Abstract
Background & Aims
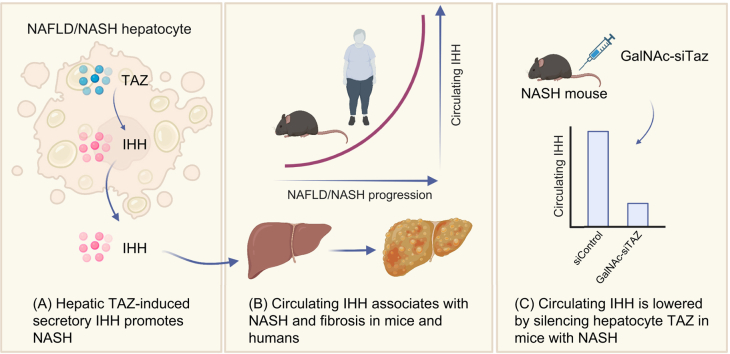
Non-alcoholic steatohepatitis (NASH)-induced liver fibrosis is emerging as the most common cause of liver disease. For evaluation of therapies, there is a pressing need to identify non-invasive, mechanism-based biomarkers. A pro-fibrotic process relevant to human NASH involves a pathway in which a transcriptional regulator called TAZ (WWTR1) in hepatocytes induces the secretion of pro-fibrotic Indian hedgehog (IHH). We therefore reasoned that circulating IHH may be a useful mechanism-based marker to assess changes in NASH fibrosis.
Methods
Circulating IHH was assessed in wild-type and hepatocyte-TAZ-silenced NASH mice and in three separate cohorts of patients with mild–moderate NASH.
Results
Circulating IHH was elevated in mice with diet-induced NASH compared with chow-fed mice or with NASH mice in which hepatocyte TAZ was silenced, which is an effective means to decrease NASH fibrosis. In patients with fatty liver disease with or without NASH, NASH fibrosis was associated with increased concentrations of circulating IHH.
Conclusions
The results of these analyses support further investigation to determine whether circulating IHH may be useful as a mechanism-based indicator of target engagement in anticipated future clinical trials testing NASH fibrosis therapies that block the IHH pathway.
Impact and implications
Non-alcoholic steatohepatitis (NASH)-induced liver fibrosis is a common cause of liver disease. Circulating biomarkers that reflect liver fibrosis in NASH would be very useful to evaluate therapies. One mechanism of NASH fibrosis with potential as a therapeutic target involves a liver-secreted protein called Indian hedgehog (IHH). We report that circulating levels of IHH in experimental and human NASH associates with NASH and NASH-associated liver fibrosis, providing the premise for further investigation into using circulating IHH to evaluate anticipated future NASH therapies that block the IHH pathway in liver.
Keywords: NASH, NAFLD, Fibrosis, TAZ, IHH, Biomarker
Graphical abstract
Highlights
-
•
Plasma IHH was assayed in NASH, as hepatic TAZ-induced secretory IHH promotes NASH.
-
•
Plasma IHH is increased in experimental NASH and lowered by silencing hepatocyte TAZ.
-
•
Circulating IHH is elevated in humans with NASH and associated with fibrosis.
Introduction
Non-alcoholic steatohepatitis (NASH) is emerging as the leading cause of cirrhosis worldwide.[1], [2], [3] Disease progression from simple steatosis to NASH is caused by multiple insults that cause liver inflammation, hepatocellular death, and, most importantly, histological liver fibrosis, which correlates best with clinical outcome in NASH.4 As there are no FDA-approved drugs to treat NASH, there is a critical need for novel therapies that can halt or reverse progression to liver fibrosis.[5], [6], [7], [8] Accordingly, these efforts will require non-invasive approaches to identify patients at increased risk of NASH, assess disease progression, and monitor response to therapy.
A key process in NASH fibrosis is activation of collagen-producing hepatic stellate cells (HSCs).[8], [9], [10] One mechanism involves cholesterol-induced upregulation in hepatocytes of the transcriptional regulator TAZ (WWTR1), which induces the synthesis and secretion of the HSC activator, Indian hedgehog (IHH).11,12 This pathway is supported by showing increased TAZ and IHH in human NASH vs. steatotic liver and by causation studies using primary human and mouse hepatocytes and mouse NASH models.11,12 For example, silencing hepatocyte TAZ or IHH blocks NASH progression, and the improvement in NASH by silencing hepatocyte TAZ is abrogated by genetically restoring hepatocyte IHH.11 From a therapeutic standpoint, silencing hepatocyte TAZ using GalNAc-siTaz, which is based on a platform currently in use in humans,[13], [14], [15], [16], [17], [18] blocks the progression of liver fibrosis in experimental NASH.19
Given the importance of secretory IHH, we reasoned that circulating IHH may be a useful mechanism-based marker related to histological features of NASH and fibrosis. We show here that circulating IHH increases in NASH in mice in a hepatocyte-TAZ-dependent manner and associates with non-alcoholic fatty liver disease (NAFLD) activity score and liver fibrosis in humans with mild–moderate NASH. These findings support further investigation into whether plasma IHH may be useful as an indicator of target engagement in anticipated trials testing NASH fibrosis therapies that block the IHH pathway.
