Abstract
Introduction and importance
Colorectal cancer is a leading cause of cancer-related deaths worldwide. It is estimated that approximately 1.93 million new cases of colorectal cancer were diagnosed and almost one million global colorectal cancer-caused deaths in 2020. The incidence of colorectal cancer has been dramatically rising at alarming rates worldwide in the last decades. The most often sites of metastases are lymph nodes, liver, lung, and peritoneum.
Case presentation
We present a rare case of a 63-year-old male patient presenting with a nodule in the penis after being treated for cancer in the hepatic flexure of the colon. Biopsy showed colorectal cancer recurrence in the penis.
Clinical discussion
Metastasis from colorectal cancer to the penis is rare and poorly discussed, with scarce data in the literature.
Conclusion
A high level of suspicion should be adopted for the correct diagnosis and early treatment.
Keywords: Neoplasm metastasis, Penis, Penile neoplasm, Colonic neoplasm
Highlights
-
•
Colorectal cancer is a leading cause of cancer-related deaths worldwide.
-
•
Metastasis from colorectal cancer to the penis is rare and poorly discussed.
-
•
A high level of suspicion should be adopted for the correct diagnosis.
1. Introduction
Colorectal cancer is a leading cause of cancer-related deaths worldwide [1]. It is estimated that approximately 1.93 million new cases of colorectal cancer were diagnosed and almost one million global colorectal cancer-caused deaths in 2020 [1]. The incidence of colorectal cancer has been dramatically rising at alarming rates worldwide in the last decades. Currently, it is the third leading cause of cancer-related mortality in men and women [2].
Oncologic staging is one of the major driving issues regarding long-term survival [3]. The treatment modalities choices depend on a large scale on the pretreatment clinical staging, comprising chemotherapy, radiotherapy, immunotherapy, and surgical or endoscopic resection [4]. Consequently, great efforts and progresses have been made to better understand the colorectal cancer pathways for dissemination.
The most common sites for colorectal cancer metastasis are the liver, lung, peritoneum, and bones [5]. Other less common areas include the ovaries, lymph nodes, and other organs such as the pancreas, brain, and skin [5]. The spread of colorectal cancer to these distant sites occurs through the bloodstream or lymphatic system [5]. The likelihood of metastasis can be influenced by several factors, including the size, grade of cellular differentiation, primary tumor stage, the presence of specific genetic mutations, and the patient's overall health [6].
Penile metastasis rarely occurs, and there are few reports in the literature [7], [8], [9]. Consequently, the investigational and management of these cases are poorly debated. We present a case of penile metastasis, a rare site of recurrence in colorectal cancer. The work has been reported in line with the SCARE criteria [10].
2. Presentation of case
A 63-year-old male patient, white, complained of 15 kg weight loss and asthenia over eight months. The patient had no significant history and no family history of cancer. The patient was not circumcised.
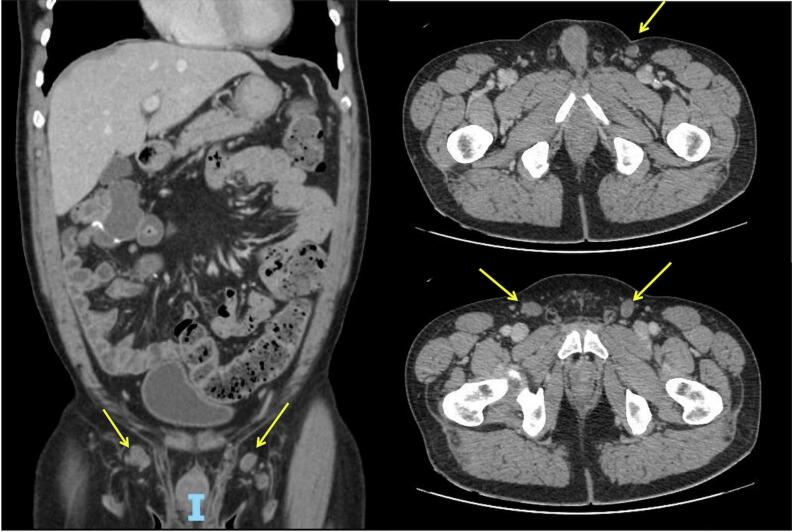
A colonoscopy revealed a mass in the hepatic flexure of the colon. The biopsy showed a colorectal adenocarcinoma with a moderate grade of differentiation. Computed tomography (CT) showed large irregular parietal thickening in the right colon, with a hypoattenuating component (mucinous or necrotic component). There were prominent ileocecal lymph nodes measuring up to 2.2 × 1.3 cm (see Fig. 1). No distant metastases were found in the staging exams. Carcinoembryonic antigen serum (CEA) level was at 2.22 μg/L.
