Abstract
Objective
To evaluate the quality of evidence, potential biases, and validity of all available studies on dietary sugar consumption and health outcomes.
Design
Umbrella review of existing meta-analyses.
Data sources
PubMed, Embase, Web of Science, Cochrane Database of Systematic Reviews, and hand searching of reference lists.
Inclusion criteria
Systematic reviews and meta-analyses of randomised controlled trials, cohort studies, case-control studies, or cross sectional studies that evaluated the effect of dietary sugar consumption on any health outcomes in humans free from acute or chronic diseases.
Results
The search identified 73 meta-analyses and 83 health outcomes from 8601 unique articles, including 74 unique outcomes in meta-analyses of observational studies and nine unique outcomes in meta-analyses of randomised controlled trials. Significant harmful associations between dietary sugar consumption and 18 endocrine/metabolic outcomes, 10 cardiovascular outcomes, seven cancer outcomes, and 10 other outcomes (neuropsychiatric, dental, hepatic, osteal, and allergic) were detected. Moderate quality evidence suggested that the highest versus lowest dietary sugar consumption was associated with increased body weight (sugar sweetened beverages) (class IV evidence) and ectopic fatty accumulation (added sugars) (class IV evidence). Low quality evidence indicated that each serving/week increment of sugar sweetened beverage consumption was associated with a 4% higher risk of gout (class III evidence) and each 250 mL/day increment of sugar sweetened beverage consumption was associated with a 17% and 4% higher risk of coronary heart disease (class II evidence) and all cause mortality (class III evidence), respectively. In addition, low quality evidence suggested that every 25 g/day increment of fructose consumption was associated with a 22% higher risk of pancreatic cancer (class III evidence).
Conclusions
High dietary sugar consumption is generally more harmful than beneficial for health, especially in cardiometabolic disease. Reducing the consumption of free sugars or added sugars to below 25 g/day (approximately 6 teaspoons/day) and limiting the consumption of sugar sweetened beverages to less than one serving/week (approximately 200-355 mL/week) are recommended to reduce the adverse effect of sugars on health.
Systematic review registration
PROSPERO CRD42022300982.
Introduction
As an important component of the human diet, sugars have been shown to be harmfully associated with a variety of risk factors for decades, mainly including obesity,1 2 3 diabetes,4 5 6 cardiovascular disease,7 8 9 10 hyperuricaemia,11 gout,11 12 13 ectopic fatty accumulation,14 15 16 dental caries,17 and some cancers.18 19 20 21 According to the latest report of the World Health Organization and the Food and Agriculture Organization of the United Nations, sugars include monosaccharides, disaccharides, polyols, and free sugars, of which free sugars are identified as all monosaccharides and disaccharides added to foods by the manufacturer, cook, or consumer and sugars naturally present in honey, syrups, and fruit juices.3 22 In addition, another important group of sugars, added sugars, has been proposed in the Dietary Guidelines for Americans and has been defined as all monosaccharides and disaccharides used in processed and prepared foods and drinks and sugars added to foods but not naturally occurring sugars such as in fruits and fruit juices (table 1).23
Table 1.
| Class | Principal components |
|---|---|
| Monosaccharides | Glucose, fructose, galactose |
| Disaccharides | Sucrose, lactose, maltose, trehalose |
| Polyols | Sorbitol, mannitol, lactitol, xylitol, erythritol, isomalt, maltitol |
| Free sugars | All monosaccharides and disaccharides added to foods by the manufacturer, cook, or consumer; sugars naturally present in honey, syrups, and fruit juices |
| Added sugars | All monosaccharides and disaccharides used in processed and prepared foods and drinks; sugars added to foods but not naturally occurring sugars such as in fruits and fruit juices |
In recent years, many studies have focused on the adverse effects of sugar sweetened beverages on human health, given the substantial contribution of these drinks to total added sugar or free sugar intake and the rapidly increasing rate of their consumption.24 25 26 Generally, sugar sweetened beverages are the largest source of added sugars, including carbonated and noncarbonated soft drinks, fruit drinks, and sports and energy drinks.27 Previous surveys have shown that consumption of sugar sweetened beverages is declining in many developed countries, although consumption levels remain high.27 28 However, the consumption of sugar sweetened beverages is still increasing in many developing countries, which may be attributed to their increased availability accompanied by economic development.29 The 2007 annual report of the Coca-Cola company revealed that the consumption of sugar sweetened beverages in India and China increased by 14% and 18%, respectively, in one year.30 In 2018 a cross sectional survey conducted among Chinese primary and junior high school students showed that sugar sweetened beverages provide 10-15% of the total calorie consumption of school students.31 Data from the National Health and Nutrition Examination Survey (NHANES) showed that, in 2009-10, sugar sweetened beverage consumption contributed 8% and 6.9% of daily energy intake among children/adolescents and adults, respectively, in the US.32 Additionally, a global survey conducted in 2010 reported that a total of 180 000 adiposity associated deaths could be attributed to the consumption of sugar sweetened beverages around the world.33 All of these findings promote the development of policies worldwide to limit sugar consumption, including sugars taxes, food labelling laws, and restrictions on advertising and marketing.34 35 36 37 Meanwhile, national and international organisations such as WHO, the US Department of Agriculture, and the US Department of Health and Human Services have recommended reducing the consumption of free sugars or added sugars to less than 10% of total daily energy intake.23 38
Although many meta-analyses of observational studies and randomised controlled trials focused on the associations between sugar consumption and a range of health outcomes have been published in recent decades, deficiencies in the study design, varying measurements of dietary sugar consumption, inconsistent findings, and different definitions of exposure make drawing definitive conclusions difficult. Therefore, before developing detailed policies for sugar restriction, the quality of existing evidence on the associations of dietary sugar consumption with all health outcomes needs to be comprehensively evaluated. To evaluate the quality of evidence, potential biases, and validity of all studies available on dietary sugar intake and any health outcomes, we did an umbrella review of meta-analyses on this topic.
Methods
Umbrella review methods
We systematically searched, extracted, and analysed large amounts of data from published systematic reviews and meta-analyses that research the associations between various health outcomes and dietary sugar consumption.39 40 Generally, dietary sugar consumption could be measured through the specific proportions of sugars in foods or a percentage of total energy and combined in meta-analyses.3 Therefore, we excluded simple systematic reviews without meta-analyses from our umbrella review. We prospectively registered this umbrella review in PROSPERO (CRD42022300982) (https://www.crd.york.ac.uk/PROSPERO/).
Literature search
We searched PubMed, Embase, Web of Science, and the Cochrane Database of Systematic Reviews from inception through January 2022 (last update) for systematic reviews and meta-analyses of randomised controlled trials and observational studies. We searched the databases through a combination of Medical Subject Headings terms, keywords, and variations of text words associated with sugars following the Scottish Intercollegiate Guidelines Network’s guidance for literature searching: (sugars OR sugar) AND (systematic review OR meta-analysis).41 Two authors (YH and ZYC) separately conducted electronic searches to screen the titles and abstracts retrieved from the databases and identified meta-analyses that met the inclusion criteria by full text reading. Any discrepancy in the literature screening between the two reviewers was resolved by a third author (LRL). We hand searched meta-analyses and reviews from the reference lists of all included articles to identify studies that might have been missed.
Eligibility criteria
We identified dietary sugar consumption as the intake of total sugars and the consumption of a component of total sugars (monosaccharides, disaccharides, polyols, free sugars, or added sugars), which are expressed in absolute amounts or as a percentage of total energy, or the intake of sugar sweetened beverages or foods (table 1).3 We included systematic reviews and meta-analyses of randomised controlled trials, cohort studies, case-control studies, or cross sectional studies that evaluated dietary sugar consumption in humans free from acute or chronic diseases. Meta-analyses were eligible for inclusion when they compared the effects of different dietary sugar consumption on the same health outcome through relative risks, odds ratios, hazard ratios, weighted mean differences, or standardised mean differences. We included meta-analyses when the exposure was total sugars, monosaccharides, disaccharides, polyols, free sugars, added sugars, or sugar sweetened beverages or foods. We extracted data on individual outcomes separately if two or more health outcomes were reported in a study. If more than one study published more than 24 months apart was conducted on the same dietary sugar exposure and health outcomes, we included the most recent study for data extraction, which is generally the study with the largest sample size. If more than one study was conducted within the same 24 month period, we included the meta-analysis with the largest number of prospective cohort studies and randomised controlled trials (a study with a higher AMSTAR score was included if the number of prospective studies was equal).42 43 Furthermore, if the most recent study did not do dose-response analysis, whereas another study did, we included both studies for data extraction.
The exclusion criteria for these umbrella reviews included meta-analyses of the association between carbohydrates, non-nutritive sweeteners, and artificially sweetened beverages and health outcomes; meta-analyses evaluating the therapeutic or metabolic effects of short term sugar supplementation; meta-analyses that evaluated the effects of dietary sugar consumption on health outcomes in certain disease populations; randomised controlled trials that aimed to achieve isoenergetic replacement of sugars with other forms of carbohydrate; studies with insufficient data to evaluate sugar consumption from sugar containing foods (such as honey, apples, chocolate, ice cream, 100% fruit juice); and non-English studies and animal and cell culture studies.
Data extraction
Two reviewers (YH and ZYC) independently extracted the following information from each eligible study: first author’s name, publication year, type of dietary sugar consumption (total sugars, monosaccharides, disaccharides, polyols, free sugars, added sugars, sugar sweetened beverages or foods), measurement of dietary sugar consumption, health outcome, number of included studies, number of cases and total participants, study design (cross sectional, case-control, cohort, and randomised controlled trial), comparison (high versus low, never/low versus moderate/high, any versus none, or extra increment of sugars per day (or week) versus none), and estimated summary effect (risk ratio, odds ratio, weighted mean difference, and standardised mean difference with 95% confidence intervals). Furthermore, we extracted the model of effect (random and fixed), heterogeneity (I2 statistic and Cochran’s Q test P value), and publication bias assessment (P value of Egger’s test or funnel plot). If dose-response analysis and subgroup analysis were conducted, we also extracted the non-linearity tests’ P value and results of subgroup analysis in meta-analyses. If a meta-analysis was conducted on both cohort and case-control/cross sectional studies and stratification analysis was conducted through study design, we selected the cohort design subanalysis results for data extraction or reanalysed. Any disagreement was determined by a third author (LRL).
Quality assessment of methods and evidence
Two reviewers (YH and ZYC) evaluated the methodological quality of the included articles by using AMSTAR (a measurement tool to assess systematic reviews), a valid and dependable measurement tool in assessing the quality of systematic reviews and meta-analyses.42 44 In addition, according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE), we evaluated evidence of each health outcome and graded it as “high,” “moderate,” “low,” or “very low” quality to draw conclusions.45 Additionally, we classified evidence of outcomes into four categories following the evidence classification criteria: class I (convincing evidence), class II (highly suggestive evidence), class III (suggestive evidence), class IV (weak evidence), and NS (non-significant).46 47 48 Table 2 shows detailed criteria of evidence classification.
