Graphical abstract
Chemical compounds studied in this article: 4-Methylumbelliferyl alpha-d-glucopyranoside; Alpha-glucosidase substrate (PubChem CID 87330); Bile acid (PubChem CID 439520); 2,2-Diphenyl-1-picrylhydrazyl (PubChem CID 2735032); Acarbose (PubChem CID 444254); Ascorbic Acid (PubChem CID 54670067); Inulin (PubChem CID 24763); Hemin chloride (PubChem CID 53627695)
Keywords: Clitocybe squamulosa polysaccharides, In vitro digestion, Fecal fermentation, Physico-chemical properties, Gut microbiota
Highlights
-
•
CSFP2 can be partially degraded during simulated saliva-gastrointestinal digestion.
-
•
CSFP2 significantly increases the amount of short chain fatty acids in a fermentation broth.
-
•
CSFP2 can significantly regulate the composition and abundance of gut microorganisms.
-
•
CSFP2 has potential prebiotic activity useful in the development of functional foods.
Abstract
The aim of this study was to establish a human digestion model in vitro to explore the degradation characteristics of a novel high-purity polysaccharide from Clitocybe squamulosa (CSFP2). The results showed that the content of reducing sugars (CR) of CSFP2 increased from 0.13 to 0.23 mg/mL, the molecular weight (Mw) of CSFP2 decreased significantly during the saliva-gastrointestinal digestion. The constituent monosaccharides of CSFP2, including galactose, glucose, and mannose, were stable during in vitro digestion, but their molar ratios were changed from 0.023: 0.737: 0.234 to 0.496: 0.478: 0.027. The surface of CSFP2 changes from a rough flaky structure to a scattered flocculent or rod-shaped structure after the gastrointestinal digestion. However, the apparent viscosity of CSFP2 was overall stable during in vitro digestion. Moreover, CSFP2 still maintains its strong antioxidant capacity after saliva-gastrointestinal digestion. The results showed that CSFP2 can be partially decomposed during digestion. Meanwhile, some physico-chemical properties of the fermentation broth containing CSFP2 changed significantly after gut microbiota fermentation. For example, the pH value (from 8.46 to 4.72) decreased significantly (p < 0.05) after 48 h of fermentation. the OD600 value increased first and then decreased (from 2.00 to 2.68 to 1.32) during 48-h fermentation. In addition, CSFP2 could also increase the amounts of short-chain fatty acids (SCFAs) (from 5.5 to 37.15 mmol/L) during fermentation (in particular, acetic acid, propionic acid, and butyric acid). Furthermore, the relative abundances of Bacteriodes, Bifidobacterium, Catenibacterium, Lachnospiraceae_NK4A136_group, Megasphaera, Prevotella, Megamonas, and Lactobacillus at genus level were markedly increased with the intervention of CSFP2. These results provided a theoretical basis for the further development of functional foods related to CSFP2.
1. Introduction
Edible fungi, knowns as mushrooms, are a type of large fungi that can form large fleshy fruiting bodies or sclerotium-like tissues. They have attracted extensive attention among researchers because of their delicious taste, rich nutrition and various medicinal values when used in the treatment of disease (Valverde, Hernández-Pérez, & Paredes-López, 2015). The various functions of mushrooms are mainly attributed to their abundant bioactive substances comprising peptides, lectins, proteins, polysaccharides and so on (Geng et al., 2016, Yin et al., 2020). It is worth mentioning that polysaccharides have been considered one of the most important components of mushrooms. At present, polysaccharides extracted from edible fungi have many potential functions such as anti-cancer treatment (Wu et al., 2012), regulation of the immune system (Wang et al., 2015b), antibacterial activity (Mirzadeh, Arianejad, & Khedmat, 2020), and preventing hyperglycemia and increased cholesterol (Zhu et al., 2013). It is therefore necessary to study mushroom-derived polysaccharides in depth.
Polysaccharides ingested by human body could be metabolized and degraded into small molecular compounds through the contraction and peristalsis of digestive system, and then absorbed and utilized by the body (Lovegrove et al., 2017). Interestingly, some structural and processing characteristics (molecular weight distribution, viscosity, particle size and rheological properties) of polysaccharides were affected by digestive enzymes, bile salts, pH and gut microflora in the gastrointestinal digestion (Yuan et al., 2020b, Zhu et al., 2019). For example, snow chrysanthemum polysaccharide can be partially degraded under gastrointestinal digestive conditions (Wu et al., 2021b). Similarly, the Mw of oyster polysaccharide also decreased significantly with the extension of gastrointestinal digestion time, which was closely related to the acidic environment of the stomach (Ma, Jiang, & Zeng, 2021). In addition, indigestible dietary polysaccharides will be further utilized by the intestinal microbiota, which could regulate the intestinal microenvironment by metabolizing energy and beneficial products (such as SCFAs) (Han et al., 2020, Xu et al., 2020). For instance, research indicated that the polysaccharides from Flammulina velutipes, Pleurotus eryngii, and Lentinus edodes could significantly regulate the abundance of Actinomycetes and Bacteroides. Meanwhile, polysaccharides from Agaricus bisporus could also affect the abundance of gut microflora, such as Fusobacteria, Firmicutes, Bacteroides, and Proteobacteria (Zhao, Yang, Pei, Zhao, & Hu, 2018). Increasing research evidence shows that gut microbiota is closely related to human physiological activities and metabolism. This research result will prompt people to improve their health through the development of microbiology-related interventions (Payling et al., 2020).
Clitocybe squamulosa grows on Wutai Mountain, Shanxi Province, China. The unique geographical location of Wutai Mountain endows it with diverse biological resources and enriches it with economically important mycolora. According to the local saying that long-term consumption of C. squamulosa can prevent arteriosclerosis and improve the immune system. In the early stage, a water-soluble crude polysaccharide (CSFP) was obtained from C. squamulosa fruiting body, and its physico-chemical properties and structure-activity relationship were systematically studied (Guo et al., 2022). However, due to the low purity of CSFP, it was difficult to judge the specific regulatory components that exert biological effects. In this study, CSFP was further separated and purified by DEAE-52 anion exchange chromatography, and a CSFP2 with a polysaccharide purity of 94.28% was determined. This study will systematically explore the changes of structural characteristics, biological activity, and gut microbial of CSFP2 before and after digestion in vitro. This result will help us better understand the potential digestion and fermentation mechanism of mushroom-derived polysaccharides.
2. Materials and methods
2.1. Materials and reagents
Materials: The C. squamulosa fruiting bodies were purchased from Shanxi Engineering Research Center for Edible Fungi (Shanxi Province, China).
Reagents: Pancreatin (trypsin activity: ≥4000 U/g, amylase activity: ≥7000 U/g, lipase activity: ≥4000 U/g), ascorbic acid (Vc), inulin, phosphate buffered saline (PBS 0.01 M, pH 6.8), vitamin K, yeast extract, and hemin chloride were obtained from Yuanye Bio-Technology Co., Ltd (Shanghai, China). Porcine stomach pepsin (lyophilized powder: ≥2500 U/mg protein), Acarbose (Ac) and alpha-glucosidase substrate were purchased from Sigma-Aldrich (St Louis, MO, USA). ABTS and ·OH assay kit were purchased from Solarbio Science (Beijing, China). All other chemicals used were of analytical reagent grade from Tianjin Kaitong Chemical Reagent Factory (Tianjin, China).
2.2. Preparation of polysaccharides from C. squamulosa (CSFP2)
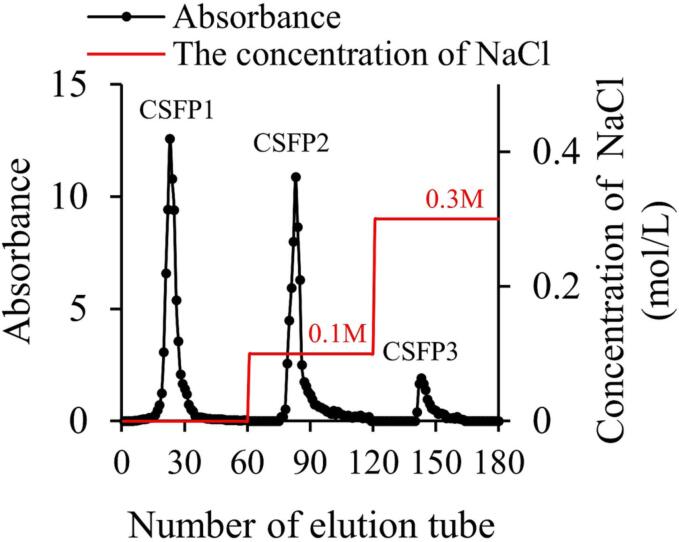
Hot-water extraction of CSFP followed a published method (Guo et al., 2022). In brief, the powder of C. squamulosa was mixed with ultrapure water in the ratio of 1:30 (w/v). The extraction temperature and time were set to 80 °C and 3.6 h, respectively. After extraction, the protein was removed by a zinc acetate potassium ferrocyanide method. Meanwhile, the supernatant was dialyzed by ultrapure water (3500 Da molecular retention), which was followed by ethanol precipitation, and freeze-drying. Collection of samples (CSFP): subsequently, CSFP solution (10 mg/mL) was loaded to a DEAE-52 Sepharose flow column (28×330 mm) and eluted with NaCl buffer (0, 0.1, and 0.3 mol/L) at a flow rate of 4 mL/min. After the eluent was collected by the fully-automated collector (Shanghai Huishi Instrument Co., Ltd, China), the total sugar content was measured tube-by-tube following the phenol-sulfuric acid method (Huang et al., 2019). Then the CSFP2 eluents were collected, concentrated, dialyzed, and freeze-dried (Fig. 1).
Fig. 1.
Elution profiles of DEAE-52 of CSFP.
2.3. In vitro digestion of CSFP2
2.3.1. Simulated saliva-gastrointestinal process
Follow the published method (Zhu et al., 2019), fresh saliva was collected from six healthy volunteers (aged 25–28 years, three male and three female). The volunteers were required to be free from oral and gastrointestinal diseases and did not receive antibiotic treatment within three months. Saliva was collected at 3500 g in a centrifuge for 10 min. Meanwhile, the supernatant was used to detect salivary amylase activity. The research shows that the activity of salivary amylase of different volunteers was (118±4 D U/mL), which was within the internationally accepted range of human salivary amylase activity (18−208 D U/mL). This finding indicated that the human saliva collected in the experiment was effective and reliable and could be used as a simulated human oral digestive fluid. In addition, research also showed that saliva was colorless and tasteless, with pH close to neutral. Its composition was mainly 99.4% water and only 0.6% solid material, mainly including mucin, salivary amylase, lysozyme, and inorganic salt. Briefly, simulated saliva digestion proceeded as follows: 10 mL 5.0 mg/mL CSFP2 solution was mixed with 1.0 mL saliva juice, incubated at a constant temperature of 37 °C; 2 mL samples were collected at 0, 5, 10, 20, and 30 min and high temperature (100 °C, 10 min) inactivated the enzyme. Simulated gastric digestion process was described as follow: The pH of the mixture of salivary digestive residue (10.0 mL) and artificial simulated gastric juice (4.0 mL) was maintained at 2.0, and the mixture was incubated at 37 °C. 2 mL digested samples were collected at different times (0.5, 1.0, 2.0, 4.0, and 6.0 h), and high temperature (100 °C, 10 min) inactivated the enzyme. Simulated small intestinal digestion process was elucidated as follow: the mixture of gastric digestive residue (10 mL) and artificial intestinal juice (4 mL) was incubated at 37 °C and maintained at a pH of 7.0. Thereafter, 2 mL digested samples were collected at different times (0.5, 1.0, 2.0, 4.0, and 6.0 h), and high temperature (100 °C, 10 min) inactivated the enzyme. The reducing sugar contents (CR) in digestive juice were determined by using 3,5-dinitrosalicylic acid method (Hu, Liu, Wu, Sui, & Zhang, 2020).
