Abstract
Kidney disease (KD) is a life-threatening disease characterized by high morbidity and mortality in clinical settings, which can be caused by many reasons, and the incidence increases with age. However, supportive therapy and kidney transplantation still have limitations in alleviating KD progression. Recently, mesenchymal stem cells (MSCs) have shown great potential in repairing injury through their multidirectional differentiation and self-renewal ability. Of note, MSCs serve as a safe and effective therapeutic strategy for treating KD in preclinical and clinical trials. Functionally, MSCs ameliorate KD progression by regulating the immune response, renal tubular cell apoptosis, tubular epithelial–mesenchymal transition, oxidative stress, angiogenesis, and so on. In addition, MSCs exhibit remarkable efficacy in both acute kidney injury (AKI) and chronic kidney disease (CKD) through paracrine mechanisms. In this review, we outline the biological characteristics of MSCs, discuss the efficacy and mechanisms of MSCs-based therapy for KD, summarize the completed and ongoing clinical trials, as well as analyze limitations and new strategies, aiming to provide new ideas and approaches for the preclinical experiments and clinical trials of MSCs transplantation for KD.
Keywords: kidney diseases, mesenchymal stem cell, regeneration, paracrine
Introduction
Kidney disease (KD) is a global public health problem that affects more than 750 million of the global population and causes 5 to 10 million deaths each year1,2, with the main common diseases being acute kidney injury (AKI) and chronic kidney disease (CKD). Previous studies have shown that the development and progression of KD were associated with obesity 3 , diabetes 4 , hepatitis B virus infection 5 , and so on. Meanwhile, KD progression is a risk factor for cardiovascular diseases 6 . In addition, clinical studies have found that the high incidence and poor prognosis of KD were relevant to clinical care and huge economic costs 7 , with approximately $10 billion spent annually on treating AKI 8 and more than $80 billion spent on caring for CKD without kidney replacement therapy 9 in the United States. Currently, the common therapeutic methods for KD include drugs, hemodialysis and peritoneal dialysis, and renal transplantation 10 . However, the expected efficacy was still not achieved due to the irreversibility of kidney injury, the toxic effects of drug therapy, the inconvenience of dialysis, and the shortage and high cost of kidney transplant donors 11 . Therefore, there is an urgent need to explore new therapeutic strategies to slow down the progression of KD.
In recent years, stem cells have been used as a new regenerative therapy for a variety of diseases, including KD 12 . Mesenchymal stem cells (MSCs), as one of the important members of the stem cell family, can be obtained from a variety of tissues such as bone marrow, adipose, umbilical cord, and peripheral blood and have powerful biological properties of immunomodulation, anti-inflammation, and tissue repair 13 . Preclinical and clinical trials have shown that MSCs possess reparative and protective effects on kidney injury14,15. Functionally, MSCs exert anti-apoptotic, antioxidant, anti-inflammatory, anti-fibrotic, and immunomodulatory activities through secreting trophic factors and delivering extracellular vesicles (EVs)16,17. Overall, MSCs are considered to be the most promising stem cell population for the treatment of KD.
In this review, we will focus on the current research advances and challenges in the use of MSCs for the treatment of KD. Preliminarily, this review provides an overview of the origin and biological properties of MSCs. Subsequently, we summarize the underlying mechanisms and clinical translational applications of MSCs in the treatment of KD. Finally, we analyzed the obstacles encountered in the use of MSCs for the treatment of KD and proposed corresponding strategies to cope with the limitations of MSCs in KD. Of note, this review aims to provide new ideas and directions for the treatment of KD with MSCs in preclinical experiments and clinical trials.
Biological Characteristics of MSCs
Mesenchymal stem cells are a class of adult stem cells with self-renewal and differentiation potential 18 . They have become a new means of treating KD because of their multidirectional differentiation potential, high proliferative capacity, immune regulation, and self-replication 19 . They can be obtained from a variety of tissues, including bone marrow, adipose tissue, umbilical cord, placenta, amniotic fluid, and dental pulp 20 (Fig. 1). Although MSCs are derived from different sources [eg, bone marrow mesenchymal stem cells (BMMSCs), adipose-derived mesenchymal stem cells (ADMSCs), and umbilical cord mesenchymal stem cells (UCMSCs)], they have similar differentiation and biological functions 21 . Numerous studies have confirmed that MSCs can differentiate a variety of cells (eg, osteoblasts, myoblasts, cardiomyocytes, and renal parenchymal cells) with different functional characteristics under different induction conditions 22 . Previous studies have shown that MSCs regulated immune activity and enhanced the expansion and differentiation potential of host cells through direct cell–cell contact or paracrine mechanisms, thus promoting the recovery of injured tissues 23 . Importantly, preclinical experiments and clinical trials have confirmed that treatment with MSCs significantly improved the progression of KD24,25. Functionally, the main regulatory roles of MSCs are as follows: (1) The recruited MSCs differentiate into functional cells to replace damaged cells, and (2) as a response to inflammatory cytokines, MSCs produce large amounts of cytokines, chemokines, growth factors, and exosomes, which stimulate angiogenesis, prevent cell apoptosis and epithelial–mesenchymal transformation (EMT) process, block oxidative stress, promote extracellular matrix (ECM) remodeling, and induce differentiation of tissue stem cells 26 . In recent years, several studies have proved that MSCs-secreted exosomes (MSCs-Exo) via paracrine mechanisms not only have the same effects as MSCs but also have the advantages of low transplantation risk, easy storage management, high controllability, low immunogenicity, high safety, high repairability, and so on 27 . The above studies suggest that MSCs exhibit great therapeutic effects on injury repair and immune-characterized diseases, and serve as shining stars of stem cells in the field of cell therapy and regenerative medicine.
