Abstract
This research examined the effects of feeding phytosomal green tea on broilers infected with coccidia. To provide phytosome, green tea extract was loaded into soy lecithin. Groups of chicks included uninfected and untreated control (NC), infected and untreated control (PC), infected and treated with salinomycin control (SC), infected and treated with 300 and 400 mL of green tea extract (GTE300, GTE400), infected and treated with 200, 300, 400 and 500 mL of green tea phytosome (GTP200, GTP300, GTP400, and GTP500). At 14-days posthatch, chickens were orally gavaged, except the NC group with a coccidia vaccine 30 times larger than the approved dose. Body weight (BW), feed intake (FI), and feed conversion ratio (FCR) were determined at 7, 14, 20, 28, 35, and 42 d. The characteristics of the carcass, internal organs and intestinal morphology were assessed on d 42. Applying overdose of coccidiosis vaccine showed experimental Eimeria infection, led to decrease in FI and BW, and increased FCR compared to PC group (P < 0.001). Meanwhile salinomycin, green tea extract, and green tea phytosome compensated the negative effects of Eimeria infection on growth performance. The treatments did not affect carcass, breast, and thigh relative weights. Interestingly, abdominal fat percent was significantly lower in chickens fed GTP300, GTP400, and GTP500 than in those fed GTE300, GTE300, and GTP200 (P < 0.0001). In comparison to the basal diet plus green tea extract forms and NC groups, the PC group increased the relative weights of the liver, spleen, bursa, and pancreas (P < 0.05). The highest values of villus height and villus height to crypt ratio were obtained in duodenum, jejunum and ileum in GTP300 group (P < 0.0001), while, villi diameter in duodenum and ileum decreased the most in GTP300 and GTP500, respectively (P < 0.0001). Consequently, as natural anticoccidial drug delivery systems, 300 mL of green tea phytosome can be introduced as the optimal dose to maximize the benefits of phytosome for intestinal integrity and reduce the consumption of green tea extract.
Key words: broiler, green tea phytosome, Eimeria infection, intestine integrity
INTRODUCTION
The integrity of the digestive system of broiler, with its nutritional, microbial, immune, and physiological functions, are among the important indicators of poultry production (Adedokun and Olojede, 2019; Zhu et al., 2021). Coccidiosis is a prevalent intestinal illness in broilers caused by single-celled protozoan parasites of the genus Eimeria, resulting in approximately $14 billion in yearly economic losses globally (Blake et al., 2020). The disease cycle starts from the ingestion of environmentally resistant Eimeria oocysts by the bird, and then parts of the digestive system are affected, which leads to severe inflammation and necrosis of submucosa. Innumerable oocysts multiply in the intestine and are excreted through feces, which results in contamination of the entire farm, more mortality and poor growth performance of chickens (Wickramasuriya et al., 2022).
Three approaches to control of the disease, including anticoccidial drugs (Chapman and Rathinam, 2022), vaccines (Soutter et al., 2020; Lee et al., 2022), and natural products (Muthamilselvan et al., 2016; El-Shall et al., 2022; Jamil et al., 2022) have introduced in the literature so far. The disadvantages of using coccidiosis drugs include: 1) Development of drug resistance, 2) Increasing vulnerability of chickens when discontinuing the medication against uncontrolled infection, 3) Increasing consumer concern about chemical residues in carcasses, 4) Present regulatory differences in different countries for export, and 5) Increasing drug residues in nontarget organisms. The use of vaccines (weakened and live weak) is much safer and healthier, however, there are considerations, as: 1) The high cost of production and application, 2) Species-specific immunity, 3) Differences in poultry production systems, 4) The risk of introducing new strains to the farm, 5) Determining the appropriate dosage for use in the herd, and 6) Short shelf life and the need for cold transfer chain have led to more limited use of this option in many countries (Attree et al., 2021).
In recent years, the use of natural materials of plant origin to treat coccidiosis has emerged as a novel and promising approach owing to the wide range of advantages, including broad dose range, absence of grace period, natural origin, and appetite-stimulating activity (El-Shall et al., 2022). Green tea is the type of tea plant (Camellia sinensis) that contains high levels of polyphenols along with caffeine and L-theanine. Green tea is made in such a way that most of the chemical components present in the green leaves remain unchanged during processing. To make green tea, freshly picked tea leaves are heat treated immediately after picking to inactivate polyphenol oxidase and peroxidases enzymes (Rana and Kumar, 2020). A review of studies reveals that green tea has anticoccidial properties (Jang et al., 2007; Molan and Faraj, 2015; Abbas et al., 2017; Seidavi et al., 2020; Zhang et al., 2020; Jelveh et al., 2022). The anticoccidiosis effects of green tea may be attributed to the antioxidant, anti-inflammatory, and antimicrobial properties of polyphenols and L-theanine. Polyphenols compose 20 to 35% of the dry matter of fresh green tea leaves. In addition, L-theanine makes up 50% of green tea's amino acids (Saeed et al., 2020). As a result of the large multiring structure of polyphenols and their lipid solubility, as well as their low water content, which act as primary barriers to crossing the outer membrane of gastrointestinal cells, the widespread use of powders and extracts of green tea is restricted (Lu et al., 2019; Thomas et al., 2022).
Encapsulation involves structuring systems capable of preserving the chemical, physical, and biological properties of active compounds as well as releasing or delivering them under desired conditions. The process of encapsulation involves coating either a single or a mixture of bioactive materials with another one or a combination of materials. A protective layer encapsulates the active ingredients in nanometer to millimeter-sized capsules (Sonawane et al., 2020). Phytosome is made using encapsulating standardized plant extracts that are linked to phospholipids, specifically phosphatidylcholine from soy, to create a lipid-compatible complex (Pal et al., 2021). Phosphatidylcholine can be dissolved in both water and lipid environments, and when taken orally, it is well absorbed. This novel drug delivery system approach delivers herbal drugs at a predetermined rate, at the site of action. It reduces toxic effects, increases drug bioavailability, and regulates drug distribution by either incorporating the drug into the carrier system or altering the molecular structure of the drug (Usman, 2021). Green tea phytosome is composed of green tea extract and phospholipids. Therefore, it is expected that phytosome technology is potentially effective in delivering green tea extract to eliminate barriers related to solubility and improve bioavailability in the poultry industry.
Previous research typically only showed the effect of green tea on performance, carcasses quality, and intestinal health. Still, a few authors have studied these parameters when challenged with Eimeria. This study aimed to compares free and phytosome forms of green tea extract with anticoccidial drug on growth performance and intestinal morphology in an experimental coccidiosis infection challenge in broilers.
MATERIALS AND METHODS
Broilers, Management, and Experimental Treatment
A total of 1,260 one-day-old male Ross 308 broilers (41 ± 05 g) from a commercial hatchery (Sepidmakian Co., Rasht, Iran) were randomly allocated to 1 of 9 treatments and 5 replicates (28 birds per replicate. Throughout the whole trial, all birds were provided with feed and fresh water ad libitum. Chickens were vaccinated against Newcastle disease at 10, 17, and 27 d of age. The humidity and temperature schedule followed the Ross 308 standard (Aviagen, 2018b). The broilers were exposed to 24-h light for the first 2 d, followed by 20-h light and 4-h darkness for the remainder of the experiment. The 3 periods of the experiment included starter (d 1–10, crumble), grower (d 12–21, pellet), and finisher (d 22–42, pellet).The treatments include: 1) noninfected and untreated control (NC), 2) infected and untreated control (PC), 3) infected and treated with Salinomycin (SC), 4) infected and treated with 300 mL green tea extract (GTE300), 5) infected and treated with 400 mL green tea extract (GTE400), 6) infected and treated with 200 mL green tea phytosome (GTP200), 7) infected and treated with 300 mL green tea phytosome (GTP300), 8) infected and treated with 400 mL green tea phytosome (GTP400), 9) infected and treated with 500 mL green tea phytosome (GTP500). Table 1 shows the diet ingredient content based on corn-soybean and Ross 308 standard nutrient recommendations (Aviagen, 2022a).