Materials and methods
Animal studies
Male wild-type C57BL/6J mice (10 weeks old) were obtained from Jackson Laboratory (#000664; Bar Harbor, ME, USA) and assigned randomly to receive chow diet (PicoLab Rodent Diet 20, #5053; Lab Diet, St Louis, MO) or a NASH-inducing diet rich in fructose, palmitate, and 1.25% cholesterol (FPC; TD.160785, Envigo Teklad Diets; Madison, WI; with drinking water containing 23.1 g/L fructose and 18.9 g/L glucose).11 In view of the poor absorption of dietary cholesterol in these mice,20 1.25% cholesterol is required to mimic the cholesterol content of human NASH liver.11 This is important, as increased liver cholesterol contributes to NASH progression,[21], [22], [23] including promoting the increase in the pro-NASH TAZ-IHH pathway.12 The feeding period was 16 or 28 weeks, with tail vein injection of AAV8-H1-shTaz virus or control AAV8-H1-shControl virus (Vector Biolabs, Malvern, PA; 2 × 1011 genome copies/mouse) at 8 weeks for the 16-week protocol. Plasma was collected and livers were harvested and snap-frozen or formalin fixed as previously described.11,12,24 Experiments complied with guidelines of the Columbia Animal Care and Use Committee.
Human samples
Three separate cohorts were included. Patients gave informed consent, and protocols were approved by the institutional review board of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milan (cohorts 1 and 2) or the University of Missouri (cohort 3) and conducted according to the World’s Medical Association Declaration. Patient records were pseudo-anonymised and de-identified. See Supplementary information for description of tissue and blood collection and analyses.
Results
Plasma IHH is increased in experimental NASH and lowered by silencing hepatocyte TAZ
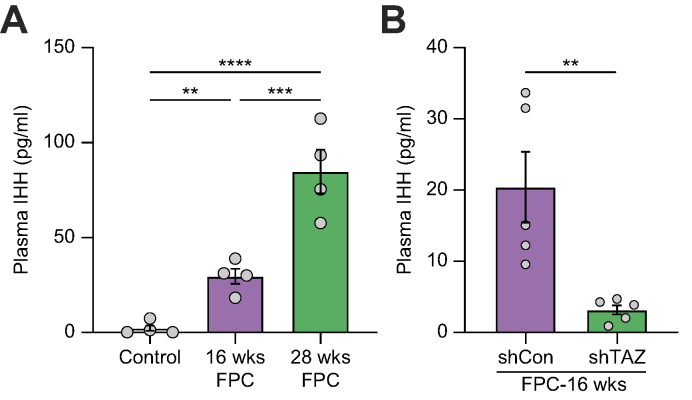
We assayed plasma IHH in mice fed the NASH-inducing FPC diet for 16 or 28 weeks and in mice in which hepatocyte TAZ was silenced between Weeks 8 and 16. Metabolic, biochemical, and histologic data for the mice have been previously published.11 Plasma IHH levels were very low in chow-fed mice but became markedly increased in mice fed the FPC diet for 16 and 28 weeks (Fig. 1A). Further, in 16-week-fed mice, silencing hepatocyte TAZ between Weeks 8 and 16, which lowers liver fibrosis and IHH,11 markedly reduced plasma IHH (Fig. 1B). Thus, in experimental NASH, plasma IHH is elevated, and it is lowered by silencing hepatocyte TAZ.
Fig. 1.
Plasma IHH is increased in experimental NASH and lowered by silencing hepatocyte TAZ.
(A) Plasma IHH of mice fed chow or FPC diet for 16 and 28 weeks (n = 4 mice/group; ∗∗p <0.01, ∗∗∗p <0.001, ∗∗∗∗p <0.0001). Data were analysed using one-way ANOVA. (B) Plasma IHH of mice fed the FPC diet for 16 weeks, with AAV8-H1-shTaz or control (shCon) vector injected at Week 8 (n = 5 mice/group, ∗∗p <0.01). Data were analysed using a two-tailed Student's t test. FPC, fructose, palmitate, and cholesterol; IHH, Indian hedgehog; NASH, non-alcoholic steatohepatitis.
