Abstract
Atherosclerosis is a significant risk factor for coronary heart disease (CHD) and myocardial infarction (MI). Atherosclerosis develops during foam cell generation, which is caused by an imbalance in cholesterol uptake, esterification, and efflux. LOX-1, SR-A1, and CD36 all increased cholesterol uptake. ACAT1 and ACAT2 promote free cholesterol (FC) esterification to cholesteryl esters (CE). The hydrolysis of CE to FC was aided by nCEH. FC efflux was promoted by ABCA1, ABCG1, ADAM10, and apoA-I. SR-BI promotes not only cholesterol uptake but also FC efflux. Circular RNAs (circRNAs), which are single-stranded RNAs with a closed covalent circular structure, have emerged as promising biomarkers and therapeutic targets for atherosclerosis due to their highly tissue, cell, and disease state-specific expression profiles. Numerous studies have shown that circRNAs regulate foam cell formation, acting as miRNA sponges to influence atherosclerosis development by regulating the expression of SR-A1, CD36, ACAT2, ABCA1, ABCG1, ADAM10, apoA-I, SR-B1. Several circRNAs, including circ-Wdr91, circ 0004104, circRNA0044073, circRNA_0001805, circDENND1B, circRSF1, circ 0001445, and circRNA 102682, are potential biomarkers for atherosclerosis to better evaluate cardiovascular risk. It is difficult to deliver synthetic therapeutic circRNAs to the desired target tissues. Nanotechnology, such as GA-RM/GZ/PL, may be an important solution to this problem. In this review, we focus on the potential role and mechanism of circRNA/miRNA axis in foam cell formation in the hopes of discovering new targets for the diagnosis, prevention, and treatment of atherosclerosis.
Keywords: Atherosclerosis, CircRNA/miRNA axis, Cholesterol uptake, Cholesterol esterification, Cholesterol efflux
1. Introduction
Atherosclerosis is one of the leading causes of disease and death worldwide, such as myocardial infarction (MI), coronary heart disease (CHD), acute coronary syndromes (ACS), cerebral infarction, and stroke [1,2]. Thus, atherosclerosis is the most dangerous stimulator of cardiovascular diseases. The incidence of atherosclerosis is associated with the recruitment of monocytes and their differentiation to macrophages, which take up lipids to become foam cells. Foam cell formation is a hallmark of atherosclerosis and has a central role in the pathogenesis of atherosclerosis [3,4]. Lipid metabolism disorder is the initiating factor of foam cell formation. Low-density lipoprotein cholesterol (LDL-C) accumulates in the endothelial cells (ECs) of blood vessels damaging the ECs integrity. LDL-C can be oxidized to oxidized low-density lipoprotein (ox-LDL). Circulating monocytes engulf large amounts of ox-LDL and transform into foam cells that adhere to and invade endothelial cells, forming lipid streaks [5,6]. Therefore, foam cell formation plays an important role in the pathological progress of atherosclerosis.
Circular RNAs (circRNAs), which are single-stranded RNAs with a closed covalent circular structure, are the newly discovered endogenous noncoding RNAs with covalently linked 3′ and 5′ ends. CircRNAs regulate the expression of downstream target genes via a variety of mechanisms, including acting as miRNA sponges, forming complexes with RNA binding proteins (RBPs), regulating protein interactions, acting as a protein sponge or scaffold, encoding protein or peptide, and participating in ribosomal RNA processing and translation [7,8]. CircRNA evolution is conserved, and its sequence is highly consistent in different species. CircRNA expression profiles are highly tissue, cell, and disease state-specific. These favorable conditions make circRNAs become promising biomarkers and therapeutic targets for diseases such as atherosclerosis, and cancer, attracting increasing interest from scientific research and pharmaceutical companies. Many studies have shown that circRNAs regulate foam cell formation to influence atherosclerosis development [[7], [8], [9]]. The formation, classification and function of circRNA and miRNA have been reviewed by other groups [[10], [11], [12], [13]]. In this review, we focused on the role and mechanism of circRNAs in regulating the gene expression of foam cell formation to affect atherosclerosis and provided some potential targets for the diagnosis, prevention, and treatment of atherosclerosis.
2. The mechanism of foam cell formation
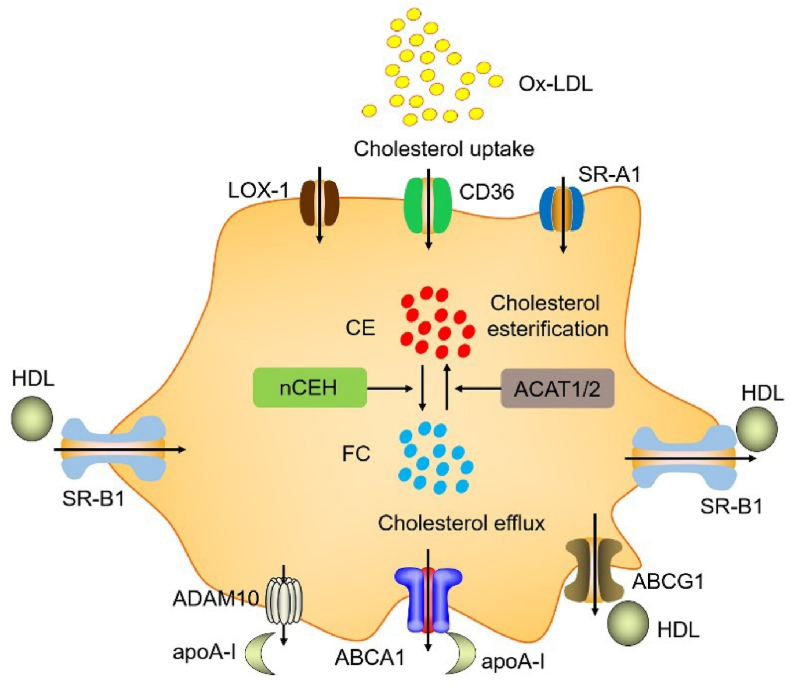
Foam cell formation is caused by cholesterol accumulation due to an imbalance of cholesterol uptake, cholesterol esterification, and free cholesterol (FC) efflux [14]. Cholesterol uptake is controlled mainly by oxidized low-density lipoprotein receptor 1 (LOX-1), macrophage scavenger receptor 1 (SR-A1, also named MSR1), and luster of differentiation 36 (CD36). LOX-1, SR-A1, and CD36 promote ox-LDL uptake. CD36 and SR-A1 account for approximately 75%–90% of ox-LDL uptake in macrophages [15]. Cholesterol esterification is important in determining intracellular FC and cholesteryl esters (CE). Cholesterol esterification is mainly controlled by acetyl-CoA acetyltransferase 1/2 (ACAT1/2) and neutral cholesteryl ester hydrolase (nCEH). ACAT1 is expressed in all the macrophages of the atherosclerotic aorta, while ACAT2 is expressed in approximately 70%–80% of macrophage [16]. ACAT1/2 promotes foam cells formation by promoting the conversion of cytoplasmic FC into the storage form of CE. In contrast, nCEH promotes the hydrolysis of CE to FC, suggesting that ACAT1/2 and nCEH are key rate-limiting genes that maintain FC efflux [17,18]. FC efflux to the extracellular is dominated by ABCA1 and ABCG1, membrane transporters abundant in macrophages. ABCA1 is required for high-density lipoprotein (HDL) particle biogenesis and uses ATP as energy to promote FC efflux to apolipoprotein A-I (apoA-I). A disintegrin and metalloprotease 10 (ADAM10) have also recently been found to promote the FC efflux to apoA-I [19,20]. ABCG1 promotes FC efflux to HDL particles. HDL reduces atherosclerosis procession by transferring cholesterol from the peripheral cells with the blood to the liver for biliary excretion, the reverse cholesterol transport (RCT) procession. ABCA1 and ABCG1 dominate approximately 50% and 20% of cholesterol efflux, respectively [[21], [22], [23]]. However, ABCA1 plays a crucial role in the initial steps of RCT and HDL generation. Scavenger receptor BI (SR-BI) promotes not only cholesterol uptake but also cholesterol efflux. However, how much SR-BI contributes to cholesterol metabolism is unclear [24]. Previous studies from our laboratory and others have shown that ABCA1, ABCG1, and SR-B1 promoted cholesterol efflux in macrophages, whereas suppressing these transporters promoted foam cell formation and the development of atherosclerosis [[25], [26], [27], [28], [29], [30]]. Our laboratory and others have also shown that apoA-I is expressed in hepatocytes and enterocytes and monocyte-macrophage cells, dendritic cells (DCs), and T cells [31]. Endogenous apoA-I in macrophages has anti-inflammatory and cholesterol efflux effects [28,31]. Therefore, foam cell formation is dominated by multiple genes, including LOX-1, SR-A1, CD36, ACAT1, ACAT2, nCEH, ABCA1, ABCG1, ADAM10, SR-B1, apoA-I (Fig. 1).
Fig. 1.
The mechanism of foam cell formation. Foam cell generation is induced by imbalance of the cholesterol uptake, esterification, and efflux. LOX-1, SR-A1, and CD36 promoted cholesterol uptake. ACAT1 and ACAT2 promote esterification of free cholesterol (FC) to cholesteryl esters (CE). nCEH promoted the hydrolysis of CE to FC. ABCA1 and ADAM10 promoted FC efflux to apoA-I. ABCG1 promoted FC efflux to HDL. SR-BI promotes not only cholesterol uptake but also FC efflux. Under the atherogenic conditions, increasing cholesterol uptake and esterification and/or reducing cholesterol efflux could lead to excessive accumulation of CE in in the cytoplasm as lipid droplets, which promotes foam cells formation.
3. The potential role and mechanism of circRNAs in foam cell formation
3.1. SR-A1: circRNA/miR-378a-5p axis
Circ-Wdr91 expressed in atherosclerotic plaque of apoE−/− mice [32]. Tanshinone IIA (TAN) suppressed lipid accumulation and atherosclerosis by suppressing circ-Wdr91 expression. Circ-Wdr91 increased SR-A1 expression by acting as a sponge of miR-378a-5p in RAW264.7 cells [32], suggesting that circ-Wdr91 promoted cholesterol uptake and foam cell formation by regulating miR-378a-5p/SR-A1 axis. Circ_000373 also acts as a sponge of miR-378a-5p [33]. Thus, circ-Wdr91 and circ_000373 promoted foam cell formation by regulating miR-378a-5p/SR-A1 axis.
3.2. CD36
3.2.1. CircRNA/miR-378b, miR-6516, miR-11986b, miR-345, and miR-502b axis
CircEZH2 (also named hsa_circ_0006357) promoted fatty acid uptake by promoting the expression of CD36, lipoprotein lipase (LPL), fatty acid desaturase 1 (FADS1), and stearoyl-CoA desaturase 1 (SCD1) in HC11 cells [34]. LPL is responsible for the hydrolysis of triglycerides to glycerol and free fatty acids and is a key factor in fatty acid uptake. CD36 promoted not only cholesterol uptake but also fatty acid uptake. FADS1 and SCD1 mainly promoted unsaturated fatty acids synthesis [[35], [36], [37]]. CD36 and LPL are the target gene of miR-378b. CircEZH2 promoted CD36 and LPL expression by serving as the miR-378b sponge [34]. Given that CD36 promotes cholesterol uptake and foam cell formation, we hypothesized that circEZH2 promoted foam cell formation by regulating miR-378b/CD36 axis and their mediated cholesterol uptake. Interestingly, circZNF609 [38] and circ_0078710 [39] could also serve as a sponge of miR-378b. Therefore, we hypothesized that these circRNAs may promote foam cell formation by regulating the miR-378b/CD36 axis. However, more studies are needed. In fact, many circRNA/miRNA/CD36 axis may promote cholesterol uptake and foam cell formation, including circFCHSD2/miR-6516/CD36 axis, circHNRNPLL/miR-11986b/CD36 axis, circKANSL1/miR-345 or miR-502b or miR-6516/CD36 axis, circMAP7/miR-11986b or miR-345/CD36 axis [40]. Taken together, circEZH2/miR-378b axis, circZNF609/miR-378b axis, circ_0078710/miR-378b axis, circFCHSD2/miR-6516 axis, circHNRNPLL/miR-11986b axis, circKANSL1/miR-345 or miR-502b or miR-6516 axis, circMAP7/miR-11986b or miR-345 promoted cholesterol uptake to foam cell formation by targeting CD36 3′UTR.