Fig. 1.
Computed tomography showed a massive neoplasm in the hepatic flexure of the colon and prominent ileocecal lymph nodes.
The patient was then submitted to a laparoscopic right colectomy with ileocolic anastomosis. Surgical specimen analysis showed an anatomopathological tubular adenocarcinoma with mucinous differentiation and negative margins. There were metastases in 6 out of 33 resected lymph nodes (pT3pN2a, 8th Edition of the UICC TNM). The postoperative course was uneventful, and the patient was discharged on the third postoperative day.
Adjuvant chemotherapy was proposed initially with XELOX. After 2cycles, the chemotherapy regimen was changed due to gastrointestinal toxicity. Six cycles of FOLFOX were then completed.
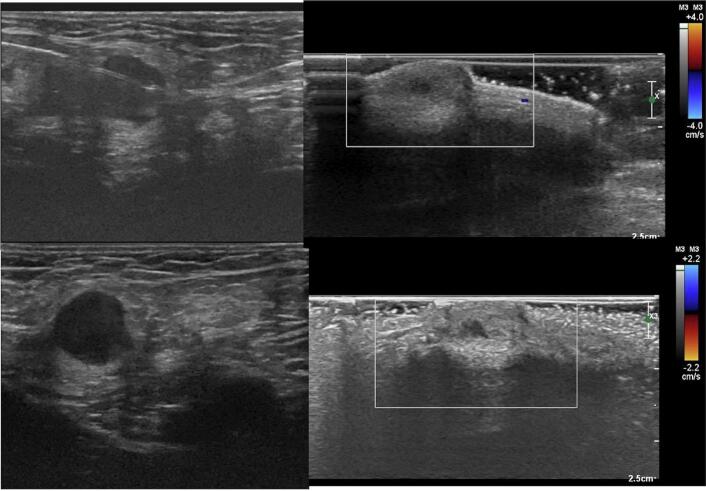
The patient was followed every three months with clinical examination, CEA, and CT scans to monitor for disease recurrence. After ten months of follow-up, the patient returned to the clinic complaining of a lump in the penile body and bilateral inguinal pain. Penile ultrasonography showed a small cystic formation, with slightly thickened walls, without flow on the color study, located in the subcutaneous tissue, measuring 0.7 × 0.5 cm (see Fig. 2). Computed tomography and magnetic resonance imaging (MRI) showed enlarged and heterogeneous inguinal lymph nodes (see Fig. 3, Fig. 4). MRI could not detect any penile tumor.
Fig. 2.
Penile ultrasonography showed a small thick cystic formation, with slightly thickened walls, without flow on the color study, located in the subcutaneous tissue.
Fig. 3.
Computed tomography showed enlarged and heterogeneous bilateral inguinal lymph nodes.
Fig. 4.
Magnetic resonance imaging showed enlarged and heterogeneous bilateral inguinal lymph nodes. No penile tumor could be detected with this imaging method.
