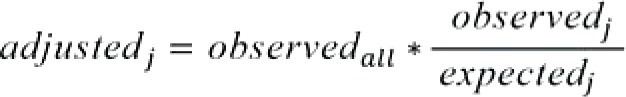Abstract
Background
In this observational study, patient-reported outcomes and short-term clinical outcome parameters in patients with colorectal cancer were studied 12 months after the start of treatment. Outcomes were also compared across German Certified Colorectal Cancer Centers.
Methods
Data were collected from 4239 patients with colorectal cancer who had undergone elective tumor resection in one of 102 colorectal cancer centers and had responded to a quality-of-life questionnaire before treatment (EORTC QLQ-C30 and -CR29). 3142 (74.1%) of these patients completed a post-treatment questionnaire 12 months later. Correlation analyses were calculated and case-mix adjusted comparisons across centers were made for selected patient-reported outcomes, anastomotic insufficiency, and 30-day mortality.
Results
At 12 months, mild improvements were seen in mean quality-of-life scores (66 vs. 62 points), constipation (16 vs. 19), and abdominal pain (15 vs. 17). Worsening was seen in physical function (75 vs. 82) and pain (22 vs. 19). Better patient-reported outcomes at 12 months were associated with better scores before treatment. Better results in at least three of the five scores were associated with male sex, higher educational level, higher age, and private health insurance. Major worsening of fecal incontinence was seen among patients with rectal cancer without a stoma. The largest differences across centers were found with respect to physical function. Anastomotic insufficiency was found in 4.3% of colon cancer patients and 8.2% of rectal cancer patients. 1.9% of patients died within 30 days after their resection.
Conclusion
Clinicians can use these findings to identify patients at higher risk for poorer patient-reported outcomes. The differences among cancer centers that were found imply that measures for quality improvement would be desirable.
With around 60 000 new cases each year, colorectal cancer is the third most commonly occurring malignancy among men in Germany and the second-ranking form of cancer in women (1). Apart from survival, the essential goal of the German clinical practice guideline is minimization of symptoms and functional impairments ([2], e.g., the recommendations in section 7.5 ff.). Patient-reported outcomes (PRO) are eminently suitable for the documentation of symptoms and functional impairments (3, 4). PRO measure “patients’ perceptions of their health status, clinical outcomes, mobility and quality of life” (5, page 25). An example of a PRO is fecal incontinence, which is an item in the questionnaire EORTC QLQ-CR29—also used in this study—with the single question “During the past week: Have you had leakage of stools from your back passage/stoma bag?” and the possible responses “Not at all,” “A little,” “Quite a bit,” and “Very much” (7). Randomized studies have shown that PRO are beneficial for treatment planning or monitoring of patients with cancer: better functional outcomes and longer survival were found after intensified PRO monitoring (8– 10), and amelioration of impaired quality of life resulted from a tailored interdisciplinary PRO intervention (4, 11).
Despite being intended when PRO were first developed and recommended by many professional groups (e.g., 13, 14), to date PRO have not been used extensively for assessment of outcome quality or comparison of treating facilities. The symptoms and functional impairments that occur during treatment are so significant, however, that PRO should be viewed as an important patient-centered complement to quality assurance and quality development procedures (16). There are already a number of promising initiatives in other countries: In the USA, for instance, standardized documentation of PRO in physiotherapy offices enables cross-office comparisons (17). In the UK, the National Health Service (NHS) has the National Prostate Cancer Audit (18). Examples in Germany are the QS-Reha project (19) and the PCO study in prostate cancer (20). Overall, there is still too little emphasis on PRO in German quality research (13).
This article presents results from the EDIUM Study (“Ergebnisqualität bei Darmkrebs: Identifikation von Unterschieden und Maßnahmen zur flächendeckenden Qualitätsentwicklung”—“Outcome in Colorectal Cancer: Identification of Differences and Measures for Nationwide Quality Development”), in which PRO and short-term clinical outcome quality parameters from over 100 Certified Colorectal Cancer Centers are compared. The study was funded by the Innovation Committee of the Federal Joint Committee (G-BA) from July 2018 to December 2021 (grant number VSF1_2017–169). To begin with, associations between patient and center characteristics and selected dimensions of EORTC QLQ-C30 and EORTC QLQ-CR29 (7, 21) are described. This is followed by case-mix-adjusted comparisons among centers based on these analyses. Furthermore, findings regarding the short-term clinical parameters anastomotic insufficiency and 30-day mortality are presented.
Method
Patient cohort and data acquisition
Each year, almost half of the patients with newly diagnosed colorectal cancer in Germany are treated in centers certified by the German Cancer Society (22). From these centers we drew a stratified random sample with a move-up procedure. At the 106 centers that initially took part, all primary colorectal cancer patients scheduled for elective tumor resection or nonsurgical palliative care in 2019 were invited to join the study before the intervention; in 6 centers, recruitment started as a pilot phase in October 2018. The analysis presented here is limited to the patients who underwent elective tumor resection, with or without (neo)adjuvant therapy. Emergency patients were excluded because they could not complete the pre-treatment questionnaire. The patients who received nonsurgical palliative care were analyzed separately and the findings were communicated to the funding body (the G-BA) in a publicly available results report. Before the commencement of treatment (tumor resection or neoadjuvant therapy), the participants completed the baseline questionnaire (T0; EORTC QLQ and sociodemographic data) either on paper or online, according to preference. However, not all centers offered the web-based option. Completion of the post-treatment questionnaire (T1; EORTC-QLQ) followed 12 months after tumor resection. Furthermore, as stipulated in the catalogue of requirements for certification the centers documented characteristics of the clinical findings and treatment, which were then linked with survey data by means of the software OncoBox at each center. The individual center data sets were sent to the certification institute OnkoZert for quality assurance and to the principal investigator for analysis. The study protocol (DRKS00008724) stipulates the EORTC dimensions at 12 months as primary endpoints and anastomotic insufficiency (measured separately for cancer of the colon and cancer of the rectum) and 30-day mortality as secondary endpoints. For reasons of space, this article reports on only a selection of EORTC dimensions together with anastomotic insufficiency and 30-day mortality. In line with the protocol, the remaining endpoints are presented in the publicly available results report.
Variables and statistical analyses
First, five dimensions relevant to colon cancer and rectal cancer (“global health status/QoL,” “physical function,” “pain,” “constipation,” “abdominal pain”) from both EORTC QLQ-C30 and EORTC QLQ-CR29 were investigated. Based on a survey of the participating centers, the scores were then selected according to the relevance of the dimensions for treatment planning (23). Scoring proceeded in line with the manuals, with scores from 0 to 100 possible. Cut-off points for clinical relevance are available for the three C30 dimensions “physical function” (cut-off: 83), “pain” (cut-off: 25), and “constipation” (cut-off: 50) (24). On functional scales (“global health status/QoL,” “physical function”) higher scores indicate better function, but on symptom scales (“pain,” “constipation,” “abdominal pain”) higher scores mean more severe symptoms. Because of the importance of incontinence particularly in rectal cancer (23), changes in “fecal incontinence” from T0 to T1 were also taken into consideration. In contrast to the procedure for the other five scores, owing to the peculiarities of the score this was accomplished purely by means of contingency tables according to site (colon/rectum) and presence or absence of stoma (eMethods). The secondary endpoints anastomotic insufficiency (in patients with an anastomosis) and 30-day mortality after resection were documented as stipulated in the requirements for certification. The covariates used for the association analyses and case-mix adjustment are listed in the eMethods and described, together with others, in Table 1 and Table 2.