3.2.2. CircRNA/miR-100 axis
Circ_0004104 is a potential biomarker for atherosclerosis. The plasma level of circ_0004104 is the potential marker in patients with the coronary atherosclerotic burden [41]. Circ_0004104 promoted ox-LDL-induced vascular EC injury and atherosclerosis development by regulating miR-100/tumor necrosis factor-α-induced protein 8 (TNFAIP8) axis [42] and miR-328-3p/tripartite motif 14 (TRIM14) axis [43]. MiR-100 suppressed cholesterol uptake and lipid accumulation by suppressing CD36 3′UTR in vitro and in vivo [44,45], suggesting that circ_0004104 promoted cholesterol uptake and foam cell formation by regulating miR-100/CD36 axis. Circ-0072309 [[46], [47], [48]] and circ_0006168 [49] could also serve as a sponge of miR-100. Therefore, we hypothesized that these circRNAs may promote foam cell formation by regulating miR-100/CD36 axis and cholesterol uptake. However, more studies are needed.
3.2.3. CircRNA/miR-107 axis
CircRNA-0044073 is also a potential biomarker for atherosclerosis. circRNA-0044073 expression was increased in atherosclerotic blood cells from patients with atherosclerosis, while miR-107 was decreased [50]. CircRNA-0044073 [50], circRNA PPP1CC <b> [51],</b> and circ_UBR4 [52] increased the proliferation and invasion of vascular smooth muscle cells (VSMCs) and atherosclerosis development by serving as a sponge of miR-107. CD36 is a target gene of miR-107 [53], suggesting that circRNA-0044073, circRNA PPP1CC, and circ_UBR4 promoted cholesterol uptake and foam cell formation by regulating the miR-107/CD36 axis. Many circRNAs could serve as a sponge of miR-107, including circMETTL3 [54], circCFL1 [55], circ-SFMBT2 [56], circ0041103 [57], circHIPK3 [58,59], circ-ZFR [60], circTGFBR2 [61], circFGFR4 [62], circ_0049472 [63], circ-LTBP1 [64], circTCF25 [65], circ_BICD2 [66], circRNA ITGA5 [67], circ_0005033 [68], circCCT3 [69], circular RNA-cTFRC [70], CircSLC7A6 [71], suggesting that these circRNAs promoted foam cell formation by regulating miR-107/CD36 axis. Furthermore, by acting as a sponge for miR-637 [72], and miR-106a-5p [73], circHIPK3 increased VSMC proliferation, calcification, and atherosclerosis development, implying that circHIPK3 not only promoted foam cell formation but also VSMC proliferation and calcification.
3.3. ACAT2: circRNA/miR-1233 axis
Circ_RPL23A (also named hsa_circ_0092360) is located in potential regulatory roles for ribosomal protein L23a (RPL23A). Circ_RPL23A increased ACAT2 expression and suppressed clear cell renal cell carcinoma development by activating a sponge of miR-1233 (miR-1233-3p) [74]. Given that ACAT2 promotes cholesterol esterification and foam cell formation, we hypothesized that circ_RPL23A promotes foam cell formation by regulating the miR-1233/ACAT2 axis. Many circRNAs could serve as a sponge of miR-1233, including circTP53 [75], circEHMT1 [76], circ-0007766 [77], circ_0004050 [78], suggesting these circRNAs promoted cholesterol esterification and foam cell formation by regulating miR-1233/ACAT2 axis. However, many studies are needed.
3.4. ABCA1
3.4.1. CircRNA/miR-106a-5p axis
CircRNA_0001805 expression was downregulated in high-fat diet (HFD)-fed mice. ABCA1 is the target of miR-106a-5p [79]. Carnitine palmitoyltransferase 1 (CPT1) is the target of miR-320a. CircRNA_0001805 reduced lipid metabolism disorder and inflammation by targeting the miR-106a-5p/ABCA1 axis and miR-320a-CPT1 axis [80]. In free fatty acid (FFA)-treated primary hepatocytes, circRNA_0001805 overexpression with adenoviral vectors suppressed lipid accumulation (triglycerides (TG) and total cholesterol (TC) levels) by increasing ABCA1 and CPT1 expression. CircRNA_0001805 overexpression with adenovirus vectors also reduced TG and TC levels in hepatic tissues by increasing ABCA1 and CPT1 expression in HFD-fed mice. However, circRNA_0001805 did not change the expression of CD36, acetyl-CoA Carboxylase 1 (Acc1), proliferator-activated receptor-α (PPARα), fatty acid synthase (Fas), SCD1, and fatty acid transporter 1 (FATP1), suggesting that circRNA_0001805 reduced lipid accumulation by promoting ABCA1-mediated cholesterol efflux but not CD36-mediated cholesterol uptake [80].
3.4.2. CircRNA/miR-17-5p axis
CircDENND1B expression is negatively correlated with foam cell formation and atherosclerosis progression [81]. The expression of circDENND1B was decreased in the aortas of HFD-fed apoE−/− mice. CircDENND1B increased cholesterol efflux by regulating miR-17-5p/ABCA1 axis in RAW264.7 macrophage-derived foam cells [81]. Interestingly, circDENND1B not only increased the expression of ABCA1 mRNA and LPL mRNA but also their protein expression. circDENND1B also increased the expression of ABCG1. However, it is not clear whether circDENND1B affects the expression of ABCG1 protein. In addition, circDENND1B did not affect the expression of ACAT1, SR-B1, SR-A1 mRNA, suggesting that circDENND1B increased expression of ABCA1, LPL, ABCG1 at the transcriptional or post-transcriptional level. However, the mechanism of circDENND1B on the expression of LPL and ABCG1 has unclear. Interestingly, miR-17-5p suppressed PPARγ and liver X receptor α (LXRα) expression in the peritoneal macrophages of apoE−/− mice. LPL and ABCG1 are the target genes of PPARγ/LXRα pathway, suggesting that circDENND1B promoted LPL and ABCG1 expression by regulating miR-17-5p/PPARγ/LXRα axis [81]. MiR-17-5p increased macrophage inflammation in the plaques of apoE−/− mice by enhancing interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and IL-1β expression [82], suggesting that circDENND1B suppressed macrophage inflammation by regulating miR-17-5p/IL-6, TNF-α, and IL-1β axis.
IL-1β monoclonal antibody (IL-1β mAb) reduced local inflammation of aortic roots and the development of atherosclerotic plaques in HFD-fed apoE−/− mice. IL-1β mAb also increased circDENND1B in the aortas of HFD-fed apoE−/− mice and RAW264.7 macrophage-derived foam cells. Blocking IL-1β with mAbs suppressed ABCA1 expression and cholesterol efflux by stimulating circDENND1B/miR-17-5p axis in RAW264.7 macrophage-derived foam cells. IL-1β also suppressed ABCA1 expression and cholesterol efflux by stimulating the reactive oxygen species (ROS)-nuclear transcription factor-kappa B (NF-kB) pathway, suggesting that IL-1β suppressed ABCA1 expression through two mechanisms, including circDENND1B/miR-17-5p axis and ROS-NF-kB pathway. In addition, IL-1β mAb increased the expression of ABCG1 mRNA, and LPL mRNA but did not affect CD36, SR-B1, SR-A1, ACAT1 RAW264.7 macrophage-derived foam cells, suggesting that IL-1β mAb increased cholesterol efflux but not change cholesterol uptake and cholesterol esterification. However, the expression and function of circDENND1B are not investigated in humans, including macrophages. It is worth mentioning that circDENND1B is highly conserved with the hsa_circ_0111650 (human circRNA) in the brain, suggesting that mice circDENND1B is also named hsa_circ_0111650 [81]. However, the function of hsa_circ_0111650 has not been investigated.
3.4.3. CircRNA/miR-30a-3p, miR-30e-3p, and miR-5581-3p axis
CircPCMTD1 (also named as circ-0001801) increased cholesterol efflux by stimulating miR-30a-3p/ABCA1 axis, miR-30e-3p/ABCA1 axis, miR-5581-3p/ABCA1 axis in THP-1 macrophage-derived foam cells [83]. PPARα promoted lipid accumulation by suppressing the expression of ACC, phospho-glycogen synthase kinase-3β (p-GSK-3β), and fatty acid synthase (FASN, also named FAS) and increasing the expression of CPT1, phosphorylated AMP-activated protein kinase (p-AMPK), and uncoupling protein 2 (UCP2). MiR-30a-3p reduced lipid accumulation by targeting PPARα 3′UTR and regulating its downstream target gene [84], suggesting that circPCMTD1 reduced lipid accumulation by regulating miR-30a-3p/PPARα axis. circPCMTD1 also served as the miR-224-5p sponges [85]. MiR-224-5p expression was downregulated in the plasma and plaque of atherosclerotic mice. MiR-224-5p prompted ox-LDL-induced endothelial injury by binding and suppressing phosphatase and tensin homolog (PTEN) expression [86], suggesting that circPCMTD1 reduced endothelial injury by regulating miR-224-5p/PTEN axis. CircLDLR could serve as the miR-30a-3p sponges [87]. Give that miR-30a-3p suppressed cholesterol efflux by targeting ABCA1, we hypothesized that circLDLR may increase cholesterol efflux by stimulating miR-30a-3p/ABCA1 axis. CircLDLR also suppressed the proliferation and apoptosis of VSMCs in coronary artery disease (CAD) by regulating miR-26-5p/lysine-specific demethylase6A (KDM6A) axis. CircNFIC could serve as the miR-30e-3p sponges [88]. Give that miR-30e-3p suppressed cholesterol efflux by targeting ABCA1, we hypothesized that circNFIC increased cholesterol efflux by stimulating the miR-30e-3p/ABCA1 axis. However, more studies are needed. Taken together, circPCMTD1 suppressed foam cell formation by regulating miR-30a-3p/ABCA1 axis, miR-30e-3p/ABCA1 axis, miR-5581-3p/ABCA1 axis, miR-30a-3p/PPARα axis. CircLDLR suppressed foam cell formation by stimulating miR-30a-3p/ABCA1 axis and cholesterol efflux. CircNFIC increased cholesterol efflux by stimulating miR-30e-3p/ABCA1 axis.
3.4.4. CircRNA/miR-3918 and miR-139-5p axis
CircBRWD1 increased cholesterol efflux by stimulating miR-3918/ABCA1 axis, miR-139-5p/ABCA1 axis, miR-3918/SR-B1 axis in THP-1 macrophage-derived foam cells [83]. Give that circ-BIRC6 could serve as the miR-3918 sponge. We hypothesized that circ-BIRC6 may increase cholesterol efflux by stimulating the miR-3918/SR-B1 axis [89]. In addition, many circRNAs could serve as the miR-139-5p sponges, including circMAP2K4 [90], circPTPRM [91], circRNF13 [92], circDNER [93], circ-STAT3.46 [94], circEIF3K [95], circ_0077109 [96], circ_0002594 [97], circTHBS1 [98], circBACH2 [99], circ_0001610 [100]. Therefore, we hypothesized that these circRNAs increased cholesterol efflux by stimulating miR-139-5p/ABCA1 axis. However, miR-139-5p expression was reduced in arterial tissues of patients with atherosclerosis. Overexpression miR-139-5p reduced serum TC, TG, LDL-C, and proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6) levels by suppressing signal transducer and activator of transcription 1 (STAT1) expression and then suppressed the formation of atherosclerotic plaques in apoE−/− mice [101], suggesting that these circRNAs (including circBRWD1) stimulated cholesterol efflux is not sufficient to resist the development of atherosclerosis. These circRNAs may have proatherogenic development in vivo.
3.4.5. CircRNA/miR-186-3p, and miR-140-3p axis
CircRNA UDP-glucose glycoprotein glucosyltransferase 2 (circUGGT2), also named hsa_circ_0008274. CircUGGT2 increased cholesterol efflux by stimulating miR-186-3p/ABCA1 axis in THP-1 macrophage-derived foam cells [83]. CircUGGT2 also served as the miR-140-3p sponges and suppressed AMPK/mammalian target of rapamycin (mTOR) signaling pathway [102,103]. Interestingly, miR-140-3p could suppress cholesterol efflux by suppressing ADAM10 3′UTR [104]. Thus, circUGGT2 increased cholesterol efflux by stimulating miR-186-3p/ABCA1 axis and miR-140-3p/ADAM10 axis, thereby reducing intracellular cholesterol accumulation and foam cell formation.
