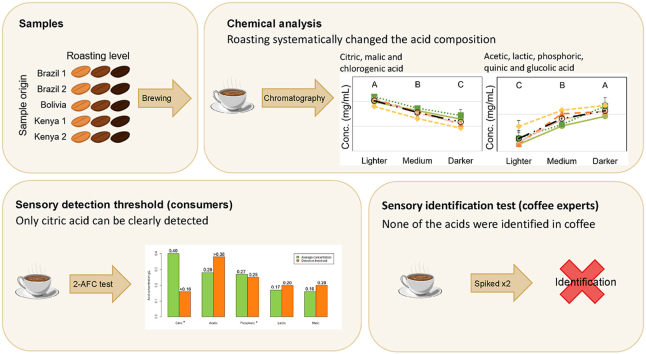
Abstract
Coffee brewed on light, and very light-roast coffee beans have emerged as a recent trend among specialty coffee drinkers. The acidity of such light-roast coffee, and coffee in general, is an important sensory characteristic, as there is demonstrated a clear correlation between the roast level and perceived acidity in brewed coffee. The acidity is believed to be strongly linked to the content and composition of organic acids in coffee. Still, there is limited literature on acid content in brewed coffee and on the relevance of specific acid concentrations to sensory perception. In this study, we determined concentrations of acids and sugars in French-press brewed specialty coffee. We used varying roast degrees in the light to very light range using five coffees from different geographical locations (Brazil, Bolivia, and Kenya) and determined the sensory detection threshold and recognition for selected acids. The concentration of all individual acids except one (formic) either significantly decreased (citric, malic, and chlorogenic acid) or increased (acetic, lactic, phosphoric, quinic, and glycolic acid) systematically with an increasing roast degree, while no systematic trends were found between the different coffee samples. The sugar content decreased with an increasing roast degree. The sensory detection threshold for malic, acetic, and lactic acid was determined to be above the actual concentration of said acids in the coffee and just below for phosphoric acid, indicating that these compounds are unlikely to individually be perceived in coffee. Only citric acid can be clearly detected in the threshold test (not identified by experts in coffee) in concentrations above the measured concentrations, as the detection threshold was below (<0.16 g/L) the concentration found in the investigated coffees (0.23–0.60 g/L). The measured citric acid concentration was found to be much higher for the Brazil coffees (0.49 ± 0.08 g/L) compared to the Bolivia coffee (0.40 ± 0.11 g/L), and the Kenya coffees (0.30 ± 0.07 g/L). Furthermore, none of the acids added to the coffee were correctly recognized by coffee experts when spiked with measured average concentrations. Combined, the results question the direct relation between individual organic acids and acidity in coffee and point towards a more complex understanding of perceived acidity.
Keywords: Coffee, Organic acids, Brew, Roasting, Origin, Sugar, C. arabica, Sensory, Detection threshold
Graphical abstract
Highlights
-
•
Acids in brewed specialty coffee varying in roasting and origin were quantified.
-
•
Roasting systematically changed the acid composition.
-
•
No general or systematic changes were found between sample origin and acid composition.
-
•
Only citric acid could be sensorially detected above the concentration measured in the brewed coffee.
-
•
None of the tested acids could be identified by coffee experts when spiked into brewed coffee.
1. Introduction
Acids are recognized as a key contributor to the sensory experience of coffee (Ginz et al., 2000), and coffee experts highly value this taste and adjust their roast accordingly (Thomas et al., 2017). Acids give rise to taste and flavor themselves but also function as flavor precursors for other quality descriptors of coffee (Borém et al., 2016; Ginz et al., 2000; Woodmann, 1985). It is widely accepted that acids contribute to the perceived acidity of coffee, which is an important part of evaluating coffee quality, and one of the main categories that coffee experts use to score coffee quality. Acidity in coffee is considered particularly important for the evaluation of coffee brewed using beans that are roasted much lighter than traditional light roast coffees, which has gained recent interest among specialty coffee drinkers. Acids in coffee are generally divided into organic acids (OAs) and chlorogenic acids (CGAs), while inorganic acids such as phosphoric acid can also be present in coffee. Multiple studies have looked at CGAs in coffee, while fewer studies have looked at OAs in coffee, particularly in brewed coffee (Yeager et al., 2021). Further, it is believed that CGAs do not contribute to the perceived acidity, although this is not well studied in the literature (Yeager et al., 2021). The main acids in green coffee beans are chlorogenic, quinic, citric, and malic acid; the specific composition of acids varies with multiple factors such as species, cultivar, geographical origin, growth altitude, and post-harvest processing (Brollo et al., 2008; Farah, 2020), where post-harvest processing is the most dominant factor in green coffee, followed by origin and altitude (Amalia et al., 2021).
During roasting, the acid composition of the coffee beans changes greatly (Belitz et al., 2009; Farah, 2020; Ginz et al., 2000). Chlorogenic, citric, and malic acid are degraded during roasting, while quinic acid increases in concentration due to the degradation of chlorogenic acid (Belitz et al., 2009; Bennat et al., 1994; Ginz et al., 2000). Additionally, OAs such as acetic, formic, lactic, and glycolic acid are formed due to the thermal degradation of soluble carbohydrates present in green coffee beans, mainly simple sugars such as sucrose, glucose, and fructose (Ginz et al., 2000). The primary acids in roasted coffee, therefore, are chlorogenic, quinic, citric, malic, acetic, formic, lactic, glycolic, and phosphoric acid, and occasionally tartaric acid is also found (Belitz et al., 2009; Engelhardt and Maier, 1985; Ginz et al., 2000).
Soluble carbohydrates and sugars function as precursors in the formation of both acids and other important aroma compounds (de Maria et al., 1996; Poisson et al., 2017). However, the sugars are generally considered to be present in such low amounts in the roasted coffee, that they do not themselves contribute to the perception of sweetness of brewed coffee. Alstrup et al. (2020) demonstrated that modulations of the roast changed the perceived sweetness, but not the sugar content. On the other hand, the sugar concentration might be important if the coffee is roasted so light that not all sugars are degraded.
Based on the recent review by Yeager et al. (2021), twenty-seven scientific papers from 1959 to 2020 have reported about OAs in coffee (green, roasted, and brewed). Further three studies were identified in our search, giving a total of 30 studies. Of those, nine studies only looked at OAs in green coffee. Of the remaining 21 studies, one was not peer-reviewed, one did not show their own data, four studies used chemicals to dissolve the roasted coffee beans and one study only looked at Robusta (C. canephora) coffee and not C. arabica which accounts for the largest share of coffee production. The remaining 14 studies, ten looked at the OAs in roasted coffee, by making a coffee extract where the roasted and grounded coffee (0.07–6.3g) was mixed with (700 μm–100 ml) hot water (80–100 °C), and were mixed using different techniques (stirred, reflux, mechanical shaking, or centrifuge for up to 30 min). By contrast, OAs composition in brewed coffee has received much less attention in the literature, as only four scientific papers have reported their own data on the OAs in brewed coffee, using different brewing methods cold brew (Ahmed et al., 2019), espresso (Khamitova et al., 2020), according to ISO standard (Rodrigues et al., 2007) and different brewing conditions (grind size, brew temperature and brew time) (ICO, 1991).
While the scientific literature has yet to better characterize acid compositions and the roles of acids in coffee, coffee acidity is an inherent part of evaluating coffee among experts – especially in the “specialty coffee” segment of the industry. Certification bodies such as the Coffee Quality Institute (CQI), the Specialty Coffee Association (SCA), and Coffee Science Certificate (CSC) allocate a significant amount of time for the students to discriminate and/or identify specific OAs in pure solution and in coffee in the training and examination procedures for coffee ‘cuppers’ (Lingle and Menon, 2017).1 SCA focuses on citric, malic, lactic, and tartaric, and CQI focuses on citric, malic, acetic, and phosphoric. Based on qualitative differences in the acidity of OAs and difference in the coffee's chemical acid composition certified coffee specialists are purportedly able to distinguish between the coffee's geographical origin (Rivera, 2020). For instance, it is believed that Kenyan coffee contains lower levels of citric acid and higher levels of malic acid than coffee from Central America (Balzer, 2001; Rivera, 2020). The common wisdom amongst coffee experts is that coffee from Kenya is especially known for its high, fine, and citrus-like acidity (Thomas et al., 2017; Vitzthum, 1976). However, this relation between acids and their origin has not been reported or systematically investigated in chemical studies of acids in coffee. Furthermore, neither the detection threshold nor recognition of the acids found in coffee is thus far reported in the scientific literature. In this study, we investigate acids (incl. OAs) and sugars in coffee brewed with a French press method, which due to the simplicity of the brewing process makes it easy to standardize the brewing procedure. Further, the French press method is the most similar to the industry standard for coffee evaluation called ‘cupping’ where hot water is poured on ground beans. We used beans of different (but limited) geographical origins: Kenya, Brazil, and Bolivia. As it is beyond the scope of this study to make a complete map of organic acids in coffees worldwide, but rather look at the general relationship between acids and perceived acidity. Therefore, we chose a rather small but relevant sample set based on the common wisdom in the specialty coffee community that Kenya coffees are amongst the coffees with the highest perceived acidity and Brazilian coffees are the lowest. Further, we systematically varied the roasting degree to three different levels (lighter, medium, and darker). The research hypothesis of the study is that acid concentrations alone are insufficient to distinguish between the geographical origins of brewed coffees as other parameters such as roasting degree will have a higher influence on the acid concentrations. Further, we hypothesize that not all acids in coffee are present in concentrations relevant to the human detection threshold. The first aim of this paper is to investigate trends based on the OAs differences and similarities primarily between roast level and geographical origin. The second aim of this paper is to find the detection threshold of five acids (citric, malic, acetic, lactic, and phosphoric) in brewed coffee and whether coffee experts can identify the acids in water and in brewed coffee. From a practical application perspective, we aim to support and improve future sensory training for coffee experts by providing chemical data on the concentration and detection threshold values of OAs.