Plasma IHH is increased in humans with NASH fibrosis
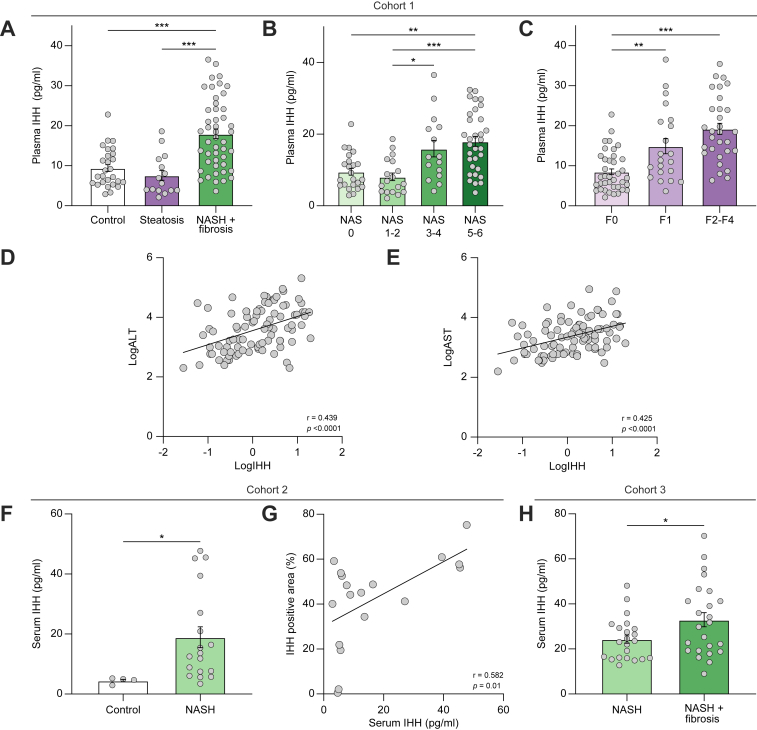
In cohort 1, which included 96 patients who underwent liver biopsy for suspected NASH, plasma IHH was approximately twofold higher in participants with histologically confirmed NASH and mild–moderate fibrosis vs. simple steatosis or without NAFLD (Fig. 2A and Table S1). Further, plasma IHH was higher in participants with higher NAFLD activity score (Fig. 2B) and in participants with NASH with liver fibrosis vs. participants with NASH without liver fibrosis (Fig. 2C). Plasma IHH also correlated positively with serum alanine aminotransferase and aspartate aminotransferase (Fig. 2D and E). The finding of increased circulating IHH in participants with NASH vs. control participants was reproduced in cohort 2, which included patients (n = 22) who were overweight or obese and referred for liver biopsy to diagnosis NASH (Fig. 2F and Table S2). In addition, there was a positive correlation between IHH-positive area in immunostained liver sections and serum IHH (Fig. 2G). In cohort 3, which consisted of patients (n = 48) with morbid obesity undergoing bariatric surgery as described,25 serum IHH was higher in participants with NASH with histologically confirmed mild–moderate liver fibrosis vs. participants with NASH without fibrosis (Fig. 2H and Table S3). Although the wedge liver biopsies from these bariatric surgery patients may contain loci of capsular and subcapsular fibrosis, these findings support our findings in cohorts 1 and 2 that plasma and serum IHH is elevated in the setting of NASH fibrosis.
Fig. 2.
Plasma IHH is increased in patients with NASH and NASH-associated liver fibrosis.
(A) Plasma IHH concentrations in control participants (n = 29), participants with steatosis (n = 17), and participants with NASH + fibrosis (n = 50) from cohort 1. (B and C) Data from (A) were stratified based on NAS and fibrosis score. (D and E) Pearson R correlation of the relationship between plasma IHH (LogIHH) and serum ALT (LogALT) or AST (LogAST) in cohort 1. (F and G) Serum IHH in participants without NAFLD (control; n = 4) or with NASH (n = 18) and Pearson R correlation of the relationship between serum and liver IHH in participants from cohort 2. (H) Serum IHH in obese participants with NASH without or with fibrosis (n = 22 and 25, respectively) from cohort 3. Grouped data were analysed using one-way ANOVA. For (A)–(C), (F), and (H), the data are expressed as mean ± SE relative to the first group in each graph (∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001). ALT, alanine aminotransferase; AST, aspartate aminotransferase; IHH, Indian hedgehog; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis.
