Abstract
Introduction
The most common cause of death in patients with amyotrophic lateral sclerosis (ALS) is respiratory failure, often in the period of 2–5 years, with a small percentage of patients surviving up to 10 years or more. The aim of the study was to evaluate the significance of pulmonary function tests in prediction of mortality and definition of indications for noninvasive mechanical ventilation (NIMV).
Material and methods
This retrospective-prospective study was performed at the Clinic of Pulmonology, Clinical Centre of Serbia in the period from January 2015 to December 2017. Patients with diagnosis of ALS established according to El Escorial criteria were included.
Results
The study included 76 patients with ALS, 50 (65.85%) with spinal and 26 (34.2%) with bulbar form of disease. Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) were higher in spinal form of ALS, and the difference was statistically significant when compared to bulbar form. Form of disease, FVC < 70%, maximum inspiratory pressure (PImax) < 50 and maximum expiratory pressure (PEmax) < 50 were significant factors for survival. The patients with bulbar form of disease had 2.174 (95.0% CI: 1.261–3.747) higher risk for death.
Conclusions
Our study points to the significance of timely application and early start of NIMV in patients with ALS as an important approach to defer functional impairment, which would mean that the criteria, in our country, for application of these devices must be changed, not only regarding the value of current functional diagnostic tests used in everyday practice in patients with ALS but also in regard to the introduction of new diagnostic tests, such as sniff nasal inspiratory pressure and/or polysomnographic testing.
Keywords: pulmonary function, amyotrophic lateral sclerosis, noninvasive ventilation
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neuromuscular disease that causes skeletal muscle weakness, including muscles involved with respiration. Amyotrophic lateral sclerosis belongs to a group of neurological diseases, and affects the upper and lower motor neurons. It is one of the most common motor neuron diseases, and can occur individually or as a hereditary family illness. The most common cause of death in these patients is respiratory failure, often in the period of 2–5 years, with a small percentage of patients surviving up to 10 years and more. Accordingly, monitoring respiratory status is critical to ALS management. Pulmonary function tests (PFTs) are used for evaluation of respiratory impairment and decision-making including the timing of noninvasive ventilation. Recognizing different respiratory tests in relation to disease progression and survival may help determine which tests are most suitable [1].
Poor prognosis and decreased survival time of patients with ALS correlate with their nutritional status. Various studies have evaluated weight, body mass index (BMI), survival time and ALS Functional Rating Scale-Revised (ALSFRS-R) in order to determine the best nutrition management methods for this patient population [2].
The aim of the study was to determine the significance of pulmonary function tests in prediction of mortality and define indications for noninvasive mechanical ventilation (NIMV).
Material and methods
This cross-sectional retrospective study was performed at the Clinic of Pulmonology, Clinical Centre of Serbia in the period from January 2015 to December 2017. Approval granted by the local ethics committee was not required. Patients with a diagnosis of ALS established according to El Escorial criteria were included [3]. The diagnosis of disease was made at the Clinic of Neurology, Clinical Centre of Serbia.
The following PFTs were performed in all patients: spirometry, impulse oscillometry, maximal inspiratory and expiratory mouth pressures, and arterial blood gases analysis. Spirometry was performed by means of a Lilly’s pneumotachometer (MasterScreen Pneumo, Viasys Healthcare, Germany), impulse oscillometry and maximal inspiratory and expiratory pressures (PImax and PEmax) at the mouth during occlusion were performed using commercial systems (MasterScreen IOS, Viasys Healthcare, Germany, and MasterScreen Body, Viasys Healthcare, Germany, respectively). The analysis of the arterial blood gasses was also performed on a commercial analyzer (ABL5, Radiometer, Denmark). All PFTs were performed according to European Respiratory Society/American Thoracic Society joint recommendations [4–8].
Informed consent was not obtained because all study procedures were routinely performed in all patients with ALS at our clinic.
