This cohort study evaluates the association between novel metabolic subtypes in pregnant women and the risk of early childhood obesity in offspring.
Key Points
Question
Can metabolic phenotyping of pregnant women, beyond prepregnancy obesity or gestational diabetes status, improve assessment of adiposity risk in offspring?
Findings
In this cohort study of mother-offspring pairs (1325 women and 727 offspring), an unsupervised clustering of maternal biomarkers measured in blood in midpregnancy yielded 5 subgroups of women whose children had increased fat mass percentage and risk of obesity at approximately 5 years of age. Classification to the dyslipidemic–high free fatty acid and insulin resistant–hyperglycemic subgroups had a greater association with offspring adiposity compared with classifying women on the basis of prepregnancy obesity or gestational diabetes.
Meaning
These findings suggest metabolic phenotyping via conventional metabolic biomarkers measured during pregnancy may improve risk stratification of early childhood obesity.
Abstract
Importance
The in utero metabolic milieu is associated with offspring adiposity. Standard definitions of maternal obesity (according to prepregnancy body mass index [BMI]) and gestational diabetes (GDM) may not be adequate to capture subtle yet important differences in the intrauterine environment that could be involved in programming.
Objectives
To identify maternal metabolic subgroups during pregnancy and to examine associations of subgroup classification with adiposity traits in their children.
Design, Setting, and Participants
This cohort study included mother-offspring pairs in the Healthy Start prebirth cohort (enrollment: 2010-2014) recruited from University of Colorado Hospital obstetrics clinics in Aurora, Colorado. Follow-up of women and children is ongoing. Data were analyzed from March to December 2022.
Exposures
Metabolic subtypes of pregnant women ascertained by applying k-means clustering on 7 biomarkers and 2 biomarker indices measured at approximately 17 gestational weeks: glucose, insulin, Homeostatic Model Assessment for Insulin Resistance, total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, free fatty acids (FFA), HDL-C:triglycerides ratio, and tumor necrosis factor α.
Main Outcomes and Measures
Offspring birthweight z score and neonatal fat mass percentage (FM%). In childhood at approximately 5 years of age, offspring BMI percentile, FM%, BMI in the 95th percentile or higher, and FM% in the 95th percentile or higher.
Results
A total of 1325 pregnant women (mean [SD] age, 27.8 [6.2 years]; 322 [24.3%] Hispanic, 207 non-Hispanic Black [15.6%], and 713 [53.8%] non-Hispanic White), and 727 offspring with anthropometric data measured in childhood (mean [SD] age 4.81 [0.72] years, 48% female) were included. We identified the following 5 maternal metabolic subgroups: reference (438 participants), high HDL-C (355 participants), dyslipidemic–high triglycerides (182 participants), dyslipidemic–high FFA (234 participants), and insulin resistant (IR)–hyperglycemic (116 participants). Compared with the reference subgroup, women in the IR-hyperglycemic and dyslipidemic–high FFA subgroups had offspring with 4.27% (95% CI, 1.94-6.59) and 1.96% (95% CI, 0.45-3.47) greater FM% during childhood, respectively. There was a higher risk of high FM% among offspring of the IR-hyperglycemic (relative risk, 8.7; 95% CI, 2.7-27.8) and dyslipidemic–high FFA (relative risk, 3.4; 95% CI, 1.0-11.3) subgroups; this risk was of greater magnitude compared with prepregnancy obesity alone, GDM alone, or both conditions.
Conclusions and Relevance
In this cohort study, an unsupervised clustering approach revealed distinct metabolic subgroups of pregnant women. These subgroups exhibited differences in risk of offspring adiposity in early childhood. Such approaches have the potential to refine understanding of the in utero metabolic milieu, with utility for capturing variation in sociocultural, anthropometric, and biochemical risk factors for offspring adiposity.
Introduction
Childhood obesity is a global health concern that results in premature morbidity across the life course.1 A large literature on the in utero origins of childhood obesity suggests that maternal obesity and hyperglycemia may have a role in programming offspring risk of adiposity starting in early life.2,3 Observational studies have found independent associations of maternal glucose and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) with offspring adiposity and metabolic traits, even below thresholds of gestational diabetes (GDM).4,5,6,7,8,9,10,11,12,13,14,15 These data suggest other glycemic, as well as nonglycemic metabolic factors, such as lipids and free fatty acids (FFAs),16,17,18,19,20,21 may also play important roles in fetal programming that may operate across the continuum of maternal weight status and glucose metabolism in pregnancy.18,19
Several studies have examined the association of individual or classes of metabolic biomarkers such as glucose, insulin, lipoproteins, and FFAs with offspring adiposity.16,22,23,24,25,26 However, this approach does not account for potential interrelated effects of biomarkers across classes of compounds, which is a limitation that can be overcome by using data reduction or classification techniques.24,27 Studying maternal profiles of metabolic compounds during pregnancy can help to capture the context between biomarkers that both do and do not demonstrate individual statistical associations with offspring adiposity.27
In this analysis, we used an unsupervised classification approach that applies algorithms to discover hidden patterns in data to generate metabolic subgroups using 9 biomarkers relevant to metabolic health that were measured during midpregnancy. Next, we tested associations of maternal subgroup classification with offspring adiposity at 2 time points in early life. We hypothesized that the unsupervised classification approach would yield distinct subgroups that are associated with differences in offspring adiposity traits (eg, weight, fat mass, and obesity) measured in the neonatal period and early childhood.
Methods
All participants provided written informed consent, and the study was approved by the Colorado Multiple Institutional Review Board. The Healthy Start Study is registered as an observational study.58 This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.
Setting and Participants
The Healthy Start Study is an observational, prebirth cohort that enrolled pregnant women from 2010 to 2014. Inclusion criteria were being aged 15 years or older, no history of stillbirths, being less than 24 weeks’ gestation, singleton birth, and no preexisting serious chronic disease. In-person visits were completed during midpregnancy (median [IQR], 17 [5.4] weeks), late pregnancy (median [IQR], 27 [3.9] weeks), at delivery, and in early childhood (median [IQR] age, 4.6 [0.5] years). A detailed participant flow diagram is included in the eFigure in Supplement 1. Briefly, this analysis included data from 1325 women with assays from blood collected at midpregnancy and offspring anthropometry data collected in the neonatal period (1116 participants) or in childhood (727 participants). The proportion of women who had offspring without neonatal or childhood anthropometric data was not different between the maternal metabolic subgroups (eTable 1 in Supplement 1). Women with offspring who did not have neonatal or childhood anthropometric data were on average younger, with less educational attainment, a lower diet quality, and more likely to have smoked in pregnancy.
