Abstract
Background:
Ambient particulate matter (PM) air pollution is a leading cause of global disability and accounts for an annual 2.9 million deaths globally. PM is established as an important risk factor for cardiovascular disease, however the evidence supporting a link specifically between long-term exposure to ambient PM and incident stroke is less clear. We sought to evaluate the association of long-term exposure to different size fractions of ambient PM with incident stroke (overall and by etiologic subtypes) and cerebrovascular deaths within the Women’s Health Initiative, a large prospective study of older women in the US.
Methods:
We studied 155,410 postmenopausal women without previous cerebrovascular disease enrolled into the study between 1993–1998, with follow-up through 2010. We assessed geocoded participant address-specific concentrations of ambient PM (fine [PM2.5], respirable [PM10] and coarse [PM10–2.5]), as well as nitrogen dioxide [NO2]) using spatiotemporal models. We classified hospitalization events into ischemic, hemorrhagic, or other/unclassified stroke. Cerebrovascular mortality was defined as death from any stroke etiology. We used Cox proportional hazard models to calculate hazard ratios (HR) and 95% confidence intervals, adjusting for individual and neighborhood-level characteristics.
Results:
During a median follow-up time of 15 years, participants experienced 4,556 cerebrovascular events. The hazard ratio for all cerebrovascular events was 2.14 (95% CI: 1.87, 2.44) comparing the top versus bottom quartiles of PM2.5. Similarly, there was a statistically significant increase in events comparing the top versus bottom quartiles of PM10 and NO2 (HR: 1.17; 95% CI: 1.03, 1.33 and HR:1.26; 95% CI: 1.12, 1.42). The strength of association did not vary substantially by stroke etiology. There was little evidence of an association between PMcoarse and incident cerebrovascular events.
Conclusions:
Long-term exposure to fine (PM2.5) and respirable (PM10) particulate matter as well as NO2 was associated with a significant increase of cerebrovascular events among postmenopausal women. Strength of the associations were consistent by stroke etiology.
Keywords: air pollution, particulate matter, stroke, cerebrovascular disease, ischemic stroke, hemorrhagic stroke
INTRODUCTION
Ambient particulate matter (PM) air pollution is a leading cause of disability and accounts for an estimated 2.9 million deaths per year globally1. Estimates from the Global Burden of Disease suggest that 977,000 (approximately one-third) of these excess deaths are due to ischemic heart disease with an additional 184,000 (6.3%) excess deaths due to ischemic stroke and 226,000 (7.7%) due to intracerebral hemorrhage.1 An earlier analysis by Global Burden of Disease investigators reported that ambient air pollution was a leading cause of global stroke-related disability-adjusted life-years, accounting for an estimated 16% of all stroke-related disability-adjusted life-years.2,3
These estimates of population attributable numbers or fractions explicitly assume the presence of a relationship between exposure and specific health end points. PM has been established as an important risk factor for cardiovascular disease with extensive evidence of adverse health effects of both long-term exposures (over the course of months to years, on which the Global Burden of Disease estimates are based) and short-term exposures (on the order of hours to days).4–6 On the other hand, the evidence supporting a link specifically between long-term exposure to ambient PM and incident stroke is less clear.7 For example, prospective cohort studies in North America8,9, Europe10,11, and Asia12–14, provide important evidence supporting an association between long-term exposure to ambient fine (PM2.5) and/or respirable (PM10) particulate matter and either incident stroke or cerebrovascular mortality. However, a number of other studies report either no association or suggestive positive associations with wide confidence intervals that include the null hypothesis of no association.10,15–20 A few additional studies have only found associations among specific subgroups of participants such as women16, those with specific stroke subtypes, particularly those that examine ischemic stroke events21,22, or other subsets of the study population.16–18
This heterogeneity across prior studies suggests the need for additional prospective cohort studies to examine the effects of long-term exposure to air pollution across different cerebrovascular outcomes. While the pathophysiologic mechanisms that link air pollution to stroke and cerebrovascular events are still largely unknown, mechanisms may differ by etiologic sub-type. 7 Accordingly, our goal was to evaluate the association between long-term exposure to PM2.5 and PM10 and incident stroke (overall and by etiologic subtypes) and cerebrovascular deaths within the context of the Women’s Health Initiative, a large prospective cohort study of post-menopausal women across the United States with 17 years of follow-up data and more than 4,400 documented cerebrovascular events. We hypothesized that long-term average concentrations of ambient PM2.5 and PM10 at the residence would be associated with incident cerebrovascular events. In secondary analyses we additionally considered the association between ambient concentrations of coarse particulate matter (PMcoarse) and NO2 (a marker of traffic pollution) and cerebrovascular events.
METHODS
Study population
The Women’s Health Initiative (WHI) enrolled post-menopausal women aged 50 to 79 into either the WHI Observational Study (WHI-OS) or one or more of three WHI Clinical Trials (WHI-CT) between 1993 and 1998, as previously described.23–25 Briefly, the WHI-OS is a longitudinal cohort designed to examine causes of morbidity and mortality in postmenopausal women.23 The WHI-CT examined the effects of menopausal hormone therapy (HT), calcium and vitamin D supplementation (CaD), and a low-fat dietary modification (DM).24
Of the 93,676 WHI-OS and 68,132 WHI-CT participants enrolled, we included all participants except those with history of cerebrovascular events at enrollment (n=2,156), those with missing stroke etiology (n=56), and those missing exposure data (n=4,176). Our final analytical sample included 155,410 women.
