Abstract
In order to combat an evolving, multidimensional disease such as cancer, research has been aimed at synthesizing more efficient and effective versions of popular chemotherapeutic drugs. Despite these efforts, there remains a necessity for the development of suitable delivery vehicles that can both harness the chemotherapeutic effects meanwhile reducing some of the known issues when using these drugs such as unwanted side-effects, acquired drug resistance, and associated difficulties with drug delivery. Synthetic drug discovery approaches focusing on modification of the native structure of these chemotherapeutic drugs often face challenges such as loss of efficacy, as well as a potential worsening of side-effects. Synthetic chemists are then left with increasingly narrow choices for possible chemistry they could implement to achieve the desired therapy. The emergence of targeted therapies using controlled-release nanomaterials can provide many opportunities for conventional chemotherapeutic drugs to be delivered to specific target sites, ultimately leading to reduced side-effects and improved efficacy. Logically, it may prove advantageous to consider nano-delivery systems as a likely candidate for circumventing some of the barriers associated with creating viable drug therapies. In this review, we summarize controlled release nanoformulations of the three most widely used and approved chemotherapeutics, doxorubicin, paclitaxel, and cisplatin as an alternative therapeutic approach against different cancer types.
Keywords: Cancer therapy, Nanotherapeutic platform, Clinical trials, Doxorubicin, Cisplatin, Taxol®
1. Introduction
Chemotherapy saw its origins unfold amidst unconventional times, specifically during World War II. Inspired from an earlier observation that mustard gas served as a formidable suppressor of hematopoiesis (Krumbhaar and Krumbhaar, 1919), scientists thought to study mustard-compounds as potential medicines to treat lymphomas (Fenn and Udelsman, 2011). The result of this work was the development of the first chemotherapeutic drug, mustine, and the beginning of a multibillion-dollar industry. The fields of cancer research and the accompanying pharmacological responses advanced forward, drugs with the ability to treat a wide variety of cancers came into popular view: doxorubicin, cisplatin, and paclitaxel.
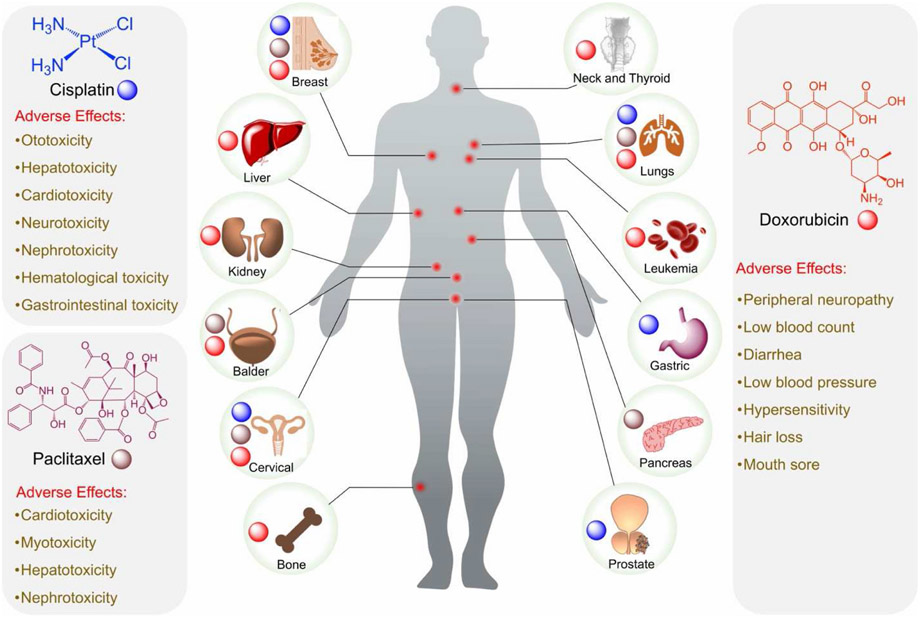
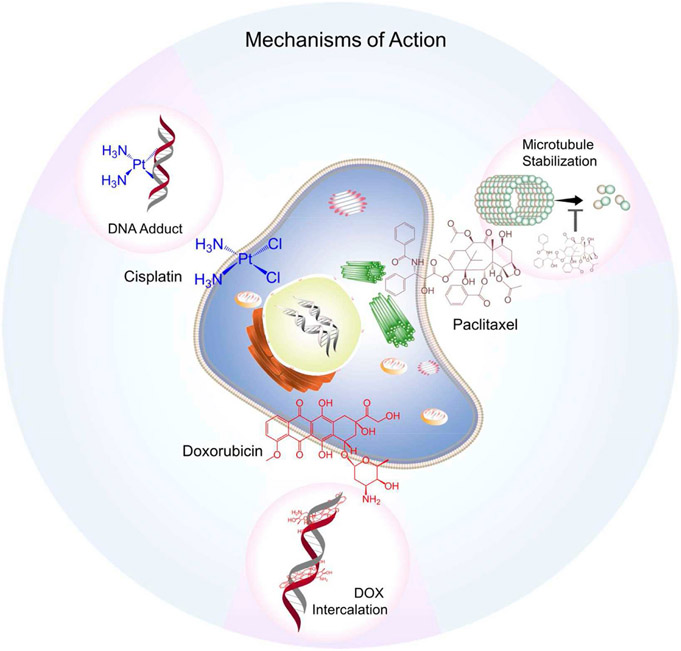
Approved by the U.S. Food and Drug Administration (FDA) in 1974, doxorubicin (Fig. 1) (DOX) also known as adriamycin, is a fluorescent anthracycline molecule containing a hydroxy-substituted anthraquinone and an aminoglycosidic side chain which is hydrophilic and imparts a net amphiphilicity to doxorubicin. This chemo agent works by intercalating into the DNA, preventing proper DNA replication and thus induces cell death (Fig. 2) (Jawad et al., 2019). DOX has also been shown to inhibit topoisomerase II, which further inhibits vital DNA replication (Pommier et al., 2010). Primarily it is used in the treatment of breast cancer at various stages, but has also been used to treat ovarian cancer, bladder cancer, stomach cancer, soft-tissue sarcomas, and leukemias (Tacar et al., 2013). Obstacles hindering DOX’s employment stem from acquired drug resistance and increased cellular efflux mechanisms. Drug resistance becomes a problem when repeated dosing results in undesired increases in cytotoxicity, largely cardiotoxicity, as well as subsequent irreversible cardiomyopathy with increasing dosages, nephrotoxicity, increased risk to severe allergic reaction, bone marrow suppression, inflammation of the mouth, and damage to tissue at the site of injection (Tacar et al., 2013).
Fig. 1.
Schematic representation of structures of cisplatin, paclitaxel, and doxorubicin, use in different cancer types, and listed adverse effects.
Fig. 2.
Mechanisms of action of Cisplatin, Paclitaxel and Doxorubicin.
Paclitaxel (PTX) also known as Taxol® (Fig. 1), was first approved by the FDA to treat ovarian cancer and is formed from a diterpene with a taxane ring and a four-membered oxetane ring having an ester side chain at its C-13 position (De Weger et al., 2014). The drug works by promoting the stabilization of microtubules which induces mitotic arrest, it meanwhile permits the mitotic checkpoints to remain active causing a delay in the separation of chromosomes which eventually leads to cell death (Fig. 2) (De Weger et al., 2014). Paclitaxel is able to treat a wide range of cancers including ovarian cancer, cervical cancer, breast cancer, pancreatic cancer, lung cancer, esophageal cancer, and Kaposi sarcoma (De Weger et al., 2014). It suffers from low solubility in water and thus requires formulating dosages with polyethoxylated castor oil and dehydrated ethanol, but this poses several risks for patients using this drug as the castor oil from the formulation can likely lead to severe allergic reactions, peripheral neuropathy as well as neurotoxicity, hyperlipidemia (De Weger et al., 2014). Like doxorubicin, paclitaxel is susceptible to multi-drug resistance cellular efflux pathways (Yusuf et al., 2003).