Figure 1.
Sources and application of MSCs. MSCs: mesenchymal stem cells.
Efficacy and Mechanisms of MSCs Therapy in KD
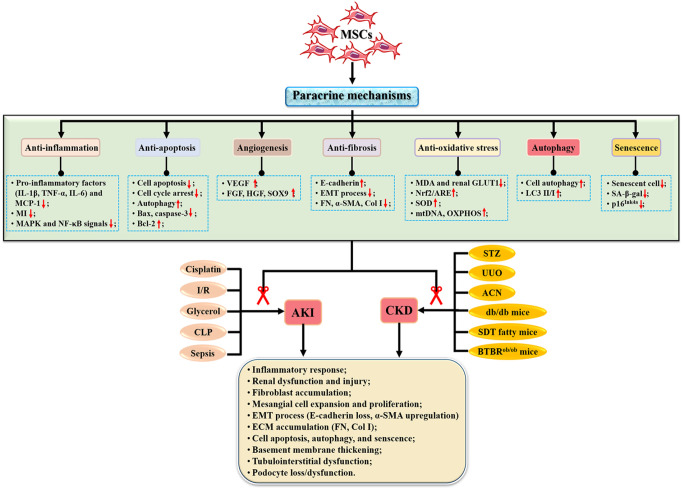
Currently, MSCs are considered as new therapeutic tools for the treatment of KD because of their multidirectional differentiation, migration and homing, and paracrine effects17,28. Previous studies have shown that MSCs are effective and safe when used to treat organ injury29,30. Importantly, MSCs therapy promoted the recovery of renal function after renal pathogenesis through various mechanisms 31 (Fig. 2) such as anti-inflammation, anti-apoptosis, angiogenesis, anti-oxidative stress, anti-fibrosis, regulating autophagy, and senescence (Table 1).
Figure 2.
Mechanisms of MSCs in the treatment of kidney diseases. ACN: aristolochic acid nephropathy; AKI: acute kidney injury; BTBRob/ob: Black and Tan Brachyury (BTBR) leptin deficiency; CKD: chronic kidney disease; CLP: cecal ligation and puncture; Col I: type I collagen; ECM: extracellular matrix; EMT: epithelial–mesenchymal transformation; FGF: fibroblast growth factor; FN: fibronectin; GLUT1: glucose transporter type 1; I/R: ischemia–reperfusion; HGF: hepatocyte growth factor; IL-1β: interleukin-1β; MAPK: mitogen-activated protein kinase; MDA: malondialdehyde; MI: macrophage infiltration; mtDNA: mitochondrial DNA; NF-κB: nuclear factor–kappa B; Nrf2/ARE: nuclear factor erythronid 2-related factor 2/antioxidant response elements; OXPHOS: oxidative phosphorylation; SDT: Spontaneously Diabetic Torii; α-SMA: alpha-smooth muscle actin; SOD: superoxide dismutase; SOX9: sex-determining region Y-box 9; STZ: streptozotocin; TNF-α: tumor necrosis factor–alpha; UUO: unilateral ureteral obstruction; VEGF: vascular endothelial growth factor.
Table 1.
Mechanisms of MSCs and Extracellular Vesicles in the Treatment of KD According to Published Studies From 2017 to 2022.
