Abstract
Background
There are gaps in our understanding of the epidemiology of atopic dermatitis (AD) in adults.
Objective
To evaluate the prevalence and severity of AD in adults from countries/regions within Asia, Eurasia, Latin America, Middle East, and Russia.
Methods
This international, web-based survey was performed in Argentina, Brazil, China, Colombia, Egypt, Hong Kong, Israel, Malaysia, Mexico, Russia, Kingdom of Saudi Arabia (KSA), Singapore, Taiwan, Thailand, Turkey, and United Arab Emirates. Questionnaires were sent to adult members of online respondent panels for determination of AD and assessment of severity. A diagnosis of AD required respondents to meet the modified United Kingdom (UK) Working Party criteria and to self-report they had a physician diagnosis of AD. Severity of AD was determined using Patient-Oriented Scoring of Atopic Dermatitis (PO-SCORAD), Patient-Oriented Eczema Measure (POEM), and Patient Global Assessment (PGA).
Results
Among respondents by country/region the prevalence of AD ranged from 3.4% in Israel to 33.7% in Thailand. The prevalence was generally higher in females versus males. Severity varied by scale, although regardless of scale the proportion of respondents with mild and moderate disease was higher than severe disease. PGA consistently resulted in the lowest proportion of severe AD (range 2.4% China – 10.8% Turkey) relative to PO-SCORAD (range 13.4% China – 41.6% KSA) and POEM (range 5.1% China – 16.6% Israel).
Conclusions
This survey highlights the importance of AD in adults, with high prevalence and high morbidity among respondents and emphasizes that AD is not just a disease of childhood—there is disease persistence and chronicity in adults.
Keywords: Atopic dermatitis, Epidemiology, POEM, PO-SCORAD, Prevalence, Severity
Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory systemic diseases requiring long-term management, such as frequent application of emollients, topical medications and a close follow-up by healthcare professionals, which may have negative consequences on the mental health of patients with AD.1 It is estimated that up to 30% of patients whose AD was diagnosed during childhood will have persistent disease throughout adulthood.2 A minority of patients will also develop AD as adults.3 A further concern is access to physicians and treatment in developing countries.4 A lower socioeconomic status may have a negative impact on access to care and treatment in developing countries. The pathophysiology of AD is complex and involves T-cell driven inflammation, epidermal dysfunction, and genetic predisposition, and AD is associated with increased risk of multiple comorbidities, including asthma, allergic rhinitis, and food allergy.5 Comorbidities also extend well beyond atopic conditions to include other skin diseases, bowel, joint, and cardiovascular abnormalities. There are important differences in the characteristics of AD among different ethnicities from various geographical regions and age groups, which may impact upon how AD is diagnosed.6 It is therefore important that we understand the geographical and age-related variations in the disease burden of AD.7
The European Community Respiratory Health Survey attempted to measure the epidemiology of AD in the adult population in 11 European countries and the United States of America by relying on the self-diagnosis of the disease by patients.8 The resulting prevalence over the 12-month period varied significantly across countries, from 0.3% in Switzerland to 6.2% in Estonia. Other sources confirm that the prevalence of AD in the adult population lies in the lower range of 1%–3%.9 The differences in the estimation of prevalence among adult patients comes from the absence of a common diagnostic tool and a further challenge is estimating the severity of AD among patients. AD in adults is also underdiagnosed by healthcare professionals and more awareness is needed among physicians to identify cases in adults.
The most common tools to assess the severity of AD include the Patient-Oriented Scoring of Atopic Dermatitis (PO-SCORAD),10 the Eczema Area and Severity Index (EASI), and the Patient-Oriented Eczema Measure (POEM).11,12 EASI and POEM are core outcomes proposed by the Harmonizing Outcome Measures for Eczema (HOME) initiative.13 These tools are frequently used in clinical trials, but rarely in epidemiological studies. Consequently, as different tools are used, it is difficult to compare studies estimating prevalence of mild AD with those estimating the prevalence of moderate or severe forms of the disease.
Our survey provides comparable data on the prevalence of AD among adult patients across a number of countries in Asia, Eurasia, Latin America, and the Middle East, based on the most extensively validated diagnostic criteria of the United Kingdom (UK) Working Party and its translation in a self-reporting tool for patients in the International Study of Asthma and Allergies in Childhood.14,15 Another aim of our survey was to provide a comparable and robust estimation of the prevalence of the severity of AD in participating countries.
Methods
Study design
This was a cross-sectional general population web-based survey carried out in Argentina, Brazil, China, Colombia, Egypt, Hong Kong, Israel, Kingdom of Saudi Arabia (KSA), Malaysia, Mexico, Russia, Singapore, Taiwan, Thailand, Turkey, and United Arab Emirates (UAE). A large and representative sample of adult residents in each country/region answered a web-based questionnaire.
The identification of the respondents suffering from AD relied on the self-reporting of the condition by the respondent, based on criteria of the UK Working Party, and on the respondent's confirmation that the diagnosis of AD had been made by a physician. Once identified as diagnosed with AD, the current disease severity of the participant was self-reported using the PO-SCORAD, the POEM, and the Patients Global Assessment (PGA).
Survey population
The survey was sent to a representative sample of the adult population in each country. The sampling plan was based on the Quota Methods of apportionment.16 Inclusion criteria included individuals aged 18–65 years, who were able to read and write the local language and were members of an online panel. For China, Turkey, and Egypt, the >55-year-old age group was removed from the final analysis as the data were deemed not to be representative of the adult population based on inconsistencies in the proportion of respondents compared with the known populations in these countries; therefore, only results from the 18–54-year-old population were analyzed for these countries.
Participant recruitment
Kantar Health (Paris, France) was responsible for the recruitment of the survey respondents, who were recruited through several online panels. Panelists who met the inclusion criteria received an email invitation from the panel to participate in the survey. Invitation emails were sent according to the recruitment quotas on gender, age group and region based on the demographic information registered in the panelists’ profile. To reduce selection bias, panelists were blinded to the research topic when invited.
The survey was compliant with the United States Health Insurance Portability and Accountability Act. Participation in the survey was voluntary and responses remained confidential. All participants provided informed consent to participate before they could access the survey portal. If potential respondents agreed to participate in the survey after reading the statement of informed consent, they selected an “I agree to participate” option in the electronic survey. No further instructions, coaching or assistance was provided to respondents in answering questions in the electronic survey.
Outcomes
The measurement of the prevalence of AD was based on the diagnostic criteria of the UK Working Party17 and the self-reporting of the receipt of a diagnosis of AD by a physician.
