 | 20 | −40 | −20 | −8 | −100 | −22 | −14 | ||||
| Enterodiol | 1.5 | 0.04–320 | 5.4 | Q | 301 | 253 | 20 | −60 | −33 | −10 | −95 | −33 | −17 |
| C | 301 | 106 | 20 | −60 | −49 | −7 | −95 | −47 | −14 | ||||
| IS | 304 | 256 | 20 | −60 | −33 | −7 | −115 | −33 | −13 | ||||
| Enterolactone | 2.0 | 0.1–3300 | 6.8 | Q | 297 | 189 | 20 | −51 | −30 | −7 | −120 | −31 | −17 |
| C | 297 | 93 | 20 | −51 | −47 | −8 | −120 | −49 | −13 | ||||
| IS | 300 | 255 | 20 | −55 | −29 | −7 | −114 | −29 | −14 | ||||
| Genistein | 2.3 | 0.2–730 | 5.0 | Q | 269 | 133 | 20 | −41 | −49 | −9 | −120 | −50 | −15 |
| C | 269 | 224 | 20 | −41 | −36 | −10 | −120 | −36 | −17 | ||||
| IS | 272 | 63 | 20 | −60 | −59 | −9 | −120 | −58 | −22 | ||||
| Umbelliferone | 1.2 | IS | 175 | 133 | 20 | −150 | −30 | −10 | −150 | −30 | −10 | ||
Q quantitation, C confirmation, IS internal standard
Sample preparation
A 200-μL aliquot of each urine sample (patient specimens and QC pools) was transferred to a 1-mL, 96-well conical bottom plate (Nalge Nunc International, Rochester, NY, USA). Each aliquot was then amended in order with 50 μL of a 5× water dilution of the stable isotope-labeled internal standard solution, 10 μL of 20× water dilution of the deconjugation internal standard solution, 20 μL of ammonium acetate buffer (2.5 mol/L, pH 5.0), and 10 μL (120 U) of β-glucuronidase/sulfatase. Calibrators, blanks, and double blanks were prepared in solutions of synthetic urine and processed in an identical manner. The plate was covered with a pre-slit silicone plate seal (Thermo-Fisher Scientific, Fair Lawn, NJ, USA); mixed by use of a gentle rocking motion; and all specimens, standards, and blanks were incubated overnight (≥12 h) at 45 °C. After incubation, 100 μL of methanol was added to each specimen, calibrator, blank, and double blank and mixed. Finally, the contents of each well were transferred to a 96-well, 10-kDa size exclusion filter (Millipore, Billerica, MA, USA) and filtered by centrifugation for at least 1 h at 3,000×g, with the contents collected into a 1-mL, 96-well conical bottom plate. The well plate was then sealed with a pre-slit silicone plate seal and the contents were ready for LC-MS/MS analysis.
LC-MS/MS conditions
Methods were developed for two different triple quadrupole mass spectrometers (API 4000 and API 5000, AB Sciex, Foster City, CA, USA) equipped with the same APPI source. In both cases, chromatographic separation was achieved using the same 600-bar, 1260 HPLC system (Agilent, Santa Clara, CA, USA) consisting of a binary pump and degasser (model G1312A), an autosampler (G1367D) and autosampler thermostat module (model G1330B), and a thermostatted column compartment (model G1316B). Compounds were separated by use of a solidcore C18 analytical column (Kinetex C18, 50×2.1-mm ID, 2.6-μm particle diameter; Phenomenex, Torrance, CA, USA) with an in-line filter (Krud Katcher Ultra, 0.5-μm porosity, 102-μm ID; Phenomenex). A gradient consisting of water (solvent A) and methanol (solvent B) with a constant flow rate of 0.6 mL/min was used. The solvent gradient was as follows (expressed as percent solvent B): 0 min, 35%; 0.5 min, 35%; 2.5 min, 95%, 4.5 min, 95%; 5 min, 35%; 7 min, 35%. Injection volumes of 5 μL (API 5000) and 20 μL (API 4000) were used throughout. An automated six-port switching valve was used to direct eluent flow. From 0.75 min to completion of the gradient program, the eluent flow was directed to the mass spectrometer to capture analyte elution; from 0 to 0.75 min, the eluent flow was diverted to waste.
A summary of LC-MS/MS conditions for both instruments is shown in Table 1. Potential analyte and internal standard MS/MS transitions were first identified and optimized by manual tuning. Candidate MS/MS transitions were then incorporated into a preliminary LC-MS/MS method used to analyze a random subset of human urine samples. The analyte transition that was found to yield a chromatographic signal with the most favorable analyte sensitivity and signal-to-noise characteristics, accompanied by the least amount of background and concomitant peak contributions, was selected as the primary “quantitation” transition from which the concentration data would be reported; the second most favorable transition was used as a “confirmation” transition. The same approach was used in identifying the transition to be used for internal standardization, selecting whenever possible the same transition used for quantitation when the 13C3 labeling is taken into account. Toluene (0.2 mL/min flow) was introduced into the APPI source as a dopant by use of an isocratic pump (Agilent, model G1310A). Ionization interface potential (−875 V), temperature (450 °C), and gas flow rates [nebulizing gas, 50 psi (API 4000), 80 psi (API 5000); lamp gas, 60 psi (API 4000), 80 psi (API 5000)] were optimized to maximize chromatographic peak height signal-to-noise ratio. Nitrogen was used as the interface gas (nebulizing gas, lamp gas) as well as the curtain and collision gas. All LC-MS/MS components were controlled and data analysis was performed by use of Analyst software (version 1.4.2, AB/Sciex). Analytes were quantified by interpolation of peak area ratios for MS/MS transitions against a nine-point calibration curve (1/x weighting).
Validation
QC pools were prepared at three distinct concentrations levels approximating the 25th (“low” QC), 50th (“medium” QC), and 75th (“high” QC) percentiles observed previously in the US population. Pools were prepared using varying amounts of the specimens collected from anonymous volunteers. Specimens were screened by use of the LC-MS/MS method and pooled based on their endogenous concentrations to meet target concentrations. No direct analyte spiking was required. All QC pools were run at the beginning and end of each analysis batch. Data from the first 20 independent runs (days) were used to establish the QC limits as part of a multirule procedure for determining whether instrument runs were in or out of control [30]. These QC pools were also used for specific validation tests, as indicated. The within-run and between-run coefficients of variation from the 20-day duplicate analysis of the three QC pools were also used to assess within-run and between-run imprecision, respectively.