Cisplatin (Fig. 1) is the only complete inorganic chemotherapeutic which is FDA approved and can be used to treat more than 50% of all types of cancers (Surnar et al., 2018). Cisplatin is a square planer coordination complex cis-[Pt(NH3)2Cl2]. Once inside the cell, utilizing the trans effect, the drug undergoes stepwise aquation, releasing the two chloride ligands that are facilitated by the low chloride concentration at the intracellular level. The resulting species binds to N7 of guanine bases that causes inhibition of DNA transcription and translation, prompting cellular apoptosis (Fig. 2) (Fuertes et al., 2003). Cisplatin can treat a wide variety of cancers such as testicular cancer, bladder cancer, ovarian cancer, cervical cancer, breast cancer, neuroblastomas, lung cancer, head and neck cancers, as well as mesotheliomas amongst others (Tsang et al., 2009). Issues arising from the use of cisplatin include ototoxicity, electrolyte disturbance, nausea and vomiting, neurotoxicity, nephrotoxicity, hepatoxicity and even hemolytic anemia, all unintended side-effects (Tsang et al., 2009).
Rapid success and utilization, however, has yet to be fully realized in any ensuing synthetic drug discovery trials surrounding the chemotherapeutic drugs. Nanomedicine, more specifically, nano-delivery systems, have found themselves skyrocketing in applicability and effectiveness as was seen in the case with the rapidly developed and employed COVID-19 mRNA vaccines which utilized lipid nanoparticle (NP) technologies (Chauhan et al., 2020). This begs the question, should nano-delivery systems be considered as an alternative solution, if not a better means to resolving barriers associated with conventional chemotherapeutic drugs? In this review, we present strategies afforded by nanotechnology to circumvent challenges faced with traditional drug discovery, within the lens of controlled release nanoplatforms.
2. Passive targeting
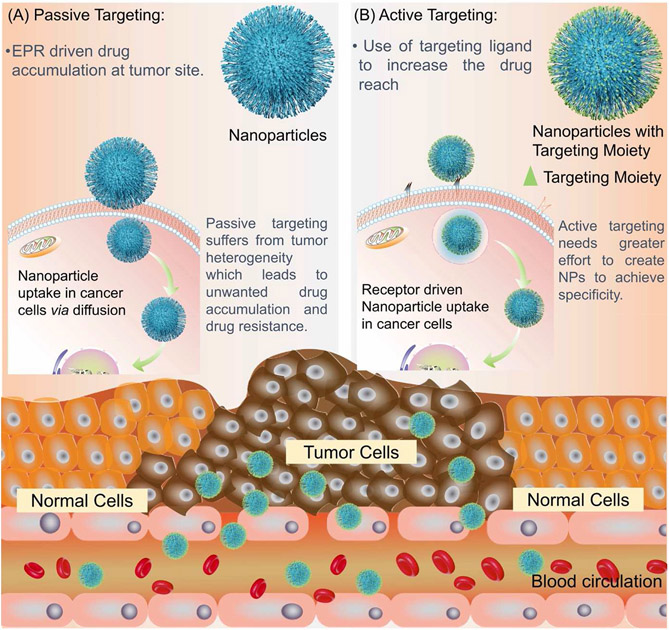
Passive targeting refers to the ability of nanoparticles to accumulate at the site of a tumor without any ligands on the surface of the nanoparticle to assist in accumulation. This is done by taking advantage of the characteristics of tumor such as enhanced permeability and retention (EPR) effect that are not present around healthy tissue (Bazak et al., 2014).
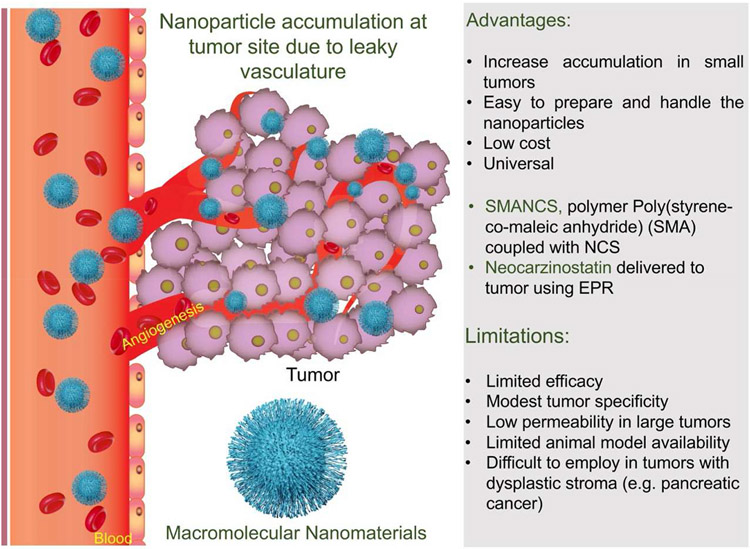
Before the discovery and usage of “passively targeted” nano delivery systems (PTS), conventional chemotherapeutics and similar free form pharmacological agents would suffer in their ability to home in on, as well differentiate between, malignant and healthy tissues, usually exhibiting poorer biodistribution and pharmacokinetic properties with increased risks to unintended side-effects. It was later thought that the use of polymeric nanoformulations could alleviate some of these challenges associated with conventional drug delivery by having polymers serve as nanocarriers of these drugs through either direct conjugation or physical entrapment. Drug therapies utilizing polymers saw improvements upon pharmacological goals, such as improved drug solubility for lower MW compounds, as well as increased blood residence; they could provide stability to the drugs and were able to reduce the immunogenicity of the drugs while offering protection from biomolecules poised to degrade or sequester them (Liechty et al., 2010). However, chemotherapeutics and polymer-based nanotechnologies would merge and see a rise in popularity after the documentation of what soon became known as the EPR effect (Matsumura and Maeda, 1986) by Maeda in 1986, and PTS, taking the world of macromolecular therapeutics by storm.
It had previously been seen that a drug named SMANCS (Kobayashi et al., 1991), composed of poly(styrene-co-maleic acid) conjugated to an anticancer protein neocarzinostatin (NCS) in a lipid contrast medium preferentially accumulated and was retained in tumor vasculature and nearby capillary spaces for prolonged periods of time as opposed to just NCS itself (Maeda et al., 1979; Maeda et al., 1984, 1985). Later it was confirmed that macromolecules within the rough molecular weight range of 15,000–70,000 Da (Matsumura and Maeda, 1986) seemed to emulate this same tumoritropic EPR effect due to some hallmark characteristics of solid tumors (Fig. 3).
Fig. 3.
Schematic representation of a nanomaterial targeting to tumor site through EPR effect and characteristics of the phenomenon.
Tumors exhibit a unique physiology that distinguishes them from peripheral tissues and organs and allows for the EPR effect to take hold. Biological traits such as of increased vascular density coupled with enhanced vascular permeability is seen with most tumor types due to their demand for a constant blood supply and nutrients to sustain their growth. Tumors can achieve this hypervascular state through secreting angiogenesis inducing factors such as vascular endothelial growth factor (VEGF) in response to the tumor’s need for more nutrients. VEGF is not alone, as other inflammatory permeability-enhancing factors like bradykinin, nitric oxide, prostaglandins, and cytokines have been found to be upregulated at tumor sites and all contribute to an enhanced permeability of the tumor neovasculature, which is known to be fenestrated, abnormally branched, accompanied by irregular perfusions and structural disorganization (Fang et al., 2011; Nel et al., 2017). The thought is that these permeability enhancing factors, although supplying the tumor with blood vessels which also feature a greater ability to bring in nutrients through extravasation, additionally allow for the passively selected uptake and diffusion of macromolecules of a certain weight threshold into tumors (Matsumura and Maeda, 1986). The same effect is not seen with non-solid tumors and non-malignant tissues, hence making the EPR effect tumor tropic.
Another pivotal characteristic commonly seen with tumor biology, causal to the EPR effect, is impaired lymphatic drainage (Fang et al., 2011). A common pathway for metastasis of cancer cells from a tumor site is through expansion of its nearby lymph system, infiltration of cancer cells into these lymphatic vessels, and subsequent spread through draining lymphatic nodes (Podgrabinska and Skobe, 2014; Padera et al., 2016). Because of this, tumors tend to secrete lymphangiogenic growth factors (VEGF-C and VEGF-D) (Karpanen et al., 2001; Stacker et al., 2001) which leads to the proliferation of functional lymphatic vessels near the peritumoral space and adjacent normal tissues, whereas intratumorally the lymph vessels are nonfunctional because of high interstitial pressures within the tumor, due to abnormalities of the neovasculature and altered extracellular matrices, causing their collapse and impairing lymphatic drainage (Heldin et al., 2004; Hanahan and Weinberg, 2011). The idea is, with this inability of tumors to drain macromolecules once in the intratumoral lymph spaces you would see a retention of these potential molecular therapies for prolonged periods of time, as opposed to normal tissues with functional lymphatic clearance, and this was seen to be the case (Matsumura and Maeda, 1986).