The primary correlation analyses for the five EORTC scores specified above ensued, in line with the procedure for case-mix adjustment, as a series of multivariable linear regressions, presented in the form of forest plots (eTable 1). Case-mix adjustment was performed according to the NHS procedure (25). For each score, only those centers were included which contributed post-treatment questionnaires from at least ten patients. Graphically the adjusted values were provisionally set in relation to minimally important differences (MID) (26– 28). An MID is the smallest change in a score that is perceived as important by the patient. Details of the statistical procedure for the primary and secondary endpoints and of further analyses (mixed and tobit models) can be found in the eMethods.
Results
Sample
Between October 2018 and December 2019, 4239 patients from 102 centers completed the baseline questionnaire prior to tumor resection (table 1). Just short of 7% of the participants completed the questionnaire online, while the rest used the paper version. The median response rate of the centers in the study as a whole was 51%. The association analyses were based on the 3142 patients (74.1%) who took part in the post-treatment survey. The drop-out rate was 25.9%. Around a third of the drop-outs were accounted for by documented deaths, while the reasons for the remaining cases are unknown (Table 1, eFigure 1).
Table 1. Characteristics of the sample.
| Colon (n = 2859) | Rectum (n = 1380) | Total (N = 4239) | |
| Age | |||
| Mean (SD) | 70.8 (11.6) | 67.7 (11.2) | 69.8 (11.5) |
| Median (IQR) | 73 (63–79) | 68 (60–77) | 71 (62–79) |
| Age (grouped) | |||
| ≤ 39 | 35 (1.2%) | 9 (0.7%) | 44 (1.0%) |
| 40–49 | 94 (3.3%) | 59 (4.3%) | 153 (3.6%) |
| 50–59 | 357 (12.5%) | 275 (19.9%) | 632 (14.9%) |
| 60–69 | 686 (24.0%) | 413 (29.9%) | 1099 (25.9%) |
| 70–79 | 983 (34.4%) | 385 (27.9%) | 1368 (32.3%) |
| ≥ 80 | 704 (24.6%) | 239 (17.3%) | 943 (22.2%) |
| Sex | |||
| Male | 1580 (55.3%) | 891 (64.6%) | 2471 (58.3%) |
| Female | 1279 (44.7%) | 489 (35.4%) | 1768 (41.7%) |
| ASA score | |||
| ASA 1 | 113 (4.0%) | 57 (4.1%) | 170 (4.0%) |
| ASA 2 | 893 (31.2%) | 439 (31.8%) | 1332 (31.4%) |
| ASA 3 | 707 (24.7%) | 331 (24.0%) | 1038 (24.5%) |
| ASA 4 | 43 (1.5%) | 10 (0.7%) | 53 (1.3%) |
| ASA 5 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Missing | 1103 (38.6%) | 543 (39.3%) | 1646 (38.8%) |
| Nationality | |||
| German | 2729 (95.5%) | 1321 (95.7%) | 4050 (95.5%) |
| Other | 112 (3.9%) | 43 (3.1%) | 155 (3.7%) |
| Missing | 18 (0.6%) | 16 (1.2%) | 34 (0.8%) |
| Health insurance | |||
| Statutory | 2449 (85.7%) | 1171 (84.9%) | 3620 (85.4%) |
| Private | 367 (12.8%) | 183 (13.3%) | 550 (13.0%) |
| Other/not insured | 20 (0.7%) | 10 (0.7%) | 30 (0.7%) |
| Missing | 23 (0.8%) | 16 (1.2%) | 39 (0.9%) |
| Education level | |||
| General secondary school | 1312 (45.9%) | 596 (43.2%) | 1908 (45.0%) |
| Intermediate secondary school | 560 (19.6%) | 306 (22.2%) | 866 (20.4%) |
| Polytechnic secondary school | 152 (5.3%) | 99 (7.2%) | 251 (5.9%) |
| Qualification for university of applied sciences | 249 (8.7%) | 114 (8.3%) | 363 (8.6%) |
| Qualification for university | 443 (15.5%) | 202 (14.6%) | 645 (15.2%) |
| Other | 67 (2.3%) | 27 (2.0%) | 94 (2.2%) |
| No qualification | 25 (0.9%) | 16 (1.2%) | 41 (1.0%) |
| Missing | 51 (1.8%) | 20 (1.4%) | 71 (1.7%) |
| UICC stage | |||
| UICC I | 812 (28.4%) | 498 (36.1%) | 1310 (30.9%) |
| UICC II | 961 (33.6%) | 332 (24.1%) | 1293 (30.5%) |
| UICC III | 773 (27.0%) | 395 (28.6%) | 1168 (27.6%) |
| UICC IV | 313 (10.9%) | 155 (11.2%) | 468 (11.0%) |
| Stoma (initially created) | |||
| Yes | 192 (6.7%) | 1010 (73.2%) | 1202 (28.4%) |
| No | 2667 (93.3%) | 370 (26.8%) | 3037 (71.6%) |
| Stoma (still present at 12 months) | |||
| No | 2025 (70.8%) | 632 (45.8%) | 2657 (62.7%) |
| Yes | 102 (3.6%) | 325 (23.6%) | 427 (10.1%) |
| Missing | 732 (25.6%) | 423 (30.7%) | 1155 (27.2%) |
| Deceased | |||
| Not deceased/unknown | 2607 (91.2%) | 1265 (91.7%) | 3872 (91.3%) |
| Deceased | 252 (8.8%) | 115 (8.3%) | 367 (8.7%) |
| Deceased (30 days) | |||
| Survived > 30 days | 2800 (97.9%) | 1359 (98.5%) | 4159 (98.1%) |
| Survived ≤ 30 days | 59 (2.1%) | 21 (1.5%) | 80 (1.9%) |
Missing values are given only for variables where missing values occurred.
ASA, American Society of Anesthesiologists; IQR, interquartile range; SD, standard deviation;
UICC, Union Internationale Contre le Cancer
Patient-reported outcomes
As shown in Table 2, the mean global health status/QoL was slightly better after 12 months than at the time of the baseline survey (score 66 versus 62 points), and both constipation (16 versus 19) and abdominal pain (15 versus 17) were on average less severe. The mean scores worsened for physical function (75 versus 82) and pain (22 versus 19). The arithmetic means after treatment for the three dimensions for which cut-offs were available were 75 (physical function), 22 (pain), and 16 (constipation). Therefore, a mean impairment of physical function was identified that could have relevance for treatment planning (24; 2).
eTable 2 shows that fecal incontinence became much worse in patients with cancer of the colon who had a stoma (mean change from 8 to 26 points, n = 63) and in patients with rectal carcinoma who did not have a stoma (mean change from 14 to 27 points, n = 522).