Exposure Derivation: k-Means Clustering and Clustering Inputs
We used unsupervised k-means clustering to identify metabolic subgroups of women. The inputs for k-means clustering comprised 7 biomarkers measured from fasting blood samples collected at approximately 17 gestational weeks, and 2 biomarker indices that have been implicated in in utero metabolic programming. The biomarkers were glucose; total cholesterol (TC); high-density lipoprotein cholesterol (HDL-C); triglycerides (TGs); and FFAs, which were measured using enzymatic kits and the AU400e Chemistry Analyzer (Olympus America Inc); insulin, measured by radioimmunoassay (Millipore); and tumor necrosis factor α (TNF-α), measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc). The indices were the ratio of TGs:HDL-C calculated as an indicator of an atherogenic lipid profile28,29 and the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).30 We scaled the input variables using min-max scaling to reduce algorithmic selection according to different units of measure and ranges of values.
We then implemented unsupervised k-means clustering on the 9 variables (glucose, insulin, TGs, TC, HDL-C, FFAs, TNF-α, TGs:HDL-C, HOMA-IR) using clusterR.31 The optimal number of clusters was determined by the gap statistic which was computed using the factoExtra32 algorithm with the following specifications: 25 centroid initializations, 2 to 10 cluster considerations in increments of 1, and 10 Monte Carlo simulated reference data sets. From here forward, we use the term metabolic subgroup to refer to the clusters.
Outcome Measures: Offspring Adiposity
Neonatal body composition, including fat mass (FM) and fat free mass (FFM), was measured by PEA POD (Life Measurement, Inc) air displacement plethysmography. Weight at delivery and gestational age were used to calculate birthweight z score and large-for-gestational age status (≥90th percentile) according to national reference data.33 In early childhood, FM and FFM were measured using whole body air displacement plethysmography (BodPod, Life Measurement, Inc) with the pediatric option.34 The neonatal and childhood fat mass percentage (FM%) was calculated as FM / FFM. Early childhood (mean [SD] age, 4.81 [0.72] years) height was measured to the nearest 0.1 cm by stadiometer, weight was measured to the nearest 0.1 kg using an electronic scale, and age-specific BMI percentiles were calculated according to the US Centers for Disease Control and Prevention reference.35
Covariates: Maternal Demographics and Childhood Obesity Risk Factors
The collection and calculation of maternal perinatal characteristics have been described in detail elsewhere.22,36,37 In brief, maternal race and ethnicity, educational attainment, parity, dietary intake, and prenatal smoking status were self-reported via questionnaire. We combined American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, and more than 1 race into a single category of non-Hispanic other. Across all analyses, we consider race and ethnicity as social constructs rather than biological determinants of health.38 Race and ethnicity have been associated with prenatal risk, and therefore we included these constructs in the current analysis to assess differences across maternal metabolic subgroup. We calculated maternal prepregnancy BMI using prepregnancy weight and measured height. Gestational weight gain was estimated by subtracting prepregnancy weight from the last clinically measured weight during pregnancy and categorized according to the Institute of Medicine 2009 Guidelines.39 All women were screened for GDM at 24 to 28 weeks and GDM diagnosis was abstracted from medical records and according to Carpenter-Coustan criteria.
Statistical Analysis
First, we examined the distribution of maternal demographic, lifestyle variables, and prenatal risk characteristics across maternal metabolic subgroups to aid in their interpretation. Within each subgroup, we also examined differences in prevalence of women who met criteria for clinically relevant atherogenic dyslipidemia40,41,42 and elevated glucose,40,43,44 as well as sample-specific cutoffs of the 75th percentile for biomarkers measured at approximately 17 gestational weeks. We assessed statistical significance of differences using a Wald test for continuous variables, and a χ2 test for categorical variables.
Next, we examined the association of subgroup membership with continuous neonatal (birthweight z score and FM%) and childhood adiposity (BMI and FM%) using linear regression. We estimated the association of subgroup membership and relative risk (RR) of early childhood obesity (BMI ≥95th percentile35), and high FM% (FM% ≥95th percentile of the study sample) using linear regression with a Poisson distribution, log link, and repeated subject statement to obtain robust standard errors.45 Across all models, we focus on unadjusted estimates given that the maternal subgroups capture metabolic differences related to lifestyle, weight status, and GDM. Accordingly, including these variables as covariates would likely remove meaningful variation from the maternal metabolic subgroups.46,47 However, as a sensitivity analysis, we sequentially adjusted multivariable models that account for maternal race and ethnicity, education, parity, smoking, age, prepregnancy BMI, and GDM. To assess whether the association of maternal metabolic subgroup with offspring obesity was predominately explained by maternal obesity or GDM, we excluded women with these conditions in an exploratory analysis.
We performed k-means clustering in R version 4.0.2 (R Project for Statistical Computing). All other statistical analyses were performed with SAS version 9.4 (SAS Institute). We considered a P value less than .05 from a 2-sided test to be significant.
Results
Participants
The mean (SD) maternal age of participants was 27.8 (6.2) years; 322 (24.3%) were Hispanic, 207 (15.6%) were non-Hispanic Black, 713 (53.8%) were non-Hispanic White; 578 (43.6%) had a college or graduate degree; 53 (4.4%) had diagnosis of GDM; 259 (19.6%) had obesity before pregnancy; and 604 (45.7%) experienced excessive weight gain in pregnancy (Table 1). Approximately half (48.1%) of offspring were female. Mean (SD) birthweight was 3100 (500) grams, and 1.9% were large-for-gestational age. At the early childhood visit the mean (SD) age of offspring was 4.8 (0.7) years, mean (SD) BMI for age percentile was 48.6 (28.6), and 6.2% had obesity.
Table 1. Characteristics of 1325 Pregnant Women in the Healthy Start Study, Overall and by Metabolic Subgroup Membership.