The supernatant was precipitated with four volumes (v/v) of absolute ethanol, the subject to dialysis and freeze-drying. The digested samples were named thus: salivary digestion sample (CSFP2-S), gastric-digested sample (CSFP2-G), and small intestine digested sample (CSFP2-I), respectively (Guo et al., 2022; Liu et al., 2020a; Minekus et al., 2014, Yuan et al., 2020c). Preparation for simulated gastric electrolyte: H2O (1 L), NaCl (94.4 mmol/L), NaHCO3 (50 mmol/L), KCl (13.8 mmol/L), KH2PO4 (1.8 mmol/L), (NH4)2CO3 (1 mmol/L), CaCl2 2H2O (0.3 mmol/L), and MgCl2 6H2O (0.24 mmol/L) were used. We adjusted the pH of the gastric electrolyte with HCl (0.1 mol/L). The artificial simulated gastric juice consisted of 3.6 mL of gastric electrolyte and 0.4 mL of pepsin solution (pepsin (30 mg) dissolved in CH3COONa (1 mol/L 1 mL)). Simulated intestinal electrolyte included: H2O (1 L), KCl (13.6 mmol/L), KH2PO4 (1.6 mmol/L), NaHCO3 (170 mmol/L), NaCl (76.4 mmol/L), MgCl2 6H2O (0.66 mmol/L), CaCl2 2H2O (1.2 mmol/L). The simulated small intestine solution consisted of 2.0 mL of intestine electrolyte solution, 1.0 mL of trypsin solution (0.25 g of trypsin dissolved in 1 mL of electrolyte) and 1.0 mL of bile salt solution (40 mg of bile acid salt dissolved in 1 mL of electrolyte).
2.3.2. Chemical composition
Following the former methods, the digestibility of CSFP2 was calculated (Wu et al., 2021a), the total polysaccharides, proteins, and phenol contents of CSFP2 and their digested samples were measured by the phenol–sulfuric acid method, Coomassie brilliant blue method, and Folin-Ciocalteu method respectively (Hu et al., 2020, Huang et al., 2019, Yuan et al., 2020b).
2.3.3. Structural analysis
2.3.3.1. Scanning electron microscopy (SEM)
CSFP2 and their digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were sprayed with gold for 90 s under a current of 15 mA. The different samples were then examined using an SEM (Zeiss Merlin, Germany) to observe their morphological characteristics under different magnifications with a 20.0 kV accelerating voltage (Liu et al., 2020b).
2.3.3.2. Monosaccharide composition
Monosaccharide compositions were measured using ion chromatography (Thermo Fisher Scientific, USA) equipped with DionexTMCarbopacTMPA20 (3 mm × 150 mm) chromatographic column. The electrochemical detector was used to detect changes in electrical signals of substances at the flow rate of 0.3 mL/min at a column temperature of 30 °C (Wang, Yu, & Wei, 2012).
2.3.3.3. Ultraviolet–visible absorption spectra (UV)
CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I were respectively prepared into 1-mg/mL solutions with ultrapure-water, respectively. Then, the samples were assayed using a UV–VIS spectrophotometer (Agilent, USA) in the range of 190–800 nm (Qiao, Ye, Cai, Li, & Liu, 2022).
2.3.3.4. Fourier transform infrared spectroscopy (FT-IR)
Following the published methods (Wang, Pei, Ma, Cai, & Yan, 2014), The FT-IR spectra of the CSFP2 and their digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were determined by Fourier transform infrared spectrophotometer (Thermo Fisher Scientific FT-IR, USA). All samples were scanned across the entire band from 400 to 4000 cm−1.
2.3.3.5. Molecular weight (Mw) distribution
The values of the absolute Mw of CSFP2, digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were determined by using the HPGPC (Wyatt Technology, Santa Barbara, USA). Meanwhile, the column was eluted with double-distilled water, at a flow rate of 1.0 mL/min (Zheng et al., 2021).
2.3.3.6. Mean particle size and zeta potential
According to the former methods (Feng et al., 2020), different digested sample solutions were diluted with distilled water to a certain multiple and determined with a light scattering instrument (Malvern Instruments, Worcestershire, UK).
2.3.3.7. Rheological characterization
The rheological behavior of CSFP2 and their digested samples could be measured by rotational rheometer (MCR102, Anton Paar, Graz, Austria). The apparent viscosity of samples solution was measured in the range of 0.1–1000 s−1 (Nie et al., 2019). Meanwhile, the variations in storage modulus (G') and loss modulus (G'') were also studied in the range of 0.1–100 rad/s (Yuan et al., 2020a).
2.3.4. Bioactivities analysis
2.3.4.1. Antioxidant and enzymatic inhibitory activities
The antioxidant activities (DPPH, ·OH, and ABTS radical-scavenging capacities) of CSFP2 and digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were determined by the related test kit (Solarbio Science, Beijing, China). The reducing powers of CSFP2 and their digested samples were also measured by using former methods (Liu et al., 2018d; Zhao et al., 2016). Meanwhile, enzyme inhibitory activities (α-glucosidase, α-amylase) of CSFP2, and digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were determined by published methods (Floris et al., 2021, Xu et al., 2018).
2.4. In vitro fermentation of CSFP2
2.4.1. Simulated fermentation process
The fresh feces were collected from six healthy humans (19–24 years old, three male and three female) who did not take related antibiotics within three months. The feces were fully mixed with PBS (30% w/v) and filtered with sterile gauze. We mixed 9 mL of basic nutrient medium (contain 15 mg/mL CSFP2 or 15 mg/mL Inulin or no carbon source) with 1.0 mL of 10% fecal slurry and incubated these specimens in an anaerobic system at 37 °C. According to the experimental requirements, the fermentation products were collected at different time periods for analysis. The basic medium included: 2 g/L yeast peptone, 2 g/L extract, 0.5 g/L l-cysteine hydrochloride, 0.5 g/L bile salt, 0.1 g/L chloride sodium, 0.02 g/L hemin, 0.04 g/L K2HPO4, 0.01 g/L MgSO4⋅7H2O, 0.01 g/L calcium chloride, 2 mL/L Tween-80, 1 mL/L 1% resazurin, and 10 μg/L vitamin K1. The original fecal suspension (OR group), the carbon free source (BLK group), CSFP2 experimental group (CSFP2 group), and inulin experimental group (INL group) were established.
2.4.2. Physico-chemical properties
Fermentation samples were collected at 0, 6, 12, 24, 48 h and centrifuged at 3000 rpm at 4 °C for 10 min. The pH, OD600, CR and total sugar of supernatant of a fermentation broth were measured by published methods (Guo et al., 2022).
2.4.3. Short-chain fatty acids (SCFAs)
The SCFA contents of fermentation samples were determined by gas chromatography (GC, Thermo Scientific, Germany) which was equipped with an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm). In addition, other parameters were set based on a previous method (Zhou et al., 2018).
2.4.4. Gut microbiota analysis
The total bacterial DNA was extracted by using an EZNA DNA Kit after 48 h of fermentation. Then, sequencing of 16S rRNA genes of V4 region was performed by Nuohe Zhiyuan Biotechnology Co., Ltd (Beijing, China).
2.5. Statistical analysis
Data were plotted using MS-Excel® and R language. All data were presented as the mean ± standard deviation. Duncan’s test was used for comparison of the significance of data, and statistical significance was deemed at p < 0.05.
3. Results and discussion
3.1. Effect of digestion on chemical composition of CSFP2
3.1.1. The change of CR
The breaking of glycosidic bonds and the degradation of Mw in polysaccharides will affect their CR during digestion (Chen et al., 2012). Salivary digestion will not influence the CR of CSFP2 (Table 1). The result showed that most non-starch polysaccharides could not be utilized by saliva (Chen et al., 2018). However, the CR of CSFP2 significantly increased (p < 0.05) (0.20±0.01 to 0.22±0.01 mg/mL) after gastric digestion for 6 h. The study found that there were abundant digestive enzymes dissolved in the gastrointestinal fluid environment, which played a special role in the degradation of high Mw polysaccharides into glucose or oligosaccharide monomers by cooperating with the gastrointestinal fluid (Liu et al., 2020a). At the same time, the hydrolysis of gastrointestinal digestive fluid also plays a key role in the release of reducing sugar in polysaccharides. They complement each other, cooperate with each other and play a digestive role together.
Table 1.
The concentration of reducing sugar (CR) of CSFP2 during in vitro digestion.
| Process | Time (h) | CR (mg/mL) |
|---|---|---|
| Saliva digestion | 0 | 0.13±0.02d |
| 0.083 | 0.13±0.01d | |
| 0.166 | 0.13±0.01d | |
| 0.333 | 0.13±0.01d | |
| 0.5 | 0.13±0.01d | |
| Gastric juice digestion | 0.5 | 0.20±0.01c |
| 1 | 0.21±0.01bc | |
| 2 | 0.22±0.02abc | |
| 4 | 0.22±0.01abc | |
| 6 | 0.21±0.01bc | |
| Small intestinal juice digestion | 0.5 | 0.22±0.01abc |
| 1 | 0.22±0.02abc | |
| 2 | 0.23±0.02abc | |
| 4 | 0.24±0.01a | |
| 6 | 0.23±0.02ab | |
Values represent mean ± standard deviation, different lowercase letters denote statistical significance (p < 0.05).
3.1.2. The changes of digestibility, total polysaccharides, protein, and phenols
The chemical compositions and digestibility of CSFP2, digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were summarized (Table 2). The digestibility of CSFP2 after salivary digestion, gastric digestion, and intestinal digestion was 1.12%, 7.98%, and 10.49, respectively, indicating that CSFP2 could be decomposed by the stomach juice (pH = 3). Meanwhile, the polysaccharide, protein and phenol contents in CSFP2 were 94.28%, 1.26%, and 0.0024%, respectively. Besides, the polysaccharide and protein contents in CSFP2 were remarkably decreased (p < 0.05) to 86.27% and 0.73% after gastrointestinal digestion, respectively. This phenomenon may be related to the synergistic effect of electrolytes and enzymes in the gastrointestinal tract (Hu, Nie, Min, & Xie, 2013; Liu et al., 2018c). In conclusion, the digestion could significantly affect the chemical composition of CSFP2.
Table 2.
Changes of digestibility, total polysaccharides content, total proteins content, total phenol content, constituent monosaccharides and molar ratio of CSFP2 during digestion.
| CSFP2 | CSFP2-S | CSFP2-G | CSFP2-I | |
|---|---|---|---|---|
| Digestibility | – | 1.12% | 7.98% | 10.49% |
| Total polysaccharides | 94.28±1.80a | 93.54±2.89a | 87.33±2.15b | 86.27±2.25b |
| Total proteins | 1.26±0.06a | 1.20±0.04b | 0.81±0.08c | 0.73±0.04c |
| Total phenol | 0.0024 | 0.0023 | 0.0022 | 0.0022 |
| Constituent monosaccharides and molar ratios | ||||
| Galactose | 0.023 | 0.092 | 0.041 | 0.496 |
| Glucose | 0.737 | 0.603 | 0.686 | 0.478 |
| Mannose | 0.234 | 0.298 | 0.266 | 0.027 |
Values represent mean ± standard deviation, and different superscript lowercase letters indicated significance (p < 0.05) in each row.