Astaxanthin (AST, also known as ASX) is a strong antioxidant. Clinical trials have shown that AST suppressed the oxLDL and increased HDL-C and adiponectin levels. Astaxanthin increased RCT by enhancing ABCA1, ABCG1, SR-B1 expression in apoE−/− mice [105]. Astaxanthin increased the expression of circUGGT2, circPCMTD1, and circBRWD1, and then increased cholesterol efflux by stimulating ABCA1, ABCG1, and SR-B1 expression in THP-1 macrophage-derived foam cells [83]. Astaxanthin also increased the promoter of ABCA1 and ABCG1 in an LXR-independent manner in RAW264.7 macrophage-derived foam cells [106] and increased ABCA1 and ABCG1 expression by inducing PPARγ/LXRα in thioglycollate-elicited peritoneal macrophages from C57BL/6j mice [107]. Therefore, astaxanthin can promote cholesterol efflux through multiple mechanisms, including circRNA/miRNA/ABCA1, ABCG1, SR-B1 axis, the promoter of ABCA1 and ABCG1, PPARγ/LXRα pathway.
3.4.6. CircRNA/miR-758, and miR-135b-5p axis
CircRSF1 (also called hsa_circ_0000345) is derived from remodeling and spacing factor 1 (RSF1). Serum circRSF1 expression was decreased in patients with atherosclerosis and ox-LDL-induced human aortic endothelial cells (HAECs) injury model, whereas miR-758 (miR-758-3p) was increased. CCND2 is an enhancer of proliferation in vascular ECs and SMCs in atherosclerosis procession. CircRSF1 could serve as the miR-758 sponge and reduce EC injury by regulating miR-758/cyclin D2 (CCND2) axis [108]. MiR-758 suppressed foam cell formation and atherosclerosis development by targeting ABCA1 3′UTR [109,110], suggesting that circRSF1 increased cholesterol efflux to suppress foam cell formation by regulating miR-758/ABCA1 axis. CircRSF1 also could serve as the miR-135b-5p sponge and reduce vascular EC apoptosis (Bax: Bcl-2 ratio and cleaved-caspase-3) and inflammation (IL-1β, IL-6, TNF-α, and IL-8) by regulating miR-135b-5p/HDAC1 axis [111]. MiR-135b-5p also suppressed foam cell formation by targeting ABCA1 [112], suggesting that circRSF1 increased cholesterol efflux by regulating miR-135b-5p/ABCA1 axis. Taken together, circRSF1 suppressed foam cell formation by regulating miR-758/ABCA1 axis and miR-135b-5p/ABCA1 axis. In fact, many circRNAs could serve as a sponge of miR-758, including circ_0002483 [113], circ_0003221 [114], circRBM33 [115], circ_0093887 [116], circ_0008500 [117], circ_0002360 [118], circNFIX [119]. Therefore, we hypothesized that these circRNAs may suppress foam cell formation by regulating miR-758/ABCA1 axis and cholesterol efflux. However, more studies are needed.
3.4.7. CircRNA/miR-145, miR-326, and miR-543 axis
Circ-PRMT5 could serve as a sponge of miR-145 [120]. Give that miR-145 reduced ABCA1 expression and cholesterol efflux by targeting the ABCA1 3′UTR and then promoted foam cell formation and atherosclerosis development [121]. We hypothesized that circ-PRMT5 may suppress foam cell formation by regulating miR-758/ABCA1 axis and cholesterol efflux. However, more studies are needed. Circ_0092317 and circ_0003546 regulated ABCA1 expression and cholesterol efflux by regulating miR-326/phosphodiesterase type 3B (PDE3B) axis [122]. In contrast, circ_0028198 and circ_0092317 regulated ABCA1 expression and cholesterol efflux by regulating miR-543/PDE3B axis, suggesting that PDE3B play a key role in circ_0092317, circ_0003546, circ_0028198 and circ_0092317 mediated ABCA1 expression and cholesterol efflux [123].
3.4.8. CircRNA/miR-33, and miR-34a axis
CircFASN [124] and circ_SATB2 [125] reduced triglyceride accumulation by serving as a sponge of miR-33. MiR-33 promoted cholesterol efflux by targeting the ABCA1 and ABCG1 3′UTR then suppressed foam cell formation and atherosclerosis development [126], suggesting that CircFASN and circ_SATB2 suppressed cholesterol efflux by regulating miR-33-ABCA1/ABCG1 axis. CircRNA_0046366 [127] and circRNA_0046367 [128] increased PPARα expression by serving as a sponge of miR-34a. As mentioned earlier, PPARα also suppressed foam cell formation by regulating ABCA1 and ABCG1, suggesting that circRNA_0046366 and circRNA_0046367 suppressed foam cell formation by regulating miR-34a/PPARα axis.
3.4.9. CircRNA/miR-1635 axis
CircRNA Z: 54674624|54755962 is a novel endogenous RNA of chicken. circRNA Z:54674624|54755962 could regulate lipid metabolism by regulating the expression of ABCA1, mannosyl (beta-1,4-)-glycoprotein beta-1,4-Nacetylglucosaminyltransferase (MGAT3), arylacetamide deacetylase (AADAC) as the gga-miR-1635 sponge [129]. CircRNA Z: 54674624|54755962 is located in Talpid3. Talpid3 is also expressed in humans (KIAA0586) and mouse (2700049A03Rik). However, the role of circRNA Z: 54674624|54755962 in humans and mouse is unclear.
3.5. ABCG1: circRNA/miR-208b-5p axis
Circ_0001445 (also named circSMARCA5) is located in the exons 15 to 16 of SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 5 (SMARCA5, encoded SNF2H protein), which is a member of the ISWI family of chromatin remodelers. Circ_0001445 is highly expressed in the human heart. Serum circ_0001445 was decreased in 200 patients with a higher coronary atherosclerotic burden. Circ_0001445 is expressed in human coronary SMCs and is decreased by atherosclerotic conditions such as native LDL (nLDL) or Aggregated LDL (agLDL), implying that the plasma level of Circ_0001445 could be used as a marker in patients with coronary atherosclerotic burden [130]. In another study, serum circ_0001445 and ABCG1 expression were decreased in 44 patients with a higher coronary atherosclerotic burden and ox-LDL-triggered HAECs injury model, while miR-208b-5p was increased, suggesting that the expression of circ_0001445 and ABCG1 were negatively correlated with miR-208b-5p [131]. Circ_0001445 promoted ABCG1 expression by sponging miR-208b-5p. Circ_0001445 reduced ox-LDL-induced endothelial injury in HAECs by regulating miR-208b-5p/ABCG1 axis. Circ_0001445 also increased matrix metalloproteinase 13 (MMP13) and a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS4) expression and suppressed TNF-α, IL-6 and Collagen II levels in ox-LDL-stimulated HAECs by regulating miR-208b-5p/ABCG1 axis [131]. Circ_0001445 suppressed ox-LDL-induced HUVECs inflammation (such as TNF-α, IL-1β, and IL-16), oxidative stress, and apoptosis by serving as the miR-640 sponge [132]. Therefore, circ_0001445 reduced endothelial injury and inflammation by regulating miR-208b-5p/ABCG1 axis and miR-640 axis. However, the role of circ_0001445 in cholesterol efflux by regulating miR-208b-5p/ABCG1 axis has not been investigated.
3.6. ADAM10: circRNA/miR-140-3p, miR-212-3p, miR-7-5p, miR-143-3p, miR-515-5p, miR-30a-3p, and miR-153 axis
CircRNA_ABCA1 (also named circRNA_36781) is located in the exonic of ABCA1. circRNA_ABCA1 expression was increased both in aortic vessels of HFD-induced ApoE−/− mice and H2O2-induced mouse aortic endothelial cells (MAECs) injury model, while miR-140-3p and MKK6 (protein encoded by MAP2K6) expression were reduced and increased in these two models, respectively [133]. CircRNA_ABCA1 could bind and suppress miR-140-3p, while miR-140-3p could bind and suppress MAP2K6. MKK6 is a protein kinase and could specifically activate p38MAPK pathway. Many studies have shown that MKK6/p38MAPK signaling pathway plays a key role in endothelial injury and lipid metabolic disorder. miR-140-3p also reduced endothelial injury, suggesting that circRNA_ABCA1 may increase vascular endothelial injury and atherosclerosis by regulating miR-140-3p-MAP2K6 axis [133]. However, the role of circRNA_ABCA1 in ABCA1 expression and cholesterol metabolism has unclear. As mentioned above, miR-140-3p also suppressed ADAM10 by targeting ADAM10 3′UTR, suggesting that circRNA_ABCA1 promoted cholesterol efflux by regulating miR-140-3p/ADAM10 axis. Thus, CircRNA_ABCA1 regulated foam cell formation and vascular endothelial injury by regulating miR-140-3p/ADAM10 axis and miR-140-3p/MAP2K6 axis. In fact, many circRNA/miRNA/ADAM10 axis may promote cholesterol efflux and suppress foam cell formation, including circ-FNDC3B/miR-143-3p/ADMA10 axis [134], circ-PRKCH (also named hsa_circ_0032131)/miR-140-3p/ADMA10 axis [104], circNFIX/miR-212-3p/ADAM10 axis [135], circ-SFMBT2/miR-7-5p/ADAM10 axis [136], circFOXM1/miR-515-5p/ADAM10 axis [137], circ_0088194/miR-30a-3p/ADAM10 axis [138], circ_0000977/miR-153/ADAM10 axis [139]. Together together, the circRNA_ABCA1/miR-140-3p axis, circ-PRKCH/miR-140-3p axis, circ-FNDC3B/miR-143-3p axis, circNFIX/miR-212-3p axis, circ-SFMBT2/miR-7-5p axis, circFOXM1/miR-515-5p axis, circ_0088194/miR-30a-3p axis, circ_0000977/miR-153 axis promoted cholesterol efflux to suppress foam cell formation by targeting ADAM10 3′UTR.
3.7. ApoA-I: circRNA/miR-520h axis
The serum circRNA_102682 expression in gestational diabetes mellitus (GDM) was correlated with the TG, apoA-I, HDL-C, apoB, and blood glucose, suggesting that circRNA_102682 regulated lipid metabolism. Specifically, the expression of circ_0088212 and apoA-I were reduced in osteosarcoma tissues and cells, while miR-520h was increased. Circ_0088212 suppressed osteosarcoma cell growth and migration by regulating miR-520h/apoA-I in vitro and in vivo [140]. As mentioned above, apoA-I plays an irreplaceable role in ABCA1-mediated cholesterol efflux [141,142]. Thus, circ_0088212 increased cholesterol efflux and suppressed foam cell formation by regulating miR-520h/apoA-I axis. In fact, many circRNAs could serve as the miR-520h sponge, including circCSPP1 [143], circ_0001860 [144], circSETD3 [145], circTAOK1 [146], circNDRG1 [147], suggesting that these circRNAs increased cholesterol efflux and suppressed foam cell formation by regulating miR-520h/apoA-I axis (Table 1).
Table 1.