| No. | Type of source | Model & doses | Treatment effect | Mechanism | Ref. |
|---|---|---|---|---|---|
| AKI | |||||
| 1 | BMMSCs | ♦ In vitro: NRK-52E cells subjected to
H/R ♦ Dose: co-culture (BMMSCs:NRK-52E) ratio 1:1 ♦ In vivo: I/R-induced AKI mice model ♦ Dose: 5 × 105 BMMSCs |
✓ Renal macrophage infiltration and inflammation, and tubular
apoptosis ↓; ✓ Tubular proliferation ↑; ✓ Superoxide formation, DNA damage, and lipid peroxidation ↓; ✓ Increased antioxidant expression ↑; ✓ Expression of IL-1β, Bax, and caspase 3 ↓; ✓ Expression of autophagy-related LC3B, Atg5 and Beclin 1 ↑; |
Anti-oxidative stress Anti-apoptosis |
Tseng et al. 32 |
| 2 | BMMSCs | ♦ In vitro: HK-2 cells treated with
LPS ♦ Dose: 1 × 104 BMMSCs ♦ In vivo: sepsis-induced AKI rat model ♦ Dose: 1 × 106 BMMSC |
✓ Tubular injury score ↓; ✓ Levels of serum creatinine and nitrogen ↓; ✓ Levels of TNF-α, IL-6, and IL-1β ↓; ✓ Mitophagy in RTECs of kidney tissues and HK-2 cells ↑; ✓ Cell apoptosis and pyroptosis ↓; ✓ Expression of NLRP3, ASC, Caspase-1 ↓; ✓ SITR1/Parkin pathway ↑; |
Anti-apoptosis | Guo et al. 33 |
| 3 | BMMSCs | ♦ In vivo: I/R-induced AKI rat
model ♦ Dose: 5 × 105 BMMSCs |
✓ Expression of α-SMA, collagen I/III ↓; ✓ Interstitial fibrosis and infiltration of inflammatory cells ↓; ✓ Levels of VEGF, HGF, and PGE2 ↑; |
Anti-fibrosis | Ishiuchi et al. 34 |
| 4 | BMMSCs | ♦ In vivo: I/R-induced AKI ♦ Dose: 1 × 106 BMMSCs |
✓ Levels of serum creatinine and nitrogen ↓; ✓ Levels of TNF-α, IL-1β, and IL-6 ↓; ✓ Cell apoptosis ↓; |
Anti-inflammation Anti-apoptosis |
Wang et al. 35 |
| 5 | BMMSCs | ♦ In vivo: I/R-induced AKI ♦ Dose: 1 × 106 BMMSCs |
✓ Macrophages infiltration and pro-inflammatory cytokines (TNF-α
and IL-1β) ↓; ✓ C5a and C5aR expression ↓; ✓ NF-κB pathway ↓; |
Anti-inflammation | Tang et al. 36 |
| 6 | BMMSCs | ♦ In vivo: adriamycin-induced
AKI ♦ Dose: 5 × 106 BMMSCs |
✓ Tubular fibrosis, serum creatinine, and nitrogen
↓; ✓ Profibrotic PECs ↓; |
Anti-fibrosis | Aslam et al. 37 |
| 7 | ADMSCs | ♦ In vivo: gentamicin-induced
AKI ♦ Dose: 1 × 106 ADMSCs |
✓ Levels of serum creatinine and nitrogen ↓; ✓ Expression of Grp78, Atf6, Ire1, Perk, Chop, Caspase12, and Xbp1 ↓; ✓ ER stress ↓; |
Anti-ER stress | He et al. 38 |
| 8 | ADMSCs | ♦ In vivo: I/R-induced AKI ♦ Dose: 2 × 106 ADMSCs |
✓ Number of apoptotic cells ↓; ✓ Levels of total urinary protein and serum creatinine, pro-inflammatory cytokines (eg, IL-6, TNF-α, IL-1β, IFN-γ, IFN-γ, and TGF-β), and the inflammation-associated proteins (eg, HGF and SDF1) ↓; ✓ Expression of the anti-inflammatory cytokine (IL-10) and Bcl-2 ↑; |
Anti-inflammation Anti-apoptosis |
Zhang et al. 39 |
| 9 | ADMSCs | ♦ In vivo: cisplatin-induced AKI ♦ Dose: 2.5 × 107 ADMSCs |
✓ Necrosis or epithelial cells damage ↓; ✓ Levels of serum creatinine and nitrogen ↓; ✓ Expression of TNF-α and TGF-β1 ↓; |
Anti-inflammation Anti-apoptosis |
Begum et al. 40 |
| 10 | ADMSCs-Exo | ♦ In vivo: sepsis-induced AKI ♦ Dose: 100 μg ADMSCs-Exo |
✓ Levels of BUN and Scr ↓; ✓ Levels of MCP-1, IL-6, TNF-α ↓; ✓ NF-κB pathway ↓; |
Anti-inflammation | Gao et al. 41 |
| 11 | ADMSCs-Exo | ♦ In vivo: sepsis-induced AKI ♦ Dose: 2 mg/kg body weight ADMSCs-Exo |
