Abstract
Background and Objectives
Although the diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is based on serum MOG antibodies (MOG-Abs) positivity, patients with coexisting or restricted MOG-Abs in the CSF have been reported. The aim of this study is to characterize the relevance of CSF MOG-Abs positivity in clinical practice.
Methods
Eleven medical centers retrospectively collected clinical and laboratory data of adult and pediatric patients with suspected inflammatory CNS disease and MOG-Abs positivity in serum and/or CSF using live cell-based assays. Comparisons were performed using parametric or nonparametric tests, as appropriate. Potential factors of unfavorable outcomes were explored by Cox proportional hazard models and logistic regression.
Results
The cohort included 255 patients: 139 (55%) women and 132 (52%) children (i.e., <18-year-old). Among them, 145 patients (56.8%) had MOG-Abs in both serum and CSF (MOG-Abs seropositive and CSF positive), 79 (31%) only in serum (MOG-Abs seropositive and CSF negative), and 31 (12%) only in CSF (MOG-Abs seronegative and CSF positive). MOG-Abs seronegative and CSF positive predominated in adults (22% vs 3% of children), presented more commonly with motor (n = 14, 45%) and sensory symptoms (n = 13, 42%), and all but 4 (2 multiple sclerosis, 1 polyradiculoneuritis, and 1 Susac syndrome) had a final diagnosis compatible with MOGAD. When comparing seropositive patients according to MOG-Abs CSF status, MOG-Abs seropositive and CSF positive patients had a higher Expanded Disability Status Scale (EDSS) at nadir during the index event (median 4.5, interquartile range [IQR] 3.0–7.5 vs 3.0, IQR 2.0–6.8, p = 0.007) and presented more commonly with sensory (45.5% vs 24%, p = 0.002), motor (33.6% vs 19%, p = 0.021), and sphincter symptoms (26.9% vs 7.8%, p = 0.001) than MOG-Abs seropositive and CSF negative. At the last follow-up, MOG-Abs seropositive and CSF positive cases had more often persistent sphincter dysfunction (17.3% vs 4.3%, p = 0.008). Compared with seropositive patients, those MOG-Abs seronegative and CSF positive had higher disability at the last follow-up (p ≤ 0.001), and MOG-Abs seronegative and CSF positive status were independently associated with an EDSS ≥3.0.
Discussion
Paired serum and CSF MOG-Abs positivity are common in MOGAD and are associated with a more severe clinical presentation. CSF-only MOG-Abs positivity can occur in patients with a phenotype suggestive of MOGAD and is associated with a worse outcome. Taken together, these data suggest a clinical interest in assessing CSF MOG-Abs in patients with a phenotype suggestive of MOGAD, regardless of the MOG-Abs serostatus.
Myelin oligodendrocyte glycoprotein (MOG) has been identified as a target of circulating serum antibodies (Abs) in patients with a distinctive demyelinating CNS condition named MOG antibodies (MOG-Abs)-associated disease (MOGAD). The development of highly sensitive live cell-based assays (CBAs), displaying a very good interassay agreement,1 has allowed the clinical-MRI spectrum of this disorder to be defined and differentiated from multiple sclerosis (MS) and aquaporin-4-antibody-positive neuromyelitis optica spectrum disorder (NMOSD).2 MOGAD can have a monophasic or relapsing course, manifesting most often with optic neuritis and/or myelitis in adults and acute disseminated encephalomyelitis (ADEM) or optic neuritis in children. Non-ADEM encephalitis, brainstem, or cerebellar syndromes can also occur.3-15 The prognosis is usually good, but moderate-severe disability has been reported, emphasizing the need to identify predictors of long-term outcome.16-18
Currently, a diagnosis of MOGAD requires the presence of MOG-Abs in the serum of patients with a compatible clinical-MRI phenotype.2,3,19 Accordingly, MOG-Abs are thought to be primarily produced in the periphery and may mediate their pathogenic effect in the CNS after crossing the blood–brain barrier in concomitance with T cell activation.20-23 However, recent studies reported paired serum and CSF positivity in 41%–61% of patients23,24 and also some cases with isolated CSF MOG-Abs positivity,7,25-28 suggesting intrathecal MOG-Abs production. Isolated CSF MOG-Abs cases are rare and have clinical and pathologic findings similar to seropositive patients. However, there are some reports of CSF isolated positivity in patients with MS in both adults and in children.7,27
The aim of our study was to evaluate the frequency and the clinical utility of CSF MOG-Abs positivity in both adults and children with suspected inflammatory CNS disorders.
Methods
Study Subjects
This study includes patients with suspected inflammatory demyelinating diseases of the CNS and MOG-Abs positivity in serum and/or CSF retrospectively enrolled from 11 centers (Italy, Spain, France, Austria, Germany, Switzerland, Australia, and the United States, eTable 1, links.lww.com/WNL/C527). Only patients with available paired serum and CSF obtained within a month interval from each other were included.
To determine the specificity of CSF-only MOG-Abs in relation to MOGAD, a control group of patients with a final diagnosis of MS according to the updated diagnostic criteria29 and with available paired serum and CSF samples was also included.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was part of the research protocol approved by the Ethics Committees of the enrolling centers: prog. 1052CESC Verona-Rovigo approved by the Ethics Committee of Verona University Hospital (Italy), COOLIN-BRAIN CER-VD—approval number: 2018-01622 for Lausanne University Hospital (Switzerland), EK 1123/2015 for the Medical University of Vienna (Austria), AN4095 approved by the Ethics Committee of the Medical University of Innsbruck (Austria), 12/CHW/295 approved by the human research ethics committee at the Sydney Children's Hospitals Network (Australia), protocol approved by the institutional review board at Mayo Clinic College of Medicine, Rochester (MN, USA), PR(AG)398/2020 for Cemcat, Barcelona (Spain), and HCB/2014/0297 approved by the Ethic Committee of the Hospital Clinic of Barcelona (Spain). Samples from the Hospital Clinic of Barcelona are deposited in the registered biobank of Institut d'Investigació Biomèdica August Pi I Sunyer (IDIBAPS). Informed consent for storage and use of these samples for research purposes was obtained from all patients.