Exposure Assessment
Participant’s addresses were recorded at the time of enrollment, confirmed at each follow-up visit, and reviewed at least annually with participants as part of cohort retention activities. Addresses were then geocoded using a previously described protocol.26 For each address, annual average geocoded participant address-specific concentrations of different size fractions of particulate matter (PM2.5, PM10) were estimated. Estimates from 1993–1998 were obtained using a national spatiotemporal model of annual average concentrations of pollutants. For years 1999–2013, a national model was built using partial least squares and universal kriging to predict average concentrations of PM2.5.27,28 Predictions were made using data collected in the continental United States from IMPROVE and CSN monitors, and geographic covariates such as distance to roadway, population density, and Normalized Difference Vegetation Index. PM10 was based on a national model similar to that of PM2.5, described above. We calculated PMcoarse (PM10–2.5) from the difference between estimates of PM10 and PM2.5.29 We additionally estimated annual average geocoded participant address-specific concentrations of NO2, which were derived from similar national models with the addition of station monitor data. Residential addresses were updated at each annual follow-up, and time-varying estimates of pollutant exposure were calculated annually for each participant as an average of the current and previous year pollutant predictions weighted by time spent at each address during each year measured.
Outcome Assessment
We followed participants for clinical outcomes through the end of the WHI Extension I study (December 2010) or the date of the first stroke event. Hospitalization events were first identified using medical records and potential stroke events were adjudicated by physicians and classified into ischemic stroke, hemorrhagic stroke, or other/unclassified stroke. The WHI criteria for clinical endpoints were adapted from standardized criteria as previously reported.30
We defined cerebrovascular mortality as death from any stroke etiology and first obtained as part of routine participant follow-up that included reports from family or next of kin, obituaries, and data linkage with the National Death Index.30 Death certificates and hospital records were obtained when possible, and all events were adjudicated by trained reviewers. Cerebrovascular events included cerebrovascular death and all-cause stroke hospitalizations.
Covariate data
We collected sociodemographic characteristics, lifestyle factors, medical history, and health status using self-reported standardized questionnaires at enrollment. We defined race-ethnicity as White, African American/Black, and Other (where ‘other’ included Hispanic or Latina, Asian, Pacific Islander, American Indian, and Other). Education was defined as completing a college degree versus having less than a college degree. Household income was obtained through self-report. Employment status was dichotomized into working outside the home versus not working outside the home at baseline. We categorized smoking status as never smoker (<100 lifetime cigarettes) and ever smoker (≥ 100 lifetime cigarettes). Alcohol consumption was reported as servings per week, where servings were defined as 12 oz. of beer, 6 oz. of wine, or 1.5 oz. of liquor. High cholesterol and diabetes were defined as a self-reported physician diagnosis, and hypertension at enrollment was defined as using antihypertensive medication or elevated blood pressure (systolic≥140 or diastolic≥90 mmHg). We calculated body mass index (BMI, in kg/m2) at baseline and subsequent follow-up visits. Physical activity was defined as Metabolic Equivalent (MET) hours per week, calculated using a questionnaire which collects frequency, duration, and pace of self-reported activities.31
We calculated neighborhood socioeconomic status (NSES) as a summary z-score derived at the census tract level as a neighborhood measure of wealth, education, and occupation,32 based on data came the American Community Survey Crosswalk from the US Census.
Statistical Methods
We used multiple imputation by chained equations in order to include in the analyses participants with missing covariate data on income (missing 6.7%), education (missing 0.7%), race-ethnicity (missing 0.2%), high cholesterol (missing 5.9%), hypertension (missing 0.8%), alcohol use (missing 0.3%), smoking (missing 0.8%), MET (missing 4.7%), diabetes (missing 0.1%), marital status (missing 0.5%), family history of stroke (missing 5.7%), employment status (missing 6.6%), NSES z-score (missing 0.01%), and BMI (missing 0.1%). All covariates and pollutants were included in the imputation model regardless of whether they were missing any values, while outcome was not included in the imputation model. We implemented this approach using the R mice package (version 2.46.0)33 and used 10 imputations.
To estimate the hazard ratio (HR) and 95% confidence interval (CI) for incident cerebrovascular events associated with an IQR shift in each pollutant we used time-varying Cox proportional hazards models. In all models, air pollution was considered a time-varying exposure. We adjusted for potential confounders including age, race, smoking, education, income, marital status, employment status, BMI, high cholesterol, diabetes, and hypertension at enrollment, family history of stroke, alcohol consumption, physical activity, WHI study component (HT or OS), and WHI center, all which were considered time-fixed at enrollment and not allowed to vary over time. We allowed NSES measures to be time-varying as to reflect address changes over time. We additionally adjusted for PM2.5 in models with PMcoarse as the exposure. Pollutant estimates were modeled as continuous variables (per interquartile range (IQR)) and repeated using quartiles of pollutants. Quartiles were calculated using pollution data at enrollment due to the overall decreasing trend in pollutant concentrations over time.
Additionally, we looked to see whether the association between ambient air pollution and cerebrovascular outcomes varied across strata of age, race, education, BMI, region, smoking, MET, high cholesterol, hypertension, and diabetes by adding interaction terms to our main models. Interaction terms with a p-value <0.10 were considered potentially statistically significant.