Table 2.
| Evidence class | Description |
|---|---|
| Class I: convincing evidence | >1000 cases (or >20 000 participants for continuous outcomes); statistical significance at P<10−6 (random effects); no evidence of small study effects and excess significance bias; 95% prediction interval excluded null value; no large heterogeneity (I2<50%) |
| Class II: highly suggestive evidence | >1000 cases (or >20 000 participants for continuous outcomes), statistical significance at P<10−6 (random effects), and largest study with 95% confidence interval excluding null value |
| Class III: suggestive evidence | >1000 cases (or >20 000 participants for continuous outcomes) and statistical significance at P<0.001 |
| Class IV: weak evidence | Remaining significant associations with P<0.05 |
| NS: non-significant | P>0.05 |
Data analysis
We reanalysed the risk ratio, odds ratio, weighted mean difference, or standardised mean difference with 95% confidence intervals through random or fixed effects models and calculated the I2 statistic, P value of Cochran’s Q test for heterogeneity, and P value of Egger’s regression test (at least 10 studies were included) for small study effects in each included meta-analysis that reported the metric, number of cases, and participants of the included original studies.49 50 51 For outcomes classified as class I or II, we did sensitivity analysis if sufficient data were available to assess whether the credibility of the evidence varied when some component studies were excluded. We also extracted dose-response associations between dietary sugar consumption and various health outcomes from the included meta-analyses, if available. Moreover, if the latest meta-analysis did not include the original articles that were included by other meta-analyses, we combined the data of these meta-analyses and did a reanalysis. We assessed agreement statistics between two authors (YH and ZYC) regarding study selection by using Cohen’s κ statistics and associated 95% confidence interval. We interpreted magnitude of agreement by following guidelines reported by Landis and Koch: slight (0.00-0.20), fair (0.21-0.40), moderate (0.41-0.60), substantial (0.61-0.80), and almost perfect agreement (0.81-1.00).52 In addition, if a meta-analysis reported the estimated effect by combining observational studies with randomised controlled trials, we reanalysed the estimated effects for observational studies and randomised controlled trials separately. If we could not do a reanalysis from a meta-analysis, we extracted summary data and assessed heterogeneity and publication bias from the meta-analysis as far as possible. We identified a P value <0.10 as statistically significant for heterogeneity tests. For other tests, we considered a P value <0.05 to be significant. We used Review Manager version 5.3 for evidence synthesis, Stata version 12.1 for Egger’s test and sensitivity analysis, and IBM SPSS Statistics version 25 for Cohen’s κ statistics.
Patient and public involvement
Patients and the public were not involved in the planning, design, and implementation of the study, as this study used secondary data. No patients were asked to advise on interpretation or writing up of the manuscript.
Results
Characteristics of meta-analyses
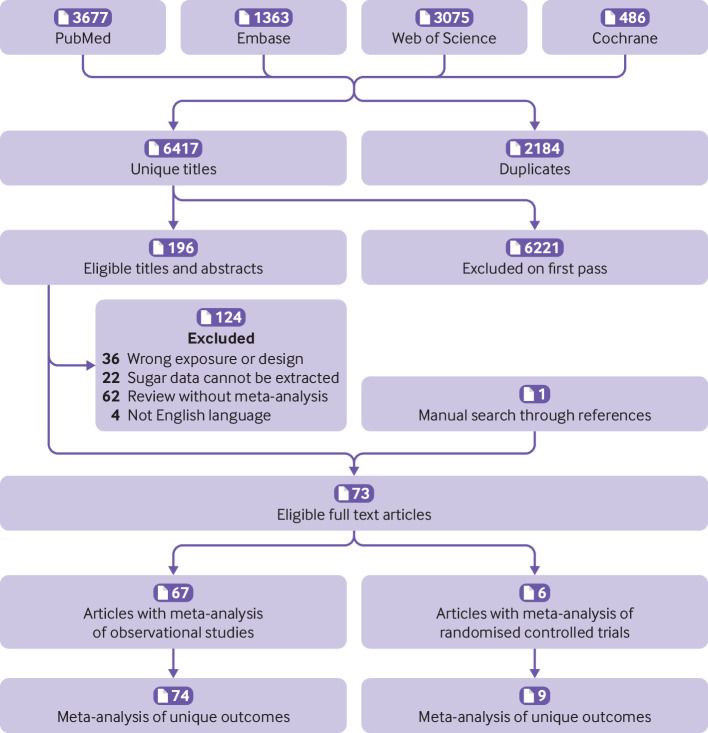
Figure 1 shows the flowchart of the literature search and selection process. After a systematic literature search, we identified 8601 unique articles. Application of our inclusion criteria yielded total of 73 meta-analyses, including 67 meta-analyses of observational studies and six meta-analyses of randomised controlled trials. Agreement between the two reviewers (YH and ZYC) for study selection was almost perfect (κ=0.906, 95% confidence interval 0.859 to 0.953; P<0.001). We extracted 74 unique outcomes in meta-analyses of observational studies and nine unique outcomes in meta-analyses of randomised controlled trials. Meta-analyses of randomised controlled trials included only change in body weight (sugar sweetened beverages), liver fat accumulation, muscle fat accumulation, change in body mass index, change in body weight (fructose), postprandial triglycerides, serum uric acid, intrahepatocellular lipids, and alanine aminotransferase. Figure 2 shows the significant dose-response relations between dietary sugar consumption and multiple health outcomes. The other forest plots show the significant non-dose-response relations between dietary sugar consumption and endocrine/metabolic (fig 3), cardiovascular (fig 4), cancer (fig 5), and other outcomes (fig 6). The full versions of the associations between dietary sugar consumption and each outcome are shown in supplementary tables A-D.
Fig 1.
Flowchart of systematic search and selection process
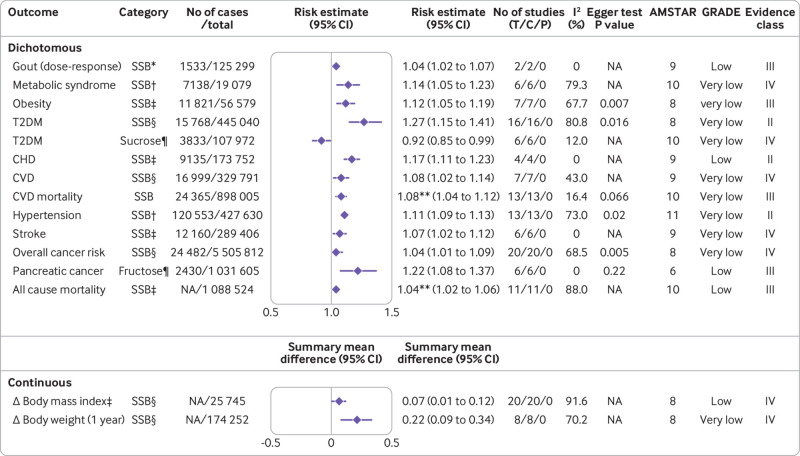
Fig 2.
Significant dose-response relations between dietary sugar consumption and multiple health outcomes. Estimates are relative risks, summary mean difference is weighted mean difference, and effect models are random unless noted otherwise. Δ=final value – baseline value; AMSTAR=a measurement tool to assess systematic reviews; C=cohort studies; CHD=coronary heart disease; CI=confidence interval; CVD=cardiovascular disease; GRADE=Grading of Recommendations Assessment, Development and Evaluation; NA=not available; P=population based case-control and/or cross sectional studies; SSB=sugar sweetened beverage; T=total No of studies; T2DM=type 2 diabetes mellitus. *1 serving/week increment. †355 mL/d increment. ‡250 mL/d increment. §1 serving/d increment. ¶25 g/d increment. **Hazard ratio. †Children
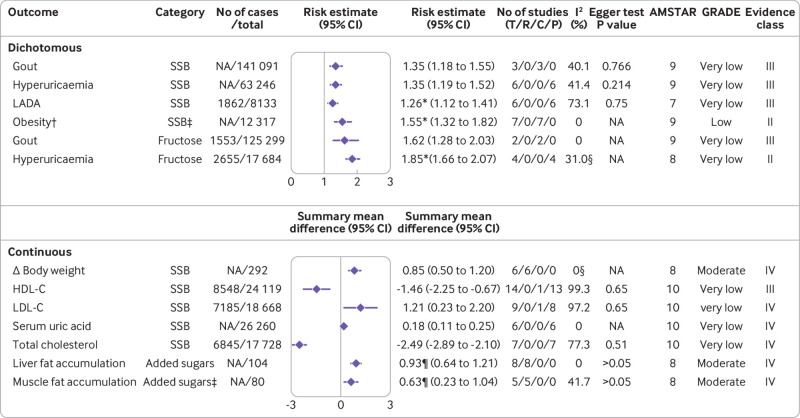
Fig 3.
Significant non-dose-response relations between dietary sugar consumption and endocrine and metabolic outcomes. Comparisons are highest versus lowest, estimates are relative risks, summary mean difference is weighted mean difference, and effect models are random unless noted otherwise. Complete associations between dietary sugar consumption and endocrine and metabolic outcomes are shown in supplementary table A. Δ=final value – baseline value; AMSTAR=a measurement tool to assess systematic reviews; C=cohort studies; CI=confidence interval; GRADE=Grading of Recommendations Assessment, Development and Evaluation; HDL-C=high density lipoprotein cholesterol; LADA=latent autoimmune diabetes in adults; LDL-C=low density lipoprotein cholesterol; NA=not available; P=population based case-control and/or cross sectional studies; R=randomised controlled trials; SSB=sugar sweetened beverage; T=total No of studies. *Odds ratio. †Children. ‡Any versus none. §Fixed effects model. ¶Standardised mean difference
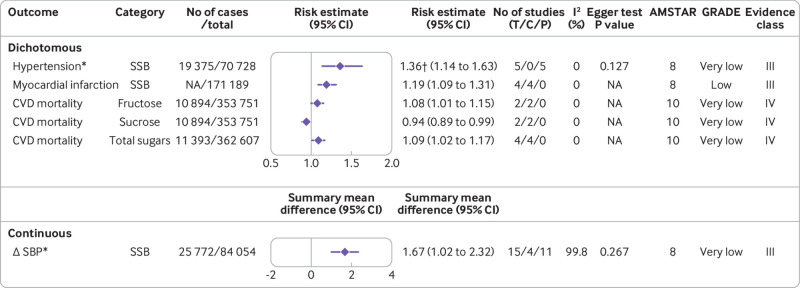
Fig 4.
Significant non-dose-response relations between dietary sugar consumption and cardiovascular outcomes. Comparisons are highest versus lowest, estimates are relative risks, summary mean difference is weighted mean difference, and effect models are random unless noted otherwise. Complete associations between dietary sugar consumption and cardiovascular outcomes are shown in supplementary table B. Δ=final value – baseline value; AMSTAR=a measurement tool to assess systematic reviews; C=cohort studies; CI=confidence interval; CVD=cardiovascular disease; GRADE=Grading of Recommendations Assessment, Development and Evaluation; NA=not available; P=population based case-control and/or cross sectional studies; SBP=systolic blood pressure; SSB=sugar sweetened beverage; T=total No of studies. *Children and adolescents. †Odds ratio
Fig 5.
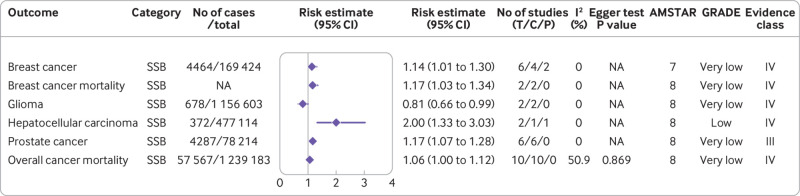
Significant non-dose-response relations between dietary sugar consumption and cancer outcomes. Comparisons are highest versus lowest, estimates are relative risks, and effect models are random unless noted otherwise. Complete associations between dietary sugar consumption and cancer outcomes are shown in supplementary table C. AMSTAR=a measurement tool to assess systematic reviews; GRADE=Grading of Recommendations Assessment, Development and Evaluation; C=cohort studies; CI=confidence interval; NA=not available; P=population based case-control and/or cross sectional studies; SSB=sugar-sweetened beverage; T=total No of studies
Fig 6.