Table 1.
Ingredient composition and calculated values of the corn-soybean-based diet (as-fed basis %).
| Ingredients (%) | Starter | Grower | Finisher |
|---|---|---|---|
| Corn | 51.3 | 58.8 | 63.9 |
| Soybean meal | 39.5 | 35 | 30 |
| Corn gluten meal | 3 | 1.032 | 0.73 |
| Soybean oil | 1.40 | 1.01 | 1.47 |
| Dicalcium phosphate | 1.93 | 1.42 | 1.3 |
| Shell | 0.47 | 0.48 | 0.5 |
| Bentonite | 0 | 0.15 | 0.15 |
| Salt | 0.3 | 0.2 | 0.2 |
| Sodium bicarbonate | 0.13 | 0.1 | 0.1 |
| HCL-lysine | 0.45 | 0.4 | 0.41 |
| DL-methionine | 0.39 | 0.35 | 0.31 |
| L-threonine | 0.13 | 0.10 | 0.10 |
| Sodium sulfate | 0.05 | 0.035 | 0.035 |
| Choline chloride | 0.06 | 0.06 | 0.051 |
| L-Valine | 0.05 | 0.055 | 0.055 |
| L-Arginine | 0.015 | 0.01 | 0 |
| Toxin binder | 0.3 | 0.3 | 0.3 |
| Phytase 10000 | 0.001 | 0.001 | 0.001 |
| Vit/Min. premix1 | 0.5 | 0.5 | 0.45 |
| Calculated nutrient values | |||
| Crude protein (%) | 23.34 | 20.86 | 18.89 |
| Metabolizable energy (kcal/kg) | 2850 | 2945 | 3030 |
| Lysine (%) | 1.3 | 1.17 | 1.07 |
| Methionine (%) | 0.649 | 0.607 | 0.557 |
| Met + Cys (%) | 0.95 | 0.869 | 0.8 |
| Threonine (%) | 0.871 | 0.77 | 0.681 |
| Tryptophan (%) | 0.247 | 0.229 | 0.205 |
| Arginine (%) | 1.38 | 1.249 | 1.106 |
| Iso-leucine (%) | 0.871 | 0.779 | 0.701 |
| Leucine (%) | 1.841 | 1.584 | 1.456 |
| Valine (%) | 1 | 0.913 | 0.835 |
| Calcium (%) | 0.96 | 0.87 | 0.82 |
| Ava. phosphorus | 0.48 | 0.435 | 0.41 |
| Crude fiber (%) | 2.98 | 2.93 | 2.82 |
Provided per kilogram of diet: 12,000 IU of vitamin A (retinol); 5,000 IU of vitamin D3; 80 IU of vitamin E (tocopheryl acetate); 3.2 mg of vitamin K3; 3 mg of thiamin; 8.6 mg of riboflavin; 4.3 mg of vitamin B6 (pyridoxine); 0.017 mg of vitamin B12 (cyanocobalamin); 65 mg of niacin; 2.2 mg of folic acid; 0.22 mg of biotin; 20 mg of pantothenic acid; 1,700 mg of choline chloride.110 mg of Zn; 120 mg of Mn; 20 mg of Fe; 16 mg of Cu; 1.25 mg of I; 0. 3 mg of Se.
Preparation of Green Tea Extract and Green Tea Phytosomes
A total of 300 g of dried green tea powder was added to a 70% ethanol/water solvent. The resulting mixture was stirred, placed in a dark place for 7 d and then filtered. The filtered mixture was centrifuged (ROTOFIX 32 A, Hettich Zentrifugen, Tuttlingen, Germany) for 3 min (3,000 rpm), and the filtered surface liquid was collected and concentrated using a rotary (RV 10 digita v-c, IKA, Staufen, Germany) evaporator at 45°C. Finally, green tea extract completely dried and powdered using freeze dryer (121550 PMMA, Christ, Berlin, Germany). The green tea powder was stored in dark containers in the freezer at −25°C. Phytosomes of green tea were prepared as described by Direito et al. (2019) with a little modification. In brief it was prepared by adding 0.5 g green tea extract in 20 mL of ethanol in Erlenmeyer where slowly dissolved. In a separate Erlenmeyer a mixture of 0.5 g of lecithin in 0.5 mL of dichloromethane was dissolved, and gently added to the green tea extract + ethanol using magnetic stirrer at 190 rpm. After 2 h and achieving uniformity, 40 mL of 2% acetic acid was added. Then, Solvents was separated in a rotary evaporator and homogenized the size of phytosomes by ultrasonic probe (BANDELIN, Berlin, Germany) with 20% power at the temperature of 28°C for 10 min.
Phytosome Characterization Evaluation
Using a JSM5600 scanning electron microscope (JEOL, Tokyo, Japan), the materials' surface morphology was scanned. Furthermore, the mean particle size of phytosomes was calculated using ZETACHECK (Microtrac, Osaka, Japan) at 25°C. Additionally, FTIR spectra were acquired using a JASCO 4700 FTIR spectrometer (JASCO, Tokyo, Japan).Each sample was scanned within the wavelength 400 to 4,000 cm−1.
Eimeria Infection of Chicks
On d 14, birds in all treatment groups (except NC) orally received a 30-fold dose of commercial Livacox T vaccine (Biopharm Co., Prague, Czech Republic) to infect them with coccidiosis. Each dosage of the vaccine contain 300 to 500 sporulated oocysts of 3 Eimeria strains including Eimeria maxima, Eimeria tenella, and Eimeria acervuline, in 0.01 mL of distilled water. With 0.5 cc of distilled water, birds from NC were fictitiously injected.
Anticoccidial Efficacy Evaluation
Broiler chicks were individually weighted at 7th, 14th, 28th, 35th, and 42nd days of age to record the body weight (BW), feed intake (FI) and feed conversion ratio (FCR). In the end of experiment, 2 birds from each replicate were randomly chosen and were slaughtered to assess characteristics of the carcass, internal organs and intestinal morphology.
Intestinal Histomorphological Analysis
The duodenum, jejunum, and ileum were removed from the digestive system and divided into 3 sections, and washed 3 times with cold phosphate buffer solution before being fixed in 10% formalin. The fixed tissues were dehydrated in progressively higher alcohol concentrations (70–100%), xylene infiltrated, embedded in paraffin, and then submitted for processing. The tissue blocks were sectioned at 5 µm cross-section using a DS4055 microtome (Didsabz, Orumieh, Iran), placed on a glass slide, and stained with hematoxylin and eosin. Two images were taken per section and captured using an optical microscope Medic M-107 BN (Wincom, Changsha Hunan, China). The villus height, crypt depth, villus diameter, and villus height to crypt depth ratio were recorded.
Statistical Analysis
The statistical significance of data was evaluated by General Linear Model procedure of Statistical Analysis System (SAS Institute Inc, 2019). Differences in the mean values were considered significant at P < 0.05. Significant differences among the means were separated by Duncan's multiple range test.