✓ Levels of AST, ALT, BUN ↓; ✓ Levels of IL-6, IL-1β, TNF-α, MCP-1 ↓; ✓ circ_0001295 expression ↑; |
Anti-inflammation | Cao et al. 42 |
| 12 | ADMSCs-Exo BMMSCs-Exo |
♦ In vivo: LPS-induced AKI ♦ Dose: 1 × 105 and 5 × 105 BMMSCs-Exo |
✓ Renal function ↑; ✓ Oxidative stress and inflammation ↓; |
Anti-oxidative stress Anti-inflammation |
Zhang et al. 43 |
| 13 | BMMSCs-Exo | ♦ In vitro: H/R-induced HK-2
cells ♦ Dose: unknown ♦ In vivo: IRI mice model ♦ Dose: 5 × 1010 BMMSCs-Exo |
✓ Cell apoptosis ↓; ✓ Expression of cleaved caspase-3 and Bax ↓; ✓ miR-199a-3p expression ↑; ✓ AKT and ERK pathway ↑; |
Anti-apoptosis | Zhu et al. 44 |
| 14 | BMMSCs-Exo | ♦ In vivo: IRI mice model ♦ Dose: 100 μg/mouse BMMSCs-Exo |
✓ Levels of BUN and Scr ↓; ✓ Renal tubular cell apoptosis ↓; ✓ Renal fibrosis ↓; ✓ M1 macrophages infiltration and levels of IL-1β, IL-6 and TNF-α ↓; ✓ M1 macrophage to M2 macrophage ↑; |
Anti-inflammation | Xie et al. 45 |
| 15 | UCMSCs-Exo | ♦ In vivo: I/R-induced AKI ♦ Dose: 50 and 100 μg UCMSCs-Exo |
✓ Renal tubules injury ↓; ✓ Cell cycle arrest and apoptosis of TECs ↓; ✓ miR-125b-5p expression ↑; |
Anti-apoptosis | Cao et al. 46 |
| 16 | UCMSCs-Exo | ♦ In vivo: I/R-induced AKI ♦ Dose: 4 × 108 UCMSCs-Exo |
✓ Apoptosis and necroptosis ↓; ✓ Pro-inflammatory cytokines/chemokines and infiltration of macrophages ↓; ✓ NF-κB pathway ↓; |
Anti-apoptosis Anti-inflammation |
Huang et al. 47 |
| 17 | UCMSCs-Exo | ♦ In vivo: I/R-induced AKI ♦ Dose: unknown |
✓ Pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and
oxidative stress (malondialdehyde) ↓; ✓ Levels of BUN, Scr, urinary albumin and CR, 8-isoprostane ↓; ✓ IL-10 level ↑; |
Anti-inflammation | Zhang et al. 48 |
| 18 | UCMSCs-Exo | ♦ In vivo: sepsis-induced AKI ♦ Dose: 120 μg UCMSCs-Exo |
✓ Levels of BUN and Scr ↓; ✓ Level of cleaved caspase-3 protein ↓; ✓ Levels of IL-1β and TNF-α ↓; ✓ NF-κB pathway ↓; MiR-146b ↑; |
Anti-inflammation | Zhang et al. 49 |
| 19 | UCMSCs-Exo | ♦ In vitro: cisplatin-induced NRK-52E
cells ♦ Dose: 200 μg/mL UCMSCs-Exo ♦ In vivo: cisplatin-induced AKI ♦ Dose: 200 μg UCMSCs-Exo |
✓ Cell proliferation ↑; ✓ Levels of Scr and BUN ↓; ✓ The protein levels of caspase-3 and Bax ↓; ✓ Expression of LC3B, ATG5 and ATG7 ↑; ✓ Levels of TNF-α, IL1-β, and IL6 ↓; |
Autophagy Anti-apoptosis |
Wang et al. 50 |
| 20 | MSCs-Exo | ♦ In vivo: I/R-induced AKI ♦ Dose: 1.5 × 105 MSCs-Exo |
✓ Levels of IL-6, TNF-α, IFN-γ ↓; ✓ Levels of caspase-9, cleaved caspase-3, and Bax ↓; |
Anti-inflammation Anti-apoptosis |
Li et al. 51 |
| CKD | |||||
| 1 | UCMSCs | ♦ In vitro: HK2 and NRK-52E cells treated with
high glucose ♦ Dose: 25, 50, and 100 μg/mL UCMSCs ♦ In vivo: STZ-induced DN ♦ Dose: 2 × 106/500 μL UCMSCs |
✓ Serum urea nitrogen and CR ↓; ✓ The 24-hour urinary protein and urinary albumin/CR ratio ↓; ✓ Kidney weight/kidney weight index ↓; ✓ Levels of IL-6, IL-1β, TNF-α, and TGF-β ↓; ✓ Expression of EGF, FGF, HGF, and VEGF ↑; |
Anti-inflammation Anti-fibrosis |
Xiang et al. 52 |
| 2 | UCMSCs | ♦ In vitro: HK2 cells treated with high glucose
and rhTNF-α ♦ Dose: co-culture at a 5:1 ratio (HK2: UCMSCs) ♦ In vivo: STZ-induced rhesus macaque model of DN ♦ Dose: 2 × 106 UCMSCs |