The current criteria for assigning the noninvasive mechanical ventilation (NIMV) in our country are forced vital capacity (FVC) less than 50% of the predicted, and global respiratory failure or elevated bicarbonate levels (more than 27–28 mmol/l) in arterial blood gases analyses. The criteria were determined by the Republic Fund of Health Insurance, which has been providing non-invasive mechanical ventilation devices for home treatment to insured persons since 2007. Since that time, these criteria have been widely accepted as indications for home ventilation in our country.
Blood samples were collected from patients for laboratory analysis (urea (normal values 2.5–7.5 mmol/l), creatinine (normal values 45–84 μmol/l), creatine kinase (CK) (normal values 0–150 U/l), CK-MB (normal values < 25 U/l)); all tests were performed in an Accredited Medical Laboratory, ATC03-001, SRPS EN 150 15189-2014.
Primary outcome was the time from diagnosis to the establishment of indications for NIMV, as well as the secondary outcome – the time from diagnosis to death.
The outcome was determined by direct contact with patients or their families during regular clinical visits or by telephone contact or with family members when returning the NIMV device.
Statistical analysis
Descriptive statistics was calculated for the baseline demographic and clinical features. Normality of distribution was tested using graphical and mathematical methods. Continuous variables were presented as means with standard deviations or median with 25th–75th percentile, if required. Categorical variables were reported in numbers and percentages. Differences between groups were analyzed using Student’s t-test for normally distributed continuous variables or the Mann-Whitney test for non-normally distributed continuous variables and Pearson’s χ2 test for categorical variables.
Survival was computed by means of Kaplan-Meier survival analysis, and two groups were compared using the log-rank test. Cox-regression analysis was used to determine factors influencing survival. The level of significance was set at 0.05. Statistical analysis was performed using the IBM SPSS 21 (Chicago, IL, 2012) package.
Results
The clinical characteristics of patients are presented in Table I. The study included 76 patients with ALS, 50 (65.8%) with spinal and 26 (34.2%) with bulbar form of disease. There was no difference in age, gender or BMI between these two groups. Forced vital capacity and forced expiratory volume in 1 s (FEV1) were higher in spinal form of ALS, and the difference was statistically significant when compared to bulbar form. There was no difference in R5HZ, R20HZ, PImax or PEmax between the groups. Laboratory parameters – urea, creatinine and creatine kinase (CK) – did not differ between the groups. CK-MB was higher in spinal form, and the difference was statistically significant in comparison with bulbar form.
Table I.
Clinical characteristics of amyotrophic lateral sclerosis patients
| Factor | Spinal n = 50 Mean ± SD | Bulbar n = 26 Mean ± SD | P-value |
|---|---|---|---|
| Age | 54.3 ±8.3 | 53.9 ±10.2 | 0.870 |
| Sex (male)* | 26 (52.0%) | 10 (38.5%) | 0.262 |
| BMI | 25.7 ±6.6 | 27 ±8.5 | 0.484 |
| FVC | 93.8 ±31.2 | 78.7 ±26.7 | 0.039 |
| FEV1 | 94.6 ±32.2 | 77.4 ±27.9 | 0.024 |
| R5HZ | 83.4 ±41.8 | 93.3 ±43 | 0.336 |
| R20HZ | 73.4 ±34.6 | 73.7 ±28.4 | 0.976 |
| PImax | 37.3 ±17.4 | 32.6 ±12.1 | 0.173 |
| PEmax | 39.7 ±25.2 | 42 ±25.5 | 0.707 |
| Urea | 5.1 ±1.5 | 5 ±1.4 | 0.832 |
| Creatinine | 58.6 ±15.9 | 58.6 ±19.7 | 0.997 |
| CK | 153.5 ±60.4 | 131.8 ±55.8 | 0.131 |
| CK-MB | 4.6 ±1.3 | 4 ±1.3 | 0.047 |
Data are presented as n (%).
BMI – body mass index, FVC – forced vital capacity, FEV1 – forced expiratory volume in 1 s, PImax – maximum inspiratory pressure, PEmax – maximum expiratory pressure, CK – creatine kinase.
Mean follow-up time was 31.1 (minimum–maximum 2.0–81.0) months. Overall median survival was 32.0 (95% CI: 28.1–35.9) months from ALS diagnosis to outcome.