MOG-Abs Testing
Serum and CSF samples were tested for MOG-Abs through live CBA quantified by either flow cytometry (FACS) or microscopic visual score evaluation in immunofluorescence in the reference laboratory for each recruiting center. When a live CBA was not available, a reference center (the Neuropathology and Neuroimmunology Laboratory, University of Verona, Italy) performed the analysis. MOG-Abs positivity in serum and CSF was defined according to the cut-off previously established in each reference laboratory (eTable 1, links.lww.com/WNL/C527). Patients were classified according to paired serum/CSF MOG-Abs results into (1) isolated CSF positive (MOG-Abs seronegative and CSF positive), (2) isolated serum positive (MOG-Abs seropositive and CSF negative), and (3) paired serum and CSF positive (MOG-Abs seropositive and CSF positive). All MOG-Abs seronegative and CSF positive samples were independently retested in a blinded manner in a second expert laboratory (Neurologic Research Laboratory, Medical University of Innsbruck, Austria) for confirmation.1
Demographic and Clinical Information
Clinical and paraclinical data were retrospectively collected in a dedicated database by different referring physicians from the involved centers. Information comprised (1) demographic data (sex and age at onset, defining 2 groups: adults ≥18-year-old and children <18-year-old); (2) dates of different clinical episodes; (3) visual acuity (collected through the Snellen Chart, in case of bilateral visual loss the value of the worst eye was considered) and disability at nadir of the clinical episode and at the last follow-up (Expanded Disability Status Scale [EDSS], or Pyramidal Functional System Score); (4) CSF information (protein concentration, cell count, oligoclonal bands presence, and IgG index); (5) acute treatment including intravenous corticosteroids, plasma exchange, and intravenous immunoglobulins (IVIg); and (6) maintenance therapy including azathioprine, mycophenolate mofetil, rituximab, other MS-disease-modifying drugs, and other treatments (cyclophosphamide, methotrexate, mitoxantrone, IVIG, tocilizumab).
A clinical attack was defined as the occurrence of new symptoms or exacerbation of existing symptoms persisting for at least 24 hours in the absence of fever and infection.
Statistical Analysis
For descriptive statistics, quantitative variables are expressed as median (interquartile ranges [IQRs]) or mean (SD) and categorical variables as percentages. For group comparisons (MOG-Abs seropositive and CSF positive/MOG-Abs seropositive and CSF negative/MOG-Abs seronegative and CSF positive), parametric (t-test or χ2) or nonparametric (Kruskal-Wallis, Wilcoxon, or exact Fisher) tests were performed, as appropriate.
To evaluate time to first relapse and the risk of disability MOG-Abs seropositive and CSF positive or MOG-Abs seropositive and CSF negative patients were both considered seropositive and compared with MOG-Abs seronegative and CSF positive cases. First, to evaluate time to first relapse, a Kaplan-Meier curve was performed. Second, to assess disability (defined as reaching EDSS ≥3.0 at the last follow-up), univariate binary logistic regression models were performed according to baseline covariates (age at onset, sex, disability at onset measured by EDSS, oligoclonal bands, and CSF pleocytosis), and treatment received over the follow-up. Variables resulting from the univariate analysis with a p-value ≤ 0.20 were included in a multivariate binary regression model. The model was adjusted by time of follow-up. The results were expressed as odds ratio (OR) with 95% of CI. A p-value of 0.05 was considered statistically significant. All statistical analyses were performed with STATA-12 software (64-bit, StataCorpi, College Station, TX).
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Demographic Data and Cohort Subdivision
The study included 255 patients: 139 (54.5%) were women, median age at onset was 16 years old [IQR 6–39], and 132 (51.8%) were pediatric patients. Most patients were tested within 3 months from onset or relapse (69.6% and 11.3%, respectively), and 59.6% received acute treatment before sampling. Among them, 145 cases (56.8%) were MOG-Abs seropositive and CSF positive, 79 (31%) MOG-Abs seropositive and CSF negative, and 31 (12.2%) MOG-Abs seronegative and CSF positive. MOG-Abs seronegative and CSF positive status was more common in adults than in children (27/123, 22% vs 4/132, 3.1%, p < 0.001), and MOG-Abs seronegative and CSF positive cases were older at onset (median age 32, IQR [19.0–50.0 vs 13 [IQR 5.3–36]) in comparison to seropositive patients (p ≤ 0.001). None in the consecutive control cohort of 90 adult patients with MS referred to the Verona Neurology Unit tested positive for MOG-Abs in serum or CSF.
Description of MOG-Abs Seronegative and CSF Positive Cases
MOG-Abs were detected in CSF only in 31 patients, with no discordant results on confirmatory analysis. Of these, 20 (64.5%) were women, median age at onset was 32 years old [IQR 19–47], and 4 (12.9%) were children. Detailed clinical and paraclinical data are reported in Table 1, whereas representative radiologic findings are described in Figure 1. At the index event, patients presented more commonly with motor (n = 14, 45.2%) and sensory symptoms (n = 13, 41.9%), suggestive for myelitis or visual symptoms (n = 10, 32.5%), with a median EDSS at nadir of 3.5 [IQR 3.0–5.0]. None of adult patients displayed MRI findings suggestive of MS. On CSF analysis, 19 (61.3%) patients displayed pleocytosis (>5 white blood cells) and 14 (51.9%) had CSF restricted oligoclonal bands. After a median follow-up of 9 months [IQR 2–18], 10 (32.3%) patients experienced relapses. Median EDSS at the last evaluation was 2.0 [IQR 1.0–4.0], but 13 (41.9%) patients had an EDSS ≥3.0, and 8/29 (27.6%) patients had residual bladder/bowel dysfunction.
Table 1.
