Abstract
Introduction
Percutaneous coronary interventions (PCI) in bifurcations are still challenging and are associated with higher risks of periprocedural complications as well as restenosis and stent thrombosis. The aim of this paper was to summarize 12 months of clinical results of the prospective, first-in-man registry assessing the BiOSS LIM C stent (Balton, Poland).
Material and methods
In the prospective two-center registry we enrolled patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) and stable coronary artery disease. Provisional T-stenting was the default treatment strategy. The primary endpoint was defined as the rate of cardiac death, myocardial infarction (MI) and clinically driven target lesion revascularization (TLR) in 12-month follow-up.
Results
The study population consisted of 95 patients (mean age: 66.8 ±9.8 years, 17.9% were females). A BiOSS LIM C stent was implanted in the left main (LM) in 53 (55.8%) cases. There were 25.2% of patients with NSTE-ACS, 33.7% with diabetes, 90.5% with hypertension, and 53.7% had previous MI. The device success rate was 100%. An additional regular drug-eluting stent was deployed in the side branch in 18.9% of cases. Proximal optimization technique and final kissing balloon (FKB) technique were used in 53.7% and 30.5% of cases, respectively. MI type 4a was registered in 4 (4.2%) cases. At 12 months the MACE rate was 9.5%, cardiac death 1.1%, MI 2.1% and clinically driven TLR 6.3%. All incidents, apart from one TLR, appeared in the LM subgroup.
Conclusions
Our registry might suggest that PCI using the BiOSS LIM C in coronary bifurcations is feasible and might be an option for percutaneous revascularization.
Keywords: coronary bifurcation, left main, dedicated bifurcation stent, drug-eluting stent, sirolimus-eluting stent
Introduction
Percutaneous coronary interventions (PCI) in bifurcations are still challenging and are associated with higher risks of periprocedural complications as well as in-stent restenosis and stent thrombosis [1, 2]. Dedicated bifurcation stents were proposed as one of the resolutions to these issues [3]. However, few of them have been tested in large randomized clinical trials [4, 5].
In response to this interest, the Polish dedicated bifurcation stent was designed. The BiOSS Clinical Program started in 2008. Initially, the BiOSS stent was the only stainless steel stent, but shortly after in 2010 a paclitaxel-eluting stainless steel stent, the BiOSS Expert stent, was developed. Moreover, going with the times, in 2012 the sirolimus-eluting stainless steel BiOSS LIM stent was released. We gathered a lot of data coming from both randomized clinical trials as well as from real world evidence (RWE) studies [6–14]. The data were acceptable and encouraging, but still a way for enhancement was sought. In consequence, a cobalt-chromium sirolimus-eluting version was created – the BiOSS LIM C stent. Research on an animal model met the expectations as to the safety profile and effectiveness, and thus the BiOSS LIM C stent started the phase of clinical trials [15].
Previously, we presented 3-month results of the interim analysis of the BiOSS LIM C Registry [16]. The aim of this research was to summarize 12 months’ complete clinical results of the prospective, first-in-man BiOSS LIM C registry.
Material and methods
Study population and device description
Between August 2016 and December 2017 we enrolled consecutive patients meeting exclusion and inclusion criteria with stable coronary artery disease or non-ST-elevation acute coronary syndrome (NSTE-ACS). Procedures were performed in two centers in Poland (Warsaw, Olsztyn). Prior to the procedure, written, informed consent was obtained. The Local Ethics Committee approved the registry protocol (No. 84/2016).