The potential role and mechanism of circRNAs in foam cell formation.
| CircRNAs | Axis | Function | References |
|---|---|---|---|
| circ-Wdr91 | miR-378a-5p/SR-A1 | cholesterol uptake | [32] |
| circ_000373 | miR-378a-5p/SR-A1 | cholesterol uptake | [32,33] |
| circEZH2 | miR-378b/CD36 | cholesterol uptake | [34] |
| circZNF609 | miR-378b/CD36 | cholesterol uptake | [34,38] |
| circ_0078710 | miR-378b/CD36 | cholesterol uptake | [34,39] |
| circFCHSD2 | miR-6516/CD36 | cholesterol uptake | [40] |
| circHNRNPLL | miR-11986b/CD36 | cholesterol uptake | [40] |
| circKANSL1 | miR-345/CD36 | cholesterol uptake | [40] |
| miR-502b/CD36 | |||
| miR-6516/CD36 | |||
| circMAP7 | miR-11986b/CD36 | cholesterol uptake | [40] |
| miR-345/CD36 | |||
| circ_0004104 | miR-100/CD36 | cholesterol uptake | [42,44,45] |
| circ-0072309 | miR-100/CD36 | cholesterol uptake | [[44], [45], [46], [47], [48]] |
| circ_0006168 | miR-100/CD36 | cholesterol uptake | [44,45,49] |
| circRNA-0044073 | miR-107/CD36 | cholesterol uptake | [50,53] |
| circRNA PPP1CC | miR-107/CD36 | cholesterol uptake | [51,53] |
| circ_UBR4 | miR-107/CD36 | cholesterol uptake | [52,53] |
| circMETTL3 | miR-107/CD36 | cholesterol uptake | [53,54] |
| circCFL1 | miR-107/CD36 | cholesterol uptake | [53,55] |
| circ-SFMBT2 | miR-107/CD36 | cholesterol uptake | [53,56], |
| circ0041103 | miR-107/CD36 | cholesterol uptake | [53,57] |
| circHIPK3 | miR-107/CD36 | cholesterol uptake | [53,58,59] |
| circ-ZFR | miR-107/CD36 | cholesterol uptake | [53,60] |
| circTGFBR2 | miR-107/CD36 | cholesterol uptake | [53,61] |
| circFGFR4 | miR-107/CD36 | cholesterol uptake | [53,62] |
| circ_0049472 | miR-107/CD36 | cholesterol uptake | [53,63] |
| circ-LTBP1 | miR-107/CD36 | cholesterol uptake | [53,64] |
| circTCF25 | miR-107/CD36 | cholesterol uptake | [53,65] |
| circ_BICD2 | miR-107/CD36 | cholesterol uptake | [53,66] |
| circRNA ITGA5 | miR-107/CD36 | cholesterol uptake | [53,67] |
| circ_0005033 | miR-107/CD36 | cholesterol uptake | [53,68] |
| circCCT3 | miR-107/CD36 | cholesterol uptake | [53,69] |
| circular RNA-cTFRC | miR-107/CD36 | cholesterol uptake | [53,70] |
| circSLC7A6 | miR-107/CD36 | cholesterol uptake | [53,71] |
| circ_RPL23A | miR-1233/ACAT2 | cholesterol esterification | [74] |
| circTP53 | miR-1233/ACAT2 | cholesterol esterification | [74,75] |
| circEHMT1 | miR-1233/ACAT2 | cholesterol esterification | [74,76] |
| circ-0007766 | miR-1233/ACAT2 | cholesterol esterification | [74,77] |
| circ_0004050 | miR-1233/ACAT2 | cholesterol esterification | [74,78] |
| circRNA_0001805 | miR-106a-5p/ABCA1 | cholesterol efflux | [80] |
| circDENND1B | miR-17-5p/ABCA1 | cholesterol efflux | [81] |
| circPCMTD1 | miR-30a-3p/ABCA1 | cholesterol efflux | [83] |
| miR-30e-3p/ABCA1 | |||
| miR-5581-3p/ABCA1 | |||
| circLDLR | miR-30a-3p/ABCA1 | cholesterol efflux | [83,87] |
| circNFIC | miR-30e-3p/ABCA1 | cholesterol efflux | [83,88] |
| circBRWD1 | miR-139-5p/ABCA1 | cholesterol efflux | [83] |
| miR-3918/SR-B1 | |||
| circ-BIRC6 | miR-3918/SR-B1 | cholesterol efflux | [83,89] |
| circMAP2K4 | miR-139-5p/ABCA1 | cholesterol efflux | [83,90] |
| circPTPRM | miR-139-5p/ABCA1 | cholesterol efflux | [83,91] |
| circRNF13 | miR-139-5p/ABCA1 | cholesterol efflux | [83,92] |
| circDNER | miR-139-5p/ABCA1 | cholesterol efflux | [83,93] |
| circ-STAT3.46 | miR-139-5p/ABCA1 | cholesterol efflux | [83,94] |
| circEIF3K | miR-139-5p/ABCA1 | cholesterol efflux | [83,95] |
| circ_0077109 | miR-139-5p/ABCA1 | cholesterol efflux | [83,96] |
| circ_0002594 | miR-139-5p/ABCA1 | cholesterol efflux | [83,97] |
| circTHBS1 | miR-139-5p/ABCA1 | cholesterol efflux | [83,98] |
| circBACH2 | miR-139-5p/ABCA1 | cholesterol efflux | [83,99] |
| circ_0001610 | miR-139-5p/ABCA1 | cholesterol efflux | [83,100] |
| circUGGT2 | miR-186-3p/ABCA1 | cholesterol efflux | [83,103,104] |
| miR-140-3p/ADAM10 | |||
| circRSF1 | miR-758/ABCA1 | cholesterol efflux | [[108], [109], [110]] |
| miR-135b-5p/ABCA1 | [111,112] | ||
| circ_0002483 | miR-758/ABCA1 | cholesterol efflux | [109,110,113] |
| circ_0003221 | miR-758/ABCA1 | cholesterol efflux | [109,110,114] |
| circRBM33 | miR-758/ABCA1 | cholesterol efflux | [109,110,115] |
| circ_0093887 | miR-758/ABCA1 | cholesterol efflux | [109,110,116] |
| circ_0008500 | miR-758/ABCA1 | cholesterol efflux | [109,110,117] |
| circ_0002360 | miR-758/ABCA1 | cholesterol efflux | [109,110,118] |
| circNFIX | miR-758/ABCA1 | cholesterol efflux | [109,110,119] |
| miR-212-3p/ADAM10 | [135] | ||
| circ-PRMT5 | miR-145/ABCA1 | cholesterol efflux | [120,121] |
| circ_0092317 | miR-326/PDE3B/ABCA1 | cholesterol efflux | [123] |
| circ_0003546 | miR-326/PDE3B/ABCA1 | cholesterol efflux | [123] |
| circ_0028198 | miR-543/PDE3B/ABCA1 | cholesterol efflux | [123] |
| circ_0092317 | miR-543/PDE3B/ABCA1 | cholesterol efflux | [123] |
| circFASN | miR-33/ABCA1 | cholesterol efflux | [124,126] |
| miR-33/ABCG1 | |||
| circ_SATB2 | miR-33/ABCA1 | cholesterol efflux | [125,126] |
| miR-33/ABCG1 | |||
| circRNA_0046366 | miR-34a/PPARα/ABCA1 | cholesterol efflux | [84,127] |
| miR-34a/PPARα/ABCG1 | |||
| circRNA_0046367 | miR-34a/PPARα/ABCA1 | cholesterol efflux | [84,128] |
| miR-34a/PPARα/ABCG1 | |||
| circRNA Z: 54674624|54755962 | miR-1635/ABCA1 | cholesterol efflux | [129] |
| circ_0001445 | miR-208b-5p/ABCG1 | cholesterol efflux | [131] |
| circRNA_ABCA1 | miR-140-3p/ADAM10 | cholesterol efflux | [104,133] |
| circ-PRKCH | miR-140-3p/ADMA10 axis | cholesterol efflux | [104] |
| circ-FNDC3B | miR-143-3p/ADMA10 | cholesterol efflux | [134] |
| circ-SFMBT2 | miR-7-5p/ADAM10 | cholesterol efflux | [136] |
| circFOXM1 | miR-515-5p/ADAM10 | cholesterol efflux | [137] |
| circ_0088194 | miR-30a-3p/ADAM10 axis | cholesterol efflux | [138] |
| circ_0000977 | miR-153/ADAM10 | cholesterol efflux | [139] |
| circ_0088212 | miR-520h/apoA-I | cholesterol efflux | [140] |
| circCSPP1 | miR-520h/apoA-I | cholesterol efflux | [140,143] |
| circ_0001860 | miR-520h/apoA-I | cholesterol efflux | [140,144] |
| circSETD3 | miR-520h/apoA-I | cholesterol efflux | [140,145] |
| circTAOK1 | miR-520h/apoA-I | cholesterol efflux | [140,146] |
| circNDRG1 | miR-520h/apoA-I | cholesterol efflux | [140,147] |
4. Nanotechnology: the potential method ways to deliver circRNAs
The effective delivery of synthetic therapeutic circRNAs to the desired target tissues is challenging. Nanotechnology may be an important method to solve this problem. In fact, nanotechnology has been successfully used in siRNA, which has a significant anti-cancer therapeutic effect [148,149]. A novel metalorganic framework nanocarrier (GZ) was successfully prepared from glycyrrhizic acid (GA) and zinc ions (Zn2+). Glycyrrhizic acid-RBC membrane/loaded with the circRNA_0001805 plasmid to construct a nanocore (GA-RM/GZ/PL) has good blood compatibility, suggesting that GA-RM/GZ/PL can circulate with the blood to the tissues in vivo. Interestingly, GA-RM/GZ/PL avoids phagocytosis by reducing the recognition ability of macrophages and the clearance ability of the immune system. GA-RM/GZ/PL can be endocytosed into hepatocytes and hepatic tissues. GA-RM/GZ/PL carried GA and overexpression circRNA_0001805. GA-RM/GZ/PL reduced lipid accumulation in FFA-stimulated primary hepatocytes by regulating the miR-106a-5p/ABCA1 axis and the miR-320a/CPT1 axis by increasing circRNA_0001805 expression, which is greater than glycyrrhizic acid, GZ, and GZ/PL. GA-RM/GZ/PL also reduced TG and TC levels of hepatic tissues by increasing ABCA1 and CPT1 expression in the HFD-fed mice. In fact, GA also suppressed lipid accumulation and inflammation. GA-RM/GZ/PL alleviated liver function impairment by reducing Aspartate transaminase (AST) and Alanine aminotransferase (ALT) levels in HFD-mice and did not change erythrocyte, hemoglobin, white blood cell, platelets, and a renal function index. GA-RM/GZ/PL also did not induce pathological changes in tissues, including heart, spleen, lung, and kidney tissues, suggesting that the safety of GA-RM/GZ/PL is relatively high. CircRNA_0001805 with adenovirus vectors and GA-RM/GZ/PL also reduced proinflammatory cytokines and chemokine levels by suppressing NF-κB pathway in FFA-treated primary hepatocytes and hepatic tissues of the HFD-fed mice, including TNF-α, IL-6, IL-1β, and CCL2. Given the superior safety and efficacy of GA-RM/GZ/PL over adenovirus vectors, we hypothesized that GA-RM/GZ/PL might be a potential new drug for the treatment of atherosclerosis [80]. However, many studies are needed, such as multiple animal models, drug stability, pharmacokinetics, and systems toxicology.
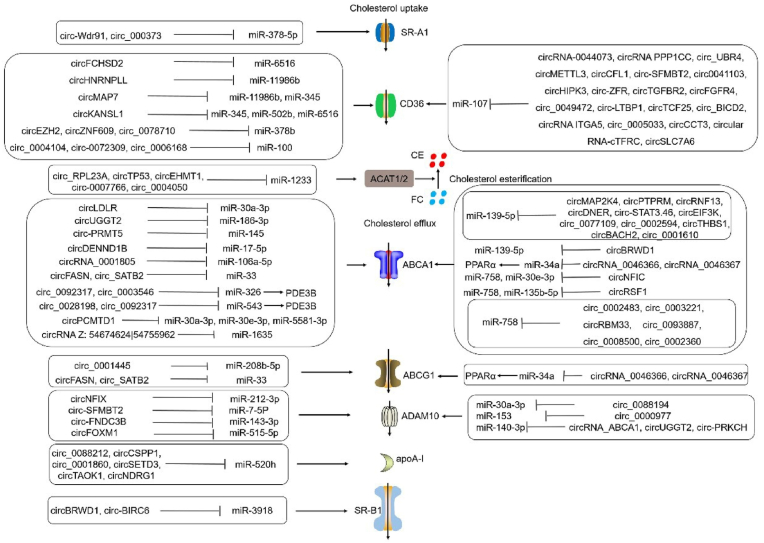
5. Summary
Atherosclerosis is a major factor in CHD and MI. Atherosclerosis occurs during foam cell generation, a process induced by imbalance of the cholesterol uptake, esterification, and efflux [150,151]. LOX-1, SR-A1, and CD36 promoted cholesterol uptake. ACAT1 and ACAT2 promoted esterification of FC to CE. nCEH promoted the hydrolysis of CE to FC. ABCA1, ABCG1, ADAM10, and apoA-I promoted FC efflux. SR-BI promotes not only cholesterol uptake but also FC efflux. Many circRNAs regulate foam cell formation, acting as miRNA sponges to influence atherosclerosis development by regulating the expression of SR-A1, CD36, ACAT2, ABCA1, ABCG1, ADAM10, apoA-I, SR-B1 (Fig. 2). Interestingly, many circRNAs promoted cholesterol uptake and efflux by multiple mechanisms. Such as, circKANSL1 promoted cholesterol uptake by regulating miR-345/CD36 axis, miR-502b/CD36 axis, miR-6516/CD36 axis. CircMAP7 promoted cholesterol uptake by regulating miR-11986b/CD36 axis and miR-345/CD36 axis. CircBRWD1 promoted cholesterol efflux by regulating miR-139-5p/ABCA1 axis and miR-3918/SR-B1 axis. CircUGGT2 promoted cholesterol efflux by regulating miR-186-3p/ABCA1 axis and miR-140-3p/ADAM10 axis. CircRSF1 promoted cholesterol efflux by regulating miR-758/ABCA1 axis and miR-135b-5p/ABCA1 axis. CircNFIC promoted cholesterol efflux by regulating miR-30e-3p/ABCA1, miR-758/ABCA1, miR-212-3p/ADAM10. In addition, several interesting and critical questions remain unanswered. (1) It is important to determine whether the effects of these circRNAs in the same cell are competitive, antagonistic, synergistic, or noninteracting, particularly when they target the same miRNA, such as miR-107, miR-139-5p, miR-758, or miR-33. (2) circRNAs promoted cholesterol uptake or efflux by acting as a sponge of multiple miRNAs, such as circKANSL1, circMAP7, circPCMTD1, and circRSF1. However, the competition, synergy, and antagonism among these miRNAs remains unknown. (3) Up to the present, only miRNAs have been found to play an important role in circRNAs-medicated gene expression of foam cell formation. However, circRNAs also regulated the downstream gene expression by forming complexes with RBPs and protein interactions. Whether circRNAs have other mechanisms to regulate the gene expression of foam cell formation is unclear. (4) The effective delivery of synthetic therapeutic circRNAs to the desired target tissues is a challenge that should be addressed. (5) Nanotechnology is an important method for solving the delivery and stability of circRNA drugs, such as GA-RM/GZ/PL. However, the pharmacokinetics and systems toxicology of GA-RM/GZ/PL has unclear. (6) Many circRNAs are used as an independent class of potential clinical biomarkers for atherosclerosis to better evaluate cardiovascular risk in combination with a “biomarker” score, including circ-Wdr91, circ_0004104, circRNA-0044073, CircRNA_0001805, circDENND1B, circRSF1, circ_0001445, circRNA_102682. However, despite the numerous studies on these biomarkers, their clinical translation is still being investigated. Biomarkers should be sensitive and specific for diagnosing the disease state. A better understanding of the mechanisms underlying circRNAs-mediated foam cell formation, coupled with improvements in drug delivery technology, will enable circRNAs to open a new avenue in atherosclerosis treatment.