A non-invasive ventilation machine (NIVM) was introduced after a median of 23.0 (12.0–31.0) months in spinal and 13.5 (8.0–23.0) months in bulbar form of disease (p < 0.05).
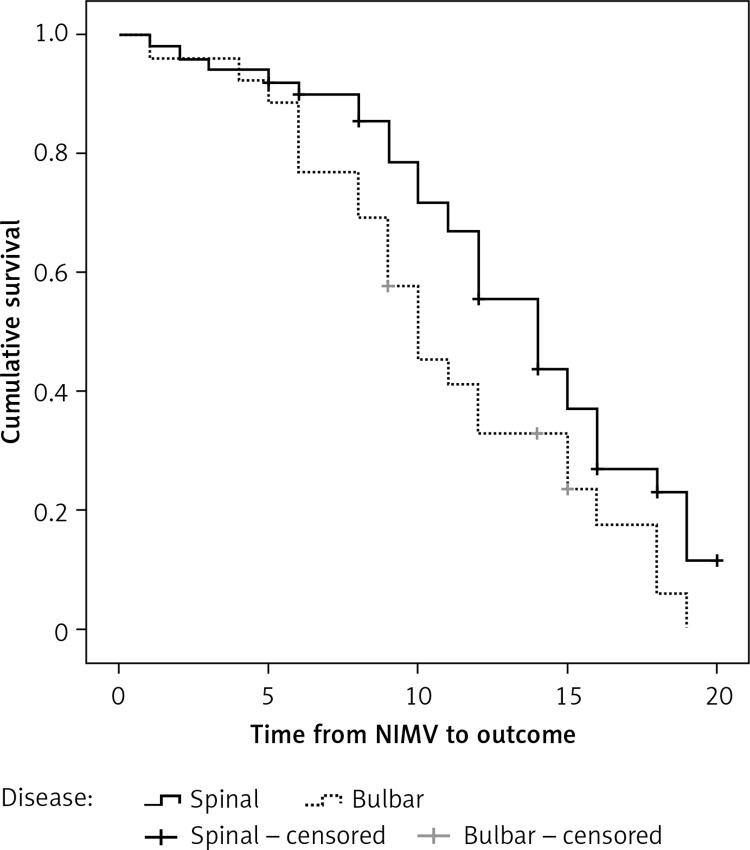
Median survival from NIMV introduction to outcome was 12.0 (95% CI: 9.98–14.03) months. Median survival from NIMV introduction to final outcome (death) in patients with spinal form was 14.0 (95% CI: 11.36–16.64), and 10.0 (95% CI: 7.64–12.35) months in patients with bulbar form of disease (Figure 1). According to the log-rank test, this difference was statistically significant (p < 0.05).
Figure 1.
Survival from noninvasive mechanical ventilation (NIMV) between the spinal and bulbar form
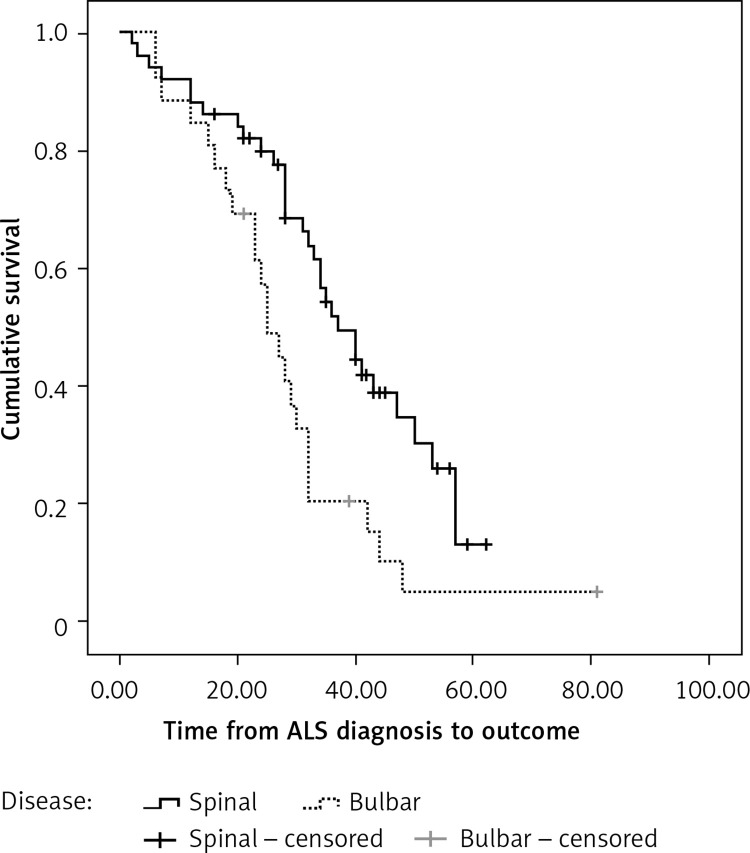
Median survival from diagnosis to final outcome (death) in patients with spinal form was 37.0 (95% CI: 29.74–44.26) months, and 25.0 (95% CI: 20.20–29.80) months in patients with bulbar form of disease (Figure 2). According to the log-rank test, this difference was statistically significant (p < 0.05).
Figure 2.
Survival from amyotrophic lateral sclerosis (ALS) diagnosis between the spinal and bulbar form
Cox-regression analysis was performed to determine the effect of pulmonary function parameter values recorded at the time of making the ALS diagnosis to survival, the results being presented in Table II.
Table II.
Factors influencing survival at the time of amyotrophic lateral sclerosis diagnosis
| Variable | HR | 95% CI for HR | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Bulbar form | 2.174 | 1.261 | 3.747 | 0.005 |
| FVC < 70% | 1.945 | 1.019 | 3.713 | 0.044 |
| R20HZ < 120 | 0.739 | 0.315 | 1.736 | 0.488 |
| R5HZ < 120 | 0.669 | 0.371 | 1.206 | 0.181 |
| PImax < 50 | 1.957 | 1.014 | 3.775 | 0.045 |
| PEmax < 50 | 2.156 | 1.117 | 4.162 | 0.022 |
FVC – forced vital capacity, PImax – maximum inspiratory pressure, PEmax – maximum expiratory pressure.
