Abstract
Prior case studies suggest that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its vaccines may unmask CNS neuroinflammatory conditions. We present a case of relapsing steroid-responsive encephalomyelitis after SARS-CoV-2 infection and subsequent COVID-19 vaccination. We also characterize the frequency of CNS neuroinflammatory events reported in the literature after both SARS-CoV-2 infection and COVID-19 vaccination.
Case Presentation
A 47-year-old man with a history of psoriasis and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection presented with 3 months of subacute lower extremity weakness, erectile dysfunction, and gait instability with falls. His symptoms started 2 weeks after receiving the second of his 2-shot primary mRNA vaccine series for coronavirus disease 2019 (COVID-19) vaccination, which he received 3 months after a mild case of COVID-19. He had no other relevant medical or family history and no recent travel. After evaluation by a urologist, he was prescribed a course of cefdinir for presumed prostatitis. His weakness and gait dysfunction worsened over the ensuing 3 months despite this treatment and prompted his presentation to our hospital.
On initial examination, his vital signs and general medical examination were normal. Neurologic examination demonstrated upbeat nystagmus on vertical gaze. On motor exam, he had increased tone in right lower extremity, 4/5 strength with right knee extension, 4/5 strength with bilateral ankle dorsiflexion and plantar flexion. Reflex testing showed lower extremity hyperreflexia with extensor plantar responses. On sensory exam, he had moderately impaired vibration at the ankles, with positive Romberg test with eye closure but no sensory level. Coordination testing revealed right-sided dysmetria with finger to nose. He had an unsteady gait and only able to walk a few steps without assistance, with complete inability to perform tandem gait.
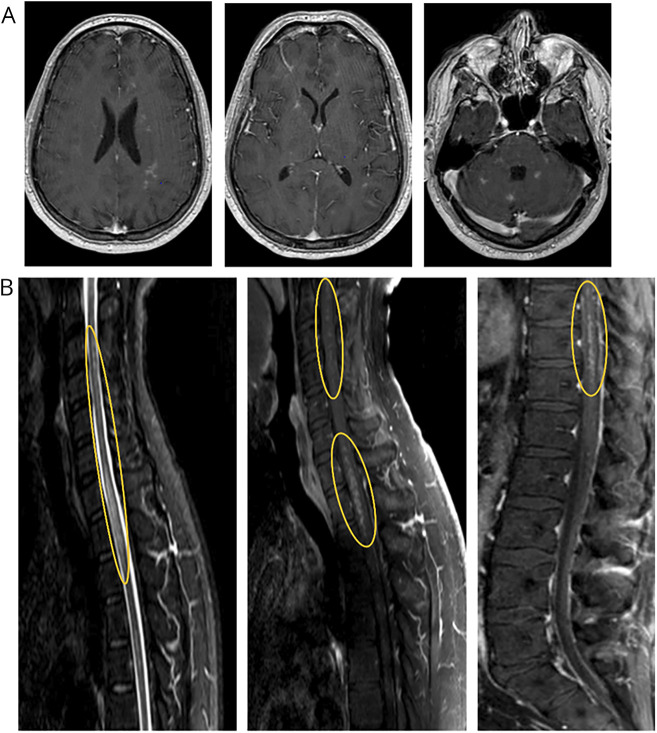
MRI of the brain and complete spine (Figure 1) demonstrated diffuse innumerable punctate enhancing lesions involving the subcortical white matter, basal ganglia, thalami, brainstem, cerebellum, and the entire spinal cord parenchyma extending to the conus medullaris. There were no obvious T2 hyperintense, T1 hypointense, or fluid-attenuated inversion recovery hyperintense lesions in the brain. There were patchy short tau inversion recovery changes throughout the spinal cord following the same distribution as the enhancing lesions.
Figure 1. Pretreatment MRI Demonstrating Multifocal Enhancing Lesions.