RESULTS
Phytosome Structural Characterization
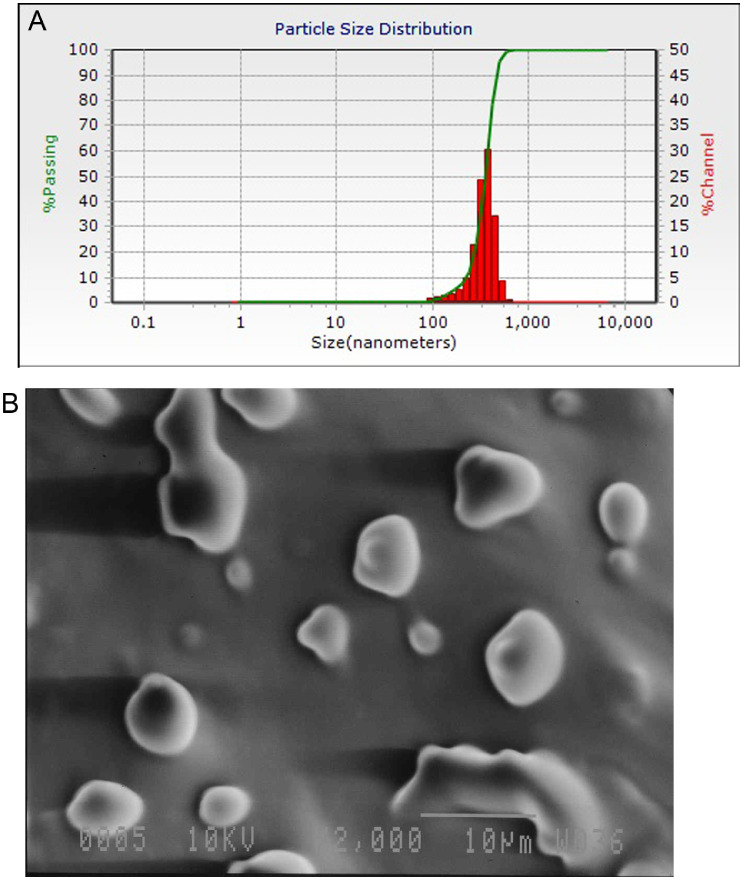
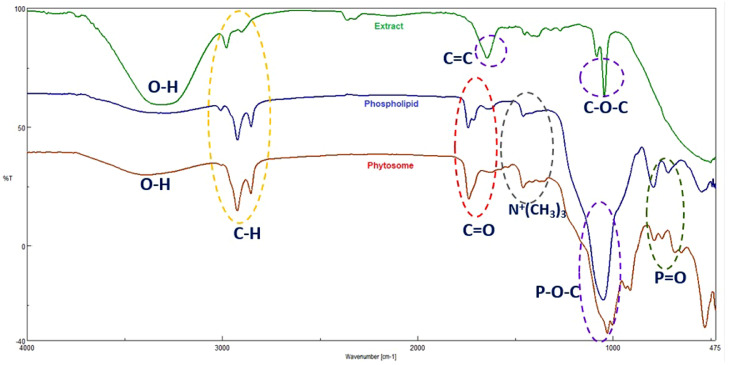
Dynamic light scattering (DLS) is a physical method to determine particle size distribution. According to the results, the average particle size and poly dispersity index (PDI) value of green tea phytosome were 344 ± 82.5 nm and 0.86 (Figure 1A), respectively. Furthermore, morphological analysis phytosome using SEM is shown in Figure 1B. IR spectroscopic analysis was performed to characterization of the phytosome and electrostatic interactions formed between extract and phospholipid (Figure 2). The FT-IR spectrum of phospholipid shows the characteristic signals at 2,922.59 cm−1 and 2,853.17 cm−1, associated with the presence of C–H stretching bonds in the long fatty acid chain. Also, the signals were observed at 1,741.41 cm−1 (C=O stretching in fatty acid ester), 1,460.81 cm−1 (–N+ (CH3)3) asymmetric bending, 1,050.05 cm−1 (P–O–C stretching), and P–O stretching in 793.56 cm−1. In the FT-IR spectrum of the green tea extract, the broad peak at 3,330 cm−1 corresponds to the free hydroxyl groups of phenolic compounds (Senthamilselvi et al., 2013). The absorption peaks located at around 2,981 cm−1 and 2,898 cm−1 are related to aromatic and aliphatic C–H stretching vibrations, respectively (Vanitha et al., 2017). The absorption peak at 1,644 cm−1 indicates the stretching vibration of C=C in aromatic rings. (Sambandam et al., 2016). The peaks appeared at 1,064 cm−1 are attributed to C–O–C stretching vibration in heterocyclic benzopyran ring (Sambandam et al., 2016). According to Figure 2 and the comparison of IR spectra of green tea extract, phospholipid and phytosome, it can be observed that stretching frequency of hydroxyl phenolic groups present in the extract spectrum is shifted from 3,330 to 3,393 cm−1 in the complex spectrum, resulting from the formation of weak intermolecular interactions during the synthesis of phytosome. Therefore, it can be concluded that the hydrogen bonds between the O–H groups of bioactive compounds and the polar groups of phospholipids has been formed. Furthermore, the FT-IR spectrum of phytosome shows the characteristic signals at 2,923, 2,854 cm−1 (C–H stretching bonds), 1,738 cm−1 (C=O stretching), and 789 cm−1 (P=O stretching) confirming the new interaction like H-bonds formed between extract and phospholipid. The area bonds of 1,237.68 to 1,335.75 show the stretching and bending resonances of the C–O–H groups of the carboxyl phenolic groups.
Figure 1.
(A) Dynamic light scattering graph, and (B) scanning electron microscope of green tea phytosome.
Figure 2.
The FT-IR spectra of green tea extract, phospholipid, and green tea phytosome.
Growth Performance
Table 2 shows the effects of salinomycin, green tea extract, and phytosome on the average BW, average FI, and average FCR of broilers challenged with the coccidiosis infection. Throughout the first 14 d of the rearing period, none of the dietary treatments had an impact on BW, FI, and FCR. Chickens fed diets containing either salinomycin or green tea were able to make up for the growth losses brought on by Eimeria and then improved BW, FI, and FCR (P < 0.01) after 7-days postinfection challenge with Eimeria protozoa on d 21, 28, 35, and 42 compared to the PC group, which had significantly worsened growth performance parameters. There were no significant differences between the Salinomycin and green tea groups in BW, FI, and FCR. Moreover, before infection challenge with Eimeria protozoa, there were no significant differences in BW and FCR among treatments containing phytosome. From d 1 to 42, the BWG in GTP300 was numerically higher than in all green tea and phytosome groups but better (P < 0.01) than the values in birds in NC and PC.
Table 2.
Growth performance of broiler chicken at 7, 14, 21, 28, 35, and 42 d of age fed different treatment diets.
