Abstract
Background and Objectives
Perfusion imaging can identify adult patients with salvageable brain tissue who would benefit from thrombectomy in later time windows. The feasibility of obtaining hyperacute perfusion sequences in pediatric stroke is unknown. The aim of this study was to determine whether contrast perfusion imaging delayed time to treatment and to assess perfusion profiles in children with large vessel occlusion stroke.
Methods
The Save ChildS retrospective cohort study (January 2000–December 2018) enrolled children (1 month–18 years) with stroke who underwent thrombectomy from 27 European and U.S. stroke centers. This secondary analysis included patients with anterior circulation occlusion and available imaging for direct review by the neuroimaging core laboratory. Between-group comparisons were performed using the Wilcoxon rank-sum exact test for continuous variables or Fisher exact test for binary variables. Given the small number of patients, evaluation of perfusion imaging parameters was performed descriptively only.
Results
Of 33 patients with available neuroimaging, 15 (45.4%) underwent perfusion (CT perfusion n = 6; MR perfusion n = 9); all were technically adequate. The median time from onset to recanalization did not differ between groups {4 hours (interquartile range [IQR] 4–7.5) perfusion+; 3.4 hours (IQR 2.5–6.5) perfusion-, p = 0.158}. Target mismatch criteria were met by 10/15 (66.7%) patients and did not correlate with reperfusion status or functional outcome. The hypoperfusion intensity ratio (HIR) was favorable in 11/15 patients and correlated with older age but not NIHSS, time to recanalization, or stroke etiology. Favorable HIR was associated with better functional outcome at 6 months (Pediatric Stroke Outcome Measure 1.0 [IQR 0.5–2.0] vs 2.0 [1.5–3.0], p = 0.026) and modified Rankin Scale 1.0 [0–1] vs 2.0 [1.5–3.5], p = 0.048) in this small sample.
Discussion
Automated perfusion imaging is feasible to obtain acutely in children and does not delay time to recanalization. Larger prospective studies are needed to determine biomarkers of favorable outcome in pediatric ischemic stroke and to establish core and penumbral thresholds in children.
Automated perfusion imaging provides real-time quantification of irreversibly infarcted stroke core and hypoperfused penumbral tissue in adults with acute stroke due to large vessel occlusion (LVO).1 Randomized trials have shown that the presence of a significant perfusion mismatch between core and penumbra can identify patients who stand to benefit from clot removal with mechanical thrombectomy.2 Perfusion imaging is now recommended as part of acute stroke triage in guidelines for adult patients presenting with LVO between 6-24 hours of last known well (LKW).3
Parameters defining core and penumbra have been optimized over a series of preliminary studies in adults. Relative cerebral blood flow (rCBF) <30% and apparent diffusion coefficient (ADC) < 620 × 10−6 mm2/s are accepted thresholds defining irreversibly infarcted tissue on CT perfusion and MR perfusion, respectively.4,5 Time to maximum tissue residue function (Tmax) >6 seconds on either CT or MR perfusion defines critically hypoperfused tissue doomed to infarct if not reperfused.6,7 Perfusion mismatch-based approaches hold promise in patient selection for late window thrombolysis and thrombectomy >24 hours.8,9 The stroke landscape is thus evolving from a one-size-fits-all time cutoff-based selection approach to a more individualized tissue-based approach, expanding the ability to offer reperfusion therapy to a substantial number of stroke victims who would otherwise have been denied.
Late window stroke therapy is a compelling opportunity for children with stroke, who often present beyond the previously accepted 4.5-hour intravenous (IV) alteplase or 6-hour thrombectomy time window.10,11 Thrombectomy is increasingly being performed in children up to and beyond 24 hours12; however, because thrombectomy trials excluded patients younger than 18 years, it is not yet known whether the same thresholds defining core and penumbra in adults are applicable to children. There is hesitancy to use perfusion techniques in pediatric patients given the concern for possible adverse effects, unfamiliarity with perfusion software, concern for potential time delay with added sequences, and lack of prospective pediatric trials showing benefit. We analyzed a subset of the retrospective, multicenter Save ChildS study cohort to assess feasibility of obtaining technically interpretable acute perfusion imaging in children with LVO and to assess and describe perfusion profiles for children undergoing mechanical thrombectomy.