✓ Blood glucose level and daily insulin requirement
↓; ✓ Expression of FN, SGLT2, IL-1β, TNF-α ↓; ✓ Interstitial fibrosis ↓; ✓ NF-κB pathway ↓; |
Anti-inflammation Anti-fibrosis |
An et al. 53 |
| 3 | UCMSCs | ♦ In vitro: HK2 cells treated with
LPS ♦ Dose: RAW264.7 plus MSCs at a ratio of 2:1 (MSCs: RAW264.7 cells) ♦ In vivo: STZ-induced mice model of DN ♦ Dose: 5 × 105 UCMSCs |
✓ Plasma CR and BUN ↓; ✓ Levels of desmin, α-SMA, FN1, Kim-1, and Lcn2 ↓; ✓ Expression of arginase-1 ↑; ✓ Expression of IL-1β, TNF-α, IL-6 ↓; |
Anti-inflammation | Lee et al. 54 |
| 4 | UCMSCs | ♦ In vivo: STZ-induced DN rat
model ♦ Dose: 2 × 106 UCMSCs |
✓ 24-hour urinary total protein, urinary albumin to CR ratio,
Scr, and blood urea nitrogen ↓; ✓ Renal cell apoptosis ↓; ✓ Apoptosis signal-regulating kinase 1 and P38 MAPK ↑; |
Anti-apoptosis | Chen et al. 55 |
| 5 | ADMSCs | ♦ In vivo: SDT fatty rat ♦ Dose: 6.0 × 106 cells/mL ADMSCs |
✓ Kidney engraftment ↑; ✓ Glomerular injury ↓; ✓ Urinary levels of TNF-α and IL-6 ↓; |
Anti-inflammation 31622047 |
Takemura et al. 56 |
| 6 | BMMSCs | ♦ In vitro: LPS-induced peritoneal
macrophages ♦ Dose: 3 × 104 BMMSCs ♦ In vivo: STZ-induced rat model of DN ♦ Dose: 5 × 106 BMMSCs |
✓ Renal macrophage infiltration and inflammatory cytokine
secretion ↓; ✓ Serum anti-inflammatory cytokines IL-10 and EGF ↑; ✓ Levels of IL-6, MCP-1, TNF-α, and IL-1β ↓; |
Anti-inflammation | Li et al. 57 |
| 7 | BMMSCS | ♦ In vitro: HG-induced glomerular mesangial
cells ♦ Dose: co-culture at a 1:5 ratio (BMMSCs: glomerular mesangial cells) ♦ In vivo: BTBRob/ob mice ♦ Dose: 1 × 106 BMMSCs |
✓ Mitochondrial ROS accumulation ↓; ✓ Cell apoptosis ↓; ✓ Mesangial expansion ↓; ✓ Renal cleaved caspase-3 ↓; |
Anti-oxidative Anti-apoptosis 33557007 |
Sávio-Silva et al. 58 |
| 8 | BMMSCs | ♦ In vitro: LPS-induced peritoneal
macrophages ♦ Dose: 3 × 104 BMMSCs ♦ In vivo: STZ-induced rat model of DN ♦ Dose: 5 × 105 BMMSCs |
✓ Expression of FN, α-SMA, Bax ↓; ✓ Lysosome-autophagy, M2 polarization, IL-10 and TFEB expression ↑; ✓ Levels of MCP-1, IL-1β, and TNF-α ↓; ✓ AMPK pathway ↑; |
Anti-inflammation | Yuan et al. 59 |
| 9 | BMMSCs | ♦ In vitro: rat glomerular mesangial cells
treated with high glucose ♦ Dose: 400,000 cells/well BMMSCs ♦ In vivo: STZ-induced rat model of DN ♦ Dose: 5 × 106 BMMSCs |
✓ Lipoxin A4 expression ↑; ✓ Renal fibrosis ↓; ✓ Levels of TNF-α, IL-6, IL-8, and IFN-γ ↓; ✓ TGF-β/Smad pathway ↓; |
Anti-inflammation | Bai et al. 60 |
| 10 | BMMSCs | ♦ In vivo: STZ-induced DN rat
model ♦ Dose: 100 μg BMMSCs |
✓ BUN and Scr, blood lipid–related indicators of total
cholesterol and triglyceride ↓; ✓ Cell apoptosis ↓; ✓ Expression of USP22, caspase-3, and Bax ↓; ✓ miR-let-7a ↑; |
Anti-apoptosis | Mao et al. 61 |
| 11 | BMMSCs | ♦ In vivo: UUO mice model ♦ Dose: 2 × 106 BMMSCs |
✓ CD68-positive macrophage, renal tubulointerstitial injury and
fibrosis ↓; ✓ Proliferation of myofibroblasts ↓; |
Anti-inflammation Anti-fibrosis |
Xing et al. 62 |
| 12 | BMMSCs | ♦ In vivo: UUO mice model ♦ Dose: 1 × 106 BMMSCs |
✓ Expression of E-cadherin ↑; ✓ Expression of TGF-β1, α-SMA and TNF-α ↓; |
Anti-fibrosis | Saberi et al. 63 |