Discussion
The premise for this study was based on recently published preclinical work, backed by studies of human NASH liver and human hepatocytes, suggesting that targeting the TAZ-IHH pathway in hepatocytes NASH may be a therapeutic option to block the progression of NASH fibrosis.11,12,19 To summarise, TAZ and IHH are upregulated in human and experimental NASH; TAZ-induced secretory IHH is a direct activator of fibrosis-inducing HSCs; and silencing TAZ or IHH in hepatocytes blocks HSC activation and liver fibrosis in experimental NASH.11,12 Moreover, we have shown that genetically targeting hepatocyte TAZ in NASH mice lowers liver fibrosis by blocking the IHH pathway,11 and we obtained similar results by treating NASH mice with GalNAc-siTaz,19 which uses a platform in use in humans for other diseases.[13], [14], [15], [16], [17], [18] These preclinical studies and analyses of human NASH liver provide the rationale for future clinical trials to evaluate the effectiveness and safety26,27 of this type of therapy in humans. Accordingly, we reasoned that a mechanism-based biomarker of target engagement involving the measurement of circulating IHH, that is, based on the fact that IHH is a secretory protein, might be useful as investigators begin to consider such clinical trials. The combination of the preclinical and clinical data herein, namely, showing that plasma IHH is markedly decreased by silencing hepatocyte TAZ in NASH mice and that circulating IHH in humans associates with NAFLD activity score, mild–moderate fibrosis, and liver IHH in humans with mild–moderate NASH, provides preliminary support for our idea and provides the rationale for further work in this area. However, the ultimate value of plasma IHH as a marker of target engagement will not be known until such trials are initiated.
As to whether plasma IHH might be useful as a more general marker of liver fibrosis in NASH cannot be addressed by this study, as we do not have the data to compare plasma IHH with other plasma or imaging markers of NASH. Moreover, the cohorts were relatively small and did not include enough participants with advanced NASH to compare different fibrosis stages, and we did not have certain clinical data that would allow us to correct for all confounding factors, such as medications at the time of biopsy. One interesting direction for a future prospective study would be to determine if plasma IHH, when added to another scoring scheme based on different mechanisms, may be able to improve the sensitivity and/or specificity for predicting the progression of early to advanced NASH.
Conclusions
Circulating IHH is elevated in mice with diet-induced NASH and lowered by silencing hepatocyte TAZ, which blocks IHH-induced liver fibrosis in NASH. In humans, increased concentrations of circulating IHH associates with mild–moderate NASH fibrosis. Although these results should be confirmed in larger cohorts across the full spectrum of NASH and NASH-associated fibrosis, they provide the premise for further investigation into using circulating IHH as a mechanism-based indicator of target engagement in anticipated future trials testing NASH fibrosis-targeting therapies that lower liver IHH.
Financial support
This work was supported by NIH grants R01DK116620 and R01DK133694 (to IT); an American Liver Foundation Liver Scholar Award (to XW); and a grant from the Ines Mandl Research Foundation (to MPM). PD and MM are supported by the Italian Ministry of Health (Ricerca Corrente 2022 to PD and GR-2019-12370172 to MM). LV is supported by the Italian Ministry of Health (Ministero della Salute), Ricerca Finalizzata RF-2016-02364358 and Rete Cardiologica ‘CV-PREVITAL’; Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Ricerca corrente; Fondazione IRCCS Ca’ Granda ‘Liver BIBLE’ (PR-0391); Gilead_IN-IT-989-5790; and several European Union (EU) grants: Horizon 2020 research and innovation programme, EFPIA Programme Horizon 2020 under grant agreement no. 777377 for the project LITMUS, and programme ‘Photonics’ under grant agreement ‘101016726’. NIH grant R01DK113701 supported RSR, EJP, and JAI and also the collection of human samples included in this study (see cohort 3).
Authors’ contributions
Involved in the study conception and experimental design: MPM, XW, IT. Conducted mouse experiments, liver analyses, and plasma and serum assays: XW, MPM, HS. Were involved in conducting and analysing the human studies and providing important intellectual contributions: MM, AC, LR, EJP, JAI, RSR, LV, PD. Drafted the manuscript: MM, XW, IT. Revised the manuscript and approved the final version: all authors.
Data availability statement
Further information and requests for resources, reagents, and data should be directed to, and will be fulfilled by, the lead contact, IT (iat1@cumc.columbia.edu).