Methods
This analysis used data from Save ChildS, a retrospective, observational, multicenter cohort study that enrolled children with stroke who underwent endovascular thrombectomy at 27 European and U.S. stroke centers between January 2000 and December 2018. Detailed inclusion and exclusion criteria and methods for Save ChildS have been published previously.13 Patients were included in this secondary analysis if they had either MR or CT perfusion imaging with contrast performed before thrombectomy that was available for central review. Posterior circulation vessel occlusions were excluded.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Ethics Committee of the University of Muenster for central data processing and by the ethics committees of the participating centers with local requirements. This study was performed in accordance with the Declaration of Helsinki. This study is registered within the German Clinical Trials Register (DRKS00016528) and is completed.
Imaging Transfer and Analysis
DICOM images were deidentified and either mailed on disk directly to the neuroimaging core laboratory or uploaded through a secure cloud-based server. Every image submitted by participating centers underwent central review by 2 neuroradiologists (M.W. and B.J.) with expertise in pediatric stroke imaging who were blinded to all clinical data.
Assessment of cerebrovascular collaterals, initial and final stroke volumes, and infarct growth has been previously published.14 In brief, baseline angiographic imaging was graded according to the binary Tan scale, where favorable collaterals demonstrate >50% filling of the vascular territory distal to the occluded artery and unfavorable collaterals demonstrate ≤50% filling.15 The Miteff score is a 3-point scale based on degree of postocclusion filling in relation to the Sylvian fissure.16 Early infarct growth rate was defined as the ratio of the absolute ischemic core volume over the number of hours from stroke onset to initial image, and absolute infarct growth was calculated by the difference between the final and initial stroke volumes.
For patients who underwent CT or MR perfusion imaging not using RAPID software, the source perfusion images were postprocessed using RAPID (iSchemaView, Mountain View, CA) to avoid heterogeneity. Automated values for penumbral Tmax were generated using >4, >6, >8, and >10 seconds threshold. Ischemic core was defined on MR perfusion by the volume of ADC <620 × 10−6 mm2/s lesion, and on CT perfusion by the volume of rCBF< 30%, in milliliters. Automated values for rCBF core at different thresholds were generated automatically by using RAPID software.
Mismatch volume, defined as the difference between the Tmax >6 and core volumes, and mismatch ratio, defined as the ratio of Tmax>6 volume to core volume, were generated automatically by the RAPID software and the hypoperfusion intensity ratio (HIR), defined as the ratio of [Tmax>10 volume]/[Tmax>6 volume] and correlated with Tan collateral scores. The Target Mismatch (TMM) profile was defined per DEFUSE 3 criteria: (1) ischemic core volume <70 mL, (2) mismatch volume >15 mL, and (3) mismatch ratio >1.7. Favorable HIR was defined as <0.4 per adult data, unfavorable HIR ≥0.4.17 Evaluation of perfusion thresholds was performed descriptively only.
Baseline Characteristics, Treatment, and Outcome Measures
Assessment of baseline characteristics, treatment details, and clinical outcome measures in the Save ChildS cohort has been previously reported.13 In brief, the pediatric National Institutes of Health Stroke Scale (pNIHSS) was assessed on admission, after 12–24 hours, and after 7 days. Recanalization rates were classified as complete, partial, or no recanalization by the responsible neuroradiologists according to the modified Treatment in Cerebral Infarction (mTICI) score.