In addition to the impaired lymphatic drainage and the leaky vasculature exhibited by tumors, many other factors contribute to the EPR effect. Once the NPs escape the vasculature, they interact with the tumor microenvironment (TME) where they can attach to cells of the TME and avoid clearance by the macrophages of the mononuclear phagocyte system (Shi et al., 2017). Designing NPs to avoid such clearance systems also contributes to the overall therapeutic success of the NP.
This landmark discovery introduced the ‘gold-standard’ for modeling anti-cancer therapies and is now widely regarded as a clinically relevant result. Nonetheless, it should be acknowledged there is still a need for review on systemic factors that affect presentation of the EPR effect as it can vary from tumor-to-tumor xenografts implanted at the same site (Yuan et al., 1994), and from site-to-site following implantation of the same tumor (Jain et al., 1998). For example, a clinical study showed a difference of 2.7%–53% injected dose (ID)/kg for the accumulation of PEGylated liposomes in solid tumors with locally advanced cancers of the breast, lung, head and neck (Harrington et al., 2001).
Passive targeting nano-delivery systems are possible by utilizing this same principle (Clemons et al., 2018). Regardless of the heterogeneity of the EPR effect, additional, biologically relevant, measures are considered when formulating any targeted nano-delivery system, as well as inherent luxuries only afforded by using a nano delivery vehicle that prove the advent of passive targeting superior over traditional chemotherapies with free form pharmacological agents.
2.1. Doxorubicin nanoformulations
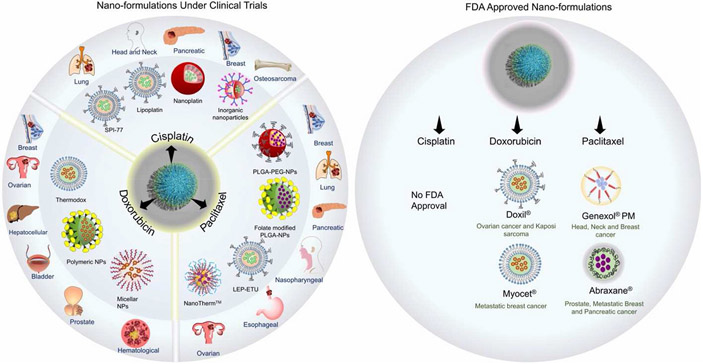
A prime example is FDA approved Doxil®. Currently the FDA has approved a total of three passively doxorubicin containing targeted nanoparticulate nanomedicines, Doxil ®, Myocet ®, and Lipo-Dox ®. In pending for passively targeted nanoparticulate systems remains one liposomal formulation by the name of ThermoDox which is in Phase I/IIII. ThermoDox ® uses thermally sensitive liposomal carriers that degrade once heated past 40 °C - 45 °C to release the doxorubicin payload into the tumor site (Thai Thanh Hoang et al., 2021).
Doxil and Lipo-Dox are PEGylated formulations, which help in increasing their circulation half time. They both contain 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine-Polyethylene Glycol-2000 (DSPE-PEG-2000), cholesterol, and Doxil has hydrogenated soy phosphatidylcholine (HSPC), while Lipo-Dox has distearoylphosphatidylcholine (DSPC). Myocet on the other hand is not PEGylated. It is composed of a mixture of egg phosphatidylcholine and cholesterol (Chang and Yeh, 2012). PEGlyation increased the circulation half-life of Doxil and Lipo-Dox, 55 h and 65 h respectively compared to that of Myocet which was 2.5 h (Hong and Tseng, 2001; Tomkinson et al., 2003). DSPC liposomes have higher stability than HSPC liposomes due to its high phase transition temperature of lipids, resulting in low rigid packing and low energy of random motion. Although Doxil and Lipo-Dox have higher circulation half-lives than Myocet, all three have better half-life than free DOX which has a half-life of 0.2 h. The difference in circulation half-life indicates the effectiveness of the nano-formulation, and how it can lead to better therapeutic outcomes.
2.2. Paclitaxel nanoformulations
As for passively targeted NPs using paclitaxel as the payload, the FDA currently has approved two micellar formulations, and one using a polypeptide; respectively, Genexol® PM, Nanoxel® M, and Abraxane®.. There are four passively targeted nanoparticle-based medicines in progress for FDA approval using paclitaxel by the names of EndoTAG-1, LEP-ETU, Paclical®, and NK105. EndoTAG-1 is a cationic liposomal complex which has completed Phase II trials and is thought to distribute to tumor tissues relying mainly on the EPR effect, with the added bonus that once at tumor sites it will stick slightly better to developing endothelial cells due to the negatively charged surfaces they exhibit and the nature of the lipids complexed to paclitaxel (Eichhorn et al., 2010; Löhr et al., 2012). LEP-ETU is short for Liposome Entrapped Paclitaxel – Easy to Use and is reportedly in Phases I/II/IV. The idea behind this formulation is to avoid commonly cited issues with using paclitaxel such as side effects of its use due to excipients, so with this liposomal formulation it should retain the anti-tumoral efficacy of paclitaxel without having the worser side effects of using an excipient such as castor oil (Bernabeu et al., 2017). Paclical ® has completed Phase III and is paclitaxel conjugated to poly(L-glutamic acid) (Oldham et al., 2000). The use of Paclical is to maintain anti-tumoral efficacy meanwhile preferably having enhanced pharmacokinetic and pharmacodynamic properties with the use of the new carrier. NK105 has completed Phase III and is a micellar nanoparticle formulation which uses paclitaxel as its payload and was designed to overcome the same commonly reported issues with traditional methods of paclitaxel delivery using excipients such as castor oil (Hamaguchi et al., 2005).
2.3. Cisplatin nanoformulations
Cisplatin has yet to feature a passively targeted nanoparticle-based delivery system that has been FDA approved. But in progress are two nanoparticulate systems named LiPlaCis and SPI-077. LiPlaCis, in Phase I/II, designed to treat solid tumors, skin and metastatic breast cancer, is a liposomal formulation of cisplatin with the goal of delivering the cisplatin payload to tumor sites only to have the carrier be degraded by the abundant source of phospholipase 2 commonly found at tumor sites, releasing cisplatin afterwards (Thai Thanh Hoang et al., 2021). SPI-077 is in Phase I/II/III and is designed to treat ovarian cancer, as well as relapsed or progressive osteosarcomas metastatic to the lung. This liposomal formulation of cisplatin is designed to reach and accumulate at tumor sites with the EPR effect meanwhile exhibiting high deliverance capabilities and enjoying the benefits of encapsulating cisplatin in a stealth liposome like increased blood circulation and better drug solubility (Thai Thanh Hoang et al., 2021).