Fig. 2.
The role and mechanism of circRNAs in regulating the gene expression of foam cell formation.
Funding
The authors are grateful for the financial support provided by the Qingdao Major Scientific and Technological Project for Distinguished Scholars (20170103), the Laoshan Major Scientific and Technological Project for Distinguished Scholars (20181030), the Natural Science Foundation of Shandong Province (ZR2020MH369, ZR2020MH242, ZR2022MH218), Youth Research Fund of Affiliated Hospital of Qingdao University in 2021 (QDFYQN202101012).
Declaration of competing interest
The authors have no conflicts of interest to declare in association with this paper.
Acknowledgments
We thank colleagues in Dr. DX's and XW's laboratory for their technical help and the stimulating discussions provided during this investigation.
Contributor Information
Xiaodan Xu, Email: 974206395@qq.com.
Dongming Xing, Email: xdm_tsinghua@163.com.
Xiaolin Wu, Email: fyqs01@126.com.
References
- 1.Chen W., Xing J., Liu X., Wang S., Xing D. The role and transformative potential of IL-19 in atherosclerosis. Cytokine Growth Factor Rev. 2021;62:70–82. doi: 10.1016/j.cytogfr.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Rox K., Rath S., Pieper D.H., Vital M., Bronstrup M. A simplified LC-MS/MS method for the quantification of the cardiovascular disease biomarker trimethylamine-N-oxide and its precursors. J Pharm Anal. 2021;11(4):523–528. doi: 10.1016/j.jpha.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu F., Zhu X., Jiang X., Li S., Lv Y. Transcriptional control by HNF-1: emerging evidence showing its role in lipid metabolism and lipid metabolism disorders. Genes Dis. 2022;9(5):1248–1257. doi: 10.1016/j.gendis.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z., Yu G.L., Zhu X., Peng T.H., Lv Y.C. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: implications in lipid metabolic disorders. Genes Dis. 2022;9(1):51–61. doi: 10.1016/j.gendis.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rachmawati E., Sargowo D., Rohman M.S., Widodo N., Kalsum U. miR-155-5p predictive role to decelerate foam cell atherosclerosis through CD36, VAV3, and SOCS1 pathway. Noncoding RNA Res. 2021;6(2):59–69. doi: 10.1016/j.ncrna.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonhogen I.G.C., Mohseni Z., Winkens B., Xiao K., Thum T., Calore M., da Costa Martins P.A., de Windt L.J., Spaanderman M.E.A., Ghossein-Doha C. Circulating miR-216a as a biomarker of metabolic alterations and obesity in women. Noncoding RNA Res. 2020;5(3):144–152. doi: 10.1016/j.ncrna.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y., Zhang C., Xiong J., Ren H. Emerging important roles of circRNAs in human cancer and other diseases. Genes Dis. 2021;8(4):412–423. doi: 10.1016/j.gendis.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X., Xiang F., Pei Z., Miao J., Wu P., Song X., Li Y., Zhang Y. Circ-Smad5 retards the G1/S transition of cell cycle via inhibiting the activity of wnt/lef/cyclind1 signaling in JB6 cells. Genes Dis. 2021;8(3):364–372. doi: 10.1016/j.gendis.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X., Zhao Y., Zhou H., Li Y. Circular RNAs in atherosclerosis. Clin. Chim. Acta. 2022;531:71–80. doi: 10.1016/j.cca.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Farooqi A.A., Gulnara K., Mukhanbetzhanovna A.A., Datkhayev U., Kussainov A.Z., Adylova A. Regulation of RUNX proteins by long non-coding RNAs and circular RNAs in different cancers. Noncoding RNA Res. 2021;6(2):100–106. doi: 10.1016/j.ncrna.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao S., Lin X., Mo C. Integrated analysis of circRNA-miRNA-mRNA regulatory network identifies potential diagnostic biomarkers in diabetic foot ulcer. Noncoding RNA Res. 2020;5(3):116–124. doi: 10.1016/j.ncrna.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q., Li Y., Wu Q., Huang L., Xu J., Zeng Q. Pathogenic role of microRNAs in atherosclerotic ischemic stroke: implications for diagnosis and therapy. Genes Dis. 2022;9(3):682–696. doi: 10.1016/j.gendis.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafarinejad-Farsangi S., Jazi M.M., Rostamzadeh F., Hadizadeh M. High affinity of host human microRNAs to SARS-CoV-2 genome: an in silico analysis. Noncoding RNA Res. 2020;5(4):222–231. doi: 10.1016/j.ncrna.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josefs T., Boon R.A. The long non-coding road to atherosclerosis. Curr. Atherosclerosis Rep. 2020;22(10):55. doi: 10.1007/s11883-020-00872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan Y., Yang J., Li H., Li H., Zhang S., Li X., Song Y., Dang W., Liu L., Cao X., Wang X., Nandakumar K.S., Shen X., You Y. WITHDRAWN: SNX10 deficiency restricts foam cell formation and protects against atherosclerosis by suppressing CD36-Lyn axis. Can. J. Cardiol. 2020;(20):30456–30460. doi: 10.1016/j.cjca.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Sakashita N., Miyazaki A., Chang C.C., Chang T.Y., Kiyota E., Satoh M., Komohara Y., Morganelli P.M., Horiuchi S., Takeya M. Acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) is induced in monocyte-derived macrophages: in vivo and in vitro studies. Lab. Invest. 2003;83(11):1569–1581. doi: 10.1097/01.lab.0000095687.17383.39. [DOI] [PubMed] [Google Scholar]
- 17.Iatan I., Palmyre A., Alrasheed S., Ruel I., Genest J. Genetics of cholesterol efflux. Curr. Atherosclerosis Rep. 2012;14(3):235–246. doi: 10.1007/s11883-012-0247-y. [DOI] [PubMed] [Google Scholar]
- 18.Jones A.C., Irvin M.R., Claas S.A., Arnett D.K. Lipid phenotypes and DNA methylation: a review of the literature. Curr. Atherosclerosis Rep. 2021;23(11):71. doi: 10.1007/s11883-021-00965-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X., Yin R., Shi H., Wang X., Shen D., Wang X., Pan C. LncRNA ZFAS1 confers inflammatory responses and reduces cholesterol efflux in atherosclerosis through regulating miR-654-3p-ADAM10/RAB22A axis. Int. J. Cardiol. 2020;315:72–80. doi: 10.1016/j.ijcard.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 20.Li H., Han S., Sun Q., Yao Y., Li S., Yuan C., Zhang B., Jing B., Wu J., Song Y., Wang H. Long non-coding RNA CDKN2B-AS1 reduces inflammatory response and promotes cholesterol efflux in atherosclerosis by inhibiting ADAM10 expression. Aging (Albany NY) 2019;11(6):1695–1715. doi: 10.18632/aging.101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jing L., Shu-Xu D., Yong-Xin R. A review: pathological and molecular biological study on atherosclerosis. Clin. Chim. Acta. 2022;531:217–222. doi: 10.1016/j.cca.2022.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Gui Y., Zheng H., Cao R.Y. Foam cells in atherosclerosis: novel insights into its origins, consequences, and molecular mechanisms. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.845942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang P., Blanchard V., Francis G.A. Smooth muscle cell-macrophage interactions leading to foam cell formation in atherosclerosis: location, location, location. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.921597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe R.J., Abe J.I., Nguyen M.T.H., Olmsted-Davis E.A., Mamun A., Banerjee P., Cooke J.P., Fang L., Pownall H., Le N.T. Free cholesterol bioavailability and atherosclerosis. Curr. Atherosclerosis Rep. 2022;24(5):323–336. doi: 10.1007/s11883-022-01011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen X., Zhang S., Guo Z., Xing D., Chen W. The crosstalk of ABCA1 and ANXA1: a potential mechanism for protection against atherosclerosis. Mol. Med. 2020;26(1):84. doi: 10.1186/s10020-020-00213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W., Zhang S., Wu J., Ye T., Wang S., Wang P., Xing D. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin. Chim. Acta. 2020;507:236–241. doi: 10.1016/j.cca.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Chen W., Wang S., Xing D. New horizons for the roles and association of APE1/ref-1 and ABCA1 in atherosclerosis. J. Inflamm. Res. 2021;14:5251–5271. doi: 10.2147/JIR.S330147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W., Li L., Wang J., Zhang R., Zhang T., Wu Y., Wang S., Xing D. The ABCA1-efferocytosis axis: a new strategy to protect against atherosclerosis. Clin. Chim. Acta. 2021;518:1–8. doi: 10.1016/j.cca.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Jiang H., Qin X., Wang Q., Xu Q., Wang J., Wu Y., Chen W., Wang C., Zhang T., Xing D., Zhang R. Application of carbohydrates in approved small molecule drugs: a review. Eur. J. Med. Chem. 2021;223 doi: 10.1016/j.ejmech.2021.113633. [DOI] [PubMed] [Google Scholar]
- 30.Zhang R., Liu W., Zeng J., Meng J., Jiang H., Wang J., Xing D. Niemann-Pick C1-Like 1 inhibitors for reducing cholesterol absorption. Eur. J. Med. Chem. 2022;230 doi: 10.1016/j.ejmech.2022.114111. [DOI] [PubMed] [Google Scholar]
- 31.Chen W., Wu Y., Lu Q., Wang S., Xing D. Endogenous ApoA-I expression in macrophages: a potential target for protection against atherosclerosis. Clin. Chim. Acta. 2020;505:55–59. doi: 10.1016/j.cca.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Chen W., Guo S., Li X., Song N., Wang D., Yu R. The regulated profile of noncoding RNAs associated with inflammation by tanshinone IIA on atherosclerosis. J. Leukoc. Biol. 2020;108(1):243–252. doi: 10.1002/JLB.3MA0320-327RRR. [DOI] [PubMed] [Google Scholar]
- 33.Yu M., Huo S., Sun L., Gao J., Liu Y., Yu J., Liu F., Sheng S., Nie X., Nan Q., Tian Y. Epidermal growth factor receptor mutation mechanisms in nonsmall cell lung cancer by transcriptome sequencing. Cancer Biother. Radiopharm. 2022;37(7):560–568. doi: 10.1089/cbr.2020.4049. [DOI] [PubMed] [Google Scholar]
- 34.Wang D., Zhao Z., Shi Y., Luo J., Chen T., Xi Q., Zhang Y., Sun J. CircEZH2 regulates milk fat metabolism through miR-378b sponge activity. Animals. 2022;12(6) doi: 10.3390/ani12060718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury M., McCrindle B.W. The rationale, indications, safety, and use of statins in the pediatric population. Can. J. Cardiol. 2020;36(9):1372–1383. doi: 10.1016/j.cjca.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Mury P., Dupuis J., Thorin E. A novel molecular pathway of plaque vulnerability reveals a cholesterol-independent effect of statins and supports inflammation as a therapeutic target. Can. J. Cardiol. 2020;36(11):1710–1713. doi: 10.1016/j.cjca.2020.02.068. [DOI] [PubMed] [Google Scholar]
- 37.Vikulova D.N., Trinder M., Mancini G.B.J., Pimstone S.N., Brunham L.R. Familial hypercholesterolemia, familial combined hyperlipidemia, and elevated lipoprotein(a) in patients with premature coronary artery disease. Can. J. Cardiol. 2021;37(11):1733–1742. doi: 10.1016/j.cjca.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z., Li G., Han Y., Li Y., Ji Z., Guo R., Yu X. Circular RNA ZNF609 enhances proliferation and glycolysis during glioma progression by miR-378b/SLC2A1 axis. Aging (Albany NY) 2021;13(17):21122–21133. doi: 10.18632/aging.203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Q., Guo H., Zong Y., Zhao X. Curcumin restrains hepatocellular carcinoma progression depending on the regulation of the circ_0078710/miR-378b/PRIM2 axis. J. Recept. Signal Transduct. Res. 2022;42(3):313–324. doi: 10.1080/10799893.2021.1936554. [DOI] [PubMed] [Google Scholar]