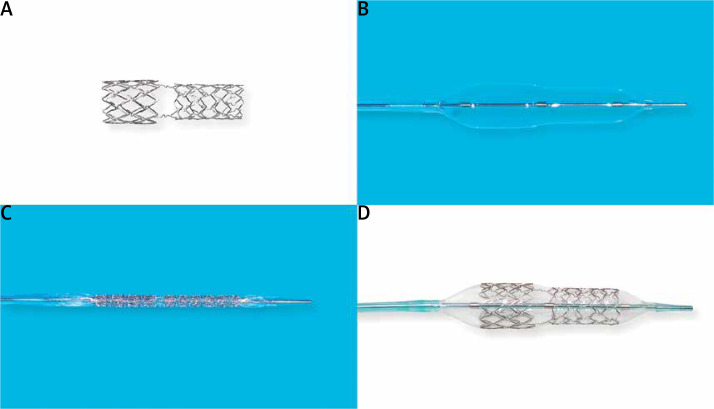
The BiOSS LIM C is a balloon-expandable sirolimus-eluting stent made of cobalt-chromium (strut thickness 70 μm). Sirolimus (1.4 μg/mm2) is released from the biodegradable coating built of a copolymer of lactic and glycolic acids (Figure 1). The BiOSS LIM C stent can be characterized as having two parts different in the diameter. The proximal part is joined with the distal part by only two struts. The details of patient population/device description were presented in detail previously [16].
Figure 1.
A – BiOSS LIM C stent alone, B – bottle balloon catheter, C – BiOSS LIM C crimped on the balloon, D – the fully deployed BiOSS LIM C
Interventional procedure and concomitant medications
Provisional T-stenting (PTS), i.e. single stent deployment in the main vessel-main branch across a side branch, was the default strategy in all patients. Medina classification was used to assess bifurcation lesions, where the value of 1 indicated stenosis greater than 50% (visual estimation) [17]. No restriction was applied in terms of lesion length. If necessary, an additional regular sirolimus-eluting stent was deployed. A drug-eluting stent was deployed in a side branch only if proximal residual stenosis was greater than 70% after balloon dilatation and/or significant blood flow disturbances after main vessel–main branch stenting and/or blood flow limiting dissection were observed.
The stent deployment protocol was based on the recent European Bifurcation Club guidelines and it was as follows [18]:
wiring of both branches,
main vessel predilatation and/or side branch predilatation according to the operator’s judgment,
stent deployment (inflation for at least 20 s),
proximal optimization technique (POT; highly recommended, but at operator’s judgment),
side branch postdilatation/side branch stent deployment if required,
final kissing balloon inflation after 2nd stent implantation or at operator’s judgment in other cases,
re-POT after final kissing balloon technique.
Proximal optimization technique is a technique in which a short balloon is expanded in the proximal part of the stent in the main vessel. This ensures full expansion and complete apposition of the stent as well as facilitating re-crossing into the side branch. Final kissing balloon technique is mandatory when two stents are implanted, and is based on simultaneous inflation of two balloons – one located in the main vessel/main branch, and one located in the side branch.
In patients with NSTE-ACS a loading dose of P2Y12 inhibitor was given (clopidogrel 600 mg or ticagrelor 180 mg), and if necessary, also a loading dose of acetylsalicylic acid (ASA) was administered (300 mg). In planned cases 72 h before the procedure patients received ASA (75 mg/day) and clopidogrel (75 mg/day) or ticagrelor (90 mg b.i.d). All percutaneous coronary interventions (PCIs) were performed via radial or femoral access using 6 Fr or 7 Fr guiding catheters, and periprocedurally unfractionated heparin was administered (100 IU/kg). Dual antiplatelet therapy was recommended for 12 months.
All patients had TnI, CK and CK-MB levels determined before PCI, and 6 and 24 h after. Periprocedural myocardial infarction (MI) (type 4a) was defined according to the third universal definition [19].
Endpoints
The composite primary endpoint (major adverse cardiovascular events – MACE) was defined as the rate of cardiac death, MI and clinically driven target lesion revascularization (TLR) in 12-month follow-up. Secondary endpoints were as follows: death rate, MI rate, stent thrombosis (ST) rate as well as the device, angiographic and procedure success. Clinically driven TLR was defined as the reintervention within the target lesion because of a symptomatic ≥ 50% diameter stenosis within 12 months after the index procedure. Further details were presented previously [16].