Demographic and Clinical Data of CSF-Only MOG-Abs Positive Patients
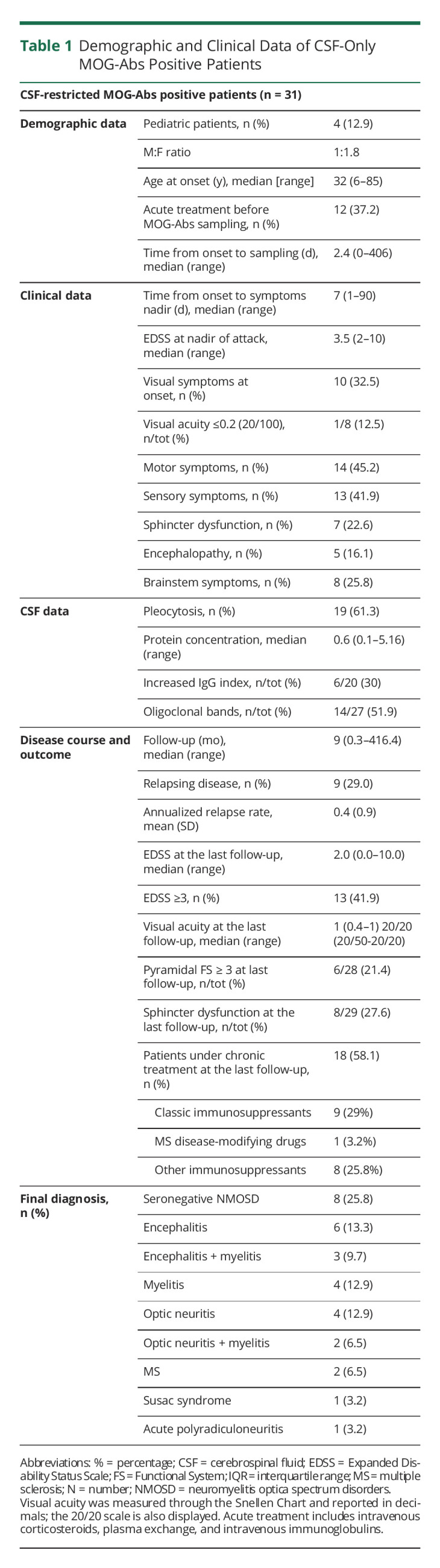
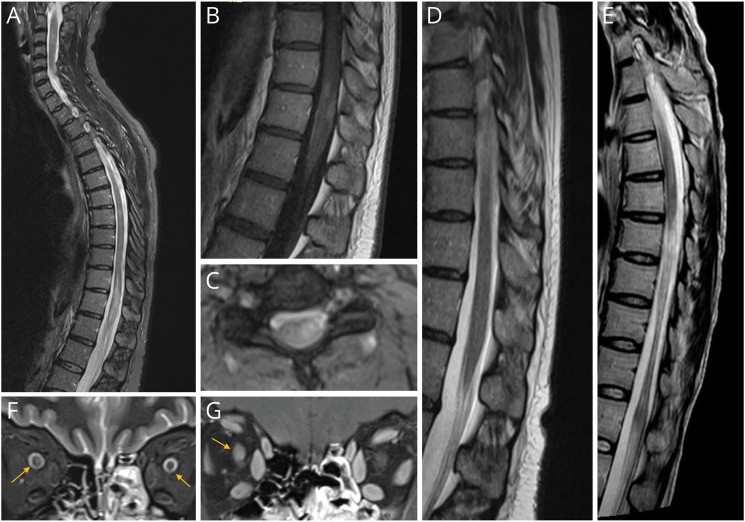
Figure 1. Radiologic Findings of MOG-Abs Seronegative and CSF Positive Patients.
Spinal cord MRI displayed short T2-hyperintense lesions in the cervical and thoracic spinal cord (C2-C3, C6, T8) and a longitudinally extensive lesion (T11-L1) involving conus medullaris (A) that had patchy postcontrast enhancement (B). Transversal section on the cervical spinal cord showed T2-hyperintensity, more evident on the left (C). The follow-up MRI performed 7 months after the clinical episode and intravenous steroids treatment showed an almost complete normalization (D). A follow up MRI of a MOG-Abs seronegative and CSF positive patient who had a LETM showing marked spinal cord atrophy of the thoracic segment (E). Orbital MRI of a patient with bilateral optic neuritis showed bilateral anterior optic nerve thickening (F) and postcontrast enhancement (bilateral, left optic nerve enhancement not shown, G).
The final diagnoses were compatible with MOGAD in 27/31 cases (87.1%). Phenotypes at the last follow-up were seronegative NMOSD (n = 8, 25.8%), encephalitis (n = 9, 23.0%, in 3 cases in association with myelitis), isolated optic neuritis (n = 4, 12.9%), isolated myelitis (n = 4, 12.9%), and combined optic neuritis and myelitis (n = 2, 6.5%). %). In addition, 1 adult had Susac syndrome (3.2%), 1 pediatric patient acute polyradiculoneuritis (n = 1, 3.2%), and 2 pediatric patients with MS (n = 2, 6.5%).
Comparison of Seropositive Patients According to MOG-Abs CSF Status (MOG-Abs Seropositive and CSF Negative/MOG-Abs Seropositive and CSF Positive)
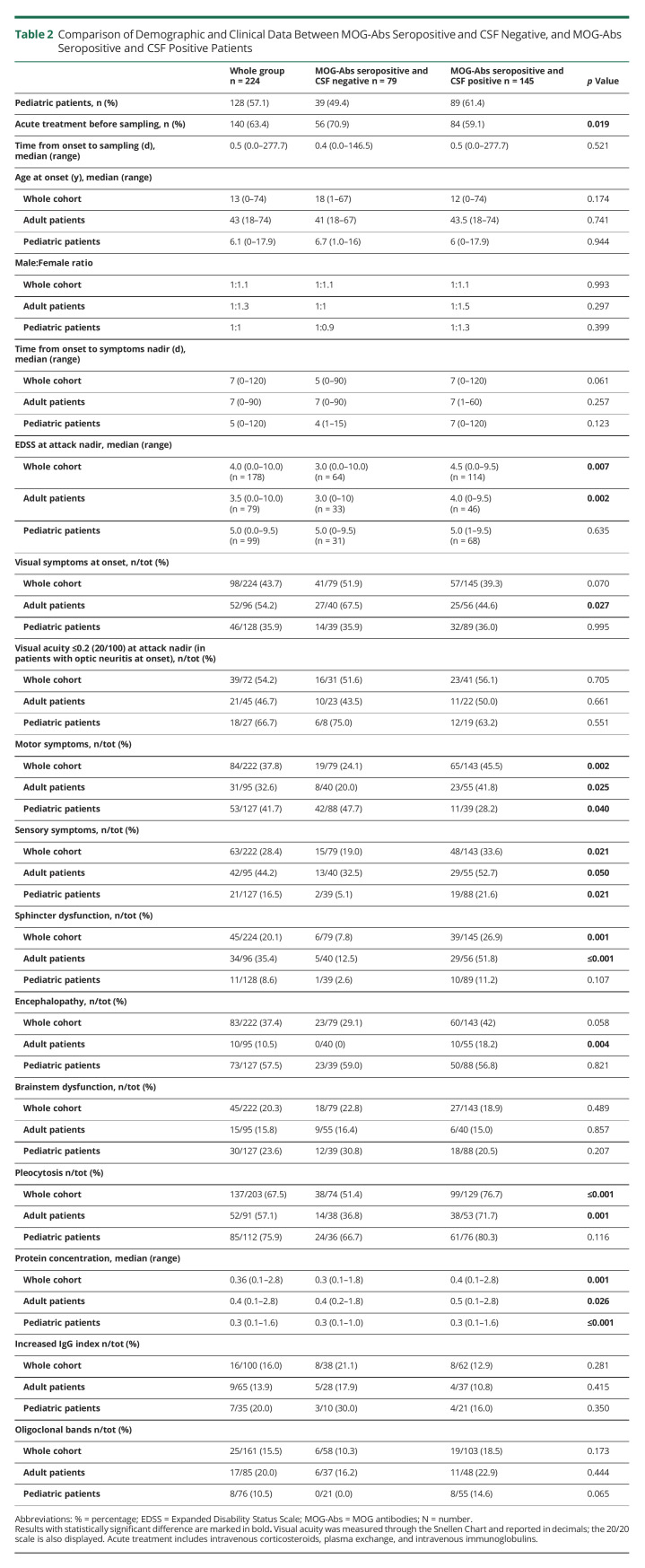
Among 224 MOG-Abs seropositive patients, 119 (53.1%) were women, median age at onset was 13 years old [IQR 5.3–36], and 128 cases (57.1%) were children. Of these, 145 (64.7%) patients were MOG-Abs seropositive and CSF positive and 79 (35.3%) MOG-Abs seropositive and CSF negative, being MOG-Abs seropositive and CSF positive the most common profile in both adults and children. Acute treatment before sampling was more frequently administered in MOG-Abs seropositive and CSF negative (70.9% vs 59.1%). When comparing demographic data between MOG-Abs seropositive and CSF negative and MOG-Abs seropositive and CSF positive patients, no significant differences emerged (for more details see Table 2).