- 40.Wang D., Chen Z., Zhuang X., Luo J., Chen T., Xi Q., Zhang Y., Sun J. Identification of circRNA-Associated-ceRNA networks involved in milk fat metabolism under heat stress. Int. J. Mol. Sci. 2020;21(11) doi: 10.3390/ijms21114162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L., Shen C., Wang Y., Zou T., Zhu H., Lu X., Li L., Yang B., Chen J., Chen S., Lu X., Gu D. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis. 2019;286:88–96. doi: 10.1016/j.atherosclerosis.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Ji P., Song X., Lv Z. Knockdown of circ_0004104 alleviates oxidized low-density lipoprotein-induced vascular endothelial cell injury by regulating miR-100/TNFAIP8 Axis. J. Cardiovasc. Pharmacol. 2021;78(2):269–279. doi: 10.1097/FJC.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C., Wang L., Shen Y. Circ_0004104 knockdown alleviates oxidized low-density lipoprotein-induced dysfunction in vascular endothelial cells through targeting miR-328-3p/TRIM14 axis in atherosclerosis. BMC Cardiovasc. Disord. 2021;21(1):207. doi: 10.1186/s12872-021-02012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smolka C., Schlosser D., Hohnloser C., Bemtgen X., Janich C., Schneider L., Martin J., Pfeifer D., Moser M., Hasselblatt P., Bode C., Grundmann S., Pankratz F. MiR-100 overexpression attenuates high fat diet induced weight gain, liver steatosis, hypertriglyceridemia and development of metabolic syndrome in mice. Mol. Med. 2021;27(1):101. doi: 10.1186/s10020-021-00364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smolka C., Schlosser D., Koentges C., Tarkhnishvili A., Gorka O., Pfeifer D., Bemtgen X., Asmussen A., Gross O., Diehl P., Moser M., Bode C., Bugger H., Grundmann S., Pankratz F. Cardiomyocyte-specific miR-100 overexpression preserves heart function under pressure overload in mice and diminishes fatty acid uptake as well as ROS production by direct suppression of Nox4 and CD36. Faseb. J. 2021;35(11) doi: 10.1096/fj.202100829RR. [DOI] [PubMed] [Google Scholar]
- 46.Yuan F., Zhang S., Sun Q., Ye L., Xu Y., Xu Z., Deng G., Zhang S., Liu B., Chen Q. Hsa_circ_0072309 enhances autophagy and TMZ sensitivity in glioblastoma. CNS Neurosci. Ther. 2022;28(6):897–912. doi: 10.1111/cns.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T., Shao S., Li W., Liu Y., Cao Y. The circular RNA hsa-circ-0072309 plays anti-tumour roles by sponging miR-100 through the deactivation of PI3K/AKT and mTOR pathways in the renal carcinoma cell lines. Artif. Cells, Nanomed. Biotechnol. 2019;47(1):3638–3648. doi: 10.1080/21691401.2019.1657873. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y., Li J., Li J., Xu L., Lian W. The decreased circular RNA hsa_circ_0072309 promotes cell apoptosis of ischemic stroke by sponging miR-100. Eur. Rev. Med. Pharmacol. Sci. 2020;24(8):4420–4429. doi: 10.26355/eurrev_202004_21024. [DOI] [PubMed] [Google Scholar]
- 49.Shi Y., Guo Z., Fang N., Jiang W., Fan Y., He Y., Ma Z., Chen Y. hsa_circ_0006168 sponges miR-100 and regulates mTOR to promote the proliferation, migration and invasion of esophageal squamous cell carcinoma. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109151. [DOI] [PubMed] [Google Scholar]
- 50.Shen L., Hu Y., Lou J., Yin S., Wang W., Wang Y., Xia Y., Wu W. CircRNA0044073 is upregulated in atherosclerosis and increases the proliferation and invasion of cells by targeting miR107. Mol. Med. Rep. 2019;19(5):3923–3932. doi: 10.3892/mmr.2019.10011. [DOI] [PubMed] [Google Scholar]
- 51.Liu J., Wang Y., Liao Y., Zhou Y., Zhu J. Circular RNA PPP1CC promotes Porphyromonas gingivalis-lipopolysaccharide-induced pyroptosis of vascular smooth muscle cells by activating the HMGB1/TLR9/AIM2 pathway. J. Int. Med. Res. 2021;49(3) doi: 10.1177/0300060521996564. 300060521996564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Zhang C., Chen Z., Wang M. Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis. Open Life Sci. 2021;16(1):419–430. doi: 10.1515/biol-2021-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Yao J., Shi H., Gao B., Zhang L. LncRNA TINCR/microRNA-107/CD36 regulates cell proliferation and apoptosis in colorectal cancer via PPAR signaling pathway based on bioinformatics analysis. Biol. Chem. 2019;400(5):663–675. doi: 10.1515/hsz-2018-0236. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F., Su T., Xiao M. RUNX3-regulated circRNA METTL3 inhibits colorectal cancer proliferation and metastasis via miR-107/PER3 axis. Cell Death Dis. 2022;13(6):550. doi: 10.1038/s41419-022-04750-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Chen X., Xie X., Zhou W. CircCFL1/MiR-107 Axis targeting HMGB1 promotes the malignant progression of diffuse large B-cell lymphoma tumors. Cancer Manag. Res. 2020;12:9351–9362. doi: 10.2147/CMAR.S263222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang Z., Fu Y., Jia Y., Gao M., Song L., Zhang W., Zhao R., Qin Y. Circ-SFMBT2 drives the malignant phenotypes of esophageal cancer by the miR-107-dependent regulation of SLC1A5. Cancer Cell Int. 2021;21(1):495. doi: 10.1186/s12935-021-02156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen L.Q., Yi C.L., Liu D.C., Wang P., Zhu Y.F., Yuan L.P. Hsa_circ_0041103 induces proliferation, migration and invasion in bladder cancer via the miR-107/FOXK1 axis. Eur. Rev. Med. Pharmacol. Sci. 2021;25(3):1282–1290. doi: 10.26355/eurrev_202102_24832. [DOI] [PubMed] [Google Scholar]
- 58.Wei J., Xu H., Wei W., Wang Z., Zhang Q., De W., Shu Y. circHIPK3 promotes cell proliferation and migration of gastric cancer by sponging miR-107 and regulating BDNF expression. OncoTargets Ther. 2020;13:1613–1624. doi: 10.2147/OTT.S226300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Hong W., Zhang Y., Ding J., Yang Q., Xie H., Gao X. circHIPK3 acts as competing endogenous RNA and promotes non-small-cell lung cancer progression through the miR-107/BDNF signaling pathway. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/6075902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu T., Liu S., Xu Y., Shu R., Wang F., Chen C., Zeng Y., Luo H. Circular RNA-ZFR inhibited cell proliferation and promoted apoptosis in gastric cancer by sponging miR-130a/miR-107 and modulating PTEN. Cancer Res Treat. 2018;50(4):1396–1417. doi: 10.4143/crt.2017.537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Li W., Lu H., Wang H., Ning X., Liu Q., Zhang H., Liu Z., Wang J., Zhao W., Gu Y., Li H., Sun X., Hu L., Wang D. Circular RNA TGFBR2 acts as a ceRNA to suppress nasopharyngeal carcinoma progression by sponging miR-107. Cancer Lett. 2021;499:301–313. doi: 10.1016/j.canlet.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Yu C., Li L., Xie F., Guo S., Liu F., Dong N., Wang Y. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc. Res. 2018;114(1):168–179. doi: 10.1093/cvr/cvx180. [DOI] [PubMed] [Google Scholar]
- 63.Zeng C., Xing H., Chen M., Chen L., Li P., Wu X., Li L. Circ_0049472 regulates the damage of Abeta-induced SK-N-SH and CHP-212 cells by mediating the miR-107/KIF1B axis. Exp. Brain Res. 2022;240(9):2299–2309. doi: 10.1007/s00221-022-06401-y. [DOI] [PubMed] [Google Scholar]
- 64.Li C., Zhang L., Bu X., Wang J., Li L., Yang Z. Circ-LTBP1 is involved in doxorubicin-induced intracellular toxicity in cardiomyocytes via miR-107/ADCY1 signal. Mol. Cell. Biochem. 2022;477(4):1127–1138. doi: 10.1007/s11010-022-04360-0. [DOI] [PubMed] [Google Scholar]
- 65.Zhong Z., Lv M., Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci. Rep. 2016;6 doi: 10.1038/srep30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu L.S., Wang Y.L., Li R., Xu X.T., Li K.Y., Zuo C.R. circ_BICD2 acts as a ceRNA to promote tumor progression and Warburg effect in oral squamous cell carcinoma by sponging miR-107 to enhance HK2. Am J Transl Res. 2020;12(7):3489–3500. [PMC free article] [PubMed] [Google Scholar]
- 67.Huang G., Ma J., Zhang L. Integrin subunit alpha 5 (ITGA5) gene circular RNA sponges microRNA-107 in colorectal carcinoma cells and tissues and regulates the expression of the forkhead box J3 (FOXJ3) gene. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.920623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong L., Chen J., Jiang X. Circ_0005033 is an oncogene in laryngeal squamous cell carcinoma and regulates cell progression and Cisplatin sensitivity via miR-107/IGF1R axis. Anti Cancer Drugs. 2022;33(3):245–256. doi: 10.1097/CAD.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 69.Li J., Lu R., Yang K., Sun Q. circCCT3 enhances invasion and epithelial-mesenchymal transition (EMT) of non-small-cell lung cancer (NSCLC) via the miR-107/wnt/FGF7 Axis. JAMA Oncol. 2022;2022 doi: 10.1155/2022/7020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su H., Tao T., Yang Z., Kang X., Zhang X., Kang D., Wu S., Li C. Circular RNA cTFRC acts as the sponge of MicroRNA-107 to promote bladder carcinoma progression. Mol. Cancer. 2019;18(1):27. doi: 10.1186/s12943-019-0951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J., Hao Y., Gao X., Wu Y., Ding Y., Wang B. CircSLC7A6 promotes the progression of Wilms' tumor via microRNA-107/ABL proto-oncogene 2 axis. Bioengineered. 2022;13(1):308–318. doi: 10.1080/21655979.2021.2001204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Kang L., Jia H., Huang B., Lu S., Chen Z., Shen J., Zou Y., Wang C., Sun Y. Identification of differently expressed mRNAs in atherosclerosis reveals CDK6 is regulated by circHIPK3/miR-637 Axis and promotes cell growth in human vascular smooth muscle cells. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.596169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.W.B. Zhang, Y.F. Qi, Z.X. Xiao, H. Chen, S.H. Liu, Z.Z. Li, Z.F. Zeng, H.F. Wu, CircHIPK3 regulates vascular smooth muscle cell calcification via the miR-106a-5p/MFN2 Axis, J Cardiovasc Transl Res 15(6) (2022) 1315-1326. [DOI] [PubMed]
- 74.Cheng L., Cao H., Xu J., Xu M., He W., Zhang W., Dong L., Chen D. Circ_RPL23A acts as a miR-1233 sponge to suppress the progression of clear cell renal cell carcinoma by promoting ACAT2. J. Bioenerg. Biomembr. 2021;53(4):415–428. doi: 10.1007/s10863-021-09901-8. [DOI] [PubMed] [Google Scholar]
- 75.Ma W., Zhao P., Zang L., Zhang K., Liao H., Hu Z. CircTP53 promotes the proliferation of thyroid cancer via targeting miR-1233-3p/MDM2 axis. J. Endocrinol. Invest. 2021;44(2):353–362. doi: 10.1007/s40618-020-01317-2. [DOI] [PubMed] [Google Scholar]
