Abstract
Background and Objectives
Sleep traits can have implications for ischemic stroke recovery in observational studies. The purpose of our present study was to explore the relationship between genetically predicted sleep traits and poststroke functional outcomes with Mendelian randomization (MR) method.
Methods
Instrumental variables for insomnia and sleep duration were adopted from genome-wide association studies data of European ancestry individuals. Summary data for functional outcome after ischemic stroke were retrieved from the Genetics of Ischemic Stroke Functional Outcome network. Inverse-variance weighted approach was adopted as the main analyses. Alternative MR approaches were used in sensitivity analyses. I2 and Q value statistics were used to appraise the heterogeneity among genetic variants.
Results
In univariable analysis, genetic liability to insomnia was significantly associated with worse functional outcome (modified Rankin Scale ≥3) after ischemic stroke (odds ratio [OR] = 1.30; 95% CI: 1.10–1.54, p = 0.002). Genetic liability to short sleep, long sleep, and continuous sleep duration were not associated with poststroke functional outcome (all p > 0.05). Sensitivity analyses without adjustment for stroke severity also supported that insomnia was causally associated with poor functional outcome (OR = 1.25; 95% CI: 1.08–1.44, p = 0.003). In the multivariable MR analysis adjusting for potentially confounding traits including body mass index, depression, type 2 diabetes, smoking, and alcohol consumption, the overall patterns between genetic liability to insomnia and poststroke outcome remained (all p < 0.05).
Discussion
This MR study supports potential adverse effects of liability to insomnia on functional outcome after ischemic stroke. Interventions that address insomnia may offer a therapeutic target to improve recovery after ischemic stroke and warrant exploration in a clinical context.
Unfavorable sleep habits have tremendous public health implications around the world. Over the past 2 decades, numerous studies have explored the association between sleep traits (e.g., insomnia and sleep duration) and risk of ischemic stroke.1,2 During a 10-year follow-up in the China Kadoorie Biobank (n = 487,200), insomnia symptoms were associated with a higher risk of ischemic stroke.1 In the Women's Health Initiative Observational Study (n = 93,175), both short and long sleep duration was related with increased risk of ischemic stroke.2 For functional outcomes after ischemic stroke, observational study also indicated that insomnia may negatively affect neurologic recovery and was associated with poor functional outcome of stroke.3 Given that the main evidences on sleep traits and functional outcome after ischemic stroke were from observational studies, the causality of these findings could not be fixed as reverse causation and residual confounding might bias the results. Furthermore, randomized controlled trials investigating this are unfeasible.
Mendelian randomization (MR) uses single nucleotide polymorphisms (SNPs) as instrumental variables for studying the effect of modifying an exposure (e.g., sleep traits), facilitating causal inference.4 The MR method has the potential to reduce residual confounding because SNPs are randomly assorted during conception and thus unlikely to be associated with environmental confounding factors. In addition, MR could also decrease the potential for reverse causation bias because the SNPs are fixed from conception.4,5 Therefore, if a SNP that modifies the liability to sleep traits is also associated with functional outcome after stroke, this would provide evidence to support that the sleep traits are of causal relevance in affecting poststroke outcomes. To our knowledge, the associations between sleep traits and poststroke outcome have not been evaluated using MR. Thus, we used MR framework to ascertain the causal associations of sleep traits with poststroke outcome.