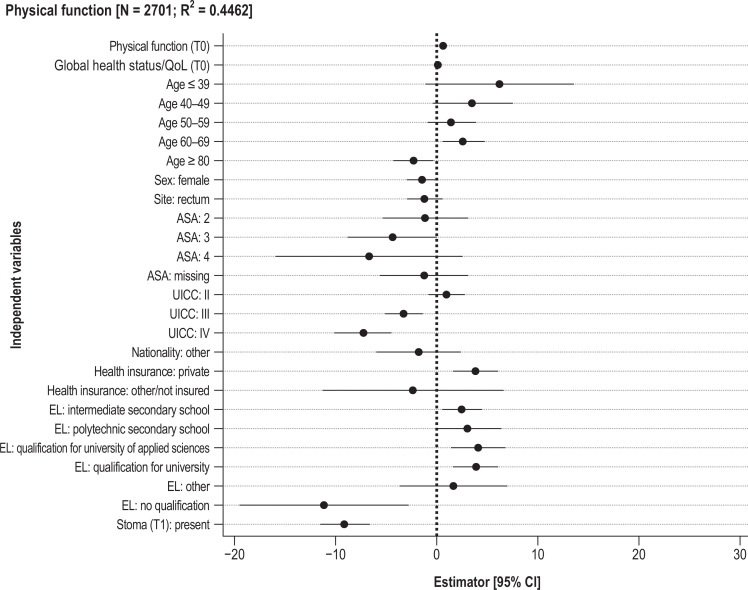
The multivariable linear regression analyses (Figure 1, eTable 1, eFigure 2) explain 11–45% of the variance (R²) for the dependent variables.
Figure 1.
Results of multivariable linear regression analyses: estimator (95% confidence intervals [95% CI])
ASA, American Society of Anesthesiologists; EL, Education level; UICC, Union Internationale Contre le Cancer
The pre-treatment scores were positively associated with the PRO in all models. Better PRO at 12 months were associated with better general health status (global health status/QoL, pain), cancer of the colon (constipation, global health status/QoL), higher level of education (pain, physical function, global health status/QoL), private health insurance (constipation, pain, physical function, global health status/QoL), male sex (abdominal pain, physical function, constipation), and a lower Union Internationale Contre le Cancer (UICC) stage for physical function and global health status/QoL but a higher UICC stage for abdominal pain and constipation. The age group over 79 years had worse PRO than the reference group of 70- to 79-year-olds for two scores, while the 50–59 age group had worse PRO for four scores. Presence of a stoma 12 months after treatment was associated with restriction of physical function and global health status/QoL but with a better score for constipation. With regard to distribution of residuals, testing of the model assumptions showed deviations from normal distribution, especially for constipation and abdominal pain. Tobit models showed larger estimators for the baseline scores but otherwise yielded similar results (eTable 3). Owing to singularity, multilevel models could be calculated only for global health status/QoL: the proportion of variance was small (ICC < 1%) at center level, and center characteristics failed to explain the variance in the sole converging multilevel model (eTable 4).
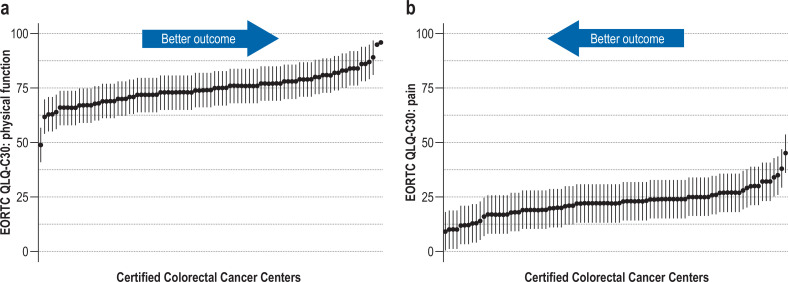
Comparison of the centers after case-mix adjustment revealed differences in scores (Figure 2 [illustrating pain and physical function], eFigure 3; observed and adjusted data available on request). The greatest interquartile range (69–79, larger than an MID) was found for the physical function score. For the other scores the interquartile ranges (4, 7, 5, and 7 points) were smaller than an MID (7, 10, 10, 9). Some centers had outcomes that were clearly below or above average, especially for physical function. Descriptively, there were no clear patterns with regard to associations between the centers’ adjusted scores and the following center characteristics: surgical case numbers, hospital funding type, teaching status, community size (eTable 5– 8).
Anastomotic insufficiency and 30-day mortality
Of the patients who had an anastomosis, 4.3% (colon, 121/2783) and 8.2% (rectum, 87/1061) experienced anastomotic insufficiency; 1.9% (80/4239) died within 30 days of resection. The multivariable regression analyses of the short-term clinical endpoints anastomotic insufficiency and 30-day mortality yielded R² < 0.03. The findings are illustrated in eFigure 4 and eFigure 5. The case-mix-adjusted results for the secondary endpoints are not shown because of the small degree of variance explained.
Discussion
This study examined the associations between patient characteristics and both PRO and short-term clinical outcome quality parameters 12 months after elective resection of colorectal cancer, comparing the results across Certified Colorectal Cancer Centers. The association analyses provided evidence of previously little investigated correlations of post-treatment PRO. These can be used by clinicians to identify patients with a higher risk of poor PRO a year after surgery. The sometimes strong associations with regard to the socioeconomic characteristics are particularly striking. For example, global health status/QoL, controlled for clinical characteristics and baseline status, is 5 points higher for persons with private than for those with statutory health insurance and 4 points higher for persons with a qualification for university entrance than for those whose education went no further than general secondary school (year 9 lower secondary school certificate). Evidence for associations between outcome quality and socioeconomic status has already been shown for Germany (29). With regard to the American Society of Anesthesiologists (ASA) score, as can be expected PRO were predominantly worse with severe disease. It is striking that four scores were worse in 50- to 59-year-olds than in the 70–79 age group.
Comparison of the centers showed that the interquartile ranges mostly differed to the extent of about an MID, although the distribution was wide. Case-mix adjustment and graphic presentation of the data enable the identification of some centers with distinctly worse outcomes than would be expected from the case mix. The largest differences were in the score for physical function (figure 2), where the interquartile range was 10 points, larger than an MID. This score was based on five items, so it may differentiate better than the other scores, which were calculated from either one or two items. The items are not specific to colorectal cancer and are of central relevance to the patients: An example is provided by the item “Do you have any trouble taking a long walk?,” with the possible responses “Not at all,” “A little,” “Quite a bit,” “Very much”. The center characteristics examined in the additional analyses contributed only slightly or not at all to explanation of the variance.
Figure 2.
Case-mix adjustment—point estimator of the centers’ adjusted post-treatment patient-reported outcome (PRO) scores. Only centers with at least 10 post-treatment questionnaires are included. Each dot represents an anonymized center.
a) Function score (the higher the score, the better the outcome): physical function (75, 69–79)
b) Symptom score (the lower the score, the better the outcome): pain (23, 19– 26)
The vertical lines (“antennae”) extending upward and downward represent provisional minimally important differences (MID) as one third of the standard deviation at patient level: physical function 7, pain 10
With regard to the change from before surgery to 12 months thereafter, results from the Netherlands point in a similar direction (30, 31). There too, only small differences were found between baseline and 12 months, although some of the differences for physical function were pronounced. Altogether, there is little in the way of comparable data from countries other than Germany.
The frequency of the short-term clinical endpoints anastomotic insufficiency and 30-day mortality in our sample is almost identical to that in the certified centers overall (22). Apart from sex and tumor site (for anastomotic insufficiency) and age and ASA score (for 30-day mortality), there were no factors associated with the short-term clinical endpoints, and overall the amount of variance explained by the models is low. Some risk factors known from the literature, e.g., body mass index, were not measured in this study. The procedure described here therefore seems unsuitable for case-mix-adjusted comparison of the short-term clinical endpoints; additional adjustor variables, for instance, would be needed for this purpose.