Form of disease, FVC < 70%, PImax < 50 and PEmax < 50 were significant factors for survival. R20HZ < 120 and R5HZ < 120 were not significant factors for survival in patients with ALS. The patients with bulbar form of disease had 2.17 (95% CI: 1.26–3.75) higher risk for death. The patients with FVC < 70% of disease had 1.94 (95% CI: 1.02–3.71), PImax < 50 and PEmax < 50 1.96 (95% CI: 1.01–3.77) higher risk.
Discussion
Our study showed that the patients with FVC less than 70% at the time of making their diagnosis as compared to the expected values carried two times higher risk of a lethal outcome. In addition, it was noted that the values of PImax and PEmax lower than 80% in relation to the expected values represented a twice-higher risk of lethal outcome. On the other hand, impulse oscillometry parameters did not show any significant association with the length of survival of these patients.
A large number of former cross-section studies have pointed to FVC limitations in detecting an early respiratory dysfunction. It is questionable whether the employment of the FVC value is a relevant criterion as well as whether its value should be confined to the decision on timely application of NIMV [9–12]. In the study by Gordon et al., 50 patients having required tracheotomy underwent spirometry tests and their FVC values were recorded 30 days prior to the aforesaid event; FVC was 50% of the expected value or higher in 11 patients, 60% of the expected value or higher in 7 patients and 70% of the expected value or higher in 5 patients. All the aforementioned data indicate a possible disadvantage of FVC as a tool for evaluation of patient survival [13].
Longitudinal follow-up of ALS patients by Polkey et al. confirmed that the differences in FVC values showed low amplitudes either until death occurred or need for NIMV appeared, while the values of respiratory muscle tests manifested significant amplitudes; accordingly, it directly suggested that the measurement of respiratory muscle strength could be a more beneficial marker for assessing the most optimal timing of NIMV application [14].