Method accuracy was assessed by analyte spike recovery and comparative analysis of quantitation results by use of different MS/MS transitions (“quantitation” vs. “confirmation” transitions), different instruments (API 4000 vs. API 5000), and different methods (APPI vs. ESI). Analyte spike recovery was performed by use of the “low” and “medium” QC pools. Each pool was amended in triplicate with analyte additions approximating 50%, 100%, and 200% of its endogenous concentration. The spike recovery was calculated as the measured concentration difference between the spiked and unspiked pools divided by the nominal concentration of the analyte spike. The average spike recovery was calculated across all QC pools, spiking levels, and replicates. Comparative analysis of the quantitation results was performed across several days using randomly selected unknown patient samples. For each analyte, the correlation (Pearson r), the error in variables regression (Deming), and the relative bias (Bland−Altman) were calculated, employing significance testing at the 95% confidence interval. The number of samples and days of analysis were as follows: 225 samples over 3 days for the comparison of different MS/MS transitions and different instruments and 328 samples over 5 days for the comparison of different methods.
Method selectivity was assessed by preparing synthetic urine samples individually spiked with each analyte and internal standard and then analyzing these samples for spurious signal contributions across all MS/MS transitions used in the method. The analyte concentrations used were equivalent to the upper limit of the calibration range, and internal standard concentrations were approximately double what the method protocol calls for.
Method sensitivity was objectively determined by calculating the limit of detection (LOD) and the lower limit of quantitation (LLOQ) for each analyte. Serial dilution of the “low” QC pool with synthetic urine was performed and the standard deviation at a concentration of zero (σ0) was estimated by extrapolating repeat analyte measurements (n=8) made near the detection limit in these dilutions [31]. The LOD and LLOQ were defined as 3σ0 and 10σ0, respectively. Ion suppression was also used as a means of evaluating the susceptibility of the method to matrix-induced sensitivity changes. Post-column analyte and internal standard infusion was performed to elevate the baseline of each MS/MS transition to approximately 1×105 ion counts per second. Synthetic urine blanks, calibrators, and QC samples were injected as test specimens. The degree of ion suppression was evaluated as the relative deviation of the baseline observed in the elution time window for each analyte and internal standard.
Method robustness was evaluated by identifying critical method parameters and evaluating the effects of intentionally varying these conditions on the observed concentrations in the QC pools. The parameters tested were (method specification, test condition 1, test condition 2): β-glucuronidase/sulfatase (120, 60, and 240 U); incubation time (12, 4, and 24 h); ammonium acetate buffer pH (5.0, 4.5, and 5.5); ammonium acetate buffer concentration (2.5, 0.25, and 1.25 M); and time elapsed from complete sample preparation (<1, 3, and 7 days).
Results and discussion
Method development
The development of an APPI-based LC-MS/MS method for quantifying urinary concentrations of phytoestrogenic isoflavones and lignans was a significant departure from our past ESI- or APCI-based procedures. We found that optimal ionization conditions were highly dependent upon both dopant flow rates and gas flow rates, with gas flow settings requiring more critical optimization in APPI as compared to our experiences with both ESI and APCI. Mobile phase composition also had a far more profound effect on ionization. For example, we observed that the presence of ammonium acetate buffer in the mobile phase selectively and completely inhibited equol ionization—even when the buffer concentration was reduced to sub-millimolar levels—whereas the effect on the ionization efficiency of other analytes was not nearly as severe. Removing the acetate buffer from the mobile phase restored the equol signal to levels proportionate to the other analytes. This type of phenomenon is not unheard of in APPI; analyte-dependent suppression of ionization efficiency due to the addition of ammonium acetate or other buffers has been reported previously [32–34]. Solvent selection is also known to greatly influence APPI efficiency, a phenomenon we also observed. For instance, we found that a water/methanol gradient yielded better signal intensity than water/acetonitrile.
The adverse effects of mobile phase buffers on APPI efficiency presented a unique challenge in terms of chromatographic separation. Mobile phase buffering is a preferred means of ensuring consistent chromatographic separation by controlling analyte protonation. Since the use of available mobile phase buffers was not practical if we intended to use APPI, we performed experiments to see whether the addition of buffer to the sample alone was sufficient to reliably control protonation and yield reproducible chromatography. We found that a pH 5 acetate buffer at a concentration of approximately 170 mmol/L in the sample was sufficient for reproducible chromatography in all urine samples analyzed in our laboratory. At lower concentrations (e.g., 50 mmol/L), we occasionally encountered evidence of analyte deprotonation in the urine samples in which analyte elution—in particular for daidzein and genistein—would occur in the void volume. To minimize sample dilution, we added a small (20 μL) aliquot of a relatively concentrated (2.5 M) buffer to the urine sample. The only limitation we found with this approach was that the order in which reagent additions were performed was critical; addition of the buffer aliquot prior to the addition of the β-glucuronidase/sulfatase enzyme was effective, whereas adding the enzyme solution first would often result in its denaturation.
Hydrolysis of the glucuronidated forms of urinary isoflavones and lignans by β-glucuronidase from H. pomatia is well documented in the literature and has been studied in depth by Taylor et al. [35]. In the course of our own method development, we tested a variety of incubation times (1–24 h) and enzyme concentrations (<100 to >1,000 U) for hydrolyzing the glucuronide and sulfate conjugates of the urinary isoflavones and lignans studied. In designing our experiments, we sought to limit the amount of enzyme used in order to minimize the potential contribution of isoflavones and lignans endogenous to the enzyme [35]. Through the use of our QC pools for testing, initial experiments showed that a 200-μL urine sample treated with 120 U of enzyme at 37 °C appeared to undergo complete hydrolysis for all analytes in 4 h on the basis of the appearance of concentration vs. time curves. We also found that synthetic urine blanks treated with 120 U of enzyme did not show any detectable evidence of background isoflavones or lignan concentrations. However, replicate experimentation revealed that concentrations were on average 2–7% higher when hydrolysis was allowed to proceed for at least 12 h. In contrast, Taylor et al. [35] reported that urine sample hydrolysis was complete in 2 h (37 °C, pH 5). It is worth noting that the amount of enzyme used by Taylor et al. (2,410 U) was 20 times higher than the amount used in our experimental design for the same volume of urine (200 μL). It is possible that this concentration difference may be a contributing factor to the time difference as well as to the methodological differences in how complete hydrolysis was calculated. Additionally, we used type H-1 β-glucuronidase from H. pomatia, whereas Taylor et al. [35] used type HP-2, and this difference may have also been a contributing factor in the differences observed in enzyme performance. Increasing the hydrolysis temperature to 45 °C as a means of accelerating the deconjugation step [35] was tested and found to have no negative effect on enzyme performance. In the end, we adopted a 45 °C overnight hydrolysis (≥12 h) as a judicious approach to ensure the complete conversion of all analyte conjugates to aglycones.