The UK Working Party diagnostic criteria require that the respondent have an itchy skin condition in the past 12 months and meet at least 3 of the following 5 criteria: onset of the itchy rash before 2 years of age; history of flexural involvement; history of generalized dry skin; history of asthma; visible flexural dermatitis.
These criteria were adapted from the International Study of Asthma and Allergies of Childhood (ISAAC)14 study to create a self-diagnosis tool for patients.18 The UK Working Party criteria were therefore covered by the questions from the eczema section of the ISAAC questionnaire and by 2 questions from the patient questionnaire from the UK Working Party study covering the diagnostic criteria. Among the criteria above, the visible flexural dermatitis was originally meant to be confirmed by a clinician upon examination of the patient. In our survey, it was determined by a series of questions presenting pictures that illustrated the signs of visible dermatitis.
Other survey questions included whether a respondent was seeing a physician for the management of AD and whether they had a history of atopic comorbidities: asthma, hay fever, rhinitis, food allergies, and keratoconjunctivitis.
Variables used to report severity of atopic dermatitis
Patient-Oriented Scoring of Atopic Dermatitis (PO-SCORAD)10
Respondents were asked to assess the severity of their AD over the last 3 days using the AD self-assessment scoring system, PO-SCORAD. The assessment was performed in 3 sections: first, the extent of the body area involved; second, the intensity of symptoms, where participants were asked to score six symptoms corresponding to AD (dryness, redness, swelling, oozing/scabs, scratch marks, and thickening of skin), using a 4-point visual analog scale19 (0 = absence; 3 = severe); and third, subjective symptoms of pruritus and sleep loss, where participants were asked to rate the intensity of their itching and their sleep quality using a visual analog scale19 ranging from 0 (no itching/no insomnia) to 10 (unbearable itching/total insomnia).
The PO-SCORAD severity score was determined by the following: a). The sum of the percentage corresponding to the body areas affected by AD; b). The sum of the points from the responses regarding each of the six symptoms; c). The sum of the answers to the 2 visual analog scales. The total PO-SCORAD score = A/5 + (7∗B)/2 + C. Severity groups were defined based on the total score, where respondents with a PO-SCORAD score of <25 were assigned to the mild group, 25–50 to the moderate group, and >50 to the severe group.10
Patient-Oriented Eczema Measure (POEM)
The eczema severity monitoring tool, POEM, was used to measure the frequency of seven symptoms of eczema over the last week on a 5-point scale (0 = no day; 4 = every day) (questions are listed in Supplementary Table 1). The POEM scoring algorithm consisted of summing the points of each of the seven symptoms and, based on the total score, the severity groups were defined as follows: combined score of 0–7 were assigned to the mild group, 8–16 to the moderate group, and >16 to the severe group.20
Patients Global Assessment (PGA)
Respondents self-assessed the severity of their disease using the PGA scoring system, by checking 1 answer that best described the intensity of their AD over the previous week as either mild, moderate, or severe.
Statistical analyses
Means, standard deviations, and medians were reported for continuous variables and number and proportions for categorical variables. Differences between variables were analyzed by the Z-test, and differences between continuous variables by the Student t-test. The analyses were carried out using DAISIE version 2.4.82. A weighting was applied to each country's sample so that the structure of the weighted sample matched the structure of the overall adult population. All percentages reported in this report are for the weighted sample.
Results
Survey population
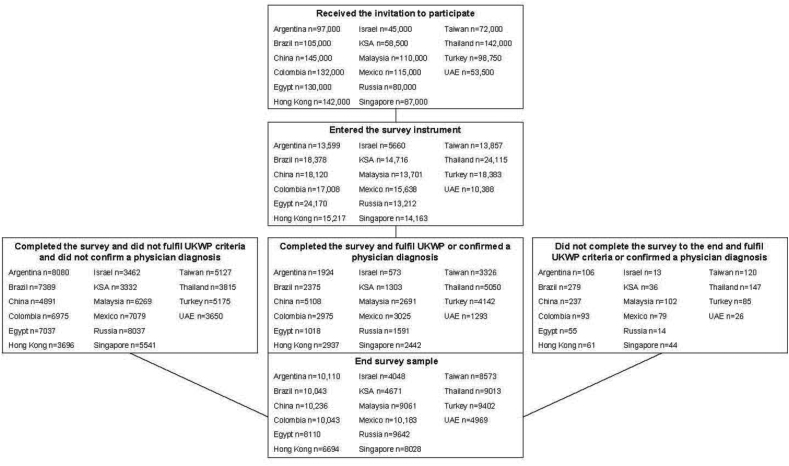
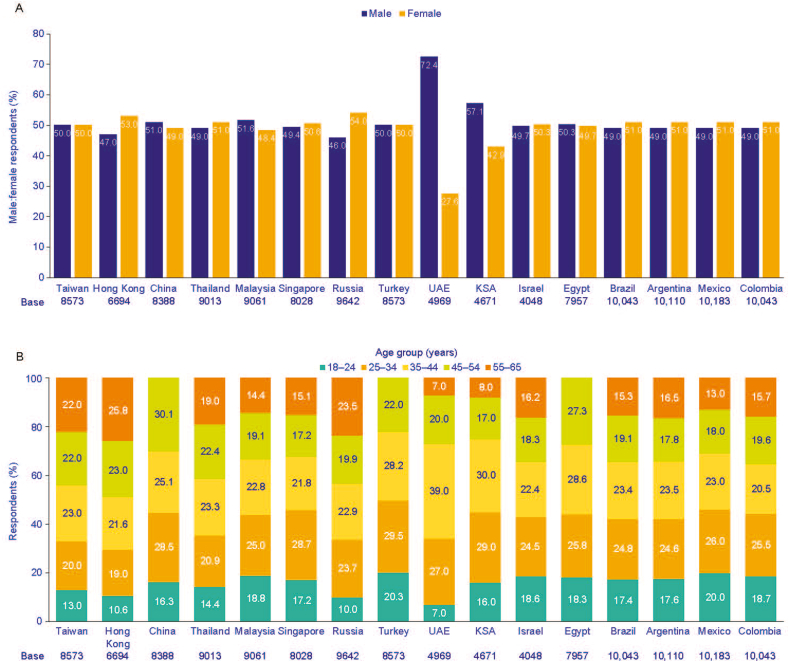
The recruitment of respondents is shown in Fig. 1. Overall, 1 612 750 adults were invited to participate in the survey, of which 250 325 (15.5%) entered the survey and 132 826 (8.2%) completed the survey. In China, Egypt, and Turkey, the >55-year-old age group was removed from the analysis as the data was deemed to be not representative of the adult population, leaving a survey population of 129 996 respondents. Response rates in the survey population ranged from 4.7% (6694/142 000) in Hong Kong to 12.1% (9642/80 000) in Russia. The gender distribution of the final sample was well balanced across most participating countries, except for the Saudi Arabia and the United Arab Emirates where there were more male respondents, and for Hong Kong and Russia where there was a slightly higher proportion of females (Fig. 2A). The age distribution of the final sample is shown in Fig. 2B.