| 13 | UCMSCs | ♦ In vivo: STZ-induced DN mice
model ♦ Dose: 1.0 × 104 MuMSCs |
✓ Levels of glomerular volume ↓; ✓ Expression of FN, α-SMA, vimentin ↓; ✓ TGF-β1/Smad2/3 pathway ↓; |
Anti-fibrosis | Li et al. 64 |
| 14 | ADMSCs-Exo | ♦ In vitro: hypoxia/serum deprivation injury
models ♦ Dose: 100 μg/mL ADMSCs-Exo ♦ In vivo: UUO mice model ♦ Dose: 1 × 103 ADMSCs-Exo |
✓ Peritubular capillary rarefaction and renal fibrosis
↓; ✓ Cell migration and angiogenesis ↑; ✓ SIRT1/eNOS signaling pathway ↑; |
Angiogenesis | Chen et al. 65 |
| 15 | ADMSCs-Exo | ♦ In vitro: high glucose–induced MPC5
cells ♦ Dose: 25 μg/mL of ADMSCs-Exo ♦ In vivo: C57BL/KsJ db/db ♦ Dose: unknown |
✓ Levels of Scr, BUN, and podocyte apoptosis ↓; ✓ Cell viability and autophagy flux ↑; ✓ Cell apoptosis and podocyte injury ↓; ✓ miR-486 and mTOR pathway ↑; |
Autophagy Anti-apoptosis |
Jin et al. 66 |
| 16 | ADMSCs-Exo | ♦ In vivo: adenine-containing diet to induce
CKD ♦ Dose: 50 and 100 μg ADMSCs-Exo |
✓ Pro-inflammatory cytokines, BUN, and Scr ↓; ✓ Aquaporin 2 and 5 levels ↑; ✓ Renal fibrosis ↓; |
Anti-fibrosis Anti-inflammation |
Yea et al. 67 |
| 17 | BMMSCs-Exo | ♦ In vitro: TGF-β1-induced HK-2
cells ♦ Dose: 100 μg/mL BMMSCs-Exo |
✓ EMT process ↓; ✓ Cell autophagy ↑; |
Anti-fibrosis | Yin et al. 68 |
| 18 | BMMSCs-Exo | ♦ In vivo: postmenopausal CKD ♦ Dose: 100 μg/mL BMMSCs-Exo |
✓ Body weight, drastic reduction of estrogen and progesterone
levels ↓; ✓ MDA levels and pro-inflammatory cytokines ↓; ✓ GPx SOD, and CAT in kidney tissue ↑; |
Anti-inflammation | Alasmari et al. 69 |
| 19 | BMMSCs-Exo | ♦ In vivo: isogenic/allograft kidney
transplantation mouse model ♦ Dose: 100 μg/mL BMMSCs-Exo |
✓ Treg cell differentiation in kidney transplantation mice
↑; ✓ Inflammatory response, CD4+ T-cell infiltration, SCr, and plasma rejection–related factors’ expression ↓; ✓ lncRNA DANCR expression ↑; |
Anti-inflammation | Wu et al. 70 |
| 20 | BMMSCs-Exo | ♦ In vivo: STZ-induced DN mice
model ♦ Dose: 100 μg BMMSCs-Exo |
✓ Levels of LC3 and Beclin-1 ↑; ✓ Fibrotic marker expression ↓; |
Autophagy | Ebrahim et al. 71 |
| 21 | BMMSCs-Exo | ♦ In vivo: UUO mice model ♦ Dose: 1 mg/kg BMMSCs-Exo ♦ In vitro: TGF-β1-induced NRK-52E cells ♦ Dose: 20 μM BMMSCs-Exo |
✓ ECM deposition and renal fibrosis ↓; ✓ EMT process ↓; ✓ let-7i-5p ↓; ✓ TSC1/mTOR pathway ↑; |
Anti-fibrosis | Jin et al. 72 |
| 22 | BMMSCs-Exo | ♦ In vitro: TGF-β1-induced HK-2
cells ♦ Dose: 1 × 105 BMMSCs-Exo ♦ In vivo: UUO mice model ♦ Dose: 1 × 106 BMMSCs-Exo |
✓ Expression of α-SMA, collagen 1α1, and fibronectin
↓; ✓ mTOR signaling and autophagy ↓; ✓ Renal fibrosis ↓; ✓ miR-122a ↑; |
Anti-fibrosis Autophagy |
Li et al. 73 |
| 23 | BMMSCs-Exo | ♦ In vivo: 5/6 nephrectomy + high phosphate
diet-induced CKD mice model ♦ Dose: 75 μg BMMSCs-Exo |
✓ Cellular apoptosis ↓; ✓ Levels of Bax and caspase-3 ↓; ✓ Levels of Scr and BUN ↓; ✓ miR-381-3p expression ↑; |
Anti-apoptosis | Liu et al. 74 |
| 24 | BMMSCs-Exo | ♦ In vivo: 5/6 subtotal nephrotomy rat
model ♦ Dose: 150 μg/week BMMSCs-Exo ♦ In vitro: TGF-β1-induced human renal proximal tubular epithelial cells ♦ Dose: 100 μg/mL BMMSCs-Exo |
✓ Renal fibrosis ↓; ✓ Expression of fibronectin, collagen I, α-SMA ↓; |