Excisional biopsy of the penile protuberance showed infiltration of fibrous connective tissue by epithelial neoplasia with an acinar pattern and extensive mucinous differentiation, presence of mucin lakes, areas of desmoplasic reaction, and intermingled chronic inflammatory infiltrate. The margins of resection were free (see Fig. 5). The immunohistochemical panel was suggestive of colorectal cancer metastasis, with Cytokeratin 20, CDX-2, and SATB2 positive (see Supplementary File 1). A multidisciplinary team gathered and opted for surgery. The patient underwent bilateral inguinal lymphadenectomy (retroperitoneal and iliac lymph nodes were not included), performed 15 months after primary colon surgery, with no complications. The surgical specimen showed cancer cells with mucin lakes in lymph nodes (3 metastasis out of 9 lymph nodes on the left and one metastasis out of 13 lymph nodes on the right). The immunohistochemical panel matched the one of the penile tumor.
Fig. 5.
Penile tumor histologic examination showed infiltration of the fibrous connective tissue by epithelial neoplasia with an acinar pattern, extensive mucinous differentiation, mucin lakes, areas of desmoplasic reaction, and intermingled chronic inflammatory infiltrate. Negative margins of resection.
Supplementary File 1.

The penile tumor immunohistochemistry study suggested colorectal cancer metastasis, with Cytokeratin 20, CDX-2, and SATB2 positive.
After surgery, the patient was proposed to FOLFOXIRI, considering the previous good tolerance to the FOLFOX regimen, the bad tolerance with capecitabine, and the institutional availability of chemotherapy and access to anti-cancer drugs. The patient was under monitoring for recurrence every three months with clinical examination, CEA, and CT scans in the first two years and then every six months. He is currently being followed with no evidence of recurrence and no symptoms after four years of follow-up.
3. Discussion
Penile metastasis from colorectal cancer is quite rare. The colon lymph runs through the mesenteric system, and the venous flow runs through the portomesenteric axis, completely away from the penile vascular and lymphatic systems [11], [12]. Consequently, colon cancer cells migration to penile tissues is extremely unlikely.
The form of dissemination to the penis is still unknown. Low rectal cancers may have an easier route to the penis than colon cancer. The lower rectum venous system drains to the iliac vein, and a retrograde venous cell migration could explain how cancer cells reach the pudendal veins and the dorsal vein of the penis [13], [14]. In fact, most colorectal cancer with dissemination to the penis previously described was located in the rectum [7]. However, some colon cancers with penis dissemination were also reported. Triki et al. [8] reported a patient with a T4N0 left colic flexure adenocarcinoma that recurred after two years of colon resection. Ruano Mayo et al. [9] described another patient with advanced-stage (T4N0) colorectal cancer located in the right colon cancer, with metastasis to the penis. Analysis of the surgical specimen showed a mucinous pattern neoplasm, as well as our case. Mucinous adenocarcinoma corresponds to 6 % of all colorectal cancers and has particular genetic and pathological profiles [15]. Theoretically, the mucus produced by the neoplasm could facilitate cancer cell dissemination, worsening prognosis and facilitating unusual sites of recurrence [16], [17]. Both previously described cases were locally advanced, similar to our report, which may have contributed to the risk of distant site recurrence. However, it is even more unlikely that a non-rectal colon cancer disseminates through a retrograde iliac route than rectal cancer.
The way colorectal cancer cells, out of the rectum, could reach penile tissues is an enigma. Theoretically, in advanced cancer, micrometastasis could run through systemic circulation and achieve and lodge any organ or tissue, such as subcutaneous and penis. However, this setting would suggest a highly advanced disease, making the disease cure unlikely. There is still no consensus in the literature about the benefit of rescue surgical treatment in these cases, and further studies are needed. The surgery is generally palliative for symptom control.
The current case report was proposed for resectioning inguinal lymph nodes both by symptom control (inguinal pain) and for curative purposes. The potential benefit of resection is highly uncertain since there is a high chance of circulating micrometastasis that could seed anywhere. However, the patient had a resectable disease and a load of tumors limited to groins. After the surgery, the patient underwent systemic chemotherapy for micrometastasis control. Fortunately, the curative strategy seems to have worked since the patient has been followed for four years, and currently, there is no sign of recurrence and good symptom control. It is important to note that a multidisciplinary team conducted all the treatment strategies, and all the possible outcomes were extensively discussed with the patient and family, avoiding frustrations in case of any new recurrence.