2. Materials and method
2.1. Coffee samples and roasting
Five different high-quality C. arabica samples: two from Brazil (Minas Gerais), one from Bolivia, and two from Kenya (Embu and Nyeri), were studied. Table 1 presents specifications on the coffees: producer, species, growth altitude, and processing method. Each coffee was roasted to three different roasting levels (relative scale, see Table 1 for specifications): lighter (L), medium (M), and darker (D), chosen to be relevant for the Specialty coffee industry which is generally less dark than in the commodity segment. Our “medium” roast is, therefore, more equivalent to some roasts defined as “light” in earlier literature, and our “darker” are in the range reported in the literature as a medium roast. Roasting was performed on a Stronghold S7 ProX (Stronghold Technology, Seoul, South Korea). The roast profiles were designed to imitate specialty coffee roast times (Münchow et al., 2020). To standardize the roasting, all lighter, medium, and darker samples were roasted on the same profile and further, the roasting was terminated at 169 °C, 174 °C, and 180 °C,2 accordingly. The main roast profile parameters (times and temperature) are reported in Table 1. The Roast color of the coffee was measured with Lighttells CM-100 plus (Lighttells, Zhubei, Taiwan) on ground coffee, and Agtron numbers in Table 1 are averages of triplicate measurement for each sample. As Agtron reading on ground coffee depends on the grind size, we chose the finest grind size that still not caused any clumping of the surface of the ground coffee of any of the samples, when distributed evenly with a thin ruler oriented perpendicularly in the reading plate to avoid any compression of the sample during distribution.
Table 1.
Coffee and roasting data for the coffee samples.
| SAMPLE NAME | GEOGRAPHICAL ORIGIN | PRODUCER | C. ARABICA CULTIVAR | ALTITUDE (m) | PROCESSING | ROAST CATEGORY | COLOR(Agtron number)a | TOTAL ROAST TIME (s) | DEVELOPMENT TIMEb (s) | END TEMP (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| BRAZIL 1_D | Minas Gerias | Fazenda | Icatú | 1250 | Pulped Natural | Darker | 62 | 624 | 117 | 180.2 |
| Brazil | São Silvestre | |||||||||
| BRAZIL 1_M | Minas Gerias | Fazenda | Icatú | 1250 | Pulped Natural | Medium | 80 | 601 | 61 | 174 |
| Brazil | São Silvestre | |||||||||
| BRAZIL 1_L | Minas Gerias | Fazenda | Icatú | 1250 | Pulped Natural | Lighter | 109 | 505 | 10 | 169 |
| Brazil | São Silvestre | |||||||||
| BRAZIL 2_D | Minas Gerias | Daterra | Bourbon | 1200 | Pulped Natural | Darker | 61 | 630 | 120 | 180.1 |
| Brazil | Full Bloom | |||||||||
| BRAZIL 2_M | Minas Gerias | Daterra | Bourbon | 1200 | Pulped Natural | Medium | 81 | 518 | 83 | 174.1 |
| Brazil | Full Bloom | |||||||||
| BRAZIL 2_L | Minas Gerias | Daterra | Bourbon | 1200 | Pulped Natural | Lighter | 110 | 505 | 0 | 168.6 |
| Brazil | Full Bloom | |||||||||
| BOLIVIA_D | Taypiplaya | Villa Imperial | Caturra, Catuai, Criolla | 1600 | Washed | Darker | 54 | 648 | 168 | 180.4 |
| Bolivia | ||||||||||
| BOLIVIA_M | Taypiplaya | Villa Imperial | Caturra, Catuai, Criolla | 1600 | Washed | Medium | 77 | 601 | 49 | 174 |
| Bolivia | ||||||||||
| BOLIVIA_L | Taypiplaya | Villa Imperial | Caturra, Catuai, Criolla | 1600 | Washed | Lighter | 115 | 505 | 5 | 169.3 |
| Bolivia | ||||||||||
| KENYA 1_D | Nyeri | Kieni | SL28, SL34, Ruiru11, Batian | 1800 | Washed | Darker | 51 | 629 | 174 | 180 |
| Kenya | ||||||||||
| KENYA 1_M | Nyeri | Kieni | SL28, SL34, Ruiru11, Batian | 1800 | Washed | Medium | 74 | 578 | 88 | 174 |
| Kenya | ||||||||||
| KENYA 1_L | Nyeri | Kieni | SL28, SL34, Ruiru11, Batian | 1800 | Washed | Lighter | 102 | 496 | 28 | 169 |
| Kenya | ||||||||||
| KENYS 2_D | Embu | Gakundu | SL28, SL34 | 1680 | Washed | Darker | 50 | 627 | 172 | 180.2 |
| Kenya | Coffee Factory | |||||||||
| KENYA 2_M | Embu | Gakundu | SL28, SL36 | 1680 | Washed | Medium | 71 | 594 | 89 | 174 |
| Kenya | Coffee Factory | |||||||||
| KENYA 2_L | Embu | Gakundu | SL28, SL35 | 1680 | Washed | Lighter | 102 | 491 | 21 | 169 |
| Kenya | Coffee Factory |
Measure indicating the roast degree of the roast (smaller numbers indicate darker roasts).
Defined as time elapsed from ’first crack’ (i.e., the moment when the accumulated steam pressure causes the beans to crack) to the end of the roasting process (Münchow et al., 2020).
2.2. Grinding and brewing
For the chromatographic measurements, the coffees were ground using a Sage Dose Control™ Pro (Breville, Sydney, Australia). Due to the different roast degrees, the grinding of the coffee was adjusted per sample, so that the final brew all had the same Total Dissolved Solid (TDS) around 1.1 (L: 1.07 ± 0,04, M: 1.11 ± 0.02, D: 1.20 ± 0.04) and (Brazil 1: 1.08 ± 0.01, Brazil 2 1.1 ± 0.06, Bolivia: 1.13 ± 0.07, Kenya 1: 1.16 ± 0.06, Kenya 2: 1.14 ± 0.09). To avoid cross-contamination between coffees, the grinder was pre-treated with 10 g of the relevant coffee before the ground coffee was collected. According to an adaptation of the Golden cup standard (SCAA, 2015), 30 g ( ± 0.0g) coffee beans were weighed and placed in a 500 ml Bodum Colombia “French Press”. Then 500 g ( ± 2g) of chemically controlled water was poured over the coffee. The water used was controlled by running through a BWT Bestaqua 14 ROC Coffee filter system (Best Water Technology, Mondsee, Austria), with the following chemical and coffee brewing technically important specifications: 4 DH/71.2 ppm, 3 CDH/65.4 ppm alkalinity, pH 7.1, 62 μs/cm/43 ppm TDS, 1 ppm sodium, 1,5 ppm chloride. The brew time started when the first water touched the coffee. After 3.30 min the coffee crust was broken with three strokes of a spoon, and the coffee was defoamed. After 4 min the stamp was pressed down. Before chemical analysis, the coffee samples were filtered using a 0.22 μm filter.
2.3. TDS and pH measurements
TDS and pH were measured after 0.22 μm filtration and were done at room temperature (20 °C). TDS was measured using a VST LAB Coffee II-refractometer (VST Inc, Cambridge, MA, US), while the pH was measured with a Mettler Toledo SevenCompact S220 pH-meter (Mettler Toledo, Glostrup, Denmark) using an InLab® Routine Pro probe.
2.4. Chromatographic measurements
2.4.1. Organic acids
OAs and phosphoric acid were quantified on Dionex ICS-3000 ion exchange chromatographic system, consisting of an AS1 autosampler, DC-2 Detector/Chromatography system equipped with an AMMS-ICE 300 suppressor, and a DP-2 pump (Thermo Fisher Scientific, Hvidovre, Denmark). 10 μL of the sample was separated on an IonPac ICE-AS6 IC ion exclusion column of size 250 mm × 9 mm, 8 μm particle size (Thermo Fisher Scientific, Hvidovre, Denmark) by an isocratic flow of 0.2 mM HCl. The column was kept at 35 °C and the organic acid was quantified by electrochemical detection. The organic acids content was calculated from a standard curve prepared in a suitable concentration range and all samples were measured in triplicate. Spike-recovery samples were done by spiking with 2 mg/mL of the relevant acid.
2.4.2. Chlorogenic acids
Due to its chemical structure, CGA was not suitable for the ion exchange method but determined by reverse phase high-performance liquid chromatography (HPLC) on an Agilent HPLC consisting of a G1379B degasser, a 1312A binary pump, a G1312A autosampler with a G1330A cooling unit, a G1316A column oven, and a G1315D PDA detector (Agilent, Glostrup Denmark). The samples were analyzed on a Phenomenex Luna C18 150 × 4.6 mm column with 3 μm particles by a gradient of water and acetonitrile, both with 0.1% of formic acid. The gradient was kept at 5% MeCN for 2 min and then changed to 25% MeCN over the next 20 min and subsequently kept there for 5 min. The flow rate was 0.6 mL/min. The concentration was calculated as the total amount of chlorogenic acid. Relevant peaks were identified by comparison of the UV-spectrum of the peak and pure standard of Chlorogenic acid. The Chlorogenic acid standard was also used for the quantification of all isomers of chlorogenic acid. The chromatogram at 330 nm was used for quantification. All samples were measured in triplicate.