3.2. Effect of digestion on structural characteristics of CSFP2
3.2.1. SEM analysis
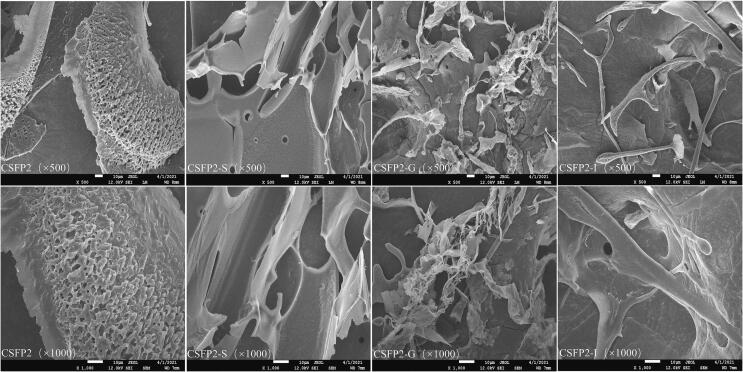
SEM is the most direct way to understand the complex structure of polysaccharides. As shown in Fig. 2, the surface of CSFP2 was rough and showed a compact and neatly arranged sheet structure; however, after the saliva-gastric digestion, CSFP2 presented flocculent or rod-shaped structures with disorderly interweaving. Previous studies also found that gastrointestinal digestion could remarkably change the apparent morphology of polysaccharides, making them tend to be disordered (Guo et al., 2022). The extremely acidic conditions in the stomach will damage the complex internal structure of polysaccharides, resulting in changes in the apparent morphology (Li et al., 2020, Yuan et al., 2020c). For example, Yuan et al. found that Sargassum pallidum polysaccharide showed many irregular fragment structures after gastrointestinal digestion. The author speculated that this phenomenon was caused by environmental degradation of gastrointestinal fluid (Yuan et al., 2020c).
Fig. 2.
SEM images of CSFP2 and their digested fractions at 500 and 1000× magnification.
3.2.2. Analysis of monosaccharide composition
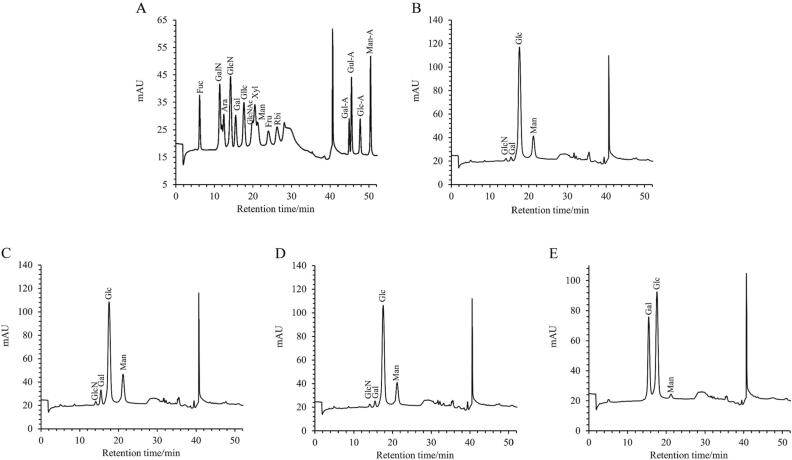
Monosaccharide composition is the natural basic unit of polysaccharides and plays a crucial role in the structural characterization of polysaccharides. (Wang et al., 2022) stated that fungal polysaccharides are mainly composed of galactose (Gal), glucose (Glc), and mannose (Man), and their molar ratios also showed significant differences due to different raw materials, and extraction and purification methods. The results of monosaccharide composition of CSFP2 during digestion were detected by ICS5000, as shown in Fig. 3. The sixteen standard monosaccharides were successfully separated within 52 min. The results indicated that the monosaccharide composition types of CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I were similar. They were also all composed of galactose, glucose, and mannose; however, after saliva-gastrointestinal digestion, the molar ratio of galactose, glucose, and mannose in CSFP2 changed from 0.023: 0.737: 0.234 to 0.496: 0.478: 0.027. which was mainly manifest by a decrease in glucose content and an increase in galactose content.
Fig. 3.
Changes in monosaccharide composition of CSFP2 during digestion. (A) Standard curve; (B) CSFP2; (C) CSFP2-S; (D) CSFP2-G; (E) CSFP2-I. Fuc-Fucose; GalN-Galactosamine hydrochloride; Rha-Rhamnose; Ara-Arabinose; GlcN-Glucosamine hydrochloride; Gal-Galactose; Glc-Glucose; GlcNAc-N-acetyl-D glucosamine; Xyl-Xylose; Man-Mannose; Fru-Fructose; Rib-Ribose; GalA-Galacturonic acid; GulA-Guluronic acid; GlcA-Glucuronic acid; ManA-Mannuronic acid.
Yuan et al. found that gastrointestinal digestion significantly changed the physico-chemical properties of okra polysaccharide, especially the breaking of the glycosidic bond therein led to the change of molecular weight. In addition, the molar ratio of rhamnose, galactose, galacturonic acid, glucose, glucuronic acid, mannose, and arabinose in the monosaccharide composition of okra polysaccharide changed from 1.00: 1.83: 1.41: 1.13: 0.24: 0.12: 0.46 to 1: 2.23: 1.19: 0.85: 0.28. The author also found that Ara disappeared, and speculated that Ara was a pentose, which was unstable and located in the branching chain of oyster polysaccharide, resulting in its ready degradation during gastrointestinal digestion (Yuan et al., 2020b). In addition, Liu et al. investigated the digestion and fermentation behavior of Craterellus cornucopioides polysaccharides (CCPs) and found that in vitro digestion can significantly reduce the molecular weight of CCPs and release a certain amount of glucose (Liu et al., 2020a).
Meanwhile, we inferred that saliva-gastrointestinal digestion will destroy the internal glycosidic bond, change the degree of branching, and break the chain conformation of CSFP2 to a certain extent, resulting in the release of glucose on the internal branch chain of CSFP2 to the digestive solution, which thus changes the molar ratio of glucose, mannose, and galactose in freeze-dried CSFP2. In conclusion, in vitro simulated digestion will also affect the monosaccharide composition of CSFP2.
3.2.3. UV and FT-IR analysis
No obvious absorption peaks of nucleic acid and protein residues were found at 260 and 280 nm in CSFP2 and its digested samples (Fig. 4A). Furthermore, the FT-IR spectra of CSFP2, and digested samples (CSFP2-S, CSFP2-G, and CSFP2-I) were similar, indicating that the digestion in vitro will not significantly influence the functional group structure of CSFP2.
Fig. 4.
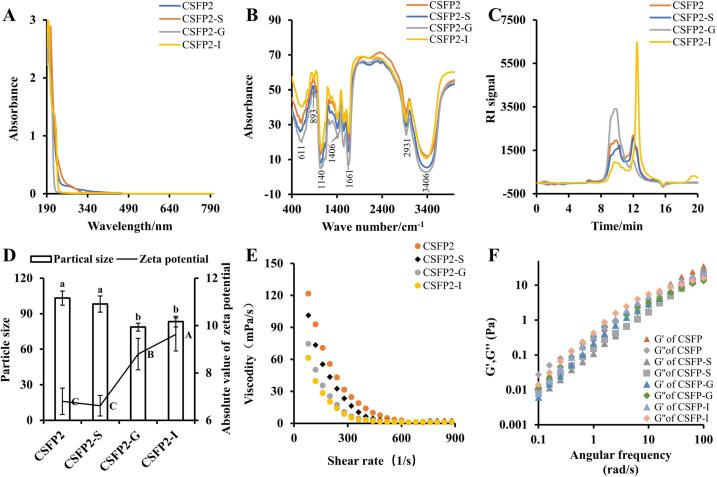
Changes in characteristics of CSFP2 during digestion. (A) UV spectra; (B) FT-IR spectra; (C) Molecular weight; (D) Particle size and zeta potential (absolute value); (E) Rheological properties of apparent viscosity on the shear rate and (F) Dynamic oscillating shear properties. Data are expressed as the mean ± standard deviation (n = 3), and different lowercase letters denote statistical significance (p < 0.05).
Briefly, the bands at 3406 and 2931 cm−1 were recognized as the stretching vibration of O—H and C—H (Chen et al., 2016; Yuan et al., 2019a). The band at 1661 cm−1 was recognized as stretching vibration of COO– (Yuan et al., 2019b). The signal at 1406 cm−1 corresponded to the C—H or O—H. The protein band of 1555 cm−1 suggested that there were no protein residue in CSFP2 and their digested samples (Yan, Wu, Qiao, Cai, & Ma, 2019). The signal at 1140 cm−1 was attributed to the pyranose ring. The signal at 893 cm−1 indicated the possible presence β-glycosidic bond in CSFP2 and these digested samples (Fig. 4B) (Yuan et al., 2020b).
3.2.4. Mw analysis
Studies have found that the bioactivities of macromolecular polysaccharides extracted from natural biomaterials were closely related to their Mw distribution (Wu et al., 2018). All HPGPC chromatograms of CSFP2 and their digested samples showed that they were heteropolysaccharides. The Mw of CSFP2 did not change significantly (ranged from 252 to 5,002,194 Da) after the saliva digestion, suggesting that CSFP2 was not degraded by salivary amylase. However, the Mw and relative peak areas (%) of CSFP2 changed significantly after gastrointestinal digestion (ranged from 2827 to 92,964 Da) (Table 3 and Fig. 4C). Results showed that gastrointestinal digestion could gradually degrade high Mw polysaccharides into relatively small fragments. Consistent with the previous study, the glycosidic bonds of most natural polysaccharides could be degraded in the gastrointestinal tract, resulting in the reduction of Mw (Carnachan et al., 2012, Chen et al., 2016).
Table 3.
Molecular weight changes of CSFP2 during digestion.
| Samples | Retention time | Mw (Da) | Percentage (%) |
|---|---|---|---|
| CSFP2 | 6.473 | 5039761.271 | 1.5137 |
| 9.28 | 152137.6361 | 17.8377 | |
| 9.905 | 69784.41818 | 32.8385 | |
| 11.973 | 5294.217596 | 32.1256 | |
| 12.396 | 3124.062689 | 15.6846 | |
| CSFP2-S | 6.479 | 5002194.496 | 1.5087 |
| 9.407 | 129854.7658 | 14.2595 | |
| 10.091 | 55338.49567 | 17.6666 | |
| 10.268 | 44378.26531 | 18.2369 | |
| 12.005 | 5087.116731 | 47.5582 | |
| 14.412 | 252.8858047 | 0.77 | |
| CSFP2-G | 9.882 | 71814.88342 | 82.3191 |
| 11.967 | 5333.977482 | 17.6787 | |
| CSFP2-I | 9.675 | 92964.77884 | 27.7892 |
| 12.476 | 2827.447373 | 72.21 | |
3.2.5. Particle size and zeta potential analysis
The charge of hydrodynamic particles and potential could characterize the stability of a solution or colloid (Feng et al., 2020). The particle size of CSFP2 and their digested samples ranged from 83 to 104 nm. CSFP2-G presented the smallest particle size (78.69±3.35 nm) while CSFP2 exhibited the largest particle size (103.17±6.02 nm) (p < 0.05) in different digested samples. Studies have confirmed that the smaller the particle size, the stronger the adsorption capacity, the better the stability of the lotion system (Li, Kong, Liu, Xia, & Chen, 2017). In addition, the zeta potential absolution of CSFP2-I was the largest (9.62±0.70), followed by CSFP2-G, CSFP2, and CSFP2-S (Fig. 4D). The increase of zeta potential indicated that the electrostatic repulsion between emulsion droplets increased, and the stability of emulsion system was predicted to increase. The increase of zeta potential will enhance the electrostatic repulsion between lotion droplets, leading to the stability enhancement of lotion system. Therefore, the samples after gastrointestinal digestion were easier to be absorbed and utilized by the body.