- 76.Lu M., Wu Y., Zeng B., Sun J., Li Y., Luo J., Wang L., Yi Z., Li H., Ren G. CircEHMT1 inhibits metastatic potential of breast cancer cells by modulating miR-1233-3p/KLF4/MMP2 axis. Biochem. Biophys. Res. Commun. 2020;526(2):306–313. doi: 10.1016/j.bbrc.2020.03.084. [DOI] [PubMed] [Google Scholar]
- 77.Xu W., Zhou B., Wu J., Jiang P., Chen H., Yan F. Circular RNA hsa-circ-0007766 modulates the progression of Gastric Carcinoma via miR-1233-3p/GDF15 axis. Int. J. Med. Sci. 2020;17(11):1569–1583. doi: 10.7150/ijms.46261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y., Zang R.K., Du Y.N. HSA_CIRC_0004050 on proliferation and apoptosis of A549 cells through ERK/JNK signaling pathway. J. Biol. Regul. Homeost. Agents. 2020;34(6):2037–2047. doi: 10.23812/20-543-A. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y., Li X., Cheng S., Wei W., Li Y. MicroRNA-106a confers cisplatin resistance in non-small cell lung cancer A549 cells by targeting adenosine triphosphatase-binding cassette A1. Mol. Med. Rep. 2015;11(1):625–632. doi: 10.3892/mmr.2014.2688. [DOI] [PubMed] [Google Scholar]
- 80.Li J., Qi J., Tang Y., Liu H., Zhou K., Dai Z., Yuan L., Sun C. A nanodrug system overexpressed circRNA_0001805 alleviates nonalcoholic fatty liver disease via miR-106a-5p/miR-320a and ABCA1/CPT1 axis. J. Nanobiotechnol. 2021;19(1):363. doi: 10.1186/s12951-021-01108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu F., Shen L., Chen H., Wang R., Zang T., Qian J., Ge J. circDENND1B participates in the antiatherosclerotic effect of IL-1beta monoclonal antibody in mouse by promoting cholesterol efflux via miR-17-5p/abca1 Axis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.652032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan L., Liu L., Jiang Z., Hao X. Inhibition of microRNA-17-5p reduces the inflammation and lipid accumulation, and up-regulates ATP-binding cassette transporterA1 in atherosclerosis. J. Pharmacol. Sci. 2019;139(4):280–288. doi: 10.1016/j.jphs.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 83.Liu J., Wei Y., Lin Y., Zhang P., Zhang Z., Huang H., Wu H., Zou T. Expression of the circular RNAs in astaxanthin promotes cholesterol efflux from THP-1 cells based on RNA-seq. Genes Nutr. 2021;16(1):13. doi: 10.1186/s12263-021-00693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang D.R., Wang B., Yang M., Liu Z.L., Sun J., Wang Y., Sun H., Xie L.J. Suppression of miR-30a-3p attenuates hepatic steatosis in non-alcoholic fatty liver disease. Biochem. Genet. 2020;58(5):691–704. doi: 10.1007/s10528-020-09971-0. [DOI] [PubMed] [Google Scholar]
- 85.Zheng S.Q., Qi Y., Wu J., Zhou F.L., Yu H., Li L., Yu B., Chen X.F., Zhang W. CircPCMTD1 acts as the sponge of miR-224-5p to promote glioma progression. Front. Oncol. 2019;9:398. doi: 10.3389/fonc.2019.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhai C., Sun Y., Qian G., Pan H., Xie S., Sun Z., Zhang S., Hu H. LncRNA AK087124/miR-224-5p/PTEN axis modulates endothelial cell injury in atherosclerosis through apoptosis and AKT signaling pathway. Arch. Biochem. Biophys. 2021;705 doi: 10.1016/j.abb.2021.108916. [DOI] [PubMed] [Google Scholar]
- 87.Wang R., Wang J., Chen Y., Chen Y., Xi Q., Sun L., Zhang X., Zhang G., Ding X., Shi T., Chen W. Circular RNA circLDLR facilitates cancer progression by altering the miR-30a-3p/SOAT1 axis in colorectal cancer. Cell Death Dis. 2022;8(1):314. doi: 10.1038/s41420-022-01110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y., Wang Z., Chen X., Peng X., Nie Q. CircNFIC balances inflammation and apoptosis by sponging miR-30e-3p and regulating DENND1B expression. Genes. 2021;12(11) doi: 10.3390/genes12111829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang G., Wang X., Liu B., Lu Z., Xu Z., Xiu P., Liu Z., Li J. circ-BIRC6, a circular RNA, promotes hepatocellular carcinoma progression by targeting the miR-3918/Bcl2 axis. Cell Cycle. 2019;18(9):976–989. doi: 10.1080/15384101.2019.1601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chi F., Cao Y., Chen Y. Analysis and validation of circRNA-miRNA network in regulating m(6)A RNA methylation modulators reveals CircMAP2K4/miR-139-5p/YTHDF1 Axis involving the proliferation of hepatocellular carcinoma. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.560506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang Z., Zhao J., Zou H., Cai K. CircRNA PTPRM promotes non-small cell lung cancer progression by modulating the miR-139-5p/SETD5 Axis. Technol. Cancer Res. Treat. 2022;21 doi: 10.1177/15330338221090090. 15330338221090090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu X., Zhou L., Chen Y., Jiang X., Jiang J. CircRNF13 promotes the malignant progression of pancreatic cancer through targeting miR-139-5p/igf1r Axis. JAMA Oncol. 2021;2021 doi: 10.1155/2021/6945046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li J., Zhu T., Weng Y., Cheng F., Sun Q., Yang K., Su Z., Ma H. Exosomal circDNER enhances paclitaxel resistance and tumorigenicity of lung cancer via targeting miR-139-5p/ITGB8. Thorac Cancer. 2022;13(9):1381–1390. doi: 10.1111/1759-7714.14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shi Y., Chen X., Xi B., Qin Y., Sun L., Hu J., Xu J., Yu X., Ouyang J., Wei L. STAT3 activation regulated circ-STAT3.46 promote expression of IGF1R by sponging of miR-139-5p in human colon cancer. Transl. Cancer Res. 2019;8(7):2593–2601. doi: 10.21037/tcr.2019.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yao L., Xu B., Li X. Neisseria gonorrhoeae-induced salpingitis is targeted by circular RNA EIF3K via miR-139-5p and regulating MAPK/NF-kappaB signaling pathway to promotes apoptosis and autophagy bacterial cells. Microb. Pathog. 2020;142 doi: 10.1016/j.micpath.2020.104051. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L., Liu M. Circ_0077109 sponges miR-139-5p and upregulates HOXD10 in trophoblast cells as potential mechanism for preeclampsia progression. Am. J. Reprod. Immunol. 2022 doi: 10.1111/aji.13609. [DOI] [PubMed] [Google Scholar]
- 97.Quan L., Ren G., Liu L., Huang W., Li M. Circular RNA circ_0002594 regulates PDGF-BB-induced proliferation and migration of human airway smooth muscle cells via sponging miR-139-5p/TRIM8 in asthma. Autoimmunity. 2022;55(5):339–350. doi: 10.1080/08916934.2022.2062596. [DOI] [PubMed] [Google Scholar]
- 98.Jiang C., Xu M., Zhu J., Yang D., Xue B. CircTHBS1 facilitates the progression of interstitial cystitis depending on the regulation of miR-139-5p/MFN2 axis. Drug Dev. Res. 2022;83(2):351–361. doi: 10.1002/ddr.21864. [DOI] [PubMed] [Google Scholar]
- 99.Cai X., Zhao Z., Dong J., Lv Q., Yun B., Liu J., Shen Y., Kang J., Li J. Circular RNA circBACH2 plays a role in papillary thyroid carcinoma by sponging miR-139-5p and regulating LMO4 expression. Cell Death Dis. 2019;10(3):184. doi: 10.1038/s41419-019-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu X., Shi Y., Dong M., Jiang L., Yang J., Liu Z. Exosomal transfer of tumor-associated macrophage-derived hsa_circ_0001610 reduces radiosensitivity in endometrial cancer. Cell Death Dis. 2021;12(9):818. doi: 10.1038/s41419-021-04087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng X., Zhao X., Han Z., Chen K. Enhancer of zeste homolog 2 participates in the process of atherosclerosis by modulating microRNA-139-5p methylation and signal transducer and activator of transcription 1 expression. IUBMB Life. 2021;73(1):238–251. doi: 10.1002/iub.2423. [DOI] [PubMed] [Google Scholar]
- 102.Zhou G.K., Zhang G.Y., Yuan Z.N., Pei R., Liu D.M. Has_circ_0008274 promotes cell proliferation and invasion involving AMPK/mTOR signaling pathway in papillary thyroid carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018;22(24):8772–8780. doi: 10.26355/eurrev_201812_16644. [DOI] [PubMed] [Google Scholar]
- 103.Gao C., Wen Y., Jiang F., Gu X., Zhu X. Circular RNA circ_0008274 upregulates granulin to promote the progression of hepatocellular carcinoma via sponging microRNA -140-3p. Bioengineered. 2021;12(1):1890–1901. doi: 10.1080/21655979.2021.1926195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao J., Li T., Luo W. Silencing of circ-PRKCH protects against lipopolysaccharide (LPS)-evoked chondrocyte damage and extracellular matrix loss by the miR-140-3p/ADAM10 axis. Gen. Physiol. Biophys. 2021;40(2):89–101. doi: 10.4149/gpb_2021001. [DOI] [PubMed] [Google Scholar]
- 105.Zou T.B., Zhu S.S., Luo F., Li W.Q., Sun X.R., Wu H.F. Effects of astaxanthin on reverse cholesterol transport and atherosclerosis in mice. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/4625932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iizuka M., Ayaori M., Uto-Kondo H., Yakushiji E., Takiguchi S., Nakaya K., Hisada T., Sasaki M., Komatsu T., Yogo M., Kishimoto Y., Kondo K., Ikewaki K. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. 2012;58(2):96–104. doi: 10.3177/jnsv.58.96. [DOI] [PubMed] [Google Scholar]
- 107.Inoue M., Tanabe H., Matsumoto A., Takagi M., Umegaki K., Amagaya S., Takahashi J. Astaxanthin functions differently as a selective peroxisome proliferator-activated receptor gamma modulator in adipocytes and macrophages. Biochem. Pharmacol. 2012;84(5):692–700. doi: 10.1016/j.bcp.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 108.Wei Z., Ran H., Yang C. CircRSF1 contributes to endothelial cell growth, migration and tube formation under ox-LDL stress through regulating miR-758/CCND2 axis. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118241. [DOI] [PubMed] [Google Scholar]
- 109.Mandolini C., Santovito D., Marcantonio P., Buttitta F., Bucci M., Ucchino S., Mezzetti A., Cipollone F. Identification of microRNAs 758 and 33b as potential modulators of ABCA1 expression in human atherosclerotic plaques. Nutr. Metabol. Cardiovasc. Dis. 2015;25(2):202–209. doi: 10.1016/j.numecd.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 110.Ramirez C.M., Davalos A., Goedeke L., Salerno A.G., Warrier N., Cirera-Salinas D., Suarez Y., Fernandez-Hernando C. MicroRNA-758 regulates cholesterol efflux through posttranscriptional repression of ATP-binding cassette transporter A1. Arterioscler. Thromb. Vasc. Biol. 2011;31(11):2707–2714. doi: 10.1161/ATVBAHA.111.232066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang X., Lu J., Zhang Q., Luo Q., Liu B. CircRNA RSF1 regulated ox-LDL induced vascular endothelial cells proliferation, apoptosis and inflammation through modulating miR-135b-5p/HDAC1 axis in atherosclerosis. Biol. Res. 2021;54(1):11. doi: 10.1186/s40659-021-00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sung H.Y., Choi E.N., Han J., Chae Y.J., Im S.W., Kim H.S., Park E.M., Ahn J.H. Protective role of ABCA1 in ischemic preconditioning is mediated by downregulation of miR-33-5p and miR-135-5p. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-91982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao Y., Ming X., Wu J. Hsa_circ_0002483 regulates miR-758-3p/MYC axis to promote acute myeloid leukemia progression. Hematol. Oncol. 2021;39(2):243–253. doi: 10.1002/hon.2829. [DOI] [PubMed] [Google Scholar]