2.4.3. Sugars
Sugars were quantified on a Dionex Ultimate 3000 HPLC system consisting of an LPG 3400 pump, a WPS 3000 SL autosampler, a TCC-3000SD column oven, andana Idex RefractoMax 521 refractive index detector (Thermo Fisher Scientific, Hvidovre, Denmark). Before measurement, organic acids were removed from the samples by passing them through a strong anion exchange column (Biotage EVOLUTE AX 50 μm 100 mg/3 mL, Biotage, Uppsala, Sweden), which was conditioned with 3 mL of methanol followed by 3 mL ultrapure water. Before passing the sample through the anion exchange column, 25 μL of 1 M NaOH was added to the sample to ensure full deprotonation of the acids. 5 μL of the purified samples were then separated by an isocratic flow of ultrapure water through a 150 × 4.6 mm Phenomenex Rezex RHM-Monosaccharide H+ column (Phenomenex, Værløse, Denmark). The flow rate was 0.4 mL/min and the column was kept at 79 °C. The sugar content was calculated from a standard curve prepared in a suitable concentration range and all samples were measured in triplicate.
2.5. Sensory detection threshold
The threshold for the acids was found using a two-alternative ascending Forced-Choice Method (2-AFC) of limits based on the standard method designated ASTM E679-19 and the method presented by Ennis and Jesionka (2011).
The samples were prepared according to (ISO 3972, 2011), for the basic taste of acidic, and brewed coffee (a mix of medium roasted Brazil 2 and Kenya 2) at room temperature was spiked with each acid. Based on pilot studies and the average concentration of acids found from chromatographic measurements four different concentrations were determined and were set at 0.31 g/L, 0.25 g/L, 0.20 g/L, and 0.16 g/L. Each concentration was presented together with the control sample and assessors were instructed to choose the more acidic of the two. As suggested by de Bouillé (2017) assessors were also asked to indicate their level of confidence using a 3-point scale (1 = Not confident, 2 = Fairly confident, 3 = Completely confident).
The samples were served every 2 min with a 5-min break between each pair. White toast bread and water were provided as palate cleansers after each sample.
A total of 40 untrained assessors (mean age = 25, 48% women) evaluated each three of the five acids in a randomized incomplete block design where each acid was evaluated 24 times. The number of evaluations was set a priori in order to achieve a statistical power of 80% considering a d’ of 1 (small sensory difference), a significance level of α = 0.05, and a guessing probability of 50% for the 2-AFC test.
2.6. Sensory identification test
The identification of the five acids in water and brewed coffee were found by adding 0.50 g/L of lactic acid and 0.40 g/L of citric, malic, acetic, and phosphoric acids in water (according to the training from SCA), and the average measured concentrations (see Fig. 6) in brewed coffee. The samples were prepared as already described in section 2.4.
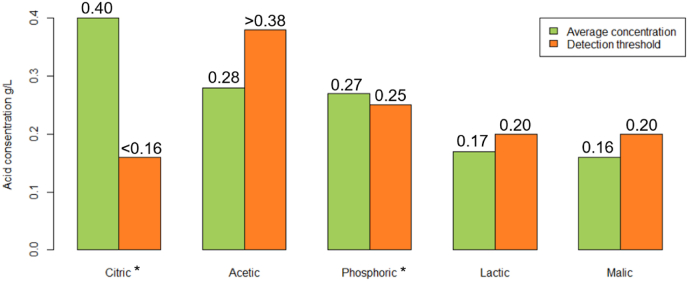
Fig. 6.
The measured average concentrations of all 15 samples and the sensory detection thresholds for five organic acids in brewed coffee. * Indicates that the threshold level is above the measured mean concentration.
A total of 13 coffee experts (mean age 29, 46% women), whom all have coffee as their profession, were used. They are all regularly trained and calibrated on sensory evaluations of coffee including organic acids. The experts were therefore also the main target group for testing the identification of organic acids in coffee. The experts evaluated each of the five acids in water and in brewed coffee. Immediately before evaluating the samples, they underwent a 30-min intense training session in detecting and memorizing the five acids in water. The coffee experts tasted the water solutions and coffee samples based on the identification of tastes test from ISO standard 3972:2011, which is a similar test setup used in the SCA and CQI training on organic acids. They tasted the samples one at a time in a complete randomized block design and were asked to name the specific acid added out of the five possible acids while being allowed to keep their personal descriptive notes for each acid from the training session.
2.7. Statistical analysis
Data from total acids were analyzed using 1-way ANOVA for the different roast levels. Data for individual acids and sugars were analyzed using a full factorial 2-way ANOVA design with roast level as a fixed factor, while sample origin was considered a random factor. Post-hoc analysis of significant differences was done by Tukey's HSD with a significance level (α = 0.05). Differences are indicated with letters, and the error bars given are standard deviations. Analyses of all chromatographic were conducted with JMP 14 software (SAS Inst., Cary, US).
For the sensory threshold data, the number of correct answers in the 2-AFC test was analyzed by an exact binomial test to determine differences between the control and the samples containing the acids at different concentrations. Sensory intensity differences between the samples were estimated based on the number of correct responses using the thurstonian model for the 2-AFC test (Christensen et al., 2012) to aid in the interpretation. The sensory data were analyzed in R (R Core Team, 2021) using functions from the sensR package (Christensen et al., 2018). The recognition test was analyzed with a chi-square test.
3. Results and discussion
3.1. Acid composition in brewed coffee
Samples of French press brewed light roast specialty coffee of varying roast levels (lighter, medium, darker) were subject to chromatographic separation of their acids. The chromatography revealed nine to twelve separate peaks depending on the coffee sample. Using spike-recovery testing across all three roasting degrees, the following nine peaks were identified: phosphoric, citric, malic, quinic, glycolic, formic, lactic, succinic, and acetic acid. The mean recovery was 98.7 ± 8.7% and the recovery range was 87.5–119.2%. The unidentified peaks were only present in some coffees and only in insignificant amounts; furthermore, succinic acid was found in amounts below the quantification limit and was not included in further analyses. Tartaric acid was also run as a standard but was not found in quantifiable amounts in any of the coffee samples.
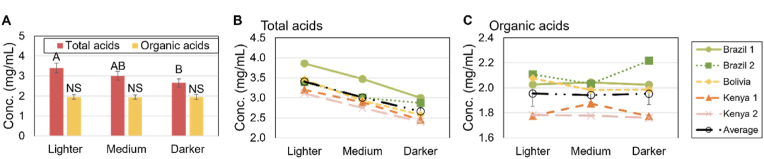
The mean concentration of total acids (sum of all identified acids) and mean concentration of all OAs (all acids excluding chlorogenic and phosphoric acid) for the varying roast degrees can be seen in Fig. 1. As seen, there is a decrease in the mean total acid concentration with increased roast level, while no significant change in the mean concentration is seen when only considering the OAs.
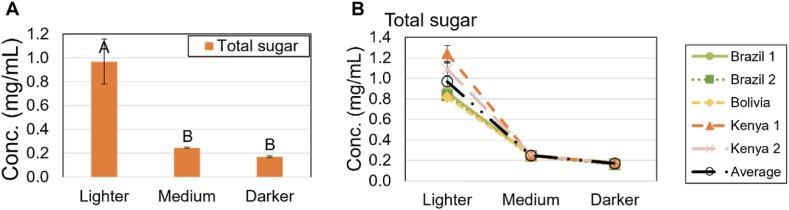
Fig. 1.
Total acid concentration. A) Mean acid concentrations for total acids (all acids, red) and for total organic acids (all acids except chlorogenic acid and phosphoric acid, yellow) for varying roast levels. Letters indicate significant differences (p < 0.05) between roast levels within each series (total acids and total organic acids). B) Total acids and C) total OAs for each sample (Brazil 1, light green, Brazil 2, dark green, Bolivia, yellow, Kenya 1, orange, Kenya 2, pink, and the mean for all coffees, black) for varying roast levels.
The observed difference in total acids is mainly attributed to the expected decrease in chlorogenic acid concentration due to thermal degradation during roasting. This decrease is also confirmed as the chlorogenic acid concentration decreases by approx. 0.4 mg/mL for all coffees when changing the roast level from lighter to medium, and a further 0.4 mg/mL decrease is observed from medium to darker (Fig. 2A).
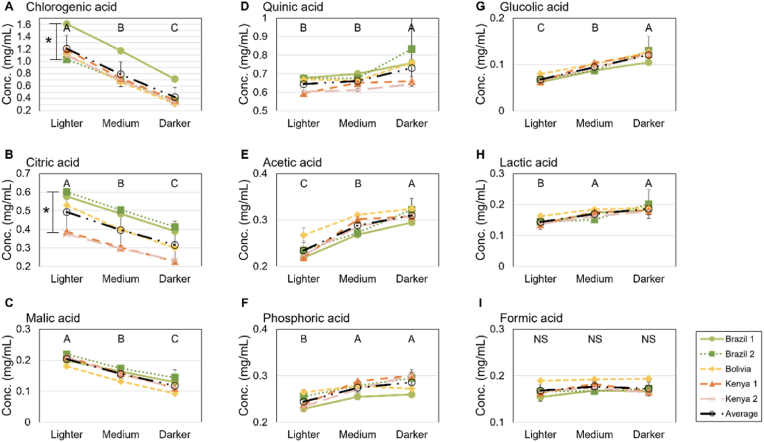
Fig. 2.
Acid concentrations for A) Chlorogenic acid, B) Citric acid, C) Malic acid, D) Quinic acid, E) Acetic acid, F) Phosphoric acid, G) Glycolic acid, H) Lactic acid, and I) Formic acid for measured coffee samples (Brazil 1, light green, Brazil 2, dark green, Bolivia, yellow, Kenya 1, orange, Kenya 2, pink, and the mean for all coffees, black) for varying roast levels. Letters indicate significant differences (p < 0.05) between roast levels. Please note that the scales are different (horizontal lines are separated by 0.1 mg/mL for all acids). * on graphs for chlorogenic and citric acid denotes p < 0,001 for sample difference.
