Abstract
Introduction:
Illicit opioids, consisting largely of fentanyl, novel synthetic opioids, and adulterants, are the primary cause of drug overdose fatality in the US. Xylazine, an alpha-2 agonist and veterinary tranquilizer, is being increasingly detected among decedents following illicit opioid overdose. Clinical outcomes in non-fatal overdose involving xylazine are unexplored. Therefore, among emergency department (ED) patients with illicit opioid overdose, we evaluated clinical outcome differences for patients with and without xylazine exposures.
Methods:
This multicenter, prospective cohort study enrolled adult patients with opioid overdose who presented to one of nine US EDs between September 21, 2020, and August 17, 2021. Patients with opioid overdose were screened and included if they tested positive for an illicit opioid (heroin, fentanyl, fentanyl analog, or novel synthetic opioid) or xylazine. Patient serum was analyzed via liquid chromatography quadrupole time-of-flight mass spectroscopy to detect all current illicit opioids, novel synthetic opioids, xylazine and adulterants. Overdose severity surrogate outcomes were: (a) cardiac arrest requiring CPR (primary); and (b) coma within four hours of arrival (secondary).
Results:
321 patients met inclusion criteria: 90 tested positive for xylazine and 231 were negative. The primary outcome occurred in 37 patients, and the secondary outcome occurred in 111 patients. Using multivariable regression analysis, patients positive for xylazine had significantly lower adjusted odds of cardiac arrest (aOR 0.30, 95% CI 0.10–0.92) and coma (aOR 0.52, 95% CI 0.29–0.94).
Conclusions:
In this large multicenter cohort, clinical outcomes for ED patients with illicit opioid overdose were significantly less severe in those testing positive for xylazine.
Keywords: Opioids, Fentanyl, Adulterants, Xylazine, Toxicosurveillance
Introduction
An unprecedented increase in US opioid overdose mortality has been observed since 2014, driven by the near ubiquitous presence of synthetic opioids in the illicit opioid supply 1–4. Polypharmacy implicated deaths, which include combinations of opioids, stimulants, and benzodiazepines, have also surged 5–8. Recently, xylazine has been reported in drug materials and overdose deaths linked to illicit fentanyl proliferation 9. However, patient clinical outcomes following non-fatal illicit opioid overdose with the presence of xylazine have not been described.
Xylazine, a potent central alpha-2 agonist used in veterinary medicine with ketamine or opioids, is used for large-animal anesthesia or pain management 10. Xylazine is structurally related to clonidine (Figure 1), resulting in central nervous system (CNS) depressant effects (sedation) and cardiovascular side effects (bradycardia, hypotention, and cardiac arrest)10. By bolstering alpha-2 adrenergic receptor activity, xylazine decreases norepinephrine presynaptic release, subsequently decreasing an adrenergic physiologic response10. Animal studies have demonstrated xylazine activity at mu-opioid receptors11.
Figure 1.
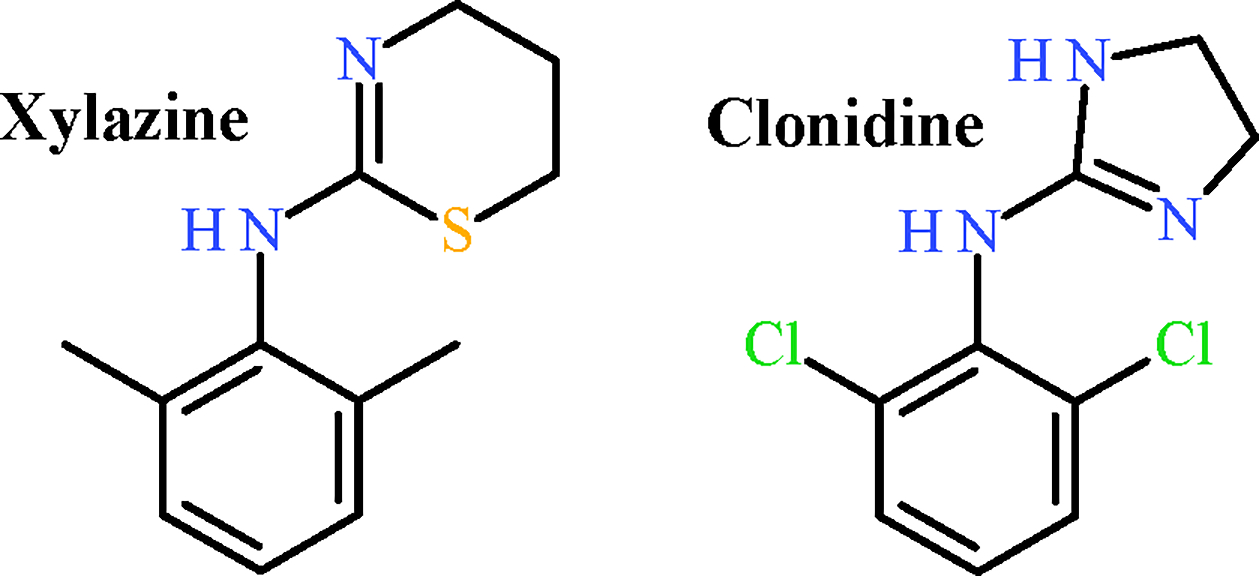
Chemical structures of xylazine and clonidine.