(A) Contrast-enhanced brain MRI demonstrating punctate enhancing lesions scattered throughout the brain parenchyma including the subcortical white matter, basal ganglia, cerebellum, and brainstem. (B) Highlighted in the yellow ovals are the prominent areas of punctate, perivascular appearing enhancement throughout the spinal cord.
Serologic workup revealed negative testing for anti-aquaporin-4 immunoglobulin G (AQP4-IgG), myelin oligodendrocyte glycoprotein (MOG)-IgG, paraneoplastic panel (Mayo Clinic Laboratories, which included amphiphysin, antiglial nuclear [AGNA-1], antineuronal nuclear [ANNA-1, ANNA-2, and ANNA-3], collapsin response mediator protein 5 [CRMP5] neuronal voltage-gated potassium channel, P/Q-type calcium channel, and Purkinje cell cytoplasmic [PCA-Tr, PCA-1, and PCA-2] antibodies), rheumatologic markers, and infections.
CSF testing revealed a lymphocytic pleocytosis (10 cells/μL, 88% lymphocytes), mildly elevated protein (61 mg/dL), and matched oligoclonal bands in the CSF and serum. Flow cytometry and cytology, along with the CSF autoimmune myelopathy panel (Mayo Clinic Laboratories, which included amphiphysin, AGNA-1, ANNA-1, ANNA-2, ANNA-3, CRMP-5, dipeptidyl-peptidase-like protein-6, glutamic acid decarboxylase 65, glial fibrillary acidic protein, metabotropic glutamate receptor 1 protein, neuronal intermediate filament, AQP4-IgG, PCA-Tr, PCA-1, and PCA-2) were negative. CSF was also tested for infectious etiologies, including West Nile virus, herpes simplex virus, cytomegalovirus, syphilis, Epstein-Barr virus, Lyme, and nextgeneration sequencing metagenomic studies, all of which were negative. In addition, CSF cytokine profiling was unrevealing without elevation of interleukin 6 or tumor necrosis factor-α. CSF SARS-CoV-2 antibody testing was not available at the time.
Additional diagnostic imaging including a full-body fluorodeoxyglucose-PET with CT scan and scrotal ultrasound did not reveal systemic disease or malignancy. Ocular coherence tomography demonstrated no evidence of optic neuropathy.
Differential Diagnosis
This patient presented with a subacute encephalomyelitis that involved the brain parenchyma, brainstem, cerebellum, and full length of the spinal cord. The differential diagnosis for such a clinicoradiologic syndrome is broad, including infections such as Lyme disease or tuberculosis, neoplasm including lymphoma, or glioma. Immune-mediated causes include neuromyelitis optica spectrum disorders (NMOSD), myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD), rheumatologic conditions (e.g., Behcet disease and Sjogren syndrome), neurosarcoidosis, Bickerstaff encephalitis, vasculitic syndromes, and paraneoplastic disease. Of these, neurosarcoidosis, Bickerstaff encephalitis, neuro-Behcet, and paraneoplastic brainstem encephalitis are conditions that particularly target the brainstem.1 The overall pattern of radiologic involvement, temporal course of symptoms, and workup ruled out most of the aforementioned conditions. Notably, certain conditions such as smoldering lymphomas or lymphomatoid granulomatosis can demonstrate an initial presentation of encephalomyelitis, and these are often only confirmed by biopsy, which was not performed in this patient.
Final Diagnosis and Treatment
The patient was diagnosed with immune-mediated encephalomyelitis. The primary cause was felt to be immune-mediated given the relapsing nature of the condition, steroid responsiveness, and absence of other identifiable causes to date, in addition to objective findings of inflammation, including the enhancing MRI lesions, and pleocytosis on CSF testing.
The patient was treated with 5 days of high-dose IV methylprednisolone (1,000 mg), with drastic improvement in gait, strength, and fatigue. He was subsequently discharged on a course of oral prednisone at 80 mg daily and slowly tapered down over several weeks. Two weeks later, his neurologic examination demonstrated near resolution of his neurologic deficits, and repeat MRI of the brain and complete spinal cord also demonstrated resolution of the enhancement.