| Maternal characteristicsa | No. (%) | P value | |||||
|---|---|---|---|---|---|---|---|
| Full sample (N = 1325) | Reference (n = 438) | High HDL-C (n = 355) | Dyslipidemic–high TG (n = 182) | Dyslipidemic–high FFA (n = 234) | IR-hyperglycemic (n = 116) | ||
| Age, mean (SD), y | 27.8 (6.2) | 27.7 (6.2) | 28.9 (5.9) | 28.2 (5.9) | 26.5 (6.3) | 26.6 (6.2) | <.001 |
| Race and ethnicity | |||||||
| Hispanic | 322 (24.3) | 71 (16.2) | 66 (18.6) | 58 (31.9) | 83 (35.5) | 44 (37.9) | <.001 |
| Non-Hispanic | |||||||
| Black | 207 (15.6) | 85 (19.4) | 46 (13.0) | 10 (5.5) | 41 (17.5) | 25 (21.6) | |
| White | 713 (53.8) | 257 (58.7) | 220 (62.0) | 100 (55.0) | 100 (42.7) | 36 (31.0) | |
| Otherb | 83 (6.3) | 25 (5.7) | 23 (6.5) | 14 (7.7) | 10 (4.3) | 11 (9.5) | |
| Education | |||||||
| High school or less | 428 (32.3) | 130 (29.7) | 73 (20.6) | 71 (39.0) | 95 (40.6) | 59 (50.9) | <.001 |
| Some college/associate's degree | 319 (24.1) | 88 (20.1) | 87 (24.5) | 50 (27.5) | 59 (25.2) | 35 (30.2) | |
| College graduate | 292 (22.0) | 100 (22.8) | 91 (25.6) | 38 (20.9) | 46 (19.7) | 17 (14.7) | |
| Graduate degree | 286 (21.6) | 120 (27.4) | 104 (29.3) | 23 (12.6) | 34 (14.5) | 5 (4.3) | |
| Nulliparous | 636 (48.0) | 197 (45.0) | 202 (56.9) | 74 (40.7) | 118 (50.4) | 45 (38.8) | <.001 |
| Smoked during pregnancy | 121 (9.1) | 48 (11.0) | 12 (3.4) | 27 (14.8) | 19 (8.1) | 15 (12.9) | <.001 |
| Healthy Eating Index, mean (SD) | 54.0 (13.6) | 54.4 (14.0) | 57.0 (13.4) | 53.4 (14.4) | 51.5 (12.6) | 49.6 (11.8) | <.001 |
| Prepregnancy body mass indexc | |||||||
| Mean (SD) | 25.7 (6.1) | 23.9 (4.4) | 23.7 (4.3) | 27.3 (5.2) | 27.1 (6.7) | 33.1 (8.7) | <.001 |
| ≥30.0 | 259 (19.6) | 48 (11.0) | 26 (7.3) | 51 (28.2) | 66 (28.3) | 68 (59.1) | <.001 |
| Gestational diabetes | 53 (4.4) | 6 (1.5) | 8 (2.4) | 12 (7.5) | 12 (5.7) | 15 (14.6) | <.001 |
| Gestational weight gaind | |||||||
| Insufficient | 347 (26.3) | 118 (27.0) | 81 (22.8) | 51 (28.2) | 65 (27.9) | 32 (27.8) | .11 |
| Adequate | 370 (28.0) | 133 (30.4) | 100 (28.2) | 55 (30.4) | 62 (26.6) | 20 (17.4) | |
| Excessive | 604 (45.7) | 186 (42.6) | 174 (49.0) | 75 (41.4) | 106 (45.5) | 63 (54.8) | |
| Biomarkers at approximately 17 gestational weekse | |||||||
| Glucose ≥95 mg/dL | 19 (1.4) | 3 (0.7) | 1 (0.3) | 1 (0.6) | 0 | 14 (12.1) | <.001 |
| Insulin ≥25 uIU/mL | 112 (8.5) | 1 (0.2) | 1 (0.3) | 12 (6.6) | 3 (1.3) | 95 (81.9) | <.001 |
| HOMA-IR ≥2.9 | 347 (26.2) | 54 (12.3) | 37 (10.4) | 87 (47.8) | 53 (22.7) | 116 (100.0) | <.001 |
| TGs:HDL-C ≥2.5 | 366 (27.6) | 41 (9.4) | 19 (5.4) | 181 (99.5) | 68 (29.1) | 57 (49.1) | <.001 |
| TGs ≥150 mg/dL | 320 (24.2) | 15 (3.4) | 55 (15.5) | 168 (92.3) | 35 (15.0) | 47 (40.5) | <.001 |
| Total-C ≥200 mg/dL | 398 (30.0) | 10 (2.3) | 252 (71.0) | 85 (46.7) | 31 (13.3) | 20 (17.2) | <.001 |
| HDL-C ≤50 mg/dL | 284 (21.4) | 90 (20.6) | 0 | 81 (44.5) | 69 (29.5) | 44 (37.9) | <.001 |
| FFAs ≥472 μEq/L | 337 (25.4) | 1 (0.2) | 44 (12.4) | 51 (28.0) | 197 (84.2) | 44 (37.9) | <.001 |
| TNF-α ≥1.36 pg/mL | 333 (25.1) | 109 (24.9) | 83 (23.4) | 48 (26.4) | 66 (28.2) | 27 (23.3) | .71 |
Abbreviations: FFA, free fatty acid; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model of insulin resistance; IR, insulin resistant; TG, triglyceride; TNF-α, tumor necrosis factor-α; total-C, total cholesterol.
To convert glucose to millimoles per liter, multiply by 0.0555; to convert insulin to picomoles per liter, multiply by 6.945; to convert TGs to millimoles per liter, multiply by 0.0113; to convert HDL-C to millimoles per liter, multiply by 0.0259.
Non-Hispanic other: due to low cell sizes for some of the categories, American Indian or Alaska Native, Asian, Native Hawaiian or Pacific Islander, and more than 1 race were combined into a single category of “non-Hispanic other.”
BMI is calculated as weight in kilograms divided by height in meters squared.
Gestational weight gain categories classified according to the Institute of Medicine 2009 guidelines.39
Maternal Metabolic Subgroups
The gap statistic indicated an optimal number of 5 metabolic subgroups. We compared the percentage of women in each subgroup that met clinically relevant cutoffs for atherogenic dyslipidemia, elevated glucose, and sample-specific cutoffs to inform the naming of the subgroups. The subgroups were named as follows. The reference group (33% of the sample) included women who had biomarker levels indicative of a favorable metabolic profile. In addition, this subgroup was used as the reference because it was the largest, which provides more stable estimates in pairwise comparisons. The high HDL-C subgroup (27%) contained women who all had HDL-C levels considered to be anti-atherosclerotic and endothelial-protective (HDL-C >50 mg/dL [to convert to millimoles per liter, multiply by 0.0259]).40 The dyslipidemic–high TG (14%) was a subgroup of women of whom most had TGs:HDL-C ratio values considered to be atherogenic (TGs:HDL-C ≥2.5).42 The dyslipidemic–high FFA (18%) was a subgroup of women of whom most had FFAs in the 75th percentile or higher (≥472 μEq/L). The insulin resistant (IR)–hyperglycemic group (9%) included women who all had HOMA-IR values indicative of insulin resistance (HOMA-IR ≥2.9)43,44 (Figure).
Figure. Prevalence of Women Who Met Criteria for Atherogenic Dyslipidemia, Elevated Glucose, and High Sample-Specific Cutoffs for Biomarkers at Approximately 17 Gestational Weeks.
