Treatment guidelines and emergency use authorizations for monoclonal antibodies (mAbs) for treatment of COVID-19 have changed frequently as different SARS-CoV-2 variants have emerged. This study evaluated whether early outpatient treatment with mAbs is associated with reduced risk for hospitalization or death at 28 days.
Visual Abstract. Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19.
Treatment guidelines and emergency use authorizations for monoclonal antibodies (mAbs) for treatment of COVID-19 have changed frequently as different SARS-CoV-2 variants have emerged. This study evaluated whether early outpatient treatment with mAbs is associated with reduced risk for hospitalization or death at 28 days.
Abstract
Background:
Treatment guidelines and U.S. Food and Drug Administration emergency use authorizations (EUAs) of monoclonal antibodies (mAbs) for treatment of high-risk outpatients with mild to moderate COVID-19 changed frequently as different SARS-CoV-2 variants emerged.
Objective:
To evaluate whether early outpatient treatment with mAbs, overall and by mAb product, presumed SARS-CoV-2 variant, and immunocompromised status, is associated with reduced risk for hospitalization or death at 28 days.
Design:
Hypothetical pragmatic randomized trial from observational data comparing mAb-treated patients with a propensity score–matched, nontreated control group.
Setting:
Large U.S. health care system.
Participants:
High-risk outpatients eligible for mAb treatment under any EUA with a positive SARS-CoV-2 test result from 8 December 2020 to 31 August 2022.
Intervention:
Single-dose intravenous mAb treatment with bamlanivimab, bamlanivimab–etesevimab, sotrovimab, bebtelovimab, or intravenous or subcutaneous casirivimab–imdevimab administered within 2 days of a positive SARS-CoV-2 test result.
Measurements:
The primary outcome was hospitalization or death at 28 days among treated patients versus a nontreated control group (no treatment or treatment ≥3 days after SARS-CoV-2 test date).
Results:
The risk for hospitalization or death at 28 days was 4.6% in 2571 treated patients and 7.6% in 5135 nontreated control patients (risk ratio [RR], 0.61 [95% CI, 0.50 to 0.74]). In sensitivity analyses, the corresponding RRs for 1- and 3-day treatment grace periods were 0.59 and 0.49, respectively. In subgroup analyses, those receiving mAbs when the Alpha and Delta variants were presumed to be predominant had estimated RRs of 0.55 and 0.53, respectively, compared with 0.71 for the Omicron variant period. Relative risk estimates for individual mAb products all suggested lower risk for hospitalization or death. Among immunocompromised patients, the RR was 0.45 (CI, 0.28 to 0.71).
Limitations:
Observational study design, SARS-CoV-2 variant presumed by date rather than genotyping, no data on symptom severity, and partial data on vaccination status.
Conclusion:
Early mAb treatment among outpatients with COVID-19 is associated with lower risk for hospitalization or death for various mAb products and SARS-CoV-2 variants.
Primary Funding Source:
None.
Monoclonal antibody (mAb) treatment received emergency use authorizations (EUAs) from the U.S. Food and Drug Administration (FDA) starting in November 2020 for patients recently diagnosed with mild to moderate COVID-19 and with risk for progression to severe disease (1–4). The EUAs were granted because mAb treatment reduced SARS-CoV-2 viral load and later showed decreased rates of hospitalization and death in at-risk outpatients compared with patients who did not receive treatment (5). Early data suggested that the benefit is most pronounced in patients aged 65 years or older and those with a suppressed immune system (6–8). Before withdrawal of the EUA for bebtelovimab, more than 3.6 million mAb treatments were administered to outpatients in the United States (9).
The EUAs for the 5 mAbs that were authorized at various times during the COVID-19 pandemic (10) have all been suspended or revoked by the FDA, based on in vitro evidence of evolving loss of efficacy against new SARS-CoV-2 variants. The EUA for the last of the mAbs (bebtelovimab) was revoked on 30 November 2022 (11). Such decisions about changes to EUAs are often based on in vitro potency alone, as randomized trials and real-world data predate evolving variants. There are limited, large-scale observational clinical data for use of mAb products in infected patients, including during the current Omicron variant era (12, 13). Taken together, these realities underscore the need for near-real-time evaluation of individual mAb products as new variants emerge, with the goal of identifying patient populations most likely to benefit from treatment.