Methods
Outcome Data Sources
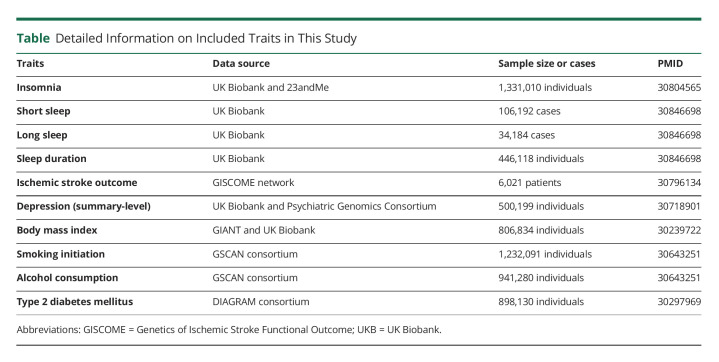
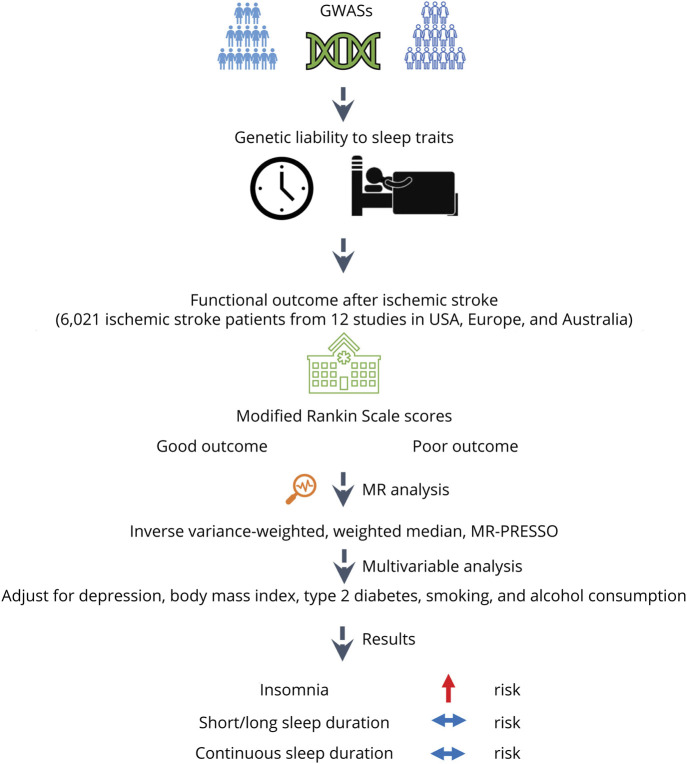
We obtained the summary data for functional outcome after ischemic stroke from the Genetics of Ischemic Stroke Functional Outcome (GISCOME) network (Table).6 The GISCOME network included 12 studies from the United States, Europe, and Australia (6,021 patients, Figure 1).6 All the participants were of European ancestry. The modified Rankin Scale (mRS) near 3 months after ischemic stroke was selected to evaluate functional outcome. Lower mRS score (0–2) represents better outcome, whereas higher score (3–6) represents worse outcome (Figure 1). The results were adjusted for age, sex, ancestry, and baseline stroke severity as evaluated by the NIH Stroke Scale (NIHSS) in the primary analysis. As a comparison, analysis without adjustment for baseline NIHSS were also performed.
Table.
Detailed Information on Included Traits in This Study
Figure 1. Study Design Overview.
GWAS = genome-wide association study; MR-PRESSO = Mendelian Randomization Pleiotropy Residual Sum and Outlier.