Modified Rankin Scale (mRS) score and Pediatric Stroke Outcome Measure (PSOM)18 were assessed at discharge, 6 months, and 24 months poststroke. Given the small absolute number of cases and unclear missingness patterns, we performed complete case analyses for all outcomes and report number of missing values; for this analysis, there were no missing 6-month outcomes. Cause of stroke was assessed using the Childhood Arterial Ischemic Stroke Standardized Classification and Diagnostic Evaluation (CASCADE) classification.19
Statistical Analysis
Owing to the retrospective and feasibility-driven nature of this study, all analyses were conducted on an exploratory basis. Continuous variables were summarized as median (interquartile range [IQR]) and binary variables as counts (proportions). Between-group comparisons were performed using the Wilcoxon rank-sum exact test for continuous variables or Fisher exact test for binary variables. Given the small number of patients, evaluation of perfusion imaging parameters was performed descriptively only. Receiver operating characteristic (ROC) curve analysis was performed to assess the optimal Tmax/HIR correlation with collateral score. Youden J-statistic was calculated to determine the value with optimal sensitivity and specificity for favorable Tan collaterals over a range of HIR values. All computations were performed with R, version 3.6.2 (R Foundation for Statistical Analysis), or IBM SPSS Statistics 28. Findings were considered significant at p < 0.05, and all tests were 2-sided.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Perfusion Imaging Characteristics
Imaging was available for direct review in 33/73 (45.2%) patients. Of those, nearly half (n = 15, 45.5%) underwent perfusion imaging before endovascular treatment: CT perfusion was performed in 6 patients and MR perfusion in 9 patients. Of the 10 Save ChildS centers that contributed to direct imaging analysis, 8 had at least 1 patient who underwent perfusion imaging. Five patients underwent perfusion with RAPID software acutely; the remaining 10 were successfully postprocessed with RAPID software. All perfusion scans were technically adequate and free from significant artifact that might confound interpretation, and no sites reported complications with contrast administration. The median time from onset to recanalization was 4 hours (IQR 4–7.5) in the perfusion imaging group and 3.4 hours (IQR 2.5–6.5) in the nonperfusion group; the time from LKW to admission and recanalization did not differ between groups. In addition, there were no differences in baseline clinical or imaging characteristics between children who underwent perfusion compared with those who did not (Table 1).
Table 1.
Comparison of Perfusion Imaging+ Vs Perfusion Imaging- Patients
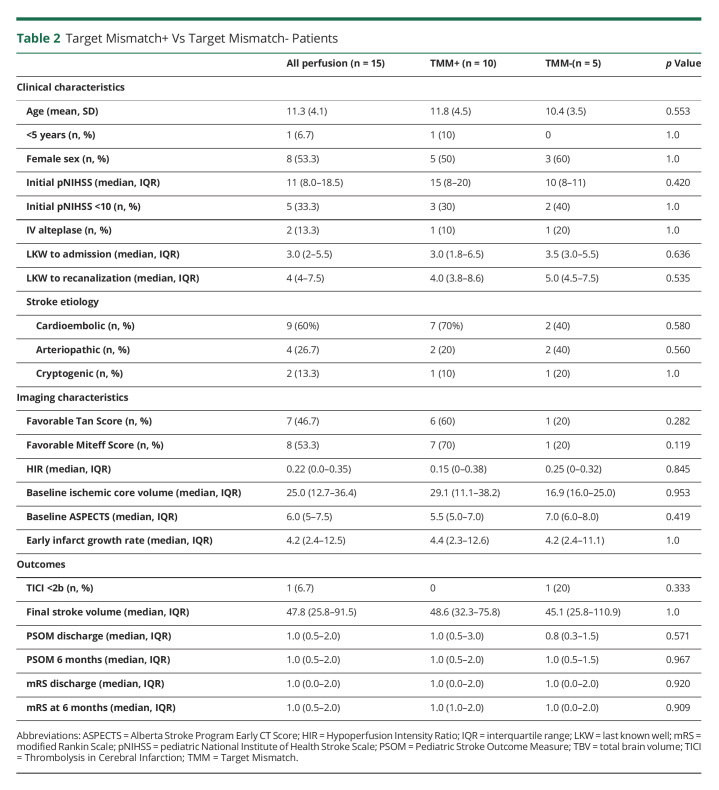
Overall, when defined by DEFUSE 3 criteria, 10/15 (66.7%) patients met TMM criteria on perfusion imaging. Of the 5 patients not meeting TMM criteria, 2 did not qualify because of large baseline core volumes (70 and 77 mL) and all 5 had an unfavorable mismatch ratio <1.4. Two additional patients would not have met DEFUSE 3 criteria because of a low pNIHSS score <6; one of those had an otherwise favorable TMM profile. TMM criteria were met in 2/4 patients treated >6 hours, compared with 6/9 patients treated <6 hours (Figure 1). When TMM was redefined as the ratio of [core volume]/[Tmax >4 volume], 4/5 became favorable. The one patient who remained TMM- by either Tmax>6 or Tmax>4 thresholds was unsuccessfully recanalized and reported mRS 3 at follow-up (Figure 2). There were no differences in baseline parameters between patients who met TMM or DEFUSE 3 criteria and those who did not. The presence of TMM did not correlate with mTICI score or functional outcome in this small sample (Table 2).
