Abstract
Background and Objectives
Chronic poststroke language impairment is typically worse in older individuals or those with large stroke lesions. However, there is unexplained variance that likely depends on intact tissue beyond the lesion. Brain age is an emerging concept, which is partially independent from chronologic age. Advanced brain age is associated with cognitive decline in healthy older adults; therefore, we aimed to investigate the relationship with stroke aphasia. We hypothesized that advanced brain age is a significant factor associated with chronic poststroke language impairments, above and beyond chronologic age, and lesion characteristics.
Methods
This cohort study retrospectively evaluated participants from the Predicting Outcomes of Language Rehabilitation in Aphasia clinical trial (NCT03416738), recruited through local advertisement in South Carolina (US). Primary inclusion criteria were left hemisphere stroke and chronic aphasia (≥12 months after stroke). Participants completed baseline behavioral testing including the Western Aphasia Battery–Revised (WAB-R), Philadelphia Naming Test (PNT), Pyramids and Palm Trees Test (PPTT), and Wechsler Adult Intelligence Scale Matrices subtest, before completing 6 weeks of language therapy. The PNT was repeated 1 month after therapy. We leveraged modern neuroimaging techniques to estimate brain age and computed a proportional difference between chronologic age and estimated brain age. Multiple linear regression models were used to evaluate the relationship between proportional brain age difference (PBAD) and behavior.
Results
Participants (N = 93, 58 males and 35 females, average age = 61 years) had estimated brain ages ranging from 14 years younger to 23 years older than chronologic age. Advanced brain age predicted performance on semantic tasks (PPTT) and language tasks (WAB-R). For participants with advanced brain aging (n = 47), treatment gains (improvement on the PNT) were independently predicted by PBAD (T = −2.0474, p = 0.0468, 9% of variance explained).
Discussion
Through the application of modern neuroimaging techniques, advanced brain aging was associated with aphasia severity and performance on semantic tasks. Notably, therapy outcome scores were also associated with PBAD, albeit only among participants with advanced brain aging. These findings corroborate the importance of brain age as a determinant of poststroke recovery and underscore the importance of personalized health factors in determining recovery trajectories, which should be considered during the planning or implementation of therapeutic interventions.
Stroke-related damage to perisylvian language-related regions is often associated with varying types and degrees of aphasia.1-3 Although some individuals with aphasia spontaneously recover in the first few months after the stroke, many stroke survivors experience persistent long-term disabling language and communication problems. Lesion size and location are important predictors of long-term language disability2,4; however, these factors only account for a proportion of the variance in aphasia severity (approximately 20%–50%5,6). In addition to lesion factors, there is considerable evidence that language impairment after stroke is more severe in older adults.7 Emerging data also support the idea that stroke age is associated with greater long-term decline, with older individuals showing poorer trajectories than their younger counterparts.8 The degree of chronic aphasic impairment is also remarkably variable across individuals, and factors that determine the likelihood and severity of long-term deficits remain incompletely understood. No current model of aphasia severity is capable of accurately predicting impairment in this heterogeneous population.
It is well documented that there are declines in multiple cognitive skills in typical aging, but there are substantial differences in the trajectory of age-related decline across individuals.9,10 This led to the emerging ideas of cognitive reserve as an explanation for why some individuals may be more resilient to the detrimental effects of aging11; however the underlying mechanisms by which the brain and cognitive reserve enable individuals to maintain cognitive performance over time are still unclear. Similarly, recent research suggests that advanced brain aging is an important factor related to declining cognitive skills among adults in general.12,13 Brain age can be estimated by comparing neuroimaging measures (e.g., regional tissue volume from gray and white matter) from one individual against a large normative database of healthy individuals. The primary measure of advanced brain aging is the difference between an individual's chronologic age and the same individual's predicted brain age based on brain tissue integrity. Advanced brain aging (higher estimated brain age than chronologic age) is now being increasingly recognized as an important factor related to lower cognition among older adults and among individuals with dementia or with risk factors for neurocognitive disorders.14,15 It may be that advanced brain aging is associated with poor cognitive reserve, and conversely, delayed brain aging (lower estimated brain age than chronologic age) may be associated with a resilience to age-related cognitive changes.
Given the known relationship between cognition and MRI-based brain aging metric, we hypothesized that brain age may be related to language impairments after a stroke beyond the effects of chronologic age and characteristics of the stroke lesion. By controlling for these known factors, our analysis sought to measure the independent predictive power of brain age in a large sample of adults with chronic stroke aphasia. The brain age measure used quantifies the structural integrity of brain tissue, irrespective of the stroke lesion, that is, not taking into context the stroke-related tissue loss, but the overall structural integrity of brain tissue that remains after the stroke.
Measuring brain age in the context of stroke can be technically challenging because existing approaches rely on tissue segmentation, which, in turn, depends on the quality of normalization (i.e., warping each individual brain into standard space). Existing approaches to brain normalization are typically designed to work with intact brains. When these methods are applied to brains with large lesion, they can fail catastrophically, providing outputs that dramatically displace relatively proximate voxel locations.16 One potential solution to this problem, which has been used successfully by multiple groups studying stroke,17,18 is to apply an enantiomorphic healing algorithm17 to damaged brains before normalization that effectively mimics healthy brains. This healing process relies on the fact that brains are generally left/right symmetrical to replace damaged left hemisphere tissue with a mirror image of homologous healthy right hemisphere tissue.
In this study, we tested the hypothesis that chronic poststroke aphasia severity is negatively influenced by advanced brain age. We investigated data from a cohort of stroke survivors with chronic aphasia, leveraging modern neuroimaging techniques (i.e., enantiomorphic healing) to adequately infer brain age from individuals with large stroke lesions and test their relationship with chronic deficits and treated recovery.
