Abstract
Background and Objectives
Brain-computer interfaces (BCIs) are being developed to restore mobility, communication, and functional independence to people with paralysis. Though supported by decades of preclinical data, the safety of chronically implanted microelectrode array BCIs in humans is unknown. We report safety results from the prospective, open-label, nonrandomized BrainGate feasibility study (NCT00912041), the largest and longest-running clinical trial of an implanted BCI.
Methods
Adults aged 18–75 years with quadriparesis from spinal cord injury, brainstem stroke, or motor neuron disease were enrolled through 7 clinical sites in the United States. Participants underwent surgical implantation of 1 or 2 microelectrode arrays in the motor cortex of the dominant cerebral hemisphere. The primary safety outcome was device-related serious adverse events (SAEs) requiring device explantation or resulting in death or permanently increased disability during the 1-year postimplant evaluation period. The secondary outcomes included the type and frequency of other adverse events and the feasibility of the BrainGate system for controlling a computer or other assistive technologies.
Results
From 2004 to 2021, 14 adults enrolled in the BrainGate trial had devices surgically implanted. The average duration of device implantation was 872 days, yielding 12,203 days of safety experience. There were 68 device-related adverse events, including 6 device-related SAEs. The most common device-related adverse event was skin irritation around the percutaneous pedestal. There were no safety events that required device explantation, no unanticipated adverse device events, no intracranial infections, and no participant deaths or adverse events resulting in permanently increased disability related to the investigational device.
Discussion
The BrainGate Neural Interface system has a safety record comparable with other chronically implanted medical devices. Given rapid recent advances in this technology and continued performance gains, these data suggest a favorable risk/benefit ratio in appropriately selected individuals to support ongoing research and development.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT00912041.
Classification of Evidence
This study provides Class IV evidence that the neurosurgically placed BrainGate Neural Interface system is associated with a low rate of SAEs defined as those requiring device explantation, resulting in death, or resulting in permanently increased disability during the 1-year postimplant period.
For many people with paralysis, the cortical substrates of motor activity, speech, and cognition are intact but functionally disconnected from the nerves and muscles that enable movement and communication. Among the most physically disabled are individuals with locked-in syndrome, who have limited or no volitional muscle control.1 Cervical spinal cord injury (SCI), brainstem stroke, muscular dystrophy, or motor neuron disease can cause similar impairments in communication and functional independence. Brain-computer interfaces (BCIs) bypass the site of pathology, transmitting information directly from cerebral cortex to an assistive technology to restore communication and improve independence.2
There are several classes of BCIs under development that use different sensors and decoding algorithms.3 Intracortical BCIs use sensors that are surgically implanted on the cortical surface and can access information-rich single neurons and local field potentials without degradation in signal content from spatial averaging and bone filtering seen with nonsurgical approaches.4 Any implanted medical device must have an adequate safety profile before clinical use so that patients, caregivers, and healthcare professionals can make informed decisions about risks and benefits.
Two reviews have addressed the safety of implanted BCIs.5,6 One is limited to endovascular stent-electrode arrays and reports primarily on electrode performance and structural characteristics of the implanted venous sinus, which are not directly related to this study.5 The other review includes an analysis of intracortical microelectrode arrays (including some data inferred from BrainGate trial participants); however, the only safety metric reported was duration of device implantation, which was used as a surrogate marker of days without major complication.6
In 2004, the first trial of the BrainGate Neural Interface System was launched.7 The objectives of the ongoing prospective, open-label, nonrandomized feasibility trial are to assess the safety of the BrainGate system and its feasibility to control assistive technology by people with paralysis. The BrainGate trial has accrued more than 12,000 participant-days of safety data, with more than 17,000 array-days (some participants received 2 arrays simultaneously), including 2 research participants who used the system for more than 5 years.8,9 In this study, we report all safety data from all BrainGate clinical trials from 2004 to December 31, 2021. We compared BrainGate's safety profile with other implanted devices approved for neurologic disorders including epilepsy and movement disorders and seek to answer the primary research question of whether the neurosurgically placed BrainGate Neural Interface System was associated with a low rate of serious adverse events (SAEs) defined as those requiring device explantation, resulting in death, or resulting in permanently increased disability during the 1-year postimplant period.
Methods
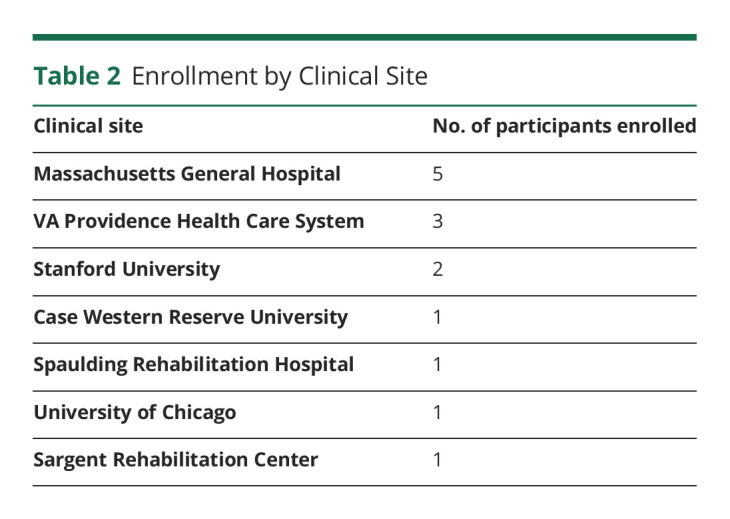
The BrainGate consortium is engaged in a multicenter, prospective, open-label, nonrandomized feasibility study (ClinicalTrials.gov Identifier: NCT00912041) of an implanted BCI system (Figure). The study is performed under an Investigational Device Exemption (IDE) from the US Food and Drug Administration and approved by the Mass General Brigham Institutional Review Board (Protocol #: 2009P000505; CAUTION: Investigational Device. Limited by Federal Law to Investigational Use). Adults aged 18–75 years with quadriparesis caused by SCI, brainstem stroke, motor neuron disease, or muscular dystrophy have been recruited primarily through referral by a neurologist or other clinician at 1 of 7 clinical sites in the United States (Tables 1 and 2). Please see the eMethods, links.lww.com/WNL/C565 section in the Supplement for additional details.