Therefore, using the framework of a hypothetical pragmatic randomized trial, we assessed the evolving real-world effectiveness of early mAb treatment for outpatients with COVID-19 who were treated in a large U.S. health care system over nearly 2 years. We examined the specific mAb products that were used, the SARS-CoV-2 variant that was presumed to be predominant, the time from SARS-CoV-2 infection to treatment, and patients' immunocompromised status. This work complements smaller, date-restricted subsets that have been published previously on the effectiveness of bamlanivimab monotherapy in 232 patients from 9 December 2020 to 3 March 2021 (8); the comparative effectiveness of subcutaneous (n = 969) or intravenous (n = 1216) casirivimab–imdevimab in patients treated from 14 July to 26 October 2021 (14); the comparative effectiveness of bamlanivimab, bamlanivimab–etesevimab, and casirivimab–imdevimab among 1935 patients treated from 10 March to 25 June 2021 (15); a randomized comparative effectiveness trial of casirivimab–imdevimab (n = 2454) versus sotrovimab (n = 1104) in the Delta variant period, coupled with a parallel propensity score–matched cohort study of mAb treatment (n = 1023) versus no mAb treatment (n = 2046) (16); and the effectiveness of bebtelovimab monotherapy in 1860 patients from 30 March to 28 May 2022 (12). We conclude by briefly discussing possible future uses of mAb therapy for emerging SARS-CoV-2 variants.
Methods
Using observational data, we emulated a hypothetical pragmatic randomized trial of outpatients with COVID-19 who met EUA eligibility criteria for mAb treatment. All treated patients verbally consented to mAb treatment and reviewed the FDA EUA fact sheets for available mAbs before treatment. Treatment was assigned via a central management system overseen by a multidisciplinary COVID-19 Therapeutics Committee (Supplement Appendix A); this system was used even when only 1 mAb was available (17). The Quality Improvement Review Committee and Institutional Review Board provided ethical review and approval of the study as an exempt protocol, and all data remained deidentified for this analysis.
Data Sources
We used health-related data captured in the electronic health record (EHR) and ancillary clinical systems, all aggregated and harmonized in a clinical data warehouse (8, 18). For all patients, we accessed sociodemographic data, medical history, and billing charges for all outpatient and inpatient encounters with diagnoses and procedures coded based on the International Classification of Diseases, Ninth and 10th Revisions (19, 20). We classified self-declared race as Black versus all others. Deaths at 28 days were identified using hospital discharge dispositions of “ceased to breathe” sourced from the inpatient medical record system; deaths after discharge were identified via the Death Master File from the Social Security Administration's 2022 National Technical Information Service (21, 22). Definitions for variables used in the analysis, as captured in the EHR, are provided in the Statistical Analysis and Subgroup Analyses sections and in Supplement Appendix B. Components of the study design are summarized in the following 3 sections, with full details provided in Supplement Appendix C.
Eligibility Criteria
The index date for patient selection and analysis was the date of a positive SARS-CoV-2 test result, as documented in the University of Pittsburgh Medical Center (UPMC) health system during the period from 8 December 2020 to 31 August 2022. All selected (eligible) patients were aged 12 years or older, had at least 1 EUA-eligible risk factor for progression to severe disease identified in the EHR on the index date, were not pregnant, were not in the emergency department (ED) or hospital on the index date, had at least 1 record in the EHR in the previous year (making them likely to access care within the system, thus allowing for ascertainment of follow-up data), and had near-complete covariate data for analysis. Patients were excluded if they received tixagevimab–cilgavimab (Evusheld [AstraZeneca]) (preexposure prophylaxis for prevention of COVID-19) before or within 28 days of the index date.
Classification of Treatment Groups
We classified and compared patients as either treated (if they received “early” mAb treatment within 2 days of the index date) or nontreated (if they never received mAb therapy or received “delayed” treatment within 3 to 10 days of the index date). In sensitivity analyses, we varied the primary 2-day early treatment grace period to 1 and 3 days. Treated patients were matched in a 1:2 ratio to nontreated patients using propensity score methods (see the Statistical Analysis section).