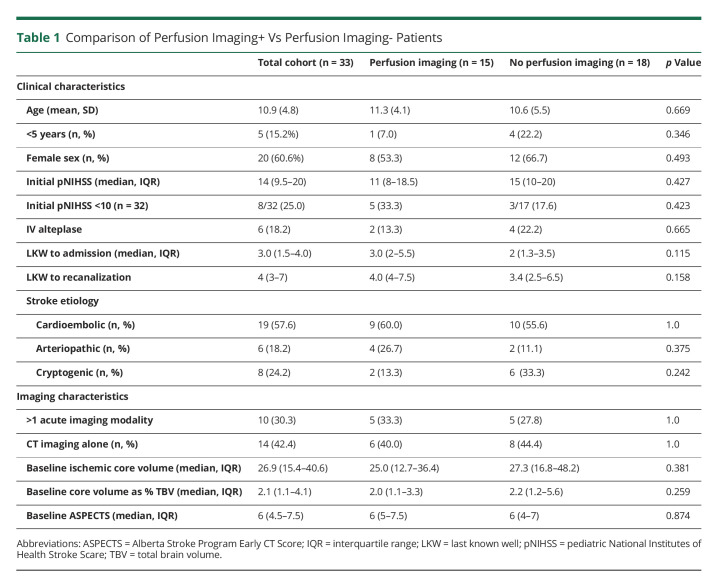
Figure 1. Example of a TMM+, Recanalized Patient.
(A) A 15-year-old boy presented 1.5 hours after last known well with NIHSS 25. Imaging notable for left M1 occlusion with a favorable mismatch ratio of 3.0. (B) Moderate-sided DWI lesion on initial imaging. (C) After TICI 2b reperfusion, final stroke volume seems similar to initial; mRS 0, PSOM 0 reported at follow-up. Abbreviations: DWI = diffusion-weighted imaging; mRS = modified Rankin Scale; TMM = target mismatch.
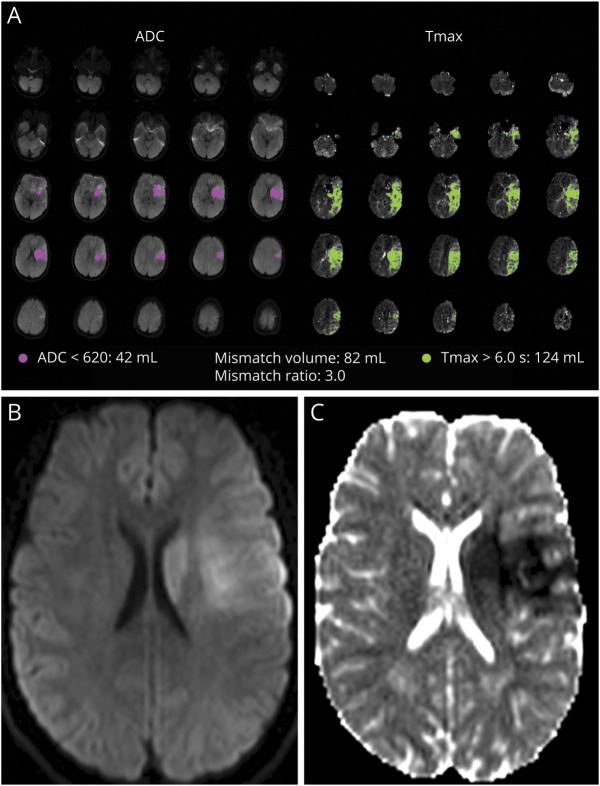
Figure 2. Example of a TMM-, Nonrecanalized Patient.
(A) An 8-year-old girl presented within 3.5 hours of last known well with right MCA-M1 occlusion, NIHSS 8. (B) TMM criteria were not met using either Tmax>6 or Tmax>4 penumbral thresholds. Core infarct of 29 mL defined by ADC< 620 × 10−6 mm2/s. (C) mTICI 1 (unsuccessful) reperfusion. (D) Final stroke volume was 25.6 mL (consistent with absent penumbra on initial RAPID maps); parenchymal hemorrhage (PH2) noted in the stroke bed. mRS 3 at 6-month follow-up. Abbreviations: ADC = apparent diffusion coefficient; mRS = modified Rankin Scale; mTICI = modified treatment in cerebral infarction; Tmax = time to maximum tissue residue function; TMM = target mismatch.