All analyses were run in R version 3.4.3 with packages survival v.2.41–3.34,35
RESULTS
At enrollment, the 155,410 post-menopausal women included in these analyses had a mean age of 63.2 ± 7.2 years (mean ± standard deviation (SD)) and were predominantly White (84%), without a college degree (60%), and married or living with a partner (62%) (Table 1).
Table 1:
Characteristics of WHI participants at study enrollment, overall and by quartiles of PM2.5.
| Characteristics | All (N=155,410) | Quartiles of PM2.5 (μg/m3) at Enrollment | |||
|---|---|---|---|---|---|
| Q1 ≤ 9.9 | Q2 (9.9,11.8] | Q3 (11.8, 13.6] | Q4 >13.6 | ||
| Age, years, mean ± SD† | 63.2 (7.2) | 63.4 (7.1) | 63.4 (7.1) | 63.0 (7.2) | 63.0 (7.4) |
| Race, % | |||||
| White | 84.1 | 88.5 | 91.0 | 81.6 | 75.2 |
| African American or Black | 9.0 | 2.1 | 3.8 | 12.7 | 17.8 |
| Hispanic or Latina | 4.0 | 6.9 | 2.6 | 2.8 | 3.6 |
| Asian or Pacific Islander | 1.2 | 0.8 | 1.1 | 1.3 | 1.7 |
| Other | 1.4 | 1.6 | 1.3 | 1.4 | 1.4 |
| Education, % | |||||
| < College degree | 59.8 | 64.8 | 59.4 | 57.1 | 57.8 |
| College graduate | 39.4 | 34.4 | 40.0 | 42.1 | 41.5 |
| Married or living with partner, % | 62.1 | 67.5 | 66.0 | 60.1 | 54.6 |
| Household income, % | |||||
| <$20,000 | 15.5 | 16.7 | 12.7 | 14.5 | 17.9 |
| $20,000–<$50,000 | 42.0 | 44.7 | 42.1 | 40.1 | 40.8 |
| ≥ $50,000 | 35.9 | 31.8 | 38.7 | 38.6 | 34.7 |
| Body mass index, % | |||||
| ≤ 25 kg/m2 | 34.7 | 34.2 | 35.6 | 34.5 | 34.3 |
| 25–<30 kg/m2 | 34.5 | 35.6 | 34.7 | 34.0 | 33.7 |
| ≥ 30 kg/m2 | 30.0 | 29.5 | 28.8 | 30.5 | 31.2 |
| Alcohol drinks/week, % | |||||
| None or < 1 | 62.0 | 60.4 | 59.7 | 62.7 | 65.3 |
| 1–6 | 25.9 | 26.5 | 27.6 | 25.7 | 23.7 |
| ≥ 7 | 11.8 | 12.6 | 12.4 | 11.4 | 10.7 |
| Ever Smoker, % | |||||
| Never | 50.2 | 51.4 | 50.6 | 49.3 | 41.5 |
| Past | 41.7 | 40.9 | 42.1 | 42.3 | 49.2 |
| Current | 6.8 | 6.3 | 6.1 | 7.1 | 7.8 |
| Currently working, % | 34.9 | 32.4 | 35.6 | 36.6 | 35.2 |
| Health insurance, % | 94.4 | 93.0 | 95.9 | 95.0 | 93.9 |
| Physical activity, % | |||||
| <3.00 MET† hr/wk | 26.4 | 26.4 | 25.9 | 26.7 | 26.6 |
| 3.00 – <11.75 MET hr/wk | 30.8 | 30.8 | 30.9 | 30.7 | 30.9 |
| ≥ 11.75 MET hr/wk | 38.1 | 39.5 | 39.2 | 37.8 | 35.8 |
| Diabetes ever, % | 5.7 | 5.6 | 5.1 | 5.8 | 6.4 |
| High cholesterol ever, % | 13.0 | 12.8 | 12.8 | 13.0 | 13.3 |
| Hypertension ever, % | 33.0 | 32.2 | 32.0 | 33.6 | 34.3 |
| WHI Study Region, % | |||||
| Northeast | 23.4 | 20.6 | 30.2 | 24.6 | 18.3 |
| South | 26.4 | 23.0 | 23.8 | 28.1 | 30.7 |
| Midwest | 22.4 | 15.1 | 24.8 | 28.3 | 21.9 |
| West | 27.8 | 41.3 | 21.2 | 19.0 | 29.2 |
Geocoded participant address-specific mean annual PM2.5 concentrations at enrollment ranged from 3.0 to 25.2 μg/m3 with a mean ± SD of 14.2 ± 2.8 μg/m3 (Table 2). Participants living in areas with the highest concentrations of PM2.5 were more likely to be African American or Black (18% vs 2% in the upper versus lower quartiles of PM2.5), college educated (41% vs 34%), current smoker (8% vs 6%), and have hypertension (34% vs 32%) (Table 1). PM2.5 concentrations were moderately correlated with PM10 (r=0.56) and NO2 (r=0.66) and uncorrelated with PMcoarse (r=0.07) (Supplemental Table S1).