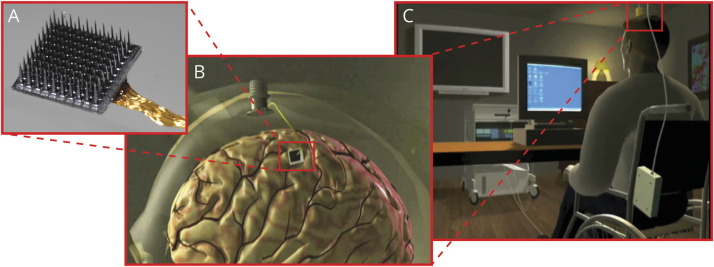
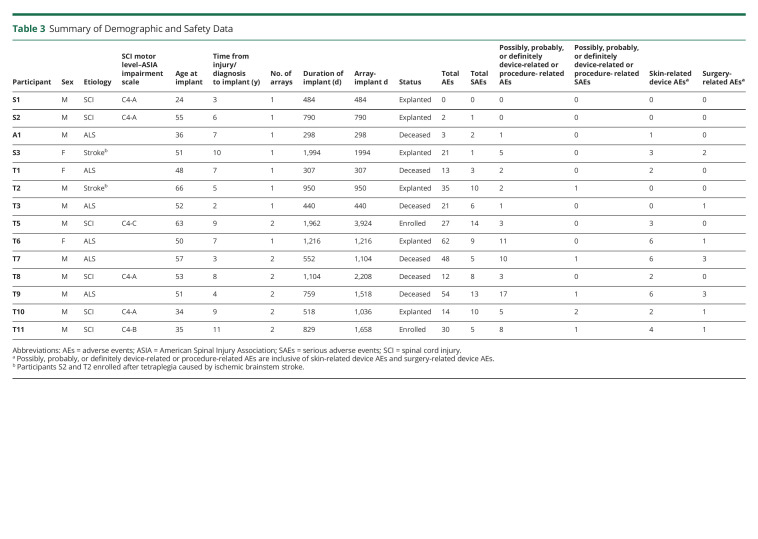
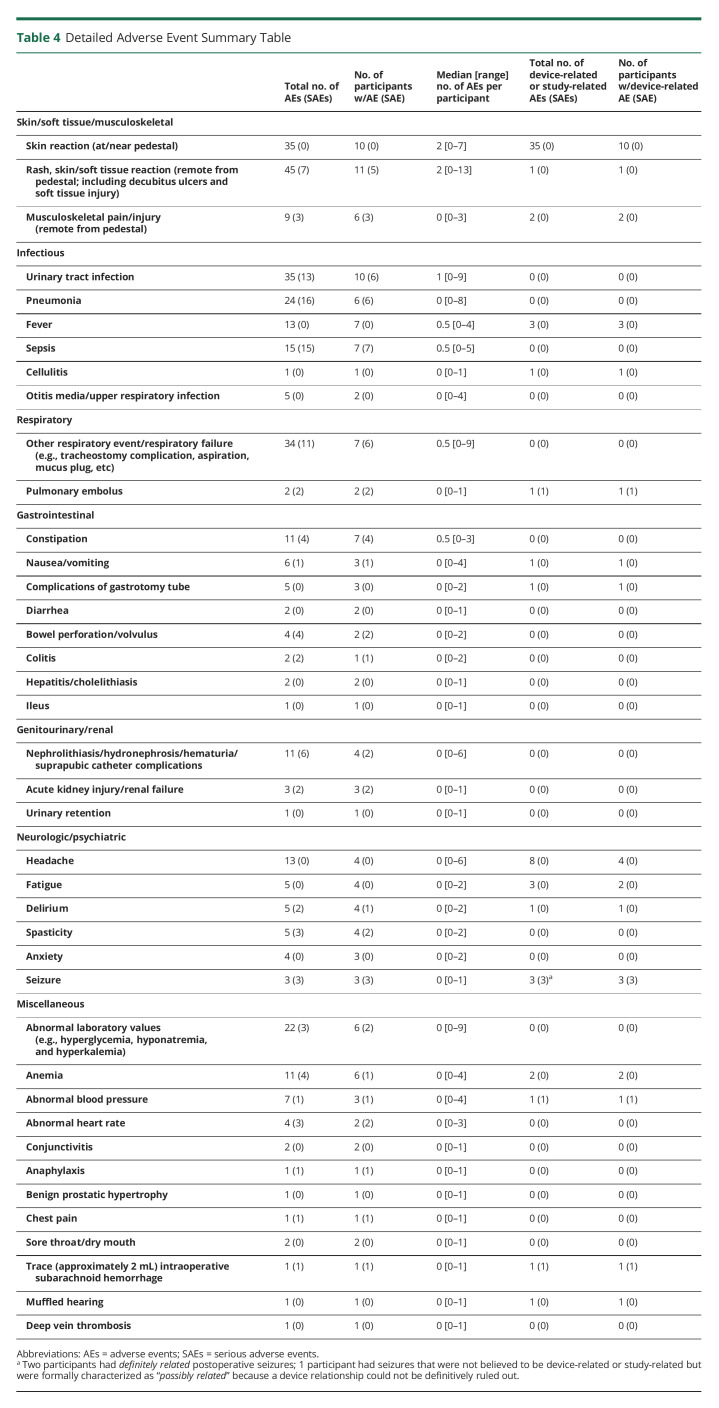
Figure. Components of the Investigational BrainGate Neural Interface System.
(A) The recording sensor is a “Utah” microelectrode array (NeuroPort, prior manufacturer: Cyberkinetics Neurotechnology Systems; present manufacturer, Blackrock Neurotech; both, Salt Lake City, UT) consisting of 96 electrodes arranged on a 4 × 4-mm platform. During surgical placement, the microelectrode array is placed onto the cortical surface of the predetermined brain region of interest and a pneumatic inserter wand is used to apply a precise amount of force to the array, inserting the microelectrodes through the pial surface and into the upper layers of cortex. (B) The microelectrode array is connected through a bundle of gold wires to a percutaneous pedestal, which is affixed to the outer table of the cranium by titanium surgical screws. When not in use, the percutaneous pedestal is covered by a disposable fitted cap. (C) Signals from the pedestal are transmitted either wirelessly10 or through a cable to a signal processor, decoding computers, and output devices. Images published with permission from the authors.
Table 1.
Clinical Trial Inclusion and Exclusion Criteria (as of December 31, 2021)
Table 2.
Enrollment by Clinical Site
Results
Between 2004 and December 2021, 14 people enrolled in the BrainGate trial (Tables 2 and 3), of whom 2 are currently engaged in research. Four people were enrolled in a first-generation BrainGate clinical trial between 2004 and 2009. One participant was enrolled in a first-generation BrainGate trial when it ended in 2009; she transitioned into and enrolled in the second-generation BrainGate trial and participated for another 2 years. In addition to that “transitional” participant, 10 people, including 2 active participants, enrolled in the second-generation BrainGate trial.
Table 3.