The presence of the β-glucuronidase/sulfatase enzyme in the samples required special consideration in the absence of using extractive procedures such as SPE and LLE. Particulate filtration media based on glass fiber, nylon, polytetrafluroethylene, polyvinyldifluoride, and polypropylene were found to be ineffective at removing the β-glucuronidase/sulfatase enzyme from the sample, necessitating the use of a different filtration approach. Size exclusion filtration presented the possibility of filtering based on a nominal molecular weight cutoff rather than a particle size cutoff. After testing several combinations of molecular weight cutoffs and substrates, we decided upon a 10-kDa nominal molecular weight cutoff filter based on a regenerated cellulose substrate. The 10-kDa cutoff represented a highly conservative approach to removing the enzyme from the sample (a β-glucuronidase tetramer is 312 kDa) and proved effective for our filtration purposes. HPLC column lifetime was found to be >2,000 sample injections with the 10-kDa size exclusion filter vs. 50–100 sample injections when sub-micrometer particle filtration was used. Column failure with particle filtration was almost always due to the high operating pressures, which we presumed to be caused by enzyme precipitation initiated by the methanol gradient. In the course of testing various size exclusion filters, we also noted that the filter substrate itself had a profound influence on analyte recovery. When testing the prepared urine samples in which only aqueous reagent additions had been made, we observed that regenerated cellulose had the lowest degree of analyte retention, with analyte-dependent recoveries ranging from 48% (genistein) to 99% (enterodiol) as compared with unfiltered samples. In contrast, polyethersulfone substrates exhibited a high degree of analyte retention, with recoveries of <10% for enterodiol and equol and <1% for genistein. We tested methanol as a solvent for recovering the analytes retained on the regenerated cellulose substrate and found it to be highly successful. Adding 100 μL of methanol to the sample prior to filtration effectively resulted in a quantitative (100%) recovery for all analytes. In addition to improving analyte recovery, adding methanol to the sample also elevated the relative methanol content of the samples to approximately the same level found in the LC gradient starting conditions (35%), and it improved the ease with which samples could be filtered.
Method performance and validation
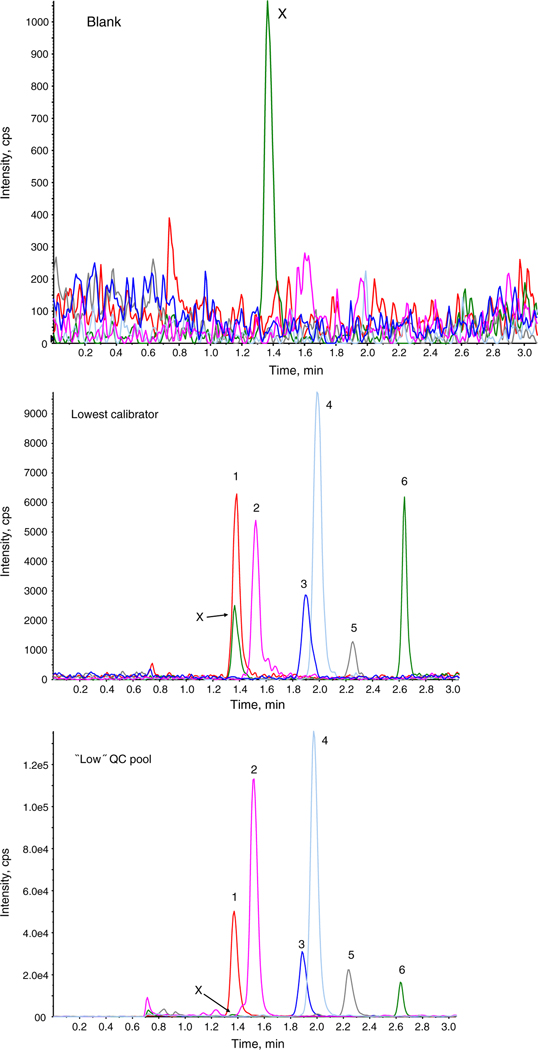
Figure 2 shows representative chromatography for the analyte quantitation transitions from a urine specimen using the blank, lowest calibrator, and low QC pool as examples. Analyte elution occurs over a retention time range of 1.25–2.75 min. Umbelliferone, the aglycone product of the 4-methylumbelliferyl glucuronide and 4-methylumbelliferyl sulfate mixture added to the samples as a means of monitoring deconjugation eluted at a retention time of 1 min. With one exception [18], the total sample run time for this method is among the shortest of the published methods for these compounds [14–17, 19–23] at only 7 min from injection to injection. Within-run retention time imprecision was typically <1% CV.
Fig. 2.
Chromatograms of quantitation MS/MS transitions (m/z of molecular/product ion) for the blank, lowest calibrator, and “low” QC urine pool. The blank and the lowest calibrator were prepared in synthetic urine. 1 Daidzein (253/117); 2 enterodiol (301/253); 3 equol (241/119); 4 enterolactone (297/189); 5 genistein (269/133); 6 O-desmethylangolensin (257/136). Peak X observed in the O-desmethylangolensin (257/136) transition at the retention time for daidzein is an M+1 contribution from the 13C3-daidzein internal standard. This peak is not observed when 13C3-daidzein is not present
Calibrators were prepared in synthetic urine at a total of nine concentration levels for each analyte. We tested calibrators prepared in both synthetic urine and aqueous solution and found that the different matrices had no discernable effect on the calibration slope or intercept. Nonetheless, we elected to use synthetic urine for calibrator preparation in the interest of good analytical practice. Calibration curves had correlation coefficients (r values) >0.995. We evaluated the accuracy (i.e., the agreement between the nominal and measured value) of each calibrator from duplicate analysis over 20 runs (Table 2) and found that the measured values of the calibrators were generally within ±5% of their nominal values throughout the calibration range. Only in the lowest concentration calibrators did we observe inaccuracies beyond ±5%.
Table 2.