Methods
Study Design and Participants
We retrospectively evaluated an existing longitudinal dataset from participants with a history of 1 or more chronic (>12 months before enrollment) left hemisphere strokes. Participants were part of the POLAR (Predicting Outcomes of Language Rehabilitation in Aphasia)19,20 clinical trial (NCT03416738). As part of the POLAR protocol, all participants underwent a baseline battery of language and neuropsychological testing at the time of enrollment. The baseline battery included the Western Aphasia Battery–Revised (WAB-R),21 Philadelphia Naming Test (PNT),22 Pyramids and Palm Trees Test (PPTT),23 and Wechsler Adult Intelligence Scale Matrices subtest (WAIS). The battery was administered by American Speech-Language-Hearing Association–certified speech and language pathologists with experience working with individuals with aphasia.
After the baseline behavioral battery, participants underwent 6 weeks of semantic and phonologic treatment using the following timeline: 3 weeks of treatment, 4 weeks of rest, then 3 weeks of the alternative treatment. Treatment order was randomized, with half of the participants receiving semantic treatment first and the other half receiving phonologic treatment first. The PNT was the primary outcome measure and was completed again 1 month after treatment (see Kristinsson et al.20 for a more detailed description of the procedure). The primary goal of POLAR was to provide information about neurobiological and demographic factors associated with aphasia treatment outcomes.
The following inclusion/exclusion criteria applied: Participants were included in the study if they (1) incurred a left hemisphere ischemic or hemorrhagic stroke to the middle cerebral artery, (2) had chronic aphasia (≥12 months after stroke), (3) were 21–80 years of age, (4) spoke English as their primary language for ≥20 years, and (5) could provide written or verbal consent. Participants were excluded if they had (1) severely limited verbal output (measured by a WAB-R Spontaneous Speech score of 0–1), (2) severely impaired auditory comprehension (measured by a WAB-R Auditory Comprehension score of 0–1), (3) bilateral or cerebellar stroke, (4) contraindications to testing with MRI, or (5) history of neurologic disorders. Individuals with multiple strokes were eligible if all lesions were confined to the left supratentorial territory.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants were recruited through local advertisement at the University of South Carolina (Columbia) or at the Medical University of South Carolina (Charleston). The study was approved by the institutional review boards of both institutions. Participants were retrospectively evaluated from an existing longitudinal clinical trial; POLAR (clinicaltrials.gov ID: NCT03416738).
Neuroimaging Acquisition and Preprocessing
All participants underwent research MRI scanning on a Siemens Trio 3T scanner equipped with a 20-channel head coil. In this study, only T1-weighted images were used for brain age estimation, and T2-weighted images were used for stroke lesion identification. These were acquired using the following parameters: T1-weighted imaging (magnetization-prepared rapid gradient-echo) sequence with 1 mm isotropic voxels, a 256 × 256 matrix size, a 9° flip angle, and a 92-slice sequence with repetition time (TR) = 2250 ms, inversion time (TI) = 925 ms, and echo time (TE) = 4.11 ms. T2-weighted scans were acquired using a 3-dimensional T2-weighted SPACE sequence covering the whole head with a resolution of 1 mm3 and a field of view = 256 × 256 mm, 160 sagittal slices, variable degree flip angle, TR = 3200 ms, and TE = 212 ms.
Chronic stroke lesions were manually drawn using each participant's T2-weighted image in native space. All lesion tracings were performed by an expert neurologist (L.B.) or by a trained study staff member (R.N.N.) and were blinded to the behavioral data. Enantiomorphic normalization was then conducted using the nii_preprocess pipeline,24 a set of MATLAB-based (R2017b, The MathWorks) scripts that leverage multiple best-of-breed programs (SPM12; Functional Imaging Laboratory, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, FSL v6.0.3,25 ASLtbx, and MRItrix) to normalize and process MRI data acquired from individuals with lesioned brains. These scripts used enantiomorphic normalization.17
To create chimeric images (i.e., healed brains) in which the damaged portion of the left hemisphere was temporarily replaced with the mirror image of intact areas from the healthy right hemisphere, using the SPM12's Clinical Toolbox leverages SPM12's unified segmentation-normalization26 method to warp this chimeric image to standard (MNI) space, and the resulting spatial transform was then applied to the native-space T1 scan and the native-space versions of the hand-drawn lesion map. This additional step (enantiomorphic normalization) ensures that segmentation-normalization methods designed for intact brains do not incorrectly warp scans with large lesions to the left hemisphere.
Brain Age Estimation
Brain age estimation was performed based solely on T1-weighted images using the BrainAgeR analysis pipeline.27 However, because the BrainAgeR analysis pipeline involves the iterative segmentation-spatial normalization routines (described below) designed to handle healthy nonlesioned brains, we used the enantiomorphic healing approach described above to remove the stroke lesions from the MRI scans to allow for adequate tissue estimation before the images were input into the BrainAgeR pipeline. By removing the stroke lesion, we ensured that the lesion did not affect the brain age calculation, and that brain age was based on the intact brain tissue outside the lesion.
As such, the lesion maps in native T1-weighted space were smoothed with a 3 mm full-width half-maximum gaussian kernel to remove sharp edges and to include the perilesional tissue. The enantiomorphic segmentation-normalization was then employed using SPM12 and custom MATLAB scripts.24 The enantiomorphic approach creates a mirrored image of the right hemisphere, which is overlayed onto the left hemisphere that contains the stroke lesion. A chimeric image is then created in which the lesioned tissue is fully replaced by tissue from the contralateral hemisphere. In other words, the stroke-related damaged area is substituted by the homologue tissue in the preserved right hemisphere. This healed brain does not include the stroke lesion, and it was this image that was input into the BrainAgeR pipeline described below.
The BrainAgeR pipeline was applied using the default protocol.27,28 First, T1-weighted images were segmented into gray matter and white matter before being normalized using nonlinear spatial registration27 and SPM12's DARTEL toolbox.26 The BrainAgeR analysis pipeline uses a customized version of FSL slicesdir to generate a directory of images and corresponding index.html files for quality controlling in a web browser. These probabilistic tissue maps were visually inspected by an expert neurologist (L.B.) to ensure quality of the segmentation. The CSF tissue was removed, and the gray and white matter probabilistic tissues were vectorized, concatenated, and subjected to a principal component analysis to reduce dimensionality. The components explaining the top 80% of the variance were used for brain age prediction. A machine learning algorithm using a pretrained gaussian regression model implemented in R package Kernlab was used to estimate brain age. This pretrained model was based on scans of 3,377 healthy individuals from 7 publicly available datasets and tested on 611 different scans of healthy individuals aged between 18 and 90 years.27 See Cole et al. for more detail on the BrainAgeR pipeline.27,28 The image processing steps are shown in Figure 1.