Over the last two decades, xylazine has emerged as a recreational drug supply adulterant (e.g., fentanyl, methamphetamine)9,12. Early xylazine detection in Puerto Rico describes patients using xylazine in combination with opioids (“anestesia de caballo”) or cocaine 13,14. Recently, xylazine, known by its street-name “tranq”, has been detected in urine, drug products and syringes with fentanyl and methamphetamine 15–17. Xylazine has also been increasingly detected among overdose fatalities in post-mortem studies 18–22. However, no studies have described clinical characteristics and outcomes for a prospective patient cohort exposed to opioids and xylazine.
Here, we investigate the effect of xylazine on clinical outcomes of emergency department (ED) patients who presented with suspected illicit opioid overdose. We performed blinded toxicological analyses and compared clinical outcomes via medical chart abstraction. We hypothesized that xylazine would be associated with worse clinical outcomes, most importantly cardiac arrest, and coma.
Methods
This multicenter, prospective cohort study enrolled consecutive patients with suspected opioid overdose who presented to a participating ED between September 21, 2020-August 17, 2021. Participating institutions were a subset of the Toxicology Investigators Consortium (ToxIC), which is an existing network of 48 U.S. hospitals in 30 U.S. cities23. Nine EDs participated across 7 states: California, Oregon, Michigan, Missouri, Pennsylvania, New York, and New Jersey. A central institutional review board (Western IRB) provided approval and a waiver of informed consent.
Inclusion/exclusion criteria
Patients at least 18 years old and who presented to the ED with suspected opioid overdose between September 21, 2020-August 17, 2021 were screened for study eligibility. Patients were eligible for study inclusion if they (1) had opioid toxicity based on chief complaint or discharge diagnosis; (2) received naloxone for overdose treatment in the ED; or (3) had self-reported opioid use resulting in an ED visit for an overdose. Patients who presented with trauma, in custody of law enforcement, or without waste specimens were excluded. Of those eligible for study inclusion, only patients testing positive for illicit opioids or xylazine were included in the final cohort. An illicit opioid included heroin, fentanyl, fentanyl analogs, nitazene analogs, or other new synthetic opioids.
Toxicological Analyses
Waste clinical specimens were collected as directed by site investigators and ToxIC staff. Serum and/or blood samples drawn in heparinized tubes obtained as part of routine clinical care were collected, de-identified, and stored at −80 °C until sent to the Center for Forensic Science Research and Education (CFSRE) for analysis. Qualitative molecular identification consisted of liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) analysis with secondary analysis by liquid chromatography tandem quadrupole mass spectrometry (LC-QqQ-MS), when necessary. Current CFSRE toxicology testing contains over 900 drugs, including therapeutics, traditional illicit drugs, novel psychoactive substances (NPS), adulterants, and other compounds. This methodology has been previously validated 24 and the molecular battery is frequently updated, as drugs in this dynamic market change frequently. Illicit opioids of interest were fentanyl, fentanyl analogs (e.g., acetylfentanyl, furanylfentanyl, carfentanil, para-fluorofentanyl), nitazene analogs (e.g., isotonitazene, metonitazene), and other new synthetic opioids (e.g., brorphine, 2-metyhl AP-237), as well as previously prevalent synthetic opioids (e.g., AH-7921, MT-45, U-47700) 5. The limit of detection for both xylazine and fentanyl was 0.1 ng/ml.
Biological samples were de-identified with a code linking the patient’s sample to the corresponding ToxIC site clinical data entry. Toxicological analyses were blinded to clinical outcomes. Results were summarized and sent to the principal investigator for linkage to clinical data for analysis. Patients were then categorized into those testing positive (i.e., xylazine group) or negative (i.e., controls) for xylazine based on LC-QTOF-MS and/or LC-QqQ-MS.
Definitions
An illicit opioid was defined as heroin, fentanyl, fentanyl analogs, nitazene analogs, or other new synthetic opioids. Patients testing positive for prescription opioids (e.g., oxycodone, methadone) without xylazine were not included in the study cohort.
Cardiovascular adverse events were defined as a ventricular arrhythmia, intraventricular conduction delay, QT prolongation, documented cardiac arrest, elevated troponin, or bradycardia (<50 beats per minute at any time). Troponin was considered elevated if above the upper limit of normal for the given hospital’s reference range.
Individual sites were grouped into three regions: West (California, Oregon), Central (Michigan, Missouri), and East (Pennsylvania, New York, New Jersey).
Data Collection
Medical record data included age, sex, past medical and psychiatric comorbidities, suspected opioid name, treatment rendered (including dose, amount, route, and duration of naloxone administration), and outcome, including the presence or absence of any organ system toxicity. Data were collected and entered in a secure, web-based software platform (Research Electronic Data Capture [RedCap]) by a trained research assistant or site investigator/toxicologist.
Outcomes
The primary outcome of cardiac arrest was defined as loss of pulse requiring CPR, as documented in the medical chart. The secondary outcome of coma was defined as unarousable unresponsiveness or the phrase ‘coma’ at any time within the first four hours of ED arrival based on medical chart documentation. Adjudication of outcomes was performed independently by each ToxIC site investigator.
Data Analysis
Descriptive statistics are reported as medians with interquartile ranges and percentages. Categorical variables were evaluated using the Chi-squared test and Fisher’s exact test (when appropriate). Continuous variables were compared via Student’s T-test. Clinical variables included age, sex, race/ethnicity, psychiatric history, initial blood pressure, total naloxone dose administered, and positive xylazine toxicology. Multivariable logistic regression analysis was used to estimate the association between the explanatory variable (xylazine) and study outcomes when controlling for confounders. Data are reported as point estimates with corresponding 95% confidence intervals. Data analysis was performed on Stata/SE (version 16.1; College Station, Texas).