Statistical analysis
Continuous variables are shown as mean ± standard deviation. Categorical data are shown as numbers (%). Continuous variables were compared using an unpaired two-sided Student t-test, and categorical data using the χ2 test or Fisher’s exact test, as appropriate. If the distribution was not normal in the Shapiro-Wilk test, the Wilcoxon signed-rank and Mann-Whitney U-tests were applied. P-values of < 0.05 were recognized as statistically significant. The time to event analysis was performed with the Kaplan-Meier estimator of survival curves. Statistical analyses were performed using R 3.0.2 software for OS (R Foundation, Vienna, Austria).
Results
The study population consisted of 95 patients. The mean age was 66.8 ±9.8 years and 17.9% (n = 17) were females. In the study population hypertension (90.5%) and dyslipidemia (81.1%) were the most common risk factors. The target lesion was located in the left main most commonly (55.8%, n = 53) (Table I). In Table II baseline clinical characteristics in subgroups LM vs. non-LM are presented. In the LM subgroup significantly more patients had diabetes (43.4% vs. 21.4%, p < 0.05) and dyslipidemia (84.9% vs. 52.4%, p < 0.05). Baseline angiographic characteristics are presented in Table III. It is noteworthy that true bifurcations were present in 52.6% (n = 50).
Table I.
Baseline characteristics
| Parameter | BiOSS group, n = 95 (%) |
|---|---|
| Age [years] | 66.8 ±9.8 |
| Women (%) | 17 (17.9) |
| Hypertension | 86 (90.5) |
| Hypercholesterolemia | 77 (81.1) |
| Diabetes type 2 | 32 (33.7) |
| Prior MI | 51 (53.7) |
| Prior PCI | 44 (46.3) |
| CABG | 14 (14.7) |
| Peripheral artery disease | 10 (10.5) |
| Chronic kidney disease | 13 (13.7) |
| History of smoking | 21 (22.1) |
| Clinical indication for PCI: | |
| Planned PCI | 70 (73.7) |
| UA | 14 (14.7) |
| NSTEMI | 10 (10.5) |
| STEMI | 0 |
| Lesion location: | |
| LM | 53 (55.8) |
| LAD | 26 (27.4) |
| LCx | 13 (13.7) |
| RCA | 3 (3.2) |
MI – myocardial infarction, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft, UA – unstable angina, NSTEMI – non-ST elevation myocardial infarction.
Table II.
Baseline characteristics in left main (LM) and non-LM subgroups
| Parameter | LM subgroup, n = 53 (%) | Non-LM subgroup, n = 42 (%) |
|---|---|---|
| Age [years] | 68.3 ±8.6 | 65.8 ±7.4 |
| Women (%) | 12 (22.6) | 5 (11.9) |
| Hypertension | 47 (88.7) | 39 (92.9) |
| Hypercholesterolemia | 45 (84.9) | 22 (52.4)* |
| Diabetes type 2 | 23 (43.4) | 9 (21.4)* |
| Prior MI | 30 (56.6) | 21 (50) |
| Prior PCI | 28 (52.8) | 16 (38.1) |
| CABG | 11 (20.8) | 3 (7.1) |
| Peripheral artery disease | 6 (11.3) | 4 (9.5) |
| Chronic kidney disease | 6 (11.3) | 7 (16.7) |
| History of smoking | 13 (24.5) | 8 (19.1) |
MI – myocardial infarction, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft,
p < 0.05 between LM and non-LM groups.
Table III.
Baseline angiographic characteristics
| Parameter | Whole population, n = 95 (%) | LM subgroup, n = 53 (%) | Non-LM subgroup, n = 42 (%) |
|---|---|---|---|
| SYNTAX score | 26.09 ±3.4 | 27.6 ±4.2 | 24.2 ±2.8 |
| EuroSCORE II | 0.86 ±0.21 | 0.94 ±0.32 | 0.76 ±0.29 |
| Medina classification: | |||
| 1.1.1 | 22 (23.2) | 12 (22.6) | 10 (23.8) |
| 1.0.1 | 13 (13.7) | 6 (11.3) | 7 (16.7) |
| 0.1.1 | 15 (15.8) | 7 (13.2) | 8 (19.1) |
| 1.0.0 | 12 (12.6) | 8 (15.1) | 4 (9.5) |
| 0.1.0 | 10 (10.5) | 6 (11.3) | 4 (9.5) |
| 1.1.0 | 23 (24.2) | 7 (13.2) | 16 (38.1) |
LM – left main.