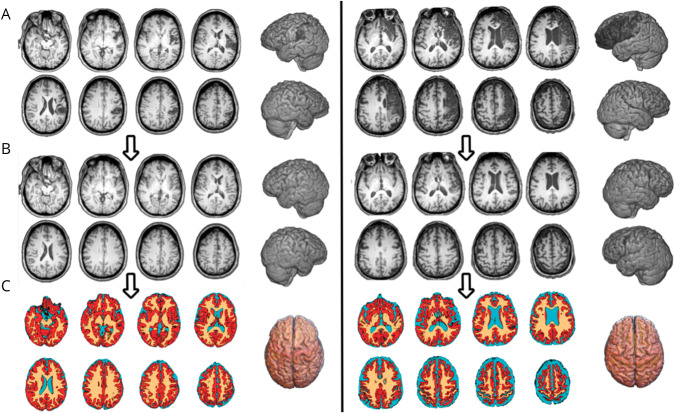
Figure 1. Steps Used to Estimate Brain Age.
This figure illustrates the steps used to estimate brain age. Images from 2 representative participants are shown. The T1-weighted images with the stroke lesion (A) were segmented using an enantiomorphic process to heal the location of the stroke lesion. The resulting images (B) were then processed using BrainAgeR. Probabilistic tissue maps (C), that is, gray matter (red), white matter (orange), and CSF (teal), were used in machine learning models trained with a large dataset of healthy individuals to estimate each participant's brain age.
The relative difference between the estimated brain and the participant's chronologic brain age was determined using the formula: (estimated brain age − chronologic age)/chronologic age.
As such, for each participant, a proportional brain age difference (PBAD) was calculated representing the proportion of the participant's chronologic age accounted for by the difference between brain age and chronologic age. More specifically, PBAD is the measure of advanced (or delayed) brain age beyond chronologic age, adjusted proportionally for chronologic age. Positive values suggest that the predicted brain age is older than the chronologic age of the participant (i.e., advanced brain aging), and negative values suggest that chronologic age is older than the predicted brain age (i.e., delayed brain aging). This measure is hereafter referred to as the PBAD.
Statistical Analyses
The normality of primary behavioral variables used for analysis was tested using a 1-sample Kolmogorov-Smirnov test. The relationship between continuous demographic variables was assessed using Pearson's or Spearman's correlations for parametrically or nonparametrically distributed variables, respectively (as indicated in the Results section).
To assess the relationship between PBAD and language/cognitive measures, we performed 2 main sets of analyses. First, we evaluated the relationship between the PBAD and baseline performance (pretreatment assessments) using multiple linear regression models in which the behavioral variable was set as the dependent variable, and the following variables were used as independent variables: PBAD, chronologic age, and lesion volume.
Second, we evaluated the relationship between PBAD and POLAR treatment outcomes, where the relative gain in language function was set as the dependent variable in multiple linear models. More specifically, the relative gain in language function was evaluated as the proportion of maximal gains according to the formula: (correct items posttherapy − correct items pretherapy)/(maximum score − correct items pretherapy).
The same independent variables were used to assess treatment outcomes: chronologic age, PBAD, and lesion volume. In addition, baseline performance (correct items pretherapy) was also included as an independent variable.
It should be noted that PBAD used in all analyses (concerning baseline behavioral measures or posttreatment recovery) was computed based on the MRI obtained at baseline, that is, before language treatments.
Data Availability
The conditions of our ethics approval do not permit sharing of the raw MRI data supporting this study with any individual outside the author team under any circumstances. However, anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Participants
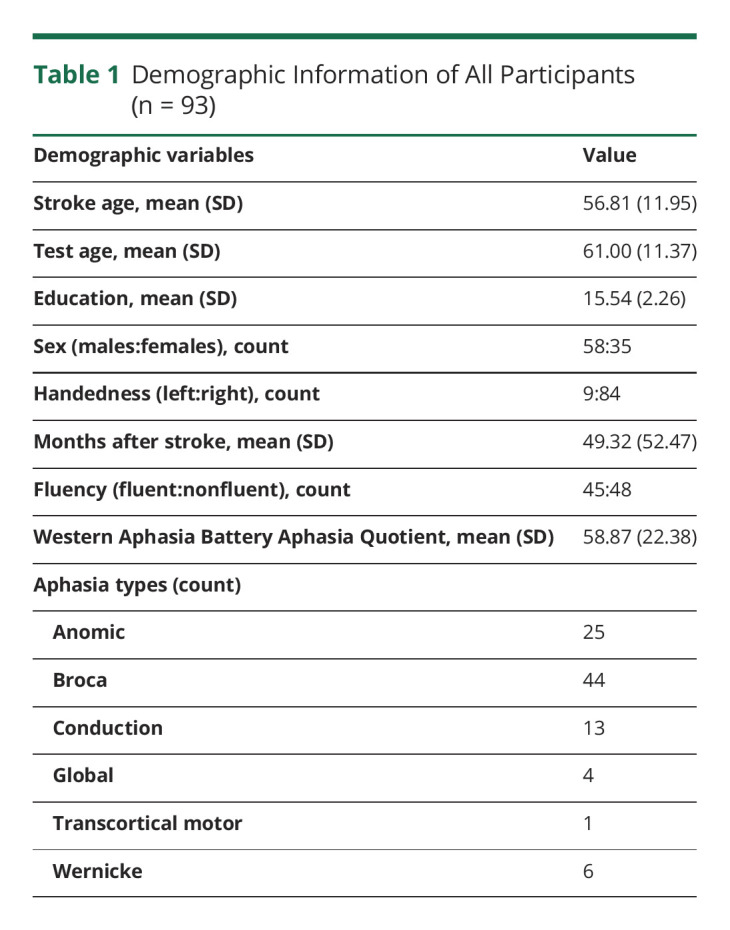
We retrospectively evaluated an existing longitudinal dataset from participants (N = 109) with a history of 1 or more chronic (>12 months before enrollment) left hemisphere strokes. After confirming that participants met our inclusion/exclusion criteria, the results cohort comprised 93 participants. Demographic information describing this sample is provided in Table 1.