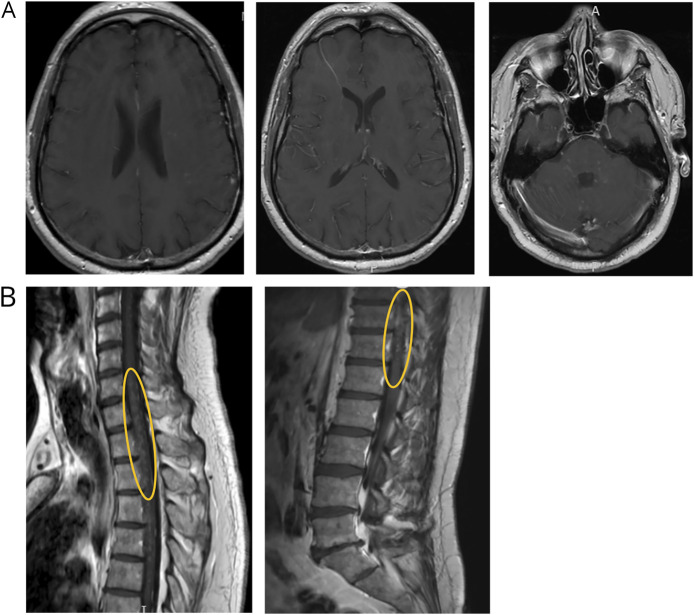
Over the course of the next 2 months, his oral prednisone was further tapered to 20 mg, at which point he noted worsening symptoms, with examination findings of horizontal and vertical nystagmus, bilateral hip flexion weakness (4/5), a T12 sensory level, and right-sided ataxia. These clinical features prompted treatment with an additional course of IV methylprednisolone (1 g daily for 5 days) and an increase of his daily oral prednisone dose back to 60 mg. A brain and spine MRI was obtained after completing his IV steroid treatment; no obvious enhancement was seen. Unfortunately, as his oral steroids were again tapered to 20 mg, he endorsed worsening symptoms and was admitted to the hospital for evaluation of suspected relapse. Repeat MRI demonstrated recurrent enhancement within the same lesion distribution as his initial presentation and persistent longitudinally extensive hyperintensities within the lateral columns of his spinal cord (Figure 2). A repeat CSF analysis again demonstrated a pleocytosis of 11 cells/μL with 99% lymphocytic predominance, but studies including SARS-CoV-2 antibodies were otherwise unrevealing in the CSF, including cytology and flow cytometry. He was treated again with 5 days of high-dose IV methylprednisolone, with partial improvement although incomplete resolution of his symptoms and deficits.
Figure 2. MRI After Steroid Taper Demonstrating Recurrence of Multifocal Enhancing Lesions.
(A) Contrast-enhanced brain MRI after weaning of oral steroids and recurrence of symptoms, demonstrating punctate enhancing lesions in many of the same areas as seen on initial presentation. (B) Highlighted in the yellow ovals are residual areas of punctate, perivascular enhancement, notably in the same regions as when the patient first obtained imaging.
Given his diagnosis of immune-mediated relapsing encephalomyelitis, he was started on mycophenolate mofetil (now on 1,000 mg twice a day) with overlapping slow tapering of his oral steroids (a few months). He has remained relapse-free on this treatment regimen. Although, he is experiencing increasing spasticity with impaired ambulation which is requiring antispasticity medication and rehabilitation interventions.