Table 2:
Exposure distribution at participant study enrollment
| Pollutant (units) | Mean (SD) | Minimum | 25th Percentile |
50th Percentile |
75th Percentile |
Maximum | IQR |
|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 14.2 (2.8) | 3.0 | 12.5 | 14.3 | 15.9 | 25.2 | 3.5 |
| PM10 (μg/m3) | 23.9 (5.5) | 7.8 | 20.3 | 23.1 | 26.5 | 56.9 | 6.2 |
| PMcoarse (μg/m3) | 9.7 (4.5) | −0.6 | 6.6 | 8.8 | 11.7 | 42.2 | 5.1 |
| NO2 (ppb) | 17.7 (7.2) | 0.9 | 12.4 | 17.3 | 21.9 | 48.4 | 9.5 |
During a median follow-up time of 14.9 years, study participants experienced 4,556 documented cerebrovascular events, including 2,946 ischemic stroke hospitalizations, 666 hemorrhagic stroke hospitalizations, 605 stroke hospitalizations of undetermined etiology, and 946 cerebrovascular deaths. In models adjusting for individual and neighborhood-level characteristics, the hazard ratio of cerebrovascular events increased monotonically with increasing quartiles of PM2.5 (Table 3). A linear association was apparent and statistically significant for all outcomes of interest. For example, the hazard ratio for all cerebrovascular events was 2.14 (95% CI: 1.87, 2.44) comparing the top versus bottom quartiles of PM2.5. In models considering PM2.5 as a linear continuous variable, the hazard ratio for an IQR (3.5 μg/m3) shift in PM2.5 ranged from 1.13 (95% CI: 1.06, 1.19) to 1.18 (95% CI: 1.11, 1.26) dependent on event type (Figure 1, Supplemental Table S2), however hazard ratios did not differ statistically significantly by cerebrovascular outcome.
Table 3:
Hazard ratios (95% confidence intervals) of the association between time-varying PM2.5 and incident stroke.
| Outcome* | Number of Events | Quartiles of PM2.5 | Ptrend | Per IQR increase in PM2.5 | |||
|---|---|---|---|---|---|---|---|
| Q1 ≤ 9.9 | Q2 (9.9, 11.8] | Q3 (11.8, 13.6] | Q4 >13.6 | ||||
| All Stroke hospitalization | 4217 | 1.0 (Ref.) | 1.37 (1.24, 1.52) | 1.75 (1.55, 1.97) | 2.15 (1.88, 2.47) | <10^–16 | 1.15 (1.13 1.18) |
| Ischemic Stroke | 2946 | 1.0 (Ref.) | 1.42 (1.26, 1.61) | 1.70 (1.47, 1.97) | 2.20 (1.87, 2.59) | <10^–16 | 1.15 (1.12, 1.18) |
| Hemorrhagic Stroke | 666 | 1.0 (Ref.) | 1.24 (0.94, 1.64) | 1.95 (1.45, 2.62) | 1.83 (1.30, 2.58) | 0.0001 | 1.13 (1.06, 1.19) |
| Unclassified Stroke | 605 | 1.0 (Ref.) | 1.29 (0.98, 1.68) | 1.70 (1.25, 2.32) | 2.26 (1.60, 3.20) | <10^–5 | 1.18 (1.11, 1.26) |
| Cerebrovascular Death | 946 | 1.0 (Ref.) | 1.20 (0.97, 1.49) | 1.42 (1.10, 1.83) | 1.92 (1.44, 2.55) | <10^–5 | 1.16 (1.10, 1.22) |
| Cerebrovascular Event | 4556 | 1.0 (Ref.) | 1.36 (1.23, 1.50) | 1.71 (1.52, 1.92) | 2.14 (1.87, 2.44) | <10^–16 | 1.15 (1.13, 1.18) |
Stroke hospitalization includes hospitalization for ischemic stroke, hemorrhagic stroke, and other/unclassified stroke. Cerebrovascular events include cerebrovascular death and any type of stroke hospitalization. All models adjusted for age, race, smoking, education, income, marital status, employment status, BMI, high cholesterol, family history of stroke, alcohol consumption, physical activity, WHI study component, neighborhood SES, diabetes and hypertension at enrollment, and WHI center.