Data Management and Quality
Site-specific medical record data were abstracted into a RedCap data collection platform without patient identifiers. Patient data were linked to corresponding biological specimens. ToxIC registry data quality assurance is maintained in accordance with current best-practices25 including database logical checks, pilot testing, procedure manuals, quality assurance personnel, paperless e-forms, automated data cleaning, data tracking, secure encryption, and data abstractor training26. RedCap platform quality assurance confirmed that >90% of pertinent data fields were completed.
Results
Figure 2 shows study patient selection. During the study period, 1006 patients were screened for eligibility and 395 patients were enrolled. 321 patients (81.3%) were identified with at least one illicit opioid of interest or xylazine present in toxicology samples. Of these patients, 90 patients (28.0%) tested positive for xylazine and 231(72.0%) tested positive for an illicit opioid without xylazine. Among patients without xylazine, 16% had heroin detected, 93.5% had fentanyl detected, 13.9% had other fentanyl analogs detected and 3.0% had a novel synthetic opioid detected. Among patients with xylazine, 25.5% had heroin detected, 98.9% had fentanyl detected, 32.2% had other fentanyl analogs detected and 2.2% had a novel synthetic opioid detected (Table 1). Only one patient tested positive for xylazine without an illicit opioid. This patient tested positive for a prescription opioid (methadone).
Figure 2.
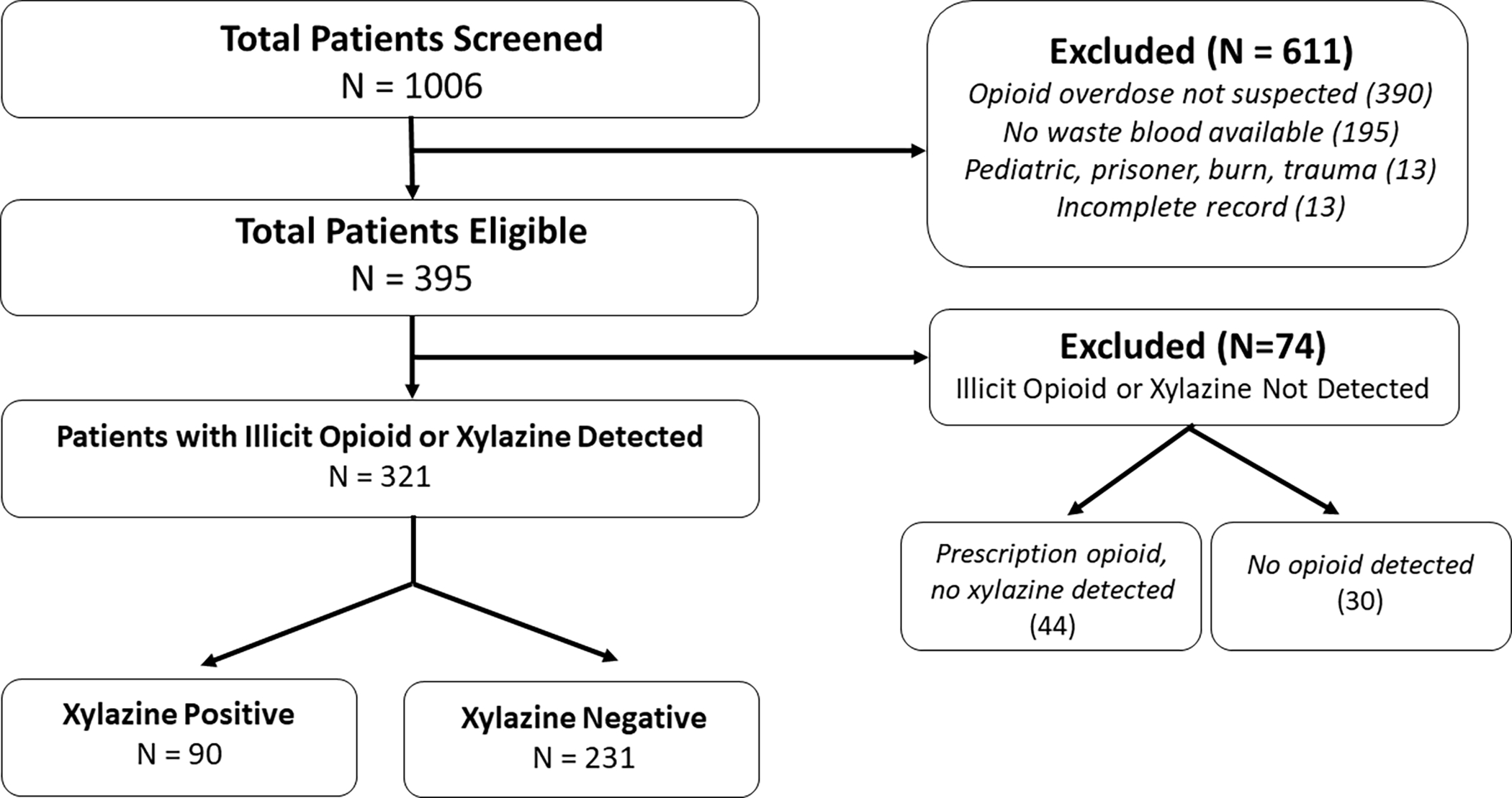
Patient eligibility and enrollment.