Fig. 1.
Survey flowchart. AD, atopic dermatitis; KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates; UKWP, United Kingdom Working Party.
Fig. 2.
Gender distribution of the final sample (A), age distribution of the final sample (B). KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates.
Prevalence of atopic dermatitis
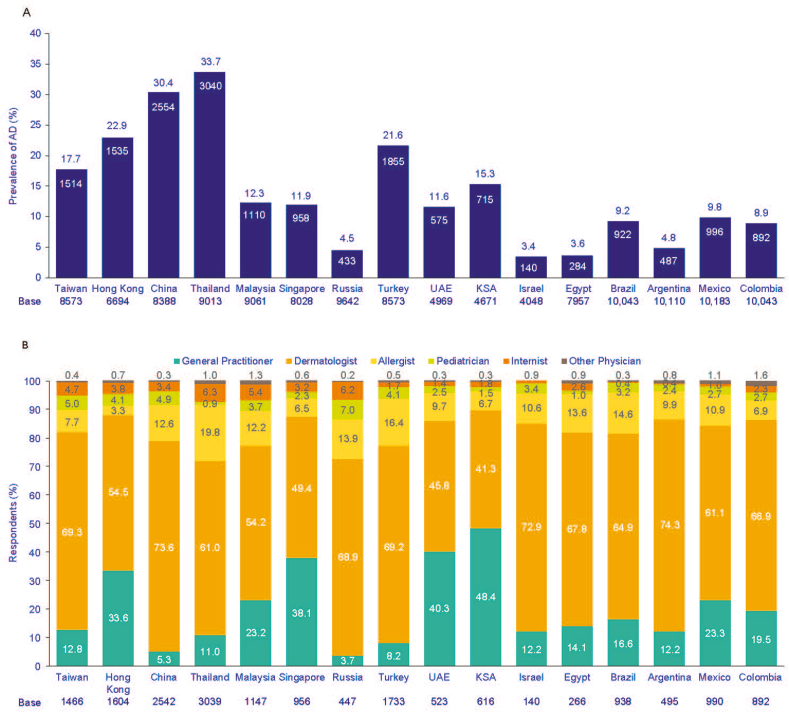
Prevalence of AD is defined as respondents who met the UK Working Party criteria and who self-reported a physician diagnosis of AD. The lowest prevalence of AD was reported in Israel (3.4%) and the highest prevalence was in Thailand (33.7%, Fig. 3A).
Fig. 3.
Prevalence of AD among adults (A), and specialists who diagnosed respondents' AD (B). In B respondents had both self-declared AD diagnosis by a physician and fulfilled the UK Working Party criteria. AD, atopic dermatitis; KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates; UK, United Kingdom.
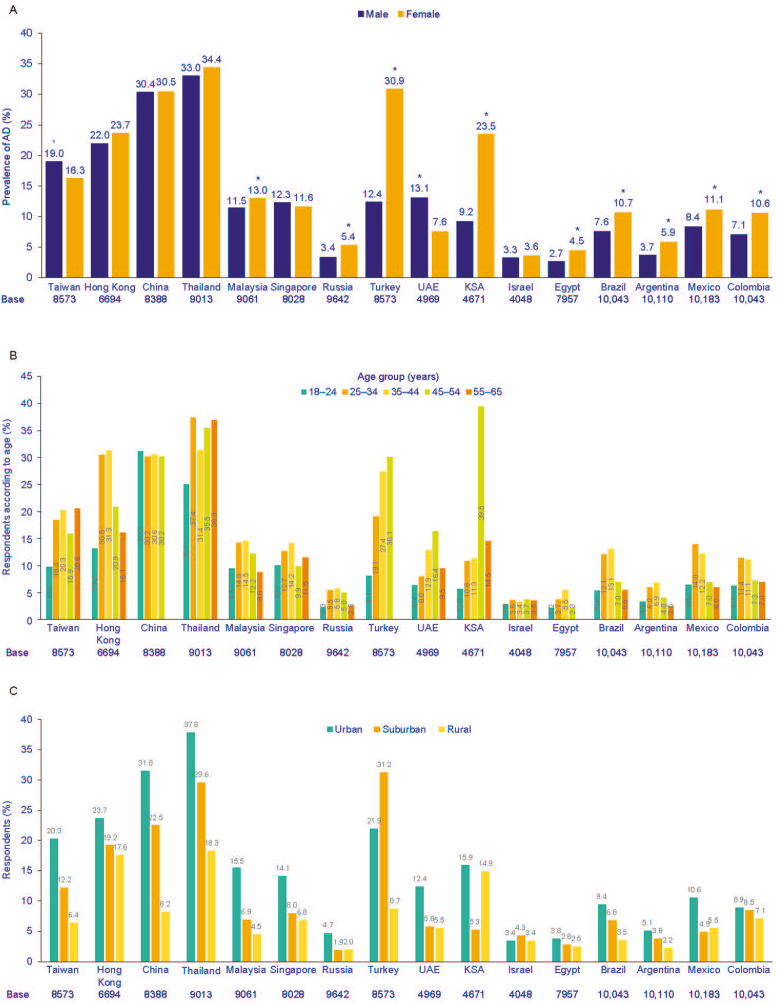
In the majority of countries, dermatologists most frequently diagnosed respondents with AD, with the exception being Saudi Arabia, where a higher proportion of general practitioners/family physicians were reported as the diagnosing specialist (Fig. 3B). The prevalence of AD was significantly higher among females in the surveyed Latin American countries, Saudi Arabia, Egypt, Malaysia, Russia, and Turkey (P < 0.05), but significantly higher in males in Taiwan and United Arab Emirates (P < 0.05), and the difference between males and females was highest in Turkey (30.9% among females vs 12.4% among males) (Fig. 4A).
Fig. 4.
Prevalence of AD across genders (A), by age group (B), by residential area (C). Asterisk indicates prevalence significantly higher between male and female (P < 0.05). AD, atopic dermatitis; KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates.
In Latin American countries, Hong Kong, Egypt, Russia, Singapore, and Malaysia, AD prevalence was highest in the 25–34 years and 35–44 years age groups (Fig. 4B). The prevalence of AD was lowest in the 55–65 years age group in Argentina (2.5%), Mexico (6.0%) and Malaysia (8.8%), and among the 18–24 years age group in Colombia, Brazil, Hong Kong, Israel, Thailand, Russia, Taiwan, Egypt, Saudi Arabia, United Arab Emirates, and Turkey. In the United Arab Emirates, Saudi Arabia, Israel, and Turkey, AD prevalence was highest for the 45–54 years age group, and in Argentina, Brazil, Egypt, Hong Kong, Malaysia, Russia and Singapore, the highest AD prevalence was observed in the 35–44 years age group.