Table 1.
Demographic Information of All Participants (n = 93)
Brain Age
The estimated brain age difference ranged from 14 years younger to 23 years older than the participant's chronologic age. The mean difference between brain age and chronologic age was 2.18 ± 7.6 years. The PBAD was 0.04 ± 0.14. Figure 2 demonstrates 2 examples highlighting (A) a participant whose estimated brain age differed substantially from the participant's chronologic age and (B) a participant whose estimated brain age and chronologic age were similar.
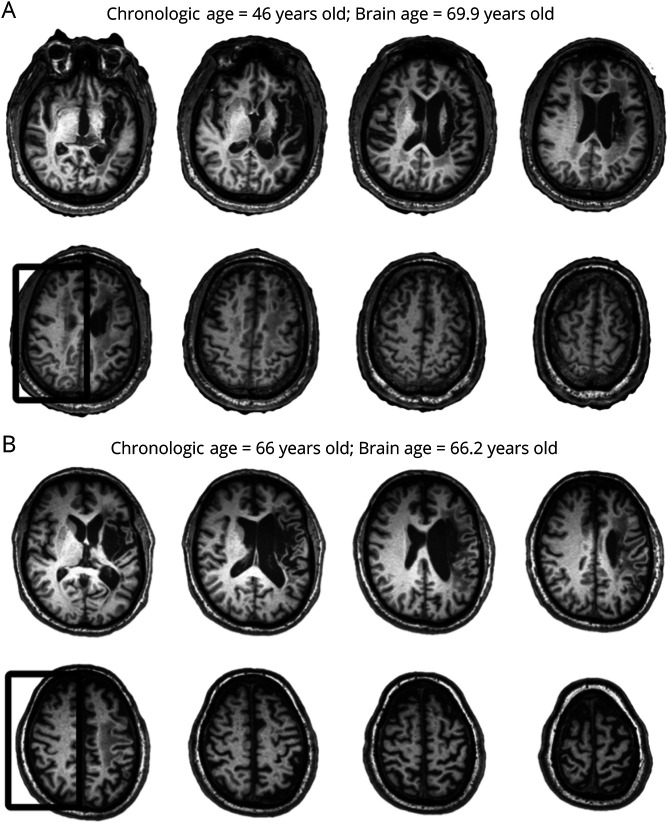
Figure 2. Example of Brain Ages.
Examples of 2 participants whose estimated brain ages were substantially higher (A) or similar (B) to the participant's age. The box outlines the noticeable gray and white matter atrophy in the participant with a large brain age difference compared with the other participant. Notice that the size of the lesion before enantiomorphic correction was overall similar.
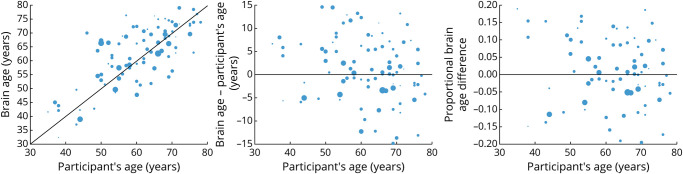
There was a significant relationship between participants' chronologic age and the estimated brain age (Spearman's r = 0.7190, p < 0.0001). Older participants had a lower difference between brain age and chronologic age (Spearman's r = −0.2775, p = 0.007), as well as a smaller PBAD (Spearman's r = −0.3317, p = 0.001). These results are shown in Figure 3.
Figure 3. Scatterplots to Show the Relationship Between Chronologic Age and Brain Age.
The size of each data point in the scatterplot is proportional to lesion volume.
There was not a significant relationship between chronologic age and lesion volume (Spearman's r = −0.1524, p = 0.15), between brain age and lesion volume (Spearman's r = −0.1757, p = 0.1), or between the PBAD and lesion volumes (Spearman's r = −0.006, p = 0.96).
Baseline Cognitive and Language Variables
PPTT
Models composed of chronologic age, PBAD, and lesion volume significant predicted performance on the PPTT (F = 7.9079, p = 0.0001). Chronologic age was not significantly associated with the PPTT scores (T = −0.6424, p = 0.5223), but higher PBAD (T = −2.5174, p = 0.0136) and larger lesion volumes (T = −4.2830, p < 0.0001) were associated with worse performance on this test.
WAIS Matrix Reasoning
A similar model showed significant associations between WAIS Matrix Reasoning scores, chronologic age, PBAD, and lesion volume (F = 4.1546, p = 0.0084). Chronologic age (T = −3.1200, p = 0.0024) and lesion volumes (T = −2.0311, p = 0.0452) were associated with lower WAIS scores, with older participants having significantly worse behavioral scores. However, PBAD was not an independent predictor (T = −1.2832, p = 0.2027).