Analytical performance
| Analyte | Calibration |
Imprecision |
Accuracy |
Sensitivity |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Level (ng/mL) | Accuracy (%) | CV (%) | QC pool (ng/mL) | Overall CV (%) | Within-run CV (%) | Between-run CV (%) | Spike recovery (%) | CV (%) | LOD (ng/mL) | LLOQ (ng/mL) | |
|
| |||||||||||
| Daidzein | 1 | 115.4 | 9.3 | 20.5 | 4.9 | 3.9 | 3.4 | 109 | 3 | 0.4 | 1 |
| 10 | 97.5 | 3.7 | 73.7 | 4.1 | 3.8 | 2.3 | |||||
| 40 | 93.1 | 3.0 | 612 | 4.1 | 3.3 | 3.3 | |||||
| 55 | 98.7 | 3.2 | |||||||||
| 70 | 99.0 | 2.5 | |||||||||
| 100 | 98.7 | 2.5 | |||||||||
| 125 | 100.2 | 2.8 | |||||||||
| 1000 | 101.7 | 2.6 | |||||||||
| 1600 | 100.0 | 2.9 | |||||||||
| O-desmethylangolensin | 0.4 | 111.6 | 9.7 | 2.6 | 3.8 | 3.8 | 3.8 | 106 | 6 | 0.2 | 0.6 |
| 1 | 101.4 | 5.8 | 13.3 | 6.0 | 4.5 | 6.0 | |||||
| 3 | 95.3 | 3.7 | 216 | 5.9 | 5.2 | 4.0 | |||||
| 4 | 95.7 | 4.3 | |||||||||
| 5 | 96.8 | 4.2 | |||||||||
| 14 | 97.1 | 4.1 | |||||||||
| 20 | 97.6 | 4.3 | |||||||||
| 110 | 101.1 | 3.9 | |||||||||
| 300 | 99.4 | 4.0 | |||||||||
| Equol | 0.3 | 105.6 | 11.0 | 5.6 | 4.3 | 3.9 | 2.5 | 103 | 3 | 0.06 | 0.2 |
| 1 | 99.6 | 4.2 | 13.8 | 4.0 | 3.6 | 2.3 | |||||
| 4 | 98.9 | 2.9 | 39.4 | 4.0 | 3.5 | 2.7 | |||||
| 6 | 99.8 | 3.0 | |||||||||
| 8 | 100.8 | 3.1 | |||||||||
| 14 | 99.3 | 2.0 | |||||||||
| 18 | 99.8 | 2.6 | |||||||||
| 60 | 101.9 | 2.1 | |||||||||
| 100 | 100.4 | 2.0 | |||||||||
| Enterodiol | 0.3 | 106.2 | 7.5 | 20 | 3.8 | 3.3 | 2.8 | 102 | 11 | 0.04 | 0.1 |
| 2 | 95.9 | 3.6 | 45.5 | 3.6 | 3.2 | 2.5 | |||||
| 25 | 98.6 | 2.7 | 212 | 4.6 | 4.1 | 2.8 | |||||
| 30 | 100.6 | 2.2 | |||||||||
| 34 | 99.6 | 2.1 | |||||||||
| 55 | 99.7 | 2.0 | |||||||||
| 75 | 100.2 | 2.1 | |||||||||
| 175 | 100.6 | 2.4 | |||||||||
| 320 | 99.5 | 2.0 | |||||||||
| Enterolactone | 2 | 97.9 | 7.3 | 70.5 | 3.5 | 3.1 | 2.3 | 108 | 3 | 0.1 | 0.3 |
| 10 | 96.2 | 3.4 | 394 | 3.8 | 3.1 | 3.0 | |||||
| 150 | 102.3 | 2.1 | 1410 | 4.9 | 4.1 | 4.0 | |||||
| 250 | 102.0 | 2.2 | |||||||||
| 315 | 101.5 | 2.1 | |||||||||
| 550 | 101.8 | 2.3 | |||||||||
| 750 | 100.4 | 2.3 | |||||||||
| 2100 | 96.2 | 3.2 | |||||||||
| 3300 | 104.3 | 4.9 | |||||||||
| Genistein | 0.4 | 102.2 | 16.0 | 15.8 | 6.3 | 5.1 | 5.7 | 112 | 7 | 0.2 | 0.6 |
| 2 | 96.1 | 7.9 | 46.7 | 7.1 | 5.8 | 5.8 | |||||
| 15 | 96.2 | 4.4 | 302 | 5.9 | 5.2 | 3.9 | |||||
| 22 | 100.7 | 4.0 | |||||||||
| 27 | 102.8 | 3.4 | |||||||||
| 55 | 103.4 | 2.8 | |||||||||
| 75 | 99.8 | 3.3 | |||||||||
| 325 | 98.9 | 3.0 | |||||||||
| 730 | 101.3 | 3.8 | |||||||||
QC pools were prepared at three distinct concentration levels (“low,” “medium,” and “high”) and were used for judging instrument run control as well as aspects of the method validation. The QC pools were prepared by mixing human urine specimens with known analyte concentrations. The QC pools were assigned target values and uncertainty limits by a characterization process (duplicate analysis over 20 days). The use of QC pools prepared from genuine biologic specimens with endogenous analyte concentrations has been employed elsewhere [20, 21]. We chose this approach over the practice of preparing QC pools by spiking analytes into synthetic or blank biologic matrices because our approach facilitated the inclusion of conjugated analyte forms and thus faithfully represented the analyte and matrix composition encountered in actual human urine specimens. Although spiking with conjugates has been performed in methods where the conjugated forms are measured [36, 37], most cases of amended QC pool preparation with blank or synthetic matrices entailed the addition of analytes solely as aglycones [16–19, 22] rather than a biologically representative mixture of aglycones and conjugates such as glucuoronides and sulfates. Because conjugated forms of the isoflavones and lignans predominate in biologic specimens, the use of QC pools with no conjugated forms present may not genuinely reflect true method performance with actual biologic samples, particularly since most of these methods quantified total aglycone concentrations following deconjugation.
Method imprecision was determined from the duplicate analysis of the three QC pools over 20 runs. The method imprecision is summarized in Table 2. Between-run imprecision was 3.1–5.8% CV and within-run imprecision was 2.3–6.0% CV, depending on the QC pool and analyte. Overall imprecision was 3.5–7.1% CV. Within-run repeatability based on replicate injections of the same sample was 1.8–4.8% CV. Of particular interest were the imprecision characteristics of our method at very low concentrations. The overall imprecision observed in the low QC pool was <5% CV, with 3.8% CV seen for 3 ng/mL O-desmethylangolensin and 4.3% CV for 6 ng/mL equol. The ability to quantify, especially at low concentrations, with a low degree of measurement imprecision is key to increasing the statistical power of population biomonitoring studies. This is especially true for the measurement of O-desmethylangolensin and equol, which are gut bacteria biotransformation products of daidzein and are often present at very low concentrations [24]. With an average overall imprecision of <5% CV across all measured analytes and concentration levels, the performance of our new method exceeded that of our previously reported SPE-ESI-based method [20] as well as other published LC-MS/MS methods [16–19, 22, 23], including those that use stable isotope-labeled analogues as internal standards for all analytes [20, 21].