Summary of Demographic and Safety Data
Three participants were female and 11 were male. Eleven participants identified as White, 1 as Asian, 1 as Black, and 1 as Other. One participant identified as Hispanic ethnicity. All participants had quadriparesis, including 6 with SCI, 6 with ALS, and 2 with brainstem stroke. The median age at array implantation was 51 years (range 24–66). For participants with SCI or brainstem stroke, the median time from injury or stroke to implant was 8.5 years (range 3–11). For participants with ALS, the median time from diagnosis to implant was 5.5 years (range 2–7); we note that people with ALS often have symptoms for months or years before receiving a formal diagnosis. The median duration of device implantation was 774 days (range 296–1,994). There were 12,203 implant-days across all 14 participants (or 17,927 array-implant-days, counting participants with 2 arrays twice). Six participants underwent elective explant of the BrainGate device at the conclusion of their participation in the trial; 1 participant requested removal of the percutaneous pedestal but not the intracranial microelectrode array. Six participants died of progression of their underlying neurologic disease while enrolled. In no case was a participant’s death related to the investigational device or clinical trial; there were no unanticipated adverse device effects. Other than transitioning in 2013 from placing 1 to 2 arrays per participant and the introduction of a wireless signal transmitter in 2017,10 there have been no substantial modifications to the underlying hardware.
Among the 14 participants, there were 342 adverse events (AEs); the median number of AEs per participant was 21, and the range was from 0–62 AEs (Table 3). Two hundred seventy-four of the 342 (80.1%) AEs were unrelated to the investigational device; most were medical events common in people with tetraplegia (e.g., urinary tract infection, pneumonia, and decubitus ulcer). There were no device-related AEs that resulted in a participant's death, a participant exiting the clinical trial, a need to explant the device, or the inability to continue participating in research sessions.
Of the 68 device-related AEs (median 2 per participant, range 0–17), approximately half (35/68) were related to irritation, sensitivity, a sense of tightness, or other changes of the skin around the surgical incision or pedestal site. In many cases, these were caused by overly enthusiastic preventive care of the pedestal site by a caretaker or family member, and these resolved after reeducating caregivers. One participant was treated with oral antibiotics for a localized skin infection around a pedestal site and required no further treatment. In more than 17,000 array-days of implant, there were no intracranial or deep tissue (e.g., bone) infections related to the investigational device or any device-related infections requiring hospitalization, intravenous antibiotics, or device removal.
Seven participants had perioperative AEs. In most cases, these were common postoperative problems such as low-grade fever, nausea, or headache. One participant had postoperative hypertension that required treatment with intravenous antihypertensives. A participant with a history of pulmonary embolism who was on chronic anticoagulation developed respiratory distress on postoperative day 3 (before the planned resumption of therapeutic anticoagulation on postoperative day 14). He was diagnosed with recurrent pulmonary embolus and restarted therapeutic anticoagulation with resolution of his symptoms. Given that anticoagulation was held for device placement, this event was classified as related to the study.
In 3 of the 5 participants who had elective array explantation after more than a year (a sixth participant had the pedestal but not the array explanted), array removal was without complications or AEs. In all participants who had elective array removal, it was noted that the arachnoid had grown over the array. For 1 participant who had the BrainGate array explanted at the conclusion of her participation in the trial, surgery revealed that the array was well adhered to the brain and that, as expected, a thin, semitranslucent layer of arachnoid had grown over the array. The arachnoid was incised to partially expose the array, which was then removed. A small piece of tissue secured within the forceps (approximately 5 × 5 × 1 mm intact; measured after dislodgement from the sensor as a triangular piece of tissue 7.0 mm in its longest dimension) was lightly adherent to the electrode side of the array. Histology confirmed that the tissue included meninges and underlying superficial cortex. There was no clinical impact on the participant, who returned to her residence and usual activities on the first postoperative day. In another participant who underwent explantation at the conclusion of trial participation, after removal of the array and all visible portions of reference wires, the surgical investigator noted that the dura was densely adherent to the brain in the area surrounding the array; it was felt unsafe to perform further dissection to retrieve the full length of the 2 15 cm × 0.127-mm diameter reference wires. Postoperative CT imaging demonstrated a short curvilinear hyperdensity within the extra-axial space, suggestive of either a short length of retained reference wire or calcification along the track of the prior location of a reference wire. There were no clinical sequelae.
Two participants, both of whom had a history of traumatic brain injury in addition to SCI, had seizures in the postimplantation period. In one case, the participant was noted intraoperatively to have a small but nonetheless atypical quantity (approximately 2 mL) of subarachnoid hemorrhage around 1 of the 2 implanted arrays. Four days later, he experienced 2 self-limited focal motor seizures, for which he was hospitalized and treated with levetiracetam. He continued levetiracetam as an outpatient, experienced no further seizures, and subsequently participated in the trial for 17 months without any other neurologic AEs. This participant had not received a prophylactic antiseizure medication postoperatively because this practice had not yet been incorporated into the study protocol. Another participant had a single seizure on postoperative day 4; he too was admitted to the hospital, started on levetiracetam, and had no further seizures. He remains enrolled in the clinical trial and has been participating in research sessions for more than 2 years. He had been prescribed a prophylactic antiseizure medication, but 2 doses were missed during the transition from hospital to home. These participants' prior traumatic brain injuries were believed to have increased their risk for seizures.
Before the 2 postoperative seizures described earlier, 1 participant experienced new-onset refractory status epilepticus (NORSE).11 This person had quadriplegia and locked-in syndrome due to a brainstem stroke 5 years before enrollment. He had no history of seizures, traumatic brain injury, or other known predisposing comorbidities. After placement of a single array, he had been participating in research sessions for more than 18 months when this SAE occurred. On the morning of the event, the participant's family awoke to a ventilator dyssynchrony alarm and found him unresponsive, with chaotic eye movements. He was transported to Massachusetts General Hospital, where EEG demonstrated status epilepticus. He was treated with phenytoin, levetiracetam, lacosamide, and phenobarbital and intravenous anesthetics (propofol, midazolam, and ketamine) to achieve a burst-suppression pattern on EEG. The pedestal site was clean, and there was no evidence of localized skin infection or irritation. A CT scan of the brain demonstrated no acute structural pathology, and CSF analysis revealed normal levels of protein and glucose, 3–5 white blood cells per microliter, and negative results for viral and bacterial testing. Ultimately, the etiology of his seizures remained unknown, although pneumonia or urinary tract infections were included in the differential diagnosis. Continuous EEG monitoring demonstrated a left temporal lobe origin of his seizures, and the epileptologists involved in his care noted that the cortical location of the BrainGate array (the “hand-knob” of the precentral gyrus of the left frontal lobe) was comparatively less involved in ictal activity. By hospital day 4, he had resolution of all seizure activity. His EEGs evolved after the period of burst-suppression and initially showed generalized slowing with frequent epileptiform discharges; over subsequent days, the background rhythm improved considerably with re-emergence of a posterior dominant rhythm and only rare low-amplitude sharp waves. His antiseizure regimen was slowly tapered down to only lacosamide, and he was discharged to a rehabilitation setting. He resumed participating in research sessions approximately 4 months after this AE and participated in research sessions for another 8 months before concluding his participation in the trial.