To our knowledge, our study is the first to compare PRO across centers in patients with colorectal cancer. Differences among treating facilities have been found for other diseases, e.g., orthopedic diagnoses (15), prostate cancer (28), and other surgical interventions (32). In some of these cases the differences were much greater. The differences across centers may be less pronounced in certified centers than they would be in a sample of other hospitals because of the relative homogeneity of the former. Certified centers are obliged to implement a whole range of quality requirements and have on average a better oncological outcome quality than uncertified institutions (33– 35). They thus represent a selection of the hospitals offering treatment for colorectal cancer. However, this is also true for prostate cancer centers, for which the differences in the PRO are larger (28). Although resection for colorectal cancer does not entail relatively frequently occurring functional impairments like the incontinence and erectile dysfunction that may follow treatment for prostate cancer, differences can by all means be anticipated owing to the challenging nature of interventions in the rectum. With this in mind, it would be beneficial to differentiate among different sites and between presence and absence of stoma in future research.
EORTC QLQ-C30 and EORTC QLQ-CR29 are recommended, for example, in the ICHOM standard set for colorectal cancer (36). However, no recommendations exist for prioritization among the more than 30 scores for patients with colorectal cancer or on whether stratified analyses are necessary. Further research efforts are required. Work is also needed to establish the significance of different surgical procedures with regard to PRO and also rehabilitation and aftercare, which can only partly be influenced by the Certified Colorectal Cancer Centers.
When interpreting the findings, the strengths and limitations of the study have to be taken into account: Unlike many other measures of care quality, questionnaire data necessitate recruitment efforts by the centers involved and active cooperation on the part of the patients. The median proportion of eligible patients who completed the baseline questionnaire was 51%, a satisfactory figure. Around one quarter of the patients did not take part in the follow-up survey. Death was documented for one third of this group. The remaining patients who did not respond at T1 had been more severely ill on average at T0 (data available on request).
The basic clinical documentation used for the purposes of this study was applied uniformly across all centers and is also obligatory for the certification process. The documentation is therefore close to complete, with simultaneous external validation by the auditors during certification. Nevertheless, a high proportion of missing data is found for the ASA classification (eMethods). Although the participating centers had high case numbers compared with German centers as a whole, low numbers in a few centers (median primary number of patients undergoing elective surgery: 76), together with the selective inclusion of patients, may distort the results. The centers with sufficient data for case-mix adjustment did not differ in hospital funding type, teaching status, and community size from those with insufficient data, but had higher primary case numbers (data available on request).
Finally, it can be stated that measurement of PRO for comparison of outcome quality may be especially useful to identify centers with results far above or below average. This gives those responsible for treatment an additional instrument to help them understand what patients need and what must be done to improve care provision further. The center-oriented data presented here may serve to stimulate steps towards quality improvement, e.g., the development of measures for the benefit of patients at high risk of reduced function; consideration in certification audits or in internal quality circles; and identification of centers with particularly good results. In this way we can all learn from one another (37).
Supplementary Material
eMethods
Center sample
A stratified random sample with move-up procedure was drawn from among the 273 German colorectal cancer centers that held a valid certificate and had first been certified no later than 2016. The 25 centers with the highest case numbers and a random sample of the remaining centers were invited to take part in the study. The study was initiated in 106 centers. The protocol foresaw that all patients at the participating centers who had colorectal cancer and were treated with elective tumor resection or nonsurgical palliative measures in 2019 (in six pilot centers, from October 2018) would be informed about the study by center staff, invited to take part, and included.
Variables and statistical analyses
The covariates for the association analyses and case-mix adjustment (CMA) at patient level were the pre-treatment symptom or function score and pretherapeutic global health status/QoL together with age, sex, stage, American Society of Anesthesiologists (ASA) score, site (colon/rectum), nationality, education level, health insurance status (see Table 1 for details), and presence of a stoma after 12 months. In additional analyses multilevel models were used to examine the center characteristics case numbers (continuous), teaching status (university hospital, academic teaching hospital, not a teaching hospital), community size (small, medium, large, ≥ 1 million population) and hospital funding type (charitable, public, private) (eTable 4, eTable 9, eTable 10). Moreover, the averages of the adjusted scores were observed according to center characteristics in contingency tables (eTable 5– 8).
Furthermore, in view of the importance of fecal incontinence, particularly for rectal cancer, we noted changes in this score from before resection to 12 months thereafter. In divergence from the procedure for the other five scores, owing to the peculiar nature of the score, stratified by site (colon/rectum) and presence of stoma (yes/no), assessment of the 12-month data was on the basis of contingency tables alone. Unlike the other scores reported, the item on fecal incontinence differentiated patients with and without a stoma, entailing separate analysis. At T0 the item was answered solely by persons without a stoma and was thus the same for all participants. The item for persons without a stoma was “Have you had leakage of stools from your back passage?,” while those with a stoma were asked “Have you had leakage of stools from your stoma bag?”. Each question could be answered with the following categories: “Not at all,” “A little,” “Quite a bit,” or “Very much.” Comparisons of the baseline and 12-month scores for “fecal incontinence” in persons with a stoma must be made with this limitation in mind.
The association analyses were based on the procedure of the UK National Health Service (NHS) for CMA and primarily took the form of multivariable linear regressions without taking account of center characteristics for each of the five scores (e1). The model assumptions (normal distribution of the residuals, linearity, multicollinearity, homogeneity of variance) were verified. The primary analyses were based on complete cases. Due to the large number of missing values for the ASA score, effects for missing values were estimated. Although the ASA score is a field in the “colorectal cancer” module of the oncological basic dataset for cancer registration (OBDS) in Germany, its documentation in the clinical cancer registries is even patchier than in the EDIUM study (information from the Arbeitsgemeinschaft Deutscher Tumorzentren [German Tumor Center Consortium]). Because of the exploratory nature of the study, there was no correction for multiple testing.
The data were presented with the aid of forest plots. The corresponding tables can be found in eTable 1. As sensitivity analyses, tobit regressions (eTable 3) and evaluations by site (colon/rectum) were carried out (eTable 11; stratified regression analyses available on request). In further sensitivity analyses (available on request), missing values for baseline PRO, nationality, health insurance status, stoma status, and education level were replaced by multiple imputation (number of imputations: maximum percentage of missing values for all variables) (e2). Because the creation and the presence of a stoma are strongly dependent on tumor site, an interaction effect was also estimated for this association (tumor site × stoma). In this regard, the characteristic stoma as surveyed at 12 months fed into the models. At that time it can be assumed, with few exceptions, that the stoma is permanent. The stoma may also have been created for another reason. The (temporary) creation of a stoma is documented in the clinical records. The stoma status at 12 months was taken into account because it is thought to be a decisive factor for the PRO at this time. Further sensitivity analyses were performed (1) to depict the multilevel structure of the data by means of linear multilevel models (adjusting for center characteristics) and (2) to take account of the censored PRO scales (possible floor and ceiling effects) by means of tobit regression (e3).The results of the mixed linear models are reported only where the variance–covariance matrix was not singular (eTable 4). For the null models and the complete mixed models (adjusted for potential influencing factors), intraclass correlation coefficients (ICC) were determined. In further sensitivity analyses the patient cohort was divided according to tumor site (colon/rectum). Moreover, a model for “global health status/QoL” was calculated in which it was determined whether the mode (online/paper) is associated with the PRO (eTable 12). The distributions of the post-treatment scores were additionally expressed as violin plots (eFigure 6). Tenfold cross-validation was performed to verify the model quality of the association analyses (e4), ensuring that patients from the same center were not separated. Model quality was established using R² (explained variance). Because EDIUM is an exploratory study, the p-values reported here are presented solely for descriptive purposes; p-values ≤ 0.05 are regarded as showing a statistically significant difference.