Method accuracy was first evaluated by performance of spike recovery experiments. In the absence of certified reference materials, method accuracy is often evaluated by use of QC pools prepared by spiking blank biologic matrices with known amounts of analyte and calculating the agreement between the nominal and measured concentrations [16–19, 22, 23]. Because our QC pools were assigned concentration values through a characterization process rather than by spiking with standards, their use in this context would not necessarily be a true indicator of method accuracy since their concentrations were not directly ascribable to a starting material of known chemical purity. Spike recovery, however, did accomplish this since the added concentrations were traceable to compounds of known chemical purity and made use of the “low” and “medium” QC pools that had analytes present in both aglycone and conjugated forms (the “high” QC pool was not used due to the likelihood that spiking would result in measurements exceeding the calibration range of the method). The spike recovery, calculated as the average across all pools, spiking levels, and replicates performed, was generally within ±10% of being quantitative (100%), with the exception of genistein (112%, Table 2). No bias or trending related to the QC pool or spiking amount was observed in the recoveries; however, the CVs of the average recoveries in the “low” QC pool when spiked with the lowest concentration (8.7–24%) were notably higher than the CVs observed in all other QC pools and spiking level combinations (1.8–11.4%). These recoveries fall within the 15% accuracy specification generally accepted when QC pools are used for determining method accuracy, and they are consistent with or surpassed those of existing methods [16–23].
As a means of further evaluating method accuracy, we performed a series of comparative analyses entailing the analysis of several hundred samples over multiple days. These experiments were designed to assess the agreement between results from several vantage points. Within-run results were compared by simultaneously measuring concentrations using both “quantitation” and “confirmation” MS/MS transitions (224 samples, 3 days), within-method results were compared by performing measurements on two different instruments (225 samples, 3 days), and between-method results were compared by performing measurements using both our new method and our existing SPE-ESI-based method [20] (328 samples, 5 days). In each case, the correlation, regression, and relative differences among results were examined. For correlation analyses, the Pearson r statistic was calculated using log-transformed data. The distributions of urinary isoflavone and lignan concentrations in the urine samples used for comparison spanned several orders of magnitude and were right-skewed. Log transformation was performed so that the right-skewed distributions of these data were transformed to yield data that were more normally distributed and better suited for the Pearson test. Regression and difference calculations, however, were done on the original data without transformation. This methodology allowed the regression slopes and intercepts to be directly representative of proportional and constant biases, respectively, and it allowed differences to be presented on a scale that could be easily interpreted. An error-in-variables (Deming) regression was used because it assumes uncertainty in both variables and is most appropriate for method comparisons [38]. Bland−Altman difference testing was done by use of a relative (in percent) scale since the distribution of differences on a relative scale better approximated a normal distribution than did the use of absolute differences [39]. Table 3 summarizes the correlation, regression, and difference testing findings for the comparative analyses. Pearson correlation tests showed a high degree of correlation among results in the three comparisons; Pearson r values were ≥0.994 and correlations were highly significant (two-tailed p<0.0001) in all three experiments. Proportional biases in the between-instrument and between-method comparisons were extremely low (within ±4% using Deming slope or Bland−Altman), as were constant biases (≤0.17 ng/mL using Deming intercept). In many cases, these differences were not statistically significant (95% CI). The biases observed within-run when different MS/MS transitions were used were marginally higher, but consistent with biases observed between instruments and methods (Table 3); the biases we found may be attributable to differences in the measurement sensitivity of the two MS/MS transitions used for each analyte. Performing a comparison between a new method and an established or reference method is a valuable practice, particularly in the absence of certified reference materials, and these comparison studies clearly demonstrate that our method is capable of measurements that are internally consistent as well as consistent across different instruments and with established methods.
Table 3.
Comparison studies
| Comparison type | Analyte | Correlation Pearson) r statistic (95% CI) | Regression weighted Deming) |
Relative (%) bias (Bland-Altman, 95% CI) | |
|---|---|---|---|---|---|
| Intercept (ng/mL, 95% CI) | Slope (95% CI) | ||||
|
| |||||
| MS/MS transitions: | Daidzein | 0.999 (0.999–0.999) | 0.2 (−0.6 to 1.1) | 0.98 (0.97–0.99) | 0.47 (0.27–0.66) |
| x: Quantitation | O-desmethylangolensin | 0.999 (0.998–0.999) | 3.3 (1.9–4.6) | 1.04 (1.03–1.05) | −0.02 (−0.06 to 0.02) |
| y: Confirmation | Equol | 0.998 0.998–0.999) | −2.5 (−3.3 to −1.7) | 0.98 (0.97–0.99) | −0.01 (−0.05 to 0.02) |
| n=225 | Enterodiol | 0.994 (0.992–0.996) | 1 (−0.5 to 2.4) | 1.02 (1–1.04) | −0.16 (−0.57 to 0.24) |
| Enterolactone | 0.999 0.998–0.999) | −2.3 (−3.3 to −1.2) | 0.98 (0.97–0.99) | 0.12 (−0.72 to 0.96) | |
| Genistein | 0.997 (0.997–0.998) | −6.2 (−7.6 to −4.7) | 0.95 (0.93–0.97) | −0.11 −0.29 to 0.08) | |
| Instruments: | Daidzein | 0.999 0.998–0.999) | 0.11 (−0.02 to 0.23) | 1.01 (1.00–1.02) | 1.5 0.4–2.6) |
| x: API 4000 | O-desmethylangolensin | 0.998 0.998–0.999) | 0.04 (0.01–0.08) | 0.98 (0.96–1.00) | −0.1 −1.7 to 1.4) |
| y: API 5000 | Equol | 0.997 0.996–0.998) | 0.01 (−0.07 to 0.09) | 1.01 (0.99–1.03) | 1.2 (0.2–2.2) |
| n=224 | Enterodiol | 0.998 0.997–0.998) | 0.15 (0.05–0.25) | 1.02 (1.01–1.03) | 2.5 1.5–3.5) |
| Enterolactone | 0.999 0.999–0.999) | 0.17 (0.02–0.32) | 1.00 (1.00–1.01) | 0.7 −0.2 to 1.6) | |
| Genistein | 0.996 0.994–0.997) | 0.16 (−0.10 to 0.42) | 1.03 (1.01–1.05) | 4.0 2.1–5.8) | |
| Methods: | Daidzein | 0.998 (0.998–0.998) | −0.03 (−0.14 to 0.09) | 1.01 (1.00–1.02) | 1.0 −0.1 to 2.0) |
| x: ESI | O-desmethylangolensin | 0.997 0.996–0.998) | 0.04 (0.01–0.07) | 0.96 (0.94–0.97) | −2.9 −4.3 to −1.6) |
| y: APPI | Equol | 0.997 0.997–0.998) | −0.04 (−0.09 to 0.01) | 0.99 (0.97–1.00) | −2.2 −3.1 to −1.4) |
| n=328 | Enterodiol | 0.998 0.997–0.998) | 0.01 (−0.06 to 0.08) | 0.96 (0.95–0.97) | −3.7 −4.6 to −2.7) |
| Enterolactone | 0.999 0.999–0.999) | −0.02 (−1.74 to 1.70) | 0.97 (0.95–0.99) | −3.2 −3.9 to −2.4) | |
| Genistein | 0.997 0.996–0.997) | −0.12 (−0.32 to 0.07) | 0.96 (0.95–0.98) | −4.9 −6.2 to −3.7) | |
Method selectivity was evaluated by spiking synthetic urine with single-compound standards of each analyte and internal standard and looking for unexpected signal contributions in the MS/MS transitions used for quantitation, confirmation, and internal standardization. We found that all six analytes gave rise to miniscule signal contributions in their respective internal standard transitions, amounting to approximately 0.06–0.2% of the analyte concentration. Since all internal standards used were 13C3 analogues of their corresponding analytes, the signal contribution observed in the internal standard transitions (+3 m/z) was consistent with the predicted relative abundances of the analytes based on natural isotopic abundance (0.16–0.27%). 13C3-enterolactone, 13C3-equol, and 13C3-enterodiol caused relative signal contributions of 0.01%, 0.06%, and 0.4%, respectively, in their corresponding analyte transitions. No analyte signal contributions were detected for 13C3-daidzein, 13C3-O-desmethylangolensin, or 13C3-genistein internal standards. When we looked for cases of signal augmentation among analytes, internal standards, and non-corresponding analyte−internal standard pairings, we found very few occurrences, and those that we saw were negligible; of a possible 120 combinations, we encountered only 8 cases where a signal contribution could be detected, and in no single case was the relative signal contribution >0.1%.