3.2.6. Rheological characterization analysis
Natural plant polysaccharides have excellent rheological properties, so they have been widely used as industrial gelling agents, thickeners, and emulsifiers (Kumar Varma & Jayaram Kumar, 2017). Research showed a series of in vitro activities of natural polysaccharides, such as antioxidant and binding properties, were also closely related to their apparent viscosity (Yuan et al., 2019b; Yuan et al., 2019a). As shown in Fig. 4E, the apparent viscosity of CSFP2 and its digested sample (CSFP2-S, CSFP2-G, and CSFP2-I) decreased with the increase of shear rate at the same concentration, showing the shear thinning behavior of a Newtonian fluid in the range of high shear rate. The study found that shear thinning behavior of polysaccharides was closely related to the dissociation of molecular chains in solution (Ghannam and Esmail, 1997, Jin et al., 2017). Moreover, compared with CSFP2, the apparent viscosity of the digested sample decreased, and the decrease became significant after gastrointestinal digestion, which might be caused by the Mw and polydispersity of the sample (Zhong et al., 2015). This finding was consistent with the previous results (Nie et al., 2019, Nie et al., 2020).
Besides, polysaccharides are a viscoelastic material with fluid properties (solid and liquid), which can be measured by dynamic measurement (Chen, Zhang, Sun, & Wei, 2014). As shown in Fig. 4F, the values of G' and G'' of CSFP2 and its digested sample (CSFP2-S, CSFP2-G, and CSFP2-I) increased with the increase of oscillation frequency. This phenomenon occurred when the number of connections between high-concentration polymer chains increased (Simas-Tosin et al., 2010). Moreover, G' and G'' are also an important basis for judging that the solution has liquid or gel behavior (Wang et al., 2018). When the loss modulus G'' of the sample was greater than the storage modulus G', it indicated that the sample showed liquid-like behavior, given the contrary, the sample demonstrated solid-like behavior. The results implied that CSFP2 and its digested samples were cross-linked at different concentrations. The crossover points in CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I occurred at 2.51, 3.98, 15.8, and 15.8 rad/s, respectively. In conclusion, in vitro simulation of saliva-gastrointestinal digestion could affect the rheological properties of CSFP2.
3.3. Effect of digestion on bioactivities of CSFP2
3.3.1. Antioxidant activities
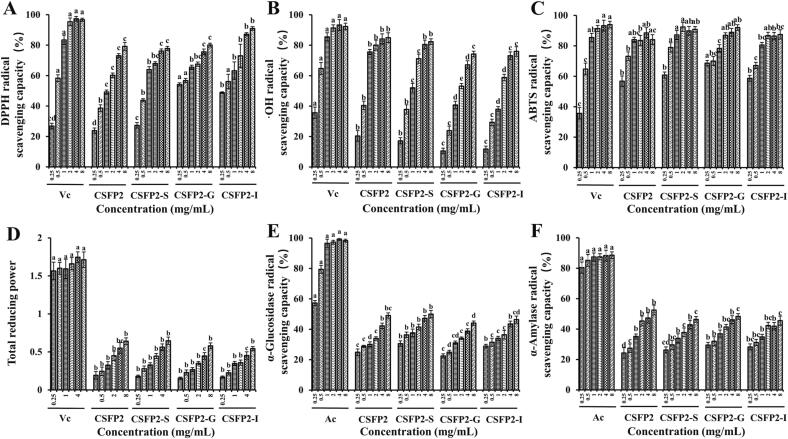
Modern studies of free radicals posited that human aging, inflammation, cardiovascular and cerebrovascular diseases, tumors and other diseases are almost all related to the increase of free radicals in the body. The mechanism of antioxidation is to eliminate superfluous free radicals in the body and maintain the dynamic balance of the body (Mirzadeh et al., 2020; Zhong et al., 2019). Studies indicated that the scavenging capacity of CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I on DPPH (IC50 ranged from 0.189 to 1.78 mg/mL), ·OH (IC50 ranged from 0.644 to 1.888 mg/mL) and ABTS (IC50 ranged from 0.069 to 0.107 mg/mL) showed a concentration gradient increasing relationship (Fig. 5A–D). Furthermore, when the concentration was 8 mg/mL, the reduction capacity of CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I could reach 0.64, 0.65, 0.58, and 0.54, respectively. Compared with the Vc, CSFP2 and its digested samples all had significant antioxidant activity. The DPPH radical-scavenging ability of CSFP2-I increased significantly after gastrointestinal digestion (IC50 value decreased from 1.082 to 0.337 mg/mL) (p < 0.05). This phenomenon is similar to research results from studies of Craterellus cornucopioides polysaccharides (CCPs): the EC50 value of DPPH free radical-scavenging ability of CCPs decreased significantly (from 283.3 to 249 μg/mL) after gastrointestinal digestion (Liu et al., 2020a). Furthermore, CSFP2 also always maintained a strong ABTS radical-scavenging ability during digestion. In general, the Mw and monosaccharide composition of natural polysaccharides play a critical role in their antioxidant activities (Duan et al., 2018, Kelishomi et al., 2016, Sheng and Sun, 2014). For example, Luo et al. confirmed that acidic polysaccharides have more significant antioxidant activity through in vitro antioxidant tests on the purified components of ginseng polysaccharides (Luo & Fang, 2008). Furthermore, when Tian et al. explored the protective effect of Ganoderma lucidum polysaccharides (GLP) with different molecular weights on ethanol-induced acute gastric injury in rats, they found that when GLP was greater than 10 KDa, the symptoms of gastric mucosal congestion and bleeding in rats were improved, and serum myeloperoxidase and inflammatory factors were reduced. Moreover, GLP with different molecular weights has a dose-dependent effect on reducing alcohol-induced gastric injury (Wang, Yin, Hu, Nie, & Xie, 2022). Meanwhile, studies also showed that the potential combination of flavonoids, polyphenols, and polysaccharides is also one of the main ways to affect the antioxidant activity of polysaccharide extracts (Ahn, Halake, & Lee, 2017; (Liu et al., 2018b, Liu et al., 2018a). In conclusion, CSFP2 still has significant antioxidant activity after digestion in vitro, so it could be developed and utilized as a potential functional-food antioxidant.
Fig. 5.
Changes of in vitro biological activities of CSFP2 during digestion. (A) DPPH radical-scavenging capacity; (B) ·OH radical-scavenging capacity; (C) ABTS radical-scavenging capacity; (D) Total reducing power; (E) α-Glucosidase inhibition capacity; (F) α-Amylase inhibition capacity. Data are expressed as the mean ± standard deviation (n = 3), and different lowercase letters denote statistical significance (p < 0.05).
3.3.2. Enzymatic inhibitory activities analysis
Inhibition in vivo α-glucosidase activity can effectively improve the symptoms of hyperglycemia induced by diabetes (Podsedek et al., 2014). At present, in clinical practice, the hypoglycemic effect is mainly achieved by injecting insulin or taking western medicine such as acarbose, metformin hydrochloride, glimepiride, etc. Studies have shown that long-term use of western medicine will have certain toxic, and side-effects on the body, so it is important to find a natural biomacromolecule active substance that can replace such medicines. Results showed that CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I possessed strong inhibitory effect on α-glucosidase (IC50: 11.74, 7.71, 18.36, and 16.41 mg/mL) in a dose-dependent manner (Fig. 5E). In addition, after gastrointestinal digestion, the α-glucosidase inhibitory effect of CSFP2 did not change significantly (p > 0.05). Meanwhile, α-amylase is also an important enzyme that decomposes complex carbohydrates into oligosaccharides in the body. The IC50 values of α-amylase inhibition of CSFP2, CSFP2-S, CSFP2-G, and CSFP2-I were 5.09, 13.14, 8.91, and 14.81 mg/mL (Fig. 5F), respectively. However, compared with acarbose, the inhibitory rate of amylase CSFP2 and its digested samples was lower.
In general, the inhibitory effect of polysaccharides on the activity of hypoglycemic enzymes in vivo is closely related to their chemical structures, such as molecular weight distribution, flavonoids, etc (Chen et al., 2014, Podsedek et al., 2014). Previous studies have shown that the crude polysaccharide (CSFP) extracted from C. squamulosa had a tendency to weaken its inhibitory enzyme activity after gastrointestinal digestion, but still maintained its stable hypoglycemic activity (Guo et al., 2022). Yang et al. separated a purified polysaccharide (HDPs-2A) from Hovenia dulcis polysaccharide, and studies found that HDPs-2A had good therapeutic effect on type-2 diabetes rat models (Yang, Luo, Sang, & Kan, 2022). In addition, Zhu et al. gavaged diabetes mice with polysaccharides from Ganoderma lucidum, and found that Ganoderma lucidum can significantly reduce fasting blood glucose levels and improve endothelium-dependent aortic relaxation (Zhu, Nie, Li, Gong, & Xie, 2014). In summary, these results indicated that CSFP2 has a potential hypoglycemic effect and shows potential for further development.
3.4. Effects of fermentation on pH, OD600, CR, and total sugar content of CSFP2
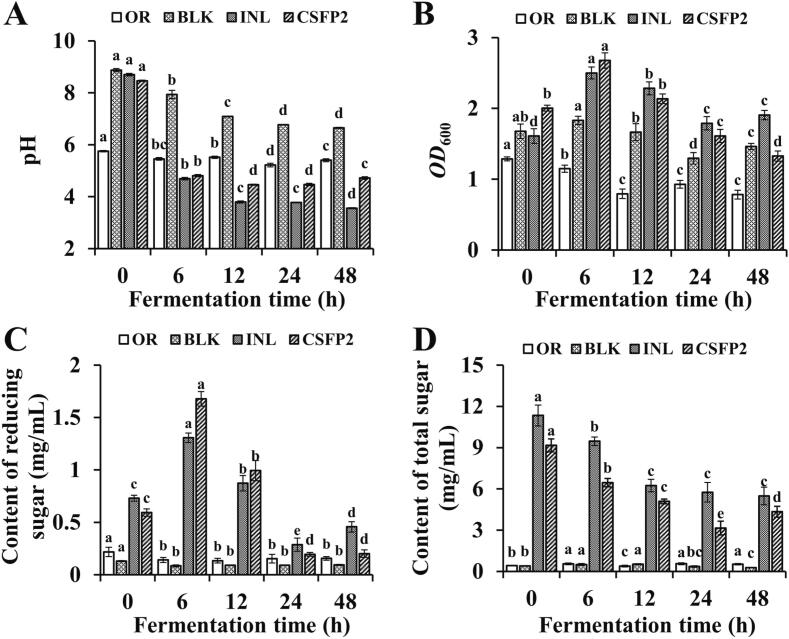
The pH in fermentation broth of BLK, INL and CSFP2 groups decreased significantly after fermentation for 6 to 48 h (range from 8.87 to 6.65, range from 8.70 to 3.56, and range from 8.46 to 4.72) (p < 0.05) (Fig. 6A). We speculated that this phenomenon may be closely related to a large amount of SCFAs produced by the fermentation broth (Li, Wang, Liu, & He, 2020; Zhou et al., 2018). In particular, results also showed that CSFP2 could improve the intestinal microbiota by slowly changing the pH of the gut microenvironment. In addition, the pH value of polysaccharides from Cyclocarya paliurus leaves also decreased significantly after simulated fecal fermentation in vitro (Min, Hu, Nie, Xie, & Xie, 2014).
Fig. 6.
Changes of in vitro fermentation characteristics of CSFP2. (A) pH value; (B) OD600; (C) Reducing sugar content; (D) Total sugar content. Data are expressed as the mean ± standard deviation (n = 3), and different lowercase letters represent statistical significance (p < 0.05).
The OD600 value of BLK, INL, and CSFP2 groups in fermentation broth reached maxima after fermentation for 6 h, at 1.83, 2.50, and 2.68, respectively. Since then, the OD600 value of different experimental groups began to decline with increasing fermentation time. Meanwhile, INL was always lower than CSFP2 in terms of its OD600 value (Fig. 6B): this may be closely related to the continuous loss of carbon sources and the lack of timely availability of carbon sources (Rosch et al., 2017).