The mean total concentration of OAs was found not to change when varying the roast degree, but as will be shown below, the relative distribution of the different AOs does change for the different roast levels, so the overall mean non-difference could be coincidental and not necessarily a systematic tendency.
Fig. 2 shows CGA and CGA-derived quinic acid are the two most abundant acids in brewed coffee with mean concentrations between 0.6 and 0.8 mg/mL, citric, acetic, and phosphoric acids have intermediate concentrations between 0.2 and 0.4 mg/mL, while the remaining acids are <0.2 mg/mL (Fig. 2, Table 2).
Table 2.
Acid concentrations and p-values. Mean acid concentration for each roast level, and p-values associated with comparison of roast level (fixed factor: lighter, medium, darker). Letters indicate significant differences (p < 0.05) between roast levels.
| ACID | ROASTING |
||||||
|---|---|---|---|---|---|---|---|
| Lighter |
Medium |
Darker |
p-value | ||||
| mean |
std |
mean |
std |
mean |
std |
||
| (mg/mL) | (mg/mL) | (mg/mL) | |||||
| Chlorogenic | 1.207 | 0.212 | 0.792 | 0.198 | 0.423 | 0.152 | |
| A | B | C | <0.0001 | ||||
| Quinic | 0.644 | 0.039 | 0.661 | 0.032 | 0.731 | 0.097 | |
| B | B | A | 0.0055 | ||||
| Citric | 0.493 | 0.100 | 0.396 | 0.092 | 0.316 | 0.085 | |
| A | B | C | <0.0001 | ||||
| Acetic | 0.235 | 0.021 | 0.288 | 0.017 | 0.310 | 0.012 | |
| C | B | A | <0.0001 | ||||
| Phosphoric | 0.244 | 0.014 | 0.274 | 0.012 | 0.286 | 0.019 | |
| B | A | A | 0.0024 | ||||
| Formic | 0.168 | 0.012 | 0.177 | 0.010 | 0.172 | 0.013 | |
| NS | NS | NS | 0.0758 | ||||
| Lactic | 0.144 | 0.013 | 0.169 | 0.013 | 0.186 | 0.020 | |
| B | A | A | 0.0006 | ||||
| Malic | 0.204 | 0.014 | 0.156 | 0.015 | 0.118 | 0.020 | |
| A | B | C | <0.0001 | ||||
|
Glycogenic |
0.067 | 0.007 | 0.094 | 0.008 | 0.121 | 0.015 | |
|
C |
B |
A |
<0.0001 |
||||
| Total acids | 3.404 | 0.240 | 3.007 | 0.223 | 2.660 | 0.204 | |
| A | AB | B | 0.0040 | ||||
| Total OAs | 1.954 | 0.112 | 1.942 | 0.102 | 1.952 | 0.134 | |
| NS | NS | NS | 0.9870 | ||||
The pH measurements of the coffee samples showed a higher acidity for the lighter roasts (L: pH 3.97 ± 0.1, M: pH 4.10 ± 0.2, and D: pH 4.25 ± 0.1). The Brazilian coffees samples have on average the lowest pH (Brazil 2: pH 4.00 ± 0.2 and Brazil 1: pH 4.08 ± 0.2), followed by Kenya 2 (pH 4.10 ± 0.1), Bolivia (pH 4.12 ± 0.1) and Kenya 1 (pH 4.23 ± 0.2) with the highest pH. Although only slight differences were found, the trend was opposite to the common belief in the industry that coffee from Brazil generally contains a lower concentration of acids than those from Kenya. According to (Belitz et al., 2009), the taste of the coffee depends on pH and Brazilian coffee would have a pH of 5.0 and that Kenyan coffee would have a pH of 4.85. Another study (Brollo et al., 2008) found, like we did, that the pH values for brewed coffee were higher for the African coffee (Kenya pH 5.21 and Ethiopia pH 5.37) than for the brewed coffee from Central America (Guatemala pH 4.96 and El Salvador 5.09 and 5.02).
The pH values that we obtained in this study are somewhat lower than those found in other studies (Belitz et al., 2009; Brollo et al., 2008; Córdoba et al., 2021; Rao and Fuller, 2018). One possible explanation for this is that the roast levels in our study are comparably lighter than those reported in the literature. However, other factors than roast degree might also play a role, e.g., different brewing methods were found of the same coffee samples were found to yield different pH (Córdoba et al., 2021; Rao and Fuller, 2018). Based on the SCA water quality handbook (SCA, n.d.) another explanation could be the use of different brew water. Since none of the other studies mention the composition of their brew water, we cannot further conclude this.
3.2. Effect of roasting degree on individual acids
To further investigate specific variations for the individual acids, their concentrations were plotted to visualize differences due to roasting levels for the different sample origins (Fig. 2). While the total mean OA concentration did not depend on the roasting degree, the roast degree significantly affected the acid concentrations of individual acids in the brewed coffee (Fig. 2, Table 2). Significant differences between all three roast levels (L, M, and D) were found for all nine compounds except formic acid. The concentration of citric, malic, and chlorogenic acids decreased with increasing roast levels (L to D), whereas the concentration of acetic, lactic, quinic, phosphoric, and glycolic acid increased (Fig. 2). Formic acid was slightly higher for M roast as compared to L and D, a trend that has also previously been observed in brewed coffee by Rodrigues et al. (2007), based on only three samples, where not all acids were recovered in all three samples. And since the three other studies looking at OAs in brewed coffee did not look at the roasting difference, our study stands alone.
The observed changes in the acid composition due to the effect of roasting are however also similar to the finding of roasted coffee (Balzer, 2001; Ginz et al., 2000; Verardo et al., 2002; Wei and Tanokura, 2015). This suggests that all acids are extracted equally well when the coffee is brewed, but this might strongly depend on the extraction method and therefore, more studies are needed to shed light on this subject.
3.3. Effect of coffee origin
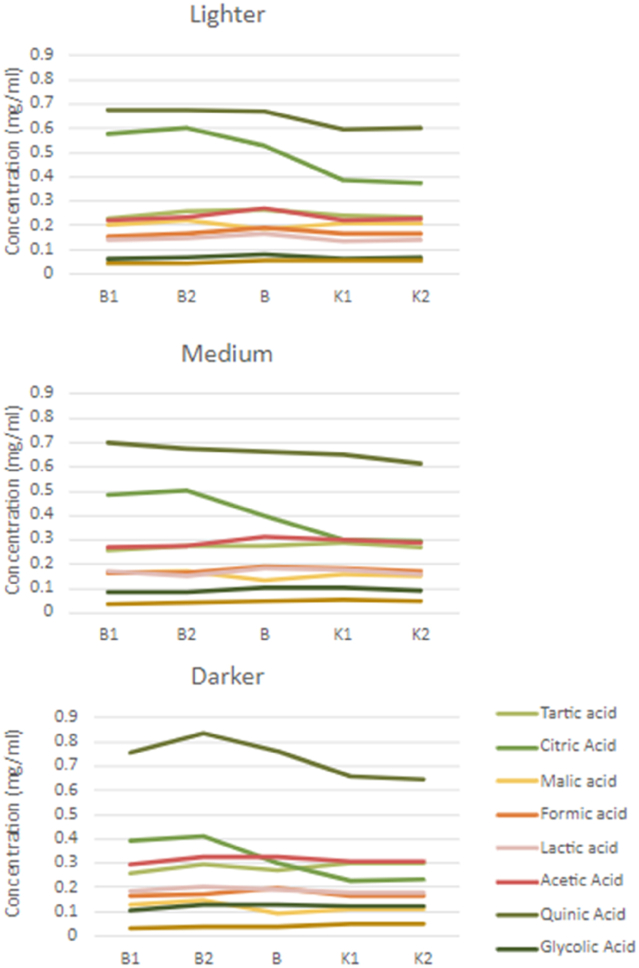
As seen in Fig. 2 (and in Appendix A), the differences in acid concentration between the five coffee samples were generally not strongly dependent on the geographical origin except for citric and chlorogenic acid. Citric acid had larger and significant (mean for all roast levels, p < 0.001) variations between the sample origins. For chlorogenic acid, Brazil 1 had a much higher concentration than the other samples (p < 0.001). The two Kenyan coffees, which are from different regions in Kenya, differed slightly in chlorogenic acid concentration (mean for all roast levels, p < 0.001), but showed no significant differences between any other acids. In contrast, the two Brazilian coffees that are from the same region were significantly different in the concentration of chlorogenic, citric, malic, phosphoric, and acetic acids (mean for all roast levels, p < 0.001). Sample Brazil 1 had the same concentration of malic acid as both Kenyan coffees and Brazil 2 did not differ in the concentration of acetic, phosphoric, formic, and glycolic acid to both Kenyan coffee samples. These findings are in line with Rodrigues et al. (2007) who also found that the acid concentration varied among samples from the same origin linked to other differences such as growth altitude, soil content, and post-harvest conditions. Amalia et al. (2021) found that post-harvest processing is a more dominant factor for the OA composition in green coffee, followed by origin and altitude. Our study was limited to a small number of samples and therefore conclusions on the effect of geographical origins should not be generalized, which would require a larger experimental design. However, based on these selected coffee samples that were chosen based on expected large differences in acid concentration based on the geographical origin, no such differences were confirmed.