Table 1:
Demographic Characteristics of Xylazine and Control Cohorts
| Demographic Variables | Xylazine (N=90) | Control (N=231) |
|---|---|---|
|
| ||
| Male (%) | 69 (76.7%) | 154 (66.7%) |
| Age; median (IQR) | 41 (32–53) | 38 (30–50) |
| Psychiatric history | ||
| Any | 58 (64.4%) | 138 (59.7%) |
| Anxiety | 19 (21.1%) | 34 (14.7%) |
| Attention Deficit Hyperactivity Disorder | 4 (4.4%) | 10 (4.3%) |
| Bipolar | 9 (10%) | 25 (10.8%) |
| Depression | 17 (18.9%) | 55 (23.9%) |
| Post-traumatic stress disorder | 4 (4.4%) | 12 (5.2%) |
| Schizophrenia | 4 (4.4%) | 10 (4.3%) |
| Geographic Region | ||
| East [removed for blind review] | 63 | 127 |
| Central [removed for blind review] | 26 | 74 |
| West [removed for blind review] | 1 | 30 |
| Naloxone | ||
| Received any Naloxone (%) | 70 (77.8%) | 195 (84.4%) |
| Initial Naloxone Dose mg; median (IQR) | 2 (0.875–4) | 2 (2–4) |
| Total Naloxone Dose mg; median (IQR) | 3.6 (1.3–4.1) | 2.8 (2–4.1) |
| Number of naloxone doses; median (IQR) | 2 (1–3); range 1–5 | 1 (1–2); range 1–9 |
| Repeat Narcan received (%)* | 39 (43.3%) | 96 (41.5%) |
| Initial ED Vital Signs | ||
| SBP; median (IQR) | 132 (114–150) | 130 (118–145) |
| DBP; median (IQR) | 84 (68–98) | 84 (70–95) |
| HR ED; median (IQR) | 95 (81–108) | 98 (84–112) |
| RR ED; median (IQR) | 18 (14–20) | 18 (15–20) |
| Opioid Analytes Detected ** | ||
| Heroin | 23 (25.5%) | 37 (16%) |
| Fentanyl | 89 (98.9%) | 216 (93.5%) |
| Other Fentanyl Analogs | 29 (32.2%) | 32 (13.9%) |
| Novel Synthetic Opioids | 2 (2.2%) | 7 (3.0%) |
Abbreviations: IQR = Interquartile range; [removed for blind review]; DBP = Diastolic blood pressure; SBP = Systolic blood pressure; DBP = Diastolic blood pressure; HR = Heart rate; RR = Respiratory rate.
Percentage of entire cohort
Samples tested for all potential analytes. Single sample may have multiple analytes and percent totals may exceed 100%.
Control = Xylazine negative.
Overall, most patients were male (69.5%). The median (IQR) age was 39 (30–50) years. Psychiatric illness was prevalent and relatively evenly distributed among patients with and without xylazine. Baseline characteristics were similar between groups, but xylazine was more prevalent in samples from the East (Table 1).
Most patients (82.6%) were treated with naloxone and received a median initial 2mg dose. Table 1 describes naloxone administration in patients with and without xylazine detected. A large patient minority (42.1%) in both groups required multiple doses of naloxone.
Cardiovascular-related clinical outcomes were uncommon and did not differ between patients who did and did not have xylazine detected (Table 2). Xylazine-negative patients were more likely to have cardiac arrest compared to xylazine-positive patients: 33 patients (14.3%) without xylazine compared to 3 patients with xylazine [(4.4%), p=0.013; 95% CI −0.16–0.036].
Table 2:
Clinical Outcomes in Xylazine vs. Control Patients
| Clinical Outcome Variables | Xylazine (N=90) | Control (N=231) | P-Value |
|---|---|---|---|
|
| |||
| Cardiovascular (CV) Outcomes | |||
| Received CPR | 4 (4.4%) | 33 (14.3%) | 0.013 |
| Bradycardia | 2 (2.2%) | 4 (1.7%) | 0.77 |
| Pulmonary Outcomes | |||
| Intubated within 4 hours | 2 (2.2%) | 13 (5.6%) | 0.193 |
| Non-invasive positive pressure within 4 hours | 1 (1.1%) | 4 (1.7%) | 0.689 |
| Any ventilatory support within 4 hours | 3 (3.3%) | 17 (7.4%) | 0.182 |
| Intubated after 4 hours | 2 (2.2%) | 11 (4.8%) | 0.298 |
| Non-invasive positive pressure after four hours | 2 (2.2%) | 2 (0.9%) | 0.327 |
| Any ventilatory support after 4 hours | 4 (4.4%) | 13 (5.6%) | 0.67 |
| Central nervous system (CNS) Outcomes | |||
| Coma within 4 hours | 24 (26.7%) | 87 (37.7%) | 0.063 |
| Coma after 4 hours | 12 (13.3%) | 35 (15.2%) | 0.682 |
| Overall Outcomes | |||
| Death | 1 (1.1%) | 5 (2.16%) | 0.528 |
| Discharged from the ED | 59 (65.6%) | 147 (63.6%) | 0.528 |
| ICU Admissions | 11 (12.2%) | 39 (16.9%) | 0.30 |
| Miscellaneous | |||
| Length of Hospitalization (hrs.); median (IQR) | 10 (5–28) | 9 (5–36) | 0.806 |
| Total Naloxone Dose (mg) | 3.68 (1.3–4.05) | 2.8 (2–4.1) | 0.448 |
Abbreviations: IQR = Interquartile range; DBP = Diastolic blood pressure; SBP = Systolic blood pressure; DBP = Diastolic blood pressure; HR = Heart rate; RR = Respiratory rate.
Percentage of entire cohort
Control = Xylazine negative.