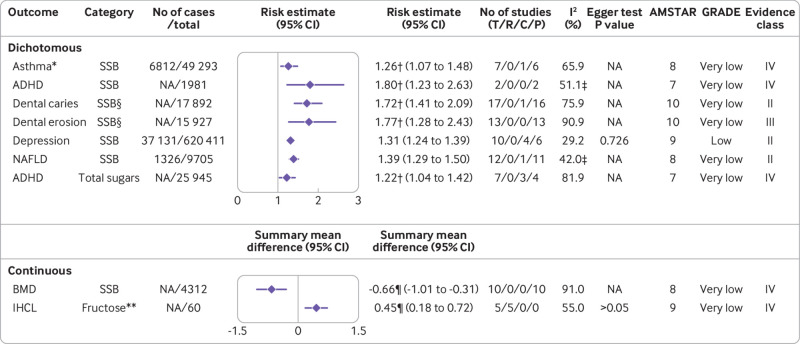
Significant non-dose-response relations between dietary sugar consumption and other outcomes. Comparisons are highest versus lowest, estimates are relative risks, summary mean difference is weighted mean difference, and effect models are random unless noted otherwise. Complete associations between dietary sugar consumption and other outcomes are shown in supplementary table D. ADHD=attention deficit/hyperactivity disorder; AMSTAR=a measurement tool to assess systematic reviews; BMD=bone mineral density; C=cohort studies; CI=confidence interval; GRADE=Grading of Recommendations Assessment, Development and Evaluation; IHCL=intrahepatocellular lipids; NA=not available; NAFLD=non-alcoholic fatty liver disease; P=population based case-control and/or cross sectional studies; R=randomised controlled trials; SSB=sugar-sweetened beverage; T=total No of studies. *Children. †Odds ratio. ‡Fixed effects model. §Never/low versus moderate/high consumption. ¶Standardised mean difference. **Any versus none
Most of the included meta-analyses focused on the associations between dietary sugar consumption and endocrine/metabolic diseases (n=28), followed by cancer (n=25), cardiovascular diseases (n=17), neuropsychiatric diseases (n=3), dental diseases (n=2), and other diseases (n=8) (fig 7). Dietary sugar exposure included sugar sweetened beverages (n=58), fructose (n=11), sucrose (n=4), lactose (n=1), added sugars (n=4), free sugars (n=1), and total sugars (n=4). Significance was reached for 45 harmful associations and four beneficial associations. The remaining 34 outcomes were either harmfully or beneficially associated but did not reach significance. After quality assessment of evidence through GRADE and evidence classification criteria, most of the 83 outcomes were classified as “low” or “very low” quality and III, IV, or NS evidence class. Only four (5%) endocrine/metabolic outcomes were classified as “moderate” quality. Three (4%) endocrine/metabolic outcomes, two (2%) cardiovascular outcomes, and three (4%) other outcomes were graded as class IIB. No “high” quality or class I evidence was found in this umbrella review.
Fig 7.
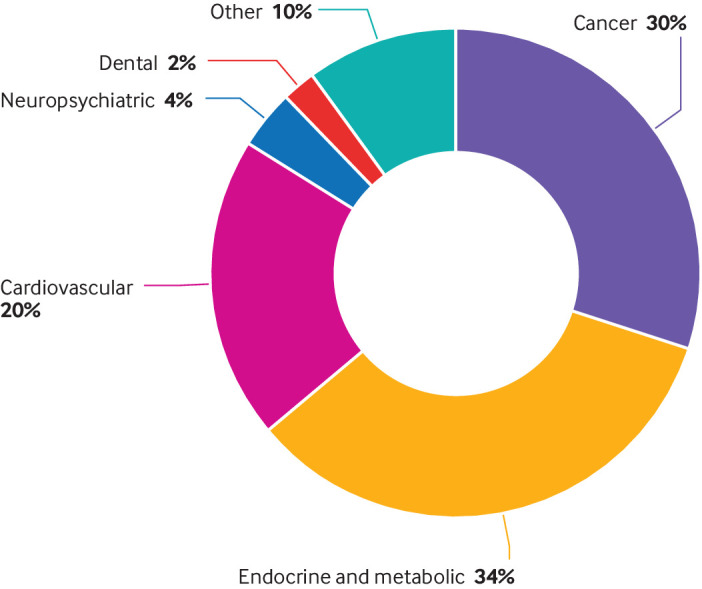
Map of outcomes associated with dietary sugar consumption
Endocrine and metabolic outcomes
Low and moderate quality evidence
A meta-analysis of six randomised controlled trials found that sugar sweetened beverage consumption was significantly associated with increased body weight for highest versus lowest consumption (weighted mean difference 0.85, 95% confidence interval 0.50 to 1.20) (moderate; IV (the quality of evidence is expressed as “GRADE, evidence class”)).53 In addition, any versus no added sugar consumption was associated with increased liver fat accumulation (standardised mean difference 0.93, 95% confidence interval 0.64 to 1.21) (moderate; IV) and muscle fat accumulation (standardised mean difference 0.63, 0.23 to 1.04) (moderate; IV).54 Another dose-response meta-analysis showed that a one serving/week increment in artificially sweetened beverages was associated with a 4% higher risk of gout (risk ratio 1.04, 95% confidence interval 1.02 to 1.07) (low; III).13 Furthermore, comparison of higher sugar sweetened beverage consumption with non-sugar sweetened beverage consumption indicated a 55% (odds ratio 1.55, 95% confidence interval 1.32 to 1.82) increased risk of obesity in children associated with higher consumption (low; II).3 Sugar sweetened beverage consumption was also linked with an increased body mass index in children.53 The authors conducted a dose-response analysis and showed that body mass index in children increased by 0.07 units for every one serving/day increment of sugar sweetened beverages (weighted mean difference 0.07, 0.01 to 0.12) (low; IV).53 Evidence from this umbrella review suggests that fructose intake was not associated with serum uric acid (moderate; NS)55 or changes in body weight (low; NS) (fig 2; fig 3).56
Very low quality evidence
Dose-response analysis based on seven cohort studies showed that a one serving/day increment of sugar sweetened beverages was associated with a 0.22 kg weight gain in one year (weighted mean difference 0.22, 0.09 to 0.34).53 Furthermore, the risk of gout increased by 35% (risk ratio 1.35, 1.18 to 1.55) for the highest versus lowest sugar sweetened beverage consumption.11 The highest versus lowest sugar sweetened beverage consumption was also significantly associated with a 35% (risk ratio 1.35, 1.19 to 1.52) higher risk of hyperuricaemia.11 In addition, another pooled analysis found that participants with the highest sugar sweetened beverage consumption had 0.18 mg/dL greater concentrations of serum uric acid than did those with the lowest consumption (weighted mean difference 0.18, 0.11 to 0.25).57 Similarly, the highest fructose intake could also increase the risk of gout (risk ratio 1.62, 1.28 to 2.03)58 and hyperuricaemia (odds ratio 1.85, 1.66 to 2.07)59 compared with the lowest consumption.
The most recent meta-analysis found a 1.46 mg/dL (weighted mean difference −1.46, −2.25 to −0.67) decrement of high density lipoprotein cholesterol for the highest versus lowest sugar sweetened beverage consumption.60 Subgroup analysis indicated that the highest versus lowest sugar sweetened beverage consumption was associated with lower high density lipoprotein cholesterol in studies conducted in the US (weighted mean difference −2.85, −4.09 to −1.61) but was associated with higher high density lipoprotein cholesterol in studies conducted in Europe/Oceania (weighted mean difference 1.65, 0.26 to 3.05).60 The highest versus lowest sugar sweetened beverage consumption was also significantly associated with increased low density lipoprotein cholesterol (weighted mean difference 1.21, 0.23 to 2.20) and decreased total cholesterol (−2.49, −2.89 to −2.10).60 After stratification by region, no significant association between sugar sweetened beverage consumption and low density lipoprotein cholesterol was detected in the US, Europe/Oceania, and Asia,60 whereas the highest versus lowest sugar sweetened beverage consumption was associated with lower total cholesterol concentrations in studies conducted in the US/Europe (weighted mean difference −2.47, −2.88 to −2.07) but not in Asia.60
The risk of metabolic syndrome was increased by 14% (risk ratio 1.14, 1.05 to 1.23) for a 355 mL/day increment of sugar sweetened beverages, with no evidence for departure from linearity.61 In addition, a meta-analysis including 56 579 participants and 11 821 incident cases of obesity showed an adverse linear dose-response association between sugar sweetened beverage consumption and the risk of obesity.1 Each 250 mL/day increment in sugar sweetened beverage consumption was associated with a 12% (risk ratio 1.12, 1.05 to 1.19) higher risk of obesity, and this association also remained after adjustment for energy intake (1.13, 1.09 to 1.18) and physical activity (1.14, 1.05 to 1.25).1 Moreover, a meta-analysis of 16 cohort studies found that with each one serving/day increment of sugar sweetened beverage consumption, the risk of developing type 2 diabetes mellitus increased by 27% (risk ratio 1.27, 1.15 to 1.41).6 By contrast, an 8% (risk ratio 0.92, 0.85 to 0.99) lower risk of type 2 diabetes mellitus for each 25 g/day increment in sucrose intake was confirmed in dose-response analysis based on six cohort studies.62 The highest versus lowest sugar sweetened beverage consumption was also significantly associated with a higher risk of latent autoimmune diabetes in adults (odds ratio 1.26, 1.12 to 1.41) (fig 2; fig3).30
We found no significant association between sugar sweetened beverage consumption and changes in body mass index in adults,63 triglycerides,60 or large waist circumference.64 Fructose intake was not associated with postprandial triglycerides or type 2 diabetes mellitus.62 65 Total sugar consumption was also not associated with type 2 diabetes mellitus (supplementary table A).62
Cardiovascular outcomes
Low quality evidence
In a single article,10 a positive association between sugar sweetened beverage consumption and the risk of coronary heart disease was observed. Dose-response analysis showed that each 250 mL/day increment of sugar sweetened beverage consumption was positively associated with a 17% (risk ratio 1.17, 1.11 to 1.23) higher risk of coronary heart disease (low; II).10 In addition, extreme category analysis showed that the highest versus lowest sugar sweetened beverage consumption was associated with an increased risk of myocardial infarction (risk ratio 1.19, 1.09 to 1.31) (low; III).66 Low quality evidence suggests that fructose intake was not associated with the risk of hypertension (low; NS) (fig 2; fig 4).67
Very low quality evidence
Except for a beneficial association between sucrose intake and cardiovascular disease mortality, all categories of dietary sugar exposure were adversely associated with various cardiovascular outcomes. A recent dose-response meta-analysis showed that each 250 mL/day increment of sugar sweetened beverage consumption was positively associated with a 7% (risk ratio 1.07, 1.02 to 1.12) higher risk of stroke.10 Another meta-analysis of seven cohort studies with 329 791 participants and 16 999 cases found that each one serving/day increment of sugar sweetened beverage consumption was linearly associated with an 8% (risk ratio 1.08, 1.02 to 1.14) increased risk of cardiovascular disease.8 For cardiovascular disease mortality, each serving/day increment of sugar sweetened beverage consumption was also linearly associated with a higher risk (hazard ratio 1.08, 1.04 to 1.12).68 However, subgroup analysis found that the association between sugar sweetened beverage consumption and cardiovascular disease mortality was not statistically significant among participants from Asia.68 In a separate meta-analysis in children and adolescents,69 the highest versus lowest sugar sweetened beverage consumption was shown to be associated with a 1.67 mm Hg (weighted mean difference 1.67, 1.02 to 2.32) increase in systolic blood pressure and a 36% (odds ratio 1.36, 1.14 to 1.63) higher risk of hypertension. In adults, the results from pooled analysis of 13 prospective cohort studies indicated a harmful dose-response association between sugar sweetened beverage consumption and incidence of hypertension.70 The risk of hypertension was increased by 11% (risk ratio 1.11, 1.09 to 1.13) for a 355 mL/day (1 serving/day) increment in sugar sweetened beverage consumption.70 Moreover, both fructose (risk ratio 1.08, 1.01 to 1.15) and total sugars (risk ratio 1.09, 1.02 to 1.17) were harmfully associated with the risk of cardiovascular disease mortality for highest versus lowest consumption,71 whereas a beneficial association between sucrose intake and cardiovascular disease mortality was observed (fig 2; fig 4).71
We observed no significant association between sugar sweetened beverage consumption and changes in diastolic blood pressure (children and adolescents)69 or heart failure.10 We also observed no significant association between sucrose intake or total sugar consumption and the risk of cardiovascular disease.71 In addition, added sugar consumption was not associated with the risk of cardiovascular disease mortality (supplementary table B).71
Cancer
Low quality evidence
A dose-response meta-analysis showed that the risk of hepatocellular carcinoma increased by 100% (risk ratio 2.00, 1.33 to 3.03) for the highest sugar sweetened beverage consumption compared with the lowest (low; IV).18 Additionally, a meta-analysis conducted by Aune and colleagues found that 25 g/day of fructose intake was linearly associated with a 22% higher risk of pancreatic cancer (risk ratio 1.22, 1.08 to 1.37) (low; III).72 The association between fructose intake and incidence of pancreatic cancer remained significant in the subgroups of studies that adjusted for smoking, body mass index, red and processed meat consumption, and energy intake, whereas the association was diminished in the subgroups of studies that adjusted for alcohol consumption, diabetes status, or physical activity (fig 2; fig 5).72
Very low quality evidence