All patients who were treated with mAbs (either early or delayed treatment) received intravenous bamlanivimab (700 mg), intravenous bamlanivimab–etesevimab (700 and 1400 mg, respectively), intravenous or subcutaneous casirivimab–imdevimab (1200 mg each before 15 June 2021 and 600 mg each from 15 June 2021 to 31 January 2022), intravenous sotrovimab (500 mg), or intravenous bebtelovimab (175 mg) in an outpatient infusion clinic for treatment of COVID-19. Per the respective EUAs, bamlanivimab was used through March 2021, bamlanivimab–etesevimab and casirivimab–imdevimab were available during March to December 2021, sotrovimab was available during July 2021 through March 2022, and bebtelovimab was used from April to August 2022. Thus, as few as 1 and as many as 3 mAb products were available on a given date.
Outcomes
The primary outcome was risk for hospitalization or death at 28 days, with secondary outcomes of hospitalization, death, ED visit without hospitalization, and a composite outcome of ED visit or hospitalization at 28 days. The 28-day follow-up period for the analysis started on the day after the index date (the date of a positive SARS-CoV-2 test result).
Statistical Analysis
We compared sociodemographic and clinical characteristics between treated and nontreated patients (before and after matching) using standardized mean differences (SMDs). We selected nontreated control patients matched to treated patients using propensity score methods (23, 24). Specifically, we used propensity scores from a logistic regression model fit with classification into the mAb treatment group as the response variable and inclusion of explanatory variables (Table 1). Separate propensity score models were fit for each treatment grace period (defined earlier). We used 1:2 propensity score greedy nearest-neighbor matching within a caliper width of 0.20 to construct the matched treated and nontreated groups (25). This included exact 1:2 matching on the presumed SARS-CoV-2 variant. Vaccination status (evidence of being vaccinated) was available in the EHR for just 21.4% of patients and thus was not included in the primary propensity score model but was included in a sensitivity analysis (value of 1 for known vaccination and 0 otherwise).
Table 1.
Comparison of Characteristics Among Treated and Nontreated Patients

From the matched groups, risks for patient outcomes at 28 days were calculated as relative risks (treated vs. nontreated) and 95% CIs. The Kaplan–Meier method was used to plot survival curves for freedom from hospitalization or death by treatment status over follow-up. Several sensitivity analyses were done, including among the subgroups of patients with nonmissing data on body mass index (BMI) or vaccination status and per different treatment grace periods. For subgroup analyses by individual mAb product and patients' immunocompromised status, separate propensity score models were fit to enhance matching of treated and nontreated patients.
Subgroup Analyses
In addition to variables used in propensity score matching (Table 1), key variables used in subgroup analyses included individual mAb product, presumed SARS-CoV-2 variant, and the patient's immunocompromised status (given its recognized priority status for treatment) (6). SARS-CoV-2 variant was not measured in each patient but was presumed from date- and region-specific prevalence estimates from the Variants and Genomic Surveillance tool in the Centers for Disease Control and Prevention (CDC) COVID Data Tracker (https://covid.cdc.gov/covid-data-tracker/#variant-proportions) as follows: pre-Alpha from 8 December 2020 to 28 February 2021, Alpha (B.1.1.7) from 1 March to 5 June 2021, Alpha and Delta (B.1.1.7 and B.1.617.2) from 6 June to 17 July 2021, Delta (B.1.617.2) from 18 July to 17 December 2021, Delta and Omicron (B.1.617.2 and B.1.1.529) from 18 December to 31 December 2021, and Omicron (B.1.1.529 and all subsequent sublineages) from 1 January to 31 August 2022. Near the end of the Omicron recruitment period (the week ending 27 August 2022), the CDC region-specific prevalence estimates of Omicron subvariants were 85.7% for BA.5, 7.9% for BA.4.6, and 4.1% for BA.4. We defined immunocompromised from a range of conditions, such as selected cancer diagnoses (for example, leukemia) within the previous year, selected autoimmune disorders (for example, lupus) within the previous year, and transplant within the previous year (Supplement Appendix D).
We used SAS, version 9.4 (SAS Institute), for all analyses. Missing BMI data (19.2% of patients) were imputed based on the mean observation for patients with known values.