The CMA procedure was based on that of the NHS (e1). Missing values in prediction were replaced by means of the k-nearest neighbor algorithm (k = 6):
Calculation of the difference between the observed (O) and expected (E; prediction of the regression model) post-treatment PRO for each patient (O-E)
Calculation of the mean of these differences for each center (performance score)
Calculation of the case-mix-adjusted center values by addition of the performance scores to the posttreatment average PRO value (ō) for all study patients (adjusted value = performance score + ō).
For the purpose of CMA, for each score only those centers were included which had contributed post-treatment questionnaires from at least ten patients. In the graphs the point estimators (adjusted values) were brought into relationship with provisional minimally important differences (MID) according to (e5, e6) (standard deviation individual data × 1/3). An MID is the smallest change in a score perceived as important by the patient. This visual orientation serves to improve the classification of the differences among centers and has proved its worth in similar reports (e7). All analyses were carried out using the software R (version 4.0.0.).
For the short-term clinical secondary endpoints of anastomotic insufficiency in the colon or rectum and 30-day mortality, multivariable logistic regressions were estimated. In the association analyses performed to explore the secondary endpoints, the following variables were selected on the basis of the information available: age group (≤ 49, 50–69, 70–89, ≥ 90), sex, site (colon/rectum), ASA score, health insurance status, nationality, education level (combining intermediate secondary school with polytechnic secondary school and qualification for university of applied sciences with general university qualification), tumor stage (Union Internationale Contre le Cancer [UICC] stage I+II/III/IV), radiotherapy (yes/no), and PRO score for physical function (at T0).
Based on the procedure of the National Healthcare Safety Network of the Center for Disease Control (NHSN) for short-term clinical complications (postoperative infections, e8), the following method was selected for CMA of the secondary outcomes (each of which was operationalized in binary fashion):
Calculation of the expected values for each patient (expected likelihood of the outcome) pj,i on the basis of the logistic regression models (outcomes: “anastomotic insufficiency” yes/no or “deceased at 30 days after surgery” yes/no; “i” stands for “patient in center j”).
Calculation of the expected events at each center j by summation of the expected values for the patients at that center:
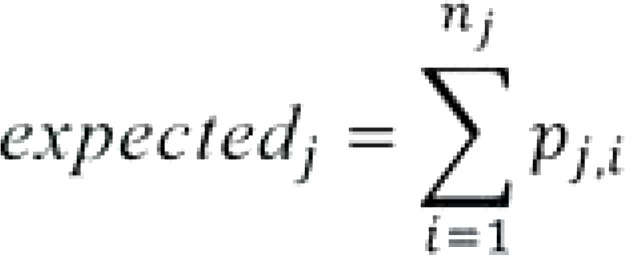
Here, “nj” stands for the number of patients treated in center j.
Calculation of the adjusted value by multiplying the ratio of actually observed events to expected events at each center by the rate of observed events at all centers:
Here, “observedall” is the number of observed events across all centers. If the value for “expectedj” is smaller than 1, “adjustedj” cannot be calculated. The results for anastomotic insufficiency and 30-day mortality are stratified by tumor site, analogous to the primary endpoints.
Notes on ASA score and model quality
ASA score is a field of the module “colorectal cancer” of the oncological basic dataset. Its documentation is even more fragmentary in clinical cancer registries than in the EDIUM study (personal communication, German Tumor Center Consortium). Improvement is needed. While the linear regression models for the PRO usually showed satisfactory predictive power, this was not the case for the models of the short-term clinical (secondary) endpoints. This could be at least partly attributable to the absence of parameters associated with these endpoints. It can be anticipated that adequate CMA for these endpoints will require further adjustor variables that will have to be additionally documented, including comorbidities, body mass index, and smoking status. This extra documentation will involve a greater demand on resources.
Table 2. EORTC scores for patients who took part in the post-treatment survey (N = 3142).
| T0 | T1 | ||
| Global health status/ QoL | n missing | 41 | 18 |
| Mean (SD) | 62 (23) | 66 (21) | |
| Median (IQR) | 67 (50–83) | 67 (50–83) | |
| Physical function | n missing | 7 | 9 |
| Mean (SD) | 82 (22) | 75 (24) | |
| Median (IQR) | 93 (73–100) | 80 (60–93) | |
| Pain | n missing | 3 | 10 |
| Mean (SD) | 19 (28) | 22 (28) | |
| Median (IQR) | 0 (0–33) | 17 (0–33) | |
| Constipation | n missing | 30 | 31 |
| Mean (SD) | 19 (31) | 16 (26) | |
| Median (IQR) | 0 (0–33) | 0 (0–33) | |
| Abdominal pain | n missing | 23 | 19 |
| Mean (SD) | 17 (27) | 15 (24) | |
| Median (IQR) | 0 (0–33) | 0 (0–33) |
IQR, Interquartile range; SD, standard deviation; T0, before commencement of treatment;
T1, 12 months after tumor resection
eTable 2. Stoma-specific score “fecal incontinence” at T0 and T1 in colon cancer and rectal cancer for the strata: stoma present after 12 months and stoma absent after 12 months (all study patients with scores at T0 and T1).