Coma was documented in 24 (26.7%) xylazine-positive patients within four hours and persisted in 12 patients (13.3%) beyond four hours. In contrast, coma was documented in 87 (37.7%) xylazine-negative patients within four hours and persisted beyond four hours in 35 patients (15.2%). However, there was no significant difference in early or late coma rates among those with and without xylazine (Table 2).
Most patients were discharged from the ED (59 [65.5%] xylazine-positive, vs. 147 [63.6%] xylazine-negative patients). One xylazine-positive patient (1.1%) died, compared with 5 (2.16%) xylazine-negative patients. The proportion of patients discharged from the ED, admitted patient average length-of-stay, and mortality rates were not significantly different between the xylazine-positive and xylazine-negative groups.
Table 3a shows multivariate logistic regression modeling results for patients developing coma within four hours of ED arrival. After controlling for age group, sex, race, prior psychiatric history, initial blood pressure and naloxone administration, xylazine exposure was associated with a significantly lower odds of developing coma within four hours of ED arrival (OR = 0.52, 95% confidence interval [CI]: 0.29 – 0.94). Blacks/African Americans (OR = 1.95, CI: 1.01–3.74), unknown race (OR = 3.64, CI: 1.63–8.16), and receiving naloxone (OR = 2.48, CI: 1.29–4.79) were associated with significantly higher odds of coma within four hours of ED arrival.
Table 3a:
Modelling Xylazine as an Independent Predictor of Coma
| Variable Name | aOR | 95% CI |
|---|---|---|
|
| ||
| Xylazine | 0.52 | 0.29–0.94 |
| Age Category | ||
| 18–29 years old | REF | REF |
| 30–39 years old | 1.52 | 0.73–3.17 |
| 40–50 years old | 0.92 | 0.41–2.05 |
| 50+ years old | 1.54 | 0.69–3.45 |
| Sex | ||
| Female | REF | REF |
| Male | 1.49 | 0.84–2.64 |
| Race Category | ||
| Non-Hispanic White | REF | REF |
| Black/African American | 1.95 | 1.01–3.74 |
| Asian | 1.00 | - |
| Hispanic | 0.51 | 0.15–1.67 |
| Other / Native American / Hawaiian / Mixed Race | 2.54 | 0.72–8.91 |
| Race - Unknown | 3.64 | 1.63–8.16 |
| Prior Psychiatric History | 0.87 | 0.49–1.56 |
| Initial ED Blood Pressure | 0.99 | 0.97–1.00 |
| Received Naloxone | 2.48 | 1.29–4.79 |
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval; ED = emergency department; REF = reference category. Variables in bold were statistically significant.
Table 3b shows multivariate logistic regression modeling results for patients with cardiac arrest. After controlling for age group, sex, race, prior psychiatric history, initial blood pressure and administration of naloxone, xylazine exposure was associated with a significantly lower odds of cardiac arrest (OR = 0.30, 95% confidence interval [CI]: 0.10 – 0.92). Black/African American race (OR = 0.23, CI: 0.06–0.84) was also associated with lower odds of cardiac arrest.
Table 3b:
Modelling Xylazine as an Independent Predictor of Cardiac Arrest
| Variable Name | aOR | 95% CI |
|---|---|---|
|
| ||
| Xylazine | 0.30 | 0.10–0.92 |
| Age Category | ||
| 18–29 years old | REF | REF |
| 30–39 years old | 1.41 | 0.57–3.50 |
| 40–50 years old | 0.78 | 0.26–2.35 |
| 50+ years old | 0.56 | 0.15–2.03 |
| Sex | ||
| Female | REF | REF |
| Male | 0.68 | 0.32–1.44 |
| Race Category | ||
| Non-Hispanic White | REF | REF |
| Black/African American | 0.23 | 0.06–0.84 |
| Asian | 1.00 | - |
| Hispanic | 1.63 | 0.51–5.23 |
| Other / Native American / Hawaiian / Mixed Race | 1.10 | 0.21–5.69 |
| Race Unknown | 0.80 | 0.24–2.67 |
| Prior Psychiatric History | 1.93 | 0.92–4.05 |
| Received Naloxone | 1.37 | 0.52–3.61 |
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval; ED = emergency department; REF = reference category. Variables in bold were statistically significant.
Discussion
This is the largest study to date analyzing xylazine overdose severity in ED patients. Our primary finding was that clinical outcomes for ED patients with illicit opioid overdose were significantly less severe in those testing positive for xylazine compared to those testing negative for xylazine. Additionally, high rates of cardiac arrest (11.5% of patients analyzed) and high total naloxone requirements (3.68 mg xylazine vs. 2.8 mg non-xylazine) were observed. Importantly, almost all xylazine patients had fentanyl/fentanyl analogs detected during toxicological analysis rather than heroin. These findings are consistent with recent reports describing a strong association between xylazine detection and fentanyl analogs in the illicit drug supply9,17,21,22.
Our findings of lower clinical severity among xylazine-adulterated opioid overdoses are consistent with, and build upon, prior studies. Previously, commonly described xylazine overdose clinical effects included CNS depression, bradycardia, and hypotension10,27,28. Xylazine overdose case reports have described respiratory depression, hyperglycemia, and hypotonia27,29. With supportive treatment, most patients recover from xylazine intoxication 27. In our study, the mortality rate overall was low, and most patients in both groups (i.e., xylazine and controls) were discharged from the ED. Both groups had similar initial ED vital signs (heart rate and blood pressure), and there was no difference in rates of bradycardia. These findings may be explained by the increasing presence of adulterants, contaminants, and other substances in illicit opioids.