The CR of INL and CSFP2 group in fermentation broth increased significantly (p < 0.05) after fermentation for 6 h (Fig. 6C). The change of CR may be attributed to the cleavage of glycosidic bonds in polysaccharides and the exposure of reducing end groups during fermentation (Hu et al., 2013). In particular, with increasing fermentation time, CR showed a downward trend. Microorganisms will consume and utilize CR in the fermentation process, leading to the decrease of the amount thereof (Rastall et al., 2005). In addition, the study also found that the CR increased sharply during the fermentation of Craterellus cornucopioides polysaccharides in human feces in vitro (Liu et al., 2020a).
The total sugar content in CSFP2 group decreased significantly with the increase in fermentation time (p < 0.05) (Fig. 6D): however, the total sugar content of OR and BLK groups remained unchanged during the fermentation process. Research has shown that human fecal bacteria could consume carbohydrates during fermentation, and the utilization efficiency depended largely on the properties of carbohydrates (Wu et al., 2021a). Previous literature reported that aloe polysaccharide and Sargassum Pallidum polysaccharides could be effectively utilized by microorganism in vitro fermentation, and the total sugar content decreased significantly (Liu et al., 2021; Liu et al., 2020a). In conclusion, the alternations of physico-chemical properties of CSFP2 by gut microbiota (acidic products, bacteria amount, and carbohydrate consumption) could provide a theoretical basis for further studying the effect of CSFP2 on intestinal function.
3.5. Effect of CSFP2 on SCFA levels
As reported, SCFAs are usually produced by the metabolism of indigestible polysaccharides by microbiota encoding various endogenous enzymes, which are then released by colonic intestinal cells (Lam and Cheung, 2013, Ringseis et al., 2020). SCFAs are also considered to be a key player in multiplying targets in the metabolism and immune processes in the host.
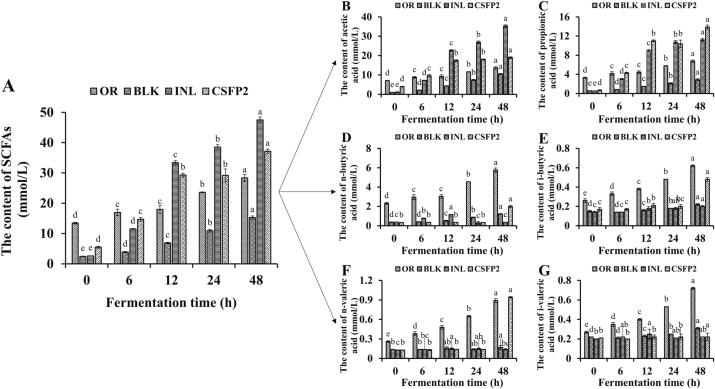
As shown in Fig. 7, SCFA concentrations of different samples (OR, BLK, INL, CSFP2) at different times (0, 6, 12, 24, 48 h) differed with the progress of fermentation. The total SCFA concentration in the CSFP2 group increased from 5.50±0.32 mmol/L to 37.15±0.72 mmol/L after fermentation for 48 h, which was significantly higher than that of BLK group (increased from 2.46±0.07 mmol/L to 15.26±0.53 mmol/L) (p < 0.05), while it was slightly lower than that of the INL group (increased from 2.62±0.01 mmol/L to 47.49±0.96 mmol/L). Furthermore, the acetic acid, propionic acid, and n-butyric acid were the main SCFAs in this study metabolized CSFP2 by gut microbiota, reaching 18.95±0.40 mmol/L, 13.88±0.42 mmol/L, and 1.98±0.13 mmol/L after fermentation for 48 h respectively (Fig. 7). Notably, the levels of acetic and propionic acids in the CSFP2 group were higher than those in the BLK group at different fermentation times.
Fig. 7.
Changes in SCFAs levels in fermentation solutions. Data are expressed as the mean ± standard deviation (n = 3), and different lowercase letters denote statistical significance (p < 0.05).
Studied showed that acetic acid is an important energy source for brain and peripheral tissue, which could activate G-protein coupled receptors, so as to regulate fat insulin signal transduction (Ferreira-Lazarte et al., 2019, Payling et al., 2020, Zhang et al., 2020). In addition, acetic acid also plays a key role in fat production and cholesterol synthesis in the body (Mateos-Aparicio, Mengibar, & Heras, 2016). Acetate is mainly produced from pyruvate via a two-step enzymatic catalysis (pyruvate formate-lyase and acetate-CoA ligase) with the metabolic intermediate being acetyl-CoA. In addition, acetogenic bacteria can also convert one molecule of glucose to three molecules of acetate to produce acetic acid (Karnholz, Küsel, Gößner, Schramm, & Drake, 2002). Combined with the determination results of intestinal microbiota and relevant references of acetic acid producing bacteria (Hayashi et al., 2007, Karnholz et al., 2002), the high production of acetate in the CSFP2 and INL group might be due to the growth of Bifidobacterium and Lactobacillus, respectively. Furthermore, propionate is also a major SCFA, which can participate in the activation of intestinal gluconeogenesis, thus regulating food intake, enhancing insulin sensitivity, and maintaining metabolic homeostasis (El Hage, Hernandez-Sanabria, Calatayud Arroyo, & Van de Wiele, 2020). In vitro analysis showed that propionic acid could enhance glucose stimulated insulin release and maintenance by inhibiting cell apoptosis; β-cell quality plays a direct role (Pingitore et al., 2017). Meanwhile, propionic acid could also slow fatty acid metabolism in the liver (Sa'ad, Peppelenbosch, Roelofsen, Vonk, & Venema, 2010). One of the most important pathways of propionate synthesis is that Bacteroides and some species of Negativicutes subclass use pentose or hexose as the substrate to synthesize these through the succinic acid route (Reichardt et al., 2014). Based on the experimental results, the increase of propionic acid content in the fermentation broth may be closely related to the fermentation of Dialister, Phascolarctobacterum, and Bifidobacterium (Xu, Zhu, Li, & Sun, 2020).
Meanwhile, butyric acid also plays an important role in energy source of colon epithelial cell (Maslowski & Mackay, 2011), T-cell proliferation and intestinal immune response (Topping & Clifton, 2001). Butyrate is most commonly produced from butyryl-phosphate via the phosphotransbutyrylase/butyrate kinase routes by Coprococcus comes and Coprococcus eutactus (Duncan et al., 2002, Louis et al., 2014). Bacteroides and Eubacterium are important to the production of butyric acid in the intestine, which is consistent with the findings of the present study. Collectively, CSFP2 has good potential to produce more SCFAs beneficial to the health of the host.
3.6. Effect of CSFP2 on gut microbiota
3.6.1. Effect of CSFP2 on microbial species
Gut microorganisms play an key role in the energy metabolism and immune system in body (Sekirov, Russell, Antunes, & Finlay, 2010). Mushroom-derive polysaccharides are a type of mild foodborne substance. After ingestion, it could affect the gut microflora of the body, thus regulating the health status of the host. Therefore, an in-depth understanding of their mechanism is very important to prevent intestinal diseases (Han et al., 2020).
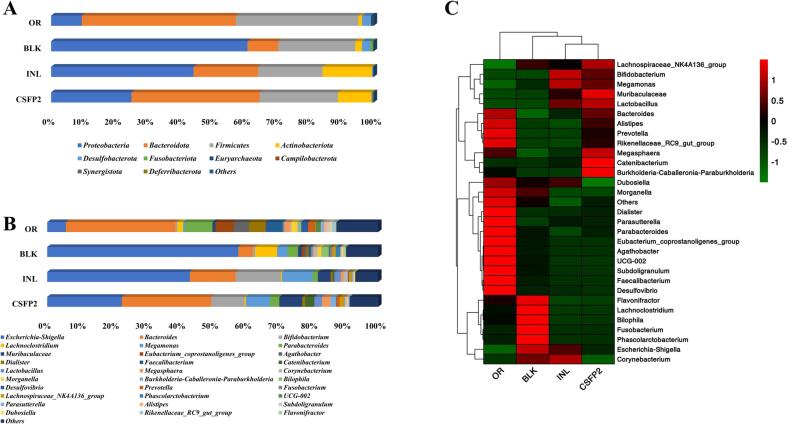
In this study, 16S rRNA high-throughput sequencing technology was used to study the effect of CSFP2 on the gut microbiota. The community structure of the original fecal bacterial solution (OR) was normal, indicating that the collected samples had experimental operability. The microbial community structure at the phylum level is shown in Fig. 8 A. Similar to the gut microbial community as previously reported, Proteobacteria, Bacteroidota, Firmicutes, and Actinobacteriota are dominant bacteria in different experimental samples (Wang et al., 2019). Compared with the BLK group, the levels of Firmicute and Bacteroidota in the CSFP2 group were significantly increased after 48 h of fermentation, while the relative abundance of Proteobacteria decreased noticeably. It was reported that some certain colonic butyric-acid-producing bacteria from Firmicutes could ferment indigestible dietary polysaccharides, promote the release of butyric acid, and improve intestinal function (Xu et al., 2019). Bacteroides are colonized resides in the distal colon and can encode many carbohydrate enzymes, such as glycosidase and polysaccharide lyase, which can hydrolyze indigestible polysaccharides to produce SCFAs (Johnson, Heaver, Walters, & Ley, 2017). In this study, the Firmicutes/Bacteroidota (F/B) of the CSFP2 and INL groups was significantly (p < 0.05) lower than that of the BLK group. Research showed that the reduction in the F/B ratio was associated with reduced energy intake, which might help to control the risk of obesity. Moreover, Proteobacteria is a group of pathogenic bacteria, mainly including Escherichia-Shigella, Salmonella, and Campylobacter. Disorder can cause mild inflammation and even chronic colitis (Zhang et al., 2020).
Fig. 8.
Effects of CSFP2 on gut microbiota. (A) Phylum level; (B) Genus level; (C) Heatmap analysis of the relative abundance of bacterial community at the genus level.
In order to further identify the specific genus of interest to metabolize CSFP2, the abundance of genus was analyzed (Fig. 8B). the relative abundances of Bacteriodes, Bifidobacterium, Catenibacterium, Lachnospiraceae_NK4A136_group, Megasphaera, Prevotella, Megamonas, and Lactobacillus were significantly increased with the intervention of CSFP2 at the genus level (Fig. 8B), while the relative abundances of Escherichia-Shigella, Dialister, Lachnoclostridium, Corynebacterium, Morganella, and Bilophila were decreased. Moreover, the clustering tree reflected similarities in bacteria composition and suggested specificity in bacteria distribution. The cluster analysis for bacterial communities revealed that the INL and CSFP2 supplementation changed the microbial composition (Fig. 8C). The main functions of gut microflora are as follows: Bacteroides could metabolize intermediates, generate SCFAs, and maintain microbial communities (De Filippo, Cavalieri, Di Paola, Ramazzotti, Poullet, Massart, Collini, Pieraccini, & Lionetti, 2010; Hu et al., 2014). Bifidobacteria have been shown to have the ability to metabolize glycans (Koropatkin, Cameron, & Martens, 2012). Catenibacterium is a potentially specific metabolic bacterium, which can produce beneficial SCFAs through carbohydrate metabolism (Yang, Martínez, Walter, Keshavarzian, & Rose, 2013). Moreover, Lachnospiraceae NK4A136 is closely associated with ulcerative colitis (Liu et al., 2019), which belongs to the butyric acid-promoting microbiota. Prevotella is a type of beneficial microorganism in the gut of healthy people that could produces acetic acid, regulate postprandial blood glucose, hydrolyze complex carbohydrates, and alleviate intestinal immunity disorders (Vandeputte et al., 2017). Megasphaera could promote bodily production of lactic acid and butyric acid (Hashizume, Tsukahara, Yamada, Koyama, & Ushida, 2003). In addition, Escherichia-Shigella are harmful bacteria that can destroy the intestinal epithelial mucosa (Thingholm, 2019). INL and CSFP2 intervention could significantly inhibit their proliferation.