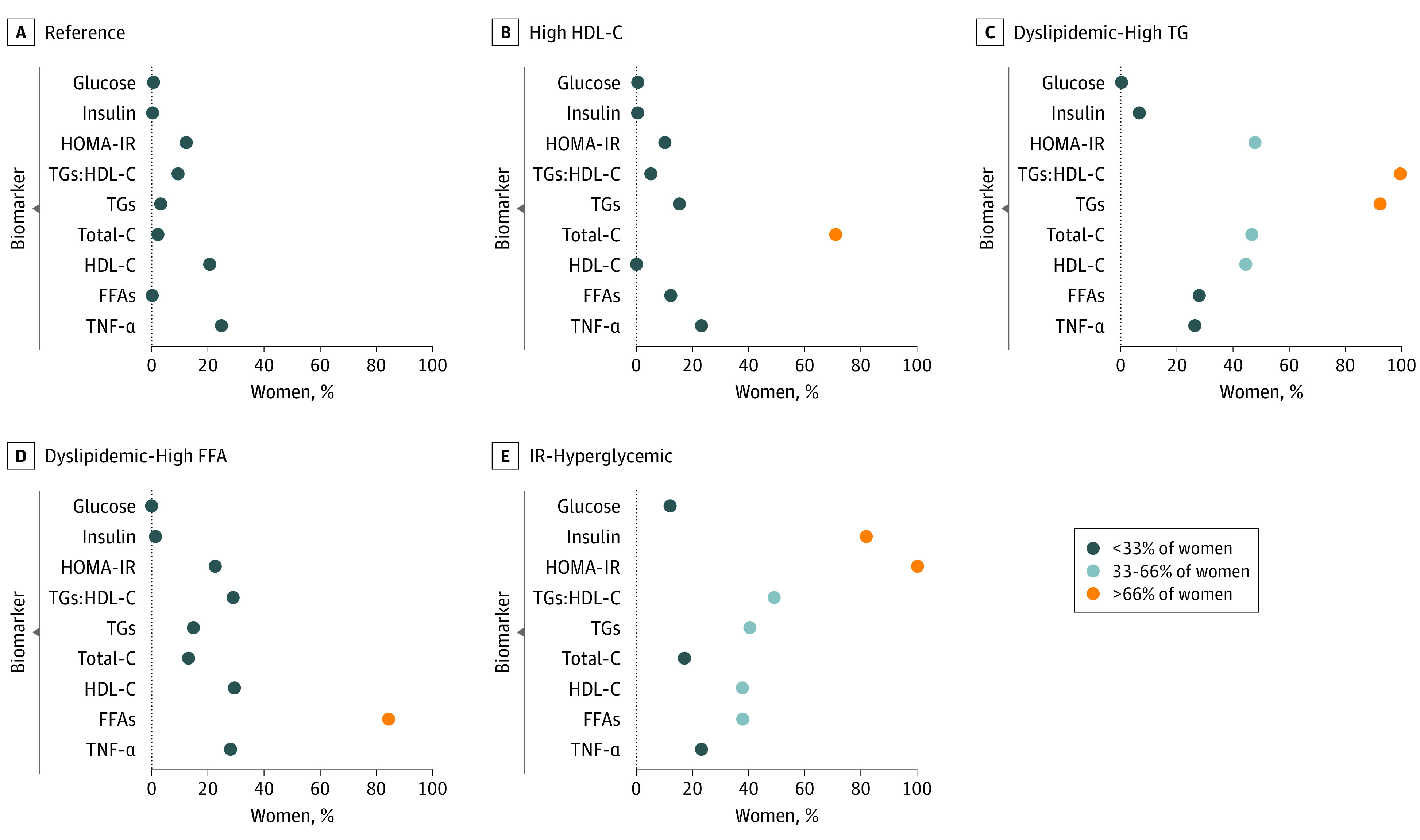
Dark blue circle: less than 33% of women, light blue: 33% to 66% of women, orange: more than 66% of women. Biomarker cutoffs: glucose greater than or equal to 95 mg/dL (to convert to millimoles per liter, multiply by 0.0555), insulin greater than or equal to 25 uIU/mL (to convert to picomoles per liter, multiply by 6.945), HOMA-IR greater than or equal to 2.942,43, TGs:HDL-C greater than or equal to 2.539,40,41, TGs greater than or equal to 150 mg/dL (to convert to millimoles per liter, multiply by 0.0113), total-C greater than or equal to 200 mg/dL (to convert to millimoles per liter, multiply by 0.0259)39, HDL-C less than or equal to 50 mg/dL (to convert to millimoles per liter, multiply by 0.0259)41, FFAs greater than or equal to 472 μEq/L, TNF-α greater than or equal to 1.36 pg/mL. FFA indicates free fatty acid; HOMA-IR, homeostatic model of insulin resistance, HDL-C, high-density lipoprotein cholesterol; IR, insulin resistant; TG, triglyceride; TNF-α, tumor necrosis factor-α; total-C, total cholesterol.
Among women in the reference subgroup, there was a low frequency of GDM (6 participants [1.5%]) and a balanced distribution of education status. Women classified to the high HDL-C subgroup had a higher quality diet (Healthy Eating Index score ≥57) and had the lowest prevalence of prepregnancy obesity (26 participants [7.3%]). Almost half of the women classified to the dyslipidemic–high TG group had high HOMA-IR (≥2.9). These women had the highest rates of prenatal smoking (27 participants [14.8%]), lower educational attainment (71 participants [39.0%] had a high school diploma or less), and older age than other subgroups. Women in the dyslipidemic–high FFA had FFAs levels in midpregnancy that were 79.2% greater than the levels of the reference subgroup in late pregnancy (eTable 2 in Supplement 1); however, the remaining biomarker levels of the dyslipidemic–high FFA subgroup were below clinical thresholds of metabolic risk. The women in this subgroup had a mean prepregnancy BMI of 27.1, and 106 partcipants (45.5%) had excessive gestational weight gain. Finally, more than a third of women in the IR-hyperglycemic subgroup had atherogenic lipid levels (TGs ≥150 mg/dL [to convert to millimoles per liter, multiply by 0.0113] and HDL-C ≤50 mg/dL),40 63 participants (54.8%) had excessive gestational weight gain, and 68 participants (59.1%) had prepregnancy obesity. The women in this subgroup had the highest rates of GDM (15 participants [14.6%]), the lowest quality diet (mean Healthy Eating Index score of 50), and the lowest prevalence of nulliparity (45 participants [38.8%]).
Associations of Maternal Metabolic Subgroups With Offspring Adiposity
Compared with the reference subgroup, offspring of the IR-hyperglycemic subgroup had higher neonatal (β, 1.34; 95% CI, 0.37-2.31) and childhood (β, 4.27; 95% CI, 1.94-6.59) FM%, and higher childhood BMI percentile (β, 16.79; 95% CI, 8.38-25.20) (Table 2). Offspring born to women in the dyslipidemic–high FFA subgroup had higher childhood FM% (β, 1.96; 95% CI, 0.45-3.47). Offspring of the dyslipidemic–high TGs had higher FM% and BMI in childhood; however, the confidence intervals crossed the null for the estimate of BMI. There was no difference in offspring outcomes between the reference and high HDL-C subgroup.