Table 2.
Comparison of Demographic and Clinical Data Between MOG-Abs Seropositive and CSF Negative, and MOG-Abs Seropositive and CSF Positive Patients
Compared with MOG-Abs seropositive and CSF negative cases, MOG-Abs seropositive and CSF positive patients had a more severe disability at nadir during their index event (p = 0.007) and more commonly motor and sensory symptoms (p = 0.002 and 0.021, respectively) consistent with myelitis. The whole MOG-Abs seropositive and CSF positive group, and in particular adults, presented more commonly with sphincter dysfunction (p = 0.001 and p ≤ 0.001, respectively). MOG-Abs seropositive and CSF positive adults also had less commonly visual symptoms (p = 0.027) and more commonly encephalopathy (p = 0.004). On CSF analysis, MOG-Abs seropositive and CSF positive subjects showed an increased protein concentration (p = 0.001) and CSF cell count (p ≤ 0.001), whereas no difference emerged for IgG index and oligoclonal bands (Table 2).
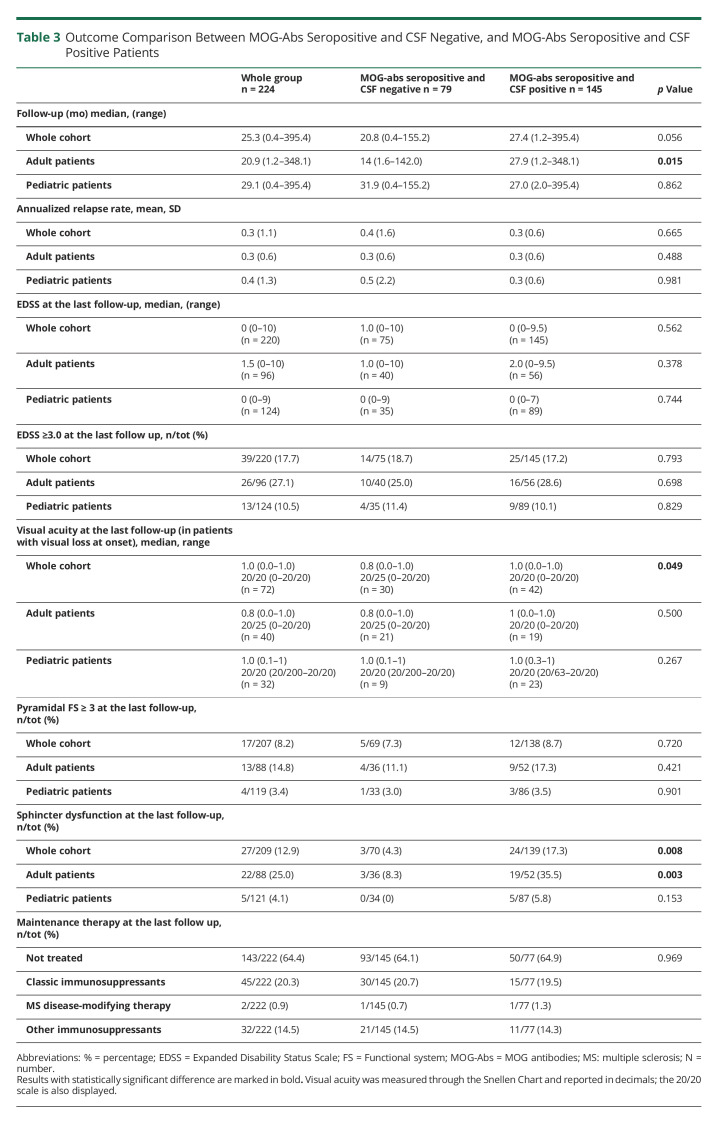
At the last follow-up (median 25.3 months [IQR 9.9–59.3]), MOG-Abs seropositive and CSF negative cases showed worse visual acuity (p = 0.049), and MOG-Abs seropositive and CSF positive patients had more commonly persistent sphincter dysfunction (p = 0.008). However, no difference emerged in annualized relapse rate (ARR) and final EDSS ≥3.0, Table 3. On univariate and multivariate analyses, the presence of CSF MOG-Abs was not related with increased relapse risk or EDSS≥3.0 at the last follow-up (eTable 2, links.lww.com/WNL/C527).
Table 3.
Outcome Comparison Between MOG-Abs Seropositive and CSF Negative, and MOG-Abs Seropositive and CSF Positive Patients
Disease Course Comparison Between Seropositive (MOG-Abs Seropositive and CSF Negative, and MOG-Abs Seropositive and CSF Positive) and Seronegative (MOG-Abs Seronegative and CSF Positive) Patients
Mean (SD) ARR was 0.3 (1.2), with no differences between the 2 groups. Median EDSS at the last follow-up was higher in MOG-Abs seronegative and CSF positive patients (2.0, [IQR 1.0–4.0]) compared with seropositive patients (0, [IQR 0–2.0], p ≤ 0.001). EDSS ≥3.0 and sphincter dysfunction were more frequently observed in MOG-Abs seronegative and CSF positive patients than in seropositive patients (21.4% vs 8.2%, p = 0.027 and 27.6% vs 12.9%, p = 0.037, respectively). Visual acuity at the last follow-up did not differ between the 2 groups (for more details see eTable 3, links.lww.com/WNL/C527).