While not an AE, we also note that for the participants who had the investigational device (array(s) and/or pedestal) electively explanted at their conclusion of participation in the trial, additional surgical consideration was required to close the well-healed scalp site(s) after removal of the pedestal(s). In participants with a thicker scalp, clean debridement and closure of the healed pedestal site was directly achieved. In a participant with a thinner scalp, additional loosening of the underlying tissue was necessary to close the site, which contributed to postoperative headaches. In addition, and while also not AEs, 2 participants in the first IDE had unexpected abrupt changes (or initial absence) of recording quality. In 1 participant, nonoperative provisional repair of an external component of the percutaneous pedestal was performed 6 months after device placement; the wire bonds had failed at the landing grid assembly within the pedestal, and conductive epoxy was manually placed on each bond site to attempt to restore connections. The initial source of this failure was attributed to the method of sterilization of the devices, which was subsequently changed. Approximately 4.5 months later, the number of useable signals recorded from the array again dropped abruptly, with moderate recovery of signals. There was no clinical change in the participant coinciding with this change in signals nor was this event believed to represent a risk to the health, safety, or welfare of the participant, and it was reported as an unexpected change in device performance. It is unknown whether this loss of signals was due to failure of the previous repair or an unrelated event, such as array dislodgement/displacement from coughing (which the patient had reported) or other participant-specific factors. After the initial device failure and discussion with the device manufacturer, modifications were made to the sterilization procedures used before delivery to investigators. In a second participant during this early period, caregivers inadvertently applied a sudden axial force to the participant's head and pedestal while placing the participant back into bed (by too forcefully contacting the headrest of the bed with the participant's head). There was a substantial return of recording quality within a month, though with considerably more day-to-day variability than previously seen. Overall, this study provides Class IV evidence that the neurosurgically placed BrainGate Neural Interface system is associated with a low rate of SAEs, defined as those requiring device explantation, resulting in death, or resulting in permanently increased disability during the 1-year postimplant period.
Discussion
The investigational BrainGate Neural Interface system has an encouraging safety record, with more than 17,000 person-array implant-days of data. All 12 participants who were enrolled for more than 1 year met the primary safety endpoint of not having the device removed for safety reasons during the 1-year postimplant evaluation. Although 6 SAEs were classified as possible, probably, or definitely device-related or procedure-related, there were no device-related SAEs that resulted in death or permanently increased disability during the 1-year postimplant evaluation period (Two participants died of complications from ALS before the 1-year evaluation). Device safety has also remained reassuring over the 2 to 5+ years that 8 participants have been enrolled.
The most common AE (Table 4) was local skin reaction and/or sensitivity around the pedestal, in most cases related to expected postsurgical changes or excessive cleaning with chlorhexadine solution as part of the infection control protocol. The most notable adverse safety events were postoperative seizures that occurred 4 days postdevice implantation in 2 participants, both of whom had a history of traumatic brain injury and had not received consistent postoperative seizure prophylaxis. Neither participant had any further seizures nor did these events affect their ability to continue to participate in the clinical trial or in their usual activities of daily living. Although preclinical data did not suggest the need for seizure prophylaxis, the trial protocol now includes 1 week of postoperative prophylactic antiseizure medication (a common practice for supratentorial craniotomies). In a participant who had a BrainGate device for more than 5 years, a small amount of tissue was adherent to the array during explantation; for 4 other participants, the array was removed without any adherent tissue. A recently published study demonstrated comparable findings in 6 Utah arrays explanted from 2 other human research participants,12 which may be an important consideration as this technology moves toward wider use.
Table 4.
Detailed Adverse Event Summary Table
The participant who experienced NORSE illustrates how medical device research can raise dilemmas at the interface of research ethics and medical ethics. Similar to many trials of novel medical devices, the ethical premise under which participants are recruited and provide informed consent to this study is that there is risk to participation and no benefit to the individual; the only benefit is that gained by society through the development of a system that could improve communication and/or mobility for people with paralysis. In this context, the NORSE SAE raised important questions that required immediate decisions. As with any clinical trial, an SAE of uncertain relationship to study procedures required rapid and comprehensive evaluation. The decision whether to remove the array was explicitly made on clinical grounds alone, and on this basis, the recommendation was to not remove the array because the neurosurgical intervention would be without a clear clinical indication and could increase the risk for additional seizures and other complications. However, during extensive discussions with the participant's family, they conveyed that he had repeatedly expressed enjoyment and altruistic fulfillment by contributing to scientific advances that could benefit people with paralysis and would be saddened to learn that he could no longer participate in the trial because the investigational device had been removed. While his family's impression of the best patient/participant–centered approach was consistent with the clinical impression for managing this SAE, the investigational team realized that the original “no benefit” premise did not fully capture the factors involved in deciding whether to remove the device. The presence of personal intangible benefits such as altruistic fulfillment might be considered in the design, review, and conduct of trials that seem to be without benefit to individuals considering enrollment.
This work has some limitations warranting discussion. The most significant, from a clinical trials standpoint, is the lack of a control arm; for myriad ethical and logistical reasons, an experimental control (e.g., surgery without device use) would not be feasible. We are nonetheless reassured by our findings that the percutaneous BrainGate system has a safety profile, to date, in a population of people with tetraplegia, which is comparable with fully implanted deep brain stimulation (DBS) devices approved for the treatment of certain movement disorders and the responsive neurostimulation (RNS) device approved for the treatment of partial-onset seizures refractory to 2 or more antiepileptic medications. Large studies with more than 100 patients undergoing DBS implantation have reported infection rates between 3% and 5% of patients,6,13-15 of which up to half have required hardware removal.15 Other complications included subcortical hemorrhage, subdural hematoma, venous infarction, and seizure; these were observed in 1%–5% of patients in case series.16,17 A meta-analysis of 1,714 patients who underwent DBS for essential tremor reported an infection rate of 3.4%, hemorrhage rate of 2.4%, and seizure rate of 2.3%.18 Device-specific complications, such as lead migration, misplaced leads, and implantable pulse generator failure have occurred at rates of approximately 3%–5%.19 In the long-term trial of the NeuroPace RNS, a chronically implanted neurostimulator used in the treatment of medication refractory epilepsy, the only device-related SAE observed in >5% of the 230 enrolled participants was implantation site infection (in 12.1%),20 with no instances of meningitis or parenchymal brain infection (although bone flap osteomyelitis was observed).21 Some patients also required operative repair of damaged leads.22 Seven patients (2.7%) had hemorrhage, 4 of which were associated with implantation surgery and had no neurologic sequaelae.20
Our clinical research experience with a percutaneously connected intracortical neural recording system has included only a single device-related skin infection and no bone or CNS infections. We ascribe this to a thorough preoperative skin cleansing protocol (consisting of 3 days of a daily pre-operative full body wash with chlorhexidine soap); careful intraoperative attention to sterile procedure; a rigorous caregiver protocol for maintaining an aseptic environment around the percutaneous pedestals; and chlorhexidine-impregnated patches (BioPatch) around the pedestal site (Table 5). However, we recognize that our study is underpowered to detect rare events such as intracranial infection. Given the low incidence in the general population (1.38 cases per 100,000 population per year in 2006–2007),23 our current study, with 33.43 person-years of observation, has a power of less than 1% to detect a doubling in the rate of intracranial infection at a 0.05 level of significance. Had we observed intracranial infections, we could query for a statistical deviation from the background rate, if present.