Table 2.
Target Mismatch+ Vs Target Mismatch- Patients
The median HIR for our perfusion cohort was 0.22 (IQR 0–0.35) and was favorable by adult standards (<0.4) in 11/15 (73.3%) patients. Favorable HIR was significantly associated with older age (mean 12.9 ± 3.2 vs 7.0 ± 3.3 p = 0.007) but did not correlate with initial pNIHSS, stroke etiology, or time to admission or recanalization; in fact, all 4 patients treated beyond 6 hours had HIR <0.4, compared with 7/9 patients treated within 6 hours. HIR did not correlate with favorable Tan collaterals in our analysis: 6/11 (54.5%) patients with favorable HIR had favorable Tan collaterals, as compared with 1/4 (25%) patients with unfavorable HIR. On the other hand, among patients with favorable Tan collaterals (n = 7), the median HIR was 0.0 (IQR 0–0.16) compared with 0.29 (IQR 0.15–0.51), p = 0.05. Our analysis found that favorable HIR was significantly associated with lower PSOM and mRS, and thus more favorable outcomes, at 6 months (Table 3). Examples of favorable and unfavorable HIR are shown in Figures 3 and 4.
Table 3.
Favorable Vs Unfavorable HIR (<0.4 vs ≥0.4)
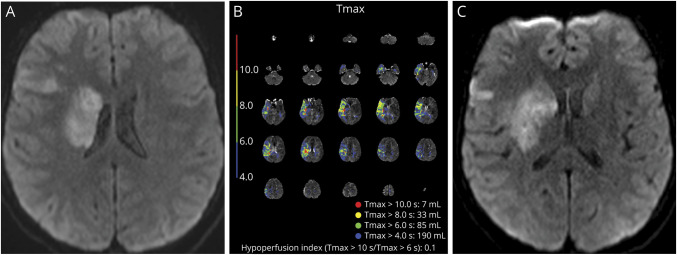
Figure 3. Example of a Patient With Favorable HIR.
(A) A 13-year-old presented with cardioembolic stroke 3 hours after last known well. Initial pNIHSS 8 and small stroke core with MR-DWI volume of 11 mL. (B) Favorable HIR 0.1 before thrombectomy, indicating good collateral status. (C) Limited stroke burden after successful thrombectomy with minimal infarct growth, calculated volume 21 mL. Abbreviations: DWI = diffusion-weighted imaging; HIR = hypoperfusion intensity ratio; pNIHSS = pediatric National Institutes of Health Stroke Scale.
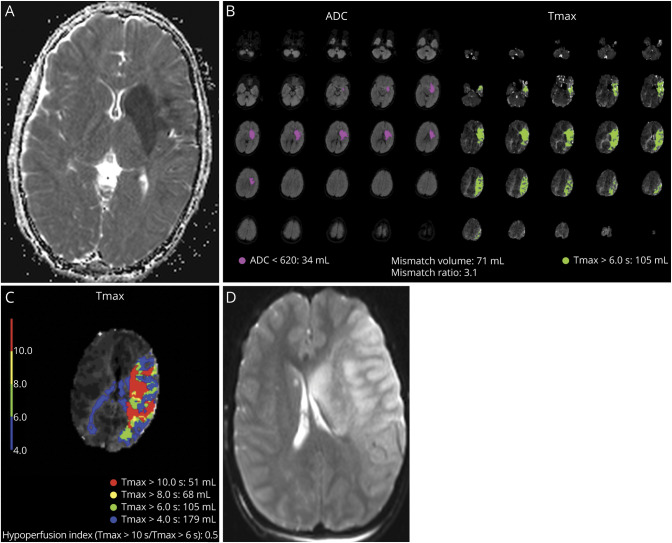
Figure 4. Example of a Patient With Unfavorable HIR.