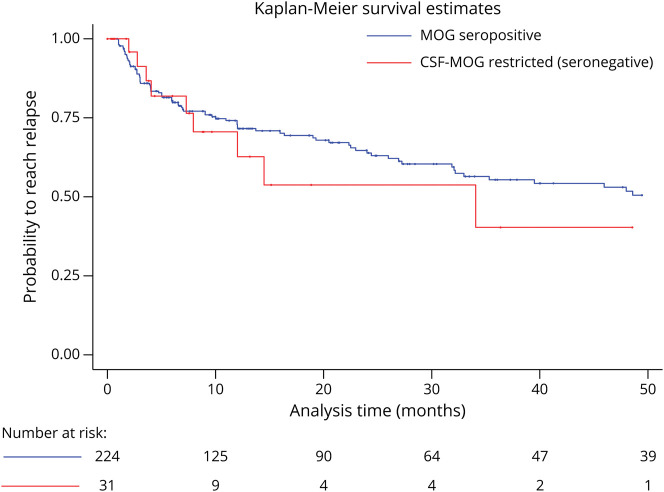
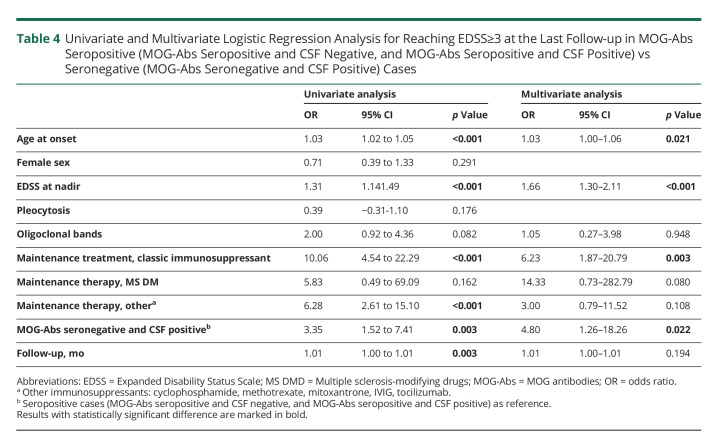
When assessing time to relapse with the Kaplan-Meier analysis, no difference was observed (Figure 2). The univariate analysis showed that MOG-Abs seronegative and CSF positive status (OR 3.35; 95% CI 1.52–7.41), older age at disease onset (OR 1.03; 95% CI 1.02–1.05), higher EDSS at nadir (OR 1.31; 95% CI 1.14–1.49), longer follow-up (OR 1.01; 95% CI 1.00–1.01), administration of classical immunosuppressants (OR 10.06; 95% CI 4.53–22.29), and other immunosuppressants (OR 6.28; 95% CI 2.61–15.10) were related to a higher risk of reaching an EDSS ≥3.0 at the last follow-up. The multivariate analysis showed that being MOG-Abs seronegative and CSF positive was an independent risk factor for reaching an EDSS ≥3.0 (OR 4.80; 95% CI 1.26–18.26). Age at onset (OR 1.03; 95% CI 1.00–1.06), EDSS at nadir (OR 1.66; 95% CI 1.30–2.11), and therapy with classical immunosuppressants (OR 6.23; 95%CI 1.87–20.79) were also independent risk factors for reaching an EDSS ≥3.0 (Table 4).
Figure 2. Kaplan-Meier Analysis Estimation of Time to Reach a First Relapse Between MOG Abs Seropositive (MOG-Abs Seropositive and CSF Negative, and MOG-Abs Seropositive and CSF Positive) and CSF MOGAbs Restricted (MOG-Abs Seronegative and CSF Positive) Patients.
Median Time to Reach the First Relapse was 6.6 months (Range 1.0–177.0) for seropositive patients and 7.3 months (Range 2.0–34.1) for seronegative patients (p = 0.970). Abbreviation: MOG-Abs = myelin oligodendrocyte glycoprotein antibodies.
Table 4.
Univariate and Multivariate Logistic Regression Analysis for Reaching EDSS≥3 at the Last Follow-up in MOG-Abs Seropositive (MOG-Abs Seropositive and CSF Negative, and MOG-Abs Seropositive and CSF Positive) vs Seronegative (MOG-Abs Seronegative and CSF Positive) Cases
Discussion
In this retrospective, multicenter study analyzing paired serum and CSF MOG-Abs in a large cohort of adult and pediatric patients, we observed that (1) CSF-restricted MOG-Abs can be found (12.2% in our cohort), particularly in adults with a phenotype suggestive of MOGAD; (2) paired serum and CSF MOG-Abs positivity occurs in more than half (56.8%) of MOGAD patients; (3) among MOG-Abs seropositive cases, patients with a paired CSF positivity have a more severe clinical presentation, more frequently symptoms compatible with myelitis, and displayed more commonly CSF pleocytosis and increased protein content; (4) compared with seropositive patients, MOG-Abs seronegative and CSF positive cases have higher risk of reaching EDSS 3.0, in particular in relation to symptoms compatible with myelitis, but display the same relapse rate.
The pathophysiology of MOGAD is not fully elucidated. The hypothesis is that an unknown trigger might elicit an immune response in the periphery with the subsequent production of MOG-Abs. The presence of intrathecal CSF MOG-Abs is thought to derive from the passive transfer of antibodies through a damaged blood–brain barrier.23,30 However, the description of cases with CSF-restricted MOG-Abs,24,27,28 which is herein confirmed, questions this model.