Table 5.
Anti-infective Procedures/Protocols
A second limitation in comparing the safety profile of this device with other chronically implanted neurotechnologies is that the people enrolled in the BrainGate trial have different neurologic conditions than the populations enrolled in other neurotechnology trials. People with quadriparesis may have greater contact with caregivers attending to health-related needs than those with epilepsy or Parkinson disease; consequently, comparisons with devices intended for ambulatory populations are imperfect. In addition, while our participants consented to the risk of worsening paralysis because of array placement on motor cortex (which has not been observed), given the significant baseline weakness required to enroll, subtle changes in motor function may occur below the threshold of clinical perception. With that said, the ongoing BrainGate trial and subsequent studies using similar research protocols and the Blackrock NeuroPort array were informed by numerous animal studies of safety, histology, and chronic device function24-27 and with the understanding that other research studies for participants with paralysis who could benefit from future intracortical BCI devices would continue to inform the field.9,28-30 In addition to our 14 participants, as of April 2022, there have been approximately 21 other human research participants who have participated in chronic intracortical BCI trials at academic centers in the United States,28,29,31-33 China,34 and Germany.35 Chronically implanted electrocorticography grids have been placed for similar research in the United States and The Netherlands,31,36 and 4 investigational Stentrode devices have been placed in Australia.37,38 Several neurotechnology companies are also preparing clinical trials of implanted BCI systems, reinforcing the growing interest in developing and deploying a clinically useful implanted BCI.
While safety is the sine qua non of any successful medical technology, quantifiable metrics of efficacy are important to establish clinical utility and to help individuals to evaluate the technology within their personal value system of risks and benefits. Our current feasibility study is being conducted partly to inform the development of metrics upon which efficacy could be evaluated in a subsequent pivotal trial. Prior publications from our group and others have demonstrated the potential for intracortical BCIs to improve the quality of life for people with paralysis. With highly accurate 2-dimensional cursor control that can be calibrated within minutes of initial use,39 BrainGate participants have controlled a point-and-click interface to communicate at 30–40 correct characters per minute (CPM).40,41 Using handwriting decoding techniques, a communication rate of 90 CPM has been demonstrated,9 approaching the range of an able-bodied individual typing on a smartphone keyboard.42 In addition to developing methods for improved communication, the investigational BrainGate system and related intracortical BCIs have been used to control assistive robotics7,28,43 and a participant's own arm and hand through functional electrical stimulation.33,44 These demonstrations suggest the potential to provide a meaningful improvement in quality of life.
While an exhaustive review of the potential benefits of the iBCI approach is beyond the scope of this article, the “risk/benefit” analysis—particularly as considered by an individual with paralysis contemplating the use of different assistive technologies—will be central to the future success of these devices. For example, some participants have entered the study frustrated, for a host of reasons, by the utility of their eye-gaze systems. Despite relative preservation of eye movements in the earlier stages of ALS, subtle changes in motor control, slowness of saccades, fatigue in supporting musculature, and progressive ophthalmoparesis can all affect performance with these systems.45 Limited data suggest communication rates of 10–20 CPM in people with ALS; in a limited head-to-head study, participants communicated faster using a manual letter board than a computerized eye-gaze system.46,47
Furthermore, for a person with severe speech and motor impairments, the ability to use an assistive technology while visually engaging with their dialog partner—which itself has communication value—is also important. Consequently, iBCIs provide not only theoretical advantages over eye-gaze systems but also over other BCIs (implanted or EEG based) that encumber eye movements specifically to navigate communication interfaces. Given iBCIs' early demonstrations of the ability to support multidimensional, dexterous reach-and-grasp movements, to restore sensation (with some further improvement in, for example, assistive robot control),29 the broad potential for iBCIs is clear. Despite the flexibility and range of effector control that iBCIs may provide in the future, the current percutaneous component and substantial external hardware will remain suboptimal features when compared with fully implanted or nonsurgery-requiring approaches. Both our group and others continue to work on components and systems that would permit iBCIs to become fully implanted, available to users around-the-clock, and incorporate a suite of design characteristics previously proposed.48
Overall, we are reassured by our findings over the past 17 years that the investigational BrainGate Neural Interface System is being deployed safely. The development of a fully implantable system, which would reduce the infection concern presented by a percutaneous component and provide more acceptable cosmesis for many users, remains an important goal of our group and others. Moreover, this trial has been conducted during and continues to benefit from a revolution in machine learning/artificial intelligence and microprocessor technology. Advances in these fields have contributed to the ongoing improvement in performance that we have observed, and we anticipate that these technical gains will only accelerate in the coming years. Of importance, these same technological advances have led to improvements in consumer assistive technology available to people with paralysis. Voice control platforms such as Siri and Alexa may allow a person with quadriplegia and intact speech naturalistic control over their consumer electronics and home environment, and head-mounted virtual reality/augmented reality technology may provide additional channels of communication for people who have lost the ability to speak. However, no one technological solution will work best for everyone. Indeed, many able-bodied individuals prefer to type or text rather than use voice recognition to maintain the privacy of their communication from the people in their immediate surroundings, and much of the drive for iBCIs is not only to restore communication but also to restore mobility through intuitively controlled functional electrical stimulation or soft/wearable robotics. Intracortical BCIs also are being developed as key components of closed-loop neuromodulation therapies that may improve the management of disorders of mood or cognition.49,50 Ultimately, the extraordinary potential utility of implanted BCIs will reflect the personal risk/benefit assessment of the people for whom these devices are being developed.
Acknowledgment
The investigators thank the participants, their families, and caregivers for their extraordinary dedication to this research. The investigators also thank their collaborators on the scientific research teams at Massachusetts General Hospital, Brown University, the Providence VA Medical Center, Stanford, and Case Western Reserve University for their ongoing contributions to this incredible research. The investigators are also grateful for John Mislow, MD, PhD, who assisted with an early draft of this manuscript. From June 2004 to May 2009, the BrainGate pilot clinical trials were sponsored and directed by Cyberkinetics Neurotechnology Systems. In 2009, those original clinical trials and the subsequent trial (“BrainGate2”) have been conducted by academic institutions with grant support from the NIH, VA, and charitable foundations. All data reported herein have been previously reported to the independent institutional review boards and to the US FDA.