A recent meta-analysis of six observational studies showed a higher risk of breast cancer for highest versus lowest sugar sweetened beverage consumption (risk ratio 1.14, 1.01 to 1.30).19 In a separate meta-analysis, Li and colleagues found that the highest sugar sweetened beverage consumption might increase the risk of breast cancer mortality by 17% (risk ratio 1.17, 1.03 to 1.34) compared with the lowest.18 Moreover, a meta-analysis of six cohort studies showed that participants with the highest sugar sweetened beverage consumption had a higher risk of prostate cancer than those with the lowest intake (risk ratio 1.17, 1.07 to 1.28). Dose-response analysis did not detect a significant association.18 However, we observed a protective association between sugar sweetened beverage consumption and glioma in our umbrella review (risk ratio 0.81, 0.66 to 0.99).18 In addition, a meta-analysis including 20 cohort studies with 5 505 812 participants observed a positive linear dose-response relation between sugar sweetened beverage consumption and overall cancer risk.18 The risk increased by 4% for every serving/day increment of sugar sweetened beverage consumption (risk ratio 1.04, 1.01 to 1.09).18 Furthermore, pooled analysis of 10 cohort studies with 1 239 183 participants found that the highest versus lowest sugar sweetened beverage consumption was significantly associated with a higher risk of overall cancer mortality (risk ratio 1.06, 1.00 to 1.12), without a significant dose-response relation.18 Stratification by region produced a positive association between sugar sweetened beverage consumption and overall cancer mortality in the North American population (odds ratio 1.08, 1.01 to 1.15) but not in Asia (0.99, 0.81 to 1.22) (fig 2; fig 5).18
We observed no significant association between sugar sweetened beverage consumption and the risk of biliary track cancer,18 bladder cancer,18 colon cancer,73 colorectal cancer,18 colorectal cancer mortality,18 endometrial cancer,18 oesophageal cancer,18 gastric cancer,18 haematological malignancy,18 kidney cancer,18 lung cancer mortality,18 nasopharyngeal carcinoma,18 pancreatic cancer,18 and prostate cancer mortality.18 In addition, added sugar consumption was not associated with the risk of colorectal cancer.74 We observed no significant association between sucrose intake and pancreatic cancer.72 Moreover, lactose intake was not associated with the risk of ovarian cancer (supplementary table C).75
Other outcomes
Low quality evidence
A recent meta-analysis of 11 cohort studies suggested that an increment in sugar sweetened beverage consumption of 250 mL/day was associated with a 4% (hazard ratio 1.04, 1.02 to 1.06) higher risk of all cause mortality (low; III).76 Moreover, a harmful association between sugar sweetened beverage consumption and the risk of depression was observed in a meta-analysis of 10 observational studies (risk ratio 1.31, 1.24 to 1.39) (low; II).77 No significant association was observed between fructose intake and alanine transaminase concentration (low; NS) (fig 2; fig 6).78
Very low quality evidence
The highest versus lowest sugar sweetened beverage consumption might increase the risk of asthma in children by 26% (odds ratio 1.26, 1.07 to 1.48).79 In a single article,80 both sugar sweetened beverage consumption (odds ratio 1.80, 1.23 to 2.63) and total sugar consumption (1.22, 1.04 to 1.42) were associated with an increased risk of attention deficit/hyperactivity disorder. In addition, the results from a meta-analysis of 10 observational studies showed a significant inverse association between sugar sweetened beverage consumption and bone mineral density in adults (standardised mean difference −0.66, −1.01 to −0.31).81 Subgroup analysis according to sex showed a significant harmful effect of sugar sweetened beverage consumption on bone mineral density in females (standardised mean difference −0.50, −0.87 to −0.13) but no association in males.81 For dental diseases, a single article found a harmful association between sugar sweetened beverage consumption and the incidence of dental caries (odds ratio 1.72, 1.41 to 2.09) and dental erosion (1.77, 1.28 to 2.43) when comparing never/low with moderate/high consumption.17 Additionally, sugar sweetened beverage consumption was positively associated with the risk of non-alcoholic fatty liver disease (risk ratio 1.39, 1.29 to 1.50).16 Fructose intake was associated with increased intrahepatocellular lipids (standardised mean difference 0.45, 0.18 to 0.72) (fig 2; fig 6).78
Sugar sweetened beverage consumption was not associated with the risk of chronic kidney disease.82 In addition, maternal increased free sugar intake during pregnancy was not associated with the risk of asthma in offspring (supplementary table D).83
Heterogeneity
We reanalysed the heterogeneity in 69% of all health outcomes by a random or fixed effects model. Reanalysis found that approximately 46% of the health outcomes that we reanalysed had significant heterogeneity (I2>50% or P value of Cochran’s Q test <0.1). The heterogeneity of most outcomes could be explained by some potential factors, including setting, region, ethnicity, sex, age, study quality, study design, sample size, duration of follow-up, and adjustment for confounding factors. Of the 26 outcomes that we could not reanalyse, approximately 54% had significant heterogeneity and 4% did not report the results of the heterogeneity evaluation.
Assessment of risk of bias
We conducted Egger’s test for 23% of the outcomes in our reanalysis, which found evidence of publication bias in three outcomes—type 2 diabetes mellitus (sugar sweetened beverages) (P=0.016), overall cancer risk (P=0.005), and hypertension in adults (sugar sweetened beverages) (P=0.02). For outcomes that we could not reanalyse, publication bias was detected for cardiovascular disease mortality (sugar sweetened beverages), non-alcoholic fatty liver disease, obesity in adults, and change in body weight (one year) by statistical test or funnel plot. The remaining outcomes did not show significant publication bias or did not report an evaluation for publication bias.
AMSTAR, GRADE, and evidence classification
The median AMSTAR score of all health outcomes was 8 (range 3-11; interquartile range 8-9.25) (supplementary table E). Supplementary table F provides the detailed AMSTAR scores for each outcome. All evidence from meta-analyses of cohorts, population based case-control studies, and cross sectional studies is graded as “low” or “very low” quality owing to the observational study design and factors for quality downgrade (significant risk of bias, inconsistency, indirectness, imprecision, and potential publication bias). Among the nine meta-analyses of randomised controlled trials, four (liver fat accumulation, muscle fat accumulation, serum uric acid (fructose), and change in body weight (sugar sweetened beverages)) were downgraded as “moderate” quality given the imprecision, and the remaining (alanine transaminase, intrahepatocellular lipids, postprandial triglycerides, change in body mass index in adults, and change in body weight (fructose)) were downgraded as “low” or “very low” owing to the risk of bias, inconsistency, indirectness, or imprecision (supplementary table E). Supplementary Table G shows the detailed GRADE classification for each outcome. In terms of evidence classification, type 2 diabetes mellitus (sugar sweetened beverages), hyperuricaemia (fructose), obesity in children (sugar sweetened beverages), coronary heart disease, hypertension in adults (sugar sweetened beverages), dental caries, depression, and non-alcoholic fatty liver disease were graded as class II. For the remaining 75 outcomes, 15 (18.1%) were graded as class III, 26 (31.3%) were graded as class IV, and 34 (41.0%) were identified as non-significant (supplementary table E). Sensitivity analyses of each outcome graded as class II did not alter the direction or significance of the association.
Discussion
Principal findings and possible explanations
Dietary sugar consumption is harmfully associated with multiple health outcomes across various measurements of exposure, including high versus low, never/low versus moderate/high, any versus none, or an extra increment of sugars per day (or week) versus none. We identified 73 meta-analyses and 83 health outcomes from 8601 unique articles, including 74 unique outcomes in meta-analyses of observational studies and nine unique outcomes in meta-analyses of randomised controlled trials.
Dietary sugar consumption had harmful associations with endocrine and metabolic outcomes, including changes in body mass index in children,53 changes in body weight,53 changes in body weight (one year),53 gout,11 13 58 high density lipoprotein cholesterol,60 hyperuricaemia,11 59 latent autoimmune diabetes in adults,30 low density lipoprotein cholesterol,60 metabolic syndrome,61 obesity in children,3 obesity in adults,1 serum uric acid,57 type 2 diabetes mellitus,6 liver fat accumulation,54 and muscle fat accumulation.54 In addition, harmful associations between dietary sugar consumption and cardiovascular outcomes were also observed, including coronary heart disease,10 cardiovascular disease,8 cardiovascular disease mortality,68 71 hypertension in children and adolescents,69 hypertension in adults,70 myocardial infarction,66 change in systolic blood pressure in children and adolescents,69 and stroke.10 Significant harmful associations between dietary sugar consumption and a higher risk of cancer were observed for breast cancer,19 breast cancer mortality,18 hepatocellular carcinoma,18 prostate cancer,18 pancreatic cancer,72 overall cancer risk,18 and overall cancer mortality.18 Finally, harmful associations existed between dietary sugar consumption and all cause mortality,76 asthma in children,79 attention deficit/hyperactivity disorder,80 bone mineral density,81 dental caries,17 dental erosion,17 depression,77 non-alcoholic fatty liver disease,16 and intrahepatocellular lipids.78
In general, no reliable evidence shows beneficial associations between dietary sugar consumption and any health outcomes, apart from glioma (sugar sweetened beverages),18 total cholesterol (sugar sweetened beverages),60 type 2 diabetes mellitus (sucrose),62 and cardiovascular disease mortality (sucrose).71 However, these favourable associations are not supported by strong evidence, and the interpretation of these results should be done with caution. For the decreased risk of glioma, evidence for this came from only two cohort studies, and no studies have shown that sugar sweetened beverage consumption is a protective factor to lower the incidence of cancer. High sugar sweetened beverage consumption was associated with lower total cholesterol concentrations. However, subgroup analysis indicated that sugar sweetened beverage consumption was associated with higher total cholesterol concentrations in studies with sugar sweetened beverage consumption >750 g/day and studies involving adolescents. Therefore, potential confounders, including region, sugar sweetened beverage dose, sample size, and sex, should be considered in explaining the association between sugar sweetened beverage consumption and total cholesterol concentrations. In terms of the protective effect of sucrose intake on type 2 diabetes mellitus and cardiovascular disease mortality, we note that sucrose tends to be found more in solid foods than in sugar sweetened beverages, including grains and grain based products, fruit and fruit products, and sweetened dairy and dairy products.84 85 86 These main sources of sucrose have shown beneficial associations (for example, whole grain cereals, fruit, and yogurt) with type 2 diabetes mellitus and cardiovascular disease mortality.87 88 89 90 91 92 Therefore, the protective association between sucrose intake and type 2 diabetes mellitus and cardiovascular disease mortality may reflect important contributions from these other food sources rather than sucrose.62 71 Further large scale, prospective studies are warranted to evaluate the association of sucrose intake with type 2 diabetes mellitus and cardiovascular disease mortality and to clarify the possible underlying mechanisms.