WAB
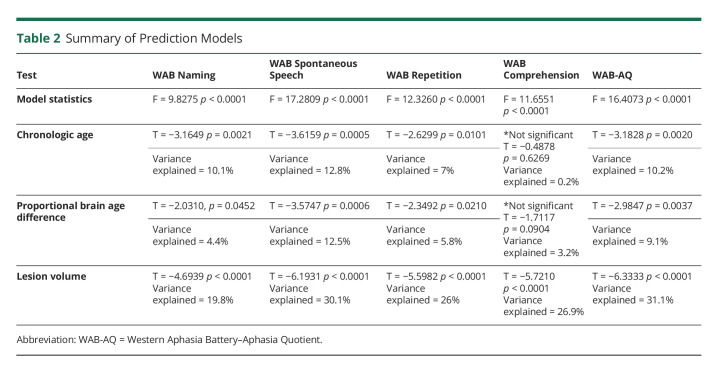
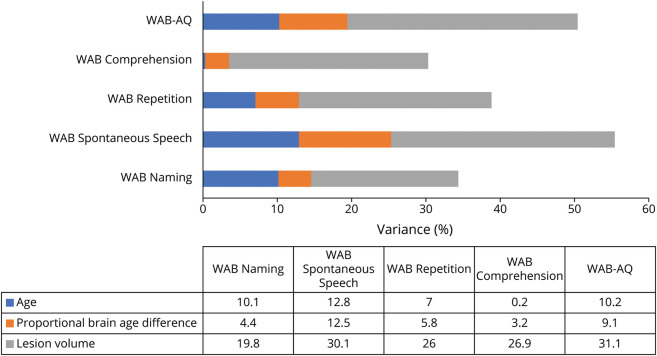
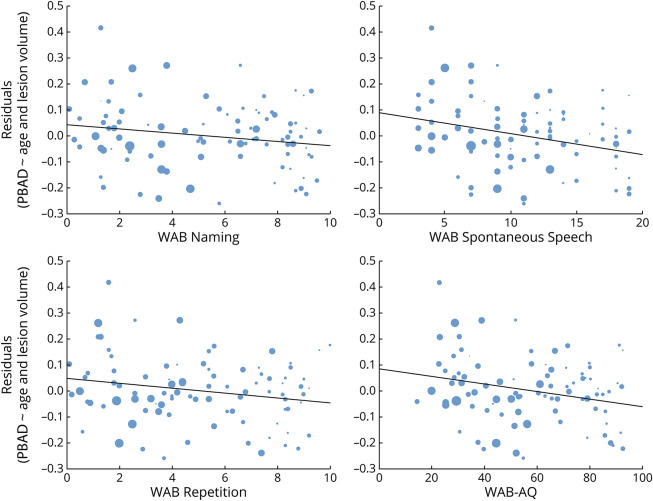
Linear modeling revealed that all WAB scores were significantly associated with chronologic age, PBAD, and lesion volume. PBAD was an independent predictor of worse scores for all measures, except for comprehension. All models are summarized in Table 2 and Figure 4. Figure 5 demonstrates the relationship between the WAB scores and PBAD scores after regressing out variance due to chronologic age and lesion volume.
Table 2.
Summary of Prediction Models
Figure 4. Graphs to Show the Percentage of Variance Explained by Each Factor.
Percentage of variance explained by chronologic age, proportional brain age difference, and lesion volume for WAB-AQ and WAB subscores. Note that only lesion volume was statistically associated with WAB Comprehension. In all other cases, significant variance in WAB scores was associated with all 3 predictors. WAB-AQ = Western Aphasia Battery–Aphasia Quotient.
Figure 5. Scatterplots to Show the Relationship Between WAB Scores and Proportional Brain Age Difference.
The relationship between WAB scores and the residualized proportional brain age difference from chronologic age and lesion volume. WAB Comprehension is not shown because it was not significantly associated with proportional brain age difference. WAB = Western Aphasia Battery.
PNT
We observed a significant relationship with the prediction model (F = 8.0514 model, p = 0.0001), with higher chronologic age (T = −2.4007, p = 0.0187) and larger lesion volume (T = −4.4976, p = 0.0000) as predictors of worse scores. PBAD was not an independent predictor (T = −1.5197, PBAD p = 0.1326). Of note, the PNT was available for only a subset (n = 82) of all participants.
Language Therapy Outcomes
The PNT was available for 78 participants at both baseline testing and testing conducted at 1 month after therapy. At baseline testing, participants had an average PNT score of 80.83 (SD = 61.77, min = 0, max = 172). At 1-month follow-up, the average PNT score was 86.76 (SD = 63.73, min = 0, max = 173). The average change in PNT scores between baseline and at 1 month was 5.93 (SD = 9.20, min = −12, max = 43.5). The average proportion of maximal gain scores was 0.13 (SD = 0.17, min = −0.11, max = 0.625).
Language improvement as the proportion of maximal gains in correctly named items in the PNT was associated with the linear model not including baseline performance (F = 6.1530, p = 0.0009), in which the chronologic age (T = −2.5217, p = 0.0138) and lesion volumes (T = −3.8274, p = 0.0003) were independent predictors of recovery. PBAD was not a significant predictor (T = −1.2824, p = 0.2037).
In the model with baseline performance included as an independent variable, there was also a significant relationship with improvement (F = 2197.9094 p < 0.0001), but only baseline performance was a significant predictor (T = 83.7965, p < 0.0001).
Forty-seven participants had an estimated brain age that was equal to or higher than the participant's chronologic age. For these participants, the average baseline PNT score was 80.42 (SD = 61.85, min = 0, max = 169). At 1-month follow-up, the average PNT score was 86.15 (SD = 63.98, min = 0, max = 171). In these participants, the average change in PNT scores between baseline and at 1 month was 5.73 (SD = 9.19, min = −12, max = 43.5). The average proportion of maximal gain scores was 0.13 (SD = 0.18, min = −0.11, max = 0.625). Among participants whose estimated brain age was equal to or higher than the participant's chronologic age (n = 47), the improvement in the PNT was independently associated with a lower PBAD. Specifically, not including baseline performance in the model, the proportion of maximal gains in correctly named items in the PNT was significantly associated (F = 3.1104, p = 0.0361) and independently predicted by a lower PBAD (T = −2.0474, p = 0.0468, 9% of variance explained) and lesion volumes (T = −2.3682, p = 0.0224). Chronologic age was nearing statistical significance but not an independent predictor (T = −1.9985, p = 0.0520, 8% of variance explained).
Notably, including baseline performance in the model, PNT improvement was associated (F = 1362.0020, p < 0.0002) and independently predicted by PBAD (T = −2.3095, p = 0.0259, 11.2% of variance explained).