Glossary
- AEs
adverse events
- BCIs
brain-computer interfaces
- CPM
characters per minute
- DBS
deep brain stimulation
- IDE
Investigational Device Exemption
- NORSE
new-onset refractory status epilepticus
- RNS
responsive neurostimulation
- SAEs
serious adverse events
- SCI
spinal cord injury
- UADEs
unanticipated adverse device effects
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. This work was supported by the Department of Veterans Affairs N2864C (L.R. Hochberg), N9228C (L.R. Hochberg), B6453R (L.R. Hochberg), A6779L (L.R. Hochberg), B6459L (J.P. Donoghue), N9271L (J. P. Donoghue), B6450L (J.P. Donoghue), A2654R (A.B. Ajiboye), and 69043W (A.B. Ajiboye); the NIH, the National Institute on Deafness and Other Communication Disorders (NIDCD) R01DC009899 (L.R. Hochberg), U01DC017844 (L.R. Hochberg), and R01DC014034 (J.M. Henderson & K.V. Shenoy); the NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) R01HD077220 (RFK), N01HD53403 (RFK), and N01HD10018 (RFK); the NIH, the National Institute of Neurologic Disorders and Stroke (NINDS) U01NS098968 (SSC), R01NS062092 (SSC), UH2NS095548 (L.R. Hochberg), and R25NS065743 (D.B. Rubin); the NINDS/NICHD 1RC1HD63931 (J.P. Donoghue); the Office of Naval Research N00014-07-1-0803 (J.P. Donoghue); Simons Foundation 543,045 (K.V. Shenoy); Howard Hughes Medical Institute (K.V. Shenoy is a Howard Hughes Investigator); Doris Duke Charitable Foundation 2008037 (L.R. Hochberg); Conquer Paralysis Now 004698 (J.D. Simeral); American Heart Association 19CSLOI34780000 (L.R. Hochberg); ALS Association 20-MALS-553 (L.R. Hochberg); Movement Disorder Foundation (Australia) (L.R. Hochberg); The American Academy of Neurology Clinical Research Training Scholarship (D.B. Rubin); L. and P. Garlick (J.M. Henderson & K.V. Shenoy), S. and B. Reeves (J.M. Henderson & K.V. Shenoy); the Massachusetts General Hospital (MGH) Deane Institute (L.R. Hochberg), The MGH Executive Committee on Research (L.R. Hochberg); Stanford University Wu Tsai Neurosciences Institute (J.M. Henderson & K.V. Shenoy), Bio-X Institute at Stanford (J.M. Henderson & K.V. Shenoy); Robert J. & Nancy D. Carney Institute for Brain Science at Brown University (L.R. Hochberg), Brown University School of Engineering (L.R. Hochberg), Brown University Office of the Vice President for Research (L.R. Hochberg); and Harvard Catalyst KL2/CMeRIT Award (D.B. Rubin). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Department of Veterans Affairs, or the United States Government.
Disclosure
D.B. Rubin serves on the External Advisory Board of Celgene/Bristol Myers Squibb; A.B. Ajiboye, L. Barefoot, and M. Bowker report no disclosures relevant to the manuscript; The MGH Translational Research Center has a clinical research support agreement with Neuralink and Synchron, for which S.S. Cash provides consultative input. S. S. Cash has stock/stock options in Beacon Biosignals; D. Chen reports no disclosures relevant to the manuscript; J.P. Donoghue reports grants/contracts support from Cyberkinetics, Inc 2003–2009 (ceased operations 2009), J.P. Donoghue reports consulting fees from Otsuka Medical Devices, Pathmaker Neurosystems, and Neurable. J.P. Donoghue reports payment/honoraria for a presentation to Mitre Corp, reports that he is an advisory board member for Neurable, a leadership role (Board Director) for Pathmaker Neurosystems, stock/stock options in Beacon Biosignals, Pathmaker Neurosystems, and Neurable, and the following patents: Microstructured arrays for cortex interaction and related methods of manufacture and use, U.S. Patent 7,212,851; Neurologic signal decoding U.S. Patent 7,392,079 (expired Nov. 14, 2021); Synergistic effector/environment decoding system, US20200104689A1. E.N. Eskandar has a patent pending for “Coherent Maser Interferometer for Recording and Modulating Brain Activity”; G. Friehs and C. Grant report no disclosures relevant to the manuscript; J.M. Henderson serves on the Medical Advisory Board of Enspire DBS. J.M. Henderson is a consultant for Neuralink and Enspire DBS, holds patents licensed to Neuralink and Blackrock Neurotech, is an inventor on several issued and pending patents on neural interfacing technology and delivery systems, and has stock or stock options in Neuralink, Enspire DBS, and Maplight Therapeutics; R.F. Kirsch, R. Marujo, and M. Masood report no disclosures relevant to the manuscript; S.T. Mernoff runs VISN 1 Neurology, a committee of all the Chiefs of Neurology for VA facilities in the 6 New England States; J.P. Miller, J.A. Mukand, and R.D. Penn report no disclosures relevant to the manuscript; J. Shefner reports research support from Amylyx, Biogen, Biotie Therapies (now Acorda Therapeutics), Cytokinetics Incorporated, Mitsubishi Tanabe Pharma America, Alexion, Medicinova, Ionis, Alector, and Orphazyme, J. Shefner reports compensation received as a consultant for Apic Biosciences, Neurosense, Cytokinetics, Denali, GSK, Mitsubishi Tanabe Pharma America, Orphazyme, Pinteon, RRD, Swanbio, and Helixsmith. J. Shefner reports royalties for serving as Neuromuscular Section Editor for UpToDate. K.V. Shenoy discloses that royalties are paid by Blackrock Neurotechnology Inc. to Stanford University and from there on to the inventors. This pertains to Patents 6, 8, 10, 12, and 13 as numbered below. Royalties are paid by Neuralink Corp to Stanford University and from there on to the inventors. This pertains to Patents 6, 8, 12, and 13 as numbered below. K.V. Shenoy is a consultant to Neuralink Corp and CTRL-Labs (Meta Platforms/Facebook), reports honoraria from a variety of universities (no corporations), reports honoraria from the NIH for reviewing grants (study section), reports support for attending meetings/travel from universities and professional societies to give invited talks, was on the Scientific Advisory Board of CTRL-Labs, Inc (now Meta Platforms/Facebook), is on the Scientific Advisory Board of MIND-X Inc, Inscopix Inc. and Heal Inc, and discloses the following patents: (1) Processed neural signals and methods for generating and using them. Patent Number: US 6,609,017 B1. Date of patent: Aug. 19, 2003. Krishna V. Shenoy, Richard A. Andersen, Sohaib A. Kureshi. California Institute of Technology. ISSUED. (2) Cognitive state machine for prosthetic systems. Publication Number: US 2003/0023319 A1. Date of patent: July 10, 2001. Richard A. Andersen, Bijan Pesaran, Partha Mitra, Daniella Meeker, Krishna V. Shenoy, Shiyan Cao, Joel W. Burdick. California Institute of Technology. ISSUED. (3) Cognitive state machine for prosthetic systems. International Publication No.: WO 03/005,934 A3. Date of patent: January 23, 2003. Richard A. Andersen, Bijan Pesaran, Partha Mitra, Daniella Meeker, Krishna V. Shenoy, Shiyan Cao, Joel W. Burdick. California Institute of Technology. ISSUED. (4) Decoding of neural signals for movement control. Patent No.: US 7,058,445 B2. Date of patent: June 6, 2001. Caleb T. Kemere, Gopal