(A) An 11-year-old boy presented with cardioembolic stroke and uncertain time of last known well. Initial pNIHSS 16 and MR-DWI volume of 29.1 mL. (B) Favorable perfusion profile on RAPID, with mismatch ratio 3.1. (C) Unfavorable HIR of 0.5 indicating poor collateral status. (D) Despite TICI 2c reperfusion, large final stroke volume 226.8 mL, final ASPECTS of 1. Abbreviations: DWI = diffusion-weighted imaging; HIR = hypoperfusion intensity ratio.
ROC curve analysis found that HIR ≤ 0.43 had the highest sensitivity (1.0) for favorable angiographic collaterals. The best combination of sensitivity and specificity based on Youden J-statistic was HIR ≤ 0.04, with a sensitivity of 0.71 and specificity of 0.88 (eFigure 1 and eTable 1, http://links.lww.com/WNL/C537). Of note, 6/15 cases had an HIR of 0.0, indicating excellent collaterals.
Discussion
Nearly half of our cohort underwent acute perfusion imaging; of those, 100% of images were technically interpretable, and times to admission and recanalization were not significantly longer compared with patients who did not undergo perfusion imaging. These findings confirm our hypothesis that perfusion sequences are feasible to obtain in the hyperacute setting and do not delay thrombectomy. Because adult guidelines now recommend the use of perfusion to help select patients for thrombectomy and because ongoing adult studies may soon extend the use of perfusion mismatch selection techniques to thrombolysis, prospective perfusion studies are urgently needed to determine the utility of these techniques in pediatric stroke. Arguably, understanding if and how perfusion imaging may inform treatment selection is of particular importance in children, given concerns for radiation and contrast exposure. Using reduced contrast protocols and performing studies at combined adult/pediatric hospitals already familiar with perfusion are potential ways to optimize feasibility and acceptability for future studies.
Although the numbers are too small to draw definitive conclusions, the presence of the TMM profile did not appear to correlate with outcomes after thrombectomy. Indeed, most children in the Save ChildS cohort had favorable outcome scores by both mRS and PSOM at follow-up. There are several possible reasons for this: First, cerebrovascular collaterals may significantly modify stroke outcomes in children with LVO (more so than in adults); second, our PSOMs may not be ideal to detect relevant differences; and finally, parameters defining irreversibly infarcted and critically hypoperfused tissue in children may differ from that in adults because of differences in the developing cerebrovascular physiology. A prior case series suggested that Tmax>4 rather than Tmax>6 seconds may be a more appropriate penumbral threshold for children because of more robust collaterals in younger patients.20 Of note, redefining penumbra by a Tmax > 4 seconds threshold increased the proportion of TMM+ patients to 14/15, and the one patient remaining TMM- did report poor outcome. However, a prospective study with an expanded cohort is warranted.
The cerebrovascular collateral circulation is an important determinant of acute ischemic stroke evolution. A prior analysis of the Save ChildS study found that favorable angiographic collaterals correlated with smaller initial and final stroke burden and slower early infarct growth rate in children undergoing thrombectomy.14 In adults, favorable collaterals are associated with greater likelihood of successful recanalization, smaller than expected final stroke size, and better clinical outcomes after reperfusion.21-23 The ESCAPE trial in adults used favorable CTA collaterals as an inclusion criterion for thrombectomy selection.24 HIR evaluates the ratio of severely delayed blood flow volume (Tmax>10) to delayed but potentially salvageable tissue (Tmax>6) within the stroke bed. Because HIR interrogates the ischemic region itself, it is considered a tissue-level marker of cerebral collateral microcirculation and may be more important to tissue fate than the visible filling of angiographic collaterals postocclusion on CTA.25,26 While over half of our cohort (8/15) had poor angiographic collaterals, by contrast, HIR was favorable in most patients; of interest, when HIR was defined as the ratio of Tmax>10/Tmax>4, all patients then qualified as favorable, suggesting that this ratio may not be an optimal marker of intrinsic collateral integrity. Reasons for the discrepancy between arterial and tissue-level collateral status in our cohort are unclear. While Tan score and HIR in adult patients has been strongly correlated, discrepancies have also been reported. Faizy et al.27 reported that patients with a mixed favorable/unfavorable “collateralome” appear to have worse radiographic and clinical outcomes than those with uniformly favorable collateral parameters.