| Colon | Rectum | Total | ||||||||||
| Stoma yes | Stoma no | Stoma yes | Stoma no | Stoma yes | Stoma no | |||||||
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| Fecal incontinence | (n = 63) | (n = 63) | (n = 1 609) | (n = 1 609) | (n = 247) | (n = 247) | (n = 522) | (n = 522) | (n = 310) | (n = 310) | (n = 2 131) | (n = 2 131) |
| Mean (SD) | 7.94 ± 19.60 | 26.45 ± 30.03 | 5.61 ± 17.26 | 10.07 ± 21.69 | 24.69 ± 32.33 | 26.85 ± 30.11 | 13.79 ± 25.15 | 26.82 ± 31.93 | 21.29 ± 30.90 | 26.77 ± 30.04 | 7.62 ± 19.80 | 14.17 ± 25.62 |
| Median (IQR) | 0 [0; 0] |
33.33 [0; 50] |
0 [0; 0] |
0 [0; 0] |
0 [0; 33.33] |
33.33 [0; 33.33] |
0 [0; 33.33] |
33.33 [0; 33.33] |
0 [0; 33.33] |
33.33 [0; 33.33] |
0 [0; 0] |
0 [0;33.33] |
IQR, Interquartile range; SD, standard deviation
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Further authors
Clara Breidenbach, Anna Hagemeier, Rebecca Roth, Thomas Seufferlein, Stefan Benz, Stefan Post, Robert Siegel, Armin Wiegering, Raphael Winkels, Stefanie Bieck-Messemer, Jörg Fahlke, Christoph Reissfelder, Martin Fuchs, Torsten Herzog, Richard Weihrauch, Julia Faber-Mertens, Hagen Rudolph, László Puskás, Kay Kohlhaw, Malgorzata Szczerbinska, Hubert Scheuerlein, Philipp-Alexander Neumann, Stephan Hollerbach, Maren Riechmann, Ernst W. Kolbe, Norbert Weigert, Jörg Köninger, Christian Klink, Shueb Mussa, Anja-Kathrin Horn, Ludger Staib, Jens Werner, Joachim Jähne, Mohamed Aly, Hubert Mörk, Robert Grützmann, Pompilio Piso, Sebastian Dieng
Affiliations of the further authors
German Cancer Society, Berlin: Clara Breidenbach
Institute for Medical Statistics and Bioinformatics, Faculty of Medicine, University Hospital Cologne: Anna Hagemeier, Dr. Rebecca Roth
University Hospital Ulm: Prof. Dr. Thomas Seufferlein
Southwest Hospital Group, Sindelfingen: Prof. Dr. Stefan Benz, Prof. Dr. Hubert Mörk
University Hospital Mannheim: Prof. Dr. Stefan Post, Prof. Dr. Christoph Reissfelder
Helios Hospital Berlin-Buch: PD Dr. Robert Siegel
University Hospital Würzburg: Prof. Dr. Armin Wiegering
Main–Taunus District Hospitals, Bad Soden: Dr. Raphael Winkels
Westpfalz Hospital, Kaiserslautern: Dr. Stefanie Bieck-Messemer
Johanniter Hospital, Stendal: Prof. Dr. Jörg Fahlke
Bogenhausen Hospital, Munich: Dr. Martin Fuchs
Catholic Hospital, Bochum: PD Dr. Torsten Herzog
Deaconesses’ Hospital, Dresden: Richard Weihrauch
St. Elisabeth Hospital, Geilenkirchen: Dr. med. Julia Faber-Mertens
Chemnitz Hospital: Dr. Hagen Rudolph
Memmingen Hospital: Dr. László Puskás
Sana Hospital, Borna: Dr. Kay Kohlhaw
Johannes Wesling Hospital, Minden: Malgorzata Szczerbinska
St. Vincenz Hospital, Paderborn: PD Dr. Hubert Scheuerlein
Rechts der Isar Hospital, Munich: PD Dr. Philipp-Alexander Neumann
Gastroenterology Hospital, Celle: Prof. Dr. Stephan Hollerbach
Sana Hospital, Hof/Saale: Dr. Maren Riechmann
Herford Hospital: Dr. Ernst W. Kolbe
St. Elisabeth Hospital, Straubing: Prof. Dr. Norbert Weigert
Katharinen Hospital, Stuttgart: Prof. Dr. Jörg Köninger
Deaconesses’ Foundation Hospital, Speyer: Prof. Dr. Christian Klink
Protestant Deaconesses’ Hospital, Leipzig: Dr. Shueb Mussa
Social Foundation Bamberg: Dr. Anja-Kathrin Horn
Esslingen Hospital: Prof. Dr. Ludger Staib
University Hospital Munich: Prof. Dr. Jens Werner
DIAKOVERE Henrietta Foundation Hospital, Hanover: Prof. Dr. Joachim Jähne
Landshut-Achdorf Hospital: Mohamed Aly
University Hospital Erlangen: Prof. Dr. Robert Grützmann
Merciful Brethren Hospital, Regensburg: Prof. Dr. Pompilio Piso
OnkoZert GmbH: Sebastian Dieng
Study registration
The study was advised and approved by the ethics committee of the Berlin Medical Association (Eth19–18) (38) and prospectively registered (DRKS-ID: DRKS00008724). All of the patients gave written consent for their participation in the study.
Data sharing statement
No access is provided to anonymized patient data (study participants’ data). The following additional data (analyses) can be supplied on request: observed and adjusted scores by center, additional analyses for imputed models and stratified models per site. Also available are the study protocol, the results as reported to the funding body after approval, patient data and consents, the questionnaire used, and the observed/adjusted data. All of these can be accessed (in German) at www.edium-studie.de or obtained from the corresponding author. The data will be available for a period of at least 2 years, starting immediately or after release by the funding body. The data will be provided to all persons with a reasonable interest in their use.
Funding
The EDIUM Study was funded by the Innovation Committee of the Federal Joint Committee (G-BA).
Conflict of interest statement
During his work on the EDIUM Study, Sebastian Dieng was employed by OnkoZert GmbH, which received a grant from the Innovation Committee of the Federal Joint Committee (G-BA) in its capacity as a study consortium partner. OnkoZert GmbH manages the certification system of the German Cancer Society (DKG) and carries out certifications of oncological centers and organ cancer centers (including Certified Colorectal Cancer Centers) for the DKG.
PD Dr. Kohlhaw has received consulting fees from the DKG and from OnkoZert GmbH.
PD Dr. Siegel has been the recipient of funding in connection with the Clinical Practice Guideline on Anal Carcinoma project in the oncology guideline program of the AWMF/DKG/German Cancer Aid.
PD Dr. Christoph Kowalski, Nora Tabea Sibert, Clara Breidenbach, and PD Dr. Simone Wesselmann are employees of the DKG.
Prof. Dr. Post is a member of the Visceral Oncology Certification Committee of the DKG and of the Guidelines Steering Committee of the DKG.
Prof. Dr. Reissfelder is chairman of the Certification Committee for Bowel Cancer Centers.
The remaining authors declare that no conflict of interest exists.
References
- 1.Robert Koch-Institut. Berlin: 2019. Krebs in Deutschland für 2015/2016. [Google Scholar]
- 2.Onkologisches Leitlinienprogramm. Berlin: 2019. S3-Leitlinie Kolorektales Karzinom. AWMF-Registernummer: 021/007OL. [Google Scholar]
- 3.Basch E, Torda P, Adams K. Standards for patient-reported outcome-based performance measures. JAMA. 2013;310:139–140. doi: 10.1001/jama.2013.6855. [DOI] [PubMed] [Google Scholar]
- 4.Klinkhammer-Schalke M, Steinger B, Koller M, et al. Diagnosing deficits in quality of life and providing tailored therapeutic options: results of a randomised trial in 220 patients with colorectal cancer. Eur J Cancer. 2020;130:102–113. doi: 10.1016/j.ejca.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 5.OECD. Recommendations to OECD ministers of health from the high level reflection group on the future of health statistics. Strengthening the international comparison of health system performance through patient-reported indicators. Ohne Ort. 2017 [Google Scholar]
- 6.Kowalski C, Hübner J. „Patient-reported outcome measures“: Reif für die Routine? Forum. 2020;35:401–405. [Google Scholar]
- 7.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 8.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis F, Lethrosne C, Pourel N, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx029. djx029. [DOI] [PubMed] [Google Scholar]
- 11.Klinkhammer-Schalke M, Koller M, Steinger B, et al. Direct improvement of quality of life using a tailored quality of life diagnosis and therapy pathway: randomised trial in 200 women with breast cancer. Br J Cancer. 2012;106:826–838. doi: 10.1038/bjc.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wintner LM, Sztankay M, Giesinger JM, et al. EORTC Quality of Life Group manual for the use of EORTC measures in daily clinical practice. Brüssel. 2016 doi: 10.1016/j.ejca.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Geraedts M, Drösler SE, Döbler K, et al. DNVF-Memorandum III „Methoden für die Versorgungsforschung“, Teil 3: Methoden der Qualitäts- und Patientensicherheitsforschung. Gesundheitswesen. 2017;79:e95–e124. doi: 10.1055/s-0043-112431. [DOI] [PubMed] [Google Scholar]