Figure 1 –
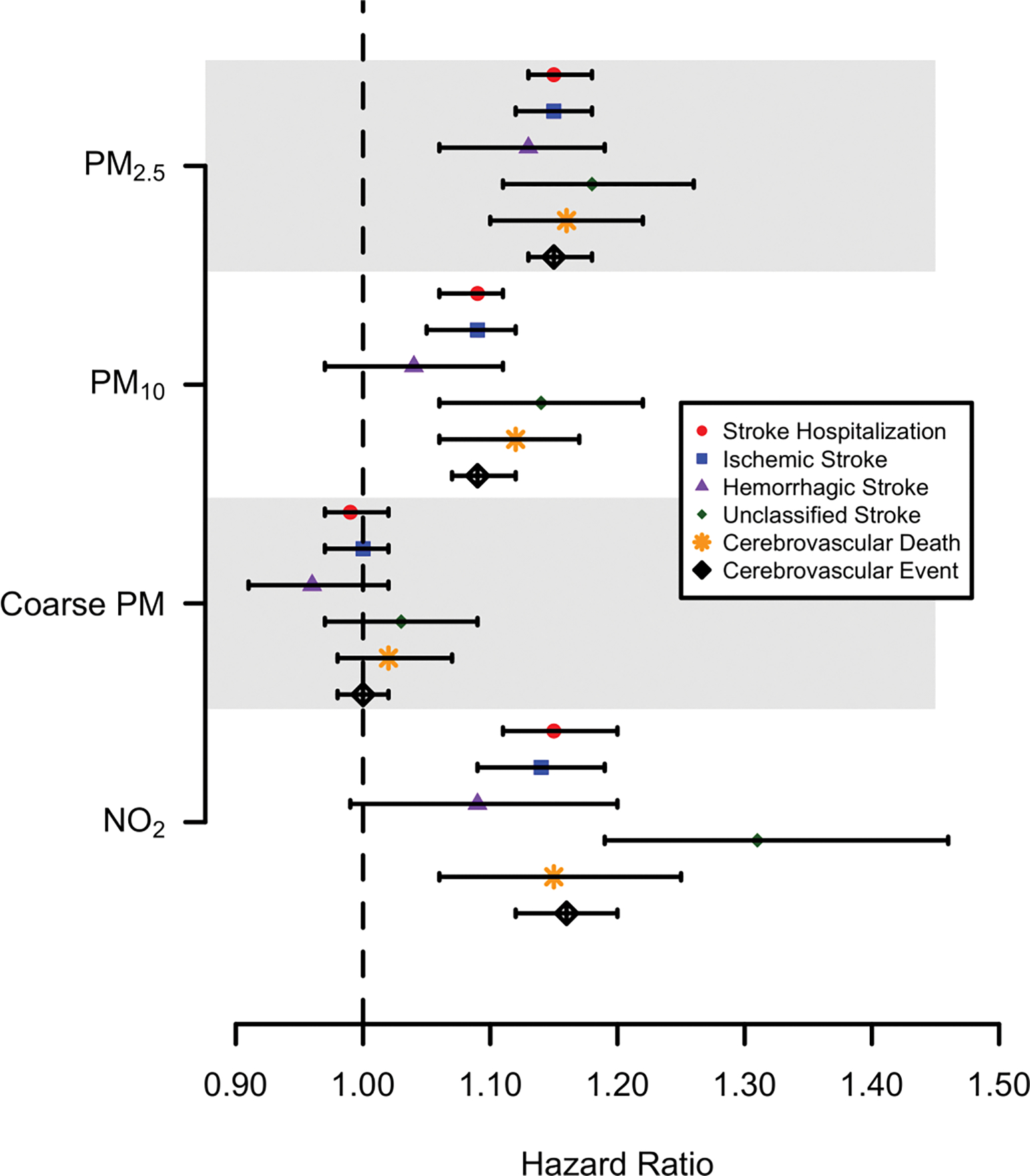
Hazard ratios for association between an IQR increase in ambient air pollutants and incident cerebrovascular events.
There were also strong associations between both PM10 and NO2 and cerebrovascular outcomes (Figure 2). The HR for all cerebrovascular events was 1.17 (95% CI: 1.03, 1.32) comparing the top versus bottom quartiles of PM10, or 1.04 (95% CI: 1.01, 1.07) per interquartile range shift in PM10 (Figure 1,Supplemental Table S2). The hazard ratio for an IQR shift in NO2 ranged from 1.02 (95% CI: 0.93, 1.11) to 1.16 (95% CI: 1.06, 1.27) depending on event type, with NO2 having the strongest association with unclassified stroke hospitalizations (Supplemental Table S3), however differences across stroke type were not statistically significant. In contrast, there was little evidence of an association between geocoded participant address-specific PMcoarse and incident cerebrovascular events (Supplemental Table S4).
We evaluated whether the association between particulate matter, specifically PM2.5 and PM10, and the hazard of all cerebrovascular events varied by the presence of key stroke risk factors. We found no evidence of statistically significant heterogeneity by age, race, education, hypertension, diabetes, BMI, smoking history, or neighborhood socioeconomic status (Supplemental Table S6).
DISCUSSION
In this national cohort of post-menopausal women, we found long-term geocoded participant address-specific concentrations of PM2.5, PM10, and NO2 to be associated with higher risk of cerebrovascular events, with the strength of association remaining relatively consistent across event types. A linear association was most apparent and statistically significant between PM2.5 and PM10 and all stroke hospitalizations, ischemic stroke hospitalizations, unclassified stroke hospitalizations, and all cerebrovascular events. Associations were weaker and not statistically significant between PM10 and hemorrhagic stroke hospitalizations. In contrast, there were no significant associations between PMcoarse and cerebrovascular events.
An earlier study done in the WHI-OS cohort with approximately 6 years of follow-up for cardiovascular events found a HR of 1.28 (95% CI:1.02–1.61) for time to first ever all-cause stroke event per 10 μg/m3 increase in PM2.5.9 Our study provided updated evidence to the prior research by extending the follow-up time to an average of 15 years, examining several exposures metrics of ambient air pollution (PM2.5, PM10, PMcoarse, and NO2), and investigating differences in associations by stroke event types. However, the pattern of results found in the Miller et al. study9 and the current analysis are similar and support the existence of an association between air pollution and cerebrovascular events.