Of interest, favorable HIR was associated with older age in our cohort. Although older children may present with more obvious stroke symptoms and thus come to medical attention earlier, notably, the time to presentation and recanalization did not differ between favorable and unfavorable HIR groups. Of the 4 patients with arteriopathic strokes who had perfusion imaging, all had favorable HIR; arteriopathic strokes may demonstrate a more favorable HIR by allowing more time for collaterals to develop in response to stenosis. However, the sample size of our cohort is too small to detect a statistical difference. Whether tissue-level response to ischemia is more robust in older children or in arteriopathic strokes remains an open question and requires further study.
We found that children with favorable HIR had more favorable PSOM and mRS scores at 6 months than those with unfavorable HIR, with no difference in baseline NIHSS, baseline ischemic core volume, or final stroke volume between groups. A previous study found that HIR also correlated with better functional outcome, controlling for size of the ischemic volume on diffusion-weighted imaging.26 While the number of patients included in our analysis is too small to draw definitive conclusions, the results suggest that tissue-level collaterals may be a relevant biomarker for favorable outcome after LVO stroke in children, and further investigation is warranted.
In addition to the small sample size, our study has several limitations. First, the retrospective design introduces the possibility of selection and ascertainment biases; however, we were able to mitigate the latter to some degree by using a central blinded neuroimaging core laboratory to perform all perfusion and imaging analyses. Moreover, the RAPID software generates automated calculations for mismatch ratio and volume, saving time and avoiding bias on the part of the interpreting provider. Second, the heterogeneity of imaging modalities obtained both acutely and in follow-up limits definitive conclusions about perfusion correlation with angiographic collateral score and perfusion parameters defining core and penumbra. Third, most patients in our study presented within 6 hours, making it difficult to draw comparisons with adult data where perfusion is most often used beyond 6 hours. Whether early window TMM- patients would still benefit from recanalization is controversial and requires further study. In addition, administration of IV tPA in the early window may affect treatment and outcome; that said, only 2/9 patients presenting <4.5 hours received IV tPA in our cohort, although the reasons for deferment are unknown. Finally, as discussed above, the definitions of favorable collaterals, TMM and HIR are based on adult studies, and it is not known whether the same definitions are relevant to children. Nevertheless, this study was intended to provide initial insights into perfusion profiles in pediatric thrombectomy patients with the goal of generating hypotheses for future investigation.
In conclusion, obtaining hyperacute perfusion imaging is technically feasible without delaying time to recanalization for children with stroke because of LVO. Most patients met TMM criteria and demonstrated a favorable HIR collateral profile, and favorable HIR correlated with better functional outcome in our cohort. Prospective, large-scale, multicenter studies are needed to confirm optimal core, penumbral, and TMM thresholds in children and determine the utility of perfusion imaging in pediatric reperfusion therapy selection.
Glossary
- ADC
apparent diffusion coefficient
- CASCADE
Childhood Arterial Ischemic Stroke Standardized Classification and Diagnostic Evaluation
- DWI
diffusion-weighted imaging
- HIR
hypoperfusion intensity ratio
- IV
intravenous
- IQR
interquartile range
- LKW
last known well
- LVO
large vessel occlusion
- mRS
modified Rankin Scale
- mTICI
modified Treatment in Cerebral Infarction
- pNIHSS
pediatric National Institutes of Health Stroke Scale
- PSOM
Pediatric Stroke Outcome Measure
- ROC
receiver-operating characteristic
- rCBF
relative cerebral blood flow
- Tmax
time to maximum tissue residue function
- TMM
Target Mismatch
Appendix. Authors
Footnotes
Editorial, page 501
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Demeestere J, Wouters A, Christensen S, Lemmens R, Lansberg MG. Review of perfusion imaging in acute ischemic stroke: from time to tissue. Stroke. 2020;51(3):1017-1024. doi: 10.1161/strokeaha.119.028337. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/nejmoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/str.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BC, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42(12):3435-3440. doi: 10.1161/strokeaha.111.618355. [DOI] [PubMed] [Google Scholar]