Discussion
In this study, we evaluated the hypothesis that chronic poststroke aphasia severity is influenced by brain age. We tested this by investigating the contribution of brain age (in addition to typical explanatory variables such as chronologic age and lesion volume) using regression models designed to predict behavioral scores and treatment trajectories. This was made possible through the application of modern neuroimaging techniques (i.e., enantiomorphic healing), which enabled us to apply brain age estimation algorithms to nonstandard (i.e., lesioned) brains. Specifically, we used output from the brain age algorithm to compute a PBAD score. Using this metric, we found that the PBAD was associated with performance on both the PPTT and well-known language tasks including WAB subscores (naming, spontaneous speech, and repetition) and overall aphasia severity (WAB-AQ). Notably, therapy outcome scores between baseline and at 1 month were also associated with PBAD, albeit only among participants whose estimated brain age was equal to or higher than their chronologic age.
Brain age predictions have been used previously in clinical populations,28 including schizophrenia29 and epilepsy.30 However, computing brain age within clinical populations with brain lesions or resections poses additional challenges.31,32 The presence of large lesions is not only methodologically cumbersome, but the large volume of damaged tissue is known to influence behavioral and cognitive outcomes substantially.2-4 This phenomenon is highlighted by the current study in which lesion volume was found to be significantly related to all behavioral scores. These results may indicate that more subtle effects of advanced brain aging are not as prominent as in otherwise healthy populations with either smaller or perhaps more diffuse brain lesions (e.g., atrophy). Furthermore, the strong relationship between stroke lesion volume and behavioral performance may explain why we did not find associations between PBAD and general cognition (measured by the WAIS), a finding that has been previously described.12,13 Thus, the current results highlight the importance of considering brain age in stroke aphasia because it was a predictor of language abilities even when accounting for lesion volume and chronologic age.
The results from this study demonstrate a relationship between PBAD and aphasia severity (measured by the WAB). Although chronologic age and lesion volume were also predictors of aphasia severity, PBAD was able to explain some of the previously unexplained (additional) variance. Although lesion volume is often reported as a reliable predictor of poststroke aphasia severity, the contribution of other health and demographic factors is less consistent. One possibility is that specific combinations of health and demographic factors influence brain age, and therefore, brain age mediates the relationship between these factors and overall aphasia severity. The association between PBAD and PPTT scores is particularly interesting because chronologic age was not a significant predictor in this case. This finding suggests the intriguing possibility that advanced aging, as defined in the current study, may preferentially affect participants' ability to access semantic information. As accessing semantic information is typically associated with the ventral language stream2,33 and the bilateral temporal lobes,34,35 it may be that advanced brain aging particularly affects these connections and regions. Future studies could investigate the spatial distribution of advanced age-related changes to identify the brain regions most affected.
Our results demonstrating that PBAD independently predicts therapy gains are of clinical relevance and have clear clinical implications. A common view suggests that aphasia recovery is limited in the chronic stage,36 with outcomes largely predicted by initial impairment.36 However, recent research casts doubt on these conclusions.8,37 Although measures of aggregate performance may appear to plateau, this may reflect different patient trajectories based on factors related to brain health (cognitive reserve), environment, and genetics.38 This suggests that aphasia recovery trajectories are highly variable, and the factors which influence therapy gains are incompletely understood.39 This view suggests that understanding these factors may prove valuable in providing accurate prognosis, recommending bespoke treatment and compensation regimens, and improving statistical power in clinical trials by matching individuals for predictable gains. Although recently health factors have been considered in models of language recovery,8,40,41 brain age has not yet been considered in these models. However, the significance of the health and integrity of the spared tissue in populations with cortical damage, such as stroke survivors, is becoming increasingly clear within the literature.41,42 The results of the current study are consistent with recent reports that the integrity of the spared tissue is related to cognitive reserve12 and capacity for therapy gains,41,42 as well as the finding that diffuse neuronal diseases, such as small vessel disease (which can result in leukoaraiosis), are associated with worse stroke outcomes,43 poststroke cognitive decline,44 and poorer language recovery.41,42 As advanced aging is thought to represent reduced cortical integrity, it is perhaps not surprising that this would affect a complex cognitive skill such as language. Notably, the lack of a relationship between therapy outcome scores when including participants with a younger brain age than chronologic age suggests that there are other factors influencing therapy outcomes, particularly in otherwise healthy individuals. These factors might include things such as age at the time of stroke, cumulative amount of therapy received since stroke, and other systemic health measures. There is a critical need to investigate these factors in combination with brain age in future research.
In this study, the estimated brain age ranged from 14 years younger to 23 years older than chronologic age, but no associations were found between lesion volume and chronologic age or PBAD. Despite the relationship between brain age and chronologic age, we observed considerable variation in brain age within participants with the same chronologic age. Previous studies have suggested that brain age in stroke patients is higher than that of chronologically age-matched controls45 at 6 weeks, 3 months, and 12 months after stroke. There are several factors that may influence brain age, including education46 and physical activity,46 and it is possible that brain age itself is a biomarker for stroke risk.45 Chronologic aging is both a risk factor for stroke47 and is associated with poorer stroke outcomes,8 as highlighted by the consistent negative relationship found between age and language scores in the current study. Similarly, because cardiovascular risk factors such as BMI, hypertension, and diabetes are associated with both poorer brain health48 and increased risk of stroke,49 these factors may also be related to advanced aging. Future studies could combine measures of brain age with other markers of overall brain health (e.g., small vessel disease) to determine the relative influence of each of these in determining the severity of poststroke aphasia and treatment outcomes. Furthermore, future studies could investigate which health or demographic factors may predict who is likely to have delayed/advanced brain aging.
Vascular mechanisms are also one of the major contributing factors to dementia in the elderly, and vascular dementia is common after stroke. As brain age is a measure of cortical integrity, advanced brain aging may be directly related to mild cognitive impairment or subclinical Alzheimer disease. Among individuals with aphasia, the ascertainment of cognitive impairment can be limited by language barriers and was not something that was directly tested in this study but is an important avenue for future research.
Although using enantiomorphic healing enabled the estimation of brain age in individuals with large stroke lesions, the natural physiologic asymmetry in brain hemispheres is therefore lost, and this is a limitation of this approach.