Table 2. Association of Maternal Metabolic Subgroup Membership With Neonatal and Offspring Adiposity During Early Childhood (Median Age 5 Years) Among Mother-Offspring Dyads.
| Offspring outcome, subgroup vs reference | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| Maternal demographics and lifestyle | Prepregnancy BMIb | GDMc | ||
| Neonatal | ||||
| Fat mass, % | ||||
| Reference | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| High HDL-C | −0.36 (−0.98 to 0.26) | −0.39 (−1.00 to 0.23) | −0.38 (−0.99 to 0.23) | −0.38 (−1.01 to 0.25) |
| Dyslipidemic–high TG | 0.03 (−0.75 to 0.81) | −0.22 (−1.00 to 0.57) | −0.39 (−1.17 to 0.39) | −0.29 (−1.12 to 0.54) |
| Dyslipidemic–high FFA | −0.39 (−1.02 to 0.25) | −0.49 (−1.14 to 0.15) | −0.69 (−1.36 to −0.03) | −0.43 (−1.10 to 0.24) |
| IR-hyperglycemic | 1.34 (0.37 to 2.31) | 1.16 (0.16 to 2.17) | 0.60 (−0.42 to 1.61) | 1.02 (−0.02 to 2.07) |
| Birthweight, z score | ||||
| Reference | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| High HDL-C | −0.02 (−0.15 to 0.12) | −0.06 (−0.19 to 0.07) | −0.06 (−0.19 to 0.07) | −0.04 (−0.17 to 0.10) |
| Dyslipidemic–high TG | −0.01 (−0.18 to 0.15) | −0.10 (−0.27 to 0.06) | −0.16 (−0.32 to 0.01) | −0.06 (−0.23 to 0.11) |
| Dyslipidemic–high FFA | −0.14 (−0.29 to 0.01) | −0.18 (−0.32 to −0.03) | −0.23 (−0.38 to −0.09) | −0.15 (−0.30 to 0.00) |
| IR-hyperglycemic | 0.15 (−0.07 to 0.38) | 0.12 (−0.10 to 0.33) | −0.04 (−0.26 to 0.18) | 0.13 (−0.09 to 0.35) |
| Childhood | ||||
| Fat mass, % | ||||
| Reference | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| High HDL-C | 0.53 (−0.74 to 1.80) | 0.52 (−0.75 to 1.78) | 0.50 (−0.77 to 1.76) | 0.44 (−0.86 to 1.73) |
| Dyslipidemic–high TG | 1.70 (0.15 to 3.25) | 1.22 (−0.38 to 2.81) | 0.96 (−0.66 to 2.58) | 1.29 (−0.37 to 2.95) |
| Dyslipidemic–high FFA | 1.96 (0.45 to 3.47) | 2.07 (0.62 to 3.53) | 1.78 (0.27 to 3.28) | 2.27 (0.81 to 3.73) |
| IR-hyperglycemic | 4.27 (1.94 to 6.59) | 4.30 (2.03 to 6.56) | 3.40 (1.15 to 5.64) | 4.18 (1.80 to 6.55) |
| BMI, percentiled | ||||
| Reference | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference |
| High HDL-C | 1.27 (−3.83 to 6.37) | 1.76 (−3.30 to 6.82) | 1.60 (−3.41 to 6.60) | 1.93 (−3.28 to 7.13) |
| Dyslipidemic–high TG | 3.96 (−2.65 to 10.58) | 0.88 (−5.52 to 7.27) | −2.41 (−8.81 to 3.99) | 2.12 (−4.45 to 8.69) |
| Dyslipidemic–high FFA | 3.72 (−2.54 to 9.98) | 1.47 (−4.65 to 7.59) | −1.87 (−8.10 to 4.37) | 2.00 (−4.30 to 8.29) |
| IR-hyperglycemic | 16.79 (8.38 to 25.20) | 13.22 (4.64 to 21.79) | 3.00 (−5.89 to 11.89) | 12.54 (3.59 to 21.49) |
Abbreviations: BMI, body mass index; FFA, free fatty acid; GDM, gestational diabetes; HDL-C, high-density lipoprotein cholesterol; IR, insulin resistant; TG, triglyceride.
Maternal demographics and lifestyle model adjusted for maternal race and ethnicity, education, parity, smoking, and age.
Prepregnancy BMI included the maternal demographics and lifestyle model plus prepregnancy BMI.
GDM included the maternal demographics and lifestyle model plus GDM.
BMI is calculated as weight in kilograms divided by height in meters squared; percentiles in childhood according to the US Centers for Disease Control and Prevention reference.35
Comparison of Metabolic Subgroup Membership With Prepregnancy Obesity or GDM in Association With Childhood Adiposity
Compared with offspring of women in the reference subgroup, those born to women in the IR-hyperglycemic subgroup had a nearly 5-fold higher risk of childhood obesity (RR, 5.3; 95% CI, 2.4-11.4) and 9-fold higher risk of high FM% (≥95th percentile) (RR, 8.7; 95% CI, 2.7-27.8). Additionally, children of women in the dyslipidemic–high FFA subgroup had over 3-fold the risk of high FM% (RR, 3.4; 95% CI, 1.0-11.3) (Table 3). The magnitude of RR of childhood obesity with respect to maternal metabolic subgroup membership was larger than that of prepregnancy obesity alone (RR, 2.1; 95% CI, 1.1-4.1), GDM alone (RR, 3.0; 95% CI, 0.9-9.2), or prepregnancy obesity and GDM (RR, 4.0; 95% CI, 1.1-14.8). After excluding women with either prepregnancy obesity or GDM (312 participants), membership to the IR-hyperglycemic subgroup vs reference subgroup remained significantly associated with risk of childhood obesity (RR, 4.9; 95% CI, 1.3-17.9). Although the estimate was somewhat attenuated, it was still greater than the estimate attributable to prepregnancy obesity, GDM, or both conditions. All estimates, regardless of whether the exposure was maternal metabolic subgroup, obesity, or GDM were somewhat attenuated after adjustment for sociodemographic and behavioral prenatal factors associated with risk; however, the association of the IR-hyperglycemic subgroup with offspring obesity remained significant.
Table 3. Associations of Maternal Metabolic Subgroup, Prepregnancy Obesity, and Gestational Diabetes (GDM) With Offspring Obesity and High Fat Mass Percentage Among Mother-Offspring Dyads.
| In utero exposure | Childhood obesity, BMI ≥95th percentile, RR (95% CI) | Childhood fat mass % ≥95th percentile, unadjusted RR (95% CI) | |
|---|---|---|---|
| Unadjusted | Adjusteda | ||
| Subgroup vs reference | |||
| Reference | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| High HDL-C | 1.0 (0.4-2.5) | 1.1 (0.4-2.8) | 2.6 (0.8-8.3) |
| Dyslipidemic–high TG | 1.3 (0.4-3.6) | 0.9 (0.3-2.4) | 1.3 (0.2-6.9) |
| Dyslipidemic–high FFA | 1.7 (0.7-4.0) | 1.2 (0.5-2.7) | 3.4 (1.0-11.3) |
| IR-hyperglycemic | 5.3 (2.4-11.4) | 3.4 (1.6-7.4) | 8.7 (2.7-27.8) |
| Maternal pregnancy complications vs none | |||
| Neither condition | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Prepregnancy obesity only | 2.1 (1.1-4.1) | 1.6 (0.8-3.2) | 3.0 (1.4-6.5) |
| GDM only | 3.0 (0.9-9.2) | 2.7 (0.8-8.9) | NAb |
| Obesity and GDM | 4.0 (1.1-14.8) | 3.3 (0.7-14.3) | 3.5 (0.5-23.9) |
Abbreviations: BMI, body mass index; FFA, free fatty acid; HDL-C, high density lipoprotein cholesterol; IR, insulin resistant; NA, not applicable; TG, triglyceride.
Adjusted model includes maternal race and ethnicity, education, parity, smoking, and age.