| Treatment | NC | PC | SC | GTE300 | GTE400 | GTP200 | GTP300 | GTP400 | GTP500 | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preinfect: D 7 | |||||||||||
| BW | 189.6 | 186.8 | 188.6 | 196.8 | 193.8 | 192.6 | 185.8 | 196.6 | 190.8 | 1.32 | 0.451 |
| FI | 139.6 | 137.4 | 138.8 | 143.6 | 142.0 | 140.8 | 137.2 | 145.6 | 139.8 | 0.832 | 0.260 |
| FCR | 0.736 | 0.736 | 0.740 | 0.728 | 0.734 | 0.732 | 0.738 | 0.740 | 0.730 | 0.002 | 0.908 |
| Infect: D 14 | |||||||||||
| BW | 525.8 | 512.4 | 512.2 | 543.8 | 525.6 | 523.8 | 515.0 | 538.2 | 518.6 | 3.46 | 0.343 |
| FI | 537.6 | 528.0 | 527.8 | 552.8 | 540.0 | 531.2 | 528.4 | 551.4 | 534.8 | 3.07 | 0.374 |
| FCR | 1.02 | 1.03 | 1.03 | 1.01 | 1.02 | 1.01 | 1.02 | 1.02 | 1.03 | 0.002 | 0.877 |
| Postinfect: D 21 | |||||||||||
| BW | 1026.8ab | 909.2c | 1019.0ab | 1041.6ab | 1009.6b | 1047.0ab | 1044.8ab | 1057.4a | 1044.4ab | 7.40 | <0.0001 |
| FI | 1224.2a | 1179.4b | 1212.8ab | 1250.6a | 1214.0ab | 1247.4a | 1214.2a | 1254.2a | 1245.4a | 5.48 | 0.012 |
| FCR | 1.19b | 1.30a | 1.18b | 1.20b | 1.20b | 1.19b | 1.18b | 1.18b | 1.19b | 0.007 | 0.0001 |
| D 28 | |||||||||||
| BW | 1703.2a | 1475.2b | 1716.8a | 1713.0a | 1713.4a | 1728.0a | 1719.8a | 1733.4a | 1701.0a | 13.31 | <0.0001 |
| FI | 2254.2 | 2236.4 | 2234.8 | 2284.8 | 2200.0 | 2282.4 | 2179.6 | 2291.8 | 2243.6 | 11.10 | 0.219 |
| FCR | 1.32b | 1.52a | 1.30b | 1.33b | 1.28b | 1.32b | 1.26b | 1.32b | 1.31b | 0.013 | <0.001 |
| D 35 | |||||||||||
| BW | 2449.2a | 2086.4b | 2413.8a | 2483.8a | 2542.0a | 2445.0a | 2573.6a | 2492.4a | 2438.6a | 25.03 | <0.0001 |
| FI | 3512.2a | 3294.4b | 3473.0a | 3583.4a | 3612.0a | 3518.6a | 3599.2a | 3541.2a | 3536.0a | 19.08 | 0.0007 |
| FCR | 1.43b | 1.55a | 1.44b | 1.44b | 1.42b | 1.44b | 1.40b | 1.42b | 1.45b | 0.008 | 0.0013 |
| D 42 | |||||||||||
| BW | 3004.2b | 2659.6c | 3046.4ab | 3039.8ab | 3162.0ab | 3045.0ab | 3180.0a | 3079.6ab | 3019.4ab | 26.28 | <0.0001 |
| FI | 4636.8ab | 4448.4c | 4690.0ab | 4732.0ab | 4808.8a | 4694.4ab | 4764.2ab | 4675.2ab | 4713.2ab | 20.54 | 0.0008 |
| FCR | 1.54b | 1.67a | 1.53b | 1.55b | 1.51b | 1.54b | 1.49b | 1.51b | 1.56b | 0.009 | <0.0001 |
Abbreviations: BW, body weight; FCR, feed conversion ratio; FI, feed intake; GTE300 and GTE400, infected with Eimeria and treated with 300- and 400-mL green tea extracts; GTP200, GTP300, GTP400, and GTP500, infected with Eimeria and treated with 200-, 300-, 400-, and 500-mL green tea phytosomes; NC, negative control, noninfected and untreated; PC, positive control, infected with Eimeria and untreated; SC, infected with Eimeria and treated with salinomycin 12%; SEM, standard error of the mean.
Means in the same row with different superscripts differ significantly (P < 0.05, 0.01, 0.001, or 0.0001).
Carcass Characteristics
The average weights of carcass, breast, and thigh did not differ among the dietary groups (Table 3). However, a numerical difference is recorded in breast percentage between GTP300 and untreated/infected treatments. Diets containing 300, 400, and 500 mL of green tea extract (GTP300, GTP400, and GTP500) showed a reduction in abdominal fat percentage of broilers (P < 0001), GTE400 group did not significantly differ between GTP300, 400, and 500. NC and SC groups showed a higher abdominal fat rate (P < 0001). Relative liver, spleen, pancreas, and bursa weights were significantly different (P < 0.05) between NC and PC; however, no significant variations were recorded between GTE and GTP groups (P > 0.05). Regarding the pancreas and bursa of fabricius, the data showed that the SC group significantly differed from those NC and green tea groups (P < 0.01).
Table 3.
Relative weight carcass traits and organs (% dressed weight) of broiler chickens in different treatment diets.
| Treatment | NC | PC | SC | GTE300 | GTE400 | GTP200 | GTP300 | GTP400 | GTP500 | SEM | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carcass, %1 | 65.49 | 65.06 | 64.84 | 65.48 | 65.21 | 66.00 | 66.09 | 65.74 | 65.52 | 0.200 | 0.907 |
| Breast, % | 26.66 | 25.36 | 25.89 | 26.71 | 26.37 | 26.13 | 27.26 | 26.30 | 26.67 | 0.163 | 0.612 |
| Thigh, % | 18.14 | 19.10 | 18.62 | 19.22 | 18.56 | 18.92 | 18.74 | 19.04 | 18.14 | 0.199 | 0.270 |
| Gizzard, % | 1.01 | 0.835 | 0.947 | 0.878 | 0.878 | 0.922 | 0.904 | 0.970 | 1.00 | 0.017 | 0.038 |
| Liver, % | 2.24bcd | 2.53a | 2.47ab | 2.26bcd | 2.23bcd | 2.35abc | 2.20cd | 2.02d | 2.17cd | 0.029 | 0.026 |
| Spleen, % | 0.110b | 0.151a | 0.140ab | 0.114b | 0.108b | 0.115b | 0.115b | 0.123ab | 0.116b | 0.003 | <0.0001 |
| Pancreas, % | 0.189b | 0.227a | 0.224a | 0.177b | 0.183b | 0.175b | 0.175b | 0.185b | 0.191b | 0.003 | 0.013 |
| Bursa, % | 0.088b | 0.144a | 0.141a | 0.081b | 0.080b | 0.114ab | 0.086b | 0.088b | 0.118ab | 0.004 | 0.005 |
| Fat, % | 1.36a | 0.874c | 1.40a | 1.12b | 0.924bc | 1.11b | 0.892c | 0.872c | 0.868c | 0.028 | <0.0001 |
Abbreviations: GTE300 and GTE400, infected with Eimeria and treated with 300- and 400-mL green tea extract; GTP200, GTP300, GTP400, and GTP500, infected with Eimeria and treated with 200-, 300-, 400-, and 500-mL green tea phytosomes; NC, negative control, noninfected and untreated; PC, positive control, infected with Eimeria and untreated; SC, infected with Eimeria and treated with salinomycin 12%; SEM, standard error of the mean.
Means in the same row with different superscripts differ significantly (P < 0.05, 0.01, 0.001, or 0.0001).
Body components were measured as the ratio to the live weight.
Gut Histomorphology
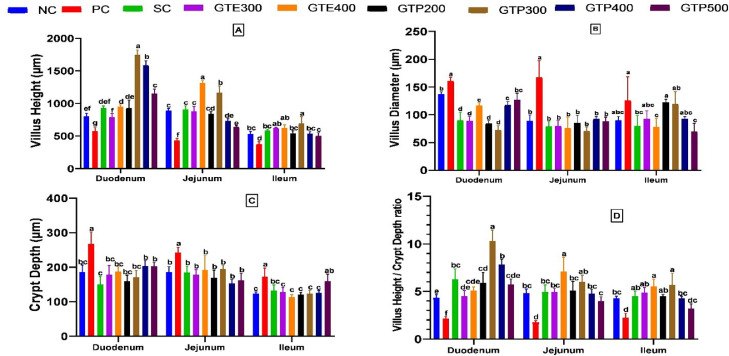
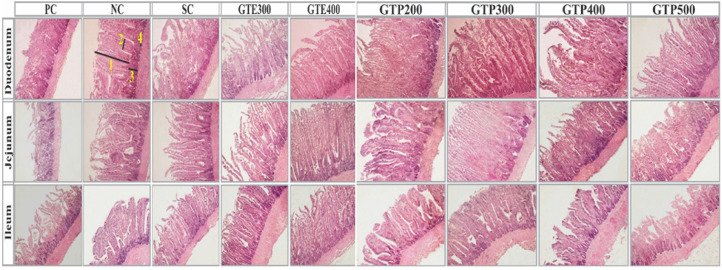
Based on the histomorphological analysis of the gut tissue samples, significant differences were evident between the experimental and control groups at villus height and diameter, crypt depth, and the ratio of villus height to crypt depth, as shown in Figures 3 and 4. In PC, coccidiosis infection substantially increased villus diameter and crypt depth while significantly decreasing villus height and the villus height/crypt depth ratio in the duodenum, jejunum, and ileum (P < 0.0001). Compared to NC treatment, duodenal villus was significantly (P < 0.0001) higher in broilers fed GTP300, GTP400, and GTP500 diets. However, NC and SC groups remained without significant difference in terms of duodenal villus height. The ileum characteristics of broiler were less affected by different treatments, and GTP300 (692 µm) showed the highest villus compared to other groups. The minimum diameter of the villus was recorded for GTP300 (72 µm) treatment. Duodenal, Jejunal, and ileal villus height to crypt depth ratios were lowest in PC (2.16) and highest in the GTP300 (10.33) group (P < 0.0001). GTP300 showed the highest duodenum, jejunum and ileum villus height compared to other groups (P < 0.0001). Villus diameter were lowest and highest in GTP300 (71 µm) and PC (167 µm) groups, respectively (P < 0.0001), compared to the NC and other treatments in the duodenum, jejunum.