The inclusion of stroke sub-type in our study builds on the existing research supporting an association between long-term exposure to ambient air pollution and incident stroke. Prior studies of long-term exposure to PM and incident all-cause stroke have generally suggested a positive association.8–14,36 However, in many other studies, the estimates of association were either null or report positive associations that have not reached statistical significance10,15–20,37–41 Similar to the aforementioned WHI study, in the international PURE study, the largest prospective cohort study on this topic to date, the authors found a HR for incident stroke of 1.07 (95% confidence interval [CI]: 1.05, 1.10) per 10 μg/m3 increase in ambient fine particles (PM2.5).36 The European ESCAPE study of 22 pooled cohorts, found increased risk of cerebrovascular disease deaths with exposure to higher levels of PM2.5, PM10, and coarse PM. 15 While this study and others have found associations between long-term exposure to pollutants and cerebrovascular mortality. 42,43 several studies have only found associations among specific subgroups of participants such as women16, those with specific ischemic stroke subtypes22, or other subsets of the study population.16–18,21
While pathophysiologic mechanisms behind the effect of ambient air pollution on stroke remain largely unknown, research demonstrates that long-term exposure to air pollution may cause systemic inflammation leading to vascular inflammation, altered sympathovagal balance causing sympathetic nervous system dominance, and altered hemostatic balance instigating a prothrombotic inflammatory state.44–48 Each mechanisms may play a different role on stroke incidence across stroke types. In our analysis, we found consistent strength of effects when looking across stroke types. These results are consistent with studies assessing the effects of short-term spikes in air pollution on stroke risk where the effects of an acute increase of air pollution were stronger in ischemic strokes compared to hemorrhagic stroke.49,50 We did see slightly attenuated effects of PM10 and NO2 among hemorrhagic stroke hospitalizations, although the differences between stroke types were not statistically significant in contrast to several earlier studies which saw stronger effects of PM on incident ischemic stroke.8,11,14 These results support the existing science which states that while all sizes of particulate matter are considered harmful to human health, the smaller particles of PM2.5 are more likely to travel deeper into the lungs where they will be trapped and become available to interact with defense mechanisms to trigger systemic inflammation, or pass directly into the circulatory system and be distributed throughout the body.51,52
The current study adds to the growing scientific evidence supporting the importance of exposure to air pollution in cerebrovascular health, although the authors acknowledge the analysis had several important limitations.
Address histories were collected from participants over the duration of follow-up, there may be potential for misclassification of exposure from inaccurate address information. Additionally, different methods were utilized to assess exposure between 1993–1998 in comparison to 1999–2013.Previous research in this cohort suggest that bias from these types of exposure misclassification will be relatively small in urban areas and potentially larger in suburban and rural areas of residence, but in both instances would bias our results towards the null.26 A second limitation is that the geocoded participant address-specific pollution estimates used here do not include data on time spent in locations outside the home. However, time spent indoors averages 19.6 hours/day at ages > 65 years.53 Moreover, ambient PM exposure-outcome associations are often biased toward the null given that ambient-personal PM correlations are driven by ambient PM concentrations. 54,55 In addition, the measurement of late-life environmental exposures does not necessarily indicate an individual’s true lifetime exposure. Lastly, the study was limited to post-menopausal women participating in either the WHI clinical trial or the WHI observational study, potentially limiting the generalizability of our findings to younger individuals, men, or the United States population in general.
Key strengths of this study include the use of a large, well characterized, geographically diverse prospective cohort with detailed clinical adjudication of cerebrovascular events and mortality. In addition, we were able to assess the differing effects of ambient air pollution on stroke type. Lastly, the comprehensive, high-quality WHI covariate data obtained longitudinally at follow-up allowed for rigorous adjustment for confounding.
CONCLUSIONS
Ambient particulate matter air pollution is a leading cause of global death and disability, with 184,000 ischemic stroke deaths and 226,000 hemorrhagic stroke deaths attributed to particulate matter each year. While PM is a well-established risk factor for cardiovascular disease, the evidence supporting an association between long-term exposure to ambient PM and incident stroke remains less clear. Our study showed that long-term exposure to PM is associated with the incidence of cerebrovascular events among postmenopausal women. The strength of associations did not vary substantially by stroke etiology. These findings speak to the need for future studies to investigate the differential effects of air pollution by stroke type in order to strengthen the evidence surrounding the association between particulate matter and stroke incidence.
Supplementary Material
Highlights.
Examined association of long-term exposure to air pollution and incident cerebrovascular events
Large, national cohort of post-menopausal women enrolled in the Women’s Health Initiative
Examined incident stroke (overall and by etiologic subtypes) and cerebrovascular deaths
Long-term PM2.5, PM10, and NO2 associated with higher risk of all cerebrovascular events
Strength of associations were consistent by stroke etiology
SOURCES OF FUNDING
This research was supported by grant R01-ES020871 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH) and grant T32HL134625 from the National Heart Lung and Blood Institute (NHLBI), NIH. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring institutions.