Overall, the prevalence of AD was higher among respondents with higher education levels (Supplementary Table 2). The largest gap between education groups was observed in Turkey, where AD prevalence ranged from 8.1% among respondents with a secondary education or lower to 39.7% for respondents with postgraduate/doctoral degrees (P < 0.05).
Across most countries, the prevalence of AD was highest among urban dwellers (Fig. 4C). The highest difference was observed in China, where the prevalence of AD was 31.5% of urban respondents compared with 8.2% of respondents from rural areas (P < 0.05). In terms of regional distribution, the prevalence was higher (compared with other regions in the same countries) in Central and South China, and in Southwest China, in the Kowloon district of Hong Kong, North East region of Thailand, Southern region of Malaysia, West region of Singapore, in the Doğu Anadolu and Güneydoğu regions of Turkey, Central Federal District and Volga Federal District of Russia, in the Madinah region of Saudi Arabia, in Abu Dhabi and Un Al Quaiwain regions of United Arab Emirates, Asyut region of Egypt, Northern district of Israel, in the Distrito Federal (DF) region of Mexico, in the Southeast region of Brazil, in the Ciudad Autónoma de Buenos Aires district of Argentina, the Eastern region of Taiwan, and the Atlantic region of Colombia (Supplementary Table 3).
Collectively, AD was highest among respondents in higher income groups of most countries/regions (Supplementary Table 4). The largest difference occurred in Turkey where prevalence was 47.5% among the highest income level versus 8.5% for the lowest income level group. The prevalence of AD was highest among smokers versus non-smokers in all countries (P < 0.05, Supplementary Fig. 1). In all countries, the prevalence of AD was higher among heavy drinkers (defined as excessive alcohol consumption affecting health, relationships, and capacity to work) versus non-drinkers (P < 0.05). The highest difference was in Turkey where the prevalence of AD reached 42.1% among heavy drinkers versus 13.2% among non-drinkers (P < 0.05, Supplementary Fig. 1). Data on drinking habits were not collected from United Arab Emirates, Saudi Arabia, and Egypt due to the religious sensitivity of these countries.
Of the UK Working Party criteria, history of generalized dry skin was the most common symptom reported in the overall adult population in all countries (Supplementary Table 5), with the proportion being the highest in China (84.9%), Hong Kong (72.8%), and Thailand (72.6%).
Patterns in physician management of atopic dermatitis
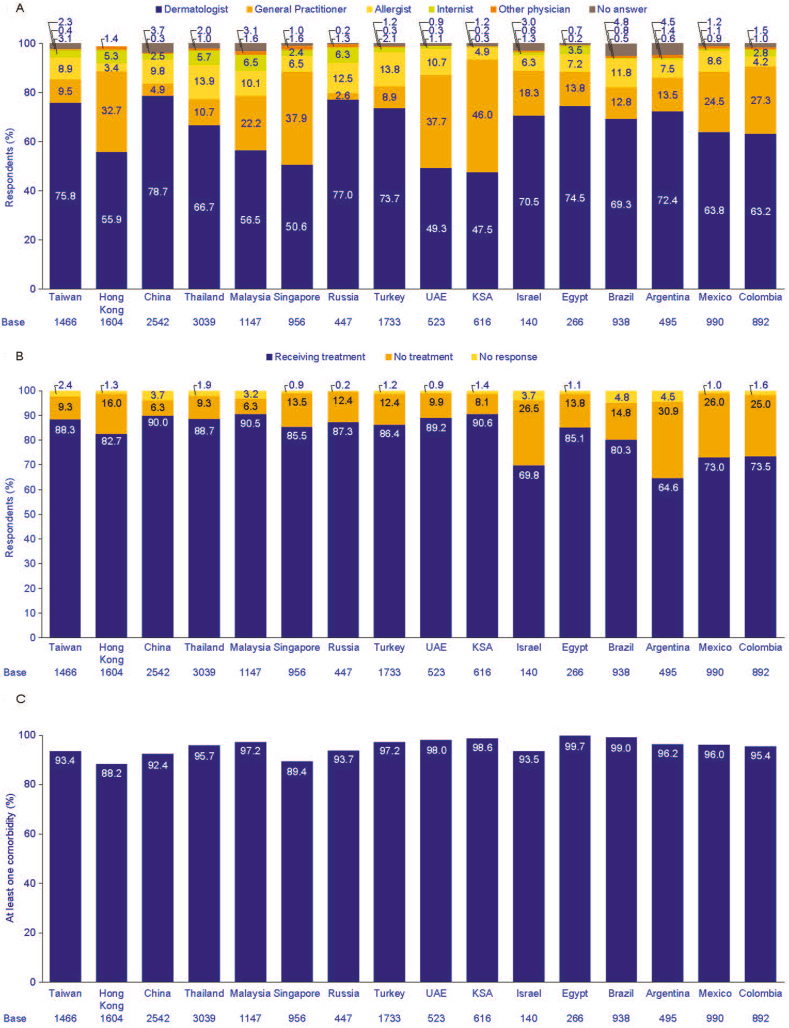
Dermatologists were the physicians who were most actively involved in the management of AD. Indeed, they are the main specialists managing AD in most of the cases: from 47.5% in Saudi Arabia to 78.7% in China (Fig. 5A), while general practitioners and allergists played a secondary role in the management of AD. Among the AD prevalent group, most respondents were receiving treatment at the time, the highest figures were observed in Saudi Arabia (90.6%), Malaysia (90.5%), and China (90.0%) respectively (Fig. 5B).
Fig. 5.