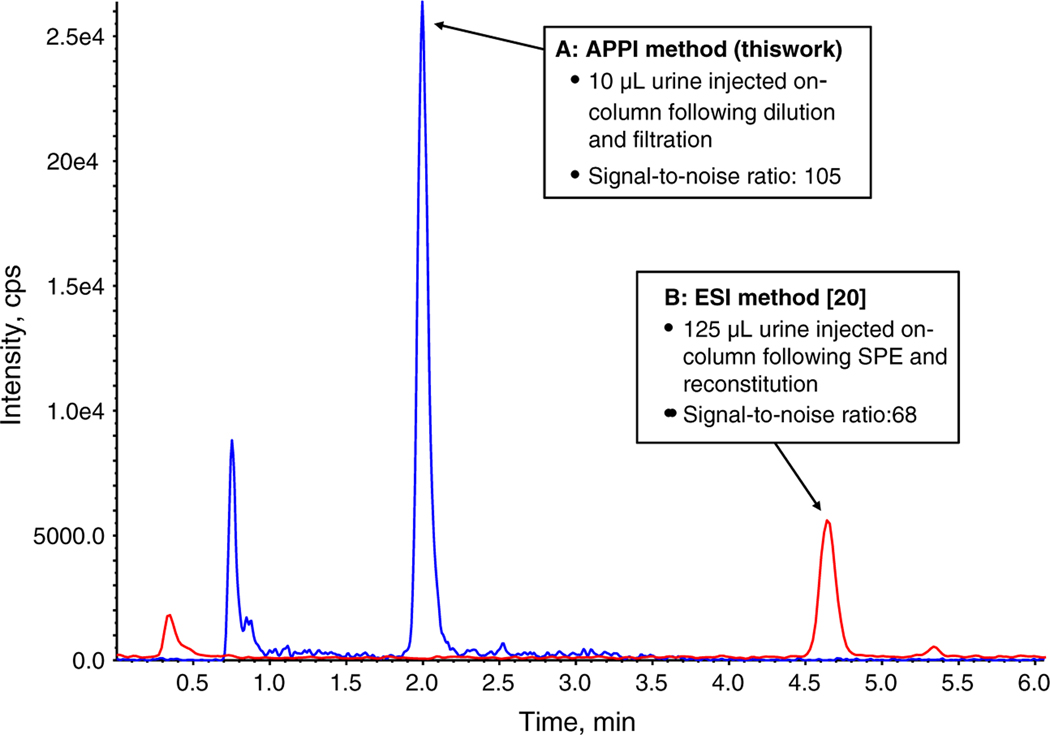
We evaluated method sensitivity by calculating the LODs and LLOQs for each analyte (Table 2). Relative LODs in urine ranged from as low as 0.04 ng/mL for enterodiol to 0.4 ng/mL for daidzein on both LC-MS/MS instruments used in this study. These LODs compare favorably with the most sensitive existing LC-MS/MS methods for measuring these compounds in urine [16, 19–21, 23]. The measurement sensitivity of our method also proved to be satisfactory for its intended purpose of biomonitoring for phytoestrogenic isoflavone and lignan exposure. We applied this method to the analysis of >2,500 specimens from the 2005–2006 NHANES, a series of continuous, nationally representative surveys designed to assess the overall health of the US population, and were able to quantify all analytes in >99% of the NHANES specimens analyzed, with the exception of O-desmethylangolensin which could be measured in >95% of the specimens. Although the relative sensitivity of our method is commensurate with the performance of other SPE- and LLE-based methods, the absolute detection power of our method is considerably greater. Absolute LODs were 0.4–4 pg when the API 4000 LC-MS/MS system was used, and the absolute LODs improved to 0.1–1 pg with the more sensitive API 5000 LC-MS/MS system. As described earlier, the use of APPI in place of ESI resulted in a substantial improvement in measurement sensitivity that permitted the development of a sample preparation protocol free of any extraction and reconstitution procedures. Consequentially, whereas SPE- and LLE-based methodologies often result in a net enrichment of analyte concentration in the sample [16–18, 20–23], our procedure results in an approximate twofold dilution. This notable difference in sample preparation accounts for the fact that, although the absolute LODs for our method far exceed those of other SPE- and LLE-based methods, the relative LODs among methods are quite similar. This is further illustrated in Fig. 3 which shows a chromatographic comparison for equol in “low” QC pool samples prepared and analyzed using both the present APPI procedure and our previous SPE-based ESI method [20]. The same instrument (API 4000) was used in both cases. In this example, the peak height signal-to-noise ratio for equol was approximately 1.5 times greater in the result obtained with the APPI method than with the ESI method; however, this modest improvement in relative measurement sensitivity was nearly a 20 times improvement in absolute measurement sensitivity when differences in sample preparation were accounted for. The possibility of further improving relative LODs for our method, either by reducing the degree of dilution in the sample preparation or by increasing the injection volume, was explored, but ultimately abandoned because it would not likely result in a substantial improvement in our ability to quantify analyte levels in low-concentration biomonitoring samples and would increase the possibility of instrument contamination and/or signal saturation at the high end of the calibration range.
Fig. 3.