Anti-fibrosis | Liu et al. 75 |
| 25 | BMMSCs-Exo | ♦ In vivo: UUO mice model ♦ Dose: 30 μg BMMSCs-Exo |
✓ Levels of α-SMA and fibronectin ↓; ✓ Levels of BUN and Scr ↓; ✓ Number of F4/80+CD86+ and F480+/CD206+ macrophages ↓; |
Anti-inflammation | Lu et al. 76 |
| 26 | BMMSCs-Exo | ♦ In vivo: postmenopausal chronic kidney
damage ♦ Dose: 100 µg BMMSCs-Exo |
✓ Levels of CR and BUN ↓; ✓ Levels of GPx, CAT, SOD ↑; ✓ Renal fibrosis, levels of α-SMA, caspase-3, and TGF-β1 ↓; ✓ Cell apoptosis ↓; |
Anti-fibrosis Anti-apoptosis |
Alasmari et al. 77 |
| 27 | BMMSCs-Exo | ♦ In vivo: UUO mice model ♦ Dose: 50 µg and 100 µg BMMSCs-Exo |
✓ Expression of fibronectin and collagen I
↓; ✓ miR-21a-5p expression ↑; |
Anti-fibrosis | Xu et al. 78 |
| 28 | UCMSCs-Exo | ♦ In vitro: TGF-β1-induced NRK-52E
cells ♦ Dose: 100 μg UCMSCs-Exo ♦ In vivo: UUO mice model ♦ Dose: 200 μg UCMSCs-Exo |
✓ Levels of BUN and Scr ↓; ✓ Cell apoptosis and oxidative stress ↓; ✓ ROS level ↓; ✓ Renal fibrosis ↓; ✓ p38MAPK/ERK1/2 pathway ↑; |
Anti-apoptosis Anti-oxidative stress |
Liu et al. 79 |
| 29 | UCMSCs-Exo | ♦ In vitro: γ-irradiation-induced renal tubular
epithelial cell senescence ♦ Dose: 2.6 × 105 UCMSCs-Exo |
✓ Senescence markers (CDKN2D, p16INK4a) and
senescence-associated secretory phenotype factors
↓; ✓ Expression of IL-6 and CCL7 ↓; ✓ SA-β-gal activity ↓; |
Senescence | Liao et al. 80 |
| 30 | UCMSCs-Exo | ♦ In vitro: high glucose–induced HK-2
cells ♦ Dose: 50 μg UCMSCs-Exo ♦ In vivo: C57BL/KsJ-db/db DN mice ♦ Dose: 10 mg/kg body weight UCMSCs-Exo |
✓ Levels of ALB, BUN, Scr ↓; ✓ Protein levels of Bax and cleaved caspase-3 ↓; ✓ Cell apoptosis ↓; ✓ Levels of N-cadherin, Snail, α-SMA ↓; ✓ Levels of E-cadherin ↑; ✓ miR-424-5p expression ↑; |
Anti-apoptosis Anti-fibrosis |
Cui et al. 81 |
| 31 | MSCs-Exo | ♦ In vitro: TGF-β1-induced NRK-52E
cells ♦ Dose: 4 × 104 MSCs-Exo ♦ In vivo: UUO mice model ♦ Dose: unknown |
✓ Level of ECM and EMT process ↓; ✓ Renal injury and fibrosis ↓; ✓ miR-186-5p expression ↑; |
Anti-fibrosis | Yang et al. 82 |
ADMSCs: adipose-derived mesenchymal stem cells; AKI: acute kidney injury; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMMSCs: bone marrow mesenchymal stem cells; BUN: blood urea nitrogen; CR: creatinine; DN: diabetic nephropathy; ECM: extracellular matrix; EGF: epidermal growth factor; EMT: epithelial–mesenchymal transformation; ER: endoplasmic reticulum; FGF: fibroblast growth factor; FN: fibronectin; HGF: hepatocyte growth factor; H/R: hypoxia/reoxygenation; I/R: ischemia–reperfusion; IFN: interferon; IL: interleukin; KD: kidney disease; LPS: lipopolysaccharide; MAPK: mitogen-activated protein kinase; MCP-1: monocyte chemoattractant protein-1; MDA: malondialdehyde; MSCs: mesenchymal stem cells; NF-κB: nuclear factor–kappa B; PGE2: prostaglandin E2; ROS: reactive oxygen species; Scr: serum creatinine; SDT: Spontaneously Diabetic Torii; α-SMA: alpha-smooth muscle actin; SOD: superoxide dismutase; STZ: streptozotocin; TGF: transforming growth factor; TNF-α: tumor necrosis factor–alpha; UCMSCs: umbilical cord mesenchymal stem cells; UUO: unilateral ureteral obstruction; VEGF: vascular endothelial growth factor.