2.4. Outlook on nanocarriers for passive targeting
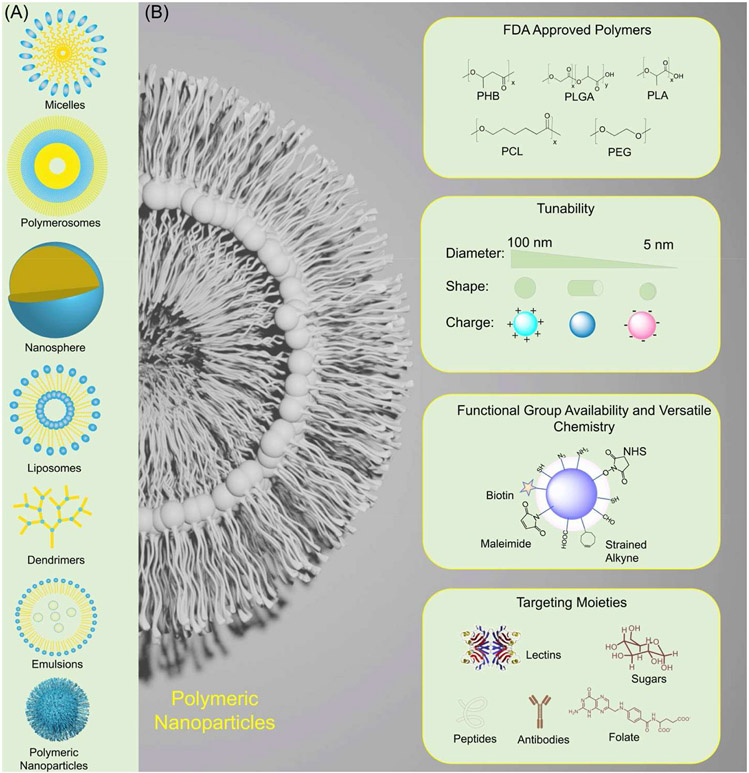
Luxuries afforded from using nanocarriers originate from having a highly tunable delivery vehicle itself. Firstly, nanocarriers can be produced from various kinds of biocompatible materials, including but not limited to polymers which can make polymeric nanoparticles, den-drimers, and micelles (Fig. 4). Nanocarriers can also be liposomal in nature and be composed of lipids almost entirely, there also are viral components that can be utilized to make viral nanoparticles, as well as organometallics that can be used to make nano rods, nanotubes, and other similar structures (Cho et al., 2008). This available variety means enhanced versatility in the design of tailored nanoparticle-based therapies for an inclusive range of pathophysiologies. Particularly polymeric materials stand out as exemplary biomaterials for drug-delivery purposes as already there exists a plethora of available, biodegradable, and highly tunable FDA approved polymers with potential for upscale with Good Manufacturing Practices (GMP) (Gagliardi et al., 2021). Such polymers include poly(D,L-lactide) (PLA), poly(lactide-co-glycolide) (PLGA), poly-Ɛ-caprolactone (PCL), poly(D,L-glycolide) (PGA), and polyalkylcyanoacrylates. These polymeric materials are fully metabolized to smaller and smaller constituents of the original parent molecules and are subsequently secreted by the body with no toll on the host’s health. Not only do these biocompatible polymers show reduced systemic toxicity when compared to other candidate nanocarrier materials, but virtually every formulation will show favorable pharmacokinetics and pharmacodynamic properties (Gagliardi et al., 2021). Natural polymers also are being used to create viable nanocarriers and feature polymers such as chitosan, albumin, and alginate to name a few (Fig. 4) (Gagliardi et al., 2021).
Fig. 4.
(A) Different types of delivery vehicles used in cancer therapy. (B) Tunability of polymeric nanomaterials to achieve enhanced drug delivery.
The main advantages, come from the ability the nanocarriers have to improve the bioavailability of these drugs through encapsulating hydrophobic drugs in a hydrophilic shell, thus improving the drug’s aqueous solubility, or vice versa and allowing for hydrophilic drugs to traverse hydrophobic barriers like cell membranes by providing the drug with a surrounding hydrophobic layer (Patra et al., 2018). Another advantage offered by nano delivery vehicles is through customizing physicochemical characteristics of the nanocarrier itself such as size, charge, and making surface modifications, as the drug vehicle will be able to better resist hepatic, or renal clearance pathways and prolong their stay in the blood which gives the therapeutic agent more time to enact effects on diseased tissues once it reaches the target site (Patra et al., 2018).
Of course, with the specificity that can be applied in the making of nano delivery systems, the pharmacokinetics of drugs can be altered by modification to the nanocarrier, thus the therapeutic index and therapeutic windows of these drug therapies can be improved to significantly better margins than are currently available on the market. This itself greatly reduces unintended side effects in non-target tissues, seen currently with conventional modes of drug therapy, and allows for the use of smaller doses of toxic drugs in cases where it previously would have been difficult, or impossible to use (Patra et al., 2018).
Biologically relevant parameters are followed as well in the design ofNP - based systems, successfully ameliorating some issues associated with drug delivery. Primarily, physicochemical factors of the NP’s are being controlled, such as charge, size, shape, composition, and surface functionalization (Fig. 4). Controlling the size of the NPs will help with evasion of blood clearance from the renal system and the innate reticuloendothelial system (RES). NP’s ranging from >6 nm in diameter, to less than 300 nm in diameter will be able to successfully evade blood clearance from the renal system and RES, respectively, thus increasing residence in the blood, consequentially increasing the likelihood that the nanodrug will reach the intended target site (Kobayashi et al., 2014; Attia et al., 2019). Charge also influences blood clearance. The negatively charged glomerular basement membrane found in the renal system is mainly responsible for filtering the plasma, thus NPs with neutral to positively charged surfaces will generally tend to be filtered out of the blood more easily. Increasing overall positive or negative charge on NP surfaces will result in increased uptake by the RES as it would allow for frequent interactions with serum proteins found in the blood, like the process of opsonization, as well as enhanced charge detection by negatively charged surface membranes on phagocytes or other cells, ultimately leading to enhanced cellular uptake of NPs and increased clearance from circulation (Xiao et al., 2011; Kobayashi et al., 2014).
Another important factor when considering formulating nano delivery systems is general shape of the NP (Canelas et al., 2009), and the degree of flexibility - “softness” or “roughness” - which can change how the NP interacts with cell membranes or other possible targets, usually more conforming “soft” NPs will filter with ease through execratory pathways (Ogawa et al., 2010). Surface functionalization and composition of the nanocarrier vehicle itself plays a role in influencing the other factors such as shape, flexibility, size, and charge depending on the ligands that are attached to the NP surface and kind of material used. PEGylation is a commonly employed surface functionalization (Fig. 4) that acquires more than one benefit for the NP (Suk et al., 2016). Polyethyleneglycol (PEG) can be made into differing lengths which are conjugated to NP surfaces to incur “stealth” properties by allowing them to evade RES clearance from the blood. PEGylating NP surfaces as well can increase the solubility of the NP in the blood because of the polar properties of PEG repeats and additionally can tune the “softness” of particles, if need be, and PEGylation itself is another technique used to effectively mask a portion of the surface charge of the NPs, thus increasing the “stealth” behavior of the NPs and overall stability in vivo.
Choosing a biocompatible, highly tunable biomaterial to make the NPs with, like liposomes, while simultaneously employing a surface functionalization such as PEGylation, were elements used in the creation of Doxil ® (Table 1). Doxil ®, is considered a passively targeted system as it does not utilize anything to accumulate at target sites other than the EPR effect. Other notable passive targeting formulations for example are Abraxane, an albumin-based NP delivery system for paclitaxel, and in Korea a polymeric micelle delivery vehicle of paclitaxel, Genexol-PM (Table 1). The field of nanomedicine has currently seen progress with PTS, and so happens to be the only form of targeting that has shown clinically reproducible effects which have further gone and warranted approval from the FDA.
Table 1.
Active and Passive targeted nanoformulations of Doxorubicin, Paclitaxel, and cisplatin.