Discussion
We report here a rare case of relapsing encephalomyelitis occurring after SARS-CoV-2 infection and subsequent COVID-19 vaccination. The unique radiologic and clinical findings of our patient could fit with a neuroinflammatory and vasculopathic condition such as the syndrome known as chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). CLIPPERS is a T cell–mediated disorder characterized by widespread punctate perivascular inflammation that is exquisitely responsive to steroid treatment.2 On MRI it appears to pepper the pons, but can also involve the supratentorial parenchyma and spinal cord via perivascular inflammation. It can also be associated with an allergic trigger, with several cases demonstrating persistently elevated serum IgE levels.3 Our patient also demonstrated multiple relapses as his corticosteroids were weaned to 20 mg daily, and multiple reports also observed no relapses when chronic corticosteroid dosages were maintained above 20 mg daily.4
However, there has been an absence of laboratory biomarkers to recognize CLIPPERS as a specific disease entity, and given the variability in presentation, multiple alternative conditions may mimic the radiologic and clinical findings. Notably, even the latest proposed diagnostic criteria for CLIPPERS, can be fraught with misdiagnoses. A subsequent case series applying these criteria found a misdiagnosis rate of 31% (13/42 cases), with the most common misdiagnosis being CNS lymphoma, followed by primary CNS angiitis.6 This raises the question of whether CLIPPERS is indeed a true isolated disease as opposed to a syndrome with overlap across multiple conditions. As such, without a biomarker to confirm a diagnosis, one must carefully exclude these other conditions before diagnosing CLIPPERS and be vigilant in monitoring for conditions that may cause such a clinicoradiologic appearance over time.
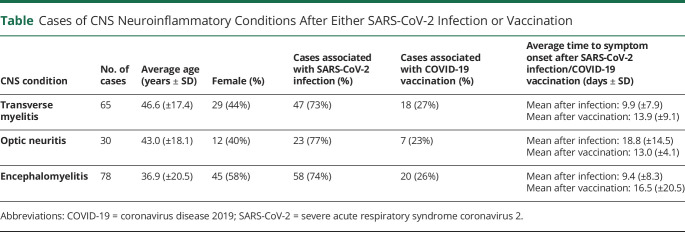
Apart from respiratory symptoms, SARS-CoV-2 infection can be associated with a variety of classic autoimmune neurologic manifestations, involving either the central or peripheral nervous system. A literature review identified 173 additional cases of CNS neuroinflammatory disease after either SARS-CoV-2 infection or vaccination, with 65 cases of transverse myelitis, 30 cases of optic neuritis, and 78 cases of encephalomyelitis (Table).7-15 Most of these cases occurred within 2–3 weeks of either the infection or vaccination as with our patient, although the vast majority of cases in the literature (>70% of each phenotype) occurred in association with infection. Transverse myelitis and encephalomyelitis tended to occur closer to the time of vaccination or infection. Some cases of encephalomyelitis occurred as late as 1 month from the time of COVID-19 or vaccinations, and the ages of patients were primarily distributed in 2 groups, with 20 of 59 patients under age 16 years and 30 of 59 patients being 40 years or older. On average, optic neuritis tended to occur later than transverse myelitis and encephalomyelitis. Notably, among the cases associated with vaccination, 60% (26/43) occurred in the setting of a positive MOG or AQP4 antibody, and these patients were eventually diagnosed with unmasking of MOGAD or NMOSD. Regardless of the type of demyelinating event, all events were far more likely (73% of transverse myelitis, 77% of optic neuritis, and 74% of encephalomyelitis) to occur in association with SARS-CoV-2 infection in comparison to vaccination.7-18 Our case was unusual in the degree of brain and spinal cord involvement, particularly the perivascular distribution of the inflammation, as well as the insidious degree of inflammation, with symptoms progressing over several months. Vascular complications and endothelial dysfunction have become increasingly identified in COVID-19, possibly mediated by SARS-CoV-2 targeting angiotensin-converting enzyme 2 receptors that are expressed in multiple organs, including the brain, although the exact pathogenesis remains unknown.19
Table.