Non-standard abbreviations and acronyms:
- PM
particulate matter
- WHI
Women’s Health Initiative
- IQR
interquartile range
- NSES
neighborhood socioeconomic status
- HR
hazard ratio
- CI
confidence interval
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
GAW has received consulting income from the Health Effects Institute (Boston, MA) and Google, LLC (Mountain View, CA). All other authors report no disclosures.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Stu. Lancet. 2018;392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, Abbasifard M, Abbasi-Kangevari M, Abd-Allah F, Abedi V, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 5.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, et al. Air Pollution and Cardiovascular Disease. Circulation. 2004;109:2655–2671. [DOI] [PubMed] [Google Scholar]
- 6.U.S. EPA. Integrated Science Assessment (ISA) for Particulate Matter (External Review Draft) [Internet]. Washington, DC: 2018. Available from: https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=341593 [Google Scholar]
- 7.Kulick ER, Kaufman JD, Sack C. Ambient Air Pollution and Stroke: An Updated Review. Stroke [Internet]. 2022. [cited 2023 Feb 2];Available from: https://pubmed.ncbi.nlm.nih.gov/36579640/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin S, Burnett RT, Kwong JC, Hystad P, Van Donkelaar A, Brook JR, Goldberg MS, Tu K, Copes R, Martin RV., et al. Ambient air pollution and the risk of atrial fibrillation and stroke: A population based cohort study. Environ. Health Perspect. 2019;127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007;356:447–458. [DOI] [PubMed] [Google Scholar]
- 10.Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, Cyrys J, de Faire U, de Hoogh K, et al. Long-Term Exposure to Ambient Air Pollution and Incidence of Cerebrovascular Events: Results from 11 European Cohorts within the ESCAPE Project. Environ. Health Perspect. 2014;122:919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amini H, Dehlendorff C, Lim YH, Mehta A, Jørgensen JT, Mortensen LH, Westendorp R, Hoffmann B, Loft S, Cole-Hunter T, et al. Long-term exposure to air pollution and stroke incidence: A Danish Nurse cohort study. Environ. Int. 2020;142:105891. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Kim J, Kim S, Kang S-H, Kim H-J, Kim H, Heo J, Yi S-M, Kim K, Youn T-J, et al. Cardiovascular Effects of Long-Term Exposure to Air Pollution: A Population-Based Study With 900,845 Person-Years of Follow-up. J. Am. Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H, Sun S, Tsang H, Wong C-M, Lee RS-Y, Schooling CM, Tian L. Fine particulate matter exposure and incidence of stroke: A cohort study in Hong Kong. Neurology. 2017;88:1709–1717. [DOI] [PubMed] [Google Scholar]
- 14.Huang K, Liang F, Yang X, Liu F, Li J, Xiao Q, Chen J, Liu X, Cao J, Shen C, et al. Long term exposure to ambient fine particulate matter and incidence of stroke: Prospective cohort study from the China-PAR project. BMJ [Internet]. 2019. [cited 2020 Aug 14];367. Available from: /pmc/articles/PMC7190010/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, Dimakopoulou K, Brunekreef B, Weinmayr G, Hoffmann B, et al. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology. 2014;25:368–78. [DOI] [PubMed] [Google Scholar]
- 16.Stockfelt L, Andersson EM, Molnár P, Gidhagen L, Segersson D, Rosengren A, Barregard L, Sallsten G. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ. Res. 2017;158:61–71. [DOI] [PubMed] [Google Scholar]
- 17.Korek MJ, Bellander TD, Lind T, Bottai M, Eneroth KM, Caracciolo B, de Faire UH, Fratiglioni L, Hilding A, Leander K, et al. Traffic-related air pollution exposure and incidence of stroke in four cohorts from Stockholm. J. Expo. Sci. Environ. Epidemiol. 2015;25:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart JE, Puett RC, Rexrode KM, Albert CM, Laden F. Effect Modification of Long-Term Air Pollution Exposures and the Risk of Incident Cardiovascular Disease in US Women. J. Am. Heart Assoc. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljungman PLS, Andersson N, Stockfelt L, Andersson EM, Sommar JN, Eneroth K, Gidhagen L, Johansson C, Lager A, Leander K, et al. Long-term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ. Health Perspect. 2019;127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson EM, Ögren M, Molnár P, Segersson D, Rosengren A, Stockfelt L. Road traffic noise, air pollution and cardiovascular events in a Swedish cohort. Environ. Res. 2020;185:109446. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi A, Nishiwaki Y, Okamura T, Milojevic A, Ueda K, Asakura K, Takebayashi T, Hasegawa S, Sairenchi T, Irie F, et al. Long-Term Exposure to Particulate Matter and Mortality from Cardiovascular Diseases in Japan: The Ibaraki Prefectural Health Study (IPHS). J. Atheroscler. Thromb. 2020;54148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crichton S, Barratt B, Spiridou A, Hoang U, Liang SF, Kovalchuk Y, Beevers SD, Kelly FJ, Delaney B, Wolfe C DA. Associations between exhaust and non-exhaust particulate matter and stroke incidence by stroke subtype in South London. Sci. Total Environ. 2016;568:278–284. [DOI] [PubMed] [Google Scholar]
- 23.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The women’s health initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann. Epidemiol. 2003;13:S107–S121. [DOI] [PubMed] [Google Scholar]
- 24.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control. Clin. Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 25.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The women’s health initiative recruitment methods and results. Ann. Epidemiol. 2003;13:S18–S77. [DOI] [PubMed] [Google Scholar]