Santhanam, Byron M. Yu, Teresa H. Meng, Krishna V. Shenoy. Stanford University. ISSUED. (5) Brain-machine interface utilizing interventions to emphasize aspects of neural variance and decode speed and angle. Pub. No.: US 2015/0245928 A1. Publication Date: September 3, 2015. Jonathan C. Kao, Chethan Pandarinath, Paul Nuyujukian, Krishna V. Shenoy. Stanford University. ISSUED. (6) Brain machine interface. Patent No.: US 8,792,976 B2. Date of patent: July 29, 2014. Vikash Gilja, Paul Nuyujukian, Cynthia A. Chestek, John P. Cunningham, Byron M. Yu, Stephen I. Ryu, Krishna V. Shenoy. Stanford University. ISSUED. (7) Brain machine interfaces incorporating neural population dynamics. Patent No.: US 9,095,455 B2. Date of patent: August 4, 2015. Jonathan C. Kao, Paul Nuyujukian, Mark M. Churchland, John P. Cunningham, Krishna V. Shenoy. Stanford University. ISSUED. (8) Brain-machine interface utilizing a discrete action state decoder in parallel with a continuous decoder for a neural prosthetic device. Patent No.: US 9,373,088 B2. Date of patent: June 21, 2016. Paul Nuyujukian, Jonathan C. Kao, Krishna V. Shenoy. Stanford University. ISSUED. (9) Task-outcome error signals and their use in brain-machine interfaces. Patent No.: US 10,779,764 B2. Date of patent: September 22, 2020. Nir Even-Chen, Krishna V. Shenoy, Jonathan C. Kao, Sergey Stavisky. Stanford University. ISSUED. (10) Multiplicative recurrent neural network for fast and robust intracortical brain-machine interface decoders. Patent No.: US 10,223,634 B2. Date of patent: Mar. 5, 2019. David Sussillo, Jonathan C. Kao, Sergey Stavisky, Krishna V. Shenoy. Stanford University. ISSUED. (11) Systems and methods for virtual keyboards for high dimensional controllers. Patent No.: US 10,949,086 B2. Publication Date: March 16, 2021. Nir Even-Chen, Krishna V. Shenoy. Stanford University. ISSUED. (12) Systems and methods for decoding intended speech from neuronal activity. Pub. No.: US 2019/0333505 A1. Publication Date: October 31, 2019. Sergey Stavisky, Krishna V. Shenoy, Jaimie M. Henderson. Stanford University. PENDING. (13) Systems and methods for decoding intended symbols from neural activity. Pub. No.: US 2021/0064135 A1. Publication Date: March 4, 2021. Krishna V. Shenoy, Jaimie M. Henderson, Frank Willett. Stanford University. PENDING; J. D. Simeral reports no disclosures relevant to the manuscript; J.A. Sweet is currently on the scientific advisory board of Koh Young Inc. and was previously on the scientific advisory board and received stock options from Helius Medical Technologies; B.L. Walter has received consulting fees from Abbott Labs. Z.M. Williams reports no disclosures relevant to the manuscript. The MGH Translational Research Center has a clinical research support agreement with Neuralink and Synchron, for which L.R. Hochberg provides consultative input. Go to Neurology.org/N for full disclosures.
References
- 1.Hochberg LR, Cudkowicz ME. Locked in, but not out? Neurology. 2014;82(21):1852-1853. doi: 10.1212/wnl.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 2.Lee B, Liu CY, Apuzzo MLJ. A primer on brain-machine interfaces, concepts, and technology: a key element in the future of functional neurorestoration. World Neurosurg. 2013;79:457-471. [DOI] [PubMed] [Google Scholar]
- 3.Martini ML, Oermann EK, Opie NL, Panov F, Oxley T, Yaeger K. Sensor modalities for brain-computer interface technology: a comprehensive literature review. Neurosurgery. 2020;86:E108–E117. [DOI] [PubMed] [Google Scholar]
- 4.Brandman DM, Cash SS, Hochberg LR. Review: human intracortical recording and neural decoding for brain-computer interfaces. IEEE Trans Neural Syst Rehabil Eng. 2017:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soldozy S, Young S, Kumar JS, et al. A systematic review of endovascular stent-electrode arrays, a minimally invasive approach to brain-machine interfaces. Neurosurg Focus. 2020;49(1):E3. doi: 10.3171/2020.4.focus20186. [DOI] [PubMed] [Google Scholar]
- 6.Bullard AJ, Hutchison BC, Lee J, Chestek CA, Patil PG. Estimating risk for future intracranial, fully implanted, modular neuroprosthetic systems: a systematic review of hardware complications in clinical deep brain stimulation and experimental human intracortical arrays. Neuromodulation. 2020;23:411-426. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164-171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 8.Simeral JD, Kim S-P, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng. 2011;8(2):025027. ii: 10.1088/1741-2560/8/2/025027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willett FR, Avansino DT, Hochberg LR, Henderson JM, Shenoy KV. High-performance brain-to-text communication via handwriting. Nat Res. 2021;593(7858):249-254. doi: 10.1038/s41586-021-03506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simeral JD, Hosman T, Saab J, et al. Home use of a percutaneous wireless intracortical brain-computer interface by individuals with tetraplegia. IEEE Trans Biomed Eng IEEE Comput Soc. 2021;68(7):2313-2325. doi: 10.1109/tbme.2021.3069119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults-infectious or not? J Neurol Sci. 2009;277(1-2):26-31. doi: 10.1016/j.jns.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Woeppel K, Hughes C, Herrera AJ, et al. Explant analysis of UT electrode arrays implanted in human cortex for brain-computer-interfaces. Front Bioeng Biotechnol. 2021;9:759711-759715. doi: 10.3389/fbioe.2021.759711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Son BC, Shon YM, Choi JG, et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg. 2016;94(3):187-197. doi: 10.1159/000446611. [DOI] [PubMed] [Google Scholar]
- 14.Lyons KE, Wilkinson SB, Overman J, Pahwa R. Surgical and hardware complications of subthalamic stimulation: a series of 160 procedures. Neurology. 2004;63(4):612-616. doi: 10.1212/01.wnl.0000134650.91974.1a. [DOI] [PubMed] [Google Scholar]
- 15.Vergani F, Landi A, Pirillo D, Cilia R, Antonini A, Sganzerla EP. Surgical, medical, and hardware adverse events in a series of 141 patients undergoing subthalamic deep brain stimulation for Parkinson disease. World Neurosurg. 2010;73(4):338-344. doi: 10.1016/j.wneu.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Umemura A, Jaggi JL, Hurtig HI, et al. Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg. 2003;98(4):779-784. doi: 10.3171/jns.2003.98.4.0779. [DOI] [PubMed] [Google Scholar]
- 17.Seijo FJ, Alvarez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson's disease. Review of 272 procedures. Acta Neurochir (Wien). 2007;149(9):867-876. doi: 10.1007/s00701-007-1267-1. [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Luo L, Liu M, et al. Outcomes and adverse effects of deep brain stimulation on the ventral intermediate nucleus in patients with essential tremor. Neural Plast. 2020;2020:2486065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doshi PK. Long-term surgical and hardware-related complications of deep brain stimulation. Stereotact Funct Neurosurg. 2011;89(2):89-95. doi: 10.1159/000323372. [DOI] [PubMed] [Google Scholar]