| Name | Drug Payload/ Nanoformulation |
Investigated Clinical Application(s) |
Status |
|---|---|---|---|
| Doxil® | Doxorubicin/Liposomal doxorubicin (PEGylated) | Ovarian cancer (secondary to platinum-based therapies), HIV-associated Kaposi’s sarcoma (secondary to chemotherapy), Multiple myeloma (secondary. Various cancers including solid malignancies, ovarian, breast, leukemia, lymphomas, prostate, metastatic, or liver | FDA Approved (1995) |
| 2B3-101 | Doxorubicin/Liposomal doxorubicin (PEGylated) | Advanced solid tumors, brain metastases, lung and breast cancers, melanoma, malignant glioma | Phase I/II Completed |
| ANTI-EGFR-IL-DOX | Doxorubicin/Doxorubicin-loaded anti-EGFR immunoliposomes | Advanced triple negative EGFR positive breast cancer, High grade gliomas | Phase II: Recruiting Phase I: Recruiting |
| MM-302 | Doxorubicin/HER2-targeted liposomal doxorubicin (PEGylated) | Breast cancer | Phase II/III: Terminated Phase I: Unknown Phase I: Withdrawn |
| MCC-465 | Doxorubicin/F(ab″)2 fragment of human mAb GAH | Metastatic stomach cancer | Phase I/II terminated |
| Abraxane | Paclitaxel/Albumin-particle bound paclitaxel | Pancreatic cancer, Metastatic breast cancer, NSCLC, Triple negative breast cancer | FDA Approved (2005, 2021, 2013, 2019) |
| LIPUSU® | Paclitaxel/Paclitaxel liposome | Advanced solid tumors, or gastric, breast cancer | Phase IV: Unknown Phase IV: Unknown Phase II: Unknown Phase IV: Not yet recruiting |
| Cynviloq | Paclitaxel/Paclitaxel polymeric micelle nanoparticle | Breast cancer | Not provided: NCT02064829 |
| Genexol-PM | Paclitaxel/Paclitaxel polymeric micelle nanoparticle | Head and neck or breast cancer | Phase II: Unknown Phase II: Completed Phase IV: Unknown Phase II: Recruiting Phase II: Recruiting |
| NK105 | Paclitaxel/Paclitaxel micelle | Breast cancer | Phase III: Completed |
| Paclical | Paclitaxel micelle | Ovarian Cancer | Phase III: Completed |
| EndoTAG-1 | Paclitaxel Liposome | Pancreatic Cancer; Breast cancers | Phase III: in progress; Phase III: in progress |
| LiPlaCis | Cisplatin/Liposomal formulated cisplatin with specific degradation-controlled drug release via phospholipase A2 (PLA2) | Advanced or refractory tumors | Phase I: Recruiting |
| Nanoplatin | Cisplatin/Polyamino acid, PEG, and cisplatin derivative micellar nanoparticle | Advanced solid tumors, lung, biliary, bladder, or pancreatic cancers | Phase I/II: Active, not recruiting Phase III: Unknown Phase II: Not yet recruiting Phase I: Completed Phase I: Unknown |
| This table is assembled based on https://clinicaltrials.gov/ and other sources mentioned in this review. | |||
As the general pathophysiology of diseases are reviewed and understood more deeply, there is likely still a host of effects that can be exploited besides the EPR effect in favor of nano delivery systems. Issues associated with using passive targeting comes from the general lack of control in cellular uptake from using PTS which could result in off-target delivery, as well as multi-drug resistance mechanisms dangerously quick. There is also the concern that some tumors don’t strongly exhibit the EPR effect enough to consider PTS viable, such as pancreatic cancer (Clemons et al., 2018). These challenges can be addressed with the advent of “active targeting”.
3. Active targeting
Active targeting uses one or more targeting ligands on the NP surface. These ligands specifically target receptors or antigens that are overexpressed or uniquely expressed on tumor cells (Pearce and O’reilly, 2019).
Further making NP-based delivery systems an extremely viable solution to conventional drug delivery with “active targeting”. As was originally the case in 1980, a monoclonal antibody was conjugated to a liposomal NP surface and an increase of NP accumulation was observed in cells previously incubated with the cognate antigen, without sacrificing the liposome’s payload (Leserman et al., 1980). The list of ligands that can be incorporated to different precisely engineered NP surfaces has since been expanded to include peptides, aptamers, synthetic groups, as well as a wide range of other functionally active ligands, that make it so actively targeted systems exhibit a wide range of affinities and physicochemical properties, opening a world of possibilities for NP-based drug therapies. Generally, the addition and functional use of one or several different targeting moieties to NP surfaces with the goal of exploiting intrinsic biological properties, like in the case of either unique or overexpression of a receptor or antigen found on certain cell membranes or extracellular matrices, can be regarded as active targeting (Fig. 5) (Pearce and O’reilly, 2019).
Fig. 5.
Illustration of passive and active tumor targeting of nanomaterials to the tumor along with advantages and disadvantages.
3.1. Doxorubicin nanoformulations
With respect to the clinical state of actively targeted nanoparticulate formulations for doxorubicin, there have been none so far that have been fully approved by the FDA. Although notably, there are three formulations in the pending status that use doxorubicin as their drug payload. 2B3-101 has completed Phase I/II trials and features a glutathione pegylated liposomal doxorubicin hydrochloride formulation as it is meant to accumulate in the brain by targeting the glutathione transporters located at the blood brain barrier (Table 1) (Gaillard et al., 2014). Another formulation is MM-302 which is a human epidermal growth factor receptor 2 (HER2) targeted antibody liposomal doxorubicin formulation is in Phase I, and it is meant to specifically target breast cancers that are HER2 positive and overexpress this receptor (Munster et al., 2018). Then there is Anti-EGFR-IL-DOX which is in Phase II and is an immunoliposomal formulation of doxorubicin which targets EGFR on triple negative breast cancers and higher-grade gliomas by virtue of the antibodies for EGFR located on the liposome carrier’s surface (Table 1) (Mamot et al., 2012).
3.2. Paclitaxel nanoformulations
As for paclitaxel, there are no FDA approved nanoparticulate formulations that are actively targeted, or pending, that use active targeting. Instead of ligand-receptor affinities, one may find formulations that rely on homing of NP’s with electrostatic interactions such as EndoTAG-1 which has completed Phase II trials and features positively charged lipids that will attach to negatively charged endothelial cells of developing tumor vasculature (Löhr et al., 2012). Paclical is another example which has completed Phase III and features poly-L-glutamic acid offering the negative charge of the glutamic acid on the peptide polymer leading towards accumulation of paclitaxel into tumors and their vasculature (Oldham et al., 2000) (Table 1).
3.3. Cisplatin nanoformulations
Cisplatin features no current FDA approved actively targeted nanoparticulate formulations and as of yet there are no clinical trials pending utilizing active targeting. We may very well assume that there will be some intricate and novel pathways explored in non-clinical laboratories that will lead to a change for the clinical landscape for cisplatin, such as Platin-M (Marrache et al., 2014) or general cisplatin prodrug formulations being utilized due to cisplatin’s labile nature and the potential added benefits of using therapeutic moieties on cisplatin (Dhar et al., 2011; Pathak et al., 2014; Pathak et al., 2017; Surnar et al., 2018).
3.4. Outlook on nanocarriers for active targeting
Regarding clinical outcome, as promising as the incorporation of actively targeted nanomedicines into a therapeutic arsenal may first appear, there are still inherent barriers associated with the translation of these precision therapies into widespread applicability. Mainly, these barriers do not occur with the design of these nanoformulations, instead we are seeing increasingly complex nanoparticle architecture with a wide plethora of bio-responsive ligands designed at enhancing delivery far above what is possible with conventional therapeutics. Challenges arise from the still pre-mature understanding we have of the pathophysiology of many disease states, as well as the heterogeneity across patients and animal models to human translatability, all which are factors pertinent to the in vivo behavior and therapeutic efficacy of NP-based delivery systems (Mitchell et al., 2021). Nevertheless, it stands to reason active targeting will remain the ideal successor upon PTS’s limitations with patient stratification improvements through more rigorously defined diagnostic criteria, and optimized nanodelivery system design (Mitchell et al., 2021).
4. What did we achieve using controlled released nanoparticles?
Historically, nano delivery systems have co-evolved from a demand to meet therapeutic challenges set forth by cancer, a disease universally known for its complications set towards an absolute, efficacious treatment (Hanahan and Weinberg, 2011). From this work and yet a more profound understanding of cancer biology, have emerged significant advances in the field of therapeutic nanomedicine, like the documentation of the clinically relevant EPR effect and a growing appreciation for the design of nano-sized delivery vehicles capable of delivering a wide range of therapeutic molecules, simultaneously equipped with properties to combat systemic barriers to small-molecule drug delivery like biodistribution, bioavailability, and toxicity. This is the basis responsible for passively targeted systems (PTS) and widely known Doxil ®. Then, seen as an adaptation to the limits set by passively targeted controlled-release nano delivery systems, came a focus on active targeting. Extensive progress in non-clinical laboratories across the globe has been observed, both in improving the complexity of nanoparticle-based delivery system design and the ligand chemistry that offers the nanoparticle’s unique targeting ability. This same trend was followed for concomitant therapeutic efficacy amongst the various diseases tested against. Despite there being evidence pointing towards active targeting being the likely successor and default clinical modality for cancer therapy, there has been limited clinical success in its application, and it is thought to be due to challenges associated with ‘lack of inclusivity’ unfortunately in therapies when unleashing precision medicines like highly tune-able nanoparticle-based delivery systems in patients’ bodies. This has left the nanomedical field to focus on patient stratification and companion diagnostics as a resource for further resolution of when a nanoparticle-based therapy will yield desirable outcome.