- 5.Purushotham A, Campbell BCV, Straka M, et al. Apparent diffusion coefficient threshold for delineation of ischemic core. Int J Stroke. 2015;10(3):348-353. doi: 10.1111/ijs.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivot JM, Mlynash M, Thijs VN, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40(2):469-475. doi: 10.1161/strokeaha.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BC, Christensen S, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43(10):2648-2653. doi: 10.1161/strokeaha.112.660548. [DOI] [PubMed] [Google Scholar]
- 8.Campbell BCV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4.5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019;394(10193):139-147. doi: 10.1016/S0140-6736(19)31053-0. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Campbell BC, Lansberg MG, et al. A Phase III, prospective, double-blind, randomized, placebo-controlled trial of thrombolysis in imaging-eligible, late-window patients to assess the efficacy and safety of tenecteplase (TIMELESS): rationale and design. Int J Stroke. 2022:17474930221088400. doi: 10.1177/17474930221088400. [DOI] [PubMed] [Google Scholar]
- 10.Rafay MF, Pontigon AM, Chiang J, et al. Delay to diagnosis in acute pediatric arterial ischemic stroke. Stroke. 2009;40(1):58-64. doi: 10.1161/strokeaha.108.519066. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan J, Miller SP, Phan TG, Mackay MT. Delayed recognition of initial stroke in children: need for increased awareness. Pediatrics. 2009;124(2):e227-e234. doi: 10.1542/peds.2008-3544. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia K, Kortman H, Blair C, et al. Mechanical thrombectomy in pediatric stroke: systematic review, individual patient data meta-analysis, and case series. J Neurosurg Pediatr. 2019:1-14. doi: 10.3171/2019.5.PEDS19126. [DOI] [PubMed] [Google Scholar]
- 13.Sporns PB, Strater R, Minnerup J, et al. Feasibility, safety, and outcome of endovascular recanalization in childhood stroke: the Save ChildS Study. JAMA Neurol. 2020;77(1):25. doi: 10.1001/jamaneurol.2019.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Jiang B, Wintermark M, et al. Cerebrovascular collateral integrity in pediatric large vessel occlusion: analysis of the Save ChildS Study. Neurology. 2021;98(4):e352-e363. doi: 10.1212/wnl.0000000000013081. [DOI] [PubMed] [Google Scholar]
- 15.Tan IY, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525-531. doi: 10.3174/ajnr.a1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132(8):2231-2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- 17.Guenego A, Fahed R, Albers GW, et al. Hypoperfusion intensity ratio correlates with angiographic collaterals in acute ischaemic stroke with M1 occlusion. Eur J Neurol. 2020;27(5):864-870. doi: 10.1111/ene.14181. [DOI] [PubMed] [Google Scholar]
- 18.Kitchen L, Westmacott R, Friefeld S, et al. The pediatric stroke outcome measure: a validation and reliability study. Stroke. 2012;43(6):1602-1608. doi: 10.1161/strokeaha.111.639583. [DOI] [PubMed] [Google Scholar]
- 19.Bernard TJ, Beslow LA, Manco-Johnson MJ, et al. Inter-rater reliability of the CASCADE criteria: challenges in classifying arteriopathies. Stroke. 2016;47(10):2443-2449. doi: 10.1161/strokeaha.116.013544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Heit JJ, Albers GW, et al. Neuroimaging selection for thrombectomy in pediatric stroke: a single-center experience. J NeuroInterv Surg. 2019;11(9):940-946. doi: 10.1136/neurintsurg-2019-014862. [DOI] [PubMed] [Google Scholar]
- 21.Bang OY, Saver JL, Buck BH, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2007;79(6):625-629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bang OY, Saver JL, Kim SJ, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42(3):693-699. doi: 10.1161/strokeaha.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab. 2020;40(10):1966-1974. doi: 10.1177/0271678x20918816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 25.Faizy TD, Heit JJ. Rethinking the collateral vasculature assessment in acute ischemic stroke: the comprehensive collateral cascade. Top Magn Reson Imaging. 2021;30(4):181-186. doi: 10.1097/rmr.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 26.Olivot JM, Mlynash M, Inoue M, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45(4):1018-1023. doi: 10.1161/strokeaha.113.003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faizy TD, Mlynash M, Kabiri R, et al. The cerebral collateral cascade: comprehensive blood flow in ischemic stroke. Neurology. 2022;98(23):e2296-e2306. doi: 10.1212/wnl.0000000000200340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.