Forced vital capacity values during the maneuver of spirometry testing with the patient assuming a recumbent position designated the preservation of diaphragmal musculature strength. Lechtzin et al. recommended applying NIMV in all patients with FVC < 70% recorded during spirometry in a lying position [12]. Polkey et al. underlined the inferiority of the FVC value in ALS patients. This study pointed to worse prognosis in patients whose value of this parameter was even higher than 80% and in whom the NIMV was not started yet [14–16].
Measurement of the maximum static inspiratory pressure that a subject can generate at the mouth (PImax) or the maximum static expiratory pressure (PEmax) is a simple way to gauge inspiratory and expiratory muscle strength. The pressure measured during these maneuvers reflects the pressure developed by the respiratory muscles (Pmus), plus the passive elastic recoil pressure of the respiratory system including the lung and the chest wall (Prs) [17–21].
When respiratory muscle weakness occurs, PImax can be more sensitive than VC because the relationship between VC and PImax is curvilinear, so that decreases in respiratory muscle strength which develop before decreases in lung volume can be identified [22, 23].
Different studies have confirmed that the strength of respiratory muscles is one of the more significant prognostic markers in ALS patients; a recent study has underscored the need for adequate physical rehabilitation for musculature strengthening. The research studies suggest that this program of respiratory physical rehabilitation is well tolerated and leads to improvement of respiratory and bulbar functions [16].
During routine pulmonary function testing of ALS patients, it has been noted that some of them have elevated R20 and R5. Our study, however, did not find any correlation between the values of impulse oscillometry and time of survival of ALS patients (p = 0.488 for R20Hz and p = 0.181 for R5Hz), in spite of our high expectations that the patients with higher values of extrathoracic and intrathoracic airways resistance would have shorter time of survival. It is particularly true if we inspect the results suggesting that the time of survival in patients with bulbar disease is significantly shorter than in patients with the spinal form of the condition. Anyway, these results call for additional research in future as well as an analysis of the potential prognostic significance of airway resistance.
Presently recommended Medicare criteria (a federal government website managed and paid for by the U.S. Centers for Medicare & Medicaid Services) for NIMV application in ALS patients include any of the following: FVC < 50% of the expected, SAT O2 < 88% during at least 5 min, and PaCO2 44 mm Hg. Upon updating the American Academy of Neurology Database, two additional criteria were added, sniff nasal inspiratory pressure (SNIP) < 40 cm H2O and orthopnea, which may justify the introduction of noninvasive ventilation. In our country, the criteria of providing home care NIMV are FVC < 50% of the expected, and global respiratory insufficiency with HCO3 > 28 mmol/l obtained from arterial blood gas analysis. The study by Vitaca et al. revealed that the NIMV, in the Italian pulmonological units, was introduced in patients with neuromuscular conditions in case of diurnal hypercapnia, nocturnal desaturation and FVC < 50% of expected. Nevertheless, a large number of centers take the position that mere presence of symptoms such as dyspnea or orthopnea are adequate criteria for starting the ventilation [24].
The above result is immensely significant given that, in our country, the indication for NIMV application is FVC below 50%. But, our results demonstrate that FVC is a powerful predictive factor.
Our study has several limitations. First of all, measurement of maximal SNIP was not performed in our patients due to lack of equipment. SNIP has been shown to be strongly correlated with maximal sniff oesophageal pressure [25], is well established as a non-invasive method for assessing inspiratory muscle strength [26], and its values better correlate with onset of respiratory failure than FVC [27]. Next, SVC measurement was also not performed in our patients, despite the fact that change in SVC predicts respiratory failure or death in patients with ALS [28]. Similarly, nocturnal hypoventilation, known to be associated with poor prognosis, has not been studied in this analysis, in spite of the fact that an increasing number of experts nowadays believe that nocturnal hyperventilation is an indication of NIMV [18]. Finally, other prognostic factors in ALS, such as delay from symptom onset to diagnosis [29], progression rate of revised ALS Functional Rating Scale (ALSFRS-R) score [30], psychological distress [31], and frontotemporal lobar dementia [32], were not taken into account. All these factors, and several more, strongly affect quality of life and have an influence on survival of patients with ALS. A couple of prognostic scores based on mentioned and other parameters were developed for patients with ALS [33, 34]. Excluding these factors from our analysis could potentially have an influence on the results of this study, despite the fact that decline in lung function, measured primarily by FVC [35–37], is a strong independent prognostic factor in ALS.