The procedure characteristics are shown in Table IV. The device and angiographic success rates were 100%. The procedure success rate was 95.8%. Main branch predilatation was performed in 80% of cases. The side branch required additional dilatation in over a third of lesions (38.9%), and in 18 cases (18.9%) the classical DES was deployed, mostly in T and protrusion (TAP) technique (n = 15, 15.8%). The proximal optimization technique was performed in 53.7% of cases, and the final kissing balloon inflation was used in 29 (30.5%) patients.
Table IV.
Procedural characteristics
| Parameter | BiOSS group N (%) | LM subgroup n = 53 (%) | Non-LM subgroup n = 42 (%) |
|---|---|---|---|
| Successful implantation | 95 (100) | 53 (100) | 42 (100) |
| Main vessel predilatation | 76 (80.0) | 45 (84.9) | 31 (73.8) |
| Side branch predilatation | 25 (26.3) | 20 (37.7) | 5 (11.9)* |
| Both branches’ predilatation | 19 (20.0) | 17 (32.1) | 2 (4.8)* |
| Nominal stent diameter in main vessel [mm] | 3.89 ±0.45 | 4.17 ±0.36 | 3.78 ±0.35* |
| Nominal stent diameter in main branch [mm] | 3.18 ±0.42 | 3.42 ±0.35 | 3.01 ±0.23* |
| Nominal stent length [mm] | 19.51 ±3.29 | 19.94 ±3.16 | 18.78 ±3.43 |
| Side branch postdilatation | 37 (38.9) | 23 (43.4) | 14 (33.3) |
| POT | 51 (53.7) | 37 (69.8) | 14 (33.3)* |
| FKB | 29 (30.5) | 22 (41.5) | 7 (16.7)* |
| Additional stent in SB | 18 (18.9) | 15 (28.3) | 3 (7.1)* |
| Dissection requiring an additional stent in main vessel | 11 (11.6) | 7 (13.2) | 4 (9.5) |
| Fluoroscopy time [min] | 19.6 ±12.4 | 21.2 ±12.1 | 17.6 ±9.5 |
| Contrast volume [ml] | 197 ±66 | 211 ±75 | 180 ±43 |
| Vascular access femoral/radial | 6.3%/93.7% | 5.7%/94.3% | 7.1%/92.9% |
| Guiding catheter 6F/7F | 95.8%/5.3% | 90.6%/9.4% | 100%/0 |
LM – left main, POT – proximal optimization technique, FKB – final kissing balloon;
p < 0.05 between LM and non-LM groups.
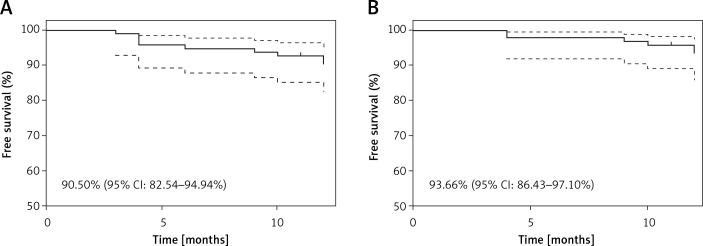
Myocardial infarction type 4a was registered in 4 (4.2%) patients and was caused by temporary side branch occlusion. In the 12-month follow-up the MACE rate was 9.5% (n = 9), including 1 (1.1%) cardiac death due to heart failure exacerbation, 2 (2.1%) MI and 6 (6.3%) clinically driven TLR (Table V). Kaplan-Meier curves are presented in the Figure 2. All incidents, apart from one TLR, appeared in the LM subgroup (Table VI). In the case of TLR 2 cases were treated with plain old balloon angioplasty (POBA), 3 with another DES and 1 with CABG (Figure 3).
Table V.