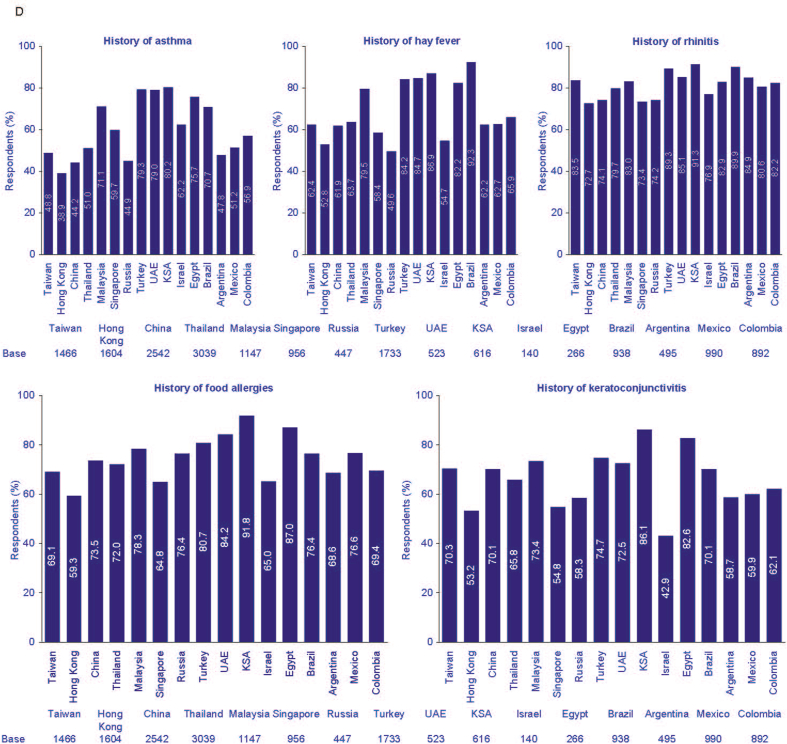
Among the patients with AD: main specialist managing AD (A), respondents currently receiving treatment (B), respondents that had at least one comorbidity (C), and specific comorbidities (D). AD, atopic dermatitis; KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates; UK, United Kingdom.
Comorbidities among respondents in the prevalence of atopic dermatitis group
Collectively, in all countries, approximately 90% of AD respondents reported at least 1 comorbidity, with the highest figure from respondents in Egypt (99.7%, Fig. 5C). Overall, rhinitis was the most common comorbidity among the AD prevalent respondents in all countries, reaching 91.3% in Saudi Arabia (Fig. 5D).
Severity of atopic dermatitis
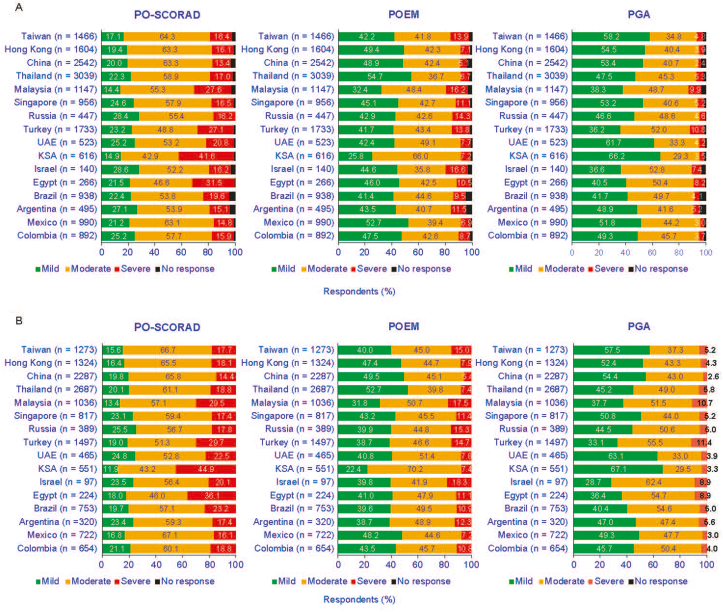
There were differences in the severity distributions according to the scale (Fig. 6A). In almost all countries, there were more severe patients identified using the PO-SCORAD and the POEM than with the PGA. This observation was most pronounced in Saudi Arabia, where, among respondents with AD, 41.6% were severe according to PO-SCORAD, 7.2% with POEM, and 3.5% with PGA. Collectively, there were slightly more severe patients according to PO-SCORAD than with POEM (except in Israel, where there were slightly more severe patients according to POEM than with the PO-SCORAD). The highest difference was also in Saudi Arabia. According to the 3 scales, while severity distributions differed between scales, within each scale severity was similar across the countries (Fig. 6A).
Fig. 6.
Severity distributions for PO-SCORAD, POEM and PGA for respondents with AD (A) and for respondents with AD receiving treatment (B). AD, atopic dermatitis; PGA, Patients Global Assessment; POEM, Patient-Oriented Eczema Measure; PO-SCORAD, Patient-Oriented Scoring of Atopic Dermatitis; KSA, Kingdom of Saudi Arabia; UAE, United Arab Emirates.
According to the PGA scale, there was a majority of mild patients in Middle East/Asian countries (up to 66.2% in Saudi Arabia and 61.7% in United Arab Emirates), except in Malaysia, where there was a majority of moderate patients according to the PGA (48.7% vs 38.3% for mild ones). According to PO-SCORAD, the majority of respondents that were receiving treatment had moderate-to-severe AD (Fig. 6B).
Discussion
This is the first study to investigate the prevalence of AD in the adult population across participating countries from Asia, Eurasia, Latin America, and the Middle East. The prevalence of AD ranged from 3.4% in Israel to 33.7% in Thailand. This compares with a prevalence of between 4.9% and 10.2% in recent United States adult studies and 4.4% in the European Union.21, 22, 23 This compares with a recent study of pediatric patients across 18 countries which reported a prevalence of AD of between 13.5% and 41.9%.24 Among most countries, more female respondents than male respondents reported having AD, which is in agreement with another survey in adults from Canada, France, Germany, Italy, Japan, Spain, United Kingdom, and United States.21
Dermatologists were the most frequent specialists who diagnosed and managed AD in all countries, except in Saudi Arabia, where general practitioners were the most common diagnosing physicians. These findings are comparable with an earlier survey, which showed that dermatologists were the main specialists involved in managing AD in France, Germany, Italy, Spain, and Japan,21 although general practitioners were the principal specialists managing AD in Canada and the United Kingdom.21
Generally, AD was highest in urban populations and lowest in rural populations, although the majority of respondents in our survey came from urban populations. Previous studies have shown that urban residency is associated with a higher prevalence of AD.25,26 Air pollution in urban environments has been reported to be a modest risk factor for the increased prevalence of AD in urban populations;27 however, the current study underrepresented the rural populations, which may be due to the lack of internet availability in some rural areas, as well as a lower level of education in some countries.
Atopic dermatitis was higher in the more highly educated respondents with AD, and the prevalence of AD was higher in those who were employed compared with those who were retired or still in education. These findings are in agreement with previous reports, which showed that higher socioeconomic status and higher level of family education were associated with an increased prevalence of AD.28, 29, 30, 31, 32, 33 Other studies have also described a positive association between household income and the prevalence of AD.29,30 The association between education, household income and prevalence of AD may be due to the higher purchasing power of this group of respondents and better access to healthcare.