Conflicts of interest
IT and XW have received research funding (unrestricted) from Takeda Pharmaceuticals. LV has been an invited speaker for MSD, Gilead, AlfaSigma, and AbbVie; consults for Gilead, Pfizer, Astra Zeneca, Novo Nordisk, Intercept pharmaceuticals, Diatech Pharmacogenetics, IONIS, Viatris, and Boehringer Ingelheim; and has received research funding (unrestricted) from Gilead.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank the volunteers and investigators involved in the clinical studies at the University of Milan, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (Sara Margarita) and the University of Missouri Health System. The graphical abstract was created with BioRender.com.
Footnotes
Author names in bold designate shared co-first authorship.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2023.100716.
Contributor Information
Xiaobo Wang, Email: xw2279@cumc.columbia.edu.
Paola Dongiovanni, Email: paola.dongiovanni@policlinico.mi.it.
Ira Tabas, Email: iat1@columbia.edu.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Cusi K., Isaacs S., Barb D., Basu R., Caprio S., Garvey W.T., et al. American Association of Clinical Endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD) Endocr Pract. 2022;28:528–562. doi: 10.1016/j.eprac.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M. Non-alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 3.Kabbany M.N., Conjeevaram Selvakumar P.K., Watt K., Lopez R., Akras Z., Zein N., et al. Prevalence of nonalcoholic steatohepatitis-associated cirrhosis in the United States: an analysis of national health and nutrition examination survey data. Am J Gastroenterol. 2017;112:581–587. doi: 10.1038/ajg.2017.5. [DOI] [PubMed] [Google Scholar]
- 4.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654.e649. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombardi R., Onali S., Thorburn D., Davidson B.R., Gurusamy K.S., Tsochatzis E. Pharmacological interventions for non-alcohol related fatty liver disease (NAFLD): an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD011640.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman S.L., Roll F.J., Boyles J., Bissell D. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci USA. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Zheng Z., Caviglia J.M., Corey K.E., Herfel T.M., Cai B., et al. Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Cai B., Yang X., Sonubi O.O., Zheng Z., Ramakrishnan R., et al. Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31:969–986.e967. doi: 10.1016/j.cmet.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald K., White S., Borodovsky A., Bettencourt B.R., Strahs A., Clausen V., et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51. doi: 10.1056/NEJMoa1609243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.J., Kim A., Miyata K., Kataoka K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv Drug Deliv Rev. 2016;104:61–77. doi: 10.1016/j.addr.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Liebow A., Li X., Racie T., Hettinger J., Bettencourt B.R., Najafian N., et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503. doi: 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machin N., Ragni M.V. An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B. J Blood Med. 2018;9:135–140. doi: 10.2147/JBM.S159297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda S., Keiser K., Nair J.K., Charisse K., Manoharan R.M., Kretschmer P., et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 18.Nair J.K., Willoughby J.L., Chan A., Charisse K., Alam M.R., Wang Q., et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Sommerfeld M.R., Jahn-Hofmann K., Cai B., Filliol A., Remotti H.E., et al. A therapeutic silencing RNA targeting Hepatocyte TAZ prevents and reverses fibrosis in nonalcoholic steatohepatitis in mice. Hepatol Commun. 2019;3:1221–1234. doi: 10.1002/hep4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz M., Davis D.L., Vick B.R., Russell D.W. Genetic analysis of intestinal cholesterol absorption in inbred mice. J Lipid Res. 2001;42:1801–1811. [PubMed] [Google Scholar]
- 21.Caballero F., Fernández A., De Lacy A.M., Fernández-Checa J.C., Caballería J., García-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol. 2009;50:789–796. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O., et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 23.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab. 2016;27:84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Zeldin S., Shi H., Zhu C., Saito Y., Corey K.E., et al. TAZ-induced Cybb contributes to liver tumor formation in non-alcoholic steatohepatitis. J Hepatol. 2022;76:910–920. doi: 10.1016/j.jhep.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore M.P., Cunningham R.P., Meers G.M., Johnson S.A., Wheeler A.A., Ganga R.R., et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022;76:1452–1465. doi: 10.1002/hep.32324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui S.T., Wang F., Stappenbeck F., French S.W., Magyar C.E., Parhami F., et al. Oxy210, a novel inhibitor of hedgehog and TGF-β signalling, ameliorates hepatic fibrosis and hypercholesterolemia in mice. Endocrinol Diabetes Metab. 2021;4 doi: 10.1002/edm2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matz-Soja M., Rennert C., Schönefeld K., Aleithe S., Boettger J., Schmidt-Heck W., et al. Hedgehog signaling is a potent regulator of liver lipid metabolism and reveals a GLI-code associated with steatosis. Elife. 2016;5 doi: 10.7554/eLife.13308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further information and requests for resources, reagents, and data should be directed to, and will be fulfilled by, the lead contact, IT (iat1@cumc.columbia.edu).