3.4. Sugar concentration and composition
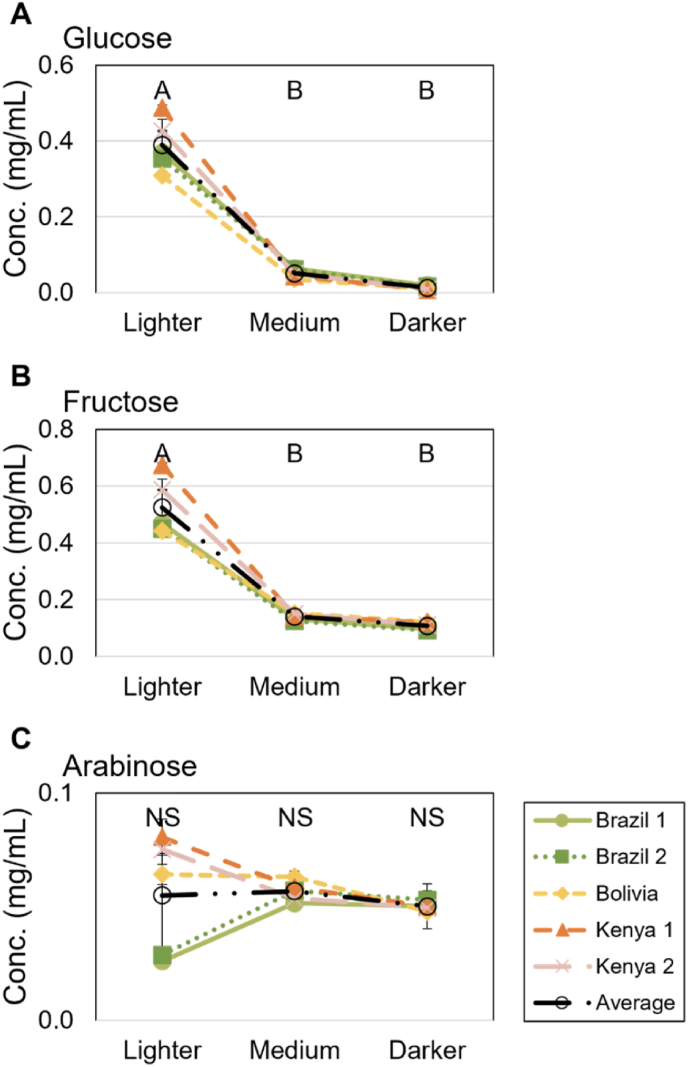
To validate if the observed changes in OAs were related to differences in sugar content, we also quantified levels of glucose and fructose in the brewed coffee. Sucrose was not measured directly due to thermal cleavage during the chromatographic process. Arabinose has also been measured as an intermediate in the roast-induced degradation of sugars to other compounds. For the M and D roast brewed coffee, the sugar concentrations were very low (total mean < 0.2 mg/mL), and although significant (Table 3), the numerical difference was small between M and D roast, and the importance of origin for glucose and fructose was low. For the L roast coffee, the total sugar concentration for all lighter roasted coffee was>0.9 mg/mL and additionally, the two Kenyan coffees contained significantly (p < 0.001) more fructose, glucose, and arabinose than the remaining coffees (Fig. 3, Fig. 4, Appendix B). The difference might be due to the difference in terroir, altitude, and cultivar (Avelino et al., 2005; Borém et al., 2016).
Table 3.
Sugar concentrations and p-values. Mean sugar concentration for each roast level, and p-values associated with comparison of roast levels (fixed factor: lighter, medium, darker). Letters indicate significant differences (p < 0.05) between roast levels.
| SUGAR | ROASTING |
||||||
|---|---|---|---|---|---|---|---|
| Lighter |
Medium |
Darker |
p-value | ||||
| mean |
std |
mean |
std |
mean |
std |
||
| (mg/mL) | (mg/mL) | (mg/mL) | |||||
| Glucose | 0.389 | 0.068 | 0.050 | 0.011 | 0.012 | 0.005 | |
| A | B | B | <0.0001 | ||||
| Fructose | 0.525 | 0.100 | 0.140 | 0.011 | 0.107 | 0.013 | |
| A | B | B | <0.0001 | ||||
|
Arabinose |
0.055 | 0.025 | 0.057 | 0.005 | 0.050 | 0.003 | |
|
NS |
NS |
NS |
0.7778 |
||||
| Total sugar | 0.969 | 0.656 | 0.247 | 0.159 | 0.170 | 0.119 | |
| A | B | B | <0.0001 | ||||
Fig. 3.
Mean total sugar concentration. A) Mean concentration of total measured sugars for varying roast levels. Letters indicate significant differences (p < 0.05) between roast levels. B) Mean concentration of total sugars for each sample (Brazil 1, light green, Brazil 2, dark green, Bolivia, yellow, Kenya 1, orange, Kenya 2, pink, and the mean for all coffees, black) for varying roast levels.
Fig. 4.
Sugar concentrations for A) Glucose, B) Fructose, and C) Arabinose for measured coffee samples (Brazil 1, light green, Brazil 2, dark green, Bolivia, yellow, Kenya 1, orange, Kenya 2, pink, and mean for all coffees, black) for varying roast levels. Letters indicate significant differences (p < 0.05) between roast levels.
Detection and recognition thresholds for sugars in coffee are not available in the literature. However, the thresholds for sucrose, fructose, and glucose dissolved in water are available, and range from 1 to 3 mg/mL and 2–6 mg/mL, respectively, but with large individual variations (Low et al., 2017; Hoehl et al., 2010). For M and D roasts, the sugars would therefore in the first iteration expectedly not directly be contributing to the taste profile, especially given that the detection threshold would be expected to be higher in coffee than pure water due to the more complex matrix. For the L roast, the sugar concentration is much closer to the threshold and could therefore affect the perceived coffee quality directly for some individuals. However, previous studies have shown that the perceived sweetness can increase even in samples with sugar concentrations below the detection threshold, pointing to the fact that perceived sweetness and sugar concentration is non-linear (Batali et al., 2020).
Further, as the sugar concentration in the L roast is significantly higher for Kenyan coffees than the Brazilian, this could indicate that the original green bean sugar level is higher. The higher sugar level is, however, not translated into a higher level of OAs for Kenyan coffee as demonstrated above. This could suggest that some of the differences experienced by coffee experts are not due to the OAs concentrations but to other thermal-induced sugar degradation compounds, which is also indicated by the higher TDS in Kenyan coffee.
3.5. Discussion of acids in brewed coffee
In brief, our results indicate that the roasting conditions do not change the total amount of OAs but rather change their relative abundance, as well as the total CGAs. As for sample origin, total acids were only higher for one of the Brazilian coffees that has much higher GCAs, whereas OAs are more abundant in the selected Brazilian coffee as compared to the Kenyan coffee, due to differences in mainly citric acid and quinic acid.
Our results agree with claims from industry experts that Kenyan coffee in general contains lower levels of citric acid than many central American coffees. However, they are in conflict with claims from the same experts that Kenyan coffee in general contains a higher level of malic acid (Balzer, 2001; Rivera, 2020), as we found no significant differences between the coffee from Brazil and Kenya even though the Brazil coffees contains slightly higher concentrations (Appendix C). In our study, we only tested coffee from South American countries, not Central America. Rodrigues et al. (2007) also found that both the Central and South American coffees contained more citric acid than African coffees and found no difference in malic acid content between African and American coffees. We found that the darker Brazilian coffees contained the same concentration of citric acid as the lighter roasted Kenyan coffees (Appendix C) and further that the concentration of malic acid was similar for both geographical origins. This conflicts with the claim made by coffee experts, that coffee origin can be distinguished on the basis of differences and similarities in the OAs concentration, where particularly citric and malic acids are said to be the most important acids for distinguishing between origins (Rivera, 2020).
Interestingly, Brazilian coffees have much higher citric acid concentration as compared to the other OAs (except quinic), while for Kenyan coffee the level of citric acids is comparable to the concentration of the other OAs.
From the meta-analysis performed by Yeager et al. (2021), it becomes clear that, even though acid concentration trends are found between the roasting levels, medium-roasted coffee is significantly more studied than light and dark-roasted coffee. Furthermore, the acid concentration from the roasted coffee extract obtained from various extraction methods and inconsistent extraction parameters were pooled with brewed coffee, perhaps because no one has, before now, questioned the comparison. In our study, based on brewed coffee, we found very different acid concentrations from those reported in roasted coffee (Yeager et al., 2021), indicating that it is not possible to compare the acid concentration found in roasted and brewed coffee. Therefore, future studies need to distinguish between acid concentration in roasted coffee and brewed coffee. For brewed coffee, the brewing method would be expected to influence the concentration of the acids in the brew. This was shown by Khamitova et al. (2020), who found very different acid yields from the same arabica coffee in espresso coffees with varying filters. Differences in brewing technique make it hard to compare acid concentrations across studies, e.g. ICO (1991) look at acid concentration using different grind sizes, water temperatures, and brew time and found all aspects to have a significant impact on the acid concentration.
Taken collectively, these results indicate that identifying coffee origin based on acid composition can be problematic. This is evident when considering that two Brazilian coffees from the same region but different cultivars are significantly different from one another in five out of nine acids (chlorogenic, citric, malic, acetic, and phosphoric) but the Brazilian coffees are only generally different from the Kenyan coffees from different regions and cultivars in three cases (chlorogenic, quinic, and citric) (Appendix A).
3.6. Sensory detection threshold and recognition of acids
Table 4 shows the results of the 2-AFC test in terms of the correct identification of the spiked samples for each concentration of the five acids. None of the samples containing acetic acid could be distinguished from the control, whereas for citric acids, all concentrations were significantly different from the control, indicating that the detection threshold for these acids in coffee must be higher than 0.31 mg/mL and lower than 0.16 mg/mL for acetic and citric acid, respectively. For the other three acids (lactic, malic, and phosphoric) the significant differences from the control depended on concentrations (Table 4), with the findings indicating a detection threshold of 0.20 mg/mL for lactic and malic acids, and 0.25 mg/mL for phosphoric.
Table 4.