In line with other studies, the prevalence of AD in the current study was higher in smokers compared with non-smokers.34, 35, 36 Furthermore, current and past cigarette smoking has previously been shown to be a risk factor for adult-onset AD.36 Our study showed that AD was higher in heavy drinkers compared with non-drinkers. Other studies have shown that alcohol intake during pregnancy significantly increased the risk of offspring developing AD in childhood compared with non-drinkers;37 however, a meta-analysis reported that there was no consistent association between maternal alcohol use and the risk of offspring developing AD in adolescents and adults.38
Across all countries, the most common UK Working Party criterion was history of generalized dry skin (range 37.9% Egypt – 84.9% China), which concurs with findings from a previous study in Europe, Japan, and North America.21 Reported comorbidities in our study were high, with on average 90.0% of respondents reporting at least 1 comorbidity, the most common being a history of rhinitis (allergic rhinitis). These 4 comorbidities were previously linked to an immunoglobulin-E mediated type 2 inflammatory response associated with the atopic march in patients with AD.39
The prevalence of rhinitis in the current study is higher than reported in other studies, which estimated the prevalence of allergic rhinitis in adults to be between 10 and 40%, worldwide;14,40, 41, 42 however, 1 study found a prevalence of acute rhinitis of 54% among 13–14-year-old adolescents in 1 region in Nigeria.43 Others have found that the occurrence of rhinitis has increased markedly in the last decade in the more affluent African countries, China, Europe, and several Middle Eastern countries.43, 44, 45, 46 Of interest, a history of food allergies ranged from 59.3% in Hong Kong to 91.8% in Saudi Arabia, which is higher than the reported prevalence of between 30% and 63% found in children with AD.47, 48, 49
Differences in the distribution of severity were observed across the scales and across countries. PGA consistently resulted in the lowest proportion of severe AD (range 2.4% China – 10.8% Turkey) relative to PO-SCORAD (range 13.4% China – 41.6% KSA) and POEM (range 5.1% China – 16.6% Israel). The modest concordance between POEM and PO-SCORAD may be attributed to these tools measuring different aspects of AD, and no single instrument fully capturing the multidimensional features of AD.50 Of interest, similar findings were reported across these scales in an earlier study from Europe, Japan, and North America.21 A large US study of 3495 adults with AD reported that 31.8% of patients had severe AD when measured by POEM, PO-SCORAD, and the numeric rating scale for itch;23 however, only 4% of patients had severe scores for all 3 assessments.
One of the limitations of the current study is that it did not determine the onset of AD in the respondents and we could not determine the proportion of respondents that developed AD in childhood compared with those who had an adult onset of AD. Also, no data were collected on the treatments that respondents were taking to alleviate their AD. The self-reported nature of the survey is also a limitation and more rigorous methods to validate comorbidities may be needed in future studies. As the AD prevalence data was higher than what was expected in some countries, it was decided to conduct a re-contact survey to understand whether this high prevalence was a result of a confusion between the words atopic dermatitis/atopic eczema and eczema in the self-reporting question of the AD diagnosis. The re-contact survey was designed to allow a sensitivity analysis on AD prevalence to be conducted. All respondents who previously reported having AD/atopic eczema were re-contacted to answer a 5-min questionnaire. Due to the low samples and the low proportion of re-contacted patients, the results of the re-contact survey were not exploitable (except for Taiwan). Despite the re-contact results, further investigation into why some countries, such as Thailand, had a higher-than-expected number of respondents with AD is warranted. The prevalence of AD estimates in our survey may be more representative of urban versus overall population in some countries where rural access to the internet may be problematic. However, the survey used a quota system to ensure that the population was representative of each country.
A further consideration is that response rates for epidemiological studies have dropped dramatically over recent decades, from rates of >90% in the 1950s to 70% or lower in recent times.51 Previously reported response rates for various web-based questionnaires/studies have ranged from 19 to 87%.52 In our study 250 325 out of 1 612 750 (15.5%) entered the survey, but only 132 826 (8.2%) completed the survey. Also, despite the caveat of a low response rate in our survey, web-based questionnaires are known to generally improve data quality, since validation checks were incorporated with prompts that alert respondents when they enter implausible or incomplete answers.53 Furthermore, respondents were blinded to the research question hence the discontinuation rate should not be due to informative bias.
In conclusion, this survey highlights the importance of AD in adults, with a high prevalence and high morbidity among respondents. Our study emphasizes that AD is not just a disease of childhood and the disease chronically persists in adults. The prevalence of comorbidities appears very high and may be due to the self-reported nature of this survey. The results from our survey investigating the prevalence of AD in the adult population in the involved countries showed that dermatologists were the specialists most frequently managing AD across most countries, and that the prevalence of AD was higher in urban respondents, in those who were more highly educated, with higher incomes, employed versus unemployed, smokers compared with non-smokers, and heavy drinkers compared with non-drinkers. Further investigation into the methodology for assessing the global epidemiology of AD, including age of onset, treatment, and more data regarding systemic comorbidities is clearly warranted.
Abbreviations
AD, atopic dermatitis; EASI, Eczema Area and Severity Index; HOME, Harmonizing Outcome Measures for Eczema; ISAAC, International Study of Asthma and Allergies of Childhood; KSA, Kingdom of Saudi Arabia; PGA, Patient Global Assessment; PO-SCORAD, Patient-Oriented Scoring of Atopic Dermatitis; POEM, Patient-Oriented Eczema Measure; UAE, United Arab Emirates; UK, United Kingdom; US, United States.
Acknowledgements
This survey was funded by Sanofi. Medical writing support was provided by John Clarke, PhD, of Elevate Medical Affairs, and funded by Sanofi.
Data availability
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access are at: https://www.vivli.org.
Author contributions
JM, MBYT, SES, and LE designed the study, analyzed and interpreted the data, and critically reviewed the manuscript. NR, ZZ, GS, JRA, MHES, AWMC, RG, IH, ST, CRP, EF, YWY, CYC, KK, OSK, AAH, and JEN analyzed and interpreted the data and critically reviewed the manuscript. LB and AT designed the study, contributed to data acquisition, analyzed and interpreted the data, and critically reviewed the manuscript. All authors read and approved the final manuscript, and are accountable for the accuracy and integrity of the data.
Ethics statement
The data were collected according to the ESOMAR and EphMRA ethical codes of conduct that guarantee the anonymity of the respondents and of the responses that they give. No identifying patient information is being collected in the web-based study instrument. Therefore, the working data files contain no identifying information apart from the panel ID number. That is, no names, addresses, or other distinguishing information. This study was compliant with HIPAA regulations. No data were obtained from any source other than directly from the patient themselves. Patients had the right to provide any personal information about themselves or their health that they choose. Patient participation in the study was completely voluntary, as noted in the informed consent statement. Patients chose to provide information about themselves and their health or not.