In conjunction with this trend arises an opportunity with NPs as the new leading controlled release platform vehicle (Fig. 5). Being explored now are many kinds of NP systems, such as nanosphere polymer-drug conjugates, dendritic polymer capsules, polymeric micelles, and more (Cho et al., 2008; Mitchell et al., 2021); particularly the polymeric NPs focused in this review are those composed of either synthetic or natural polymers which can physically entrap said drug, or are chemically conjugated to it in a polymer matrix that results in the general structure of a tunable drug capsule/sphere for loading and distribution (Cho et al., 2008). Unlike most other materials used to make nanoparticle-based delivery systems, polymeric nanoparticles can offer the most customizability, with preferable control over what NP parameter you are manipulating, as well as great biocompatibility and relatively easy formulations, to adhere to highly specific pathophysiology of patient populations currently being explored for clinical efficacy in nanomedicine-based therapeutics (Fig. 6). Currently, there are multiple synthetic methods for creating different polymeric nanoparticles including nanoprecipitation (Le et al., 2018; Zhang et al., 2019), ionic gelation (He et al., 2020), emulsification (Brown et al., 2020) and microfluidics (Zhang et al., 2020) which all produce different kinds of polymeric nanoparticles.
Fig. 6.
Nano-formulations of cisplatin, paclitaxel, and doxorubicin which are either FDA approved or under clinical trials for various malignancies. Information collected from U.S. National Library of Medicine, Clinicaltrial.gov.
Drug release from different nanoformulations has an impact on the overall efficacy of the nanoparticle which, along delivery route, drove the development of new formulations. The main controlled-release mechanisms are 1) diffusion through water-filled pores, 2) diffusion through polymer matrix, 3) osmotic pumping and 4) erosion (Kamaly et al., 2016). For most polymeric drug delivery systems, an initial rapid release of the drug cargo is observed, known as the “burst release” effect because of osmotic pressures. Poly(ester) NPs will typically undergo bulk erosion of the NP, as well as diffusion from the core. The rate of release from these will depend on the shape of the NP and the concentration of the drugs loaded (Fredenberg et al., 2011). Therapeutic efficacy of the NP will depend directly on the drug release and thus by manipulating the composition of the nano-formulation, the overall efficacy of the system can be improved (Sethi et al., 2014).
It was earlier mentioned that by modification of a NP’s biologically relevant properties such as size, charge, softness, surface functionalization and composition you could maximize the in vivo therapeutic efficacy of these NPs. This synergizes with the benefits already afforded from using a highly tunable polymeric nano delivery system over classical free form drug therapy, such as efficient drug loading or co-loading, improved biodistribution, greater bioavailability, and lower maximally required dosages with fewer unwanted toxic side-effects through their newly acquired pharmacokinetics and therapeutic indices, making NPs a real threat to well-characterized disease. PEG was then subsequently shown to be a polymer that influenced nearly every single one, if not all these aspects significantly, and is now a widely commercially used and relatively simple polymer to begin with. With not much further complexity, you can synthesize other relatively simple polymers or copolymers such as poly(lactic-co-glycolic acid) (PLGA), PGA (poly-L-glutamic acid), N-(2-hydroxypropyl)-methacrylamide copolymer (HPMA), and poly(dimethyl siloxane) (PDMS) (Cho et al., 2008; Mitchell et al., 2021), amongst others, that form the body of these polymeric-based nanoparticles, which can very effectively and with relative ease capture the physicochemical characteristics that an efficacious intelligent design for a controlled release nano delivery system currently requires, meanwhile allowing for the delivery or co-delivery of a wide range of therapeutic molecules further increasing therapeutic potential. It then begs the question, what would the landscape of nanomedical therapeutics and general pharmacological therapeutics look like if we were to further embrace these customizable nano delivery vehicle options?
With respect to three popular chemotherapeutic drugs, doxorubicin, paclitaxel, and cisplatin, polymeric nanoparticles can be leveraged to potentially revolutionize these drugs’ therapy as we know them to be. Clinically there has yet to be shown a polymeric based nanoparticle for doxorubicin, paclitaxel, or cisplatin. For doxorubicin instead we have seen various liposomal formulas such as Doxil ®, 2B3-101, ANTI-EGFR-IL-DOX, MM-302, and MCC-465 (Anselmo and Mitragotri, 2019; Pearce and O’reilly, 2019) (Table 1 and Fig. 6). An issue still concerning the use of doxorubicin is that toxicities of various tissues, particularly cardiac tissue, and acquired drug resistance. For paclitaxel, we have seen albumin-particle bound paclitaxel (Abraxane ®), as well as a liposomal formulation LIPUSU ®, and polymeric micellar formulations named Cynviloq, Genexol-PM, and NK105 (Anselmo and Mitragotri, 2019; Pearce and O’reilly, 2019) (Table 1 and Fig. 6). Main issues surrounding paclitaxel’s use stem from its low solubility, thus requiring excipients in formula that are usually allergenic such as castor oil, and paclitaxel use is associated with the enhancement of multi-drug resistance at target sites. As for cisplatin, we have observed clinical trials utilizing liposomal constituents like in LiPlaCis or derivative micellar nanoparticles like in the case for Nanoplatin (Anselmo and Mitragotri, 2019; Pearce and O’reilly, 2019) (Table 1 and Fig. 6). Repeated cisplatin use can result in multi-drug resistance pathways and multi-tissue toxicity alongside anemia and electrolyte disturbances amongst other unwanted side effects. It’s worth noting that most of the formulations did not achieve Phase III clinical trials, with most of the progress shown for those who did pass Phase III for paclitaxel, still the general trend meant unsuccessfulness in translating the therapeutic to clinically relevant status.
What if the envelope was pushed with these precision medicine polymeric nanoparticle delivery systems we have yet to see? Faithfully these failure points can be mended by employing any wide range of polymeric nanoparticle design, as their use significantly improve therapeutic indices like any other nanocarrier does, but with the added benefit that the nanoparticle delivery system itself would be the ideal precision medicine required to tackle this day and age’s evolving clinical patient stratification and understanding of disease.
5. Summary and future promise
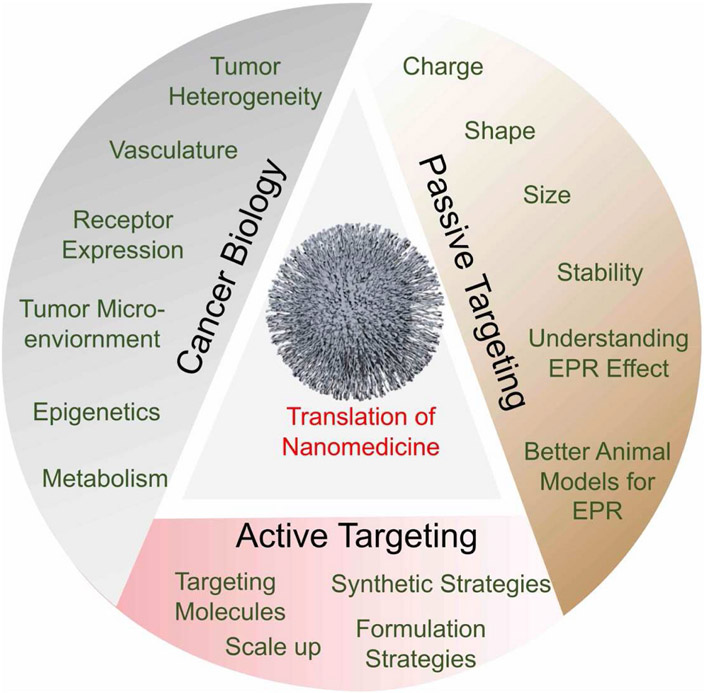
In this review, we explored notable developments and current notions in nanomedicine in the context of three major chemotherapeutics, cisplatin, paclitaxel, and doxorubicin. Nanomedicine platforms of chemotherapeutics came about into popular view after it was seen that conventional drugs suffered from major systemic barriers for drug delivery and that use of polymers and lipids had appeasing pharmacological properties like enhanced blood residence and overall better solubility and stability of drug cargoes, this along with the landmark discovery of the EPR effect. Following this work was the use of passively targeted drug delivery systems but with it came issues such as the heterogeneous presentation of the EPR effect across patients and tumoral types, and the inability to precisely control the passive accumulation of these drugs, leading to worry about lack of targeting specificity along with possibility for drug resistance (Fig. 7). To combat this, the field of nanomedicine geared towards actively targeted systems that relied on enhanced targeting specificity with surface moieties, but issues have still been observed with the clinical translation of discoveries made in non-clinical laboratories despite reported success and proof of concept (Fig. 7). The response to this has been increased patient stratification and the use of supplementary diagnostic modalities to further resolve the applicability of these nano-medicines, finally promoting the prospect of using polymeric nanoparticle-based drug delivery systems for three popular chemotherapeutic drugs doxorubicin, paclitaxel, and cisplatin.