Our umbrella review showed harmful associations between dietary sugar consumption and a range of cardiometabolic diseases, especially weight gain, ectopic fat accumulation, obesity, and cardiovascular disease, which can largely be attributed to excessive consumption of fructose containing sugars. In response to the intake of large carbohydrates, fructose could enhance hepatic lipogenic capacity by inducing hepatic master transcription factors.93 94 95 Moreover, an animal study found that dietary fructose could be converted to microbial acetate by the gut microbiota, which may enhance hepatic lipogenesis by supplying lipogenic acetyl-CoA independently of ATP citrate lyase.96 Intermediate products such as diacylglycerols generated during the process of lipogenesis may impair insulin signalling in the liver and peripheral tissues and then lead to insulin resistance.97 Subsequently, it may promote ectopic fat deposition in the liver and muscle.98 99 Dietary fructose may also inhibit fatty acid oxidation in the liver by impairing mitochondrial size and function and acetylation of the rate limiting enzyme.100 A recent animal study showed that dietary fructose improves the survival of intestinal cells and increases the length of intestinal villus in mouse models, resulting in an expanded surface area of the gut and increased nutrient absorption and adiposity in mice.101 Furthermore, fructose contained in sugar sweetened beverages is suggested to likely induce the onset of obesity by reducing resting energy expenditure and promoting leptin resistance.102 103 In addition, sugar sweetened beverages are associated with less satiety compared with solid food containing the same amount of calories, which may stimulate appetite and induce excessive calorie consumption, liver fat accumulation, and insulin resistance in the long term.104 This hypothesis is confirmed by several clinical trials conducted in healthy adults, which found that sugar sweetened beverage consumption results in more caloric intake and weight gain than artificially sweetened beverages.105 106 107 Additionally, a recent double blind, randomised controlled trial carried out in 94 healthy men suggested that consumption of sugar sweetened beverages containing fructose might induce a significant change in the low density lipoprotein particle distribution towards smaller, more atherogenic particles, partially mediating the associations of sugar sweetened beverage consumption with dyslipidaemia and cardiovascular disease.108
Another important mechanism to explain the associations between dietary sugar consumption and cardiometabolic diseases involves uric acid synthesis. Many studies have confirmed that excessive fructose consumption can promote uric acid synthesis by inducing degradation ATP to AMP, a substrate for uric acid production.109 110 111 Fructose phosphorylation in the liver uses ATP to convert fructose into fructose-1-phosphate and leads to phosphate depletion, which limits the regeneration of ATP from ADP. Then, ADP is converted to AMP and consequently induces the synthesis of uric acid.57 In addition, fructose induced hyperinsulinaemia and insulin resistance may also result in higher serum uric acid by reducing the excretion of uric acid.110 112 113 Hyperuricaemia is a precursor to gout.109 110 The positive associations between gout, hyperuricaemia, and other cardiometabolic diseases, such as hypertension, type 2 diabetes mellitus, and cardiovascular disease, have been proposed for a long time.114 115 Hyperuricaemia has been shown to precede the occurrence of type 2 diabetes mellitus and obesity.27 Mechanistically, hyperuricaemia could induce renal microvascular alteration, chronic sodium retention, reduction in nitric oxide concentrations in endothelial cells, and the activation of the renin-angiotensin system, which may account for the association between fructose containing sugar consumption and cardiovascular disease.114 116 117 118
Until now, the evidence for the association between dietary sugar consumption and the risk of cancer has remained limited and controversial.27 In 2018 the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) reported that evidence was limited for the associations between consumption of sugars and food containing sugars and the risk of colorectal cancer.119 However, at the same time, this report recommended reducing or avoiding sugar sweetened beverage consumption for the prevention of breast cancer.119 Evidence from this umbrella review supports the recommendations from the WCRF/AICR to some extent. In our study, although eight of the 25 cancer outcomes were identified as being positively associated with dietary sugar consumption (seven exposure factors were sugar sweetened beverages, and one was fructose), only evidence of hepatocellular carcinoma (sugar sweetened beverages) and pancreatic cancer (fructose) were rated as “low” quality because of the magnitude of effect or dose-response gradient, and the remaining outcomes were all rated as “very low” quality. As a result, caution is warranted when explaining the significant associations between dietary sugar consumption and some cancer risks.
The effect of dietary sugars on obesity might partly explain their association with the risk of cancer.21 As mentioned previously, dietary sugar consumption, especially sugar sweetened beverage consumption, is convincingly associated with the risk of obesity weight gain,1 3 53 which in turn is regarded as a strong risk factor for various cancers.21 119 Another pathway mediating the association between dietary sugar consumption and the risk of cancer might involve a high glycaemic index or glycaemic load. The glycaemic index has been associated with the risk of type 2 diabetes mellitus,120 which may be involved in carcinogenesis of the breast, prostate, liver, bladder, and endometrium.120 121 Moreover, excessive fructose consumption might lead to intestinal flora disturbance and intestinal barrier deterioration, which promote the development of metabolic endotoxaemia, inflammation, and lipid accumulation, finally leading to colorectal carcinogenesis.20 122 123 A recent animal study showed that high fructose corn syrup intake could induce intestinal tumourigenesis in mice by expediting glycolysis and de novo lipogenesis. The mice treated with the syrup had a substantially increased tumour size and tumour grade, independent of obesity and metabolic syndrome.124 Considering the different mechanisms of site specific cancers, further prospective studies that explore the definite associations between sugar consumption and cancer risk for diverse cancer types and ethnic groups are warranted.27
On the other hand, dietary sugar consumption has also been shown to be negatively associated with some neuropsychiatric diseases, such as depression and attention deficit/hyperactivity disorder.77 80 Several biological mechanisms might be involved in these associations. Data from an animal study showed that a high fructose diet might alter behaviour, hypothalamic-pituitary-adrenal axis function, and the hypothalamic transcriptome in male Wistar rats, inducing anxiety-like behaviour and depressive-like behaviour.125 Furthermore, sugar consumption has been suggested to stimulate the secretion of endogenous opioids in the nucleus accumbens and to stimulate the dopaminergic reward system.27 Evidence of sugar dependence in an animal model indicated that similarly to addiction to morphine and cocaine, rats with intermittent sugar intake had decreased concentrations of dopamine D2 receptor mRNA in the nucleus accumbens and showed the characteristics of addictive-like behaviours called sugar addiction.27 126
In addition, the adverse association between sugar consumption and bone mineral density might be attributed to the increased loss of urinary calcium and imbalance in calcium homoeostasis induced by high sugar intake.127 As well as the negative effect of sugars, phosphate, acidity, and caffeine contained in sugar sweetened beverages are three other major factors that affect bone metabolism.81 We note that for the link between sugar sweetened beverages and bone mineral density, stratification analysis by gender showed a significant harmful effect of sugar sweetened beverages on bone mineral density in females but not in males.81 These diverse findings indicated that sugar sweetened beverage consumption had a more detrimental effect on female bone health than on male bone health because women generally have smaller bones and lower bone strength and are therefore more susceptible to osteoporosis.128 Moreover, the high acidity of sugar sweetened beverages is also thought to be an important factor in promoting dental caries and tooth erosion.129 130 131
Of the subgroup analyses conducted in this umbrella review, the most noteworthy is the stratification according to region, as several health outcomes showed a regional discrepancy, including overall cancer mortality, high density lipoprotein cholesterol, low density lipoprotein cholesterol, and total cholesterol. Potential reasons for these discrepancies may include regional differences in sugar consumption and culture. According to the report conducted in 2010 for the quantification of global, regional, and national consumption of sugar sweetened beverages in 187 countries, consumption among Asian countries was lower than that among European and American countries.33 The consumption of sugar sweetened beverages was highest in the Caribbean and lowest in East Asia and Oceania.33 In addition, cultural factors have been shown to potentially cause different dietary quality and health inequalities by affecting food preferences or choices.132 Regional cultural diversity in lifestyle and sociodemographic factors also plays an important role in dietary sugar consumption, which may partly explain the different relations between sugar consumption and disease risk in ethnically diverse populations.132 133 On the other hand, subgroup analyses with adjustment for confounding factors should also be considered. High consumption of sugars, especially sugar sweetened beverages, may be a marker of an unhealthy diet and lifestyle.9 66 People who consumed sugar sweetened beverages more frequently were likely to ingest more total and saturated fat, carbohydrate, and sodium and less fruit, fibre, dairy products, and wholegrain foods.134 135 136 137 138 This dietary pattern was also associated with more frequent smoking and drinking, lower physical activity levels, and more time spent watching television.137 138 Therefore, the role of these confounding factors should be taken into consideration when explaining the association between sugar consumption and burden of disease.
Strengths and weaknesses of study and in relation to other studies
This umbrella review first reported a comprehensive summary of the current evidence from previous meta-analyses of observational studies and randomised controlled trials for the association between dietary sugar consumption and all health outcomes. Given the high levels of dietary sugar consumption worldwide, this study has clinical and social significance for developing preventive strategies against excessive sugar consumption, especially for children and adolescents. This study was carried out on the basis of systematic methods in which independent literature searching, study selection, and data extraction by two authors were involved. If the data were sufficient, we reanalysed the risk ratio, odds ratio, weighted mean difference, or standardised mean difference with 95% confidence intervals through random or fixed effects models and evaluated the heterogeneity and publication bias for each included meta-analysis. Furthermore, we used three standard approaches, AMSTAR, GRADE, and evidence classification criteria, to assess the methodological quality (AMSTAR), strength (GRADE) and classification (evidence classification criteria) of evidence for each health outcome and to evaluate our confidence in the estimates. Interestingly, in our umbrella review, the GRADE rating of several health outcomes was not completely consistent with the results of evidence classification. As we know, evidence classification criteria are a completely objective classification standard, whereas the GRADE rating has a certain degree of subjectivity.139 Therefore, both the GRADE rating and evidence classification criteria should be considered when evaluating evidence and making recommendations.