This case showed how necessary is anamnesis and careful clinical examination in oncologic patients. In ordinary colorectal cancer cases, restages tests do not cover the whole body, and unusual recurrence sites, such as the penis, may be little or not noticeable on these tests. A high clinical suspicion is imperative, mainly for those patients with advanced primary tumors and the mucinous subtype. Otherwise, the diagnosis would be postponed until more advanced cancer dissemination. In the current report, the high suspicion for recurrence had to be even higher since the patient did not express significant serum carcinoembryonic antigen, which could help detect recurrence.
The inguinal pain with a penile lump raised suspicion for recurrence in the current case report. However, theoretically, penile cancer dissemination could manifest as any penile neoplasm, such as discharge, ulceration, urinary retention, and priapism, due to venous outlet flow obstruction [18]. Previous reports on colorectal cancer metastasis to the penis manifested nodules, dysuria, ulcers, urethral fistula, priapism, and urination difficulties [18]. Most of these previous reports presented multiple systemic metastases alongside the penile tumor and, consequently, showed a short survival time, from weeks to a couple of months. Most previously reported cases were treated with supportive therapy, chemotherapy, or radiotherapy. Some authors proposed resection, but long-term survival was seen in a minority [19].
As said before, high clinical suspicion, good anamnesis, and a physical exam are the first steps to detecting penile metastasis properly. Ultrasonography may help to delimitate the tumor and guide biopsy. However, a histological examination cannot be dismissed on clinical suspicion. The ultrasonography in the current report showed no red signs of malignancy. Immunohistochemistry is essential to help to distinguish from mesenchymal neoplasms and to determine the site origin of the penile metastasis. In general, the lower gastrointestinal tract mucinous adenocarcinoma express CK20, whereas non-gastrointestinal mucinous adenocarcinoma express CK7 [20]. CDX-2 and SATB2 are other markers of colorectal cancer that helped to identify the site origin of penile cancer in the current case report [20].
Other diagnostic tests may also be used, but with limited application. Magnetic resonance may help to investigate, but small-sized growths may be easily dismissed between image slices. Future studies may show the benefit of using artificial intelligence to help identify patients' predictors for rare dissemination sites.
4. Conclusion
A high level of suspicion for unusual site recurrence should be adopted in colorectal cancer, mainly for those patients with advanced primary tumors and a mucinous subtype. A multidisciplinary team should conduct treatment and share the decisions with the patient and family.
The following is the supplementary data related to this article.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
The study is exempt from ethnical approval in our institution.
Sources of funding
None.
Author contribution
Study concept or design: Francisco Tustumi.
Data collection: Lucas Soares Gerbasi, Rafael Vaz Pandini.
Data interpretation: Marleny Novaes Figueiredo de Araujo.
Writing the paper: Victor Edmond Seid, Sérgio Eduardo Alonso Araujo.
Guarantor
Francisco Tustumi.
Research registration number
Not applicable.
Declaration of competing interest
None.