Figure 3.
(A) Villus height (µm), (B) villi diameter (µm), (C) crypt depth (µm), and (D) villus height/crypt depth ratio of broiler chickens at 42 d. Abbreviations: GTE300 and GTE400, infected with Eimeria and treated with 300 and 400 mL green tea extract; GTP200, GTP300, GTP400, and GTP500, infected with Eimeria and treated with 200, 300, 400, and 500 mL green tea phytosomes; NC, negative control, noninfected, and untreated; PC, positive control, infected with Eimeria and untreated; SC, infected with Eimeria and treated with salinomycin. a–gMeans in the same row with different superscripts differ significantly (P < 0.05, 0.01, 0.001, or 0.0001).
Figure 4.
Histomorphology of jejunum, duodenum, and ileum at 42 d in broiler chickens in different treatments. Abbreviations: GTE300 and GTE400, infected with Eimeria and treated with 300- and 400-mL green tea extract; GTP200, GTP300, GTP400, and GTP500, infected with Eimeria and treated with 200-, 300-, 400-, and 500-mL green tea phytosomes; NC, negative control, noninfected and untreated; PC, positive control, infected with Eimeria and untreated; SC, infected with Eimeria and treated with salinomycin.
DISCUSSION
In this study characterization and morphology of phytosomal green tea extract including particle size, PDI, and FT-IR spectroscopy were evaluated. The particle size of phytosomes is very vital as it might bear on the stability and bioavailability of phytoconstituent encapsulated systems (Rasaie et al., 2014). The average particle size and PDI of phytosome were found to be 344 nm and 0.856, respectively. The lower PDI values demonstrate a greater homogeneity of particle size (Danaei et al., 2018). The smaller size of GTP is probably due to its physicochemical properties, solubility, polarity, and size of the green tea extract. The active compounds in green tea contain charged groups that can establish a hydrogen bond or an ionic bond with the polar head of phosphatidylcholine. Also, the hydrophobic part of the active compound can be inside the phospholipid monolayer is placed and bonded with its hydrocarbon chain. In other words, the interaction between phosphatidylcholine and green tea compounds is effective in the size of phytosome particles (Rafiee et al., 2017). Also, IR spectroscopic analysis indicates to weak intermolecular interactions that considered as a specific factor in the formation of phytosome. In the current research, broilers challenged with coccidiosis infection were fed green tea extract, either free or phytosome form, at various levels to examine the impact of this phytogenic additive on the response and growth performance of the chickens. The results of some studies showed that using a high dose of live coccidiosis vaccine will lead to problems. There is a clear correlation between the severity of the Eimeria challenge and more severe intestinal lesions (Mansoori and Modirsanei, 2012; Mohiti-Asli and Ghanaatparast-Rashti, 2015; Mehdipour et al., 2020; Teng et al., 2020; Mustafa et al., 2021; Yu et al., 2021; Jeon et al., 2022). Average chicken weight before Eimeria infection in the rearing period (1–14 d) did not significantly differ between green tea-treated as compared to other groups, which is consistent with the findings reported previously (Jang et al., 2007; Khalaji et al., 2011; Farahat et al., 2016; Hrnčár and Bujko, 2017). Green tea supplement had a little but beneficial effect on weight control, according to a meta-analysis (Hursel et al., 2009). BW and FI (as the most critical indicator of Eimeria infection) were diminished in the PC treatment in current research after Eimeria infection (12% weight loss), which confirm the results reported by other researchers (Jang et al., 2007; Herrero-Encinas et al., 2021; Qaid et al., 2021, 2022; Youssef et al., 2021; Yadav et al., 2022). Such an effect is probably results from the intestinal epithelial cells damage, which subsequently leads to a decrease in the expression of digestive enzymes, nutrient transporters and the capacity of nutrients absorption (Hansen et al., 2021). In agreement with the findings of Lee et al. (2020), results reported in the present study indicate that applying an overdose of coccidiosis vaccine significantly reduced growth performance, confirming the feasibility of the experimental coccidiosis model using vaccine strain. Based on recorded data, BW and FCR in groups treated with green tea had similar effects to Salinomycin in terms of weight loss under coccidiosis challenge conditions. Zhang et al. (2020) compared 3 level of green tea extract (40, 50, and 60 mg/kg) with Toltrazuril drug and reported no significant difference in the feed conversion ratio and mortality rate. Nutritional factors can be effective in several steps of the coccidiosis disease, including as: protection factor during the pathogenesis process, and in the recovery compensation for weight loss in the broiler (Gomez-Osorio et al., 2021). The polyphenol component of green tea plays a key role in its anticoccidial activity mechanism (Molan and Faraj, 2015; El-Shall et al., 2022). Chicken coccidiosis causes inflammation and oxidative stress in intestinal tissues (Georgieva et al., 2006); oxidative stress activates nuclear factor kappa B (NF-κB), the key regulator of inflammation. NF-κB is a protein complex that is present in almost all animal cell types and, its inactive form, is bound to inhibitory proteins in the cytosol. Polyphenols prevent NF-kB activity directly by inhibiting the phosphorylation of inhibitor of nuclear factor kappa B (IκB) and scavenging reactive oxygen species (ROS). Furthermore, polyphenols can activate transcription factors, such as peroxisome proliferator-activated receptor c (PPARc), which inhibits NF-kB activation, and by activating nuclear factor erythroid 2-related factor (Nrf2). NRf2, a redox-sensitive transcription factor, stimulates the transcription of antioxidative enzymes that help to decrease ROS levels and thus reduce inflammation (Gessner et al., 2017). Due to the association between coccidial infection and lipid peroxidation of the intestinal mucosa, green tea extract has been studied as a possible treatment for Eimeria infection (Masood et al., 2013). Similarly, it is well known that L-theanine has some biological properties including antioxidants, growth promoters, immune boosters, antistress, antimicrobials, anti-inflammatory and antianxiety properties in poultry (Saeed et al., 2020; Qui, 2022).