Genetic Instruments for Sleep Traits
Genetic variants associated with insomnia at p < 5 × 10−8 were obtained from a meta-analysis of GWASs in the UK Biobank (UKB) and 23andMe (n = 1,331,010 individuals of European ancestry, Table).7 The study identified 248 independent lead genetic variants, explaining 2.6% of the variance in insomnia.7 Insomnia was evaluated with a touchscreen device in the UKB and online surveys in 23andMe.7 The associations were adjusted for age, sex, genotype array, and 10 genetic principal components in the UKB and age, sex, and the top 5 principal components in 23andMe. To consider the potential for genetic confounding through traits such as depression, type 2 diabetes (T2D), body mass index (BMI), smoking, and alcohol consumption, we further disentangled the direct effect of insomnia on poststroke outcome by adjusting for these factors using the multivariable MR approach. Summary data for these factors were adopted from corresponding GWASs (eTables 1–5, links.lww.com/WNL/C552).8-11
Sleep duration was evaluated by inquiring the following: “About how many hours sleep do you get in every twenty four hours? (include naps)” in UKB (Table).12 Summary data for short sleep (≤6 hours; 106,192 cases/305,742 controls; 27 SNPs), continuous sleep duration (number of hours sleep; 446,118 participants; 78 SNPs), and long sleep (≥9 hours; 34,184 cases/305,742 controls; 8 SNPs) at p < 5 × 10−8 were available.12 The associations were adjusted for age, sex, genotyping array, 10 principal components of ancestry, and genetic correlation matrix. For continuous sleep duration, the results were scaled to per hour increase in sleep duration. In addition, genetic instruments for obstructive sleep apnea were used.13 The genetic instrument includes 5 SNPs associated with obstructive sleep apnea in the FinnGen Study (p < 5 × 10−8).13
Statistical Analysis
The inverse-variance weighted (IVW) method was adopted as the main statistical method. Sensitivity analyses, such as the weighted median, MR-Egger, and MR-PRESSO approaches, were performed to investigate the consistency of the results when making different assumptions on the inclusion of variants with pleiotropic effects that may be violating the requisite assumptions of the IVW MR model. The weighted median affords consistent MR estimates if >50% of weight are from valid SNPs.14 The MR-Egger can detect pleiotropy through its intercept and generate pleiotropy-corrected estimates.15 However, this method typically has less statistical power.15 The MR-PRESSO approach can discern outliers and produce estimates after removing such identified outliers.16
I2 and Q value was used to appraise the heterogeneity in MR estimates among SNPs. Associations with a p < 0.01 (0.05/5 exposures) were considered statistically significant. All tests were conducted with the MendelianRandomization17 and MR-PRESSO16 packages in R and the mrrobust package18 in Stata/MP.
Standard Protocol Approvals, Registrations, and Patient Consents
This study is based on publicly available data, and the informed consent and ethical review were acquired in all of the original studies. The study is reported following the STROBE-MR statement.19
Data Availability
The data adopted for the analysis are publicly available (eTables 1–5, links.lww.com/WNL/C552).
Results
Univariable MR Analyses
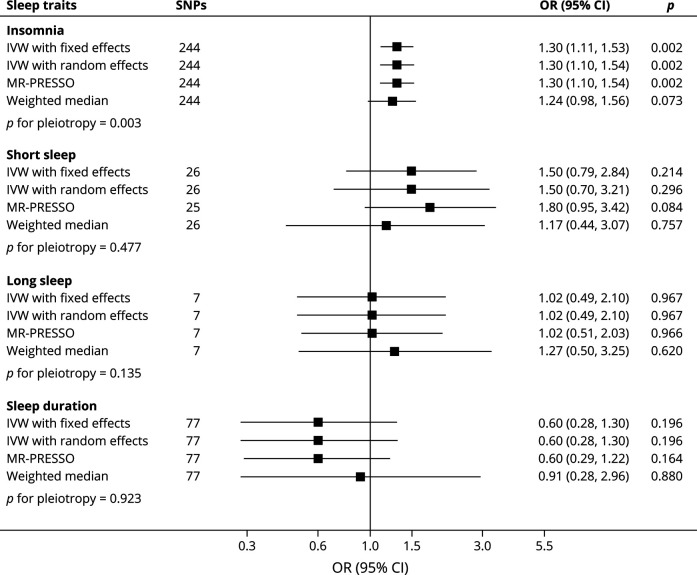
Genetic liability to insomnia was significantly associated with worse functional outcome (mRS ≥3) after ischemic stroke (random-effects IVW: odds ratio [OR] = 1.30; 95% CI: 1.10–1.54, p = 0.002; Figure 2), without evidence of marked heterogeneity in MR estimates among SNPs (I2 = 4.6%; p = 0.288). Similar results were observed in the MR-PRESSO and weighted median methods, although potentially with some bias attributable pleiotropy was observed (Figure 2). After correcting for pleiotropy in MR-Egger analysis, insomnia still showed a positive association with worse functional outcome after ischemic stroke (OR = 3.52; 95% CI: 1.79–6.92, p = 0.001).