- 26.Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, Heiss G. Accuracy of commercial geocoding: assessment and implications. Epidemiol. Perspect. Innov. 2006;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson PD, Richards M, Szpiro AA, Bergen S, Sheppard L, Larson TV, Kaufman JD. A regionalized national universal kriging model using Partial Least Squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos. Environ. (1994). 2013;75:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergen S, Sheppard L, Sampson PD, Kim S-Y, Richards M, Vedal S, Kaufman JD, Szpiro AA. A national prediction model for PM2.5 component exposures and measurement error-corrected health effect inference. Environ. Health Perspect. 2013;121:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Environmental Protection Agency. Integrated Science Assessment for Particulate Matter [Internet]. Research Triangle Park, NC: 2019. Available from: https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=347534#tab-3 [PubMed] [Google Scholar]
- 30.Curb JD, Mctiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the women’s health initiative. Ann. Epidemiol. 2003;13:S122–S128. [DOI] [PubMed] [Google Scholar]
- 31.Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-Retest Reliability of the WHI Physical Activity Questionnaire. Med. Sci. Sports Exerc. [Internet]. 2009. [cited 2023 Feb 2];41:530. Available from: /pmc/articles/PMC2692735/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diez Roux A V, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N. Engl. J. Med. 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 33.VanBuuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. SoftwareSoftware. 2011;45:1–67. [Google Scholar]
- 34.R Core Team. R: A Language and Environment for Statistical Computing. 2017;
- 35.Therneau TM. A package for Survival Analysis in R (version 2.38). 2015;
- 36.Hystad P, Larkin A, Rangarajan S, AlHabib KF, Avezum Á, Calik KBT, Chifamba J, Dans A, Diaz R, du Plessis JL, et al. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet Planet. Heal. 2020;4:e235–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sørensen M, Lühdorf P, Ketzel M, Andersen ZJ, Tjønneland A, Overvad K, Raaschou-Nielsen O. Combined effects of road traffic noise and ambient air pollution in relation to risk for stroke? Environ. Res. 2014;133C:49–55. [DOI] [PubMed] [Google Scholar]
- 38.Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Outdoor air pollution and incidence of ischemic and hemorrhagic stroke: a small-area level ecological study. Stroke. 2012;43:22–7. [DOI] [PubMed] [Google Scholar]
- 39.Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-Term Exposure to Particulate Matter Air Pollution Is a Risk Factor for Stroke: Meta-Analytical Evidence. Stroke. 2015;46:3058–3066. [DOI] [PubMed] [Google Scholar]
- 40.Johnson JYM, Rowe BH, Villeneuve PJ. Ecological analysis of long-term exposure to ambient air pollution and the incidence of stroke in Edmonton, Alberta, Canada. Stroke. 2010;41:1319–25. [DOI] [PubMed] [Google Scholar]
- 41.Chen R, Zhang Y, Yang C, Zhao Z, Xu X, Kan H. Acute effect of ambient air pollution on stroke mortality in the China air pollution and health effects study. Stroke. 2013;44:954–60. [DOI] [PubMed] [Google Scholar]
- 42.Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, Forastiere F. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect. 2013;121:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, Martin RV, van Donkelaar A, Hughes E, Shi Y, et al. Spatial analysis of air pollution and mortality in California. Am. J. Respir. Crit. Care Med. 2013;188:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, Allen G, Verrier M, Cherry R, Verrier R. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–73. [DOI] [PubMed] [Google Scholar]
- 45.Peters A, Fröhlich M, Döring A, Immervoll T, Wichmann HE, Hutchinson WL, Pepys MB, Koenig W. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur. Heart J. 2001;22:1198–204. [DOI] [PubMed] [Google Scholar]
- 46.Pope CA, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ. Health Perspect. 2004;112:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertens. (Dallas, Tex. 1979). 2009;54:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rückerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, Dailey L, Devlin RB, Diaz-Sanchez D, Koenig W, et al. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ. Int. 2014;70. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA, Investigators of the Registry of Canadian Stroke Network. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–53. [DOI] [PubMed] [Google Scholar]
- 51.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–45. [DOI] [PubMed] [Google Scholar]
- 52.Utell MJ, Frampton MW. Acute Health Effects of Ambient Air Pollution: The Ultrafine Particle Hypothesis. J. Aerosol Med. 2009;13:355–359. [DOI] [PubMed] [Google Scholar]
- 53.Spalt EW, Curl CL, Allen RW, Cohen M, Adar SD, Stukovsky KH, Avol E, Castro-Diehl C, Nunn C, Mancera-Cuevas K, et al. Time-location patterns of a diverse population of older adults: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). J. Expo. Sci. Environ. Epidemiol. [Internet]. 2016. [cited 2019 Jul 31];26:349–55. Available from http://www.ncbi.nlm.nih.gov/pubmed/25921083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.United States Environmental Protection Agency. Descriptive Statistics Tables from a Detailed Analysis of the National Human Activity Pattern Survey (NHAPS) Data [Internet]. Washington, DC: 1996. [cited 2022 Jul 26]. Available from: https://nepis.epa.gov/Exe/ZyNET.exe/30003IAZ.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1995+Thru+1999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery= [Google Scholar]
- 55.Holliday KM, Avery CL, Poole C, McGraw K, Williams R, Liao D, Smith RL, Whitsel EA. Estimating personal exposures from ambient air-pollution measures: Using meta-analysis to assess measurement error. Epidemiology [Internet]. 2014. [cited 2022 Jul 26];25:35. Available from: /pmc/articles/PMC3973436/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