Furthermore, this is a retrospective study, and 93 participants constitute a relatively small sample size for studies using brain age estimations; however, this study included participants with a broad distribution of chronologic age (29–80 years), time poststroke (12–241 months), education (12–20 years), and aphasia severity (WAB-AQ: 20.1–92.6) across 6 different aphasia subtypes.
It is important to note that it is likely that all regions of the brain do not age at the same rate, and therefore, regional distributions of aging in aphasia (and healthy aging) would be an important avenue for future research. The BrainAgeR methodology uses principal components of voxel ensembles that are responsible for most of the explained variance and are not necessarily associated with specific regions (i.e., they may involve more than 1 region or parts of regions). Therefore, future research could focus on the development of region-based aging methodologies, which would allow the identification of region-specific premature brain aging.
Overall, the current study supports the theory that brain age is an important factor for language deficits after stroke, highlighting the importance of personalized health factors beyond the characteristics of chronologic age and the stroke lesion in determining poststroke impairment and treatment response. Future studies could focus on integrating measures of brain age with other markers of brain health to better understand potential interactions between various factors affecting language outcomes after stroke.
Glossary
- TE
echo time
- TI
inversion time
- PBAD
proportional brain age difference
- PNT
Philadelphia Naming Test
- PPTT
Pyramids and Palm Trees Test
- POLAR
Predicting Outcomes of Language Rehabilitation in Aphasia
- TR
repetition time
- WAB-AQ
Western Aphasia Battery–Aphasia Quotient
- WAB-R
Western Aphasia Battery–Revised
- WAIS
Wechsler Adult Intelligence Scale
Appendix. Authors
Study Funding
National Institute on Deafness and Other Communication Disorders: R01DC014021, P50DC014664, and U01DC017521 (NIH/NIDCD).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Lukic S, Thompson CK, Barbieri E, et al. Common and distinct neural substrates of sentence production and comprehension. Neuroimage. 2021;224:117374. doi: 10.1016/j.neuroimage.2020.117374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridriksson J, Yourganov G, Bonilha L, Basilakos A, den Ouden DB, Rorden C. Revealing the dual streams of speech processing. Proc Natl Acad Sci USA. 2016;113(52):15108-15113. doi: 10.1073/pnas.1614038114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thye M, Mirman D. Relative contributions of lesion location and lesion size to predictions of varied language deficits in post-stroke aphasia. NeuroImage Clin. 2018;20:1129-1138. doi: 10.1016/j.nicl.2018.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Døli H, Andersen Helland W, Helland T, Specht K. Associations between lesion size, lesion location and aphasia in acute stroke. Aphasiology. 2021;35(6):745-763. doi: 10.1080/02687038.2020.1727838 [DOI] [Google Scholar]
- 5.Osa García A, Brambati SM, Brisebois A, et al. Predicting early post-stroke aphasia outcome from initial aphasia severity. Front Neurol. 2020;11:120. doi: 10.3389/FNEUR.2020.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridriksson J, den Ouden DB, Hillis AE, et al. Anatomy of aphasia revisited. Brain. 2018;141(3):848-862. doi: 10.1093/brain/awx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis C, Urban S. Age and aphasia: a review of presence, type, recovery and clinical outcomes. Top Stroke Rehabil. 2016;23(6):430-439. doi: 10.1080/10749357.2016.1150412 [DOI] [PubMed] [Google Scholar]
- 8.Johnson L, Basilakos A, Yourganov G, et al. Progression of aphasia severity in the chronic stages of stroke. Am J Speech Lang Pathol. 2019;28(2):639-649. doi: 10.1044/2018_AJSLP-18-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murman DL. The impact of age on cognition. Semin Hear. 2015;36(03):111-121. doi: 10.1055/S-0035-1555115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small BJ, Dixon RA, McArdle JJ. Tracking cognition–health changes from 55 to 95 years of age. J Gerontol B Psychol Sci Soc Sci. 2011;66B(Suppl 1):i153–i161. doi: 10.1093/GERONB/GBQ093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern Y, Barnes CA, Grady C, Jones RN, Raz N. Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging. 2019;83:124-129. doi: 10.1016/J.NEUROBIOLAGING.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anatürk M, Kaufmann T, Cole JH, et al. Prediction of brain age and cognitive age: quantifying brain and cognitive maintenance in aging. Hum Brain Mapp. 2021;42(6):1626-1640. doi: 10.1002/HBM.25316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott ML, Belsky DW, Knodt AR, et al. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol Psychiatry. 2019;26(8):3829-3838. doi: 10.1038/s41380-019-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke K, Gaser C. Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer's disease. GeroPsych. 2012;25(4):235-245. doi: 10.1024/1662-9647/A000074. [DOI] [Google Scholar]
- 15.Franke K, Gaser C. Ten years of BrainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front Neurol. 2019;10(JUL):789. doi: 10.3389/FNEUR.2019.00789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486-500. doi: 10.1006/NIMG.2001.0845 [DOI] [PubMed] [Google Scholar]
- 17.Nachev P, Coulthard E, Jäger HR, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage. 2008;39(3):1215-1226. doi: 10.1016/j.neuroimage.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yourganov G, Fridriksson J, Rorden C, Gleichgerrcht E, Bonilha L. Multivariate connectome-based symptom mapping in post-stroke patients: networks supporting language and speech. J Neurosci. 2016;36(25):6668-6679. doi: 10.1523/JNEUROSCI.4396-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristinsson S, Basilakos A, den Ouden DB, et al. Predicting outcomes of Language rehabilitation (POLAR). Primary results from a large-scale aphasia therapy trial. In preparation.