Figure 2. Genetically Predicted Sleep Traits With Functional Outcome After Ischemic Stroke.
IVW = inverse-variance weighted; MR-PRESSO = Mendelian Randomization Pleiotropy Residual Sum and Outlier; SNPs = single nucleotide polymorphisms.
Genetic liability to short sleep, long sleep, and continuous sleep duration were not strongly associated with poststroke outcome (all p > 0.05, Figure 2), without evidence of significant heterogeneity in MR estimates among SNPs (all p > 0.05). MR-PRESSO and weighted median methods produced similar results (Figure 2). No evidence of directional pleiotropy was identified (all p > 0.05). Genetic liability to obstructive sleep apnea was also not significantly associated with worse functional outcome after ischemic stroke (OR = 1.18; 95% CI: 0.63–2.20, p = 0.613).
Sensitivity Analyses
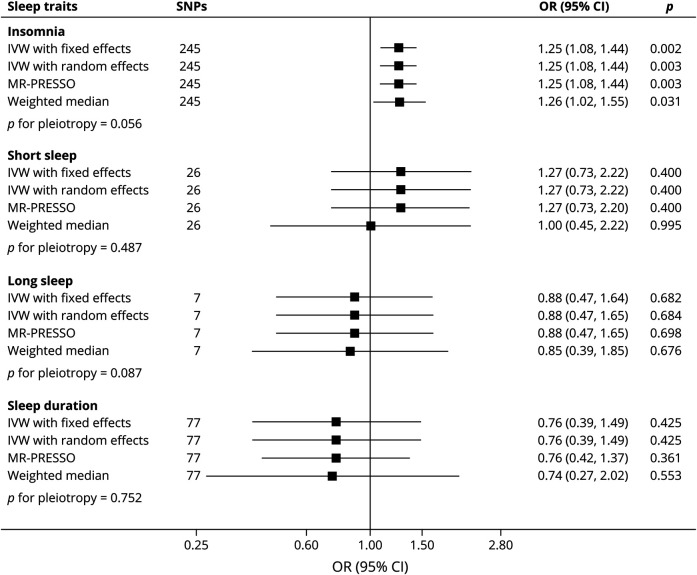
In sensitivity analyses, MR investigations using GISCOME GWAS data without adjustment for baseline NIHSS were performed. Genetic liability to insomnia was significantly related with poor functional outcome after ischemic stroke (random-effects IVW: OR = 1.25; 95% CI: 1.08–1.44, p = 0.003; Figure 3), without evidence of marked heterogeneity in MR estimates among SNPs (I2 = 2.2%; p = 0.393). Similar results were also observed in the MR-PRESSO (OR = 1.25; 95% CI: 1.08–1.44, p = 0.003) and weighted median method (OR = 1.26; 95% CI: 1.02–1.55, p = 0.031).
Figure 3. Genetically Predicted Sleep Traits With Functional Outcome After Ischemic Stroke in Models Without Adjustment for Baseline Stroke Severity.
IVW = inverse-variance weighted; MR-PRESSO = Mendelian Randomization Pleiotropy Residual Sum and Outlier; SNPs = single nucleotide polymorphisms.
Similarly to the main IVW MR analysis, genetic liability to short sleep, long sleep, and continuous sleep duration were also not related with poststroke outcome (all p > 0.05, Figure 3), without evidence of heterogeneity in MR estimates among SNPs (all p > 0.05). MR-PRESSO and weighted median methods also produced similar results (Figure 3).