In the present study, there remains a question of whether xylazine was an adulterant or desired component of the illicit opioid supply. Adulterants are pharmacologically active substances added to mirror or enhance specific drug effects30 and have been well-described in illicit drug supply studies. Adulterants in heroin have included scopolamine31 and quinine 32,33, and more recently clenbuterol34,35 and novel synthetic opioids36,37. Recent reports describe xylazine’s adulterant role as one that improves and prolongs opioid-associated euphoria9.
The explanation for lower clinical severity associated with xylazine-adulterated opioid supplies remains elusive. Xylazine does not cause the same degree of respiratory depression as opioids, especially fentanyl. It is possible that a drug sample containing both xylazine and an opioid may result in exposure to a lower opioid concentration. Alternatively, other adulterants, contaminants, or NPS in patients’ illicit opioid products may account for lower cardiac arrest and high ED discharge rates. Finally, it is possible that patients without xylazine exposure were exposed to higher total opioid amounts.
Despite similar mortality rates between groups, the xylazine group had significantly lower adjusted odds of cardiac arrest. Cardiac arrest following opioid overdose is mechanistically preceded by respiratory arrest, leading to hypercarbia, respiratory acidosis, and cardiovascular collapse. In pre-hospital settings, CPR initiation may be triggered by bystanders or emergency medical services for an apneic patient. Respiratory depression from xylazine is markedly less severe than that from opioids. Thus, the xylazine group may have had decreased risk of severe respiratory depression, and account for the lower odds of cardiac arrest.
Patients with detectable xylazine and an illicit opioid had approximately half the rate of coma within four hours of ED arrival. Due to xylazine’s known alpha-2 agonist effects, we hypothesized that the xylazine group would have a higher likelihood of developing early coma. Several factors may contribute to these results. The amount of xylazine contained in a sample may cause mild clinical CNS effects. Most case reports of xylazine exposure associated with hemodynamic or severe CNS depression/coma have described large, single-agent exposures. Also, the combination of insufficient xylazine and decreased total opioid concentration may have led to lower overall rates of coma.
Interestingly, all patients had relatively high total naloxone requirements (3.68 mg xylazine vs. 2.8 mg non-xylazine), but there was no significant difference in initial or total naloxone doses received between the groups. We hypothesized that patients in the illicit opioid only group might receive a higher total naloxone dose or more frequent repeat naloxone dosing, due to the opioid dose received or high potency of fentanyl/nitazene analogs. Again, the presence of other adulterants or contaminants may have limited the patient’s total opioid exposure. Alternatively, patients in the xylazine-opioid group may have received more naloxone due to mild xylazine-related CNS depression, which could be mistaken for opioid-related CNS depression. If ED clinicians are titrating naloxone to reverse CNS depression, frequent repeat dosing may result.
Finally, there was no association between the xylazine group and ED length-of-stay or hospital admission. Most patients in both groups were discharged from the ED. Several clinical care factors may explain this finding. The relative concentration of xylazine in a drug sample and subsequently small hemodynamic or CNS changes are easily managed with ED resuscitation, such as intravenous fluids, and standard ED observation times. Also, because xylazine is an increasingly prevalent adulterant, ED clinician disposition decision-making is likely guided by opioid and naloxone pharmacokinetic knowledge, and without consideration to monitor for xylazine’s potential clinical effects. Because the human half-life of xylazine is not known, it is difficult to assess if xylazine’s pharmacokinetics are related to patient length-of-stay.
Limitations of the present study require some consideration. Waste clinical specimens were not available for a large proportion of patients screened, leading to a large number of exclusions; this likely contributed to a higher overall overdose severity for patients included. Many screened patients did not have blood samples obtained in the ED, and patients who had blood work performed may represent a skewed overdose population. Blood sampling provided qualitative detection only; because quantitative serum concentrations were not measured, and opioid concentrations were not adjusted for, it is fraught to infer causality. Additionally, we do not know the relative timing of substance use; therefore, it is possible, however unlikely, that xylazine presence represented a prior drug exposure.
Because this study focused on ED patients, pre-hospital fatalities which were pronounced in the field were not examined; however, there were many cardiac arrests in the field which were successfully resuscitated and survived to hospital discharge. Lastly, given the severity of the US opioid epidemic, the study regions may limit generalizability especially to international locations. All participating ToxIC sites were located in large cities, and findings may not be applicable to rural communities. This study did not attempt to assess chronic dermatologic sequelae associated with xylazine because these clinical effects are not associated with acute xylazine toxicity.
Future studies should focus on measuring illicit opioid and xylazine serum concentrations to evaluate if relative serum concentrations of opioids, xylazine or other adulterants predict clinical effects and patient outcomes. Additionally, antidotal naloxone use to reverse xylazine toxicity is theoretically plausible38 but its efficacy is understudied.
Conclusions
In summary, in this large multicenter cohort study, clinical outcomes for ED patients with illicit opioid overdose were significantly less severe in those testing positive for xylazine. Confirmed illicit opioids consisted mostly of fentanyl and fentanyl analogs, rather than heroin. Overall rates of cardiac arrest and total naloxone dosing following acute opioid overdose were relatively high, consistent with the high prevalence of potent fentanyl and fentanyl analogs detected. Findings should inform the clinical and public health response to the ongoing US opioid epidemic.
Grant Funding:
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01DA048009 (PI: Manini). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest/Disclosure Statement: JSL is a research fellow in the Mount Sinai Clinical Scientist Training Program for Emergency Care Research (NIH1T32HL160513, NHLBI). BKL, AJK and SEW are employees of NMS Labs which complete the toxicologic specimen testing for this study.