Cases of CNS Neuroinflammatory Conditions After Either SARS-CoV-2 Infection or Vaccination
Our patient's steroid dependency and relapsing course suggest an unmasking of an underlying CNS neuroinflammatory condition, rather than due to direct SARS-CoV-2 CNS infection or a vaccine-related adverse event. Similar courses have been identified with post–SARS-CoV-2 infection or COVID-19 vaccination demyelinating events in patients who were eventually identified to have MS, MOGAD, and NMOSD.20 The initial neuroimaging observations of multifocality and innumerable punctate enhancement in the pons, cerebellum, and spinal cord may suggest a vasculocentric neuroinflammatory process triggered either by systemic immune reactions to SARS-CoV2 epitopes or overactivation of inflammatory responses in a genetically susceptible subject. Similar cases posit that the blood-brain barrier breakdown secondary to endothelial inflammation could facilitate antibodies to initiate and sustain the disease process, with subsequent epitope spreading leading to a relapsing course.21 This hypothesis could be supported by the temporary nature of the patient's matched oligoclonal bands on his initial CSF testing. Despite unrevealing biomarkers for a specific disease entity, the recurrent nature of his relapses with weaning of his steroid treatment necessitated a steroid-sparing agent. We ultimately chose mycophenolate mofetil because it can treat a broad spectrum of neuroinflammatory diseases.
Temporal associations of neurologic conditions with vaccinations and/or infections do not prove causality despite previous reports of such sequelae. Vaccines containing SARS-CoV-2 antigens may enhance autoimmunity by mechanisms including polyclonal activation, epitope spreading, or molecular mimicry in susceptible individuals.22 Our case highlights that the resulting inflammation may be insidious and extensive, though treatable. A literature search of post–COVID-19 vaccination-related CNS complications, as reported in the Vaccine Adverse Event Reporting System, found a total of 185 cases of either transverse myelitis or optic neuritis in association with the Pfizer, Moderna, and Janssen vaccines, underscoring its relative rarity, especially in comparison to approximately 5.2 billion total vaccine doses given by these manufacturers in that time frame (August 27, 2021).23 Alvarez et al.24 published a global report of confirmed postvaccination optic neuritis from December 2020 to June 2021 and identified a total of 55 cases, finding no difference in severity or clinical outcomes by the type of vaccine, although most cases (38/55) were associated with the AstraZeneca vaccine. Other studies have posited that there may be an association between the AstraZeneca vaccine and neurologic autoimmunity, possibly attributable to the viral vector of this vaccine, although it must be noted that these studies did not demonstrate an increase in the incidence of such events after the roll out of the mass vaccination program.16-18 Weighing the results of our findings and those in the literature, as COVID-19 constitutes a life-threatening infection in some patients, we feel that the benefits of vaccination outweigh the extremely rare event of unmasking an immune-related condition or leading to another neuroinflammatory event.
Our case represents a rare case of relapsing encephalomyelitis with the appearance of vasculocentric appearance or CLIPPERS-like radiologic findings after a one-two-punch of SARS-CoV-2 infection followed by COVID-19 vaccination. Prompt treatment of these conditions and understanding when patients may require long-term immune therapy may help prevent accrual of neurologic disability.