Number of correct answers, estimated magnitude of sensory differences (d’) and assessors’ confidence level in the 2-AFC test (n = 24) where coffee with increasing concentrations of the five organic acids. Asterisks denote statistically significant differences from control assessed by exact binomial test (*p < 0.05; **p < 0.01; ***p < 0.001).
| Acid | Concentration(g/l) | Correct answers(n = 24) | Magnitude of difference (d’) | Not confident | Fairly confident | Completely confident |
|---|---|---|---|---|---|---|
| Acetic | 0.16 | 12 | 0 | 0 | 4 | 8 |
| 0.20 | 12 | 0 | 3 | 6 | 3 | |
| 0.25 | 10 | 0 | 3 | 5 | 2 | |
| 0.31 | 14 | 0.3 | 4 | 7 | 3 | |
| Citric | 0.16 | 19** | 1.1 | 6 | 5 | 8 |
| 0.20 | 18* | 0.9 | 4 | 4 | 10 | |
| 0.25 | 19** | 1.1 | 5 | 5 | 9 | |
| 0.31 | 22*** | 1.9 | 2 | 10 | 10 | |
| Lactic | 0.16 | 13 | 0.1 | 2 | 5 | 6 |
| 0.20 | 17* | 0.8 | 4 | 8 | 5 | |
| 0.25 | 19** | 1.1 | 4 | 8 | 7 | |
| 0.31 | 17* | 0.8 | 4 | 4 | 9 | |
| Malic | 0.16 | 10 | 0 | 2 | 8 | 5 |
| 0.20 | 18* | 0.9 | 4 | 9 | 4 | |
| 0.25 | 18* | 0.9 | 4 | 2 | 12 | |
| 0.31 | 21*** | 1.6 | 3 | 5 | 13 | |
| Phosphoric | 0.16 | 16 | 0.6 | 5 | 4 | 7 |
| 0.20 | 13 | 0.1 | 3 | 4 | 6 | |
| 0.25 | 17* | 0.8 | 3 | 4 | 10 | |
| 0.31 | 19** | 1.1 | 3 | 4 | 12 |
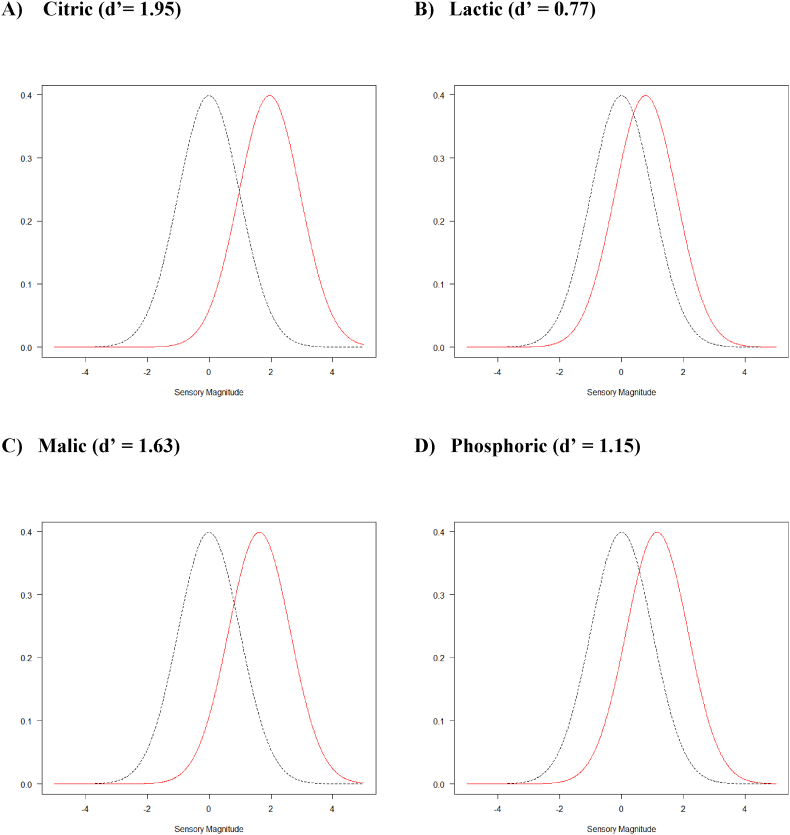
Supplemental analyses of the magnitude of difference, d’, and assessors' confidence levels (Table 4) indicated that most of the differences, while statistically significant, were small. For example, except for the highest concentrations of citric and malic, all other significant differences had d’ values ranging from 0.8 to 1, indicating very small differences (indeed, around threshold levels). Substantial overlap between the distribution of the control and the sensory intensities of even the highest concentrations was observed (Fig. 5), indicating that even at the highest concentration the samples could easily be confused (especially lactic and phosphoric).
Fig. 5.
Distribution of estimated sensory intensities for the 2-AFC tests for the four highest concentrations (0.31 mg/mL) for citric, lactic, malic, and phosphoric acid. The two distributions are estimated using a protocol-specific non-linear function. In this case, the psychometric function for the 2-AFC test (Ennis and Jesionka, 2011) through which the number of observed correct answers is directly linked to the underlying sensory difference (d’). The model is based on signal detection theory, which looks at a series of paired comparisons where a target stimulus (signal) is compared against a constant or standard stimulus (the noise) and looks at perceptual distributions (in a 2-AFC task, the proportion of times the signal is judged stronger than the noise). In this context, the black dotted line represents the distribution of the control samples, and the red continuous line that for the spiked samples. See Table 4 for numerical values for d’ for every tested concentration.
With respect to threshold estimations, a limitation of our study is that we used an incomplete design, and therefore are not able to assess the normality of the threshold distribution. If a significant heterogeneity exists, this could have affected the results. For example, for some of the compounds that were not discriminated from the control, it may be that there is a group that discriminated and one that did not, in such a way that differences in perceived acidity balance out in the population resulting in non-significant differences. For this paper, a focus on aggregate-level results was deemed sufficient. However, in the future, complete designs are advised to detect whether heterogeneity in the threshold exists. This may be important insights if, for example, such heterogeneity can be related to age, expertise, oral physiology, or other variables of interest.
The most important finding from the sensory data, however, is that most of the identified threshold values were above the actual concentrations of these compounds in coffee identified based on the chromatographic data. Fig. 6 shows this visually by comparing mean concentrations and threshold values for each acid where only citric acid is present at a concentration clearly above detectability. For the other acids, the data suggest that while they may contribute to the overall acidity, it seems unlikely that could be perceived individually and hence be the source of flavour differentiation between different coffees.
One possible caveat to the above is that the thresholds were determined based on untrained assessors and that perhaps lower threshold values might be observed with a panel of coffee experts (although that remains to be seen). In any case, in this test, we focused on detection thresholds whereas recognition thresholds (i.e., concentrations at which one would be able to correctly identify the individual acid) will necessarily be higher. Furthermore, in the subsequent identification test (described in section 2.4) we found that despite their expertise and training, none of the coffee experts successfully recognized and identified any of the five acids, in coffee spiked with the average concentration found in brewed coffee, and the only acids significantly (p > 0.001) recognized in water were acetic acid (Appendix D). With respect to the identification test, a limitation of our study is that we did not do a recognition threshold test and therefore were not able to point out at which concentration each acid could positively be identified and named. For this paper, an identification test performed similarly to the coffee certification bodies SCA and CQI was deemed sufficient, based on the results of the detection threshold test. However, in the future, a recognition threshold test is advised.
The argument behind spiking the coffee with the average concentration our study found, and doubling the concentration of each acid, was that this would make the investigated acid concentration way above the average concentration. This also factors in the possible counterargument that there might be some coffees not included in the study that contains an even higher concentration than the coffees included in this study. In terms of practical significance, from a sensory point of view, the data suggests that it makes much more sense to focus on overall coffee acidity than on individual organic acids.
3.7. Discussion of the sensory evaluation
The common wisdom amongst coffee experts is that coffee from Kenya is especially known to be perceived with high acidity. On the contrary from a chemical perspective, we found the two Kenyan samples in our study to have lower concentrations of particular importance citric acid than the two Brazilians, so we have found no evidence that the Kenyan coffees are more acidic. However, when it comes to sensory evaluation this might still be the case as multiple effects may affect perceived acidity. One example of such effects is the bitter taste of caffeine, trigonelline, and quinic acid (Farah, 2020); Brazilian coffee generally contains a significantly higher concentration of quinic acid than Kenyan coffee. The intensity and concentration of the acid and bitter components in the coffee may influence the perceived acidity and bitterness (Keast and Breslin, 2003). Another sensory study from Rubico and Mcdaniel (1992) found that the astringency and mouthfeel of the different organic and inorganic acids were more important in the description of the acids than the bitterness and sourness. Additionally, the literature indicates that different roast profiles (Alstrup et al., 2020), and different serving temperatures (Steen et al., 2017; Talavera et al., 2007), of the same coffees, generate different aroma profiles. The perceived acidity is also reported to be influenced by the storage time of the beans after roasting (Kreuml et al., 2013), the method of brewing (Belitz et al., 2009; CRI, 2006; Farah, 2020; Gloess et al., 2013), and pH and titrable acids of the coffee (Brollo et al., 2008; Gloess et al., 2013). Also, the brewing method affects the aroma, chromatographic and spectroscopic profiles significantly (Stanek et al., 2021).
Based on the findings in this study, acidity in coffee must be viewed as a more holistic concept rather than considering the perceived acidity to have a simple linear response to the acid concentration of either all acids or specific individual acids. The need for a more nuanced approach to better understand the correlation between not only the perceived acidity, but the other tastes as well, and the concentration of their respective main taste compounds is becoming more apparent. This is further backed by findings in relation e.g. to the sweetness of coffee (Batali et al., 2020) and also non-coffee products with possible mechanisms being governed by both masking effects or synergistic effects between taste and aroma perception. Cross-modal aroma-taste interaction due to e. g. a fruity aroma, as often found in e.g. Kenyan coffee, could also increase the perceived acidity. Even though the total acid content is higher in coffee from e.g. Brazil, Kenyan coffee would, in this case, be perceived as more acidic. Therefore, it can also be hypothesized that the aroma profiles of brewed coffee might also play a significant role in the perception of the actual chemical content of acids or sugars. Additional research is therefore warranted to understand these interactions from both a chemical and a sensory perspective.