- 14.Di Maio M, Basch E, Denis F, et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann Oncol. 2022;33:878–892. doi: 10.1016/j.annonc.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Gordon BE, Basak R, Carpenter WR, Usinger D, Godley PA, Chen RC. Factors influencing prostate cancer treatment decisions for African American and white men. Cancer. 2019;125:1693–1700. doi: 10.1002/cncr.31932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IQTIG—Institut für Qualitätssicherung und Transparenz im Gesundheitswesen. Berlin: 2019. Methodische Grundlagen V1.1. [Google Scholar]
- 17.Deutscher D, Werneke MW, Hayes D, et al. Impact of risk adjustment on provider ranking for patients with low back pain receiving physical therapy. J Orthop Sports Phys Ther. 2018;48:637–648. doi: 10.2519/jospt.2018.7981. [DOI] [PubMed] [Google Scholar]
- 18.National Prostate Cancer Audit. Results of the NPCA Prospective Audit in England and Wales for men diagnosed from 1 April 2018 to 31 March 2019. The Royal College of Surgeons of England. 2021 [Google Scholar]
- 19.Farin E, Jäckel WH, Schalaster V. Das Qualitätssicherungsverfahren der GKV in der medizinischen Rehabilitation: Ergebnisse und Weiterentwicklung. Gesundheitswesen. 2009;71:163–174. doi: 10.1055/s-0028-1119382. [DOI] [PubMed] [Google Scholar]
- 20.Kowalski C, Roth R, Carl G, et al. A multicenter paper-based and web-based system for collecting patient-reported outcome measures in patients undergoing local treatment for prostate cancer: first experiences. J Patient Rep Outcomes. 2020;4 doi: 10.1186/s41687-020-00224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009;45:3017–3026. doi: 10.1016/j.ejca.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Deutsche Krebsgesellschaft. Berlin: 2020. Jahresbericht der zertifizierten Darmkrebszentren. [Google Scholar]
- 23.Sibert NT, Breidenbach C, Wesselmann S, et al. Which EORTC QLQ-C30 and -CR29 scores are relevant for clinicians for therapy planning and decisions? Results of an online survey. Coloproctology. 2021;43:411–416. [Google Scholar]
- 24.Giesinger JM, Loth FLC, Aaronson NK, et al. Thresholds for clinical importance were established to improve interpretation of the EORTC QLQ-C30 in clinical practice and research. J Clin Epidemiol. 2020;118:1–8. doi: 10.1016/j.jclinepi.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 25.NHS England Analytical Team. London: 2013. Patient Reported Outcome Measures (PROMs). An alternative aggregation methodology for case-mix adjustment. [Google Scholar]
- 26.Eton DT, Cella D, Yost KJ, et al. A combination of distribution—and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 28.Sibert, NT, Pfaff H, Breidenbach C, et al. Variation across operating sites in urinary and sexual outcomes after radical prostatectomy in localized and locally advanced prostate cancer. World J Urol. 2022;40:1437–1446. doi: 10.1007/s00345-022-03985-6. [DOI] [PubMed] [Google Scholar]
- 29.Finke I, Behrens G, Maier W, et al. Small area analysis on socioeconomic inequalities in cancer survival for 25 cancer sites in Germany. Int J Cancer. 2021;149:561–572. doi: 10.1002/ijc.33553. [DOI] [PubMed] [Google Scholar]
- 30.Couwenberg AM, Burbach JPM, van Grevenstein WMU, et al. Effect of neoadjuvant therapy and rectal surgery on health-related quality of life in patients with rectal cancer during the first 2 years after diagnosis. Clin Colorectal Cancer. 2018;17:e499–e512. doi: 10.1016/j.clcc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Reudink M, Molenaar CJL, Bonhof CS, Janssen L, Mols F, Slooter GD. Evaluating the longitudinal effect of colorectal surgery on health related quality of life in patients with colorectal cancer. J Surg Oncol. 2022;125:217–226. doi: 10.1002/jso.26685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waljee JF, Ghaferi A, Finks JF, et al. Variation in patient-reported outcomes across hospitals following surgery. Med Care. 2015;53:960–966. doi: 10.1097/MLR.0000000000000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diers J, Baum P, Matthes H, Germer C-T, Wiegering A. mortality and complication management after surgery for colorectal cancer depending on the DKG minimum amounts for hospital volume. Eur J Surg Oncol. 2021;47:850–857. doi: 10.1016/j.ejso.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Trautmann F, Reißfelder C, Pecqueux M, Weitz J, Schmitt J. Evidence-based quality standards improve prognosis in colon cancer care. Eur J Surg Oncol. 2018;44:1324–1330. doi: 10.1016/j.ejso.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Nimptsch U, Mansky T. Hospital volume and mortality for 25 types of inpatient treatment in German hospitals: observational study using complete national data from 2009 to 2014. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016184. e016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ICHOM—International Consortium for Health Outcomes Measurement. Colorectal Cancer Data Collection Reference Guide. Cambridge. 2016 [Google Scholar]
- 37.Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4 doi: 10.1186/1748-5908-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breidenbach C, Sibert NT, Wesselmann S, Kowalski C. Erratum: Die Beratung durch Ethikkommissionen bei einer multizentrischen Beobachtungsstudie in Deutschland—Aufwand und Kosten. Gesundheitswesen. 2021;83 doi: 10.1055/a-1192-4946. [DOI] [PubMed] [Google Scholar]
- E1.NHS England Analytical Team. Patient Reported Outcome Measures (PROMs). An alternative aggregation methodology for case-mix adjustment. 2013 [Google Scholar]
- E2.Buuren van S, Buuren S. Flexible imputation of missing data, 2. edition. Boca Raton. 2018 [Google Scholar]
- E3.Nuttall D, Parkin D, Devlin N. Inter-provider comparison of patient-reported outcomes: developing an adjustment to account for differences in patient case mix. Health Econ. 2015;24:41–54. doi: 10.1002/hec.2999. [DOI] [PubMed] [Google Scholar]
- E4.Hastie T, Tibshirani R, Friedman JH. The elements of statistical learning: data mining, inference, and prediction: with 200 full-color illustrations. New York. 2001 [Google Scholar]
- E5.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- E6.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- E7.Sibert, NT, Pfaff H, Breidenbach C, et al. Variation across operating sites in urinary and sexual outcomes after radical prostatectomy in localized and locally advanced prostate cancer. World J Urol. doi: 10.1007/s00345-022-03985-6. DOI: 10.1007/s00345-022-03985-6. [DOI] [PubMed] [Google Scholar]
- E8.National Center for Emerging and Zoonotic Infectious Diseases, Division of Healthcare Quality Promotion. The NHSN standardized infection ratio (SIR)T—a Guide to the SIR. 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Center sample
A stratified random sample with move-up procedure was drawn from among the 273 German colorectal cancer centers that held a valid certificate and had first been certified no later than 2016. The 25 centers with the highest case numbers and a random sample of the remaining centers were invited to take part in the study. The study was initiated in 106 centers. The protocol foresaw that all patients at the participating centers who had colorectal cancer and were treated with elective tumor resection or nonsurgical palliative measures in 2019 (in six pilot centers, from October 2018) would be informed about the study by center staff, invited to take part, and included.
Variables and statistical analyses
The covariates for the association analyses and case-mix adjustment (CMA) at patient level were the pre-treatment symptom or function score and pretherapeutic global health status/QoL together with age, sex, stage, American Society of Anesthesiologists (ASA) score, site (colon/rectum), nationality, education level, health insurance status (see Table 1 for details), and presence of a stoma after 12 months. In additional analyses multilevel models were used to examine the center characteristics case numbers (continuous), teaching status (university hospital, academic teaching hospital, not a teaching hospital), community size (small, medium, large, ≥ 1 million population) and hospital funding type (charitable, public, private) (eTable 4, eTable 9, eTable 10). Moreover, the averages of the adjusted scores were observed according to center characteristics in contingency tables (eTable 5– 8).