Estimate not available because none of the women with GDM had offspring with fat mass percentages greater than or equal to the 95th percentile.
Discussion
Using prospective data from a prebirth cohort with follow-up through approximately 5 years of age, we identified 5 distinct maternal metabolic subgroups according to 7 biomarkers derived from fasting blood at approximately 17 gestational weeks and 2 biomarker indices. According to the levels of metabolic biomarkers and the prevalence of meeting clinically relevant thresholds for specific biomarkers, we refer to the subgroups as reference, high HDL-C, dyslipidemic–high TGs, dyslipidemic–high FFA, and IR-hyperglycemic. These subgroups exhibited differences in demographic, lifestyle, and prenatal risk factors for offspring obesity (eg, women with lower educational attainment, diet quality, and excessive gestational weight gain). Maternal membership to the IR-hyperglycemic subgroup was associated with the greatest risk of offspring adiposity in the neonatal and childhood periods, and membership to the dyslipidemic–high FFA or IR-hyperglycemic subgroups had a greater association with offspring FM% in the 95th percentile or higher than prepregnancy obesity alone, GDM alone, or prepregnancy obesity and GDM. These data suggest that nuances of maternal metabolism resulting from effects of interrelated biomarkers and across classes of compounds, which are not fully captured by traditional classifications of obese BMI or GDM, play a role in the in utero origins of excess offspring adiposity. In addition, women in these metabolic subgroups display different sociodemographic and lifestyle characteristics that may also be associated with an increased risk of childhood adiposity. In the following sections, we summarize sociodemographic and metabolic characteristics of each subgroup and whether or how they are associated with offspring adiposity.
Reference Subgroup
Of the 5 subgroups identified by the cluster-based algorithm, we identified a group that comprised the largest proportion of women in the study sample (33%), the majority of whom had biomarker levels below clinical thresholds for metabolic risk,40,41,42,43,44 mean prepregnancy BMI within a healthy clinical range (18.5 to 24.9), and the lowest rates of GDM diagnosis across the subgroups. Accordingly, we labeled this group as the reference category for comparisons with other subgroups.
IR-Hyperglycemic Subgroup
The IR-hyperglycemic subgroup, which comprised 9% of women, was characterized by insulin resistance and an atherogenic lipid profile (high TGs, high FFAs, low HDL-C) in midpregnancy. Classification to this subgroup was associated with higher neonatal and childhood FM%. This finding aligns with prior studies4,6,8,10,14,22,23,36 documenting the continuous relationship between maternal glucose levels and insulin resistance in pregnancy with fetal growth and fat mass accretion, including prior studies from our cohort. Furthermore, prior studies18,19,48,49,50 have shown that in the context of maternal GDM and obesity, TGs are important drivers of newborn weight48 and, at times, may even exhibit greater associations with newborn weight and adiposity than glucose.
Dyslipidemic–High TGs Subgroup
In our study, offspring of women in the dyslipidemic–high TGs subgroup, comprising 14% of women, had higher FM% and BMI in childhood, although the confidence intervals crossed the null. The association between exposure to high TGs in utero and greater offspring adiposity is consistent with prior data from women with GDM and obesity,18,19,48,49,50 and data from 2 general risk pregnancy cohorts measuring TGs in early or midpregnancy.16,17 In a subset of women from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, TGs measured at approximately 28 gestational weeks were not associated with BMI z score or sum of skinfolds in offspring at age 6 years.51 This discrepancy may be due to differences in TGs levels between studies, as the women in the dyslipidemic–high TGs subgroup had TGs that were 30% higher than women in the HAPO subset at a comparable gestational time point. Additionally, TGs in the presence of insulin resistance (see IR-hyperglycemic subgroup) increased risk of offspring adiposity; however, in the absence of higher glycemia, the extent of risk of offspring adiposity was diminished, underscoring a potential synergistic effect of metabolic compounds.
Dyslipidemic–High FFA Subgroup
The dyslipidemic–high FFA subgroup, which comprised 18% of the sample, generally had favorable levels of metabolic markers except for strikingly high levels of FFAs. In this subgroup, the FFA levels in midpregnancy were already higher than the FFA levels of women in the reference subgroup in late pregnancy, which is noteworthy considering the increase in lipolysis and circulating FFAs as gestation progresses.52 Unlike the IR-hyperglycemic subgroup, classification to the dyslipidemic–high FFA subgroup was not associated with differences in FM% in the neonatal period. However, in early childhood, offspring of the dyslipidemic–high FFA subgroup had higher FM% and higher risk for FM in the 95th percentile or higher. In our cohort, prior analyses have shown that associations of maternal lipoproteins and FFAs with offspring metabolic traits are apparent in childhood53 rather than with offspring adiposity at birth.22,36 Studies that assessed FFAs in pregnancy in association with newborn body composition have yielded conflicting results.23,26,54 However, prior studies have found similar positive associations of FFAs, as well as n-6 polyunsaturated fatty acid patterns and individual n-6 polyunsaturated fatty acids during pregnancy with offspring body composition when measured in early childhood.16,20,21,24 The differences in findings across studies demonstrate the importance of longitudinal birth cohorts and measurement of offspring adiposity at multiple time points across early life, as not all manifestations of in utero exposures are apparent at birth.
High HDL-C Subgroup
Finally, we identified a high HDL-C subgroup, which included 27% of women in the sample. The majority of women in this subgroup had biomarker levels below clinical thresholds for metabolic risk,40,41,42,43,44 mean prepregnancy BMI within the normal weight range, and lower rates of GDM diagnosis. Offspring of women in this group exhibited no difference in adiposity at birth or in early childhood from that of the reference group. The lack of differences could be due to the similarity in metabolic profiles between the women in the reference and high HDL-C subgroups, and highlights the importance of maternal metabolic health for in utero programming.
Comparison of Subgroup Membership with Traditional Prenatal Risk Factors for Childhood Obesity
Our findings support growing evidence for the importance of identifying associated compounds and interrelated biomarkers to capture meaningful metabolic heterogeneity within a population. Furthermore, differences in traditional metabolic biomarkers across the subgroups indicate the involvement of both glycemic and nonglycemic metabolic factors to fetal programming of adipogenic potential that operates across the continuum of maternal weight status and glucose metabolism in pregnancy.55
Additionally, the association of maternal subgroups with childhood obesity remained significant, even following adjustment for known sociodemographic and behavioral prenatal risk factors, including race and ethnicity, education, and prenatal smoking. On the other hand, neither maternal obesity nor GDM were associated with offspring obesity after adjustment for the same sociodemographic and lifestyle variables. These findings suggest that the subgroups characterized herein have utility for identifying pregnant women whose offspring are at elevated risk of early-life adiposity, above and beyond traditional risk factors. Together, our findings indicate that unsupervised clustering approaches of biomarkers in pregnancy have potential to capture the relative risk or relative contribution of in utero metabolic differences in pregnancy to offspring adiposity. Applications of these approaches are needed in future studies to assess whether this residual variation in the standard categories of risk is relevant for clinicians to consider in future efforts to refine risk classification in pregnancy.