Multivariable MR Analyses
We further performed multivariable MR analysis adjusting for the genetically predicted BMI, smoking, alcohol consumption, depression, and T2D. The overall patterns between genetic liability to insomnia and poststroke outcome was still significant when adjusting for the effect of genetically predicted BMI (OR = 1.24; 95% CI: 1.03–1.49, p = 0.022), T2D (OR = 1.20; 95% CI: 1.001–1.433, p = 0.049), depression (OR = 1.34; 95% CI: 1.04–1.72, p = 0.025), smoking initiation (OR = 1.31; 95% CI: 1.08–1.58, p = 0.006), and alcohol consumption (OR = 1.30; 95% CI: 1.10–1.54, p = 0.002), indicating that genetic correlation with these factors did not affect the result.
Discussion
Using the MR approach, we investigated the causal associations between sleep traits and functional outcome after ischemic stroke. Our results demonstrated that genetic liability to insomnia was causally associated with poor functional outcome after ischemic stroke. In addition, the association remained in multivariable analyses with adjustment for potential confounding factors. By comparison, genetically liability to short or long sleep, continuous sleep duration, and obstructive sleep apnea was not related with poststroke outcome.
Insomnia refers to the experience of patients who are not satisfied with sleep time and sleep quality, which may affect daytime social and intellectual function. Common features of insomnia include difficulty falling asleep, decreased sleep quality and duration, and decreased memory and concentration. Moreover, the results of traditional observational studies on insomnia and stroke outcome may also be subjected to confounding and reverse causation. In this MR study, we found that genetic liability to insomnia was significantly associated with poor functional outcome after ischemic stroke.
The detailed biological mechanisms underlying the relationship between insomnia and worse functional outcome after ischemic stroke are not fully understood. Insomnia is closely correlated with smoking, which is a risk factor for poor stroke functional outcome. In a multicenter stroke registry, smoking is related with higher risk of adverse outcomes at 90 days after ischemic stroke.20 In addition, the raised blood pressures in patients with insomnia might also partly explain the causal association between insomnia and poor functional outcome.21 High blood pressure is common after ischemic stroke and associated with poor functional outcome.22
This study has some shortcomings. First, it is noteworthy that GISCOME database did not have outcome data for different subtypes of stroke. Data regarding subtypes, extension, and localization of stroke decisively influences functional recovery in stroke survivors. The lack of these data may be an important source of bias. Thus, the relationship between sleep traits on poststroke outcome among different stroke subtypes is still unknown and needs further investigations. Second, our results might also be influenced by collider bias23 because previous MR study has indicated that genetic liability to insomnia was associated with an increased risk of ischemic stroke,24 and recovery after disease is conditional on first having had the disease. Third, we used SNPs to assess risk of insomnia as a proxy for liability to insomnia. Therefore, our results may not necessarily suggest that insomnia itself is a cause of poor stroke outcome. We cannot exclude that there may be other pathways resulting in insomnia that cause poor stroke outcome. Fourth, the diagnosis of sleep traits is made by questionnaire and may be subjective. In the future, objective measures such as polysomnography, sleep logs, and actigraphy should be used to assess the sleep traits, which could lead to better understanding of the association between sleep traits and functional outcome after ischemic stroke. Finally, this study only included subjects of European descent to reduce population stratification bias. However, the population confinement may also restrict the generalizability of our results to other populations. Thus, the associations between sleep traits and ischemic stroke outcome need to be further explored in other population groups.
In conclusion, this MR study strengthens the evidence for insomnia being a causal risk factor for functional outcome after ischemic stroke. Interventions that address insomnia may offer a therapeutic target to improve recovery after ischemic stroke and warrant exploration in a clinical context.
Acknowledgment
We thank the UK Biobank, 23andMe, GISCOME network, and ISGC Cerebrovascular Disease Knowledge Portal for providing summary statistics data for the analyses.