Role of the Funding Source
This work did not receive external funding. The U.S. federal government provided most of the mAb treatments reported in this manuscript. GlaxoSmithKline and Vir Biotechnology, Inc., donated some of the sotrovimab used in this study. The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; approval of the manuscript; or the decision to submit the manuscript for publication.
Results
Treatment Classification
The primary analysis, which used a 2-day treatment grace period, included 2571 treated patients and 5135 matched nontreated patients. For the respective 1-, 2-, and 3-day treatment grace periods, 10.3%, 6.1%, and 3.2% of patients in the nontreated (control) groups received mAb therapy after the grace period (Appendix Figure).
Appendix Figure. CONSORT (Consolidated Standards of Reporting Trials) diagram of selection of patients classified as treated or nontreated, based on time between a positive SARS-CoV-2 test result and treatment with mAb therapy.
The bottom section depicts 1:2 propensity score matching of treated and nontreated patients. mAb = monoclonal antibody.
Baseline Characteristics
Before 1:2 propensity score matching, the mean age of treated patients was 58.5 years (SD, 16.3) compared with 49.3 years (SD, 19.7) in nontreated control patients (Table 1). Before matching, the overall risk profile was higher in treated patients than in nontreated control patients, including higher prevalence of obstructive sleep apnea, diabetes, hypertension, and cancer (all SMDs ≥0.15). In response to mAb prioritization guidelines during times of drug scarcity, a higher percentage of treated patients (16.1%) were immunocompromised compared with nontreated control patients (9.3%). The proportions of patients by presumed SARS-CoV-2 variant before matching differed by treatment status. Of note, after 1:2 propensity score matching, treated patients were similar to nontreated control patients in all variables, with SMD values of 0.03 or less (Table 1). Moreover, the distributions of propensity scores were nearly identical after matching overall and by presumed SARS-CoV-2 variant (Supplement Figure 1).
Outcomes
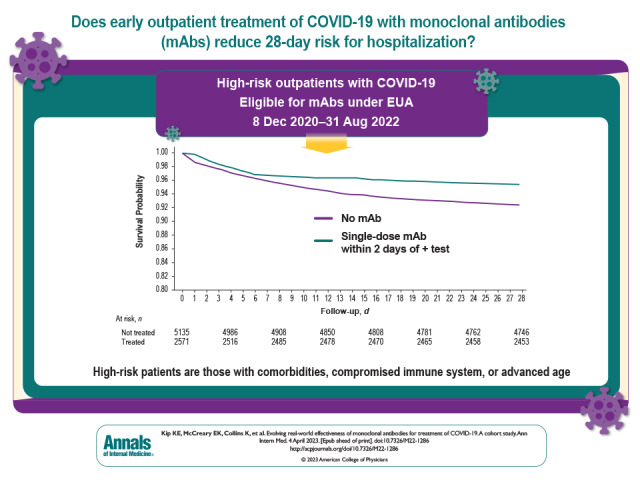
The overall risk for hospitalization or death at 28 days was 4.6% in treated patients compared with 7.6% in nontreated control patients (Table 2). This corresponded to a risk ratio (RR) of 0.61 (95% CI, 0.50 to 0.74). The divergence in freedom from death or hospitalization favoring the treated group seemed most prominent starting approximately 7 days after the index date (Figure 1). The lower RR associated with treatment was evident for risk for hospitalization at 28 days (RR, 0.74 [CI, 0.60 to 0.91]) (Table 2; Supplement Figure 2) and was especially evident for risk for death at 28 days (RR, 0.14 [CI, 0.07 to 0.26]) (Table 2; Supplement Figure 3). Treatment with mAbs was not associated with risk for ED visit without hospitalization or the composite outcome of ED visit or hospitalization (Table 2; Supplement Figures 4 and 5).
Table 2.
Risks and Risk Ratios for Outcomes at 28 Days, by Treatment Versus Nontreatment With mAbs

Figure 1. Kaplan–Meier plot of the probability of freedom from hospitalization or death over 28-day follow-up for treated patients and nontreated matched control patients.
The analysis reflects a 2-day treatment grace period from the date of a positive SARS-CoV-2 test result to monoclonal antibody treatment.