Different green tea coating systems can increase compound's shelf life and its bioavailability before and after consumption. The improvements in bioavailability can lead to stability in the digestive system by protecting it from digestion, exposing to reactive oxygen species and alkaline pH of the digestive tract, and increasing intestinal absorption (Yin et al., 2022). The supplementation of diet with green tea extract, both encapsulated and nonencapsulated, significantly enhanced body weight and feed efficiency compared to positive control. Mousapour et al. (2022) investigated the properties of encapsulated and nonencapsulated rosemary fed to poultry and concluded that the nonencapsulated form is more effective than the encapsulated one. Moreover, Amiri et al. (2021) showed that the nanoencapsulation of garlic essential oil had a negligible effect on growth performance. Although, nano-encapsulation increased garlic's antibacterial, antioxidant efficiency, and intestinal function. Therefore, it was recommended to investigate the influence of phytosomes on the antioxidant and antibacterial capabilities of green tea in Eimeria-infected broilers. The relative weight of the carcass, thigh, and breast did not change significantly between the treatment groups, which is similar with the findings reported by Hrnčár and Bujko (2017), Jelveh et al. (2018), Perricone et al. (2020), and Thomas et al. (2022). In addition, phytosome-treated groups demonstrated a decrease in abdominal fat, which did not agree with the results of Jelveh et al. (2018) but is consistent with those of Hrnčár and Bujko (2017) and Thomas et al. (2022). It is hypothesized that the decrease in abdomen fat may be attributable to the suppressive effects of green tea on feed intake, the promotion of catabolism in abdominal adipose tissue, and the enhancement of lipid metabolic use in skeletal muscle (Biswas and Wakita, 2001; Huang et al., 2013). In addition, there was no significant difference in the relative weight of the spleen, liver, bursa of fabricius, pancreas, and gizzard between the green tea and control groups, which is in agreement with the findings of Sarker et al. (2010) and Hrnčár and Bujko (2017), who found that green tea did not affect the weight of the pancreas, liver, and gizzard. Also, El-Deek et al. (2012) did not notice the relative weight of the pancreas, liver, spleen, or bursa of fabricius, with the exception of the gizzard, which shown a substantial reduction (3 g/kg diet) using green tea supplementation. Zhang et al. (2020) found a significant difference in the relative weight of the gizzard and spleen in the group treated with 4 to 6% green tea extract, whereas the liver weight dropped. Based on the results presented in this study, the green tea resulted in increased villus height in the duodenum, jejunum, and ileum under coccidiosis infection. Crypt depth was increased in PC compared to other groups. Deeper crypts indicate rapid tissue turnover to allow villus renewal in response to tissue shedding, inflammation, toxins from any pathogen, and high tissue demands (Yason et al., 1987). Although there was no significant difference between NC, the Salinomycin-treated group and the levels of green tea use, the numerical reduction shows that it is not necessary for villus renewal in terms of tissue shedding, inflammation, or toxins produced by the pathogenic agent. The highest villus diameter and the lowest villus height to crypt depth ratio were observed in the nontreated group with Eimeria infection, which indicates that Eimeria infection can lead to poor absorption of nutrients, epithelial inflammation, and destruction of villus epithelial cells as noted in a recent research (Chapman and Rathinam, 2022). The ratio of the villus height/crypt depth can decrease the absorption capacity of the intestine and an increase in the metabolic cost of intestinal epithelium. However, green tea decreased crypt depth and increased villus height to crypt depth ratio, indicating its positive effect on intestinal architecture. Hassanpour et al. (2010) and Hosseini et al. (2017) have also shown the positive effect of green tea on villus height. An increase in the villus height and diameter is associated with increased surface area, leading to better digestion and absorption (Murugesan et al., 2014). An increase in the villus height was observed when synthetic drugs were used, but it was not significantly different from the NC treatment. Moreover, villus height in duodenum increased when 300-, 400-, and 500-mL of green tea were added to diet, which revealed the effectiveness of phytosome compared to free green tea in maintaining intestinal health against Eimeria infection. The polyphenol epigallocatechin gallate (EGCG) is the most abundant catechin found in green tea with strong antioxidant characteristics and being able to significantly lessen the negative effects of cellular damage brought on by environmental stress (Abdel-Moneim et al., 2020). Due to its polyphenol structure, which contains several OH groups and renders, it high polar property to pass through the intestinal lipid membrane, EGCG is a hydrophilic molecule with limited absorption (Chacko et al., 2010). A phospholipid molecular structure with a water-soluble head and 2 fat-soluble tails makes up a phytosome. It possesses 2 solubilities and, as an emulsifier, may improve intestinal absorption to maximize the medicinal impact of green tea extract (Awasthi et al., 2011; Anwar and Farhana, 2018).
CONCLUSIONS
In conclusion, under the condition of present study, green tea phytosome at tested levels display significant anticoccidial characteristics and could enhance growth performance indices in coccidiosis-infected chickens. According to the findings of this study, 300 mL of green tea phytosome per kg of diet produced the strongest positive effects on chicken guts in terms of villus height and the ratio of villus height to crypt depth. Moreover phytosomal green tea extract is more effective than that green tea extract to prevent and improve of coccidiosis disease, and can protect the chicken's intestine at lower dosage. This issue may have a bright and promising future even for other plant extracts which, in spite of being useful and effective, have limits on their use in large volume. Further studies on the effects of green tea phytosome on the immune system and antioxidant performance of chickens are recommended.
ACKNOWLEDGMENTS
The authors extend their appreciation to Sepid Makian Co., Rasht, Iran. We are grateful to Neda Rahmaniyan, Elahe Rafizade, Niusha Effati, Adeleh Haghdoost, Pooya Jahandideh, and Amir Mehdizade who helped us during the farm experiment.
DISCLOSURES
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102627.
Appendix. Supplementary materials
REFERENCES
- Abbas A., Iqbal Z., Abbas R.Z., Khan M.K., Khan J.A., Hussain K., Mahmood M.S., Rizwan H.M. Immunomodulatory effects of Camellia sinensis against coccidiosis in chickens. J. Anim. Plant Sci. 2017;27:415–421. [Google Scholar]
- Abdel-Moneim A.M.E., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Swelum A.A., Arif M., Fayyaz M., Abd El-Hack M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020;104:1851–1866. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Olojede O.C. Optimizing gastrointestinal integrity in poultry: the role of nutrients and feed additives. Front. Vet. Sci. 2019;5:348. doi: 10.3389/fvets.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri N., Afsharmanesh M., Salarmoini M., Meimandipour A., Hosseini S.A., Ebrahimnejad H. Nanoencapsulation (in vitro and in vivo) as an efficient technology to boost the potential of garlic essential oil as alternatives for antibiotics in broiler nutrition. Animal. 2021;15 doi: 10.1016/j.animal.2020.100022. [DOI] [PubMed] [Google Scholar]
- Anwar E., Farhana N. Formulation and evaluation of phytosome-loaded maltodextrin-gum Arabic microsphere system for delivery of camellia sinensis extract. J. Young Pharm. 2018;10:s56–s62. [Google Scholar]