Glossary
- BMI
body mass index
- GISCOME
Genetics of Ischemic Stroke Functional Outcome
- IVW
inverse-variance weighted
- MR
Mendelian randomization
- mRS
modified Rankin Scale
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- SNPs
single nucleotide polymorphisms
- T2D
type 2 diabetes
- UKB
UK Biobank
Appendix. Authors
Study Funding
The authors report no targeted funding.
Disclosure
Z.Z. Zhang reports no disclosures relevant to the manuscript; M.M. Wang reports no disclosures relevant to the manuscript; D. Gill is employed parttime by Novo Nordisk; W.S. Zhu reports no disclosures relevant to the manuscript; X.F. Liu reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Zheng B, Yu C, Lv J, et al. Insomnia symptoms and risk of cardiovascular diseases among 0.5 million adults: a 10-year cohort. Neurology. 2019;93(23):e2110-e2120. doi: 10.1212/wnl.0000000000008581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008(12);39:3185-3192. doi: 10.1016/s1073-5437(09)79164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matas A, Amaral L, Patto AV. Is post-ischemic stroke insomnia related to a negative functional and cognitive outcome? Sleep Med. 2022;94:1-7. doi: 10.1016/j.sleep.2022.03.022 [DOI] [PubMed] [Google Scholar]
- 4.Davey Smith G, Holmes MV, Davies NM, Ebrahim S. Mendel's laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur J Epidemiol. 2020;35(2):99-111. doi: 10.1007/s10654-020-00622-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davey Smith G, Ebrahim S. Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1-22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 6.Soderholm M, Pedersen A, Lorentzen E, et al. Genome-wide association meta-analysis of functional outcome after ischemic stroke. Neurology. 2019;92(12):e1271-e1283. doi: 10.1212/wnl.0000000000007138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen PR, Watanabe K, Stringer S, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394-403. doi: 10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 8.Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343-352. doi: 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237-244. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulit SL, Stoneman C, Morris AP, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28(1):166-174. doi: 10.1093/hmg/ddy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505-1513. doi: 10.1038/s41588-018-0241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dashti HS, Jones SE, Wood AR, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2019;10(1):1100. doi: 10.1038/s41467-019-08917-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strausz S, Ruotsalainen S, Ollila HM, et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur Respir J. 2021;57(5):2003091. doi: 10.1183/13993003.03091-2020 [DOI] [PubMed] [Google Scholar]
- 14.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yavorska OO, Burgess S. Mendelian Randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiller W, Davies NM, Palmer TM. Software application profile: mrrobust—a tool for performing two-sample summary Mendelian randomization analyses. Int J Epidemiol. 2018;48(3):684-690. doi: 10.1093/ije/dyy195 [DOI] [Google Scholar]
- 19.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614-1621. doi: 10.1001/jama.2021.18236 [DOI] [PubMed] [Google Scholar]
- 20.Matsuo R, Ago T, Kiyuna F, et al. Smoking status and functional outcomes after acute ischemic stroke. Stroke. 2020;51(3):846-852. doi: 10.1161/strokeaha.119.027230 [DOI] [PubMed] [Google Scholar]
- 21.Jarrin DC, Alvaro PK, Bouchard MA, Jarrin SD, Drake CL, Morin CM. Insomnia and hypertension: a systematic review. Sleep Med Rev. 2018;41:3-38. doi: 10.1016/j.smrv.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 22.Bath PM, Song L, Silva GS, et al. Blood pressure management for ischemic stroke in the first 24 hours. Stroke 2022;53(4):1074-1084. doi: 10.1161/strokeaha.121.036143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paternoster L, Tilling K, Davey Smith G. Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet. 2017;13(10):e1006944. doi: 10.1371/journal.pgen.1006944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson SC, Markus HS. Genetic liability to insomnia and cardiovascular disease risk. Circulation 2019;140(9):796-798. doi: 10.1161/circulationaha.119.041830 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data adopted for the analysis are publicly available (eTables 1–5, links.lww.com/WNL/C552).