Subgroup Analyses
In subgroup analyses, patients who received mAb therapy when the Alpha and Delta variants were presumed to be predominant had estimated RRs of 0.55 (CI, 0.30 to 1.00) and 0.53 (CI, 0.41 to 0.69), respectively, compared with 0.71 (CI, 0.35 to 1.45) for the most recent Omicron variant period (Table 3). Although 95% CIs typically included unity, the RR estimates for treatment with individual mAb products were all in the direction suggesting lower risk for hospitalization or death. Among immunocompromised patients, the RR for hospitalization or death was 0.45 (CI, 0.28 to 0.71), which was modestly lower than the RR estimate of 0.58 (CI, 0.47 to 0.72) for patients who were not immunocompromised (Table 3 and Figure 2).
Table 3.
Subgroup Analyses of Risk Ratio for Hospitalization or Death at 28 Days, by Treatment Versus Nontreatment With mAbs

Figure 2. Kaplan–Meier plot of the probability of freedom from hospitalization or death over 28-day follow-up for treated patients and nontreated matched control patients, stratified by immunocompromised status.
The analysis reflects a 2-day treatment grace period from the date of a positive SARS-CoV-2 test result to monoclonal antibody treatment.
Sensitivity Analyses
The RRs for hospitalization or death using 1- or 3-day treatment grace periods were 0.59 (CI, 0.45 to 0.77) and 0.49 (CI, 0.41 to 0.59), respectively (Table 3; Supplement Figures 6 and 7). These estimates were generally similar to the RR of 0.61 observed in the primary analyses, which used a 2-day treatment grace period. Among patients with nonmissing BMI data, the RR for hospitalization or death using the primary 2-day treatment grace period was 0.63 (CI, 0.51 to 0.77). Among the subset of vaccinated patients (695 in the treated group and 954 in the nontreated group), the RR for hospitalization or death was 0.27 (CI, 0.17 to 0.41). In the propensity score–matched model that included vaccination status (with an imputed value of 0 for unknown status), the RR for hospitalization or death was 0.56 (CI, 0.46 to 0.69).
Treatment Crossover
In the primary analysis of 5135 patients in the nontreated control group, 314 (6.1%) received mAb therapy after the 2-day treatment grace period, with 159 (50.6%) receiving it on day 3, 91 (29.0%) receiving it on day 4, and the remaining 64 (20.5%) receiving it between days 4 and 9. Among the 4821 control patients who never received mAb therapy, 382 (7.9%) experienced hospitalization or death within 28 days compared with 7 of the 314 (2.2%) patients who received mAb therapy after the 2-day treatment grace period. Thus, notwithstanding the potential effect of immortal time bias, the crude (unadjusted) risk for hospitalization or death at 28 days was markedly lower in patients with delayed mAb treatment than in those who never received mAb therapy.
Discussion
In this analysis spanning nearly 2 years in a large health system, treatment with mAbs within 2 days of SARS-CoV-2 infection was associated with an estimated 39% lower risk for hospitalization or death at 28 days. Estimated risk reductions of 41% and 51%, respectively, were observed when 1- and 3-day treatment grace periods were used. Risk estimates among individual mAb products and across all presumed SARS-CoV-2 variants were in the direction favoring early treatment, including in sensitivity analyses of patients with known BMI data and those known to be fully vaccinated. Collectively, our results indicate that throughout the pandemic, early treatment with mAbs significantly reduced severity of COVID-19, which is consistent with reports in other cohorts (26, 27) and controlled trials (5, 28, 29). Of note, mAb therapy was associated with a markedly lower risk for death (RR, 0.14) than for other outcomes, such as hospitalization or ED admission. Although striking, this estimate is consistent with results from a meta-analysis of 5 randomized clinical trials (summary odds ratio, 0.16) (30).