Furthermore, in view of the importance of fecal incontinence, particularly for rectal cancer, we noted changes in this score from before resection to 12 months thereafter. In divergence from the procedure for the other five scores, owing to the peculiar nature of the score, stratified by site (colon/rectum) and presence of stoma (yes/no), assessment of the 12-month data was on the basis of contingency tables alone. Unlike the other scores reported, the item on fecal incontinence differentiated patients with and without a stoma, entailing separate analysis. At T0 the item was answered solely by persons without a stoma and was thus the same for all participants. The item for persons without a stoma was “Have you had leakage of stools from your back passage?,” while those with a stoma were asked “Have you had leakage of stools from your stoma bag?”. Each question could be answered with the following categories: “Not at all,” “A little,” “Quite a bit,” or “Very much.” Comparisons of the baseline and 12-month scores for “fecal incontinence” in persons with a stoma must be made with this limitation in mind.
The association analyses were based on the procedure of the UK National Health Service (NHS) for CMA and primarily took the form of multivariable linear regressions without taking account of center characteristics for each of the five scores (e1). The model assumptions (normal distribution of the residuals, linearity, multicollinearity, homogeneity of variance) were verified. The primary analyses were based on complete cases. Due to the large number of missing values for the ASA score, effects for missing values were estimated. Although the ASA score is a field in the “colorectal cancer” module of the oncological basic dataset for cancer registration (OBDS) in Germany, its documentation in the clinical cancer registries is even patchier than in the EDIUM study (information from the Arbeitsgemeinschaft Deutscher Tumorzentren [German Tumor Center Consortium]). Because of the exploratory nature of the study, there was no correction for multiple testing.
The data were presented with the aid of forest plots. The corresponding tables can be found in eTable 1. As sensitivity analyses, tobit regressions (eTable 3) and evaluations by site (colon/rectum) were carried out (eTable 11; stratified regression analyses available on request). In further sensitivity analyses (available on request), missing values for baseline PRO, nationality, health insurance status, stoma status, and education level were replaced by multiple imputation (number of imputations: maximum percentage of missing values for all variables) (e2). Because the creation and the presence of a stoma are strongly dependent on tumor site, an interaction effect was also estimated for this association (tumor site × stoma). In this regard, the characteristic stoma as surveyed at 12 months fed into the models. At that time it can be assumed, with few exceptions, that the stoma is permanent. The stoma may also have been created for another reason. The (temporary) creation of a stoma is documented in the clinical records. The stoma status at 12 months was taken into account because it is thought to be a decisive factor for the PRO at this time. Further sensitivity analyses were performed (1) to depict the multilevel structure of the data by means of linear multilevel models (adjusting for center characteristics) and (2) to take account of the censored PRO scales (possible floor and ceiling effects) by means of tobit regression (e3).The results of the mixed linear models are reported only where the variance–covariance matrix was not singular (eTable 4). For the null models and the complete mixed models (adjusted for potential influencing factors), intraclass correlation coefficients (ICC) were determined. In further sensitivity analyses the patient cohort was divided according to tumor site (colon/rectum). Moreover, a model for “global health status/QoL” was calculated in which it was determined whether the mode (online/paper) is associated with the PRO (eTable 12). The distributions of the post-treatment scores were additionally expressed as violin plots (eFigure 6). Tenfold cross-validation was performed to verify the model quality of the association analyses (e4), ensuring that patients from the same center were not separated. Model quality was established using R² (explained variance). Because EDIUM is an exploratory study, the p-values reported here are presented solely for descriptive purposes; p-values ≤ 0.05 are regarded as showing a statistically significant difference.
The CMA procedure was based on that of the NHS (e1). Missing values in prediction were replaced by means of the k-nearest neighbor algorithm (k = 6):
Calculation of the difference between the observed (O) and expected (E; prediction of the regression model) post-treatment PRO for each patient (O-E)
Calculation of the mean of these differences for each center (performance score)
Calculation of the case-mix-adjusted center values by addition of the performance scores to the posttreatment average PRO value (ō) for all study patients (adjusted value = performance score + ō).
For the purpose of CMA, for each score only those centers were included which had contributed post-treatment questionnaires from at least ten patients. In the graphs the point estimators (adjusted values) were brought into relationship with provisional minimally important differences (MID) according to (e5, e6) (standard deviation individual data × 1/3). An MID is the smallest change in a score perceived as important by the patient. This visual orientation serves to improve the classification of the differences among centers and has proved its worth in similar reports (e7). All analyses were carried out using the software R (version 4.0.0.).
For the short-term clinical secondary endpoints of anastomotic insufficiency in the colon or rectum and 30-day mortality, multivariable logistic regressions were estimated. In the association analyses performed to explore the secondary endpoints, the following variables were selected on the basis of the information available: age group (≤ 49, 50–69, 70–89, ≥ 90), sex, site (colon/rectum), ASA score, health insurance status, nationality, education level (combining intermediate secondary school with polytechnic secondary school and qualification for university of applied sciences with general university qualification), tumor stage (Union Internationale Contre le Cancer [UICC] stage I+II/III/IV), radiotherapy (yes/no), and PRO score for physical function (at T0).
Based on the procedure of the National Healthcare Safety Network of the Center for Disease Control (NHSN) for short-term clinical complications (postoperative infections, e8), the following method was selected for CMA of the secondary outcomes (each of which was operationalized in binary fashion):
Calculation of the expected values for each patient (expected likelihood of the outcome) pj,i on the basis of the logistic regression models (outcomes: “anastomotic insufficiency” yes/no or “deceased at 30 days after surgery” yes/no; “i” stands for “patient in center j”).
Calculation of the expected events at each center j by summation of the expected values for the patients at that center:
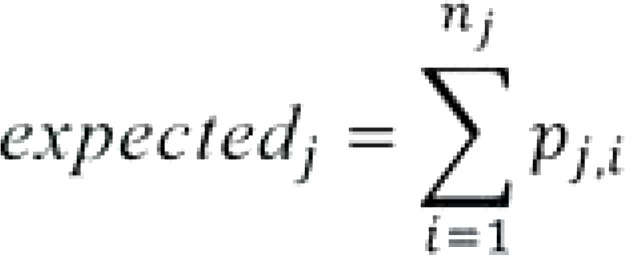
Here, “nj” stands for the number of patients treated in center j.
Calculation of the adjusted value by multiplying the ratio of actually observed events to expected events at each center by the rate of observed events at all centers:
Here, “observedall” is the number of observed events across all centers. If the value for “expectedj” is smaller than 1, “adjustedj” cannot be calculated. The results for anastomotic insufficiency and 30-day mortality are stratified by tumor site, analogous to the primary endpoints.
Notes on ASA score and model quality
ASA score is a field of the module “colorectal cancer” of the oncological basic dataset. Its documentation is even more fragmentary in clinical cancer registries than in the EDIUM study (personal communication, German Tumor Center Consortium). Improvement is needed. While the linear regression models for the PRO usually showed satisfactory predictive power, this was not the case for the models of the short-term clinical (secondary) endpoints. This could be at least partly attributable to the absence of parameters associated with these endpoints. It can be anticipated that adequate CMA for these endpoints will require further adjustor variables that will have to be additionally documented, including comorbidities, body mass index, and smoking status. This extra documentation will involve a greater demand on resources.