BiOSS LIM C clinical results
| Parameter | 1 month n = 95 (%) | 3 months n = 95 (%) | 6 months n = 95 (%) | 12 months n = 95 (%) |
|---|---|---|---|---|
| MACE | 0 | 1 (1.1) | 3 (3.2) | 9 (9.5) |
| Death | 0 | 0 | 1 (1.1)** | 2 (2.1)# |
| Cardiac death | 0 | 0 | 0 | 1 (1.1) |
| MI | 4 (4.2)* | 0 | 0 | 2 (2.1) |
| TLR clinically driven | 0 | 1 (1.1) | 3 (3.2) | 6 (6.3) |
MACE – major adverse cardiovascular event, MI – myocardial infarction, TLR – target lesion revascularization.
Periprocedural MI type 4a.
Ruptured aortic aneurysm.
Mediastinitis.
Figure 2.
Kaplan-Meier curves showing event-free survival for major adverse cardiovascular events (A) and target lesion revascularization (B), respectively
Table VI.
Clinical results of BiOSS LIM C: LM vs. non-LM
| Parameter | 12 months n = 95 (%) | LM n = 53 (%) | Non-LM n = 42 (%) |
|---|---|---|---|
| MACE | 9 (9.5) | 8 (15.1) | 1 (2.4) |
| Death | 2 (2.1)# | 2 (3.8) | 0 |
| Cardiac death | 1 (1.1) | 1 (1.9) | 0 |
| MI | 2 (2.1) | 2 (3.8) | 0 |
| TLR clinically driven | 6 (6.3) | 5 (9.4) | 1 (2.4) |
MACE – major adverse cardiovascular event, MI – myocardial infarction, TLR – target lesion revascularization.
Mediastinitis.
Figure 3.
Restenosis location in BiOSS LIM C stent
Discussion
The 12-month results of BiOSS LIM C deployment are acceptable and encouraging. The stent was successfully deployed in 100% without major difficulties, even in direct stenting. The MACE rate was 9.5%, and the clinically driven TLR rate 6.3%.
When analyzing results, a high predilatation rate was observed (80%). It could have been associated with a significant portion of true bifurcations (52.6%). However, the relatively low proportion of POT cases might be surprising. Nevertheless, one must bear in mind the POT-like effect associated with the BiOSS LIM C structure. When sizing the BiOSS stent the operator might overestimate the main vessel diameter and therefore be convinced that he obtains a POT-like effect. However, we still believe that POT should be highly recommended.
BiOSS LIM C is the improved version of BiOSS stent differing in strut thickness (70 μm vs. 120 μm). This difference might translate into better clinical outcomes. The results observed in the porcine model with the BiOSS LIM C stent seemed to confirm our hope [20]. Interestingly, Table VII presents numerical comparisons of clinical results between BiOSS LIM C, BiOSS LIM and BiOSS Expert. The results suggest that BiOSS LIM C might be superior to BiOSS Expert, but the advantages of BiOSS LIM C over BiOSS LIM need further elucidation, especially when we analyze the LM population.
Table VII.
12-month follow-up from three BiOSS Registries
| Parameter | BiOSS LIM C, n = 95 (%) | BiOSS LIM, n = 60 (%) | BiOSS Expert, n = 63 |
|---|---|---|---|
| MACE | 9 (9.5) | 3 (5) | 9 (14.3) |
| Death | 2 (2.1) | 1 (1.67) | 2 (3.2) |
| Cardiac death | 1 (1.1) | 1 (1.67) | 2 (3.2) |
| MI | 2 (2.1) | 1 (1.67) | 0 |
| TLR clinically driven | 6 (6.3) | 1 (1.67) | 7 (11.1) |
MACE – major adverse cardiovascular event, MI – myocardial infarction, TLR – target lesion revascularization.
In the BiOSS LIM LM Registry the rate of MACE rate was 9.5% (n = 7), whereas the rate of clinically driven TLR was 6.8% (n = 5) [7]. In the BiOSS LIM C Registry, these values were 15.1% (n = 8) and 9.4% (n = 5), respectively. These results are difficult to explain since the rate of true bifurcations, nominal stent parameters, and the rate of FKB were comparable or even higher in the case of the POT rate. Nevertheless, the factors negatively affecting the BiOSS LIM C results might be as follows: SYNTAX score (27.6 ±4.2 vs. 22.42 ±4.38) and the rate of additional stent implantation in the side branch (28.3% vs. 14.9%). Also, one should remember that the difference in radial force exerted by different stent platforms (cobalt-chromium vs. stainless steel) could play a significant role [21].