- Attree E., Sanchez-Arsuaga G., Jones M., Xia D., Marugan-Hernandez V., Blake D., Tomley F. Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agric. Biosci. 2021;2:1–16. doi: 10.1186/s43170-021-00056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen . Aviagen Limited; Newbridge Midlothian, Scotland, UK: 2018. Ross Broiler Management Handbook. [Google Scholar]
- Aviagen . Aviagen Limited; Newbridge Midlothian, Scotland, UK: 2022. Ross 308 Broiler: Nutrition Specification. [Google Scholar]
- Awasthi R., Kulkarni G.T., Pawar V.K. Phytosomes: an approach to increase the bioavailability of plant extracts. Int. J. Pharm. Pharm. 2011;3:1–3. [Google Scholar]
- Biswas M.A.H., Wakita M. Effect of Dietary Japanese green tea powder supplementation on feed utilization and carcass profiles in broilers. Poult. Sci. 2001;38:50–57. [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:1–14. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S.M., Thambi P.T., Kuttan R., Nishigaki I. Beneficial effects of green tea: a literature review. Chin. Med. 2010;5:1–9. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H.D., Rathinam T. Focused review: the role of drug combinations for the control of coccidiosis in commercially reared chickens. Int. J. Parasitol. Drugs Drug Resist. 2022;18:32–42. doi: 10.1016/j.ijpddr.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei M., Dehghankhold M., Ataei S., Davarani F.H., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Pharmaceutics impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direito R., Reis C., Roque L., Gonçalves M., Sanches-Silva A., Gaspar M.M., Pinto R., Rocha J., Sepodes B., Bronze M.R., Figueira M.E. Phytosomes with persimmon (Diospyros kaki l.) extract: preparation and preliminary demonstration of in vivo tolerability. Pharmaceutics. 2019;11:296. doi: 10.3390/pharmaceutics11060296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deek A.A., Al-Harthi M.A., Osman M., Al-Jassas F., Nassar R. Effect of different levels of green tea (Camellia sinensis) as a substitute for oxytetracycline as a growth promoter in broilers diets containing two crude protein levels | Einfluss des Zusatzes von grünem Tee (Camellia sinensis) in verschiedener Höhe zu. Arch. Fur Geflugelkd. 2012;76:88–98. [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat M., Abdallah F., Abdel-Hamid T., Hernandez-Santana A. Effect of supplementing broiler chicken diets with green tea extract on the growth performance, lipid profile, antioxidant status and immune response. Br. Poult. Sci. 2016;57:714–722. doi: 10.1080/00071668.2016.1196339. [DOI] [PubMed] [Google Scholar]
- Georgieva N.V., Koinarski V., Gadjeva V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006;172:488–492. doi: 10.1016/j.tvjl.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl). 2017;101:605–628. doi: 10.1111/jpn.12579. [DOI] [PubMed] [Google Scholar]
- Gomez-Osorio, L., J. Chaparro, S. Gutiérrez Lopez. 2021. Nutrition and poultry coccidiosis: causes, consequences and current strategies to modulate the disease. March 19th, 2021. DOI: 10.5772/intechopen.96995
- Hansen V.L., Kahl S., Proszkowiec-Weglarz M., Jiménez S.C., Vaessen S.F.C., Schreier L.L., Jenkins M.C., Russell B., Miska K.B. The effects of tributyrin supplementation on weight gain and intestinal gene expression in broiler chickens during Eimeria maxima-induced coccidiosis. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour H., Bahadoran S., Koosha S., Askari E., Homai S. Effect of diclazuril, semduramicin, salinomycin and maduramycin as preventive anticoccidial drugs on chicken intestinal morphology. Glob. Vet. 2010;5:1–5. [Google Scholar]
- Herrero-Encinas J., Menoyo D., Blanch M., Pastor J.J., Rochell S.J. Response of broiler chickens fed diets supplemented with a bioactive olive pomace extract from Olea europaea to an experimental coccidial vaccine challenge. Poult. Sci. 2021;100:575–584. doi: 10.1016/j.psj.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S., Chamani M., Seidavi A., Sadeghi A.A., Ansari-Pirsaraei Z. Efeito de Thymolina® em pó na dieta sobre as características de carcaça e morfologia do intestino delgado em frangos de corte ross 308. Acta Sci. - Anim. Sci. 2017;39:45–50. [Google Scholar]
- Hrnčár C., Bujko J. Effect of different levels of green tea (Camellia sinensis) on productive performance, carcass characteristics and organs of broiler chickens. Potravin. Slovak J. Food Sci. 2017;11:623–628. [Google Scholar]
- Huang J., Zhang Y., Zhou Y., Zhang Z., Xie Z., Zhang J., Wan X. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agric. Food Chem. 2013;61:8565–8572. doi: 10.1021/jf402004x. [DOI] [PubMed] [Google Scholar]
- Hursel, R., W. Viechtbauer, and M. Westerterp-Plantenga. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int. J. Obes. 33:956–961. [DOI] [PubMed]
- Jamil M., Aleem M.T., Shaukat A., Khan A., Mohsin M., Rehman T.U., Abbas R.Z., Saleemi M.K., Khatoon A., Babar W., Yan R., Li K. Medicinal plants as an alternative to control poultry parasitic diseases. Life. 2022;12:1–13. doi: 10.3390/life12030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.I., Jun M.H., Lillehoj H.S., Dalloul R.A., Kong I.K., Kim S., Min W. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. 2007;144:172–175. doi: 10.1016/j.vetpar.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Jelveh K., Rasouli B., Kadim I.T., Slozhenkina M.I., Gorlov I.F., Seidavi A., Phillips C.J.C. The effects of green tea in the diet of broilers challenged with coccidiosis on their performance, carcass characteristics, intestinal mucosal morphology, and blood constituents and ceca microflora. Vet. Med. Sci. 2022;8:2511–2520. doi: 10.1002/vms3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelveh K., Rasouli B., Seidavi A., Diarra S.S. Comparative effects of Chinese green tea (Camellia sinensis) extract and powder as feed supplements for broiler chickens. J. Appl. Anim. Res. 2018;46:1114–1117. [Google Scholar]
- Jeon Y.S., Kim Y.B., Lee H.G., Park J., Heo Y.J., Chu G.M., Lee K.W. Effect of dietary organic and inorganic sulfur on the performance of coccidiosis vaccine challenged broiler chickens. Animals. 2022;12:1–12. doi: 10.3390/ani12091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaji S., Zaghari M., Hatami K.H., Hedari-Dastjerdi S., Lotfi L., Nazarian H. Black cumin seeds, Artemisia leaves (Artemisia sieberi), and camellia L. plant extract as phytogenic products in broiler diets and their effects on performance, blood constituents, immunity, and cecal microbial population. Poult. Sci. 2011;90:2500–2510. doi: 10.3382/ps.2011-01393. [DOI] [PubMed] [Google Scholar]
- Lee K., Kim D.-H., Kim Y.-B., Jeong S.-B., Oh S.-T., Cho S.-Y., Lee K. Vaccine-challenged broiler chickens. Animals. 2020;10:1–13. doi: 10.3390/ani10030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lu M., Lillehoj H.S. Coccidiosis: recent progress in host immunity and alternatives to antibiotic strategies. Vaccines. 2022;10:215. doi: 10.3390/vaccines10020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Qiu Q., Luo X., Liu X., Sun J., Wang C., Lin X., Deng Y., Song Y. Phyto-phospholipid complexes (phytosomes): a novel strategy to improve the bioavailability of active constituents. Asian J. Pharm. Sci. 2019;14:265–274. doi: 10.1016/j.ajps.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoori B., Modirsanei M. Effects of dietary tannic acid and vaccination on the course of coccidiosis in experimentally challenged broiler chicken. Vet. Parasitol. 2012;187:119–122. doi: 10.1016/j.vetpar.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Masood S., Abbas R.Z., Iqbal Z., Mansoor M.K., Sindhu Z.U.D., Zia M.A., Khan J.A. Role of natural antioxidants for the control of coccidiosis in poultry. Pak. Vet. J. 2013;33:401–407. [Google Scholar]
- Mehdipour Z., Golian A., Nassiri-Moghadam H., Javadmanesh A. Effect of threonine supplementation on growth performance, metabolizable energy, morphological changes and immune response in broiler chickens challenged with coccidia. Poult. Sci. J. 2020;8:95–107. [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev. Vet. Med. 2015;120:195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Molan A., Faraj A.M. Effect of selenium-rich green tea extract on the course of sporulation of Eimeria oocysts. J. Dent. Med. Sci. 2015;14:68–74. [Google Scholar]