Original studies included in meta-analyses used different methods of food intake investigation, including food records, 24 hour dietary recall, food frequency questionnaires, and dietary history. All of these are associated with an unavoidable measurement bias even if validated methods are used.3 This limitation is common to all major epidemiological studies carried out worldwide in this field.21 In addition, most studies focused on beverages pre-sweetened before purchase.9 For instance, in the Nurses’ Health Study, coffee with sugars was excluded from sugar sweetened beverages, which might affect the reliability of the association.137 Similarly, another limitation of our study was that we could not evaluate sugar intake in some foods that potentially contain sugars, such as chocolate and ice cream, because of a failure to extract data on sugar consumption. Furthermore, the types of sugar sweetened beverages and dosage of their consumption varied in the original studies. In this umbrella review, most meta-analyses produced summary effects from original studies that measured exposure to dietary sugars through the number of servings a day. However, in some original studies, the number of millilitres a day, grams a day, times a day, times a week, times a month, servings a week, or servings a month were used to estimate sugar consumption, which may partly explain the origin of heterogeneity in meta-analyses. Therefore, dose-response analysis and stratification analysis by sugar sweetened beverage types were unavailable for most outcomes owing to diverse measurements of sugar sweetened beverage consumption in the original studies. Consumption of sugars in sugar sweetened beverages is generally accompanied by the ingestion of some other chemical compounds, such as 4-methylimidazole,140 141 pesticides,142 143 artificial sweeteners,144 sodium benzoate,79 and sulfites,79 which may confuse the effect of sugars and therefore should be regarded as potential confounding factors.
We reviewed details of competing interest and funding disclosures from meta-analyses included in this umbrella review. Only two meta-analyses were funded by companies that produce sugar sweetened beverages.65 145 Among them, the meta-analysis conducted by Wang and colleagues was selected for data extraction and is shown in summary tables.65 Therefore, caution is warranted when explaining the non-significant association between fructose intake and postprandial triglycerides. Another meta-analysis was not selected for data extraction,145 and the list of all meta-analyses not selected for data extraction and reanalysis are available if needed. We did not investigate the original studies included in each meta-analysis and therefore could not confirm whether these studies had a competing interest with companies associated with the sugar industry.42
The harmful association between dietary sugar consumption and multiple health outcomes observed in our umbrella review is supported by several large scale prospective cohort studies published in recent years. The first was a large prospective cohort study conducted using the results of the French NutriNet-Santé cohort (2009-17), which included 101 257 participants with an average age of 42.2.21 During the eight year follow-up period, a total of 2193 cases of cancer were reported, including 693 cases of breast cancer. A harmful association was found between sugar sweetened beverage consumption and the risk of overall cancer (hazard ratio 1.18, 1.10 to 1.27) and breast cancer (1.22, 1.07 to 1.39). No significant association was observed for sugar sweetened beverage consumption and the risk of prostate, colorectal, and lung cancer.21 In this umbrella review, however, the highest versus lowest sugar sweetened beverage consumption was associated with a 17% increased risk of prostate cancer, without a dose-response gradient. Notably, the non-significant association between sugar sweetened beverage consumption and the risk of colorectal cancer observed both in this study and in our umbrella review was inconsistent with another cohort conducted in women.20 In the Nurses’ Health Study II (1991-2015), the authors prospectively explored the association of sugar sweetened beverage consumption in adulthood and adolescence with the risk of early onset colorectal cancer among 95 464 women. A total of 109 cases of early onset colorectal cancer were confirmed during follow-up. Compared with women who consumed less than one serving a week of sugar sweetened beverages in adulthood, those who consumed two or more servings a day had a 118% higher risk of early onset colorectal cancer (risk ratio 2.18, 1.10 to 4.35). Each one serving a day increment of sugar sweetened beverage consumption was associated with a 16% (risk ratio 1.16, 1.00 to 1.36) increased risk of early onset colorectal cancer.20 In addition, another prospective cohort study showed that excessive consumption of sugars and sugar sweetened beverage during adolescence was significantly associated with the risk of colorectal adenoma (odds ratio 1.20, 1.04 to 1.39).146 Each one serving a day increase in sugar sweetened beverage consumption was associated with 11% (odds ratio 1.11, 1.02 to 1.20) and 30% (1.30, 1.08 to 1.55) higher risks of total colorectal adenoma and rectal adenoma, respectively.146 Given that the association between sugar consumption and colorectal cancer risk remains controversial, further well designed, large scale prospective studies are needed to clarify it.
The positive associations between sugar sweetened beverage consumption and the risk of mortality detected in this umbrella review were supported by a prospective cohort study of 118 363 people followed for 34 years in the US, during which time 36 436 deaths were documented.147 After adjustment for diet and lifestyle confounders, the consumption of two or more servings of sugar sweetened beverages a day was linked with a 21% (hazard ratio 1.21, 1.13 to 1.28) higher risk of total mortality, a 31% (1.31, 1.15 to 1.50) higher risk of cardiovascular disease mortality, and a 16% (1.16, 1.04 to 1.29) higher risk of cancer mortality.147 On the other hand, a prospective cohort study of 120 343 UK participants followed for 8.4 years confirmed the harmful association of added sugar consumption with the risk of type 2 diabetes mellitus.148 A dietary pattern high in added sugars was associated with a higher incidence of type 2 diabetes mellitus (hazard ratio 1.09, 1.06 to 1.12) after adjustment for confounders.148 Similar to their findings, we observed a strongly significant association between consumption of sugar sweetened beverages (one of the main sources of added sugars) and the risk of type 2 diabetes mellitus.
Conclusions and recommendations
This umbrella review shows that high dietary sugar consumption, especially intake of sugars that contain fructose, is harmfully associated with large numbers of health outcomes. Evidence for the harmful associations between dietary sugar consumption and changes in body weight (sugar sweetened beverages), ectopic fat accumulation (added sugars), obesity in children (sugar sweetened beverages), coronary heart disease (sugar sweetened beverages), and depression (sugar sweetened beverages) seems to be more reliable than that for other outcomes. Evidence of the association between dietary sugar consumption and cancer remains limited but warrants further research. In combination with the WHO and WCRF/AICR recommendations and our findings, we recommend reducing the consumption of free sugars or added sugars to below 25 g/day (approximately six teaspoons a day) and limiting the consumption of sugar sweetened beverages to less than one serving a week (approximately 200-355 mL/week).38 119 To change sugar consumption patterns, especially for children and adolescents, a combination of widespread public health education and policies worldwide is urgently needed.
What is already known on this topic
Sugar consumption could have negative effects on health, especially obesity, diabetes, cardiovascular disease, hyperuricaemia, gout, ectopic fatty accumulation, dental caries, and some cancers
Deficiencies in study design, varying measurements, inconsistent findings, and different definitions of exposure make drawing final conclusions on associations difficult
Comprehensive evaluation of the quality of existing evidence on the associations of sugar consumption with all health outcomes is needed
What this study adds
High dietary sugar consumption is generally more harmful than beneficial for health, especially in cardiometabolic disease
Evidence of the association between dietary sugar consumption and cancer remains limited but warrants further research
Existing evidence is mostly observational and of low quality, and further randomised controlled trials are needed
Acknowledgments
We thank Nanxi Yan for her linguistic assistance during the preparation and revision of this manuscript.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: YH, ZYC, BC, and, JZL are joint first authors and contributed equally to this work. QW, DHC, and LRL are joint corresponding authors and contributed equally to this work. YH, ZYC, BC, and JZL conducted study selection, data extraction, and analysis and wrote the manuscript. QW, DHC, and LRL designed the study, supervised the project, and revised the manuscript. XY, JL, WW, TTD, HYC, YW, RYW, PZW, JBG, QD, and CFL assisted with detailed statistical analysis. All authors reviewed and approved the final version of the manuscript. LRL is the guarantor. The corresponding authors (QW, DHC, and LRL) attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the National Natural Science Foundation of China (grant number 82000721) and Program from the Department of Science and Technology of Sichuan Province (grant number 2020YJ0054). The funders had no role in considering the study design or in the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Natural Science Foundation of China and Program from the Department of Science and Technology of Sichuan Province for the submitted work; no financial relationship with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patients and public communities: After publication, the findings of this review will be disseminated to appropriate audiences such as academia, clinicians, policy makers, and the general public, through various channels including blogs, press releases, and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not needed.
Data availability statement
The list of all meta-analyses not selected for data extraction and reanalysis is available if needed.
References
- 1. Qin P, Li Q, Zhao Y, et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 2020;35:655-71. 10.1007/s10654-020-00655-y [DOI] [PubMed] [Google Scholar]
- 2. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, Harindhanavudhi T. Sugar and artificially sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM 2017;110:513-20. 10.1093/qjmed/hcx068 [DOI] [PubMed] [Google Scholar]
- 3. Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012;346:e7492. 10.1136/bmj.e7492 [DOI] [PubMed] [Google Scholar]
- 4. Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477-83. 10.2337/dc10-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015;351:h3576. 10.1136/bmj.h3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng Y, Li S, Khan J, et al. Sugar- and Artificially Sweetened Beverages Consumption Linked to Type 2 Diabetes, Cardiovascular Diseases, and All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients 2021;13:2636. 10.3390/nu13082636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xi B, Huang Y, Reilly KH, et al. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr 2015;113:709-17. 10.1017/S0007114514004383 [DOI] [PubMed] [Google Scholar]
- 8. Yin J, Zhu Y, Malik V, et al. Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages and Risk of Cardiovascular Disease: A Meta-Analysis and Systematic Review. Adv Nutr 2021;12:89-101. 10.1093/advances/nmaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis 2014;234:11-6. 10.1016/j.atherosclerosis.2014.01.037 [DOI] [PubMed] [Google Scholar]
- 10. Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 2019;59:1071-90. 10.1080/10408398.2017.1392288 [DOI] [PubMed] [Google Scholar]
- 11. Ebrahimpour-Koujan S, Saneei P, Larijani B, Esmaillzadeh A. Consumption of sugar sweetened beverages and dietary fructose in relation to risk of gout and hyperuricemia: a systematic review and meta-analysis. Crit Rev Food Sci Nutr 2020;60:1-10. 10.1080/10408398.2018.1503155 [DOI] [PubMed] [Google Scholar]
- 12. Lee Y, Song GG. Association between Sugar-Sweetened Beverage Consumption and the Risk of Gout: A Meta-Analysis. J Rheum Dis 2016;23:304-10 10.4078/jrd.2016.23.5.304. [DOI] [Google Scholar]