However, regarding the true value of the BiOSS LIM C stent, the two ongoing multicenter international studies will decide. The first one, POLBOS 3 (NCT03548272), will assess the stent in non-LM coronary bifurcations, and the second, POLBOS LM (NCT03508219), will evaluate the use of the BiOSS LIM C stent in the treatment of LM distal stenosis.
And finally, we should put our registry into the perspective of the European Society of Cardiology guidelines on myocardial revascularization from 2018 [22]. They recommend PCI in LM in patients with a low (class I) or intermediate (class IIa) SYNTAX score, and they recommend PCI in three-vessel CAD without diabetes only in patients with a low SYNTAX score (class I). In patients with three-vessel disease, diabetes and a low SYNTAX score PCI has class IIb recommendations. In our study population 33.7% of patients had diabetes and the mean SYNTAX score was 26.09 ±3.4. When choosing between PCI and CABG it is also worth remembering about the patient’s preferences, prior history of CABG, comorbidities (e.g. severe chronic obstructive pulmonary disease) and coronary artery anatomy (especially in patients with diabetes), in which sometimes there is no possibility to implant a well-functioning graft [23, 24].
The number of patients is relatively small. Patients with coronary bifurcation lesions a priori planned for a two-stent strategy were excluded. Also, no identical deployment techniques were used. However, PCIs were performed by operators experienced in BiOSS stent deployment. And finally, no control group was gathered to compare the use of this dedicated bifurcation stent and stenting with other devices and techniques.
In conclusion, our registry might suggest that PCI using the BiOSS LIM C in coronary bifurcations is feasible and might be the option for percutaneous revascularization.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Chatzizisis YS, Jonas M, Coskun AU, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation 2008; 117: 993-1002. [DOI] [PubMed] [Google Scholar]
- 2.Jankowski P, Czarnecka D, Badacz L, et al. Practice setting and secondary prevention of coronary artery disease. Arch Med Sci 2018; 14: 979-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra S. Dedicated bifurcation stents – mechanistic, hardware, and technical aspects. Indian Heart J 2016; 68: 841-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrini D, Cortese B. Focus on STENTYS((R)) Xposition S Self-Apposing((R)) stent: a review of available literature. Future Cardiol 2019; 15: 145-59. [DOI] [PubMed] [Google Scholar]
- 5.Konigstein M, Srdanovic I, Gore AK, et al. Outcomes of the Tryton-dedicated bifurcation stent for the treatment of true coronary bifurcations: individual-patient-data pooled analysis. Catheter Cardiovasc Interv 2019; 93: 1255-61. [DOI] [PubMed] [Google Scholar]
- 6.Gil RJ, Bil J, Grundeken MJ, et al. Regular drug-eluting stents versus the dedicated coronary bifurcation sirolimus-eluting BiOSS LIM(R) stent: the randomised, multicentre, open-label, controlled POLBOS II trial. EuroIntervention 2016; 12: e1404-12. [DOI] [PubMed] [Google Scholar]
- 7.Gil RJ, Bil J, Grundeken MJ, et al. Long-term effectiveness and safety of the sirolimus-eluting BiOSS LIM(R) dedicated bifurcation stent in the treatment of distal left main stenosis: an international registry. EuroIntervention 2016; 12: 1246-54. [DOI] [PubMed] [Google Scholar]
- 8.Gil RJ, Bil J, Dzavik V, et al. Regular drug-eluting stent vs dedicated coronary bifurcation bioss expert stent: multicenter open-label randomized controlled POLBOS I trial. Can J Cardiol 2015; 31: 671-78. [DOI] [PubMed] [Google Scholar]