References
- 1.Xi Y., Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021;14(10) doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haggar FA, Boushey RP (n.d.). Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clinics in colon and rectal surgery. [DOI] [PMC free article] [PubMed]
- 3.Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., Ogunbiyi O.J. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stintzing S. Management of colorectal cancer. F1000prime Rep. 2014:6. doi: 10.12703/P6-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakih M.G. Metastatic colorectal cancer: current state and future directions. J. Clin. Oncol. 2015;33(16):1809–1824. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 6.Deans G.T., Parks T.G., Rowlands B.J., Spence R.A. Prognostic factors in colorectal cancer. Br. J. Surg. 1992;79(7):608–613. doi: 10.1002/bjs.1800790706. [DOI] [PubMed] [Google Scholar]
- 7.Yildirim M., Coskun A., Pürten M., Oztekin O., Ilhan E. A clinical case of the penile metastasis from the rectal carcinoma. Radiol. Oncol. 2010;44(2):121–123. doi: 10.2478/v10019-010-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triki W., Kacem A., Itami A., Baraket O., Rebai M.H., Bouchoucha S. Penile metastasis of colon carcinoma: a rare case report. Urol. Case Rep. 2019;1(24) doi: 10.1016/j.eucr.2019.100875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruano Mayo A., Bedate Nunez M., Conde Redondo C., Lara Pérez F.M., Panadero Meseguer P., Calleja Escudero J. Priapism produced by penile metastasis from colon cancer. Arch. Esp. Urol. (Ed. Impr.) 2022:572–575. doi: 10.56434/j.arch.esp.urol.20227506.84. [DOI] [PubMed] [Google Scholar]
- 10.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus Surgical Case Report (SCARE) guidelines. International Journal of Surgery. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Sakorafas G.H., Zouros E., Peros G. Applied vascular anatomy of the colon and rectum: clinical implications for the surgical oncologist. Surg. Oncol. 2006;15(4):243–255. doi: 10.1016/j.suronc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Dwyer M.E., Salgado C.J., Lightner D.J. Seminars in Plastic Surgery. Vol. 25. © Thieme Medical Publishers; 2011. Normal penile, scrotal, and perineal anatomy with reconstructive considerations; pp. 179–188. No. 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nano M., Marchisio F., Ferronato M., Breatta A.D., Solej M., Barbero S., Dei Poli M., Gandini G. Vascular anatomy of the rectal stump after total mesorectal excision. Dis. Colon Rectum. 2006;49:1897–1904. doi: 10.1007/s10350-006-0734-8. [DOI] [PubMed] [Google Scholar]
- 14.Kiyomatsu T., Ishihara S., Murono K., Otani K., Yasuda K., Nishikawa T., Tanaka T., Hata K., Kawai K., Nozawa H., Yamaguchi H. Anatomy of the middle rectal artery: a review of the historical literature. Surg. Today. 2017;47:14–19. doi: 10.1007/s00595-016-1359-8. [DOI] [PubMed] [Google Scholar]
- 15.Kruse J., Von Bernstorff W., Evert K., Albers N., Hadlich S., Hagemann S., Günther C., Van Rooijen N., Heidecke C.D., Partecke L.I. Macrophages promote tumour growth and liver metastasis in an orthotopic syngeneic mouse model of colon cancer. Int. J. Color. Dis. 2013;28:1337–1349. doi: 10.1007/s00384-013-1703-z. [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker P.H. Mucinous colorectal carcinoma. J. Surg. Oncol. 2001;77(4):282–283. doi: 10.1002/jso.1111. [DOI] [PubMed] [Google Scholar]
- 17.Numata M., Shiozawa M., Watanabe T., Tamagawa H., Yamamoto N., Morinaga S., Watanabe K., Godai T., Oshima T., Fujii S., Kunisaki C. The clinicopathological features of colorectal mucinous adenocarcinoma and a therapeutic strategy for the disease. World J. Surg Oncol. 2012;10(1):1–8. doi: 10.1186/1477-7819-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattell R.B., Mace A.J. Metastasis to the penis from carcinoma of the rectum. J. Am. Med. Assoc. 1951;146(13):1230–1231. doi: 10.1001/jama.1951.63670130010012e. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y., Shida D., Nasu K., Matsunaga H., Warabi M., Inoue S. Metachronous penile metastasis from rectal cancer after total pelvic exenteration. World J. Gastroenterol. 2012;18(38):5476. doi: 10.3748/wjg.v18.i38.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu P.G., Chung L., Weiss L.M., Lau S.K. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am. J. Surg. Pathol. 2011;35(12):1830–1836. doi: 10.1097/PAS.0b013e3182299c25. [DOI] [PubMed] [Google Scholar]