High-risk patients, including those with comorbidities or compromised immune systems, are at greater risk for severe SARS-CoV-2 infection and complications (31, 32). Because of this, COVID-19 treatment guidelines from the National Institutes of Health recommended priority selection of mAb treatment for immunocompromised persons who are not expected to mount an adequate immune response to COVID-19 vaccination or SARS-CoV-2 infection due to their underlying conditions (6). In our analysis, before propensity score matching, immunocompromised patients were more likely than nontreated control patients to be treated with mAbs, which is consistent with our prioritization guidelines. Early mAb treatment resulted in a low relative risk for hospitalization or death (0.45) for immunocompromised patients compared with no treatment. This result is consistent with findings from vaccine response studies showing decreased seropositivity in immunocompromised hosts compared with healthy volunteers, suggesting that immunocompromised patients are more likely to benefit from passive immunity (33).
Our historical results over nearly 2 years should be interpreted with the knowledge that there are currently no FDA-approved mAbs for treatment of outpatients with COVID-19. Among the SARS-CoV-2 viral proteins, the spike, envelope, membrane, and nucleocapsid proteins are the main targets of structural protein–based therapeutics for SARS-CoV-2 (34–36). All 5 of the mAb therapies we evaluated specifically targeted the spike protein (37). The spike protein mediates attachment of SARS-CoV-2 to the host cell by binding the human angiotensin-converting enzyme 2 receptor through its receptor-binding domain (38) and is thus a practical target for neutralizing antibodies (39). The high specificity and affinity of these mAbs in relation to specific variants can be expected to provide immediate immunotherapy and reduce progression and severity of disease (40), as suggested by our analyses that included selective use of different mAbs throughout the pandemic. However, with such high specificity and affinity and the propensity for the receptor-binding domain to mutate, a small change (mutation) in the epitope frequently prevents the antibody from neutralizing the target. This has been especially evident for Omicron variants and subvariants (41–43). The acquisition by SARS-CoV-2 of mutations that enable it to evade mAbs will almost certainly continue, thus affecting the utility and longevity of mAbs in managing the ongoing pandemic (41). Therefore, an mAb that targets the conserved viral epitopes is important for the development of broad-spectrum antibody therapies (37, 44).
The EUA for Evusheld, which was previously approved for preexposure prophylaxis of COVID-19 in immunocompromised persons and those for whom COVID-19 vaccination is not recommended, was revoked by the FDA on 26 January 2023. Similar to the 5 mAbs we analyzed that were previously approved for treatment in the outpatient setting, the EUA for Evusheld was revoked because this highly specific therapy is not expected to protect against SARS-CoV-2 if a person is exposed to many of the current Omicron subvariants, including XBB.1.5 (45). Again, these observations indicate a need for broader-based therapies, particularly because Omicron infections may be less clinically severe than infections with earlier variants (46). Finally, our near-real-time evaluation of ongoing treatment to evaluate effectiveness and the populations most likely to benefit shows that this approach is feasible and could be deployed for future public health crises.
Our study has limitations. First, matching of nontreated controls was based on EUA-eligible risk factors only, and we were unable to determine symptom severity (whether symptomatic or asymptomatic) in patients. Thus, many nontreated patients may have been asymptomatic and therefore at low risk for hospitalization, which could have biased the results against mAb treatment. Alternatively, nontreated patients could have had severe symptoms, biasing the results against nontreatment. Second, vaccination status was available in a minority of all patients; however, in the subset of vaccinated patients, results still indicated markedly lower risk for hospitalization or death with mAb treatment. Third, the SARS-CoV-2 variants that were presumed to be predominant were based on date- and region-specific prevalence estimates rather than patient-specific genotype analyses. Fourth, our definition of “immunocompromised” is broad, with several qualifying conditions, although it is important to note that the prevalence of selected individual qualifying conditions (Table 1) was similar in treated patients and matched nontreated control patients. Fifth, hospitalizations that occurred outside the UPMC system were not captured in our analyses. Sixth, treatment with other outpatient therapies for COVID-19 was not captured. Finally, we cannot rule out potential residual confounding of the estimated mAb treatment effects despite our close propensity score matching of treated patients and nontreated “mAb-eligible” control patients.
In conclusion, in this large study of outpatients with COVID-19, early treatment with 5 different mAb products used in accordance with prevailing authorizations and guidelines for specific SARS-CoV-2 variants was consistently associated with lower risk for hospitalization or death over nearly 2 years. The rapid evolution of new SARS-CoV-2 variants warrants timely, continuous evaluation of both mAb and non-mAb treatment approaches.