Contributor Information
Jennifer S. Love, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai, New York, NY..
Michael Levine, Department of Emergency Medicine. University of California, Los Angeles. Los Angeles, CA..
Kim Aldy, Department of Emergency Medicine. University of Texas Southwestern Medical Center. Dallas, TX. American College of Medical Toxicology, Phoenix, AZ..
Jeffrey Brent, University of Colorado School of Medicine, Aurora, Colorado..
Alex J. Krotulski, Center for Forensic Science Research and Education, Fredric Rieders Family Foundation Willow Grove, PA..
Barry K. Logan, Center for Forensic Science Research and Education, Fredric Rieders Family Foundation Willow Grove, PA..
Carmen Vargas-Torres, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai, New York, NY..
Sara E. Walton, Center for Forensic Science Research and Education, Fredric Rieders Family Foundation Willow Grove, PA..
Paul Wax, Department of Emergency Medicine. University of Texas Southwestern Medical Center. Dallas, TX. American College of Medical Toxicology, Phoenix, AZ..
Alex F. Manini, Division of Medical Toxicology, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai, Elmhurst Hospital Center, New York, NY..
Data Availability Statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Green TC, Gilbert M. Counterfeit Medications and Fentanyl. JAMA Intern Med. 2016;176(10):1555–1557. doi: 10.1001/jamainternmed.2016.4306 [DOI] [PubMed] [Google Scholar]
- 2.Macmadu A, Carroll JJ, Hadland SE, Green TC, Marshall BDL. Prevalence and correlates of fentanyl-contaminated heroin exposure among young adults who use prescription opioids non-medically. Addict Behav. 2017;68:35–38. doi: 10.1016/j.addbeh.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CM, Einstein EB, Compton WM. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010–2016. JAMA - J Am Med Assoc. 2018;319(17):1819–1821. doi: 10.1001/jama.2018.2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank RG, Pollack HA. Addressing the Fentanyl Threat to Public Health. N Engl J Med. 2017;376(7):605–607. [DOI] [PubMed] [Google Scholar]
- 5.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants — United States, 2015–2016. Am J Transplant. 2018;18(6):1556–1568. doi: 10.1111/ajt.14905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Morbidity and Mortality Weekly Report Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths-United States, 2013–2019. Morb Mortal Wkly Rep. 2021;70(6):202–207. https://www.cdc.gov/nchs/data/nvsr/nvsr61/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivolo-Kantor AM, Seth P, Gladden RM, et al. Vital Signs : Trends in Emergency Department Visits for Suspected Opioid Overdoses — United States, July 2016–September 2017. MMWR Morb Mortal Wkly Rep. 2018;67(9):279–285. doi: 10.15585/mmwr.mm6709e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoots B, Vivolo-Kantor A, Seth P. The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction. 2020;115(5):946–958. doi: 10.1111/add.14878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman J, Montero F, Bourgois P, et al. Xylazine spreads across the US: A growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend. 2022;233(December 2021):109380. doi: 10.1016/j.drugalcdep.2022.109380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Colón K, Chavez-Arias C, Díaz-Alcalá JE, Martínez MA. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: A comprehensive review of the literature. Forensic Sci Int. 2014;240:1–8. doi: 10.1016/j.forsciint.2014.03.015 [DOI] [PubMed] [Google Scholar]
- 11.Romero TRL, Pacheco DDF, Duarte IDG. Xylazine induced central antinociception mediated by endogenous opioids and μ-opioid receptor, but not δ-or κ-opioid receptors. Brain Res. 2013;1506:58–63. doi: 10.1016/j.brainres.2013.02.030 [DOI] [PubMed] [Google Scholar]
- 12.Muller AA, Osterhoudt KC, Wingert WE. Heroin: What’s in the Mix? Ann Emerg Med. 2007;50(3):352–353. doi: 10.1016/j.annemergmed.2007.03.042 [DOI] [PubMed] [Google Scholar]
- 13.Torruella RA. Xylazine (veterinary sedative) use in Puerto Rico. Subst Abus Treat Prev Policy. 2011;6(1):2–5. doi: 10.1186/1747-597X-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes JC, Negrón JL, Colón HM, et al. The emerging of xylazine as a new drug of abuse and its health consequences among drug users in Puerto Rico. J Urban Heal. 2012;89(3):519–526. doi: 10.1007/s11524-011-9662-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans A, Krause M, Leach S, Levitas M, Nguyen L, Short LC. Analysis of drug residue in needle-exchange syringes in Washington, D.C. Forensic Sci Int. 2021;329:111083. doi: 10.1016/j.forsciint.2021.111083 [DOI] [PubMed] [Google Scholar]
- 16.Bowles JM, McDonald K, Maghsoudi N, et al. Xylazine detected in unregulated opioids and drug administration equipment in Toronto, Canada: clinical and social implications. Harm Reduct J. 2021;18(1):1–6. doi: 10.1186/s12954-021-00546-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korn WR, Stone MD, Haviland KL, Toohey JM, Stickle DF. High prevalence of xylazine among fentanyl screen-positive urines from hospitalized patients, Philadelphia, 2021. Clin Chim Acta. 2021;521(May):151–154. doi: 10.1016/j.cca.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Johnson J, Pizzicato L, Johnson C, Viner K. Increasing presence of xylazine in heroin and/or fentanyl deaths, Philadelphia, Pennsylvania, 2010–2019. Inj Prev. 2021;27(4):395–398. doi: 10.1136/injuryprev-2020-043968 [DOI] [PubMed] [Google Scholar]