Collectively, after the intervention of CSFP2, the number of microorganisms related to polysaccharide metabolism and SCFA release increased, and the number of harmful microorganisms decreased, indicating that CSFP2 has the potential to maintain intestinal health.
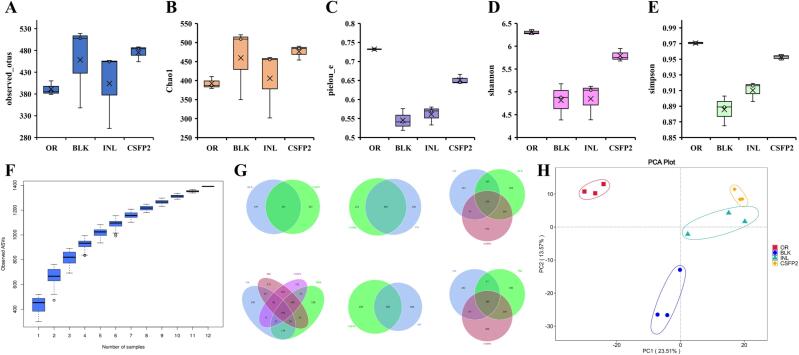
3.6.2. Effect of CSFP2 on microbial diversity
To determine whether CSFP2 affects microbial diversity, the 16SrRNA gene sequence of each group was further analyzed. The box diagram shows the changes of OTUs among different groups (Fig. 9A). The OTU is the most basic level of classification, so it is important to determine the changes of OTU levels (Wang, Tang, Zhang, Zhao, Derrien, & Rocher, 2015a). In addition, we further used the chao1 index to evaluate the number of OTUs in the sample. The larger the chao index, the more OTUs were present, indicating that the sample contained more species. The results showed that the relative number of OTUs in CSFP2 and INL groups increased significantly compared with BLK group. “Pielou e” is an indicator of phylogenetic diversity. Simpson and Shannon indices were used to assess microbial diversity. The higher the value of both, the higher the diversity of the community. As shown in Fig. 9(B – E), the microbial species, phylogenetic diversity index, and microbial diversity in CSFP2 group reached the maximum after 48 h of fermentation. Studies indicated that CSFP2 could promote the increase of microorganisms. In addition, the species accumulation curves were used to describe the changes of species with the increase of sample size. They are widely used to judge whether the sample size is sufficient and estimate species richness. The species accumulation curve shows that the position of the box diagram tends to be flat with the increase of the sample size, indicating that the number of OTUs in the flora will not change to any significant extent (Fig. 9F). The Venn diagram of different sample groups could be used to display the overlapping areas of different sample sets (Fig. 9G). Principal component analysis (PCA) analysis can help us intuitively understand the similarity between different samples. Study found that the contribution rates of PC1 and PC2 to variations were 23.51% and 13.57% respectively. Moreover, the gut microflora of CSFP2 group was different from that of BLK and INL groups. The effect of CSFP2 on microbial community composition was further confirmed. (Fig. 9H). In summary, the intervention of CSFP2 can improve the diversity and richness of intestinal communities. Previous studies have shown that the higher the diversity of intestinal flora, the lower the individual susceptibility to disease (Le Chatelier et al., 2013). Therefore, CSFP2 has played an active role in promoting the abundance of intestinal flora.
Fig. 9.
Analysis of microbial Alpha diversity. (A) Chao1; (B) Observer otus; (C) Pielou e; (D) Shannon; (E) Simpson; (F) Species accum box; (G) Venn display; (H) Principal component analysis.
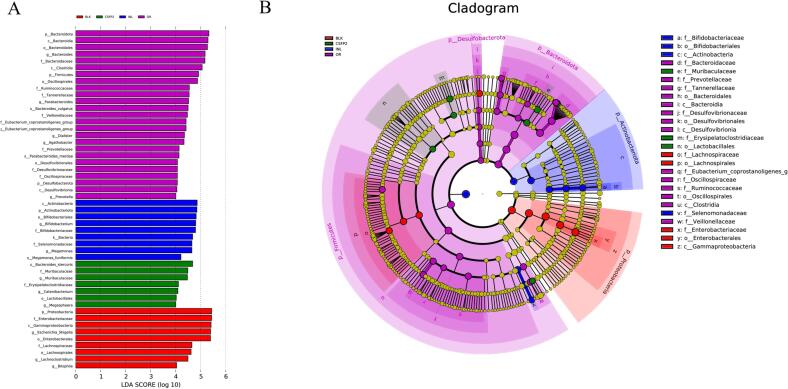
LDA can be used to analyze the gut microflora with significant difference under the intervention of samples, the higher of LDA score, the more significant the difference. As shown in Fig. 10A, since the LDA scores were between 4 and 6, there were significant differences between different microbiome groups. Then, the microbiota with significant differences were screened and explained by LEfSe (Fig. 10B). By analyzing the CSFP2 group, it was found that the number of Muribaculaceae, Bacterioides stercoris, Catenibacterium, and Lactobacillales increased significantly. Studies have shown that Bacteroides are the main beneficial gut microbiota involved in polysaccharide fermentation and utilization to produce SCFAs, which can improve body metabolism, reduce obesity, and enhance immunity (Gauffin Cano, Santacruz, Moya, & Sanz, 2012). In addition, the Muribaculaceae family associated with longevity was found to be the predominant bacterial group in the samples. These new findings are helpful when exploring the potential mechanism between microbial components and longevity (Sibai et al., 2020). In contrast, this study found that CSFP2 could reduce the number of beneficial bacteria in the intestine and reduce the risk of disease.
Fig. 10.
LDA (A) and Lefse (B) analysis of microbiota.
4. Conclusion
It was found that CSFP2 could be partially degraded after gastrointestinal digestion, and some physico-chemical properties and structural characteristics were changed. In addition, after fecal flora fermentation, CSFP2 could be significantly utilized by intestinal flora, thus regulating the composition and abundance of intestinal microorganisms, such as: Bacteriodes, Bifidobacterium, Catenibacterium, Lachnospiraceae_NK4A136_group, Megasphaera, Prevotella, Megamonas, and Lactobacillus, and then induce the production of short-chain fatty acids. The results show that CSFP2 could be developed into a functional prebiotic food providing benefits to human health.
CRediT authorship contribution statement
Xueran Geng: Supervision, Writing – review & editing. Dongdong Guo: Conceptualization, Methodology, Investigation, Writing – original draft. Tergun Bau: Writing – review & editing. Jiayu Lei: Software, Resources. Lijing Xu: Resources. Yanfen Cheng: Formal analysis, Visualization. Cuiping Feng: Resources, Formal analysis. Junlong Meng: Conceptualization, Methodology. Mingchang Chang: Resources, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was financially supported by Youth Project of Basic Research Program in Shanxi (20210302124071), Key Research and Development Program of Shanxi Province (201803D221009-1), Key Project of Coal-based Science and Technology of Shanxi Province (FT2014-03-01) and Key Team of Scientific and Technological Innovation of Edible Fungi of Shanxi Province (201805D131009).
Data availability
The data that has been used is confidential.
References
- Ahn S., Halake K., Lee J. Antioxidant and ion-induced gelation functions of pectins enabled by polyphenol conjugation. International journal of biological macromolecules. 2017;101:776–782. doi: 10.1016/j.ijbiomac.2017.03.173. [DOI] [PubMed] [Google Scholar]
- Carnachan S.M., Bootten T.J., Mishra S., Monro J.A., Sims I.M. Effects of simulated digestion in vitro on cell wall polysaccharides from kiwifruit (Actinidia spp.) Food Chemistry. 2012;133(1):132–139. doi: 10.1016/j.foodchem.2011.12.084. [DOI] [Google Scholar]
- Chen J., Liang R.H., Liu W., Liu C.M., Li T., Tu Z.C., Wan J. Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocolloids. 2012;28(1):121–129. doi: 10.1016/j.foodhyd.2011.12.01. [DOI] [Google Scholar]
- Chen G.J., Xie M.H., Wan P., Chen D., Ye H., Chen L.G.…Liu Z.H. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chemistry. 2018;244:331–339. doi: 10.1016/j.foodchem.2017.10.074. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang B., Fu X., You L.J., Abbasi A.M., Liu R.H. The digestibility of mulberry fruit polysaccharides and its impact on lipolysis under simulated saliva, gastric and intestinal conditions. Food Hydrocolloids. 2016;58:171–178. doi: 10.1016/j.foodhyd.2016.02.033. [DOI] [Google Scholar]
- Chen Y., Zhang J.G., Sun H.J., Wei Z.J. Pectin from Abelmoschus esculentus: Optimization of extraction and rheological properties. International journal of biological macromolecules. 2014;70:498–505. doi: 10.1016/j.ijbiomac.2014.07.024. [DOI] [PubMed] [Google Scholar]
- De Filippo, C., Cavalieri, D., Di Paola, M., Ramazzotti, M., Poullet, J. B., Massart, S., Collini, S., Pieraccini, G., & Lionetti, P. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14691-14696. https://doi.org/10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed]
- Duan M.Y., Shang H.M., Chen S.L., Li R., Wu H.X. Physicochemical properties and activities of comfrey polysaccharides extracted by different techniques. International journal of biological macromolecules. 2018;115:876–882. doi: 10.1016/j.ijbiomac.2018.04.188. [DOI] [PubMed] [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Applied and Environmental Microbiology. 2002;68(10):5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage R., Hernandez-Sanabria E., Calatayud Arroyo M., Van de Wiele T. Supplementation of a propionate-producing consortium improves markers of insulin resistance in an in vitro model of gut-liver axis. American Journal of Physiology Endocrinology and Metabolism. 2020;318(5):E742. doi: 10.1152/ajpendo.00523.2019. E749. [DOI] [PubMed] [Google Scholar]
- Feng H.Y., Jin H., Gao Y., Yan S.Q., Zhang Y., Zhao Q.S., Xu J. Effects of freeze-thaw cycles on the structure and emulsifying properties of peanut protein isolates. Food Chemistry. 2020;330 doi: 10.1016/j.foodchem.2020.127215. [DOI] [PubMed] [Google Scholar]
- Ferreira-Lazarte A., Moreno F.J., Cueva C., Gil-Sánchez I., Villamiel M. Behaviour of citrus pectin during its gastrointestinal digestion and fermentation in a dynamic simulator (simgi®) Carbohydrate polymers. 2019;207:382–390. doi: 10.1016/j.carbpol.2018.11.088. [DOI] [PubMed] [Google Scholar]
- Floris S., Fais A., Medda R., Pintus F., Piras A., Kumar A.…Era B. Washingtonia filifera seed extracts inhibit the islet amyloid polypeptide fibrils formations and alpha-amylase and alpha-glucosidase activity. Journal of Enzyme Inhibition and Medicinal Chemistry. 2021;36(1):517–524. doi: 10.1080/14756366.2021.1874945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauffin Cano P., Santacruz A., Moya A., Sanz Y. Bacteroides uniformis cect 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One. 2012;7(7):e41079. doi: 10.1371/journal.pone.0041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X.R., Tian G.T., Zhang W.W., Zhao Y.C., Zhao L.Y., Wang H.X., Ng T.B. A Tricholoma matsutake peptide with angiotensin converting enzyme inhibitory and antioxidative activities and antihypertensive effects in spontaneously hypertensive rats. Scientific reports. 2016;6(1):1–9. doi: 10.1038/srep24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam M.T., Esmail M.N. Rheological properties of carboxymethyl cellulose. Journal of applied polymer science. 1997;64(2):289–301. doi: 10.1002/(SICI)1097-4628(19970411)64:2<289::AID-APP9>3.0.CO;2-N. [DOI] [Google Scholar]
- Guo D.D., Lei J.Y., He C., Peng Z.J., Liu R.Z., Pan X.…Geng X.R. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulosa. International journal of biological macromolecules. 2022;208:343–355. doi: 10.1016/j.ijbiomac.2022.03.126. [DOI] [PubMed] [Google Scholar]
- Han R., Pang D.R., Wen L.R., You L.J., Huang R.M., Kulikouskaya V. In vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria Lemaneiformis. Journal of Functional Foods. 2020;64 doi: 10.1016/j.jff.2019.103652. [DOI] [Google Scholar]