4. Conclusion
This study has reported the concentration of chlorogenic, quinic, citric, acetic, phosphoric, formic, lactic, malic, and glycolic acid, pH values, and sugar content in brewed coffee varying in three different lighter specialty roasting degrees and five different sample origins. Furthermore, this study has reported the sensory detection threshold values and an identification test of citric, acetic, phosphoric, lactic, and malic acid.
The study shows that the concentration of individual acids systematically varies with the roasting degree for all acids. Significant and strong differences in acid concentration between the three roast degrees were found for all acids (except formic acid). The concentration of chlorogenic, citric, and malic acid decreased, whereas that of quinic, acetic, lactic, phosphoric, and glycolic acid increased with the increasing roast degree (lighter to darker).
Related to the origin, on average the Brazilian and Bolivian coffees contain a higher organic acid concentration than the Kenyan coffees. This was, however, mainly due to the higher amount of only two acids: citric and quinic acid. Also, the pH was lower in the Brazilian brews. This seemingly contrasts with the popular belief that Kenyan coffees are more acidic than Brazilian, and we highlight that recognizing the geographical origin of coffee based on organic acid content is at this point questionable and not supported or understood from a chemical perspective. However, as we only considered two samples from each country, further studies are needed to robustly conclude the effect of origin.
From the sensory detection threshold values found, only citric acid can clearly be detected in concentrations above average measured concentration. None of the five acids were correctly identified in coffee spiked with the average concentration found in brewed coffee by coffee experts. The other acids contribute to the overall acidity but focusing on their individual contribution to the coffee acidity appears unjustified, and indeed it appears that other factors than acids influence the perceived acidity.
Funding
This research did not receive any external funding.
CRediT authorship contribution statement
Christina J.Birke Rune: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Davide Giacalone: Conceptualization, Methodology, Validation, Writing – review & editing, Supervision. Ida Steen: Investigation, Writing – review & editing. Lars Duelund: Methodology, Investigation, Writing – review & editing. Morten Münchow: Conceptualization, Methodology, Writing – review & editing. Mathias Porsmose Clausen: Conceptualization, Validation, Formal analysis, Writing – original draft, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Jesper Alstrup (CoffeeMind) for roasting the coffee samples. Loui Baeré (CoffeeMind) for brewing the coffee prior to the chemical analysis. Rikke Klindt Müller (SDU) for performing the IC analysis. The authors would also like to thank Coffee Collective, Denmark for their great help in this study.
Handling Editor: Professor Aiqian Ye
Footnotes
Depending on the specific program, the following compounds are used in training and exam material: citric (CQI, SCA), malic (CQI, SCA), phosphoric (CQI), acetic acid (CQI only), tartaric (SCA) and lactic acid (SCA).
The termination temperature was measured by the bean probe in the roaster (not the IR probe which is also present in the Stronghold S7 ProX). Since we choose to keep the end temperature the same and not compensate the end temperature to keep the color the same, we were getting extra information on how the different beans reacts differently to the same process in the risk of getting differences between the coffees caused by difference in color. The risk would be to have less acids in the beans reacting more to the same temperature but as will be obvious in the data we saw higher concentrations of particularly the dominating Citric acids in the Kenya coffees that displayed a darker roast color (Agtron 50 and 51 on the dark roast profile) than the Brazil (Agtron 61 and 62).
Appendices.
Appendix Table A.
Acid concentrations. Means and standard deviations of acid concentration for each roast sample origin (averaged across all roast levels).]
| ACID | SAMPLE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brazil 1 |
Brazil 2 |
Boliva |
Kenya 1 |
Kenya 2 |
||||||
| mean |
std |
mean |
std |
mean |
std |
mean |
std |
mean |
std |
|
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | ||||||
| Chlorogenic | 1.163 | 0.385 | 0.696 | 0.291 | 0.701 | 0.346 | 0.763 | 0.352 | 0.712 | 0.326 |
| Quinic | 0.712 | 0.036 | 0.728 | 0.116 | 0.697 | 0.048 | 0.635 | 0.033 | 0.620 | 0.019 |
| Citric | 0.483 | 0.083 | 0.509 | 0.081 | 0.409 | 0.100 | 0.306 | 0.071 | 0.301 | 0.064 |
| Acetic | 0.261 | 0.032 | 0.274 | 0.035 | 0.303 | 0.024 | 0.275 | 0.042 | 0.274 | 0.038 |
| Phosphoric | 0.248 | 0.015 | 0.276 | 0.020 | 0.271 | 0.008 | 0.275 | 0.029 | 0.269 | 0.030 |
| Formic | 0.163 | 0.008 | 0.168 | 0.009 | 0.192 | 0.003 | 0.170 | 0.009 | 0.168 | 0.005 |
| Lactic | 0.164 | 0.022 | 0.166 | 0.036 | 0.179 | 0.013 | 0.164 | 0.021 | 0.159 | 0.018 |
| Malic | 0.164 | 0.030 | 0.179 | 0.035 | 0.135 | 0.038 | 0.160 | 0.044 | 0.158 | 0.042 |
|
Glycogenic |
0.084 |
0.019 |
0.095 |
0.032 |
0.103 |
0.020 |
0.096 |
0.026 |
0.093 |
0.024 |
| Total acids | 3.442 | 0.400 | 3.091 | 0.331 | 2.989 | 0.367 | 2.844 | 0.369 | 2.754 | 0.340 |
| Total OAs | 2.031 | 0.106 | 2.118 | 0.158 | 2.016 | 0.122 | 1.806 | 0.105 | 1.774 | 0.093 |
Appendix Table B.
Sugar concentrations. Means and standard deviations for sugar concentration for each roast sample origin (averaged across all roast levels)
| SUGAR | SAMPLE |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brazil 1 |
Brazil 2 |
Boliva |
Kenya 1 |
Kenya 2 |
||||||
| mean |
std |
mean |
std |
mean |
std |
mean |
std |
mean |
std |
|
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | ||||||
| Glucose | 0.144 | 0.159 | 0.151 | 0.156 | 0.118 | 0.144 | 0.179 | 0.233 | 0.161 | 0.200 |
| Fructose | 0.222 | 0.172 | 0.232 | 0.179 | 0.238 | 0.154 | 0.312 | 0.274 | 0.282 | 0.231 |
|
Arabinose |
0.046 |
0.013 |
0.043 |
0.013 |
0.058 |
0.008 |
0.063 |
0.016 |
0.059 |
0.014 |
| Total sugar | 0.412 | 0.268 | 0.426 | 0.280 | 0.415 | 0.272 | 0.554 | 0.365 | 0.503 | 0.330 |
Appendix Fig. C.
Measured acid concentrations for each acid in lighter, medium, and darker roast coffee samples..
Appendix Fig. D.
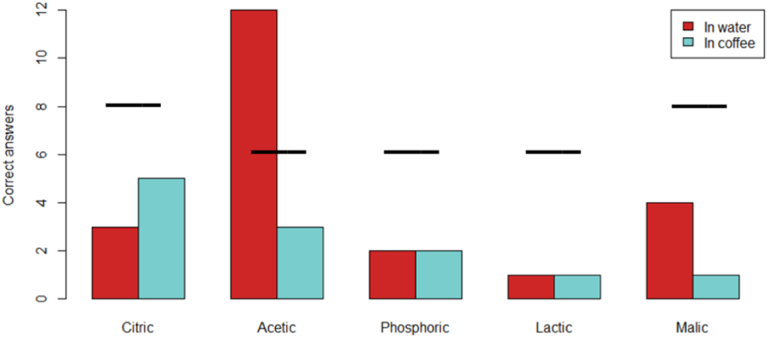
Recognition test of acids in water and coffee. The correct answers in water (red) and coffee (light blue), respectively. The black line indicates the requirements for correct answers in order to obtain significance (p < 0.05). Acetic, phosphoric, and lactic acid were each evaluated 13 times, while citric and malic acid were each evaluated 18 times. Only acetic acid in water was significantly identified.
Data availability
Data will be made available on request.