Submission declaration
We confirm that this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server. All authors consent to publication.
Declaration of competing interest
Jorge Maspero is an advisory board member and/or an investigator for Sanofi, AstraZeneca, Novartis, Immunotek, Uriach, GSK, Menarini and MSD; Norma De Paula Motta Rubini has received fees from Sanofi and is a past president and current director for the Brazilian society of Allergy and Immunology; Jianzhong Zhang is a consultant for Novartis, Pfizer, Sanofi, LEO Pharma, Lilly, GSK, Kyowa Kirin, Xian Janssen, Guanhao Biotech, Zelgen, and Conmed Biosciences and a speaker for Novartis, Pfizer, Sanofi, LEO Pharma, Xian Janssen, Guanhao Biotech, and Menarini; Gloria Sanclemente has received sponsorship from AbbVie and Sanofi Laboratories to attend scientific events related to the biological treatment of atopic dermatitis; Julio Roberto Amador has received honoraria and support for attending meetings from Sanofi; Mahira Hamdy El Sayed is a speaker and advisory board member of Novartis, Jansen, AbbVie, Pfizer, LEO, Sanofi, Amgen; Alson Chan Wai Ming Member of Sanofi Type 2 Inflammatory Diseases Steering Committee; Roni P Dodiuk-Gad is a consultant and advisory board member and has received honoraria from Janssen, Novartis, AbbVie, Pfizer and Sanofi. He has received research fees from Sanofi and travel fees from Novartis, AbbVie, and Sanofi; Issam Hamadah is on the advisory board and/or sponsored lectures: Abbvie, Amgen, Boehringer Ingelheim, GSK, Hikma, Janssen, Newbridge, Novartis, Pfizer, Sanofi.
Suganthi Thevarajah has nothing to disclose; Catalina Rincón-Perez has received honoraria and support for attending meetings from Sanofi; Elena Fedenko is a speaker for Novartis, Pfizer, Sanofi, LEO Pharma, Lilly, AbbVie, Viatris/Mylan Pharma LLC, HVD, Berlin Chemie/Menarini; Yik Weng Yew has received honoraria from AbbVie, Janssen, and Sanofi and support for attending meetings from Sanofi; Mark Tang has received speaking honoraria and participated in advisory boards for LEO Pharma, Menarini, Sanofi, Regeneron, Galderma, Bioderma, Hyphens, Kao, and GSK; Chia-Yu Chu is an investigator for AbbVie, Dermira, Lilly, Novartis, Oneness Biotech, Pfizer, Regeneron, Roche, Sanofi, and United BioPharma Inc.; a consultant for AbbVie, Lilly, Novartis, Pfizer, Roche, Sanofi, and United BioPharma Inc.; a speaker for AbbVie, Lilly, Mylan, Novartis, Pfizer, Roche, and Sanofi; and an advisory board member for Mylan, Pfizer, Roche, and Sanofi; Kanokvalai Kulthanan received institutional research support from Sanofi; Ozlem Su Kucuk Ozlem Su Kucuk has received speaking honoraria and participated in advisory boards for Sanofi; Anwar Al-Hammadi is a speaker and consultant for AbbVie, Galderma, Jansen, Lilly, Novartis, Pfizer, Sanofi; Lysel Brignoli employee of Kantar Health, a company that received funding from Sanofi to conduct the survey; Angelina Tsankova employee of Kantar Health, a company that received funding from Sanofi to conduct the survey; Sarah El-Samad is an employee and stockholder of Sanofi; Jose Eduardo Neves is an employee and stockholder of Sanofi.
Laurent Eckert is an employee and stockholder of Sanofi.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100724.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kiebert G., Sorensen S.V., Revicki D., et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151–158. doi: 10.1046/j.1365-4362.2002.01436.x. [DOI] [PubMed] [Google Scholar]
- 2.Abuabara K., Yu A.M., Okhovat J.P., Allen I.E., Langan S.M. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy. 2018;73:696–704. doi: 10.1111/all.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Napolitano M., Megna M., Patruno C., Gisondi P., Ayala F., Balato N. Adult atopic dermatitis: a review. G Ital Dermatol Venereol. 2016;151:403–411. [PubMed] [Google Scholar]
- 4.Lopez Carrera Y.I., Al Hammadi A., Huang Y.H., Llamado L.J., Mahgoub E., Tallman A.M. Epidemiology, diagnosis, and treatment of atopic dermatitis in the developing countries of Asia, Africa, Latin America, and the Middle East: a review. Dermatol Ther. 2019;9:685–705. doi: 10.1007/s13555-019-00332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langan S.M., Irvine A.D., Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–360. doi: 10.1016/S0140-6736(20)31286-1. [DOI] [PubMed] [Google Scholar]
- 6.Yew Y.W., Thyssen J.P., Silverberg J.I. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80:390–401. doi: 10.1016/j.jaad.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Laughter M.R., Maymone M.B.C., Mashayekhi S., et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. 2021;184:304–309. doi: 10.1111/bjd.19580. [DOI] [PubMed] [Google Scholar]
- 8.Harrop J., Chinn S., Verlato G., et al. Eczema, atopy and allergen exposure in adults: a population-based study. Clin Exp Allergy. 2007;37:526–535. doi: 10.1111/j.1365-2222.2007.02679.x. [DOI] [PubMed] [Google Scholar]
- 9.Larssen F., Hanifin J. Epidemiology of atopic dermatitis. Immunol Allergy Clin. 2002;22:1–25. [Google Scholar]
- 10.Stalder J.F., Barbarot S., Wollenberg A., et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy. 2011;66:1114–1121. doi: 10.1111/j.1398-9995.2011.02577.x. [DOI] [PubMed] [Google Scholar]
- 11.Scottish Intercollegiate Guidelines Network . 2011. Management of Atopic Eczema in Primary Care: A National Clinical Guideline. [Google Scholar]
- 12.Smidesang I., Saunes M., Storro O., et al. Atopic dermatitis among 2-year olds; high prevalence, but predominantly mild disease--the PACT study, Norway. Pediatr Dermatol. 2008;25:13–18. doi: 10.1111/j.1525-1470.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers J.R., Simpson E., Apfelbacher C.J., et al. Report from the fourth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative) Br J Dermatol. 2016;175:69–79. doi: 10.1111/bjd.14773. [DOI] [PubMed] [Google Scholar]
- 14.Asher M.I., Montefort S., Bjorksten B., et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 15.Asher M.I., Keil U., Anderson H.R., et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 16.Deville J.C. A theory of quota surveys. Surv Methodol. 1991;17:163–181. [Google Scholar]
- 17.Williams H.C., Burney P.G., Pembroke A.C., Hay R.J. The U.K. Working Party's diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406–416. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 18.Suarez-Varela M.M., Alvarez L.G., Kogan M.D., et al. Diet and prevalence of atopic eczema in 6 to 7-year-old schoolchildren in Spain: ISAAC phase III. J Investig Allergol Clin Immunol. 2010;20:469–475. [PubMed] [Google Scholar]