A possible explanation could be that activated peripheral B and T cells cross the blood–brain barrier early in the disease in a subgroup of patients, thus generating an immune response and antibody production within the CNS compartment. The pathogenic role of intrathecal plasma cells is well established in other CNS inflammatory diseases, such as MS31 or AQP4-Abs-seropositive NMOSD, where a fraction of CSF antibodies is produced by intrathecal B cells.32,33 Similarly, in other antibody-mediated CNS disorders such as anti-NMDAR encephalitis, intrathecal antibody production has a relevant pathogenic role and is associated with disease activity.34-38 In addition, MOG-Abs intrathecal synthesis has recently been reported in different groups of MOG-Abs seropositive and seronegative patients, supported by an increased CSF/serum MOG-IgG index in the former group and by the absence of serum MOG-Abs in the latter.26,27 Accordingly, CSF oligoclonal bands were detected in more than half of patients with CSF-restricted MOG-Abs, in agreement with previously data.27 Although oligoclonal bands generally support MS diagnosis, several studies have shown that they can be detected in a broad spectrum of neurologic diseases, including autoimmune encephalitis and other inflammatory diseases.39 Further studies are needed to assess the significance and possible persistence over time of oligoclonal bands in patients with CSF-restricted MOG-Abs. Finally, the few available pathologic studies of patients with CSF isolated MOG-Abs showed a neuropathologic phenotype compatible with MOGAD, with a minority of B cells and plasma-cells detected in the perivascular space.27,28 Taken together, these findings favor the occurrence of MOG-Abs intrathecal synthesis in a subgroup of patients.
Among patients with CSF-restricted MOG-Abs, the most common manifestations in our cohort were encephalopathy and myelitis, in accordance with a recent study.27 Of note, the clinical phenotype of MOG-Abs seronegative and CSF positive adults was compatible with MOGAD, but 2 pediatric patients had a diagnosis of MS. This finding was not unexpected because low titer MOG-Abs have already been reported in both serum and CSF of patients with MS, particularly in children,7 and serum MOG-Abs have been observed in 1% of patients with other neurologic diseases.40,41 In a recent study analyzing CSF and serum MOG-Abs in 105 patients with MS, 2 were positive in the CSF.42 Consequently, MOG-Abs CSF positivity has to be interpreted according to the clinical context and only cases with a compatible phenotype should be analyzed, as already recommended for serum MOG-Abs testing.2
Our results also confirm that CSF MOG-Abs are common in MOG-Abs seropositive patients, in agreement with previous data showing paired serum and CSF MOG-Abs positivity in 50%–70% of MOGAD.7,23,24,27 These findings are consistent with previous observations on AQP4-Abs-seropositive NMOSD, where CSF AQP4-Abs can be detected in 57%–68% of cases.23,30
According to our data, testing MOG-Abs in CSF might be of relevance also in seropositive patients and could help to identify severe cases presenting with myelitis or encephalitis. The relationship between the certain clinical phenotypes and the presence of MOG-Abs within the CSF observed in this study reinforces previous data.27 In other antibody-mediated disorders, as those associated with CASPR2-Abs, a similar phenomenon has been described, with the presence of CSF-Abs associated with limbic encephalitis and the presence of serum-Abs with neuromyotonia or Morvan syndrome.43 This observation might be explained by the susceptibility of the optic nerve to serum MOG-Abs because of the presence of a leakage in the blood–brain barrier at the level of the optic disc.44 Another proposed explanation is linked to the one-way flow from the intracranial subarachnoid space to the orbital subarachnoid space, which makes the identification of CSF antibodies in patients with isolated optic neuritis difficult.45
Of note, CSF-restricted MOG-Abs positive patients present an increased risk of reaching an EDSS 3.0 at the follow-up when compared with seropositive patients. This could reflect the association between CSF only MOG-Abs and the presence of transverse myelitis with the related tissue damage mediated by intrathecal antibody synthesis. Our results did not display any difference in relapse risk between MOG-Abs seronegative and CSF positive and seropositive patients, indicating that CSF MOG-Abs positivity does not predict a relapsing disease course.
Our study has limitations, particularly related to the retrospective design. This study included tertiary centers with a potential referral bias. Although live CBA methodology is optimal for MOG-Abs detection, this is often only available at reference centers.1,46 This could explain the higher frequency of MOG-Abs seronegative and CSF positive patients observed in our study in comparison with previous reports.24,27 Considering the wider availability of fixed CBAs, future studies comparing live and fixed assays should be performed to apply our results on a larger scale.
Moreover, because the referring laboratories used different techniques to quantify MOG-Abs (e.g., live cell-based flow cytometry assay vs live cell-based immunofluorescence assay), we were not able to perform a proper comparison between MOG-Abs titers. Of note, the inclusion of patients with paired CSF sample available potentially selected cases with a more severe phenotype, which might influence the generalization of our results. In addition, the recent introduction of MOG-Abs CSF testing might have affected the characteristics of this cohort and, in particular, the short follow-up, which might have influenced our results. In addition, data regarding MOG-Abs CSF presence in patients with other immunologic and noninflammatory disorders are scarce with few available data related to their presence and titers and should be expanded in future larger cohorts.27 Another limitation is that we did not include all consecutive patients with demyelinating diseases referred to the participating centers. For this reason, this study could not evaluate the sensitivity and specificity of MOG-Abs CSF testing.
Finally, this study was not designed to evaluate treatment efficacy and treatment was included in the analysis to mitigate bias. Even if in our analysis reaching an EDSS of 3 was associated with the administration of any/some treatments, this result should be interpreted cautiously because more disabled patients probably received more frequently immunosuppressive treatments.
In conclusion, despite the fact that few MOG-Abs seronegative and CSF positive cases did not display a clear MOGAD phenotype, our results support the relevance of MOG-Abs CSF analysis in the clinical practice, which can support, in addition to clinical and radiologic findings, the identification of patients with a more severe clinical phenotype. Future prospective multicenter studies will help to further clarify and expand our findings.
Glossary
- Abs
antibodies
- ADEM
acute disseminated encephalomyelitis
- ARR
annualized relapse rate
- CBA
cell-based assays
- CNS
central nervous system
- CI
confidence interval
- DMD
disease-modifying drug
- EDSS
Expanded Disability Status Scale
- IF
immunofluorescence
- IQR
interquartile range
- MOG
myelin oligodendrocyte glycoprotein
- MOG-Abs
MOG antibodies
- MOGAD
myelin oligodendrocyte glycoprotein antibody associated disease
- MS
multiple sclerosis
- NMOSD
neuromyelitis optica spectrum disorder
- OR
odds ratio
Appendix. Authors
Footnotes
Editorial, page 497
CME Course: NPub.org/cmelist
Study Funding
This work was supported in part by grants from Plan Nacional de I + D + I and cofinanced by the ISCIII – Subdirección General de Evaluación y Formento de la Investigación Sanitaria and the Fondo Europeo de Desarrollo Regional (ISCIII-FEDER; PI21/00,316 to TA); Fundació Marató de TV3 (37/C/2021, TA and AS), Torrons Vicens Foundation (PFNR0144 to TA); 2021 Invest AEP Grant to TA (PI047351), and 2019 Invest-AEP Support to GO from Pediatric Spanish Society. Instituto de Salud Carlos III, Spain (JR19/00,007, AC-C and JR17/00,012, EM-H). Red Española de Esclerosis múltiple (REEM) (RD16/0015/0002 to Albert Saiz). This work was also supported by a grant from Instituto de Salud Carlos III, Spain; (JR19/00,007 to Álvaro Cobo Calvo).