Fig. 7.
Future prospect of nanomedicine using the knowledge of cancer biology and taking advantage of passive and active targeting.
Stimuli responsive prodrug formulations are the next evolution in the nanomedicine field. By designing prodrugs of approved chemotherapeutics which can be released from nano formulations only in response to a specific stimulus, nanomedicine will become more precise. Currently there are nanoformulations of cisplatin that feature a prodrug conjugated to a polymer which can respond to external stimuli to release predefined ratios of a cisplatin prodrug and an anti-inflammatory agent which allows for minimizing off target effects. These formulations can make a major impact on the therapeutic options for complex diseases (Pathak et al., 2018; Pathak and Dhar, 2015). Prodrug design which can ameliorate the side effects of chemotherapeutics can be extremely beneficial in therapy. Additionally, nanoformulations which release cargo via chemical, biochemical, or physical means can increase the therapeutic payload at the site of interest. These changes can be caused by designing polymers to respond to biological factors such as pH, temperature, light, ultrasound, and other stimuli (Kamaly et al., 2016). These design factors can prevent the payload from premature release, ensuring maximum therapeutic effectiveness.
Although there are many known issues associated with the employment of these drugs with more conventional therapeutic methods, the use of controlled release NPs for these drugs can bring wide-spread clinical efficacy. We envision that as our knowledge in cancer biology continues to enrich, the scientific community will adopt and grow with this knowledge to design and optimize parameters utilizing both passive and active targeting strategies to result nanomedicine platforms with enhanced clinical translation (Fig. 7). Controlled release nanomaterials will experience a higher clinical translation rate based on the understanding of cancer biology, EPR effect, formulation strategies due to their wide variety of highly customizable structure and physicochemical parameters, coupled with their relatively less toxicity. The sheer ideal candidacy of controlled release NPs which fits the idea of increasing clinical patient stratification in efforts to resolve proper usage of tailored nanotherapeutics, as in the case of controlled release platforms like nano vehicles, begs the ultimate question of how much could be attained for the fields of cancer therapy and general medicine, ifcontrolled release NP-based delivery systems were explored as the new form of treatment. Controlled release NP-based delivery might just well be the bridge needed to wide-spread clinical efficacy and will likely circumvent issues related to current animal-model translatability, and patient to patient heterogeneity. Just as our knowledge of disease states and our own biology improves, so will these highly prospective nanoparticle-controlled release systems, historically that has always been the case.
Acknowledgement
We thank the Sylvester Comprehensive Cancer Center and Bank-head-Coley Grant (8BC10) from the Florida Department of Health to S.D. for supporting various projects related to nanoparticle-based technologies in our lab. We also acknowledge the Sylvester Comprehensive Cancer Center Support Grant 1P30CA240139. We thank Shrita Sarkar for her initial help for crafting Figs. 1 and 4.
Footnotes
Declaration of competing interest
The authors declare no competing financial interest.
References
- Anselmo AC, Mitragotri S, 2019. Nanoparticles in the clinic: an update. Bioeng. Transl. Med 4, e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF, 2019. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol 71, 1185–1198. [DOI] [PubMed] [Google Scholar]
- Bazak R, Houri M, Achy SE, Hussein W, Refaat T, 2014. Passive targeting of nanoparticles to cancer: a comprehensive review of the literature. Mol. Clin. Oncol 2, 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA, 2017. Paclitaxel: what has been done and the challenges remain ahead. Int. J. Pharm 526, 474–495. [DOI] [PubMed] [Google Scholar]
- Brown SB, Wang L, Jungels RR, Sharma B, 2020. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 101, 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canelas DA, Herlihy KP, Desimone JM, 2009. Top-down Partide Fabrication: Control of Size and Shape for Diagnostic Imaging and Drug Delivery, vol. 1. Wiley Interdiscip Rev Nanomed Nanobiotechnol, pp. 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HI, Yeh MK, 2012. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int. J. Nanomedicine 7, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan G, Madou MJ, Kalra S, Chopra V, Ghosh D, Martinez-Chapa SO, 2020. Nanotechnology for COVID-19: therapeutics and Vaccine Research. ACS Nano 14, 7760–7782. [DOI] [PubMed] [Google Scholar]
- Cho K, Wang X, Nie S, Chen Z, Shin DM, 2008. Therapeutic nanopartides for drug delivery in cancer. Clin. Cancer Res 14, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Clemons TD, Singh R, Sorolla A, Chaudhari N, Hubbard A, Iyer KS, 2018. Distinction between active and passive targeting of nanopartides dictate their overall therapeutic efficacy. Langmuir 34, 15343–15349. [DOI] [PubMed] [Google Scholar]
- De Weger VA, Beijnen JH, Schellens JH, 2014. Cellular and dinical pharmacology of the taxanes docetaxel and paditaxel–a review. Anti cancer Drugs 25, 488–94. [DOI] [PubMed] [Google Scholar]
- Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC, 2011. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc. Natl. Acad. Sci. Unit. States Am 108, 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A, Bohnenkamp H, Guenzi E, Preissler G, Michaelis U, Jauch K-W, Bruns CJ, Dellian M, 2010. Vascular targeting by EndoTAG™-l enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int. J. Cancer 126, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Fang J, Nakamura H, Maeda H, 2011. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev 63, 136–151. [DOI] [PubMed] [Google Scholar]
- Fenn JE, Udelsman R, 2011. First use of intravenous chemotherapy cancer treatment: rectifying the record. J. Am. Coll. Surg 212, 413–7. [DOI] [PubMed] [Google Scholar]
- Fredenberg S, Wahlgren M, Reslow M, Axelsson A, 2011. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems–a review. Int. J. Pharm 415, 34–52. [DOI] [PubMed] [Google Scholar]
- Fuertes MA, Alonso C, Perez JM, 2003. Biochemical modulation of Cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem. Rev 103, 645–62. [DOI] [PubMed] [Google Scholar]
- Gagliardi A, Giuliano E, Venkateswararao E, Fresta M, Bulotta S, Awasthi V, Cosco D, 2021. Biodegradable polymeric nanopartides for drug delivery to solid tumors. Front. Pharmacol 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard PJ, Appeldoorn CC, Dorland R, Van Kregten J, Manca F, Vugts DJ, Windhorst B, Van Dongen GA, De Vries HE, Maussang D, Van Tellingen O, 2014. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One 9, e82331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T, 2005. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paditaxel. Br. J. Cancer 92, 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg Robert A., 2011. Hallmarks of cancer: the next generation. Cell 144, 646–674. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JSW, 2001. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res 7, 243–254. [PubMed] [Google Scholar]
- He C, Yue H, Xu L, Liu Y, Song Y, Tang C, Yin C, 2020. siRNA release kinetics from polymeric nanopartides correlate with RNAi efficiency and inflammation therapy via oral delivery. Acta Biomater. 103, 213–222. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Rubin K, Pietras K, Östman A, 2004. High interstitial fluid pressure — an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813. [DOI] [PubMed] [Google Scholar]
- Hong RL, Tseng YL, 2001. Phase I and pharmacokinetic study of a stable, polyethylene-glycolated liposomal doxorubicin in patients with solid tumors: the relation between pharmacokinetic property and toxicity. Cancer 91, 1826–33. [PubMed] [Google Scholar]
- Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E, 1998. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc. Natl. Acad. Sci. Unit. States Am 95, 10820–10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad B, Poudel L, Podgornik R, Steimetz NF, Ching WY, 2019. Molecular mechanism and binding free energy of doxorubicin intercalation in DNA. Phys. Chem. Chem. Phys. 21, 3877–3893. [DOI] [PubMed] [Google Scholar]