- 20.Kristinsson S, Basilakos A, Elm J, et al. Individualized response to semantic versus phonological aphasia therapies in stroke. Brain Commun. 2021;3(3):fcab174. doi: 10.1093/BRAINCOMMS/FCAB174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kertesz A. WAB-R: Western Aphasia Battery-Revised. PsychCorp; 2007. [Google Scholar]
- 22.Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: scoring and rationale. Clin Aphasio. 1996;24:121-133. [Google Scholar]
- 23.Howard D, Patterson KE. The Pyramids and Palm Trees Test; 1992. [Google Scholar]
- 24.Rorden C, McKinnon E, Hanayik T, Yourganov G, Reddy D. nii_preprocess: Zenodo DOI release. 2020. doi: 10.5281/ZENODO.4027711 [DOI] [Google Scholar]
- 25.Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. doi: 10.1016/J.NEUROIMAGE.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 26.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839-851. doi: 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 27.Cole JH, Ritchie SJ, Bastin ME, et al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385-1392. doi: 10.1038/mp.2017.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole JH, Poudel RPK, Tsagkrasoulis D, et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115-124. doi: 10.1016/J.NEUROIMAGE.2017.07.059 [DOI] [PubMed] [Google Scholar]
- 29.Schnack HG, van Haren NE, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated brain aging in schizophrenia: a longitudinal pattern recognition study. Am J Psychiatry. 2016;173(6):607-616. doi: 10.1176/appi.ajp.2015.15070922. [DOI] [PubMed] [Google Scholar]
- 30.Pardoe HR, Cole JH, Blackmon K, Thesen T, Kuzniecky R. Structural brain changes in medically refractory focal epilepsy resemble premature brain aging. Epilepsy Res. 2017;133:28-32. doi: 10.1016/J.EPLEPSYRES.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Cole JH, Raffel J, Friede T, et al. Longitudinal assessment of multiple sclerosis with the brain-age paradigm. Ann Neurol. 2020;88(1):93-105. doi: 10.1002/ANA.25746 [DOI] [PubMed] [Google Scholar]
- 32.de Bézenac CE, Adan G, Weber B, Keller SS. Association of epilepsy surgery with changes in imaging-defined brain age. Neurology. 2021;97(6):e554–e563. doi: 10.1212/WNL.0000000000012289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1-2):67-99. doi: 10.1016/J.COGNITION.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 34.de Zubicaray GI, Rose SE, McMahon KL. The structure and connectivity of semantic memory in the healthy older adult brain. Neuroimage. 2011;54(2):1488-1494. doi: 10.1016/J.NEUROIMAGE.2010.08.058 [DOI] [PubMed] [Google Scholar]
- 35.Rice GE, Caswell H, Moore P, Hoffman P, Lambon Ralph MA. The roles of left versus right anterior temporal lobes in semantic memory: a neuropsychological comparison of postsurgical temporal lobe epilepsy patients. Cereb Cortex. 2018;28(4):1487-1501. doi: 10.1093/CERCOR/BHX362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breitenstein C, Grewe T, Flöel A, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. Lancet. 2017;389(10078):1528-1538. doi: 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- 37.Hope TMH, Friston K, Price CJ, Leff AP, Rotshtein P, Bowman H. Recovery after stroke: not so proportional after all? Brain. 2019;142(1):15-22. doi: 10.1093/BRAIN/AWY302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridriksson J, Elm J, Stark BC, et al. BDNF genotype and tDCS interaction in aphasia treatment. Brain Stimul. 2018;11(6):1276-1281. doi: 10.1016/J.BRS.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridriksson J, Moser D, Bonilha L, et al. Neural correlates of phonological and semantic-based anomia treatment in aphasia. Neuropsychologia. 2007;45(8):1812-1822. doi: 10.1016/J.NEUROPSYCHOLOGIA.2006.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilmskoetter J, Marebwa B, Basilakos A, et al. Long-range fibre damage in small vessel brain disease affects aphasia severity. Brain. 2019;142(10):3190-3201. doi: 10.1093/brain/awz251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basilakos A, Stark BC, Johnson L, et al. Leukoaraiosis is associated with a decline in language abilities in chronic aphasia. Neurorehabil Neural Repair. 2019;33(9):718-729. doi: 10.1177/1545968319862561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varkanitsa M, Peñaloza C, Charidimou A, Caplan D, Kiran S. White matter hyperintensities predict response to language treatment in poststroke aphasia. Neurorehabil Neural Repair. 2020;34(10):945-953. doi: 10.1177/1545968320952809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liou LM, Chen CF, Guo YC, et al. Cerebral white matter hyperintensities predict functional stroke outcome. Cerebrovasc Dis. 2010;29(1):22-27. doi: 10.1159/000255970 [DOI] [PubMed] [Google Scholar]
- 44.Liang Y, Chen YK, Liu YL, et al. Cerebral small vessel disease burden is associated with accelerated poststroke cognitive decline: a 1-year follow-up study. J Geriatr Psychiatry Neurol. 2019;32(6):336-343. doi: 10.1177/0891988719862630 [DOI] [PubMed] [Google Scholar]
- 45.Egorova N, Liem F, Hachinski V, Brodtmann A. Predicted brain age after stroke. Front Aging Neurosci. 2019;11:348. doi: 10.3389/FNAGI.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffener J, Habeck C, O'Shea D, Razlighi Q, Bherer L, Stern Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging. 2016;40:138-144. doi: 10.1016/J.NEUROBIOLAGING.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly-Hayes M. Influence of age and health behaviors on stroke risk: lessons from longitudinal studies. J Am Geriatr Soc. 2010;58(SUPPL. 2):S325–S328. doi: 10.1111/J.1532-5415.2010.02915.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Q, Huang WQ, Ma QL, et al. Incidence and risk factors of leukoaraiosis from 4683 hospitalized patients: a cross-sectional study. Medicine. 2017;96(39):e7682. doi: 10.1097/md.0000000000007682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etherton MR, Wu O, Rost NS. Recent advances in leukoaraiosis: white matter structural integrity and functional outcomes after acute ischemic stroke. Curr Cardiol Rep. 2016;18(12):123. doi: 10.1007/s11886-016-0803-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The conditions of our ethics approval do not permit sharing of the raw MRI data supporting this study with any individual outside the author team under any circumstances. However, anonymized data not published within this article will be made available by request from any qualified investigator.