Disclosure
T. Armangué received speaker honoraria from Biogen, Sanofi-Aventis, and Novartis not related to this manuscript; A. Saiz reports compensation for consulting services and speaker honoraria from Merck-Serono, Biogen-Idec, Sanofi-Aventis, Teva Pharmaceutical Industries Ltd, Novartis, Roche, Alexion, and Janssen; C. Lechner has served as a consultant for Roche but has no conflict of interest with this manuscript; I. Ayzenberg served on scientific advisory boards for Roche, Merck, and Alexion and received research support from Diamed, none related to this article; M. Sepulveda has received speaker honoraria from Biogen and Roche Pharma; E. Martínez-Hernández has received speaker honoraria from Biogen; G. Arrambide has received speaking honoraria and compensation for consulting services or participation in advisory boards from Sanofi, Merck, and Roche; travel expenses for scientific meetings from Novartis, Roche, Stendhal, and ECTRIMS; is editor for Europe of the Multiple Sclerosis Journal – Experimental, Translational, and Clinical; and is a member of the International Women in Multiple Sclerosis (iWiMS) network executive committee. F. Brilot has received research funding from the National Health and Medical Research Council (Australia), Multiple Sclerosis Research Australia, NSW Health, Novartis, and the University of Sydney. She has received speaker honoraria from Novartis, Biogen, Merck, and Limbic Neurology, has been on advisory boards for Merck and Novartis. and is a member of the International Women in Multiple Sclerosis (iWiMS) network executive committee. S. Ramanathan has received research funding from the National Health and Medical Research Council (Australia), the Brain Foundation (Australia), the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Neil Hamilton Fairley Early Career Fellowship (APP1141169). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for Biogen, EXCEMED, and Limbic Neurology. S. Ferrari received support for attending scientific meetings by Shire, Sanofi-Genzyme, Roche, and Euroimmun; E. P. Flanagan has served on advisory boards for Alexion, Genentech, and Horizon Therapeutics. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. He was a site primary investigator in a randomized clinical trial on inebilizumab in neuromyelitis optica spectrum disorder run by MedImmune/Viela-Bio/Horizon Therapeutics. He has received funding from the NIH (R01NS113828). He is a member of the medical advisory board of the MOG project. He is an editorial board member of the Journal of the Neurologic Sciences and Neuroimmunology Reports. A patent has been submitted on DACH1-IgG as a biomarker of paraneoplastic autoimmunity. M. Reindl is supported by research grants from the Austrian Science Fund (FWF project P32699), the Austrian Research Promotion Agency, Euroimmun, and Roche; consulting fees and advisory board from Roche (to institution) and payments for antibody assays (MOG, AQP4, and other autoantibodies) to institution (University Hospital and Medical University of Innsbruck, Austria). R. Marignier serves on scientific advisory board for Alexion, Horizon Therapeutics, Roche, and UCB; has received honoraria from Alexion, Biogen, Merck, Novartis, and Roche. S. Mariotto received support for attending scientific meetings by Merck and Euroimmun and received speaker honoraria from Biogen. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Reindl M, Schanda K, Woodhall M, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e674. doi: 10.1212/nxi.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarius S, Paul F, Aktas O, et al. Mog encephalomyelitis: International recommendations on diagnosis and antibody testing. J Neuroinflamm. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89-102. doi: 10.1038/s41582-018-0112-x [DOI] [PubMed] [Google Scholar]
- 4.Cobo-Calvo A, Vukusic S, Marignier R. Clinical spectrum of central nervous system myelin oligodendrocyte glycoprotein autoimmunity in adults. Curr Opin Neurol. 2019;32(3):459-466. doi: 10.1097/wco.0000000000000681 [DOI] [PubMed] [Google Scholar]
- 5.Cobo-Calvo A, Ruiz A, Rollot F, et al. Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody–associated disease. Ann Neurol. 2021;89(1):30-41. doi: 10.1002/ana.25909 [DOI] [PubMed] [Google Scholar]
- 6.Wegener-Panzer A, Cleaveland R, Wendel EM, et al. Clinical and imaging features of children with autoimmune encephalitis and MOG antibodies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e731. doi: 10.1212/nxi.0000000000000731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armangue T, Olivé-Cirera G, Martínez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies : a multicentre observational study. Lancet Neurol. 2020;19(3):234-246. doi: 10.1016/S1474-4422(19)30488-0 [DOI] [PubMed] [Google Scholar]
- 8.Waters P, Fadda G, Woodhall M, et al. Serial anti–myelin oligodendrocyte glycoprotein antibody analyses and outcomes in children with demyelinating syndromes. JAMA Neurol. 2020;77(1):82-93. doi: 10.1001/jamaneurol.2019.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease : a UK study. Brain 2017;140(12):3128-3138. doi: 10.1093/brain/awx276 [DOI] [PubMed] [Google Scholar]
- 10.Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody–associated disease. JAMA Neurol. 2018;75(4):478-487. doi: 10.1001/jamaneurol.2017.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brilot F, Dale RC, Selter RC, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. 2009;66(6):833-842. doi: 10.1002/ana.21916 [DOI] [PubMed] [Google Scholar]
- 12.Mariotto S, Ferrari S, Monaco S, et al. Clinical spectrum and IgG subclass analysis of anti - myelin oligodendrocyte glycoprotein antibody - associated syndromes: a multicenter study. J Neurol. 2017;264(12):2420-2430. doi: 10.1007/s00415-017-8635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumann M, Hennes EM, Schanda K, et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22(14):1821-1829. doi: 10.1177/1352458516631038 [DOI] [PubMed] [Google Scholar]
- 14.Sepúlveda M, Armangue T, Martinez-Hernandez E, et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol. 2016;263(7):1349-1360. doi: 10.1007/s00415-016-8147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e322. doi: 10.1212/nxi.0000000000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobo-Calvo A, Ruiz A, Maillart E, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: the MOGADOR study. Neurology. 2018;90(21):e1858-e1869. doi: 10.1212/wnl.0000000000005560 [DOI] [PubMed] [Google Scholar]