Glossary
- AGNA
antiglial nuclear antibody
- ANNA
antineuronal nuclear antibody
- CLIPPERS
chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids
- COVID-19
coronavirus disease 2019
- MOGAD
myelin oligodendrocyte glycoprotein antibody–associated disease
- NMOSD
neuromyelitis optica spectrum disorders
- PCA
Purkinje cell cytoplasmic antibody
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Jubelt B, Mihai C, Li TM, Veerapaneni P. Rhombencephalitis/brainstem encephalitis. Curr Neurol Neurosci Rep. 2011;11(6):543-552. [DOI] [PubMed] [Google Scholar]
- 2.Taieb G, Duflos C, Renard D, et al. . Long-term outcomes of CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids) in a consecutive series of 12 patients. Arch Neurol. 2012;69(7):847-855. [DOI] [PubMed] [Google Scholar]
- 3.Kastrup O, Nes J, Gasser T, Keyvani K. Three cases of CLIPPERS: a serial clinical, laboratory and MRI follow-up study. J Neurol. 2011;258(12):2140-2146. [DOI] [PubMed] [Google Scholar]
- 4.Dudesek A, Rimmele F, Tesar S, et al. . CLIPPERS: chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. Review of an increasingly recognized entity within the spectrum of inflammatory central nervous system disorders. Clin Exp Immunol. 2014;175(3):385-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobin WO, Guo Y, Krecke KN, et al. Diagnostic criteria for chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS). Brain. 2017;140(9):2415-2425. [DOI] [PubMed] [Google Scholar]
- 6.Taieb G, Mulero P, Psimaras D, et al. . CLIPPERS and its mimics: evaluation of new criteria for the diagnosis of CLIPPERS. J Neurol Neurosurg Psychiatry. 2019;90(9):1027-1038. [DOI] [PubMed] [Google Scholar]
- 7.Román GC, Gracia F, Torres A, Palacios A, Gracia K, Harris D. Acute transverse myelitis (ATM):Clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222). Front Immunol. 2021;12:653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novi G, Rossi T, Pedemonte E, et al. . Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold DM, Galetta SL. Neuro-ophthalmologic complications of coronavirus disease 2019 (COVID-19). Neurosci Lett. 2021;742:135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.François J, Collery AS, Hayek G, et al. . Coronavirus disease 2019–associated ocular neuropathy with panuveitis: a case report. JAMA Ophthalmol. 2021;139(2):247-249. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Rodríguez MS, Romero-Castro RM, Alvarado-de la Barrera C, González-Cannata MG, García-Morales AK, Ávila-Ríos S. Optic neuritis following SARS-CoV-2 infection. J Neurovirol. 2021;27(2):359-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.García-Estrada C, Gómez-Figueroa E, Alban L, Arias-Cárdenas A. Optic neuritis after COVID-19 vaccine application Clin Exp Neuroimmunol. 2022;13(2):72-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ide T, Kawanami T, Eriguchi M, Hara H. SARS-CoV-2-related myelin oligodendrocyte glycoprotein antibody-associated disease: a case report and literature review. Intern Med. 2022;61(8):1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaratnam SA, Ferdi AC, Leaney J, Lee RLK, Hwang YT, Heard R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022;22(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes, and predictors. Eur J Neurol. 2021;28(10):3491-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Netravathi M, Dhamija K, Gupta M, et al. . COVID-19 vaccine associated demyelination & its association with MOG antibody. Mult Scler Relat Disord. 2022;60:103739. doi. 10.1016/j.msard.2022.103739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis AG, Elhadd K, Camera V, et al. . Acute inflammatory diseases of the central nervous system after SARS-CoV-2 vaccination. Neurol Neuroimmunol Neuroinflamm. 2022;10(1):e200063. doi: 10.1212/NXI.0000000000200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarius S, Bieber N, Haas J, Wildemann B. MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature. J Neurol. 2022;269(10):5198-5212. doi. 10.1007/s00415-022-11194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez LM, Neo YN, Davagnanam I, et al. . Post vaccination optic neuritis: observations from the SARS-CoV-2 pandemic. 2021. Available at SSRN: https://ssrn.com/abstract=3889990 or 10.2139/ssrn.3889990. [DOI] [Google Scholar]
- 20.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107(6):1482-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail II, Salama S. Association of CNS demyelination and COVID-19 infection: an updated systematic review. J Neurol. 2022;269(2):541-576. doi. 10.1007/s00415-021-10752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powell AM, Black MM. Epitope spreading: protection from pathogens, but propagation of autoimmunity? Clin Exp Dermatol. 2001;26(5):427-433. doi. 10.1046/j.1365-2230.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 23.Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41(3):509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriwastava S, Shrestha AK, Khalid SH, Colantonio MA, Nwafor D, Srivastava S. Spectrum of neuroimaging findings in post-COVID-19 vaccination: a case series and review of literature. Neurol Int. 2021;13(4):622-639. [DOI] [PMC free article] [PubMed] [Google Scholar]