- 13. Ayoub-Charette S, Liu Q, Khan TA, et al. Important food sources of fructose-containing sugars and incident gout: a systematic review and meta-analysis of prospective cohort studies. BMJ Open 2019;9:e024171. 10.1136/bmjopen-2018-024171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wijarnpreecha K, Thongprayoon C, Edmonds PJ, Cheungpasitporn W. Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: a systematic review and meta-analysis. QJM 2016;109:461-6. 10.1093/qjmed/hcv172 [DOI] [PubMed] [Google Scholar]
- 15. Asgari-Taee F, Zerafati-Shoae N, Dehghani M, Sadeghi M, Baradaran HR, Jazayeri S. Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Nutr 2019;58:1759-69. 10.1007/s00394-018-1711-4 [DOI] [PubMed] [Google Scholar]
- 16. Chen H, Wang J, Li Z, et al. Consumption of sugar-sweetened beverages has a dose-dependent effect on the risk of non-alcoholic fatty liver disease: An updated systematic review and dose-response meta-analysis. Int J Environ Res Public Health 2019;16:2192. 10.3390/ijerph16122192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valenzuela MJ, Waterhouse B, Aggarwal VR, Bloor K, Doran T. Effect of sugar-sweetened beverages on oral health: a systematic review and meta-analysis. Eur J Public Health 2021;31:122-9. 10.1093/eurpub/ckaa147 [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Guo L, He K, Huang C, Tang S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: a systematic review and dose-response meta-analysis of observational studies. J Cancer 2021;12:3077-88. 10.7150/jca.51322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Llaha F, Gil-Lespinard M, Unal P, de Villasante I, Castañeda J, Zamora-Ros R. Consumption of Sweet Beverages and Cancer Risk. A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021;13:516. 10.3390/nu13020516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hur J, Otegbeye E, Joh HK, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021;70:2330-6. 10.1136/gutjnl-2020-323450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chazelas E, Srour B, Desmetz E, et al. Sugary drink consumption and risk of cancer: results from NutriNet-Santé prospective cohort. BMJ 2019;366:l2408. 10.1136/bmj.l2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mann J, Cummings JH, Englyst HN, et al. FAO/WHO scientific update on carbohydrates in human nutrition: conclusions. Eur J Clin Nutr 2007;61(Suppl 1):S132-7. 10.1038/sj.ejcn.1602943 [DOI] [PubMed] [Google Scholar]
- 23. Phillips JA. Dietary Guidelines for Americans, 2020-2025. Workplace Health Saf 2021;69:395. 10.1177/21650799211026980 [DOI] [PubMed] [Google Scholar]
- 24. Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274-88. 10.1093/ajcn/84.2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened Beverage Consumption Among U.S. Youth, 2011-2014. NCHS Data Brief 2017;(271):1-8. [PubMed] [Google Scholar]
- 26. Blecher E, Liber AC, Drope JM, Nguyen B, Stoklosa M. Global Trends in the Affordability of Sugar-Sweetened Beverages, 1990-2016. Prev Chronic Dis 2017;14:E37. 10.5888/pcd14.160406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malik VS, Hu FB. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat Rev Endocrinol 2022;18:205-18. 10.1038/s41574-021-00627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726-34. 10.3945/ajcn.111.018366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13-27. 10.1038/nrendo.2012.199 [DOI] [PubMed] [Google Scholar]
- 30. El-Malky AM, Naik RG, Elnouman AA. Sugary beverages consumption and latent autoimmune diabetes in adults: Systematic review and meta-analysis. Clin Diabetol 2020;9:118-27 10.5603/DK.2020.0007. [DOI] [Google Scholar]
- 31. Qin Z, Xu F, Ye Q, et al. Sugar-sweetened beverages and school students’ hypertension in urban areas of Nanjing, China. J Hum Hypertens 2018;32:392-6. 10.1038/s41371-018-0030-9 [DOI] [PubMed] [Google Scholar]
- 32. Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999-2010. Am J Clin Nutr 2013;98:180-8. 10.3945/ajcn.112.057943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh GM, Micha R, Khatibzadeh S, et al. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) . Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS One 2015;10:e0124845. 10.1371/journal.pone.0124845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ 2016;352:h6704. 10.1136/bmj.h6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taillie LS, Reyes M, Colchero MA, Popkin B, Corvalán C. An evaluation of Chile’s Law of Food Labeling and Advertising on sugar-sweetened beverage purchases from 2015 to 2017: A before-and-after study. PLoS Med 2020;17:e1003015. 10.1371/journal.pmed.1003015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Basu S, Vellakkal S, Agrawal S, Stuckler D, Popkin B, Ebrahim S. Averting obesity and type 2 diabetes in India through sugar-sweetened beverage taxation: an economic-epidemiologic modeling study. PLoS Med 2014;11:e1001582. 10.1371/journal.pmed.1001582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manyema M, Veerman LJ, Chola L, et al. The potential impact of a 20% tax on sugar-sweetened beverages on obesity in South African adults: a mathematical model. PLoS One 2014;9:e105287. 10.1371/journal.pone.0105287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization . Guideline: Sugars Intake for Adults and Children. World Health Organization, 2015. [PubMed] [Google Scholar]
- 39. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132-40. 10.1097/XEB.0000000000000055 [DOI] [PubMed] [Google Scholar]
- 40. Papatheodorou S. Umbrella reviews: what they are and why we need them. Eur J Epidemiol 2019;34:543-6. 10.1007/s10654-019-00505-6 [DOI] [PubMed] [Google Scholar]
- 41.Scottish Intercollegiate Guidelines Network. Search Filters. https://www.sign.ac.uk/what-we-do/methodology/search-filters/.
- 42. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ 2017;359:j5024. 10.1136/bmj.j5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang Y, Cao D, Chen Z, et al. Iron intake and multiple health outcomes: Umbrella review. Crit Rev Food Sci Nutr 2021;1-18. 10.1080/10408398.2021.1982861 [DOI] [PubMed] [Google Scholar]
- 44. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7:10. 10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 46. Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009;181:488-93. 10.1503/cmaj.081086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Veronese N, Solmi M, Caruso MG, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr 2018;107:436-44. 10.1093/ajcn/nqx082 [DOI] [PubMed] [Google Scholar]
- 48. Wallace TC, Bailey RL, Blumberg JB, et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci Nutr 2020;60:2174-211. 10.1080/10408398.2019.1632258 [DOI] [PubMed] [Google Scholar]
- 49. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014;348:g2035. 10.1136/bmj.g2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang Y, Cao D, Chen Z, et al. Red and processed meat consumption and cancer outcomes: Umbrella review. Food Chem 2021;356:129697. 10.1016/j.foodchem.2021.129697 [DOI] [PubMed] [Google Scholar]
- 51. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 53. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084-102. 10.3945/ajcn.113.058362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma J, Karlsen MC, Chung M, et al. Potential link between excess added sugar intake and ectopic fat: a systematic review of randomized controlled trials. Nutr Rev 2016;74:18-32. 10.1093/nutrit/nuv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang DD, Sievenpiper JL, de Souza RJ, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr 2012;142:916-23. 10.3945/jn.111.151951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sievenpiper JL, de Souza RJ, Mirrahimi A, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012;156:291-304. 10.7326/0003-4819-156-4-201202210-00007 [DOI] [PubMed] [Google Scholar]
- 57. Ebrahimpour-Koujan S, Saneei P, Larijani B, Esmaillzadeh A. Consumption of sugar-sweetened beverages and serum uric acid concentrations: a systematic review and meta-analysis. J Hum Nutr Diet 2021;34:305-13. 10.1111/jhn.12796 [DOI] [PubMed] [Google Scholar]
- 58. Jamnik J, Rehman S, Blanco Mejia S, et al. Fructose intake and risk of gout and hyperuricemia: a systematic review and meta-analysis of prospective cohort studies. BMJ Open 2016;6:e013191. 10.1136/bmjopen-2016-013191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li R, Yu K, Li C. Dietary factors and risk of gout and hyperuricemia: a meta-analysis and systematic review. Asia Pac J Clin Nutr 2018;27:1344-56. [DOI] [PubMed] [Google Scholar]
- 60. Nikniaz L, Abbasalizad-Farhangi M, Vajdi M, Nikniaz Z. The association between Sugars Sweetened Beverages (SSBs) and lipid profile among children and youth: A systematic review and dose-response meta-analysis of cross-sectional studies. Pediatr Obes 2021;16:e12782. 10.1111/ijpo.12782 [DOI] [PubMed] [Google Scholar]
- 61. Semnani-Azad Z, Khan TA, Blanco Mejia S, et al. Association of Major Food Sources of Fructose-Containing Sugars With Incident Metabolic Syndrome: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e209993. 10.1001/jamanetworkopen.2020.9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsilas CS, de Souza RJ, Mejia SB, et al. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. CMAJ 2017;189:E711-20. 10.1503/cmaj.160706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mattes RD, Shikany JM, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev 2011;12:346-65. 10.1111/j.1467-789X.2010.00755.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ardeshirlarijani E, Jalilpiran Y, Daneshzad E, Larijani B, Namazi N, Azadbakht L. Association between sugar-sweetened beverages and waist circumference in adult populations: A meta-analysis of prospective cohort studies. Clin Nutr ESPEN 2021;41:118-25. 10.1016/j.clnesp.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 65. David Wang D, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014;232:125-33. 10.1016/j.atherosclerosis.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 66. Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Clin Pract 2016;70:791-805. 10.1111/ijcp.12841 [DOI] [PubMed] [Google Scholar]
- 67. Jayalath VH, Sievenpiper JL, de Souza RJ, et al. Total fructose intake and risk of hypertension: a systematic review and meta-analysis of prospective cohorts. J Am Coll Nutr 2014;33:328-39. 10.1080/07315724.2014.916237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang YB, Jiang YW, Chen JX, Xia PF, Pan A. Association of Consumption of Sugar-Sweetened Beverages or Artificially Sweetened Beverages with Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv Nutr 2021;12:374-83. 10.1093/advances/nmaa110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farhangi MA, Nikniaz L, Khodarahmi M. Sugar-sweetened beverages increases the risk of hypertension among children and adolescence: a systematic review and dose-response meta-analysis. J Transl Med 2020;18:344. 10.1186/s12967-020-02511-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Q, Ayoub-Charette S, Khan TA, et al. Important Food Sources of Fructose-Containing Sugars and Incident Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc 2019;8:e010977. 10.1161/JAHA.118.010977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Khan TA, Tayyiba M, Agarwal A, et al. Relation of Total Sugars, Sucrose, Fructose, and Added Sugars With the Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Mayo Clin Proc 2019;94:2399-414. 10.1016/j.mayocp.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 72. Aune D, Chan DSM, Vieira AR, et al. Dietary fructose, carbohydrates, glycemic indices and pancreatic cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol 2012;23:2536-46. 10.1093/annonc/mds076 [DOI] [PubMed] [Google Scholar]
- 73. Zhang X, Albanes D, Beeson WL, et al. Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst 2010;102:771-83. 10.1093/jnci/djq107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Galeone C, Pelucchi C, La Vecchia C. Added sugar, glycemic index and load in colon cancer risk. Curr Opin Clin Nutr Metab Care 2012;15:368-73. 10.1097/MCO.0b013e3283539f81 [DOI] [PubMed] [Google Scholar]
- 75. Liu J, Tang W, Sang L, et al. Milk, yogurt, and lactose intake and ovarian cancer risk: a meta-analysis. Nutr Cancer 2015;67:68-72. 10.1080/01635581.2014.956247 [DOI] [PubMed] [Google Scholar]
- 76. Pan B, Ge L, Lai H, et al. Association of soft drink and 100% fruit juice consumption with all-cause mortality, cardiovascular diseases mortality, and cancer mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr 2022;62:8908-19. 10.1080/10408398.2021.1937040 [DOI] [PubMed] [Google Scholar]
- 77. Hu D, Cheng L, Jiang W. Sugar-sweetened beverages consumption and the risk of depression: A meta-analysis of observational studies. J Affect Disord 2019;245:348-55. 10.1016/j.jad.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 78. Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2014;68:416-23. 10.1038/ejcn.2014.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Al-Zalabani AH, Noor Elahi I, Katib A, et al. Association between soft drinks consumption and asthma: a systematic review and meta-analysis. BMJ Open 2019;9:e029046. 10.1136/bmjopen-2019-029046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Farsad-Naeimi A, Asjodi F, Omidian M, et al. Sugar consumption, sugar sweetened beverages and Attention Deficit Hyperactivity Disorder: A systematic review and meta-analysis. Complement Ther Med 2020;53:102512. 10.1016/j.ctim.2020.102512 [DOI] [PubMed] [Google Scholar]