- Kamaly N, Yameen B, Wu J, Farokhzad OC, 2016. Degradable Controlled-Release Polymers and Polymeric Nanopartides: Mechanisms of Controlling Drug Release. Chem. Rev 116, 2602–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, Alitalo K, 2001. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786. [PubMed] [Google Scholar]
- Kobayashi H, Watanabe R, Choyke PL, 2014. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 4, 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Imai K, Sugihara S, Maeda H, Konno T, Yamanaka H, 1991. Tumor-targeted chemotherapy with lipid contrast medium and maeromolecular anticancer drug (SMANCS) for renal cell carcinoma. Urology 37, 288–294. [DOI] [PubMed] [Google Scholar]
- Krumbhaar EB, Krumbhaar HD, 1919. The Blood and Bone Marrow in Yelloe Cross Gas (Mustard Gas) Poisoning: Changes produced in the Bone Marrow of Fatal Cases. J. Med. Res 40 (3), 497–508. [PMC free article] [PubMed] [Google Scholar]
- Le Z, Chen Y, Han H, Tian H, Zhao P, Yang C, He Z, Liu L, Leong KW, Mao HQ, Liu Z, Chen Y, 2018. Hydrogen-bonded tannic acid-based anticancer nanoparticle for enhancement of oral chemotherapy. ACS Appl. Mater. Interfaces 10, 42186–42197. [DOI] [PubMed] [Google Scholar]
- Leserman LD, Barbet J, Kourilsky F, Weinstein JN, 1980. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature 288, 602–604. [DOI] [PubMed] [Google Scholar]
- Liechty WB, Kryscio DR, Slaughter BV, Peppas NA, 2010. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng 1, 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhr JM, Haas SL, Bechstein WO, Bodoky G, Cwiertka K, Fischbach W, Fölsch UR, Jäger D, Osinsky D, Prausova J, Schmidt WE, Lutz MP, 2012. Cationic liposomal paclitaxel plus gemcitabine or gemcitabine alone in patients with advanced pancreatic cancer: a randomized controlled phase II trial. Ann. Oncol 23, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Maeda H, Matsumoto T, Konno T, Iwai K, Ueda M, 1984. Tailor-making of protein drugs by polymer conjugation for tumor targeting: a brief review on smancs. J. Protein Chem 3, 181–193. [Google Scholar]
- Maeda H, Takeshita J, Kanamaru R, 1979. A lipophilic derivative of neocarzinostatin. A polymer conjugation of an antitumor protein antibiotic. Int. J. Pept. Protein Res 14, 81–87. [DOI] [PubMed] [Google Scholar]
- Maeda H, Ueda M, Morinaga T, Matsumoto T, 1985. Conjugation of poly(styrene-co-maleic acid) derivatives to the antitumor protein neocarzi nostatin: pronounced improvements in pharmacological properties. J. Med. Chem 28, 455–461. [DOI] [PubMed] [Google Scholar]
- Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, Hilker C, Deuster S, Herrmann R, Rochlitz C, 2012. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 13, 1234–1241. [DOI] [PubMed] [Google Scholar]
- Marrache S, Pathak RK, Dhar S, 2014. Detouring of Cisplatin to Access Mitochondrial Genome for Overcoming Resistance. Proceedings of the National Academy of Sciences, 201405244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H, 1986. A new concept for maeromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392. [PubMed] [Google Scholar]
- Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, 2021. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov 20, 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster P, Krop IE, Lorusso P, Ma C, Siegel BA, Shields AF, Molnár I, Wickham TJ, Reynolds J, Campbell K, Hendriks BS, Adiwijaya BS, Geretti E, Moyo V, Miller KD, 2018. Safety and pharmacokinetics of MM-302, a HER2-targeted antibody–liposomal doxorubicin conjugate, in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br. J. Cancer 119, 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Ruoslahti E, Meng H, 2017. New insights into "permeability" as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano 11, 9567–9569. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Regino CAS, Marcelino B, Williams M, Kosaka N, Bryant LH, Choyke PL, Kobayashi H, 2010. New nanosized biocompatible MR contrast agents based on lysine-dendri-graft macromolecules. Bioconjugate Chem. 21, 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham EA, Li C, Ke S, Wallace S, Huang P, 2000. Comparison of action of paclitaxel and poly(L-glutamic acid)-paclitaxel conjugate in human breast cancer cells. Int. J. Oncol 16, 125–132. [PubMed] [Google Scholar]
- Padera TP, Meijer EFJ, Munn LL, 2016. The lymphatic system in disease processes and cancer progression. Annu. Rev. Biomed. Eng 18, 125–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak RK, Basu U, Ahmad A, Sarkar S, Kumar A, Surnar B, Ansari S, Wilczek K, Ivan ME, Marples B, Kolishetti N, Dhar S, 2018. A designer bow-tie combination therapeutic platform: an approach to resistant cancer treatment by simultaneous delivery of cytotoxic and anti-inflammatory agents and radiation. Biomaterials 187, 117–129. [DOI] [PubMed] [Google Scholar]
- Pathak RK, Dhar S, 2015. A nanoparticle cocktail: temporal release of predefined drug combinations. J. Am. Chem. Soc 137, 8324–8327. [DOI] [PubMed] [Google Scholar]
- Pathak RK, Mcnitt CD, Popik VV, Dhar S, 2014. Copper-free click-chemistry platform to functionalize cisplatin prodrugs. Chem. Eur J 20, 6861–6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak RK, Wen R, Kolishetti N, Dhar S, 2017. A prodrug of two approved drugs, cisplatin and chlorambucil, for chemo war against cancer. Mol. Cancer Therapeut 16, 625–636. [DOI] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS, 2018. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol 16, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce AK, O’reilly RK, 2019. Insights into active targeting of nanoparticles in drug delivery: advances in clinical studies and design considerations for cancer nanomedicine. Bioconjugate Chem. 30, 2300–2311. [DOI] [PubMed] [Google Scholar]
- Podgrabinska S, Skobe M, 2014. Role of lymphatic vasculature in regional and distant metastases. Micro vase. Res 95, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C, 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol 17, 421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi M, Sukumar R, Karve S, Werner ME, Wang EC, Moore DT, Kowalezyk SR, Zhang L, Wang AZ, 2014. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale 6, 2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Kantoff PW, Wooster R, Farokhzad OC, 2017. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG, 2001. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med 7, 186–191. [DOI] [PubMed] [Google Scholar]
- Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, 2016. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev 99, 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surnar B, Kolishetti N, Basu U, Ahmad A, Goka E, Marples B, Kolb D, Lippman ME, Dhar S, 2018. Reduction of cisplatin-induced ototoxicity without compromising its antitumor activity. Biochemistry 57, 6500–6513. [DOI] [PubMed] [Google Scholar]
- Tacar O, Sriamornsak P, Dass CR, 2013. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol 65, 157–70. [DOI] [PubMed] [Google Scholar]
- Thai Thanh Hoang T, Suys EJA, Jung Seok L, Nguyen DH, Ki Dong P, Truong NP, 2021. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines 9, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkinson B, Bendele R, Giles FJ, Brown E, Gray A, Hart K, Leray JD, Meyer D, Pelanne M, Emerson DL, 2003. OSI-211, a novel liposomal topoisomerase I inhibitor, is active in SCID mouse models of human AML and ALL. Leuk Res. 27, 1039–50. [DOI] [PubMed] [Google Scholar]
- Tsang RY, Al-Fayea T, Au HJ, 2009. Cisplatin overdose: toxicities and management. Drug Saf. 32, 1109–22. [DOI] [PubMed] [Google Scholar]
- Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS, 2011. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 32, 3435–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK, 1994. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 54, 4564–4568. [PubMed] [Google Scholar]
- Zhang CX, Cheng Y, Liu DZ, Liu M, Cui H, Zhang BL, Mei QB, Zhou SY, 2019. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J. Nanobiotechnol 17, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV, 2003. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr. Cancer Drug Targets 3, 1–19. [DOI] [PubMed] [Google Scholar]
- Zhang L, Beatty A, Lu L, Abdalrahman A, Makris TM, Wang G, Wang Q, 2020. Microfluidic-assisted polymer-protein assembly to fabricate homogeneous functional nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl 111, 110768. [DOI] [PubMed] [Google Scholar]