- 114.Xie H., Wang J., Wang B. Circular RNA Circ_0003221 promotes cervical cancer progression by regulating miR-758-3p/CPEB4 Axis. Cancer Manag. Res. 2021;13:5337–5350. doi: 10.2147/CMAR.S311242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding Y., Yuan X., Gu W. Circular RNA RBM33 contributes to cervical cancer progression via modulation of the miR-758-3p/PUM2 axis. J. Mol. Histol. 2021;52(2):173–185. doi: 10.1007/s10735-020-09933-1. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y., Chen X., Lu Z., Lai C. Circ_0093887 regulated ox-LDL induced human aortic endothelial cells viability, apoptosis, and inflammation through modulating miR-758-3p/BAMBI axis in atherosclerosis. Clin. Hemorheol. Microcirc. 2022;81(4):343–358. doi: 10.3233/CH-221445. [DOI] [PubMed] [Google Scholar]
- 117.Kong D., Shen D., Liu Z., Zhang J., Zhang J., Geng C. Circ_0008500 knockdown improves radiosensitivity and inhibits tumorigenesis in breast cancer through the miR-758-3p/PFN2 Axis. J. Mammary Gland Biol. Neoplasia. 2022;27(1):37–52. doi: 10.1007/s10911-022-09514-w. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y., Zeng S., Wang T. Circular RNA hsa_circ_0002360 promotes non-small cell lung cancer progression through upregulating matrix metalloproteinase 16 and sponging multiple micorRNAs. Bioengineered. 2021;12(2):12767–12777. doi: 10.1080/21655979.2021.1999370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liao H., Tu Q., Kang Y., Mao G., Li Z., Hu S., Sheng P., Wang X., Xu Y., Long D., Xu Y., Kang Y., Zhang Z. Cell Prolif; 2022. CircNFIX Regulates Chondrogenesis and Cartilage Homeostasis by Targeting the miR758-3p/KDM6A axis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du W., Li D., Guo X., Li P., Li X., Tong S., Tong J., Kuang L., Liang D. Circ-PRMT5 promotes gastric cancer progression by sponging miR-145 and miR-1304 to upregulate MYC. Artif. Cells, Nanomed. Biotechnol. 2019;47(1):4120–4130. doi: 10.1080/21691401.2019.1671857. [DOI] [PubMed] [Google Scholar]
- 121.Zhang S., Li L., Wang J., Zhang T., Ye T., Wang S., Xing D., Chen W. Recent advances in the regulation of ABCA1 and ABCG1 by lncRNAs. Clin. Chim. Acta. 2021;516:100–110. doi: 10.1016/j.cca.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 122.Fernandez-Tussy P., Ruz-Maldonado I., Fernandez-Hernando C. MicroRNAs and circular RNAs in lipoprotein metabolism. Curr. Atherosclerosis Rep. 2021;23(7):33. doi: 10.1007/s11883-021-00934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu G., Yang Z., Peng T., Lv Y. Circular RNAs: rising stars in lipid metabolism and lipid disorders. J. Cell. Physiol. 2021;236(7):4797–4806. doi: 10.1002/jcp.30200. [DOI] [PubMed] [Google Scholar]
- 124.Zhang C., Chen K., Wei R., Fan G., Cai X., Xu L., Cen B., Wang J., Xie H., Zheng S., Xu X. The circFASN/miR-33a pathway participates in tacrolimus-induced dysregulation of hepatic triglyceride homeostasis. Signal Transduct. Targeted Ther. 2020;5(1):23. doi: 10.1038/s41392-020-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu P., Wang M., Tang W., Li G., Gong N. Circ_SATB2 attenuates the anti-tumor role of celastrol in non-small-cell lung carcinoma through targeting miR-33a-5p/E2F7 Axis. OncoTargets Ther. 2020;13:11899–11912. doi: 10.2147/OTT.S279434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen W.J., Zhang M., Zhao G.J., Fu Y., Zhang D.W., Zhu H.B., Tang C.K. MicroRNA-33 in atherosclerosis etiology and pathophysiology. Atherosclerosis. 2013;227(2):201–208. doi: 10.1016/j.atherosclerosis.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 127.Guo X.Y., Sun F., Chen J.N., Wang Y.Q., Pan Q., Fan J.G. circRNA_0046366 inhibits hepatocellular steatosis by normalization of PPAR signaling. World J. Gastroenterol. 2018;24(3):323–337. doi: 10.3748/wjg.v24.i3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo X.Y., Chen J.N., Sun F., Wang Y.Q., Pan Q., Fan J.G. circRNA_0046367 prevents hepatoxicity of lipid peroxidation: an inhibitory role against hepatic steatosis. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/3960197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tian W., Zhang B., Zhong H., Nie R., Ling Y., Zhang H., Wu C. Dynamic expression and regulatory network of circular RNA for abdominal preadipocytes differentiation in chicken (Gallus gallus) Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.761638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vilades D., Martinez-Camblor P., Ferrero-Gregori A., Bar C., Lu D., Xiao K., Vea A., Nasarre L., Sanchez Vega J., Leta R., Carreras F., Thum T., Llorente-Cortes V., de Gonzalo-Calvo D. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: performance as a biomarker. Faseb. J. 2020;34(3):4403–4414. doi: 10.1096/fj.201902507R. [DOI] [PubMed] [Google Scholar]
- 131.Yang Z., Liang X., Yang L. Circular RNA circ_0001445 alleviates the ox-LDL-induced endothelial injury in human primary aortic endothelial cells through regulating ABCG1 via acting as a sponge of miR-208b-5p. Gen Thorac Cardiovasc Surg. 2022;70(9):779–792. doi: 10.1007/s11748-022-01799-2. [DOI] [PubMed] [Google Scholar]
- 132.Cai Y., Xu L., Xu C., Wang Y., Fan C. Hsa_circ_0001445 inhibits ox-LDL-induced HUVECs inflammation, oxidative stress and apoptosis by regulating miRNA-640. Perfusion. 2022;37(1):86–94. doi: 10.1177/0267659120979472. [DOI] [PubMed] [Google Scholar]
- 133.Li H., Liu X., Sun N., Wang T., Zhu J., Yang S., Song X., Wang R., Wang X., Zhao Y., Zhang Y. Differentially expressed circular non-coding RNAs in atherosclerotic aortic vessels and their potential functions in endothelial injury. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.657544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y., Zhong Z., Xiao L., Li W., Wang Z., Duan Z., Li X. Identification of circ-FNDC3B, an overexpressed circRNA in abdominal aortic aneurysm, as a regulator of vascular smooth muscle cells. Int. Heart J. 2021;62(6):1387–1398. doi: 10.1536/ihj.21-186. [DOI] [PubMed] [Google Scholar]
- 135.Lu J., Zhu Y., Qin Y., Chen Y. CircNFIX acts as a miR-212-3p sponge to enhance the malignant progression of non-small cell lung cancer by up-regulating ADAM10. Cancer Manag. Res. 2020;12:9577–9587. doi: 10.2147/CMAR.S272309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li C., Zhang G., Ren Z. [Effect of circ-SFMBT2 on the biological behavior of non-small cell lung cancer cells by targeting the miR-7-5p/ADAM10 axis] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2022;39(2):162–170. doi: 10.3760/cma.j.cn511374-20201221-00895. [DOI] [PubMed] [Google Scholar]
- 137.Liu G.X., Zheng T., Zhang Y., Hao P. CircFOXM1 silencing represses cell proliferation, migration and invasion by regulating miR-515-5p/ADAM10 axis in prostate cancer. Anti Cancer Drugs. 2022;33(1):e573–e583. doi: 10.1097/CAD.0000000000001183. [DOI] [PubMed] [Google Scholar]
- 138.L. Feng, W. Jing, S. Jin, B. Wang, Circ_0088194 regulates proliferation, migration, apoptosis, and inflammation by miR-30a-3p/ADAM10 Axis in rheumatoid arthritis fibroblastic synovial cells, Inflammation 46(1) (2022) 161-174. [DOI] [PubMed]
- 139.Ou Z.L., Luo Z., Wei W., Liang S., Gao T.L., Lu Y.B. Hypoxia-induced shedding of MICA and HIF1A-mediated immune escape of pancreatic cancer cells from NK cells: role of circ_0000977/miR-153 axis. RNA Biol. 2019;16(11):1592–1603. doi: 10.1080/15476286.2019.1649585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu H., Zheng X., Liu Y., Shen J., Ye M., Zhang Y. Hsa_circRNA_102682 is closely related to lipid metabolism in gestational diabetes mellitus. Gynecol. Endocrinol. 2022;38(1):50–54. doi: 10.1080/09513590.2021.1991911. [DOI] [PubMed] [Google Scholar]
- 141.Kalayci A., Gibson C.M., Ridker P.M., Wright S.D., Kingwell B.A., Korjian S., Chi G., Lee J.J., Tricoci P., Kazmi S.H., Fitzgerald C., Shaunik A., Berman G., Duffy D., Libby P. ApoA-I infusion therapies following acute coronary syndrome: past, present, and future. Curr. Atherosclerosis Rep. 2022;24(7):585–597. doi: 10.1007/s11883-022-01025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi H.Y., Ruel I., Choi S., Genest J. New strategies to promote macrophage cholesterol efflux. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.795868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu J., Zhong C., Luo J., Shu F., Lv D., Liu Z., Tan X., Wang S., Wu K., Yang T., Zhong W., Wang B., Chen Y., Li Y., Jia Z., Zou Y., Zhong W., Mao X. HnRNP-L-regulated circCSPP1/miR-520h/EGR1 axis modulates autophagy and promotes progression in prostate cancer. Mol. Ther. Nucleic Acids. 2021;26:927–944. doi: 10.1016/j.omtn.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yuan S., Zheng P., Sun X., Zeng J., Cao W., Gao W., Wang Y., Wang L. Hsa_Circ_0001860 promotes Smad7 to enhance MPA resistance in endometrial cancer via miR-520h. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.738189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang Y., Dai Y., Wen C., He S., Shi J., Zhao D., Wu L., Zhou H. circSETD3 contributes to acquired resistance to gefitinib in non-small-cell lung cancer by targeting the miR-520h/ABCG2 pathway. Mol. Ther. Nucleic Acids. 2020;21:885–899. doi: 10.1016/j.omtn.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li B., Sun G., Yu H., Meng J., Wei F. Exosomal circTAOK1 contributes to diabetic kidney disease progression through regulating SMAD3 expression by sponging miR-520h. Int. Urol. Nephrol. 2022;54(9):2343–2354. doi: 10.1007/s11255-022-03139-y. [DOI] [PubMed] [Google Scholar]
- 147.Ren L., Jiang M., Xue D., Wang H., Lu Z., Ding L., Xie H., Wang R., Luo W., Xu L., Wang M., Yu S., Cheng S., Xia L., Yu H., Huang P., Xu N., Li G. Nitroxoline suppresses metastasis in bladder cancer via EGR1/circNDRG1/miR-520h/smad7/EMT signaling pathway. Int. J. Biol. Sci. 2022;18(13):5207–5220. doi: 10.7150/ijbs.69373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schlich M., Palomba R., Costabile G., Mizrahy S., Pannuzzo M., Peer D., Decuzzi P. Cytosolic delivery of nucleic acids: the case of ionizable lipid nanoparticles. Bioeng Transl Med. 2021;6(2) doi: 10.1002/btm2.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang J., Zhou J., Xu D., Li J., Deng D. Tailoring viruslike mesoporous FeSe2 hedgehogs for controlled drug delivery and synergistic tumor suppression. ACS. Appl. Mater. Interfaces. 2020;12(42):47197–47207. doi: 10.1021/acsami.0c10888. [DOI] [PubMed] [Google Scholar]
- 150.Peng X.J., Feng C.T., Wang X., Gu H.Y., Li J.L., Zhang X.N., Zhang X.C., Yang L. Chemical composition and antioxidant activity of essential oils from barks of Pinus pumila using microwave-assisted hydrodistillation after screw extrusion treatment. Ind. Crop. Prod. 2021;166 [Google Scholar]
- 151.Wang C., Zhang Y., Wu Y., Xing D. Developments of CRBN-based PROTACs as potential therapeutic agents. Eur. J. Med. Chem. 2021;225 doi: 10.1016/j.ejmech.2021.113749. [DOI] [PubMed] [Google Scholar]