- 9.Gil RJ, Bil J, Vassiliev D, Inigo Garcia LA. First-in-man study of dedicated bifurcation sirolimus-eluting stent: 12-month results of BiOSS LIM(R) Registry. J Interv Cardiol 2015; 28: 51-60. [DOI] [PubMed] [Google Scholar]
- 10.Bil J, Gil RJ, Vassilev D, Rzezak J, Kulawik T, Pawlowski T. Dedicated bifurcation paclitaxel-eluting stent BiOSS Expert(R) in the treatment of distal left main stem stenosis. J Interv Cardiol 2014; 27: 242-51. [DOI] [PubMed] [Google Scholar]
- 11.Gil RJ, Vassilev D, Michalek A, et al. Dedicated paclitaxel-eluting bifurcation stent BiOSS(R) (bifurcation optimisation stent system): 12-month results from a prospective registry of consecutive all-comers population. EuroIntervention 2012; 8: 316-24. [DOI] [PubMed] [Google Scholar]
- 12.Bil J, Grundeken MJ, Pawlowski T, Gil RJ. Self-positioning properties of dedicated bifurcation coronary stent BiOSS LIM(R) in the eye of 3D optical coherence tomography. Minerva Cardioangiol 2017; 65: 194-6. [DOI] [PubMed] [Google Scholar]
- 13.Springler A, Hessenberger S, Reisinger N, et al. Deoxynivalenol and its metabolite deepoxy-deoxynivalenol: multi-parameter analysis for the evaluation of cytotoxicity and cellular effects. Mycotoxin Res 2017; 33: 25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salat K, Podkowa A, Malikowska N, et al. Novel, highly potent and in vivo active inhibitor of GABA transporter subtype 1 with anticonvulsant, anxiolytic, antidepressant and antinociceptive properties. Neuropharmacology 2017; 113: 331-42. [DOI] [PubMed] [Google Scholar]
- 15.Bil J, Gil RJ, Pawlowski T, Milewski KP. Assessment of vascular response to BiOSS LIM C((R)) stents vs Orsiro((R)) stents in the porcine coronary artery model. Cardiovasc Ther 2017; 35. doi: 10.1111/1755-5922.12267. [DOI] [PubMed] [Google Scholar]
- 16.Gil RJ, Bil J, Kern A, Pawlowski T. First-in-man study of dedicated bifurcation cobalt-chromium sirolimus-eluting stent BiOSS LIM C(R) – three-month results. Kardiol Pol 2018; 76: 464-70. [DOI] [PubMed] [Google Scholar]
- 17.Medina A, Suarez de Lezo J, Pan M. A new classification of coronary bifurcation lesions. Rev Esp Cardiol 2006; 59: 183. [PubMed] [Google Scholar]
- 18.Lassen JF, Holm NR, Banning A, et al. Percutaneous coronary intervention for coronary bifurcation disease: 11th consensus document from the European Bifurcation Club. EuroIntervention 2016; 12: 38-46. [DOI] [PubMed] [Google Scholar]
- 19.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020-35. [DOI] [PubMed] [Google Scholar]
- 20.Bil J, Gil RJ, Pawlowski T, Milewski KP. Assessment of vascular response to BiOSS LIM C((R)) stents vs Orsiro((R)) stents in the porcine coronary artery model. Cardiovasc Ther 2017; 35: e12267. [DOI] [PubMed] [Google Scholar]
- 21.Chichareon P, Katagiri Y, Asano T, et al. Mechanical properties and performances of contemporary drug-eluting stent: focus on the metallic backbone. Expert Rev Med Devices 2019; 16: 211-28. [DOI] [PubMed] [Google Scholar]
- 22.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87-165. [DOI] [PubMed] [Google Scholar]
- 23.Jager B, Piackova E, Haller PM, et al. Increased platelet reactivity in dyslipidemic patients with coronary artery disease on dual anti-platelet therapy. Arch Med Sci 2019; 15: 65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dziedzic EA, Gasior JS, Pawlowski M, et al. Vitamin D level is associated with severity of coronary artery atherosclerosis and incidence of acute coronary syndromes in non-diabetic cardiac patients. Arch Med Sci 2019; 15: 359-68. [DOI] [PMC free article] [PubMed] [Google Scholar]