- 17.Shor N, Deschamps R, Cobo Calvo A, et al. MRI characteristics of MOG-Ab associated disease in adults: an update. Revue Neurol. 2021;177(1-2):39-50. doi: 10.1016/j.neurol.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Chiriboga AS, Sechi E, Buciuc M, et al. Long-term outcomes in patients with myelin oligodendrocyte glycoprotein immunoglobulin G-associated disorder. JAMA Neurol. 2020;77(12):1575-1577. doi: 10.1001/jamaneurol.2020.3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Chiriboga AS, Majed M, Fryer J, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol. 2018;75(11):1355-1363. doi: 10.1001/jamaneurol.2018.1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer MC, Meinl E. Glycoproteins as targets of autoantibodies in CNS inflammation: MOG and more. Ther Adv Neurol Disord. 2012;5(3):147-159. doi: 10.1177/1756285611433772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spadaro M, Winklmeier S, Beltrán E, et al. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann Neurol. 2018;84(2):315-328. doi: 10.1002/ana.25291 [DOI] [PubMed] [Google Scholar]
- 22.Tea F, Lopez JA, Ramanathan S, et al. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol Commun. 2019;7(1):145. doi: 10.1186/s40478-019-0786-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflamm. 2016;13(1):279. doi: 10.1186/s12974-016-0717-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pace S, Orrell M, Woodhall M, et al. Frequency of MOG- IgG in cerebrospinal fluid versus serum. J Neurol Neurosurg Psychiatry. 2021;93(3):334-335. doi: 10.1136/jnnp-2021-326779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariotto S, Gajofatto A, Batzu L, et al. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology. 2019;93(20):e1867–e1872. doi: 10.1212/wnl.0000000000008479 [DOI] [PubMed] [Google Scholar]
- 26.Akaishi T, Takahashi T, Misu T, et al. Difference in the source of anti-AQP4-IgG and anti-MOG-IgG antibodies in CSF in patients with neuromyelitis optica spectrum disorder. Neurology. 2021;97(1):e1-e12. doi: 10.1212/wnl.0000000000012175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon YN, Kim B, Kim J-S, et al. Myelin oligodendrocyte glycoprotein-immunoglobulin G in the CSF clinical implication of testing and association with disability. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1095. doi: 10.1212/nxi.0000000000001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carta S, Höftberger R, Bolzan A, et al. Antibodies to MOG in CSF only : pathological findings support the diagnostic value. Acta Neuropathol. 2021;141(5):801-804. doi: 10.1007/s00401-021-02286-3 [DOI] [PubMed] [Google Scholar]
- 29.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/s1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 30.Jarius S, Pellkofer H, Siebert N, et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 1 : results from 163 lumbar punctures in 100 adult patients. J Neuroinflamm. 2020;17(1):261. doi: 10.1186/s12974-020-01824-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabatino JJ, Probstel A-K, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20(12):728-745. doi: 10.1038/s41583-019-0233-2 [DOI] [PubMed] [Google Scholar]
- 32.Kowarik MC, Dzieciatkowska M, Wemlinger S, et al. The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system-specific B cell populations. J Neuroinflamm. 2015;12:1-8. doi: 10.1186/s12974-015-0240-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowarik MC, Astling D, Gasperi C, et al. CNS Aquaporin-4-specific B cells connect with multiple B-cell compartments in neuromyelitis optica spectrum disorder. Ann Clin Transl Neurol. 2017;4(6):369-380. doi: 10.1002/acn3.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis : case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098. doi: 10.1016/s1474-4422(08)70224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malviya M, Barman S, Golombeck KS, et al. NMDAR encephalitis: passive transfer from man to mouse by a recombinant antibody. Ann Clin Transl Neurol. 2017;4(11):768-783. doi: 10.1002/acn3.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourahoui A, De Seze J, Guttierez R, et al. CSF isoelectrofocusing in a large cohort of MS and other neurological diseases. Eur J Neurol. 2004;11(8):525-529. doi: 10.1111/j.1468-1331.2004.00822.x [DOI] [PubMed] [Google Scholar]
- 37.Gresa-arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis : a retrospective study. Lancet Neurol. 2014;13(2):167-177. doi: 10.1016/s1474-4422(13)70282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guasp M, Módena Y, Armangue T, Dalmau J, Graus F. Clinical features of seronegative, but CSF antibody-positive, anti-NMDA receptor encephalitis. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e659. doi: 10.1212/nxi.0000000000000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carta S, Ferraro D, Ferrari S, Briani C, Mariotto S. Oligoclonal bands: clinical utility and interpretation cues. Crit Rev Clin Lab Sci. 2022;59(6):391-404. doi: 10.1080/10408363.2022.2039591 [DOI] [PubMed] [Google Scholar]
- 40.Held F, Kalluri SR, Berthele A, Klein AK, Reindl M, Hemmer B. Frequency of myelin oligodendrocyte glycoprotein antibodies in a large cohort of neurological patients. Mult Scler J - Exp Transl Clin. 2021;7(2):205521732110227. doi: 10.1177/20552173211022767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sechi E, Buciuc M, Pittock SJ, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol. 2021;78(6):741-746. doi: 10.1001/jamaneurol.2021.0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruciani C, Puthenparampil M, Tomas-Ojer P, et al. T-cell specificity influences disease heterogeneity in multiple sclerosis. Neurol - Neuroimmunol Neuroinflamm 2021;8(6):e1075. doi: 10.1212/nxi.0000000000001075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joubert B, Saint-Martin M, Noraz N, et al. Characterization of a subtype of autoimmune encephalitis with anti–contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol. 2016;73(9):1115-1124. doi: 10.1001/jamaneurol.2016.1585 [DOI] [PubMed] [Google Scholar]
- 44.Hofman P, Hoyng P, vanderWerf F, Vrensen GF, Schlingemann RO. Lack of blood – brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42(5):895-901. [PubMed] [Google Scholar]
- 45.Killer HE, Jaggi GP, Flammer J, Miller NR, Huber AR, Mironov A. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional. Brain 2007;130(2):514-520. doi: 10.1093/brain/awl324 [DOI] [PubMed] [Google Scholar]
- 46.Waters PJ, Komorowski L, Woodhall M, et al. A multicenter comparison of MOG-IgG cell-based assays. Neurology 2019;92(11):e1250-e1255. doi: 10.1212/wnl.0000000000007096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.