Supplementary Material
Footnotes
This article was published at Annals.org on 4 April 2023.
References
- 1. U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: November 9, 2020. 9 November 2020. Accessed at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-november-9-2020 on 22 March 2022.
- 2. U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. 21 November 2020. Accessed at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 on 22 March 2022.
- 3. U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. 9 February 2021. Accessed at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0 on 22 March 2022.
- 4. U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19. 26 May 2021. Accessed at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19 on 22 March 2022.
- 5. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81. [PMID: ] doi: 10.1056/NEJMoa2108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Institutes of Health. Prioritization of Anti-SARS-CoV-2 Therapies for the Treatment of COVID-19 in Nonhospitalized Patients When There Are Logistical Constraints. 1 December 2022. Accessed at www.covid19treatmentguidelines.nih.gov/therapies/statement-on-patient-prioritization-for-outpatient-therapies on 22 March 2022.
- 7. Lloyd EC, Gandhi TN, Petty LA. Monoclonal antibodies for COVID-19. JAMA. 2021;325:1015. [PMID: ] doi: 10.1001/jama.2021.1225 [DOI] [PubMed] [Google Scholar]
- 8. Bariola JR, McCreary EK, Wadas RJ, et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8:ofab254. [PMID: ] doi: 10.1093/ofid/ofab254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. HHS Administration for Strategic Preparedness and Response. COVID-19 Therapeutics Thresholds, Orders, and Replenishment by Jurisdiction. Accessed at https://aspr.hhs.gov/COVID-19/Therapeutics/orders/Pages/default.aspx on 27 January 2023.
- 10. Centers for Medicare & Medicaid Services. COVID-19 Monoclonal Antibodies. Accessed at www.cms.gov/monoclonal on 27 January 2023.
- 11. U.S. Food and Drug Administration. FDA Announces Bebtelovimab Is Not Currently Authorized in Any US Region. 30 November 2022. Accessed at www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region on 1 December 2022.
- 12. McCreary EK, Kip KE, Collins K, et al. Evaluation of bebtelovimab for treatment of Covid-19 during the SARS-CoV-2 Omicron variant era. Open Forum Infect Dis. 2022;9:ofac517. [PMID: ] doi: 10.1093/ofid/ofac517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dryden-Peterson S, Kim A, Joyce MR, et al. Bebtelovimab for high-risk outpatients with early COVID-19 in a large US health system. Open Forum Infect Dis. 2022;9:ofac565. [PMID: ] doi: 10.1093/ofid/ofac565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCreary EK, Bariola JR, Wadas RJ, et al. Association of subcutaneous or intravenous administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in adults with COVID-19. JAMA Netw Open. 2022;5:e226920. [PMID: ] doi: 10.1001/jamanetworkopen.2022.6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCreary EK, Bariola JR, Minnier TE, et al. The comparative effectiveness of COVID-19 monoclonal antibodies: a learning health system randomized clinical trial. Contemp Clin Trials. 2022;119:106822. [PMID: ] doi: 10.1016/j.cct.2022.106822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang DT, McCreary EK, Bariola JR, et al. Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 Delta variant surge: a cohort study and randomized comparative effectiveness trial. JAMA Netw Open. 2022;5:e2220957. [PMID: ] doi: 10.1001/jamanetworkopen.2022.20957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCreary EK, Kip KE, Bariola JR, et al. A learning health system approach to the COVID-19 pandemic: system-wide changes in clinical practice and 30-day mortality among hospitalized patients. Learn Health Sys. 2022;6:e10304. doi: 10.1002/lrh2.10304 [DOI] [PMC free article] [PubMed]
- 18. Reitz KM, Seymour CW, Vates J, et al. Strategies to Promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients. BMJ Open. 2020;10:e037690. [PMID: ] doi: 10.1136/bmjopen-2020-037690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Accessed at www.cdc.gov/nchs/icd/icd9cm.htm on 13 April 2022.
- 20. Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Accessed at www.cdc.gov/nchs/icd/icd-10-cm.htm on 13 April 2022.
- 21.National Technical Information Service website. Accessed at www.ntis.gov on 13 April 2022.