References
- Ahmed M., Jiang G.H., Park J.S., Lee K.C., Seok Y.Y., Eun J.B. Effects of ultrasonication, agitation and stirring extraction techniques on the physicochemical properties, health-promoting phytochemicals and structure of cold-brewed coffee. J. Sci. Food Agric. 2019;99(1):290–301. doi: 10.1002/jsfa.9186. [DOI] [PubMed] [Google Scholar]
- Alstrup J., Petersen M.A., Larsen F.H., Münchow M. The effect of roast development time modulations on the sensory profile and chemical composition of the coffee brew as measured by nmr and dhs-gc–ms. Beverages. 2020;6(4):1–14. doi: 10.3390/beverages6040070. [DOI] [Google Scholar]
- Amalia F., Aditiawati P., Yusianto, Putri S.P., Fukusaki E. Gas chromatography/mass spectrometry-based metabolite profiling of coffee beans obtained from different altitudes and origins with various postharvest processing. Metabolomics. 2021;17(7):1–16. doi: 10.1007/s11306-021-01817-z. [DOI] [PubMed] [Google Scholar]
- Avelino J., Barboza B., Araya J.C., Fonseca C., Davrieux F., Guyot B., Cilas C. Effects of slope exposure, altitude and yield on coffee quality in two altitude terroirs of Costa Rica, Orosi and Santa María de Dota. J. Sci. Food Agric. 2005;85(11):1869–1876. doi: 10.1002/jsfa.2188. [DOI] [Google Scholar]
- Balzer H.H. In: Coffee: Recent Developments (Pp. 18–32. Clarke R.J., Vitzthum O.G., editors. Blacwell Science; 2001. Acids in coffee. [Google Scholar]
- Batali M.E., Frost S.C., Lebrilla C.B., Ristenpart W.D., Guinard J.X. Sensory and monosaccharide analysis of drip brew coffee fractions versus brewing time. J. Sci. Food Agric. 2020;100(7):2953–2962. doi: 10.1002/jsfa.10323. [DOI] [PubMed] [Google Scholar]
- Belitz H.-D., Grosch W., Schieberle P. Food Chemistry. fourth ed. Springer; 2009. Coffee, tea and cocoa. [Google Scholar]
- Bennat C., Engelhardt U.H., Kiehne A., Wirries F.M., Maier H.G. HPLC Analysis of chlorogenic acid lactones in roasted coffee. Zeitschrift Für Lebensmittel-Untersuchung Und -Forschung. 1994;199(1):17–21. doi: 10.1007/BF01192945. [DOI] [Google Scholar]
- Borém F.M., Luisa P.F., Fabiana C.R., José H.S.T., Gerson S.G., Terezinha J.G.S. The relationship between organic acids, sucrose and the quality of specialty coffees. Afr. J. Agric. Res. 2016;11(8):709–717. doi: 10.5897/ajar2015.10569. [DOI] [Google Scholar]
- Brollo G., Cappucci R., Navarini L. Acidity in coffee : bridging the gap between chemistry and psychophysis. ASIC 22nd International Conference on Coffee Science. 2008;80:270–280. [Google Scholar]
- Christensen R.H.B., Brockhoff P.B., Kuznetsova A., Birot S., Amelia K. ’; 2018. Package ‘ sensR. [Google Scholar]
- Christensen R.H.B., Lee H.S., Brockhoff P.B. Estimation of the Thurstonian model for the 2-AC protocol. Food Qual. Prefer. 2012;24(1):119–128. doi: 10.1016/j.foodqual.2011.10.005. [DOI] [Google Scholar]
- Córdoba N., Moreno F.L., Osorio C., Velásquez S., Ruiz Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021;141 doi: 10.1016/j.foodres.2021.110141. August 2020. [DOI] [PubMed] [Google Scholar]
- CRI . Coffeeresearch.Org Science; 2006. Coffee Acidity.http://www.coffeeresearch.org/science/sourmain.htm [Google Scholar]
- de Bouillé A.G. A-Not-A test. Discrim. Test. Sens. Sci.: A Pract. Handbook. 2017;135–151 doi: 10.1016/B978-0-08-101009-9.00006-X. [DOI] [Google Scholar]
- de Maria C.A.B., Trugo L.C., Aquino Neto F.R., Moreira R.F.A., Alviano C.S. Composition of green coffee water-soluble fractions and identification of volatiles formed during roasting. Food Chem. 1996;55(3):203–207. doi: 10.1016/0308-8146(95)00104-2. [DOI] [Google Scholar]
- Engelhardt U.H., Maier H.G. The acids of coffee: proportion of individual acids in the total titratable acid. Z. Lebensm. Unters. Forsch. 1985;181:20–23. doi: 10.1007/BF01124801. [DOI] [PubMed] [Google Scholar]
- Ennis J.M., Jesionka V. The power of sensory discrimination methods revisited. J. Sensory Stud. 2011;26(5):371–382. doi: 10.1111/j.1745-459X.2011.00353.x. [DOI] [Google Scholar]
- Farah A. In: Drying and Roasting of Cocoa and Coffee. Hii L.C., Borém F.M., editors. CRC press; 2020. Flavor development during roasting; pp. 267–309. [Google Scholar]
- Ginz M., Balzer H.H., Bradbury A.G.W., Maier H.G. Formation of aliphatic acids by carbohydrate degradation during roasting of coffee. Eur. Food Res. Technol. 2000;211(6):404–410. doi: 10.1007/s002170000215. [DOI] [Google Scholar]
- Gloess A.N., Schönbächler B., Klopprogge B., D'Ambrosio L., Chatelain K., Bongartz A., Strittmatter A., Rast M., Yeretzian C. Comparison of nine common coffee extraction methods: instrumental and sensory analysis. Eur. Food Res. Technol. 2013;236(4):607–627. doi: 10.1007/s00217-013-1917-x. [DOI] [Google Scholar]
- Hoehl K., Schoenberger G.U., Busch-Stockfisch M. Water quality and taste sensitivity for basic tastes and metallic sensation. Food Qual. Prefer. 2010;21(2):243–249. doi: 10.1016/j.foodqual.2009.06.007. [DOI] [Google Scholar]
- ICO . International Coffee Organization; 1991. Sensory Evaluation of Coffee: Technical Unit Quality Series. [Google Scholar]
- ISO 3972 . 2011. Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. [Google Scholar]
- Keast R.S.J., Breslin P.A.S. An overview of binary taste-taste interactions. Food Qual. Prefer. 2003;14(2):111–124. doi: 10.1016/S0950-3293(02)00110-6. [DOI] [Google Scholar]
- Khamitova G., Angeloni S., Fioretti L., Ricciutelli M., Sagratini G., Torregiani E., Vittori S., Caprioli G. The impact of different filter baskets, heights of perforated disc and amount of ground coffee on the extraction of organics acids and the main bioactive compounds in espresso coffee. Food Res. Int. 2020;133(April) doi: 10.1016/j.foodres.2020.109220. [DOI] [PubMed] [Google Scholar]
- Kreuml M.T.L., Majchrzak D., Ploederl B., Koenig J. Changes in sensory quality characteristics of coffee during storage. Food Sci. Nutr. 2013;1(4):267–272. doi: 10.1002/fsn3.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle T.R., Menon S.N. In: The Craft and Science of Coffee. 1 ed. Folmer B., editor. Elsevier; 2017. Cupping and grading - discovering character and quality; pp. 181–203. [Google Scholar]
- Low J.Y.Q., McBride R.L., Lacy K.E., Keast R.S.J. Psychophysical evaluation of sweetness funcctions across multiple sweetners. Chem. Senses. 2017;42(2):111–120. doi: 10.1093/bjw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münchow M., Alstrup J., Steen I., Giacalone D. Roasting conditions and coffee flavor: a multi-study empirical investigation. Beverages. 2020;6(2):1–14. doi: 10.3390/beverages6020029. [DOI] [Google Scholar]
- Poisson L., Blank I., Dunkel A., Hofmann T. The Craft and Science of Coffee. Elsevier Inc; 2017. The chemistry of roasting-decoding flavor formation. [DOI] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; 2021. A Language and Environment for Statistical Computing.http://www.r-project.org/ [Google Scholar]
- Rivera J.A. Using organic acids as a training tool. Roast Magaz. 2020;4:82–86. https://www.roastmagazine.com/issues/100 [Google Scholar]
- Rao N.Z., Fuller M. Acidity and antioxidant activity of cold brew coffee. Sci. Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-34392-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues C.I., Marta L., Maia R., Miranda M., Ribeirinho M., Máguas C. Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J. Food Compos. Anal. 2007;20(5):440–448. doi: 10.1016/j.jfca.2006.08.005. [DOI] [Google Scholar]
- Rubico S.M., Mcdaniel M.R. Sensory evaluation of acids by free-choice profiling. Chem. Senses. 1992;17(3):273–289. doi: 10.1093/chemse/17.3.273. [DOI] [Google Scholar]
- SCA. (n.d.). The SCA Water Quality Handbook (second ed.).
- SCAA . SCAA; 2015. SCAA Standard | Golden Cup. [Google Scholar]
- Stanek N., Zarębska M., Biłos Ł., Barabosz K., Nowakowska-Bogdan E., Semeniuk I., Błaszkiewicz J., Kulesza R., Matejuk R., Szkutnik K. Influence of coffee brewing methods on the chromatographic and spectroscopic profiles, antioxidant and sensory properties. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-01001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen I., Waehrens S.S., Petersen M.A., Münchow M., Bredie W.L.P. Influence of serving temperature on flavour perception and release of Bourbon Caturra coffee. Food Chem. 2017;219:61–68. doi: 10.1016/j.foodchem.2016.09.113. [DOI] [PubMed] [Google Scholar]
- Talavera K., Ninomiya Y., Winkel C., Voets T., Nilius B. Influence of temperature on taste perception. Cell. Mol. Life Sci. 2007;64(4):377–381. doi: 10.1007/s00018-006-6384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E., Puget S., Valentin D., Songer P. The Craft and Science of Coffee. Elsevier Inc; 2017. Sensory evaluation-profiling and preferences. [DOI] [Google Scholar]
- Verardo G., Cecconi F., Geatti P., Giumanini A.G. New procedures for determination of acids in coffee extracts, and observations on the development of acidity upon ageing. Anal. Bioanal. Chem. 2002;374(5):879–885. doi: 10.1007/s00216-002-1561-y. [DOI] [PubMed] [Google Scholar]
- Vitzthum O.G. In: kaffee und coffein. Eichler O., editor. Springer Verlag; 1976. Chemie und Bearbeitung de Kaffees; pp. 3–64. [Google Scholar]
- Wei F., Tanokura M. Coffee in Health and Disease Prevention. Elsevier Inc; 2015. Chemical changes in the components of coffee beans during roasting. [DOI] [Google Scholar]
- Woodmann J.S. In: Coffee - Volume 1: Chemistry. Clarke R.J., Macrae R., editors. Elsevier; 1985. Carboxylic acids; pp. 266–289. [Google Scholar]
- Yeager S.E., Batali M.E., Guinard J.X., Ristenpart W.D. Acids in coffee: a review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2021;0(0):1–27. doi: 10.1080/10408398.2021.1957767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.