The increasing number of clinicians and the authors of scientific and research papers agree on the fact that the sequential diagnostic procedures, especially if used in combination with sophisticated techniques as described by Carreiro et al., could offer an alternative predictive strategy [17].
In conclusion, our study points to the significance of timely application and early start of NIMV in patients with ALS as an important approach to defer functional impairment, which would mean that the criteria, in our country, for application of these devices must be changed, not only regarding the value of current functional diagnostic tests used in everyday practice in patients with ALS but also in regard to the introduction of new diagnostic tests, such as SNIP and/or polysomnographic testing.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lechtzin N, Cudkowicz ME, de Carvalho M, Genge A, Hardiman O, Mitsumoto H. Respiratory measures in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19: 321-30. [DOI] [PubMed] [Google Scholar]
- 2.Kellogg J, Bottman L, Arra EJ, Selkirk SM, Kozlowski F. Nutrition management methods effective in increasing weight, survival time and functional status in ALS patients: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19: 7-11. [DOI] [PubMed] [Google Scholar]
- 3.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases . El Escorial revised: world Federation of Neurology Criteria for the diagnosis of amyotrophic lateral sclerosis. Amiotroph Lateral Scler Other Motor Criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319-38. [DOI] [PubMed] [Google Scholar]
- 5.Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. Eur Respir Mon 2005; 31: 72-105. [Google Scholar]
- 6.Troosters T, Gosselink R, Decramer M. Respiratory muscle assessment. Eur Respir Mon 2005; 31: 57-71. [Google Scholar]
- 7.Roca J, Rabinovich R. Clinical exercise testing. Eur Respir Mon 2005; 31: 146-65. [Google Scholar]
- 8.Hughes JMB. Pulmonary gas exchange. Eur Respir Mon 2005; 31: 106-26. [Google Scholar]
- 9.Raaphorst J, Tuijp J, Verweij L, et al. Treatment of respiratory impairment in patients with motor neuron disease in the Netherlands: patient preference and timing of referral. Eur J Neurol 2013; 20: 1524-30. [DOI] [PubMed] [Google Scholar]
- 10.Capozzo R, Quaranta VN, Pellegrini F, et al. Sniff nasal Inspiratory pressure as a prognostic factor of tracheostomy or death in amyotrophic lateral sclerosis. J Neurol 2015; 262: 593-603. [DOI] [PubMed] [Google Scholar]
- 11.Morgan RK, McNally S, Alexander M, Conroy R, Hardiman O, Costello RW. Use of sniff nasal inspiratory force to predict survival in ALS. Am J Respir Crit Care Med 2005; 171: 269-74. [DOI] [PubMed] [Google Scholar]
- 12.Lechtzin N, Wiener CM, Shade DM, Clawson L, Diette GB. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest 2002; 121: 436-42. [DOI] [PubMed] [Google Scholar]
- 13.Gordon PH, Corcia P, Lacomblez L, et al. Defining survival as an outcome measure in amyotrophic lateral sclerosis. Arch Neurol 2009; 66: 758-61. [DOI] [PubMed] [Google Scholar]
- 14.Polkey M, Lyall R, Yang K, Johnson E, Leigh N, Moxham J. Respiratory muscle strength as a predictive biomarker for survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med 2017; 195: 86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet J Rare Dis 2009; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamnegård CH, Wragg SD, Mills GH, et al. Clinical assessment of diaphragm strength by cervical magnetic stimulation of the phrenic nerves. Thorax 1996; 51: 1239-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreiro AV, Amaral PM, Pinto S, Tomás P, de Carvalho M, Madeira SC. Prognostic models based on patient snapshots and time windows: predicting disease progression to assisted ventilation in amyotrophic lateral sclerosis. J Biomed Inform 2015; 58: 133-44. [DOI] [PubMed] [Google Scholar]