- 20.Nair DR, Laxer KD, Weber PB, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):e1244-e1256. doi: 10.1212/wnl.0000000000010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degenhart AD, Eles J, Dum R, et al. Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate. J Neural Eng. 2016;13:046019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B, Zubair MN, Marquez YD, et al. A single-center experience with the NeuroPace RNS system: a review of techniques and potential problems. World Neurosurg. 2015;84:719-726. [DOI] [PubMed] [Google Scholar]
- 23.Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364(21):2016-2025. doi: 10.1056/nejmoa1005384. [DOI] [PubMed] [Google Scholar]
- 24.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil. 2005;13(4):524-541. doi: 10.1109/tnsre.2005.857687. [DOI] [PubMed] [Google Scholar]
- 25.Rousche PJ, Normann RA. Chronic intracortical microstimulation (ICMS) of cat sensory cortex using the Utah intracortical electrode array. IEEE Trans Rehabil Eng IEEE. 1999;7(1):56-68. doi: 10.1109/86.750552. [DOI] [PubMed] [Google Scholar]
- 26.Rousche PJ, Normann RA. Chronic recording capability of the Utah intracortical electrode array in cat sensory cortex. J Neurosci Methods. 1998;82:1-15. [DOI] [PubMed] [Google Scholar]
- 27.Barrese JC, Rao N, Paroo K, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6):066014. doi: 10.1088/1741-2560/10/6/066014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collinger JL, Wodlinger B, Downey JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381(9866):557-564. doi: 10.1016/s0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flesher SN, Downey JE, Weiss JM, et al. A brain-computer interface that evokes tactile sensations improves robotic arm control. Science. 2021;372(6544):831-836. doi: 10.1126/science.abd0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouton CE, Shaikhouni A, Annetta NV, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533(7602):247-250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 31.Moses DA, Metzger SL, Liu JR, et al. Neuroprosthesis for decoding speech in a paralyzed person with anarthria. N Engl J Med. 2021;385(3):217-227. doi: 10.1056/nejmoa2027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cajigas I, Davis KC, Meschede-Krasa B, et al. Implantable brain–computer interface for neuroprosthetic-enabled volitional hand grasp restoration in spinal cord injury. Brain Commun. 2021;3(4):fcab248. doi: 10.1093/braincomms/fcab248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colachis SC, Bockbrader MA, Zhang M, et al. Dexterous control of seven functional hand movements using cortically-controlled transcutaneous muscle stimulation in a person with tetraplegia. Front Neurosci. 2018;12:208-214. doi: 10.3389/fnins.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang H, Wang R, Zheng Z, et al. Short report: surgery for implantable brain-computer interface assisted by robotic navigation system. Acta Neurochir (Wien). 2022;164(9):2299-2302. doi: 10.1007/s00701-022-05235-5. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary U, Vlachos I, Zimmermann JB, et al. Spelling interface using intracortical signals in a completely locked-in patient enabled via auditory neurofeedback training. Nat Commun. 2022;13:1236-1239. doi: 10.1038/s41467-022-28859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vansteensel MJ, Pels EGM, Bleichner MG, et al. Fully implanted brain–computer interface in a locked-in patient with ALS. N Engl J Med. 2016;375(21):2060-2066. doi: 10.1056/nejmoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oxley TJ, Yoo PE, Rind GS, et al. Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: first in-human experience. J Neurointerv Surg. 2021;13(2):102-108. doi: 10.1136/neurintsurg-2020-016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell B, Lee CMS, Yoo P, et al. Long-term safety of a fully implanted endovascular brain-computer interface for severe paralysis: results of SWITCH, a first-in-human study. Paper presented at: 74th American Academy of Neurology Annual Meeting; April 2–7, 2022; Seattle, WA. [Google Scholar]
- 39.Brandman DM, Hosman T, Saab J, et al. Rapid calibration of an intracortical brain-computer interface for people with tetraplegia. J Neural Eng. 2018;15(2):026007-026026. doi: 10.1088/1741-2552/aa9ee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandarinath C, Nuyujukian P, Blabe CH, et al. High performance communication by people with paralysis using an intracortical brain-computer interface. Elife. 2017;6:185544-e18627. doi: 10.7554/elife.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacher D, Jarosiewicz B, Masse NY, et al. Neural point-and-click communication by a person with incomplete locked-in syndrome. Neurorehabil Neural Repair. 2015;29(5):462-471. doi: 10.1177/1545968314554624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palin K, Feit AM, Kim S, Kristensson PO, Oulasvirta A. How do people type on mobile devices? Observations from a study with 37, 000 volunteers. Paper presented at: 21st International Conference on Human-Computer Interaction with Mobile Devices and Services, MobileHCI 2019; October 1–4, 2019; Taipei, Taiwan. 10.1145/3338286.3340120 [DOI] [Google Scholar]
- 43.Hochberg LR, Bacher D, Jarosiewicz B, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajiboye AB, Willett FR, Young DR, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 2017;389(10081):1821-1830. doi: 10.1016/s0140-6736(17)30601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linse K, Aust E, Joos M, Hermann A. Communication matters—pitfalls and promise of hightech communication devices in palliative care of severely physically disabled patients with amyotrophic lateral sclerosis. Front Neurol. 2018;9:603. doi: 10.3389/fneur.2018.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pannasch S, Helmert JR, Malischke S, Storch A, Velichkovsky BM. Eye typing in application: a comparison of two systems with ALS patients. J Eye Mov Res. 2008;2(4):1-8. doi: 10.16910/jemr.2.4.6. [DOI] [Google Scholar]
- 47.Harris D, Goren M. The ERICA eye gaze system versus manual letter board to aid communication in ALS/MND. Br J Neurosci Nurs. 2009;5:227-230. doi: 10.12968/bjnn.2009.5.5.42128. [DOI] [Google Scholar]
- 48.Hochberg LR, Anderson K. BCI users and their needs. In: Wolpaw JR, Wolpaw E, eds. Brain-Computer Interfaces: Principles and Practice Hardcover. Oxford University Press; 2012. [Google Scholar]
- 49.Scangos KW, Khambhati AN, Daly PM, et al. Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat Med. 2021;27(10):1696-1700. doi: 10.1038/s41591-021-01480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drew L. Wiring up the brain to beat depression. Nature. 2022;608(7924):S46-S47. doi: 10.1038/d41586-022-02209-6. [DOI] [PubMed] [Google Scholar]