- Mousapour A., Salarmoini M., Afsharmanesh M., Ebrahimnejad H., Meimandipour A., Amiri N. Encapsulation of essential oils of rosemary (Rosmarinus officinalis): evaluation of in vitro antioxidant and antimicrobial properties, and effects on broiler performance. Anim. Prod. Sci. 2022;62:851–859. [Google Scholar]
- Murugesan G.R., Gabler N.K., Persia M.E. Effects of direct-fed microbial supplementation on broiler performance, intestinal nutrient transport and integrity under experimental conditions with increased microbial challenge. Br. Poult. Sci. 2014;55:89–97. doi: 10.1080/00071668.2013.865834. [DOI] [PubMed] [Google Scholar]
- Mustafa A., Bai S., Zeng Q., Ding X., Wang J., Xuan Y., Su Z., Zhang K. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express. 2021;11:1–18. doi: 10.1186/s13568-021-01299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthamilselvan T., Kuo T.F., Wu Y.C., Yang W.C. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Altern. Med. 2016;2016 doi: 10.1155/2016/2657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P., Dave V., Paliwal S., Sharma M., Potdar M.B., Tyagi A. Phytosomes—nanoarchitectures' promising clinical applications and therapeutics. Nanopharm. Adv. Deliv. Syst. 2021;2021:187–216. [Google Scholar]
- Perricone V., Comi M., Giromini C., Rebucci R., Agazzi A., Savoini G., Bontempo V. Green tea and pomegranate extract administered during critical moments of the production cycle improves blood antiradical activity and alters cecal microbial ecology of broiler chickens. Animals. 2020;10:1–13. doi: 10.3390/ani10050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaid M.M., Al-Mufarrej S.I., Azzam M.M., Al-Garadi M.A. Anticoccidial effectivity of a traditional medicinal plant, Cinnamomum verum, in broiler chickens infected with Eimeria tenella. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaid M.M., Mansour L., Al-Garadi M.A., Alqhtani A.H., Al-abdullatif A.A., Qasem M.A., Murshed M.A. Evaluation of the anticoccidial effect of traditional medicinal plants, Cinnamomum verum bark and Rumex nervosus leaves in experimentally infected broiler chickens with Eimeria tenella. Ital. J. Anim. Sci. 2022;21:408–421. [Google Scholar]
- Qui N.H. Immune-boosting role of L-theanine in broiler poultry production under stress conditions. Open Vet. J. 2022;12:250–255. doi: 10.5455/OVJ.2022.v12.i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee Z., Barzegar M., Sahari M.A., Maherani B. Nanoliposomal carriers for improvement the bioavailability of high-valued phenolic compounds of pistachio green hull extract. Food Chem. 2017;220:115–122. doi: 10.1016/j.foodchem.2016.09.207. [DOI] [PubMed] [Google Scholar]
- Rana A., Kumar S. Chemistry, pharmacology and therapeutic delivery of major tea constituents. Saneja A., Panda A.K., Lichtfouse E., editors. Chemistry, pharmacology and therapeutic delivery of major tea constituentsPages 113–129 in Sustainable Agriculture. Reviews 43: Pharmaceutical Technology for Natural Products Delivery Vol. 1 Fundamentals and Applications; Springer, Midtown Manhattan, New york City. 2020 [Google Scholar]
- Rasaie S., Ghanbarzadeh S., Mohammadi M., Hamishehkar H. Nano phytosomes of quercetin: a promising formulation for fortification of food products with antioxidants. Pharm. Sci. 2014;20:96–101. [Google Scholar]
- Saeed M., Khan M.S., Kamboh A.A., Alagawany M., Khafaga A.F., Noreldin A.E., Qumar M., Safdar M., Hussain M., Abd El-Hack M.E., Chao S. L-theanine: an astounding sui generis amino acid in poultry nutrition. Poult. Sci. 2020;99:5625–5636. doi: 10.1016/j.psj.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandam B., Thiyagarajan D., Ayyaswamy A., Raman P. Extraction and isolation of flavonoid quercetin from the leaves of Trigonella foenum-graecum and their anti-oxidant activity. Int. J. Pharm. Pharm. Sci. 2016;8:120–124. [Google Scholar]
- Sarker M., Kim G., Yang C. Effect of green tea and biotite on performance, meat quality and organ development in Ross broiler, Egypt. Poult. Sci. J. 2010;30:77–88. [Google Scholar]
- SAS Institute Inc . SAS Institute Inc.; Cary, NC: 2019. System Requirements for SAS 9.4. Foundation for Microsoft Windows for x64. [Google Scholar]
- Seidavi A., Belali M., Elghandour M.M.Y., Adegbeye M.J., Salem A.Z.M. Potential impacts of dietary inclusion of green tea (Camellia sinensis L.) in poultry feeding: a review. Agrofor. Syst. 2020;94:1161–1170. [Google Scholar]
- Senthamilselvi S., Kumar P., Prabha A.L., Govindaraju M. Green simplistic biosynthesis of anti-bacterial silver nanoparticles using Annona Squamosa leaf extract. Nano Biomed. Eng. 2013;5:102–106. [Google Scholar]
- Sonawane S.H., Bhanvase B.A., Sivakumar M., Potdar S. In: Pages 1–8 in Encapsulation of Active Molecules and Their Delivery System. Sonawane S.H., Bhanvase B.A., Sivakumar M., Potdar S., editors. Elsevier; Amesterdam, Netherland: 2020. Current overview of encapsulation. [Google Scholar]
- Soutter F., Werling D., Tomley F.M., Blake D.P. Poultry coccidiosis: design and interpretation of vaccine studies. Front. Vet. Sci. 2020;7:1–12. doi: 10.3389/fvets.2020.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Yadav S., de Castro F.L.S., Tompkins Y.H., Fuller A.L., Kim W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020;99:4203–4216. doi: 10.1016/j.psj.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D.V., Molan A.L., Singh Y., Ravindran V. Influence of green tea powder on the performance, nutrient utilisation, caecal microbiota profile and meat quality of broiler chickens. J. Appl. Anim. Nutr. 2022;10:83–90. [Google Scholar]
- Usman R.M. Phytosomes: a mini review. Biomed. J. Sci. Tech. Res. 2021;33:25507–25508. [Google Scholar]
- Vanitha V., Hemalatha S., Pushpabharathi N., Amudha P., Jayalakshmi M. Fabrication of nanoparticles using Annona squamosa leaf and assessment of its effect on liver (Hep G2) cancer cell line. IOP Conf. Ser. Mater. Sci. Eng. 2017;191 [Google Scholar]
- Wickramasuriya S.S., Park I., Lee K., Lee Y., Kim W.H., Nam H., Lillehoj H.S. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines. 2022;10:172. doi: 10.3390/vaccines10020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Teng P.Y., Singh A.K., Choi J., Kim W.K. Influence of Brassica spp. rapeseed and canola meal, and supplementation of bioactive compound (AITC) on growth performance, intestinal-permeability, oocyst shedding, lesion score, histomorphology, and gene expression of broilers challenged with E. maxima. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yason C.V., Summers B.A., Schat K.A. Pathogenesis of rotavirus infection in various age groups of chickens and turkeys: pathology. Am. J. Vet. Res. 1987;48:927–938. [PubMed] [Google Scholar]
- Yin Z., Zheng T., Ho C.T., Huang Q., Wu Q., Zhang M. Improving the stability and bioavailability of tea polyphenols by encapsulations: a review. Food Sci. Hum. Wellness. 2022;11:537–556. [Google Scholar]
- Youssef I.M.I., Abdel-Razik A.H., Aboelhadid S.M., Arafa W.M., Shany S.A., Abdel-Daim A.S.A. Comparative effects of dietary saponin and probiotic supplementation on performance, carcass traits and intestinal histomorphology of broilers challenged with Eimeria tenella. Iran. J. Appl. Anim. Sci. 2021;11:147–159. [Google Scholar]
- Yu M., Jeon J.O., Cho H.M., Hong J.S., Bin Kim Y., Nawarathne S.R., Wickramasuriya S.S., Yi Y.J., Lee H., Wan V., Ng N.K.J., Tan C.H., Heo J.M. Broiler responses to dietary 3, 4, 5-trihydroxybenzoic acid and oregano extracts under Eimeria challenge conditions. J. Anim. Sci. Technol. 2021;63:1362–1375. doi: 10.5187/jast.2021.e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Li X., Na C., Abbas A., Abbas R.Z., Zaman M.A. Anticoccidial effects of camellia sinensis (Green Tea) extract and its effect on blood and serum chemistry of broiler chickens. Pak. Vet. J. 2020;40:77–80. [Google Scholar]
- Zhu Q., Sun P., Zhang B., Kong L.L., Xiao C., Song Z. Progress on gut health maintenance and antibiotic alternatives in broiler chicken production. Front. Nutr. 2021;8:1–10. doi: 10.3389/fnut.2021.692839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.