- Hashizume K., Tsukahara T., Yamada K., Koyama H., Ushida K. Megasphaera elsdenii JCM1772T normalizes hyperlactate production in the large intestine of fructooligosaccharide-fed rats by stimulating butyrate production. Journal of Nutrition. 2003;133(10):3187–3190. doi: 10.1046/j.1365-277X.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Shibata K., Sakamoto M., Tomita S., Benno Y. Prevotella copri sp. nov. and Prevotella stercorea sp. nov., isolated from human faeces. International Journal of Systematic and Evolutionary Microbiology. 2007;57(5):941–946. doi: 10.1099/ijs.0.64778-0. [DOI] [PubMed] [Google Scholar]
- Hu L., Liu R., Wu T., Sui W.J., Zhang M. Structural Properties of Homogeneous Polysaccharide Fraction Released from Wheat Germ by Hydrothermal Treatment. Carbohydrate Polymers. 2020;240 doi: 10.1016/j.carbpol.2020.116238. [DOI] [PubMed] [Google Scholar]
- Hu J.L., Nie S.P., Min F.F., Xie M.Y. Artificial simulated saliva, gastric and intestinal digestion of polysaccharide from the seeds of Plantago asiatica L. Carbohydrate polymers. 2013;92(2):1143–1150. doi: 10.1016/j.carbpol.2012.10.072. [DOI] [PubMed] [Google Scholar]
- Hu J.L., Nie S.P., Wu Q.M., Li C., Fu Z.H., Gong J.…Xie M.Y. Polysaccharide from seeds of Plantago asiatica L. affects lipid metabolism and colon microbiota of mouse. Journal of Agricultural and Food Chemistry. 2014;62(1):229–234. doi: 10.1021/jf4040942. [DOI] [PubMed] [Google Scholar]
- Huang F., Liu Y., Zhang R.F., Bai Y.J., Dong L.H., Liu L.…Zhang M.Y. Structural characterization and in vitro gastrointestinal digestion and fermentation of litchi polysaccharide. International journal of biological macromolecules. 2019;140:965–972. doi: 10.1016/j.ijbiomac.2019.08.170. [DOI] [PubMed] [Google Scholar]
- Jin Q.W., Cai Z.X., Li X.B., Yadav M.P., Zhang H.B. Comparative viscoelasticity studies: Corn fiber gum versus commercial polysaccharide emulsifiers in bulk and at air/liquid interfaces. Food Hydrocolloids. 2017;64:85–98. doi: 10.1016/j.foodhyd.2016.11.002. [DOI] [Google Scholar]
- Johnson E.L., Heaver S.L., Walters W.A., Ley R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. Journal of Molecular Medicine. 2017;95(1):1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnholz A., Küsel K., Gößner A., Schramm A., Drake H.L. Tolerance and metabolic response of acetogenic bacteria toward oxygen. Applied and environmental microbiology. 2002;68(2):1005–1009. doi: 10.1128/AEM.68.2.1005-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishomi Z.H., Goliaei B., Mahdavi H., Nikoofar A., Rahimi M., Moosavi-Movahedi A.A.…Bigdeli B.J. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chemistry. 2016;196:897–902. doi: 10.1016/j.foodchem.2015.09.091. [DOI] [PubMed] [Google Scholar]
- Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nature Reviews Microbiology. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Varma C.A., Jayaram Kumar K. Structural, functional and pH sensitive release characteristics of water-soluble polysaccharide from the seeds of Albizia lebbeck L. Carbohydrate Polymers. 2017;175:502–508. doi: 10.1016/j.carbpol.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Lam K.L., Cheung P.C.K. Non-digestible long chain beta-glucans as novel prebiotics. Bioactive Carbohydrates and Dietary Fibre. 2013;2(1):45–64. doi: 10.1016/j.bcdf.2013.09.001. [DOI] [Google Scholar]
- Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G.…Kennedy S. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- Li P., Dhital S., Fu X., Huang Q., Liu R., Zhang B., He X.W. Starch digestion in intact pulse cotyledon cells depends on the extent of thermal treatment. Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2020.126268. [DOI] [PubMed] [Google Scholar]
- Li Y.Y., Kong B.H., Liu Q., Xia X.F., Chen H.S. Improvement of the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions by addition of zein hydrolysates. Process Biochemistry. 2017;53:116–124. doi: 10.1016/j.procbio.2016.11.010. [DOI] [Google Scholar]
- Li S.Y., Wang L.N., Liu B., He N.N. Unsaturated alginate oligosaccharides attenuated obesity-related metabolic abnormalities by modulating gut microbiota in high-fat-diet mice. Food & function. 2020;11(5):4773–4784. doi: 10.1039/C9FO02857A. [DOI] [PubMed] [Google Scholar]
- Liu C., Du P., Cheng Y.L., Guo Y.H., Hu B., Yao W.R.…Qian H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydrate Polymers. 2021;256 doi: 10.1016/j.carbpol.2020.117571. [DOI] [PubMed] [Google Scholar]
- Liu Y.T., Duan X.Y., Duan S.Q., Li C., Hu B., Liu A.P.…Wu W.J. Effects of in vitro digestion and fecal fermentation on the stability and metabolic behavior of polysaccharides from Craterellus cornucopioides. Food & Function. 2020;11(8):6899–6910. doi: 10.1039/D0FO01430C. [DOI] [PubMed] [Google Scholar]
- Liu J.Y., Hou X.X., Li Z.Y., Shan S.H., Chang M.C., Feng C.P., Wei Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. International journal of biological macromolecules. 2020;157:288–295. doi: 10.1016/j.ijbiomac.2020.04.162. [DOI] [PubMed] [Google Scholar]
- Liu Y.Y., Li T., Alim A., Ren D.Y., Zhao Y., Yang X.B. Regulatory effects of stachyose on colonic and hepatic inflammation, gut microbiota dysbiosis, and peripheral CD4+ T cell distribution abnormality in high-fat diet-fed mice. Journal of Agricultural and Food Chemistry. 2019;67(42):11665–11674. doi: 10.1021/acs.jafc.9b04731. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang X.C., Yong H.M., Kan J., Jin C.H. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. International journal of biological macromolecules. 2018;116:1011–1025. doi: 10.1016/j.ijbiomac.2018.05.149. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang X.C., Yong H.M., Kan J., Zhang N.F., Jin C.H. Preparation, characterization, digestibility and antioxidant activity of quercetin grafted Cynanchum auriculatum starch. International journal of biological macromolecules. 2018;114:130–136. doi: 10.1016/j.ijbiomac.2018.03.101. [DOI] [PubMed] [Google Scholar]
- Liu Y.G., Yang A.H., Qu Y.D., Wang Z.Q., Zhang Y.Q., Liu Y.…Wang D. Ameliorative effects of Antrodia cinnamomea polysaccharides against cyclophosphamide-induced immunosuppression related to Nrf2/HO-1 signaling in BALB/c mice. International journal of biological macromolecules. 2018;116:8–15. doi: 10.1016/j.ijbiomac.2018.04.178. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhou Y., Liu M.F., Wang Q.D., Li Y. Extraction optimization, characterization, antioxidant and immunomodulatory activities of a novel polysaccharide from the wild mushroom Paxillus involutus. International journal of biological macromolecules. 2018;112:326–332. doi: 10.1016/j.ijbiomac.2018.01.132. [DOI] [PubMed] [Google Scholar]
- Louis P., Hold G.L., Flint H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nature reviews microbiology. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- Lovegrove A., Edwards C.H., De Noni I., Patel H., El S.N., Grassby T.…Butterworth P. Role of polysaccharides in food, digestion, and health. Critical Reviews in Food Science & Nutrition. 2017;57(2):237–253. doi: 10.1080/10408398.2014.939263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D.H., Fang B.S. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. International journal of biological macromolecules. 2008;72(3):376–381. doi: 10.1016/j.carbpol.2007.09.006. [DOI] [Google Scholar]
- Ma Y.Y., Jiang S.S., Zeng M.Y. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110646. [DOI] [PubMed] [Google Scholar]
- Maslowski K.M., Mackay C.R. Diet, gut microbiota and immune responses. Nature Immunology. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- Mateos-Aparicio I., Mengibar M., Heras A. Effect of chito-oligosaccharides over human faecal microbiota during fermentation in batch cultures. Carbohydrate Polymers. 2016;137:617–624. doi: 10.1016/j.carbpol.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Min F.F., Hu J.L., Nie S.P., Xie J.H., Xie M.Y. In vitro fermentation of the polysaccharides from Cyclocarya paliurus leaves by human fecal inoculums. Carbohydrate Polymers. 2014;112:563–568. doi: 10.1016/j.carbpol.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Brodkorb A. A standardised static in vitro digestion method suitable for food - an international consensus. Food & Function. 2014;5(6):1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- Mirzadeh M., Arianejad M.R., Khedmat L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydrate Polymers. 2020;229 doi: 10.1016/j.carbpol.2019.115421. [DOI] [PubMed] [Google Scholar]
- Nie X.R., Fu Y., Wu D.T., Huang T.T., Jiang Q., Zhao L.…Qin W. Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus) Molecules. 2020;25(5):1155. doi: 10.3390/molecules25051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X.R., Li H.Y., Du G., Lin S., Hu R., Li H.Y.…Qin W. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. International journal of biological macromolecules. 2019;139:459–467. doi: 10.1016/j.ijbiomac.2019.08.016. [DOI] [PubMed] [Google Scholar]
- Payling L., Fraser K., Loveday S.M., Sims I., Roy N., McNabb W. The effects of carbohydrate structure on the composition and functionality of the human gut microbiota. Trends in Food Science & Technology. 2020;97:233–248. doi: 10.1016/j.tifs.2020.01.009. [DOI] [Google Scholar]
- Pingitore A., Chambers E.S., Hill T., Maldonado I.R., Liu B., Bewick G.…Tedford C. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Obesity and Metabolism. 2017;19(2):257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- Podsedek A., Majewska I., Redzynia M., g.,, Sosnowska,, D.,, &, KozioE,, kiewicz,, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. Journal of Agricultural and Food Chemistry. 2014;62(20):4610–4617. doi: 10.1021/jf5008264. [DOI] [PubMed] [Google Scholar]
- Qiao Y.B., Ye Y., Cai T.X., Li S., Liu X.Q. Anti-fatigue activity of the polysaccharides isolated from Ribes stenocarpum Maxim. Journal of Functional Foods. 2022;89 doi: 10.1016/j.jff.2022.104947. [DOI] [Google Scholar]
- Rastall R.A., Gibson G.R., Gill H.S., Guarner F., Klaenhammer T.R., Pot B.…Sanders M.E. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: An overview of enabling science and potential applications. FEMS Microbiology Ecology. 2005;52(2):145–152. doi: 10.1016/j.femsec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Reichardt N., Duncan S.H., Young P., Belenguer A., McWilliam Leitch C., Scott K.P.…Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. The ISME journal. 2014;8(6):1323–1335. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringseis R., Gessner D.K., Eder K. The gut-liver axis in the control of energy metabolism and food intake in animals. Annual Review of Animal Biosciences. 2020;8:295–319. doi: 10.1146/annurev-animal-021419-083852. [DOI] [PubMed] [Google Scholar]
- Rosch C., Taverne N., Venema K., Gruppen H., Wells J.M., Schols H.A. Effects of in vitro fermentation of barley beta-glucan and sugar beet pectin using human fecal inocula on cytokine expression by dendritic cells. Molecular Nutrition Food Research. 2017;61(1):1600243. doi: 10.1002/mnfr.201600243. [DOI] [PubMed] [Google Scholar]