- 81. Ahn H, Park YK. Sugar-sweetened beverage consumption and bone health: a systematic review and meta-analysis. Nutr J 2021;20:41. 10.1186/s12937-021-00698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lo WC, Ou SH, Chou CL, Chen JS, Wu MY, Wu MS. Sugar- and artificially-sweetened beverages and the risks of chronic kidney disease: a systematic review and dose-response meta-analysis. J Nephrol 2021;34:1791-804. 10.1007/s40620-020-00957-0 [DOI] [PubMed] [Google Scholar]
- 83. Gupta A, Singh A, Fernando RL, Dharmage SC, Lodge CJ, Waidyatillake NT. The association between sugar intake during pregnancy and allergies in offspring: a systematic review and a meta-analysis of cohort studies. Nutr Rev 2022;80:904-18. 10.1093/nutrit/nuab052 [DOI] [PubMed] [Google Scholar]
- 84. Brisbois TD, Marsden SL, Anderson GH, Sievenpiper JL. Estimated intakes and sources of total and added sugars in the Canadian diet. Nutrients 2014;6:1899-912. 10.3390/nu6051899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S-35S. 10.3945/jn.108.098277 [DOI] [PubMed] [Google Scholar]
- 86. Bernstein JT, Lou W, L’Abbe MR. Examining the Relationship between Free Sugars and Calorie Contents in Canadian Prepacked Foods and Beverages. Foods 2017;6:75. 10.3390/foods6090075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066-83. 10.3945/ajcn.113.059030 [DOI] [PubMed] [Google Scholar]
- 88. Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 2013;28:845-58. 10.1007/s10654-013-9852-5 [DOI] [PubMed] [Google Scholar]
- 89. Li M, Fan Y, Zhang X, Hou W, Tang Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open 2014;4:e005497. 10.1136/bmjopen-2014-005497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhan J, Liu YJ, Cai LB, Xu FR, Xie T, He QQ. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr 2017;57:1650-63. 10.1080/10408398.2015.1008980 [DOI] [PubMed] [Google Scholar]
- 91. Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ 2016;353:i2716. 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alexander DD, Bylsma LC, Vargas AJ, et al. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr 2016;115:737-50. 10.1017/S0007114515005000 [DOI] [PubMed] [Google Scholar]
- 93. Janevski M, Ratnayake S, Siljanovski S, McGlynn MA, Cameron-Smith D, Lewandowski P. Fructose containing sugars modulate mRNA of lipogenic genes ACC and FAS and protein levels of transcription factors ChREBP and SREBP1c with no effect on body weight or liver fat. Food Funct 2012;3:141-9. 10.1039/C1FO10111K [DOI] [PubMed] [Google Scholar]
- 94. Kim MS, Krawczyk SA, Doridot L, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest 2016;126:4372-86. 10.1172/JCI81993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Koo HY, Wallig MA, Chung BH, Nara TY, Cho BH, Nakamura MT. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim Biophys Acta 2008;1782:341-8. 10.1016/j.bbadis.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 96. Zhao S, Jang C, Liu J, et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 2020;579:586-91. 10.1038/s41586-020-2101-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Badin PM, Louche K, Mairal A, et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes 2011;60:1734-42. 10.2337/db10-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A 2011;108:16381-5. 10.1073/pnas.1113359108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Petersen MC, Shulman GI. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol Sci 2017;38:649-65. 10.1016/j.tips.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Softic S, Meyer JG, Wang GX, et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab 2019;30:735-753.e4. 10.1016/j.cmet.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Taylor SR, Ramsamooj S, Liang RJ, et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature 2021;597:263-7. 10.1038/s41586-021-03827-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cox CL, Stanhope KL, Schwarz JM, et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 2012;66:201-8. 10.1038/ejcn.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sundborn G, Thornley S, Merriman TR, et al. Are Liquid Sugars Different from Solid Sugar in Their Ability to Cause Metabolic Syndrome? Obesity (Silver Spring) 2019;27:879-87. 10.1002/oby.22472 [DOI] [PubMed] [Google Scholar]
- 104. Malik VS. Sugar sweetened beverages and cardiometabolic health. Curr Opin Cardiol 2017;32:572-9. 10.1097/HCO.0000000000000439 [DOI] [PubMed] [Google Scholar]
- 105. DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite 2005;44:187-93. 10.1016/j.appet.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 106. Raben A, Vasilaras TH, Møller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721-9. 10.1093/ajcn/76.4.721 [DOI] [PubMed] [Google Scholar]
- 107. Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr 2007;97:193-203. 10.1017/S0007114507252705 [DOI] [PubMed] [Google Scholar]
- 108. Geidl-Flueck B, Hochuli M, Németh Á, et al. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J Hepatol 2021;75:46-54. 10.1016/j.jhep.2021.02.027 [DOI] [PubMed] [Google Scholar]
- 109. Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ 2008;336:309-12. 10.1136/bmj.39449.819271.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in women. JAMA 2010;304:2270-8. 10.1001/jama.2010.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kanbay M, Jensen T, Solak Y, et al. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur J Intern Med 2016;29:3-8. 10.1016/j.ejim.2015.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu T, Giovannucci E, Pischon T, et al. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am J Clin Nutr 2004;80:1043-9. 10.1093/ajcn/80.4.1043 [DOI] [PubMed] [Google Scholar]
- 113. Quiñones Galvan A, Natali A, Baldi S, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995;268:E1-5. [DOI] [PubMed] [Google Scholar]
- 114. Richette P, Bardin T. Gout. Lancet 2010;375:318-28. 10.1016/S0140-6736(09)60883-7 [DOI] [PubMed] [Google Scholar]
- 115. Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 2005;1:80-6. 10.1038/ncpneph0019 [DOI] [PubMed] [Google Scholar]
- 116. Cicero AFG, Fogacci F, Desideri G, et al. Arterial Stiffness, Sugar-Sweetened Beverages and Fruits Intake in a Rural Population Sample: Data from the Brisighella Heart Study. Nutrients 2019;11:2674. 10.3390/nu11112674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Caliceti C, Calabria D, Roda A, Cicero AFG. Fructose Intake, Serum Uric Acid, and Cardiometabolic Disorders: A Critical Review. Nutrients 2017;9:395. 10.3390/nu9040395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Johnson RJ, Sanchez-Lozada LG, Nakagawa T. The effect of fructose on renal biology and disease. J Am Soc Nephrol 2010;21:2036-9. 10.1681/ASN.2010050506 [DOI] [PubMed] [Google Scholar]
- 119.World Cancer Research Fund International. Diet, nutrition, physical activity and cancer: A global perspective. A summary of the Third Expert Report. 2018. https://www.wcrf.org/dietandcancer/a-summary-of-the-third-expert-report/.
- 120. Augustin LSA, Kendall CWC, Jenkins DJA, et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr Metab Cardiovasc Dis 2015;25:795-815. 10.1016/j.numecd.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 121. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019;92:121-35. 10.1016/j.metabol.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 122. Todoric J, Di Caro G, Reibe S, et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab 2020;2:1034-45. 10.1038/s42255-020-0261-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Do MH, Lee E, Oh MJ, Kim Y, Park HY. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018;10:761. 10.3390/nu10060761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Goncalves MD, Lu C, Tutnauer J, et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 2019;363:1345-9. 10.1126/science.aat8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Harrell CS, Burgado J, Kelly SD, Johnson ZP, Neigh GN. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology 2015;62:252-64. 10.1016/j.psyneuen.2015.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32:20-39. 10.1016/j.neubiorev.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tucker KL. Dietary intake and bone status with aging. Curr Pharm Des 2003;9:2687-704. 10.2174/1381612033453613 [DOI] [PubMed] [Google Scholar]
- 128. Alswat KA. Gender Disparities in Osteoporosis. J Clin Med Res 2017;9:382-7. 10.14740/jocmr2970w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lustig RH, Schmidt LA, Brindis CD. Public health: The toxic truth about sugar. Nature 2012;482:27-9. 10.1038/482027a [DOI] [PubMed] [Google Scholar]
- 130. Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Soft drinks and dental health: a review of the current literature. J Dent 2006;34:2-11. 10.1016/j.jdent.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 131. Moynihan PJ, Kelly SA. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res 2014;93:8-18. 10.1177/0022034513508954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kouba J. Impact of Environment, Ethnicity, and Culture on Nutrition and Health. In: Touger-Decker R, Sirois DA, Mobley CC, eds. Nutrition and Oral Medicine. Humana Press, 2005:45-60 10.1385/1-59259-831-5:045. [DOI] [Google Scholar]
- 133. Pestoni G, Krieger JP, Sych JM, Faeh D, Rohrmann S. Cultural Differences in Diet and Determinants of Diet Quality in Switzerland: Results from the National Nutrition Survey menuCH. Nutrients 2019;11:126. 10.3390/nu11010126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480-8. 10.1161/CIRCULATIONAHA.107.689935 [DOI] [PubMed] [Google Scholar]
- 135. Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Intern Med 2012;27:1120-6. 10.1007/s11606-011-1968-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Eshak ES, Iso H, Kokubo Y, et al. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre-based study cohort I. Am J Clin Nutr 2012;96:1390-7. 10.3945/ajcn.112.037903 [DOI] [PubMed] [Google Scholar]
- 137. Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:1037-42. 10.3945/ajcn.2008.27140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Pereira MA, Kartashov AI, Ebbeling CB, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet 2005;365:36-42. 10.1016/S0140-6736(04)17663-0 [DOI] [PubMed] [Google Scholar]
- 139. Jaeschke R, Guyatt GH, Dellinger P, et al. GRADE Working Group . Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 2008;337:a744. 10.1136/bmj.a744 [DOI] [PubMed] [Google Scholar]
- 140. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Some chemicals present in industrial and consumer products, food and drinking-water. IARC Monogr Eval Carcinog Risks Hum 2013;101:9-549. [PMC free article] [PubMed] [Google Scholar]
- 141. Smith TJ, Wolfson JA, Jiao D, et al. Caramel color in soft drinks and exposure to 4-methylimidazole: a quantitative risk assessment. PLoS One 2015;10:e0118138. 10.1371/journal.pone.0118138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Nardin T, Barnaba C, Abballe F, Trenti G, Malacarne M, Larcher R. Fast analysis of quaternary ammonium pesticides in food and beverages using cation-exchange chromatography coupled with isotope-dilution high-resolution mass spectrometry. J Sep Sci 2017;40:3928-37. 10.1002/jssc.201700579 [DOI] [PubMed] [Google Scholar]
- 143. Albero B, Sánchez-Brunete C, Tadeo JL. Determination of organophosphorus pesticides in fruit juices by matrix solid-phase dispersion and gas chromatography. J Agric Food Chem 2003;51:6915-21. 10.1021/jf030414m [DOI] [PubMed] [Google Scholar]
- 144. Additives E. Scientific Opinion on the re‐evaluation of aspartame (E 951) as a food additive. EFSA J 2013;11:3495. [Google Scholar]
- 145. Jayalath VH, de Souza RJ, Ha V, et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015;102:914-21. 10.3945/ajcn.115.107243 [DOI] [PubMed] [Google Scholar]
- 146. Joh HK, Lee DH, Hur J, et al. Simple Sugar and Sugar-Sweetened Beverage Intake During Adolescence and Risk of Colorectal Cancer Precursors. Gastroenterology 2021;161:128-142.e20. 10.1053/j.gastro.2021.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Malik VS, Li Y, Pan A, et al. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019;139:2113-25. 10.1161/CIRCULATIONAHA.118.037401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gao M, Jebb SA, Aveyard P, et al. Associations Between Dietary Patterns and Incident Type 2 Diabetes: Prospective Cohort Study of 120,343 UK Biobank Participants. Diabetes Care 2022;45:1315-25. 10.2337/dc21-2258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
The list of all meta-analyses not selected for data extraction and reanalysis is available if needed.