- 18.Vrijsen B, Buyse B, Belge C, et al. Noninvasive ventilation improves sleep in amyotrophic lateral sclerosis: a prospective polysomnographic study. J Clin Sleep Med 2015; 11: 559-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calverley PMA. Control of breathing. In: Lung Function Testing: European Respiratory Monograph. Gosselink R, Stam H (eds.). European Respiratory Society; 2005. [Google Scholar]
- 20.Troosters T, Gosselink R, Decramer M. Respiratory muscle assessment. Eur Respir Mon 2005; 31: 57-71. [Google Scholar]
- 21.Stanojevic S, Hall GL. Reference values for spirometry: the way forward for our patients. Respirology 2011; 16: 869. [DOI] [PubMed] [Google Scholar]
- 22.Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present and future. Eur Respir J 2010; 36: 12-9. [DOI] [PubMed] [Google Scholar]
- 23.Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care 2009; 54: 1348-59. [PubMed] [Google Scholar]
- 24.Aboussouan LS, Mireles-Cabodevila E. Respiratory supportin patients with amiotrophic lateral sclerosis. Respir Care 2013; 58: 1555-8. [DOI] [PubMed] [Google Scholar]
- 25.Heritier F, Rahm F, Pasche P, Fitting JW. Sniff nasal inspiratory pressure. A non-invasive assessment of inspiratory muscle strength. Am J Respir Crit Care Med 1994; 150: 1678-83. [DOI] [PubMed] [Google Scholar]
- 26.Uldry C, Fitting JW. Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax 1995; 50: 371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyall RA, Donaldson N, Polkey MI, Leigh PN, Moxham J. Respiratory muscle strength and ventilatory failure in ALS. Brain 2001; 124: 200-13. [DOI] [PubMed] [Google Scholar]
- 28.Andrews JA, Meng L, Kulke SF, et al. Association between decline in slow vital capacity and respiratory insufficiency, use of assisted ventilation, tracheostomy, or death in patients with amyotrophic lateral sclerosis. JAMA Neurol 2018; 75: 58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Testa D, Lovati R, Ferrarini M, Salmoiraghi F, Filippini G. Survival of 793 patients with amyotrophic lateral sclerosis diagnosed over a 28-year period. Amyotroph Lateral Scler 2004; 5: 208-12. [PubMed] [Google Scholar]
- 30.Kimura F, Fujimura C, Ishida S, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 2006; 66: 265-7. [DOI] [PubMed] [Google Scholar]
- 31.McDonald E, Wiedenfeld SA, Hillel A, Carpenter CL, Walter RA. Survival in amyotrophic lateral sclerosis. The role of psychological factors. Arch Neurol 1994; 51: 17-23. [DOI] [PubMed] [Google Scholar]
- 32.Armon C, Brandstater ME. Motor unit number estimate based rates of progression of ALS predict patient survival. Muscle Nerve 1999; 22: 1571-5. [DOI] [PubMed] [Google Scholar]
- 33.Elamin M, Bede P, Montuschi A, Pender N, Chio A, Hardiman O. Predicting prognosis in amyotrophic lateral sclerosis: a simple algorithm. J Neurol 2015; 262: 1447-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westeneng HJ, Debray TPA, Visser AE, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol 2018; 17: 423-33. [DOI] [PubMed] [Google Scholar]
- 35.Chiò A, Mora G, Leone M, et al. for the Piemonte and Valle d’Aosta Register for ALS. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology 2002; 59: 99-103. [DOI] [PubMed] [Google Scholar]
- 36.Armon C, Moses D. Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. J Neurol Sci 1998; 160 (Suppl 1): S37-41. [DOI] [PubMed] [Google Scholar]
- 37.Magnus T, Beck M, Giess R, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve 2002; 25: 709-14. [DOI] [PubMed] [Google Scholar]