- Sa'ad H., Peppelenbosch M.P., Roelofsen H., Vonk R.J., Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of. Lipids. 2010;1801(11):1175–1183. doi: 10.1016/j.bbalip.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiological Reviews. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Sheng J.W., Sun Y.L. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydrate Polymers. 2014;108:41–45. doi: 10.1016/j.carbpol.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Sibai M., Altunta E., Yıldırım B., Öztürk G., Yıldırım S., Demircan T. Microbiome and longevity: High abundance of longevity-linked Muribaculaceae in the gut of the long-living rodent spalax leucodon. OMICS: A Journal of. Integrative Biology. 2020;24(10):592–601. doi: 10.1089/omi.2020.0116. [DOI] [PubMed] [Google Scholar]
- Simas-Tosin F.F., Barraza R.R., Petkowicz C.L.O., Silveira J.L.M., Sassaki G.L., Santos E.M.R.…Iacomini M. Rheological and structural characteristics of peach tree gum exudate. Food Hydrocolloids. 2010;24(5):486–493. doi: 10.1016/j.foodhyd.2009.12.010. [DOI] [Google Scholar]
- Thingholm, L. B., RГjhlemann, M. C., Koch, M., Fuqua, B., Laucke, G., Boehm, R., Bang, C., Franzosa, E. A., HГjbenthal, M., & Rahnavard, A. J. (2019). Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host & Microbe, 26(2), 252-264. https://doi.org/10.1016/j.chom.2019.07.004. [DOI] [PMC free article] [PubMed]
- Topping D.L., Clifton P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiological Reviews. 2001;81(3):1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- Valverde M.E., Hernández-Pérez T., Paredes-López O. Edible mushrooms: Improving human health and promoting quality life. International Journal of Microbiology. 2015;2015 doi: 10.1155/2015/376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte D., Kathagen G., Dhoe K., Vieira-Silva S., Valles-Colomer M., Sabino J.…Darzi Y.J.N. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551(7681):507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- Wang, J. J., Tang, H., Zhang, C. H., Zhao, Y. F., Derrien, M., Rocher, E., van-Hylckama Vlieg, J. E., Strissel, K., Zhao, L. P., & Obin, M. (2015). Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. Nature, 9(1), 1-15. https://doi.org/10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed]
- Wang K., Liao M., Zhou N., Bao L., Ma K., Zheng Z.…Wang J. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell reports. 2019;26(1) doi: 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Wang Z.B., Pei J.J., Ma H.L., Cai P.F., Yan J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydrate Polymers. 2014;109:49–55. doi: 10.1016/j.carbpol.2014.03.057. [DOI] [PubMed] [Google Scholar]
- Wang W., Tan J., Nima L., Sang Y., Cai X., Xue H.K. Polysaccharides from fungi: A review on their extraction, purification, structural features, and biological activities. Food Chemistry: X. 2022;100414 doi: 10.1016/j.fochx.2022.100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.J., Wang S., Xia Y.J., Tu M.W.L.J., Zhang L.J., Wang Y.M. Antitumor effects and immune regulation activities of a purified polysaccharide extracted from Juglan regia. International journal of biological macromolecules. 2015;72:771–775. doi: 10.1016/j.ijbiomac.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Wang X.Y., Yin J.Y., Hu J.L., Nie S.P., Xie M.Y. Gastroprotective polysaccharide from natural sources: Review on structure, mechanism, and structure-activity relationship. Food Frontiers. 2022;2022 doi: 10.1002/fft2.172. [DOI] [Google Scholar]
- Wang Y.F., Yu L., Wei X.L. Monosaccharide composition and bioactivity of tea flower polysaccharides obtained by ethanol fractional precipitation and stepwise precipitation. CyTA - Journal of Food. 2012;10(1):1–4. doi: 10.1080/19476337.2010.523901. [DOI] [Google Scholar]
- Wang L., Zhang B., Xiao J., Huang Q., Li C., Fu X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chemistry. 2018;249:127–135. doi: 10.1016/j.foodchem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Wu D.T., Guo H., Lin S., Lam S.C., Zhao L., Lin D.R., Qin W. Review of the structural characterization, quality evaluation, and industrial application of Lycium barbarum polysaccharides. Trends in Food Science & Technology. 2018;79:171–183. doi: 10.1016/j.tifs.2018.07.016. [DOI] [Google Scholar]
- Wu D.T., Nie X.R., Gan R.Y., Guo H., Fu Y., Yuan Q.…Qin W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocolloids. 2021;114 doi: 10.1016/j.foodhyd.2020.106577. [DOI] [Google Scholar]
- Wu D.T., Yuan Q., Guo H., Fu Y., Li F., Wang S.P., Gan R.Y. Dynamic changes of structural characteristics of snow chrysanthemum polysaccharides during in vitro digestion and fecal fermentation and related impacts on gut microbiota. Food Research International. 2021;141 doi: 10.1016/j.foodres.2020.109888. [DOI] [PubMed] [Google Scholar]
- Wu J., Zhou J.X., Lang Y.G., Yao L., Xu H., Shi H.B., Xu S.D. A polysaccharide from Armillaria mellea exhibits strong in vitro anticancer activity via apoptosis-involved mechanisms. International Journal of Biological Macromolecules. 2012;51(4):663–667. doi: 10.1016/j.ijbiomac.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Xu S.Y., Aweya J.J., Li N., Deng R.Y., Chen W.Y., Tang J., Cheong K.L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food chemistry. 2019;289:177–186. doi: 10.1016/j.foodchem.2019.03.050. [DOI] [PubMed] [Google Scholar]
- Xu S.Y., Chen X.Q., Liu Y., Cheong K.L. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. International Journal of Biological Macromolecules. 2020;152:748–756. doi: 10.1016/j.ijbiomac.2020.02.305. [DOI] [PubMed] [Google Scholar]
- Xu Y.Q., Niu X.J., Liu N.Y., Gao Y.K., Wang L.B., Xu G.…Yang Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chemistry. 2018;243:26–35. doi: 10.1016/j.foodchem.2017.09.107. [DOI] [PubMed] [Google Scholar]
- Xu Y.Q., Zhu Y., Li X.T., Sun B.G. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends in Food Science & Technology. 2020;100:118–130. doi: 10.1016/j.tifs.2020.02.026. [DOI] [Google Scholar]
- Yan J.K., Wu L.X., Qiao Z.R., Cai W.D., Ma H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chemistry. 2019;271:588–596. doi: 10.1016/j.foodchem.2018.08.012. [DOI] [PubMed] [Google Scholar]
- Yang B., Luo Y., Sang Y., Kan J.Q. Isolation, purification, structural characterization, and hypoglycemic activity assessment of polysaccharides from Hovenia dulcis (Guai Zao) International Journal of Biological Macromolecules. 2022;208:1106–1115. doi: 10.1016/j.ijbiomac.2022.03.211. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Martínez I., Walter J., Keshavarzian A., Rose D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013;23:74–81. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Yin C., Noratto G.D., Fan X.Z., Chen Z.Y., Yao F., Shi D.F., Gao H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydrate Polymers. 2020;250 doi: 10.1016/j.carbpol.2020.116942. [DOI] [PubMed] [Google Scholar]
- Yuan Q., He Y., Xiang P.Y., Huang Y.J., Cao Z.W., Shen S.W.…Wu D.T. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus) International Journal of Biological Macromolecules. 2020;147:1053–1063. doi: 10.1016/j.ijbiomac.2019.10.073. [DOI] [PubMed] [Google Scholar]
- Yuan Q., He Y., Xiang P.Y., Wang S.P., Cao Z.W., Gou T.…Wu D.T. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of okra polysaccharides. Carbohydrate Polymers. 2020;238 doi: 10.1016/j.carbpol.2020.116183. [DOI] [PubMed] [Google Scholar]
- Yuan D., Li C., You L.J., Dong H., Fu X. Changes of digestive and fermentation properties of Sargassum pallidum polysaccharide after ultrasonic degradation and its impacts on gut microbiota. International Journal of Biological Macromolecules. 2020;164:1443–1450. doi: 10.1016/j.ijbiomac.2020.07.198. [DOI] [PubMed] [Google Scholar]
- Yuan Y.Q., Li C., Zheng Q.W., Wu J.X., Zhu K.X., Shen X.R., Cao J. Effect of simulated gastrointestinal digestion in vitro on the antioxidant activity, molecular weight and microstructure of polysaccharides from a tropical sea cucumber (Holothuria leucospilota) Food Hydrocolloids. 2019;89:735–741. doi: 10.1016/j.foodhyd.2018.11.040. [DOI] [Google Scholar]
- Yuan Q., Lin S., Fu Y., Nie X.R., Liu W., Su Y.…Wu D.T. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus) International Journal of Biological Macromolecules. 2019;127:178–186. doi: 10.1016/j.ijbiomac.2019.01.042. [DOI] [PubMed] [Google Scholar]
- Zhang X., Aweya J.J., Huang Z.X., Kang Z.Y., Bai Z.H., Li K.H.…Cheong K.L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydrate Polymers. 2020;234 doi: 10.1016/j.carbpol.2020.115894. [DOI] [PubMed] [Google Scholar]
- Zhao Y.M., Song J.H., Wang J., Yang J.M., Wang Z.B., Liu Y.H. Optimization of cellulase-assisted extraction process and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Journal of the Science of Food and Agriculture. 2016;96(13):4484–4491. doi: 10.1002/jsfa.7662. [DOI] [PubMed] [Google Scholar]
- Zhao R.Q., Yang W.J., Pei F., Zhao L.Y., Hu Q.H. In vitro fermentation of six kinds of edible mushrooms and its effects on fecal microbiota composition. Lwt. 2018;96:627–635. doi: 10.1016/j.lwt.2018.06.012. [DOI] [Google Scholar]
- Zheng L., Ma Y.H., Zhang Y.J., Meng Q.J., Yang J.H., Wang B.L.…Shi J.G. Increased antioxidant activity and improved structural characterization of sulfuric acid-treated stepwise degraded polysaccharides from Pholiota nameko PN-01. International Journal of Biological Macromolecules. 2021;166:1220–1229. doi: 10.1016/j.ijbiomac.2020.11.004. [DOI] [PubMed] [Google Scholar]
- Zhong Q., Wei B., Wang S., Ke S., Chen J., Zhang H., Wang H. The antioxidant activity of polysaccharides derived from marine organisms: An overview. Marine drugs. 2019;17(12):674. doi: 10.3390/md17120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong K., Zhang Q., Tong L., Liu L., Zhou X., Zhou S.M. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrasonics Sonochemistry. 2015;23:75–80. doi: 10.1016/j.ultsonch.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Zhou W.T., Yan Y.M., Mi J., Zhang H.C., Lu L., Luo Q.…Cao Y.L. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from Bee collected pollen of Chinese Wolfberry. Journal of Agricultural and Food Chemistry. 2018;66(4):898–907. doi: 10.1021/acs.jafc.7b05546. [DOI] [PubMed] [Google Scholar]
- Zhu K.X., Nie S.P., Li C., Gong D., Xie M.Y. Ganoderma atrum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohydrate polymers. 2014;103:520–527. doi: 10.1016/j.carbpol.2013.12.080. [DOI] [PubMed] [Google Scholar]
- Zhu K.X., Nie S.P., Li C., Lin S., Xing M.M., Li W.J.…Xie M.Y. A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia. International Journal of Biological Macromolecules. 2013;57:142–150. doi: 10.1016/j.ijbiomac.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Zhu K.X., Yao S.W., Zhang Y.J., Liu Q.B., Xu F., Wu G.…Tan L.H. Effects of in vitro saliva, gastric and intestinal digestion on the chemical properties, antioxidant activity of polysaccharide from Artocarpus heterophyllus Lam. (Jackfruit) Pulp. Food Hydrocolloids. 2019;87:952–959. doi: 10.1016/j.foodhyd.2018.09.014. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.