Limitations
This study had limitations. We did not have childhood anthropometric data on all offspring of women included in this analysis. Women whose offspring did not have data on the adiposity outcomes of interest had a lower diet quality, educational attainment, and reported smoking in pregnancy. These characteristics have been associated with offspring obesity, and therefore the estimates in the current study may underrepresent the magnitude of risk attributable to the in utero environment. A limitation of the k-means clustering algorithm is the need to predefine a value for k, and thus classification of individuals can change according to the specified k,56 which may challenge replicability of subgroups across studies. Although there is no standard for validation of clustering classification, we used the gap statistic which captures cluster cohesion and distinctness to inform the value for k.56 In addition, we overlaid data on lifestyle and prenatal risk to capture some of the complexities between individual behaviors and metabolic compounds, which adds value to automated classification methods.57
Conclusions
The data from the current study suggest that unsupervised clustering methods may be a useful technique for identifying co-occurring patterns of metabolic markers measured during pregnancy that capture variation in sociocultural, anthropometric, and biochemical risk factors for excess adiposity in offspring. However, our findings need to be confirmed in a separate cohort. Moreover, given that we studied a group of fairly healthy pregnant women, additional research is warranted to assess validity of these subgroups in other populations with more complex obstetric presentation, and replicability of their associations with offspring adiposity.
eTable 1. Characteristics of 1,325 Pregnant Women in the Healthy Start Study by Availability of Offspring Anthropometry Data at the Early Childhood Visit
eTable 2. Mean ± SD of Metabolic Markers Among 1,325 Pregnant Women in the Healthy Start Study, Overall and According to Metabolic Subgroup Membership
eFigure. Flow Diagram of the Analytical Sample of Pregnant Women and Their Offspring in the Healthy Start Study
Data Sharing Statement
References
- 1.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011;35(7):891-898. doi: 10.1038/ijo.2010.222 [DOI] [PubMed] [Google Scholar]
- 2.Perng W, Oken E, Dabelea D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetol. 2019;62(10):1779-1788. doi: 10.1007/s00125-019-4914-1 [DOI] [PubMed] [Google Scholar]
- 3.Kaul P, Bowker SL, Savu A, Yeung RO, Donovan LE, Ryan EA. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetol. 2019;62(2):249-258. doi: 10.1007/s00125-018-4758-0 [DOI] [PubMed] [Google Scholar]
- 4.Powe CE, Allard C, Battista M-C, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052-1055. doi: 10.2337/dc15-2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis EC, Kechris K, Cohen CC, Michelotti G, Dabelea D, Perng W. Metabolomic profiles in childhood and adolescence are associated with fetal overnutrition. Metabolites. 2022;12(3):265. doi: 10.3390/metabo12030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis EC, Dabelea D, Ringham BM, Sauder KA, Perng W. Maternal blood glucose level and offspring glucose-insulin homeostasis: what is the role of offspring adiposity? Diabetol. 2021;64(1):83-94. doi: 10.1007/s00125-020-05294-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340-346. doi: 10.2337/dc07-1596 [DOI] [PubMed] [Google Scholar]
- 8.Lowe WL Jr, Scholtens DM, Lowe LP, et al. ; HAPO Follow-up Study Cooperative Research Group . Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005-1016. doi: 10.1001/jama.2018.11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Razaz N, Villamor E, Muraca GM, Bonamy AE, Cnattingius S. Maternal obesity and risk of cardiovascular diseases in offspring: a population-based cohort and sibling-controlled study. Lancet Diabetes Endocrinol. 2020;8(7):572-581. doi: 10.1016/S2213-8587(20)30151-0 [DOI] [PubMed] [Google Scholar]
- 10.Metzger BE, Lowe LP, Dyer AR, et al. ; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. doi: 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 11.Perng W, Rifas-Shiman SL, McCulloch S, et al. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metabolism. 2017;76:11-22. doi: 10.1016/j.metabol.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perng W, Ringham BM, Smith HA, Michelotti G, Kechris KM, Dabelea D. A prospective study of associations between in utero exposure to gestational diabetes mellitus and metabolomic profiles during late childhood and adolescence. Diabetol. 2020;63(2):296-312. doi: 10.1007/s00125-019-05036-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shokry E, Marchioro L, Uhl O, et al. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: results from the PREOBE cohort study. Acta Diabetol. 2019;56(4):421-430. doi: 10.1007/s00592-019-01291-z [DOI] [PubMed] [Google Scholar]
- 14.Selen DJ, Edelson PK, Corelli K, et al. Insulin resistant gestational glucose intolerance is associated with adverse perinatal outcomes. J Endocr Soc. 2021;5(suppl 1):A434-A434. doi: 10.1210/jendso/bvab048.885 [DOI] [Google Scholar]
- 15.Daraki V, Georgiou V, Papavasiliou S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea pregnancy cohort Crete, Greece. PLoS One. 2015;10(5):e0126327. doi: 10.1371/journal.pone.0126327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gademan MG, Vermeulen M, Oostvogels AJ, et al. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with offspring’s body composition at age 5-6 years: the ABCD study. PLoS One. 2014;9(4):e94594. doi: 10.1371/journal.pone.0094594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CL, Vladutiu CJ, Zikry TM, Grace MR, Siega-Riz AM. Maternal lipid levels during pregnancy and child weight status at 3 years of age. Pediatr Obes. 2019;14(4):e12485. doi: 10.1111/ijpo.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24–32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstetrics & Gynecology. 2001;97(5 Pt 1):776-780. doi: 10.1016/S0029-7844(01)01328-X [DOI] [PubMed] [Google Scholar]
- 19.Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care. 2011;34(10):2198-2204. doi: 10.2337/dc11-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr. 2011;93(4):780-788. doi: 10.3945/ajcn.110.005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries PS, Gielen M, Rizopoulos D, et al. Association between polyunsaturated fatty acid concentrations in maternal plasma phospholipids during pregnancy and offspring adiposity at age 7: The MEFAB cohort. PLEFA. 2014;91(3):81-85. doi: 10.1016/j.plefa.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 22.Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the healthy start study. J Clin Endocrinol Metab. 2015;100(4):1672-1680. doi: 10.1210/jc.2014-2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friis CM, Qvigstad E, Paasche Roland MC, et al. Newborn body fat: associations with maternal metabolic state and placental size. PLoS One. 2013;8(2):e57467. doi: 10.1371/journal.pone.0057467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voortman T, Tielemans MJ, Stroobant W, et al. Plasma fatty acid patterns during pregnancy and child's growth, body composition, and cardiometabolic health: The Generation R Study. Clinical nutrition (Edinburgh, Scotland). 2018;37(3):984-992. doi: 10.1016/j.clnu.