Comparison of APPI and ESI methods. Quantitation MS/MS transition for equol (241/119) for the “low” QC urine pool is shown. a Sample prepared and analyzed using APPI-LC-MS/MS (this work). b Sample prepared and analyzed using ESI-LC-MS/MS [20]. The same instrument was used (API 4000) in both cases
We evaluated ion suppression by examining the effect of various sample injections on post-column analyte infusion. We observed varying degrees of signal suppression for all analytes upon void volume elution; however, in all cases, full signal restoration took place prior to analyte elution and no notable ion suppression was observed in any of the sample types injected (synthetic urine blanks, calibrators, QC pools). In the course of analyzing >2,500 NHANES specimens, we did encounter rare occurrences where analyte-specific signal suppression was evident. This was primarily characterized by an internal standard signal that was greatly attenuated relative to the internal standard signals observed for the same analyte in other samples analyzed in the same instrument run. Furthermore, the internal standard response for other analytes in the affected sample typically showed no evidence of signal reduction, indicating an analyte-specific phenomenon. In addition to signal suppression, we sometimes observed an elevated baseline in both analyte and internal standard transitions, shortened retention times, and peak broadening in these cases. Although the exact cause of the signal suppression was not identified, we found that increasing the amount or concentration of the buffer had no effect on these samples, eliminating the likelihood that analyte deprotonation was the cause. We found that this issue was rectified by simply diluting the specimen prior to sample preparation by a factor of 2–8× or by reducing the injection volume by a similar factor. This phenomenon was encountered most frequently for equol in approximately 1% of NHANES specimens analyzed.
Method robustness refers to the demonstrated ability of an analytical method to withstand intentional variations in method parameters. We identified five methodological factors to test on the basis of their perceived critical nature in terms of method performance and likelihood of unintentional variation during routine operation. We performed testing on the three QC pools and identified two test conditions for each method parameter. Variables in the hydrolysis step, namely, the concentration of the β-glucuronidase/sulfatase enzyme and time permitted for deconjugation to take place, were two such examples of method parameters that were evaluated for robustness because of their direct influence on the aglycone concentration observed for each analyte. We observed subtle but important changes in method performance when these two parameters were varied. When the enzyme concentration was reduced to half the method specification (60 vs. 120 U), the concentrations measured for genistein and enterodiol were 5–7% lower than expected, with differences being more prominent at higher concentrations (i.e., in the high QC pool). Other analytes appeared to be unaffected by reducing the enzyme concentration. Analyte concentrations were 2–7% lower when the time for hydrolysis was reduced to 4 h from the method specification of 12 h. We observed no perceptible changes in analyte concentration when either hydrolysis time or enzyme concentration was increased to twice the method specification (24 h and 240 U, respectively). The ammonium acetate buffer used to modulate sample pH was also identified as a critical parameter in terms of method robustness, particularly since no buffers were used in the mobile phase. When the in-sample concentration of the buffer was reduced from the method specification of 2.5 to 1.25 M, we observed no effect on the analyte concentrations for the QC pools; however, we did observe cases with other patient samples in which analyte deprotonation and early elution would occur as described earlier, and these effects were exacerbated when the buffer concentration was further reduced to 0.25 M. Varying the buffer pH over a range of 4.5–5.5 appeared to have no effect on analyte concentration, nor did extended storage of prepared samples at 4 °C for up to 7 days.
Conclusion
We have developed and validated an LC-MS/MS method for quantifying phytoestrogenic isoflavones and lignans in urine which makes use of APPI. By doing so, we eliminated the need for the extraction and preconcentration steps inherent in other LC-MS/MS methods for these compounds. The merit of our approach is its simplicity; by eliminating the need for SPE or LLE, the time, instrument and consumable costs, safety concerns, and analytical performance issues associated with these additional procedures are no longer relevant. We have also demonstrated that this simplified approach can be used without compromise, as substantiated by the analytical performance of our method in terms of accuracy, precision, sensitivity, throughput, and robustness. We have shown that our method was fit for its intended application in population biomonitoring studies. It had performance characteristics similar to those of methods developed for pharmacokinetic studies and compliance testing. In fact, we believe that it would also be suitable for these applications. With this, the first ever method of its kind for measuring phytoestrogenic isoflavones and lignans in biologic matrices, there exist aspects of its performance that are worthy of further study. The use of this method in determining plasma or serum concentrations has not been explored up to this point, and it remains to be seen whether these samples would be amenable to our procedure, either directly or with some modification (e.g., protein denaturation). Although we were successful in using unbuffered mobile phases in our method, we acknowledge that this is not an ideal case and that the identification of mobile phase buffers that may be used without compromising APPI performance is deserving of further exploration. We observed a notable sensitivity enhancement that was directly attributable to APPI, and we demonstrated that this improvement was transferable among two MS/MS platforms from the same manufacturer; however, there are differences in the design and operation of APPI sources for LC-MS/MS systems produced by other manufacturers, and the actual performance in these cases should be tested.
Acknowledgment
We gratefully acknowledge Donna J. LaVoie, M. T. (A.S.C.P) and David T. Nguyen, B.S., who conducted many of the experiments and analyses associated with this project. We would also like to pay special tribute to Nigel P. Botting, PhD, who sadly passed away this year. We owe a good deal of our success in developing and implementing quality methods for assessing phytoestrogen exposure to Nigel and the efforts of his research group at the University of St. Andrews to provide consistently high-quality analyte and internal standards. He will be dearly missed.