- 19.Blakeway H., Van-de-Velde V., Allen V.B., et al. What is the evidence for interactions between filaggrin null mutations and environmental exposures in the aetiology of atopic dermatitis? A systematic review. Br J Dermatol. 2020;183:443–451. doi: 10.1111/bjd.18778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charman C.R., Venn A.J., Ravenscroft J.C., Williams H.C. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol. 2013;169:1326–1332. doi: 10.1111/bjd.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbarot S., Auziere S., Gadkari A., et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 22.Silverberg J.I. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283–289. doi: 10.1016/j.det.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg J.I., Gelfand J.M., Margolis D.J., et al. Atopic dermatitis in US adults: from population to health care utilization. J Allergy Clin Immunol Pract. 2019;7:1524–1532 e1522. doi: 10.1016/j.jaip.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg J.I., Barbarot S., Gadkari A., et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417–428 e412. doi: 10.1016/j.anai.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.S., Kim J.M., Seok J., Kim B.J. Correlation between socio-economic status and atopic dermatitis in Korean adults: the Korea national health and nutrition examination survey (2007-2014) J Eur Acad Dermatol Venereol. 2017;31:1509–1515. doi: 10.1111/jdv.14343. [DOI] [PubMed] [Google Scholar]
- 26.DaVeiga S.P. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc. 2012;33:227–234. doi: 10.2500/aap.2012.33.3569. [DOI] [PubMed] [Google Scholar]
- 27.Tang K.T., Ku K.C., Chen D.Y., Lin C.H., Tsuang B.J., Chen Y.H. Adult atopic dermatitis and exposure to air pollutants-a nationwide population-based study. Ann Allergy Asthma Immunol. 2017;118:351–355. doi: 10.1016/j.anai.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Chung J., Simpson E.L. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122:360–366. doi: 10.1016/j.anai.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 29.Silverberg J.I., Hanifin J.M. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132–1138. doi: 10.1016/j.jaci.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Torfi Y., Bitarafan N., Rajabi M. Impact of socioeconomic and environmental factors on atopic eczema and allergic rhinitis: a cross sectional study. EXCLI J. 2015;14:1040–1048. doi: 10.17179/excli2015-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K.S., Rha Y.H., Oh I.H., Choi Y.S., Choi S.H. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: a cross-sectional study. BMC Pediatr. 2016;16:19. doi: 10.1186/s12887-016-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters A.S., Kellberger J., Vogelberg C., et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126:590–595. doi: 10.1016/j.jaci.2010.06.020. e591-593. [DOI] [PubMed] [Google Scholar]
- 33.Shaw T.E., Currie G.P., Koudelka C.W., Simpson E.L. Eczema prevalence in the United States: data from the 2003 national survey of children's health. J Invest Dermatol. 2011;131:67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantor R., Kim A., Thyssen J.P., Silverberg J.I. Association of atopic dermatitis with smoking: a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:1119–1125. doi: 10.1016/j.jaad.2016.07.017. e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S.Y., Sim S., Choi H.G. Atopic dermatitis is associated with active and passive cigarette smoking in adolescents. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee C.H., Chuang H.Y., Hong C.H., et al. Lifetime exposure to cigarette smoking and the development of adult-onset atopic dermatitis. Br J Dermatol. 2011;164:483–489. doi: 10.1111/j.1365-2133.2010.10116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carson C.G., Halkjaer L.B., Jensen S.M., Bisgaard H. Alcohol intake in pregnancy increases the child's risk of atopic dermatitis. the COPSAC prospective birth cohort study of a high risk population. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halling-Overgaard A.S., Hamann C.R., Holm R.P., et al. Atopic dermatitis and alcohol use - a meta-analysis and systematic review. J Eur Acad Dermatol Venereol. 2018;32:1238–1245. doi: 10.1111/jdv.14814. [DOI] [PubMed] [Google Scholar]
- 39.Hill D.A., Spergel J.M. The atopic march: critical evidence and clinical relevance. Ann Allergy Asthma Immunol. 2018;120:131–137. doi: 10.1016/j.anai.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan R.A., Meltzer E.O., Derebery J., et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29:600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]
- 41.Bauchau V., Durham S.R. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24:758–764. doi: 10.1183/09031936.04.00013904. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer E.O., Blaiss M.S., Naclerio R.M., et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012;33:113–141. doi: 10.2500/aap.2012.33.3603. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 43.Katelaris C.H., Lee B.W., Potter P.C., et al. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin Exp Allergy. 2012;42:186–207. doi: 10.1111/j.1365-2222.2011.03891.x. [DOI] [PubMed] [Google Scholar]
- 44.Patil V.K., Kurukulaaratchy R.J., Venter C., et al. Changing prevalence of wheeze, rhinitis and allergic sensitisation in late childhood: findings from 2 Isle of Wight birth cohorts 12 years apart. Clin Exp Allergy. 2015;45:1430–1438. doi: 10.1111/cea.12534. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2019;11:156–169. doi: 10.4168/aair.2019.11.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X.D., Zheng M., Lou H.F., et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson H.A. The immunopathogenic role of food hypersensitivity in atopic dermatitis. Acta Derm Venereol Suppl. 1992;176:34–37. [PubMed] [Google Scholar]
- 48.Eigenmann P.A., Sicherer S.H., Borkowski T.A., Cohen B.A., Sampson H.A. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 49.Bergmann M.M., Caubet J.C., Boguniewicz M., Eigenmann P.A. Evaluation of food allergy in patients with atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1:22–28. doi: 10.1016/j.jaip.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Silverberg J.I., Gelfand J.M., Margolis D.J., et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018;121:464–468 e463. doi: 10.1016/j.anai.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Galea S., Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Kongsved S.M., Basnov M., Holm-Christensen K., Hjollund N.H. Response rate and completeness of questionnaires: a randomized study of internet versus paper-and-pencil versions. J Med Internet Res. 2007;9:e25. doi: 10.2196/jmir.9.3.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Gelder M.M., Bretveld R.W., Roeleveld N. Web-based questionnaires: the future in epidemiology? Am J Epidemiol. 2010;172:1292–1298. doi: 10.1093/aje/kwq291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report forms, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access are at: https://www.vivli.org.