- 22. Social Security Administration. Requesting SSA's Death Information. Accessed at www.ssa.gov/dataexchange/request_dmf.html on 13 April 2022.
- 23. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399-424. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. doi: 10.1093/biomet/70.1.41 [DOI]
- 25. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150-61. [PMID: ] doi: 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wynia MK, Beaty LE, Bennett TD, et al. Real-world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients. Chest. 2022. [PMID: ] doi: 10.1016/j.chest.2022.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagler AR, Horwitz LI, Jones S, et al. The impact of COVID-19 monoclonal antibodies on clinical outcomes: a retrospective cohort study. Am J Health Syst Pharm. 2022;79:2222-9. [PMID: ] doi: 10.1093/ajhp/zxac295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229-37. [PMID: ] doi: 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta A, Gonzalez-Rojas Y, Juarez E, et al; COMET-ICE Investigators. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327:1236-46. [PMID: ] doi: 10.1001/jama.2022.2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin WT, Hung SH, Lai CC, et al. The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: a systematic review and meta-analysis of randomized controlled trials. J Med Virol. 2022;94:2222-9. [PMID: ] doi: 10.1002/jmv.27623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935-41. [PMID: ] doi: 10.1158/2159-8290.CD-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-65. [PMID: ] doi: 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haidar G, Agha M, Bilderback A, et al. Prospective evaluation of coronavirus disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 Vaccination in the Immunocompromised Study (COVICS). Clin Infect Dis. 2022;75:e630-e644. [PMID: ] doi: 10.1093/cid/ciac103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta MK, Vemula S, Donde R, et al. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J Biomol Struct Dyn. 2021;39:2617-27. [PMID: ] doi: 10.1080/07391102.2020.1751300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12. [PMID: ] doi: 10.3390/v12030254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gil C, Ginex T, Maestro I, et al. COVID-19: drug targets and potential treatments. J Med Chem. 2020;63:12359-12386. [PMID: ] doi: 10.1021/acs.jmedchem.0c00606 [DOI] [PubMed] [Google Scholar]
- 37. Widyasari K, Kim J. A review of the currently available antibody therapy for the treatment of coronavirus disease 2019 (COVID-19). Antibodies (Basel). 2023;12. [PMID: ] doi: 10.3390/antib12010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727-11734. [PMID: ] doi: 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450-6. [PMID: ] doi: 10.1038/s41586-020-2571-7 [DOI] [PubMed] [Google Scholar]
- 40. Nathan R, Shawa I, De La Torre I, et al. A narrative review of the clinical practicalities of bamlanivimab and etesevimab antibody therapies for SARS-CoV-2. Infect Dis Ther. 2021;10:1933-47. [PMID: ] doi: 10.1007/s40121-021-00515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cox M, Peacock TP, Harvey WT, et al; COVID-19 Genomics UK (COG-UK) Consortium. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat Rev Microbiol. 2023;21:112-124. [PMID: ] doi: 10.1038/s41579-022-00809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tao K, Tzou PL, Kosakovsky Pond SL, et al. Susceptibility of SARS-CoV-2 Omicron variants to therapeutic monoclonal antibodies: systematic review and meta-analysis. Microbiol Spectr. 2022;10:e0092622. [PMID: ] doi: 10.1128/spectrum.00926-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Focosi D, McConnell S, Casadevall A. The Omicron variant of concern: diversification and convergent evolution in spike protein, and escape from anti-spike monoclonal antibodies. Drug Resist Updat. 2022;65:100882. [PMID: ] doi: 10.1016/j.drup.2022.100882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang P, Casner RG, Nair MS, et al. A monoclonal antibody that neutralizes SARS-CoV-2 variants, SARS-CoV, and other sarbecoviruses. Emerg Microbes Infect. 2022;11:147-157. [PMID: ] doi: 10.1080/22221751.2021.2011623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. U.S. Food and Drug Administration. FDA announces Evusheld is not currently authorized for emergency use in the U.S. 26 January 2023. Accessed at www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us on 30 January 2023.
- 46. Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in southern California. Nat Med. 2022;28:1933-43. [PMID: ] doi: 10.1038/s41591-022-01887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