- 19.Nunez J, DeJoseph ME, Gill JR. Xylazine, a Veterinary Tranquilizer, Detected in 42 Accidental Fentanyl Intoxication Deaths. Am J Forensic Med Pathol. 2021;42(1):9–11. doi: 10.1097/PAF.0000000000000622 [DOI] [PubMed] [Google Scholar]
- 20.Kariisa M, Patel P, Smith H, Bitting J. Xylazine Detection and Involvement in Drug. 2021;70(37):2019–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thangada S, Clinton HA, Ali S, et al. Notes from the Field : Xylazine, a Veterinary Tranquilizer, Identified as an Emerging Novel Substance in Drug Overdose Deaths — Connecticut, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70(37):1303–1304. doi: 10.15585/mmwr.mm7037a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chhabra N, Mir M, Hua MJ, et al. Notes From the Field: Xylazine-Related Deaths — Cook County, Illinois, 2017–2021. 2022;71(13):2021–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toxicology Investigators Consortium. American College of Medical Toxicology Toxicology Invesitgators Consortium (ToxIC). Published 2022. https://www.toxicregistry.org/
- 24.Krotulski AJ, Varnum SJ, Logan BK. Sample Mining and Data Mining: Combined Real-Time and Retrospective Approaches for the Identification of Emerging Novel Psychoactive Substances. J Forensic Sci. 2020;65(2):550–562. doi: 10.1111/1556-4029.14184 [DOI] [PubMed] [Google Scholar]
- 25.Arts DGT, De Keizer NF, Scheffer GJ. Defining and improving data quality in medical registries: A literature review, case study, and generic framework. J Am Med Informatics Assoc. 2002;9(6):600–611. doi: 10.1197/jamia.M1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirshon JM, Warner M, Irvin CB, et al. Research using emergency department-related data sets: Current status and future directions. Acad Emerg Med. 2009;16(11):1103–1109. doi: 10.1111/j.1553-2712.2009.00554.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forrester MB. Xylazine Exposures Reported to Texas Poison Centers. J Emerg Med. 2016;51(4):389–393. doi: 10.1016/j.jemermed.2015.09.051 [DOI] [PubMed] [Google Scholar]
- 28.Hoffman RJ. Methylxanthines and Selective β2-Adrenergic Agonists. In: Nelson LS, Howland MA, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, eds. Goldfrank’s Toxicologic Emergencies, 11e. McGraw-Hill Education; 2019. http://accessemergencymedicine.mhmedical.com/content.aspx?aid=1163013735 [Google Scholar]
- 29.Spoerke DG, Hall AH, Grimes MJ, Honea BN, Rumack BH. Human overdose with the veterinary tranquilizer xylazine. Am J Emerg Med. 1986;4(3):222–224. doi: 10.1016/0735-6757(86)90070-7 [DOI] [PubMed] [Google Scholar]
- 30.Smollin CG, Hoffman RS. Cocaine. In: Nelson LS, Howland MA, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, eds. Goldfrank’s Toxicologic Emergencies, 11e. McGraw-Hill Education; 2019. http://accessemergencymedicine.mhmedical.com/content.aspx?aid=1163018244 [Google Scholar]
- 31.Perrone J, Hamilton R, Nelson L, et al. Scopolamine poisoning among heroin users - New York City, Newark, Philadelphia, and Baltimore, 1995 and 1996. J Am Med Assoc. 1996;276(2):92–93. doi: 10.1001/jama.1996.03540020014008 [DOI] [Google Scholar]
- 32.Phillips KA, Hirsch GA, Epstein DH, Preston KL. Cardiac complications of unwitting co-injection of quinine/quinidine with heroin in an intravenous drug user. J Gen Intern Med. 2012;27(12):1722–1725. doi: 10.1007/s11606-012-2089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorentin TR, Krotulski AJ, Martin DM, et al. Detection of Cutting Agents in Drug-Positive Seized Exhibits within the United States. J Forensic Sci. 2019;64(3):888–896. doi: 10.1111/1556-4029.13968 [DOI] [PubMed] [Google Scholar]
- 34.Hieger MA, Emswiler MP, Maskell KF, et al. A Case Series of Clenbuterol Toxicity Caused by Adulterated Heroin. J Emerg Med. 2016;51(3):259–261. doi: 10.1016/j.jemermed.2016.05.047 [DOI] [PubMed] [Google Scholar]
- 35.Manini A, Labinson RM, Kirrane B, Hoffman RS, Rao R, Stajic M, Nelson LS. A novel neuromuscular syndrome associated with clenbuterol-tainted heroin. Clin Toxicol (Phila). 2008. Dec;46(10):1088–92. [DOI] [PubMed] [Google Scholar]
- 36.Fogarty MF, Mohr ALA, Papsun DM, Logan BK. Analysis of the Illicit Opioid U-48800 and Related Compounds by LC-MS-MS and Case Series of Fatalities Involving U-48800. J Anal Toxicol. 2022;46(1):17–24. doi: 10.1093/jat/bkaa180 [DOI] [PubMed] [Google Scholar]
- 37.Zawilska JB, Kuczyńska K, Kosmal W, Markiewicz K, Adamowicz P. Carfentanil – from an animal anesthetic to a deadly illicit drug. Forensic Sci Int. 2021;320. doi: 10.1016/j.forsciint.2021.110715 [DOI] [PubMed] [Google Scholar]
- 38.Seger D, Loden JK. Naloxone reversal of clonidine toxicity: dose, dose, dose. Clin Toxicol. 2018;56(10):873–879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