References
- 1.Nielsen ILF, Williamson G (2007) Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer 57:1–10 [DOI] [PubMed] [Google Scholar]
- 2.Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC(2005) Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr 93:393–402 [DOI] [PubMed] [Google Scholar]
- 3.Clavel T, Doré J, Blaut M (2006) Bioavailability of lignans inhuman subjects. Nutr Res Rev 19:187–196 [DOI] [PubMed] [Google Scholar]
- 4.Dong JY, Qin LQ (2010) Soy isoflavones consumption and risk ofbreast cancer incidence or recurrency: a meta-analysis of prospective studies. Breast Cancer Res Tr 125:315–323 [DOI] [PubMed] [Google Scholar]
- 5.Buck K, Zaineddin AK, Vrieling A, Linselsen J, Chang-Claude J(2010) Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr 92:141–153 [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Spitznagel EL (2009) Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 89:1155–1163 [DOI] [PubMed] [Google Scholar]
- 7.Hamilton-Reeves JM, Vazquez G, Duval SJ, Phipps WR, Kurzer MS, Messina MJ(2010) Clinical studies show no effects of soy protein or isoflavones on reproductive hormones in men: results of a meta-analysis. Fertil Steril 94:997–1007 [DOI] [PubMed] [Google Scholar]
- 8.Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A(2009) Efficacy of isoflavones in relieving vasomotor menopausal symptoms—a systematic review. Mol Nutr Food Res 53:1084–1097 [DOI] [PubMed] [Google Scholar]
- 9.Howes LG, Howes JB, Knight DC (2006) Isoflavone therapy formeno pausal flushes: a systematic review and meta-analysis. Maturitas 55:203–211 [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Ho SC, Su XY, Chen WQ, Zhang CX, Chen YM (2010) Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone 44:948–953 [DOI] [PubMed] [Google Scholar]
- 11.Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML (2010) Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev 68:571–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X (2009) Meta analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr 90:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grynkiewicz G, Ksyciańska H, Ramza J, Zagrodzka J (2005) Chromatographic quantification of isoflavones (why and how). Acta Chromatogr 15:31–65 [Google Scholar]
- 14.Prasain JK, Wang CC, Barnes S (2004) Mass spectrometric methods for the determination of flavonoids in biological samples. Free Radic Biol Med 37:1324–1350 [DOI] [PubMed] [Google Scholar]
- 15.Hoikkala AA, Schiavoni E, Wähälä K (2003) Analysis of phyto-oestrogens in biological matrices. Br J Nutr 89:S5–S18 [DOI] [PubMed] [Google Scholar]
- 16.Wyns C, Bolca S, De Keukeleire D, Heyerick A (2010) Development of a high-throughput LC/APCI-MS method for the determination of thirteen phytoestrogens including gut microbial metabolites in human urine and serum. J Chromatogr B 878:949–956 [DOI] [PubMed] [Google Scholar]
- 17.Locati D, Morandi S, Cupisti A, Ghiadoni L, Arnold A (2005) Characterization and quantification of soy isoflavone metabolites in serum of renal transplanted patients by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Comm Mass Spectrom 22:229–234 [DOI] [PubMed] [Google Scholar]
- 18.Prasain JK, Arabshahi A, Moore DR II, Greendale GA, Wyss JM, Barnes S (2010) Simultaneous determination of 11 phytoestrogens in human serum using a 2-min liquid chromatography/tandem mass spectrometry method. J Chromatogr B 878:994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Wu Q, Qaio S, Yu Z, Jin N, Yu B (2009) Simultaneous determination of phytoestrogens and key metabolites in breast cancer patients’ urine by liquid chromatography–tandem mass spectrometry. J Pharmaceut Biomed 50:939–946 [DOI] [PubMed] [Google Scholar]
- 20.Rybak ME, Parker DL, Pfeiffer CM (2008) Determination of urinary phytoestrogens by HPLC–MS/MS: a comparison of atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI). J Chromatogr B 861:145–150 [DOI] [PubMed] [Google Scholar]
- 21.Grace PB, Mistry NS, Carter MH, Leathem AJC, Teale P (2007) High throughput quantification of phytoestrogens in human urine and serum using liquid chromatography/tandem mass spectrometry (LC-MS/MS). J Chromatogr B 853:138–146 [DOI] [PubMed] [Google Scholar]
- 22.Kuijsten A, Buijsman MNCP, Arts ICW, Mulder PPJ, Hollman PCH (2005) A validated method for the quantification of enterodiol and enterolactone in plasma using isotope dilution liquid chromatography with tandem mass spectrometry. J Chromatogr B 822:178–184 [DOI] [PubMed] [Google Scholar]
- 23.Kuklenyik Z, Ye XY, Reich JA, Needham LL, Calafat AM (2004) Automated online and off-line solid-phase extraction methods for measuring isoflavones and lignans in urine. J Chromatogr Sci 42:495–500 [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Jiménez J, Hubert J, Hooper L, Cassidy A, Manach C, Williamson G, Scalbert A (2010) Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr 92:801–809 [DOI] [PubMed] [Google Scholar]
- 25.National Health and Nutrition Examination Survey (2011) U.S.Centers for Disease Control and Prevention, Atlanta. http://www.cdc.gov/nchs/nhanes.htm. Accessed 19 July 2011
- 26.Valentín-Blasini L, Blount BC, Rogers HS, Needham LL (2000) HPLC-MS/MS method for the measurement of seven phytoestrogens in human serum and urine. J Exposure Anal Environ Epidemiol 10:799–807 [DOI] [PubMed] [Google Scholar]
- 27.Bos SJ, van Leeuwen SM, Karst U (2006) From fundamentals to applications: recent developments in atmospheric pressure photoionization mass spectrometry. Anal Bioanal Chem 384:85–99 [DOI] [PubMed] [Google Scholar]
- 28.Syage JA, Short LC, Cai S-S (2008) Atmospheric pressure photoionization—the second source for LC-MS? LC GC N Am 26:286+ [Google Scholar]
- 29.Gustafsson JE, Uzaueda HR (1978) The influence of citrate and phosphate on the Mancini single radial immunodiffusion technique and suggested improvements for the determination of urinary albumin. Clin Chim Acta 90:249–257 [DOI] [PubMed] [Google Scholar]
- 30.Caudill SP, Schleicher RL, Pirkle JL (2008) Multi-rule quality control for the age-related eye disease study. Stat Med 27:4094–4106 [DOI] [PubMed] [Google Scholar]
- 31.Taylor JK (1987) Quality assurance of chemical measurement. Lewis, Boca Raton [Google Scholar]
- 32.Raffaelli A, Saba A (2003) Atmospheric pressure photoionization mass spectrometry. Mass Spectrom Rev 22:318–331 [DOI] [PubMed] [Google Scholar]
- 33.Kauppila TJ, Kuuranne T, Meurer EC, Eberlin MN, Kotiaho T, Kostianien R (2002) Atmospheric pressure photoionization mass spectrometry. Ionization mechanism and the effect of solvent on the ionization of naphthalenes. Anal Chem 74:5470–5479 [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka N, Akiyama Y, Teranishi K (2004) Rapid simultaneous determination of o-phenylphenol, diphenyl, thiabendazole, imazalil and its major metabolite in citrus fruits by liquid chromatography– mass spectrometry using atmospheric pressure photoionization. J Chromatogr A 1022:145–150 [DOI] [PubMed] [Google Scholar]
- 35.Taylor JI, Grace PB, Bingham SA (2005) Optimization of conditions for the enzymatic hydrolysis of phytoestrogen conjugates in urine and plasma. Anal Biochem 341:220–229 [DOI] [PubMed] [Google Scholar]
- 36.Hosoda K, Furuta T, Ishii K (2010) Simultaneous determination of glucuronic acid and sulfuric acid conjugated metabolites of daidzein and genistein in human plasma by high-performance liquid chromatography. J Chromatogr B 878:628–636 [DOI] [PubMed] [Google Scholar]
- 37.Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PF, Wiseman H (2002) Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography–tandem mass spectrometry with [13C3] isoflavone internal standards. Anal Biochem 309:158–172 [DOI] [PubMed] [Google Scholar]
- 38.Martin RF (2000) General Deming regression for estimating systematic bias and its confidence interval in method-comparison studies. Clin Chem 46:100–104 [PubMed] [Google Scholar]
- 39.Twomey PJ (2006) How to use difference plots in quantitative method comparison studies. Ann Clin Biochem 43:124–129 [DOI] [PubMed] [Google Scholar]