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Gingras V, Rifas-Shiman SL, Derks IPM, Aris IM, Oken E, Hivert MF. Associations of gestational glucose tolerance with offspring body composition and estimated insulin resistance in early adolescence. Diabetes Care. 2018;41(12):e164-e166. doi: 10.2337/dc18-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima RA, Desoye G, Simmons D, et al. The importance of maternal insulin resistance throughout pregnancy on neonatal adiposity. Paediatr Perinat Epidemiol. 2021;35(1):83-91. doi: 10.1111/ppe.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadakia R, Talbot O, Kuang A, et al. ; HAPO Study Cooperative Research Group . Cord blood metabolomics: association with newborn anthropometrics and c-peptide across ancestries. J Clin Endocrinol Metab. 2019;104(10):4459-4472. doi: 10.1210/jc.2019-00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbina EM, Khoury PR, McCoy CE, Dolan LM, Daniels SR, Kimball TR. Triglyceride to HDL-C ratio and increased arterial stiffness in children, adolescents, and young adults. Pediatrics. 2013;131(4):e1082-e1090. doi: 10.1542/peds.2012-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802-809. doi: 10.7326/0003-4819-139-10-200311180-00007 [DOI] [PubMed] [Google Scholar]
- 30.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191-2192. doi: 10.2337/diacare.21.12.2191 [DOI] [PubMed] [Google Scholar]
- 31.cluster: Cluster Analysis Basics and Extensions. R package version 2.1.4; 2022. Accessed March 1, 2023. https://CRAN.R-project.org/package=cluster
- 32.factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 3.1.2; 2020. Accessed March 1, 2023. https://CRAN.R-project.org/package=factoextra
- 33.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3(1):6. doi: 10.1186/1471-2431-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fields DA, Allison DB. Air-displacement plethysmography pediatric option in 2-6 years old using the four-compartment model as a criterion method. Obesity (Silver Spring). 2012;20(8):1732-1737. doi: 10.1038/oby.2012.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1-27. [PubMed] [Google Scholar]
- 36.Shapiro ALB, Schmiege SJ, Brinton JT, et al. Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the Healthy Start study. Diabetol. 2015;58(5):937-941. doi: 10.1007/s00125-015-3505-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis EC, Dabelea D, Shankar K, Perng W. Maternal diet quality during pregnancy is associated with biomarkers of metabolic risk among male offspring. Diabetol. 2021;64(11):2478-2490. doi: 10.1007/s00125-021-05533-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flanagin A, Frey T, Christiansen SL. Committee AMoS. updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326(7):621-627. doi: 10.1001/jama.2021.13304 [DOI] [PubMed] [Google Scholar]
- 39.Institute of Medicine . Weight gain during pregnancy: reexamining the guidelines. 2009. Accessed March 1, 2023. https://nap.nationalacademies.org/resource/12584/Report-Brief---Weight-Gain-During-Pregnancy.pdf
- 40.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112(17):2735-2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 41.Melmed S, Auchus RJ, Goldfine AB, Koenig R, Rosen CJ, Williams RH. Williams textbook of endocrinology. 14th edition. Elsevier; 2019. [Google Scholar]
- 42.Salazar MR, Carbajal HA, Espeche WG, et al. Relation among the plasma triglyceride/high-density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio-metabolic risk factors in men and women. Am J Cardiol. 2012;109(12):1749-1753. doi: 10.1016/j.amjcard.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 43.Ghasemi A, Tohidi M, Derakhshan A, Hasheminia M, Azizi F, Hadaegh F. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol. 2015;52(5):905-905. doi: 10.1007/s00592-015-0730-3 [DOI] [PubMed] [Google Scholar]
- 44.Lee CH, Shih AZL, Woo YC, et al. Optimal Cut-Offs of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to Identify Dysglycemia and Type 2 Diabetes Mellitus: A 15-Year Prospective Study in Chinese. PLoS One. 2016;11(9):e0163424. doi: 10.1371/journal.pone.0163424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 46.Laubach ZM, Murray EJ, Hoke KL, Safran RJ, Perng W. A biologist's guide to model selection and causal inference. Proc Biol Sci. 2021;288(1943):20202815. doi: 10.1098/rspb.2020.2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conroy S, Murray EJ. Let the question determine the methods: descriptive epidemiology done right. Br J Cancer. 2020;123(9):1351-1352. doi: 10.1038/s41416-020-1019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care. 1992;15(11):1605-1613. doi: 10.2337/diacare.15.11.1605 [DOI] [PubMed] [Google Scholar]
- 49.Barbour LA, Farabi SS, Friedman JE, et al. Postprandial Triglycerides Predict Newborn Fat More Strongly than Glucose in Women with Obesity in Early Pregnancy. Obesity (Silver Spring). 2018;26(8):1347-1356. doi: 10.1002/oby.22246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005;22(1):21-25. doi: 10.1111/j.1464-5491.2004.01336.x [DOI] [PubMed] [Google Scholar]
- 51.Thaware PK, McKenna S, Patterson CC, Casey C, McCance DR. Maternal Lipids at 28 Weeks’ Gestation and Offspring Adiposity at Age 5 to 7 Years. J Clin Endocrinol Metab. 2018;103(10):3767-3772. doi: 10.1210/jc.2018-00786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivan E, Homko CJ, Chen X, Reece EA, Boden G. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes. 1999;48(4):834-838. doi: 10.2337/diabetes.48.4.834 [DOI] [PubMed] [Google Scholar]
- 53.Cohen CC, Francis EC, Perng W, et al. Exposure to maternal fuels during pregnancy and offspring hepatic fat in early childhood: the healthy start study. Pediatr Obes. 2022;17(7):e12902. doi: 10.1111/ijpo.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rojas-Rodriguez R, Price LL, Somogie J, Hauguel-de Mouzon S, Kalhan SC, Catalano PM. Maternal lipid metabolism is associated with neonatal adiposity: A longitudinal study. J Clin Endocrinol Metab. 2022;107(9):e3759-e3768. doi: 10.1210/clinem/dgac360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyle KE, Patinkin ZW, Shapiro ALB, Baker PR II, Dabelea D, Friedman JE. Mesenchymal Stem Cells From Infants Born to Obese Mothers Exhibit Greater Potential for Adipogenesis: The Healthy Start BabyBUMP Project. Diabetes. 2016;65(3):647-659. doi: 10.2337/db15-0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J Royal Stat Soc. 2001;63(2):411-423. doi: 10.1111/1467-9868.00293 [DOI] [Google Scholar]
- 57.Grant RW, McCloskey J, Hatfield M, et al. Use of Latent Class Analysis and k-Means Clustering to Identify Complex Patient Profiles. JAMA Netw Open. 2020;3(12):e2029068. doi: 10.1001/jamanetworkopen.2020.29068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dabelea D. Healthy start: exploring the fuel-mediated programming of neonatal growth. Accessed March 9, 2023. https://clinicaltrials.gov/ct2/show/NCT02273297
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of 1,325 Pregnant Women in the Healthy Start Study by Availability of Offspring Anthropometry Data at the Early Childhood Visit
eTable 2. Mean ± SD of Metabolic Markers Among 1,325 Pregnant Women in the Healthy Start Study, Overall and According to Metabolic Subgroup Membership
eFigure. Flow Diagram of the Analytical Sample of Pregnant Women and Their Offspring in the Healthy Start Study
Data Sharing Statement