Anti-Inflammation
Previous studies have proved that activation of inflammation was an important part of the pathogenic process of KD, and macrophage infiltration and aggregation contribute to the acceleration of KD progression 83 . For example, a study by Heerspink et al. 84 showed that high plasma levels of tumor necrosis factor (TNF) receptor 1 and IL-6 were associated with the progression of diabetic KD. Martos-Rus et al. 85 found that the serum levels of inflammatory cytokines/chemokines were upregulated in patients with end-stage KD and activated the nuclear factor–kappa B (NF-κB) pathway, as well as increased peripheral monocytes and inflammatory polarization of macrophages were detected in the kidney tissue of mice with uremia model. In addition, macrophages play an important role in immune surveillance and maintaining the stability of the renal internal environment 86 , and macrophages derived from bone marrow can directly transform into myofibroblasts in the damaged kidney, accelerating the progression of pathogenic fibrosis87,88. Of note, MSCs can ameliorate kidney injury by inhibiting inflammation and promoting kidney repair 89 . For instance, treatment with UCMSCs significantly prevented the progression of diabetic nephropathy (DN) by reducing pro-inflammatory cytokines and secreting abundant epidermal growth factor and vascular endothelial growth factor (VEGF) 52 .
Recently, several studies have confirmed that MSCs-derived EVs play a major role in treating KD 15 . For example, MSCs-Exo treatment slowed the progression of ischemic–reperfusion injury (IRI) by inhibiting expressions of inflammatory factors [eg, IL-6, TNF-α, NF-κB, and interferon (IFN)-γ] 51 . Gao et al. 41 showed that ADMSCs-Exo inhibited inflammation of sepsis-related AKI by blocking the NF-κB pathway. Another study found that BMMSCs-Exo was a promising therapeutic approach for preserving CKD progression via reducing inflammation and degeneration 69 . Similarly, Song et al. 90 stated that MSCs-derived EVs serve as effective therapeutic strategies for CKD via upregulating anti-inflammatory M2 macrophages and regulatory T-cell numbers. In addition, it is well recognized that EV activity mainly involves the horizontal transfer of genetic materials91,92. For example, MSCs-EVs secrete insulin-like growth factor (IGF-1) receptor mRNA directly to renal tubular epithelial cells, as well as directly secreting IGF-1 and carrying IGF-1 receptors to promote kidney repair in AKI 93 . Several studies have confirmed that MSCs-derived exosomes enriched with miRNAs (eg, miR-15a, miR-15b, and miR-16 90 ) and/or chemokine receptors (eg, CCR2 94 and CXCR4 95 ) could ameliorate inflammation and kidney injury by reducing chemokines (eg, CX3CL1 96 and CCL2 97 ).
Anti-Apoptosis
Cell apoptosis is closely related to kidney injury and KD progression. Previous studies have shown that renal tubular epithelial cell apoptosis was detected in both animal AKI models and human kidney tissues of AKI 98 , and the expression of pro-apoptotic genes (Bax and caspase-3) was increased, while the expression of anti-apoptotic gene Bcl-2 was reduced. Of note, numerous studies have confirmed that MSCs implantation can inhibit apoptosis of renal tubular epithelial cells and thus restore renal function99,100. Guo et al. 33 confirmed that BMMSCs alleviated sepsis-induced AKI by inhibiting apoptosis and promoting mitophagy of renal tubular epithelial cells. Another study by Tseng et al. 101 found that hypoxic MSCs significantly reduced cell apoptosis in renal tubular NRK-52E cells exposed to hypoxia-reoxygenation as well as promoted renal tubular autophagy in acute renal IRI rats.
Of note, MSCs play a therapeutic role in KD through paracrine mechanisms. For example, HuMSC-Exo inhibited apoptosis of NRK-52E cells induced by cisplatin via activation of the ERK1/2 pathway 102 . Alasmari et al. 77 illustrated that exosomes derived from BMMSCs impeded the progression of CKD by interfering with fibrosis and apoptosis. Moreover, MSCs-Exo exhibited anti-apoptotic effect on KD progression by transferring miRNAs (eg, miR-199a-3p 44 and miR-424-5p 81 ). What’s more, exosomes released from MSCs preconditioned with melatonin blocked apoptosis by decreasing the levels of caspase-3 67 , which had a protective effect against CKD.
Pro-Angiogenesis
It has been reported that sparse peritubular capillaries, accompanied by reduced blood perfusion, limit the supply of interstitial oxygen to the kidney, ultimately leading to adverse consequences such as renal fibrosis and tubular atrophy, which accelerate KD progression 103 . Previous studies have shown that MSCs can survive for a long time after implantation into the injured kidney, promote renal interstitial capillary neovascularization, improve renal microcirculation, and inhibit renal fibrosis progression 104 . Meanwhile, MSCs derived from the kidney facilitated angiogenesis, vasculogenesis, and endothelial repair 105 . Numerous studies have demonstrated that MSCs transplantation increased the expression of VEGF mRNA in kidney tissues along with endothelial cell proliferation, reduced the loss of peritubular capillaries, and improved kidney function 106 .
In addition, MSCs-derived EVs ameliorated AKI progression by promoting angiogenesis (enhancing renal VEGF levels) in vivo and in vitro 107 . Eirin et al. 108 proved that the autologous ADMSCs-EVs improve the renal microvascular system in pigs with metabolic renal vascular diseases. Another study found that melatonin-stimulated MSCs-Exo isolated from patients with CKD promoted angiogenesis in ischemic diseases through the upregulation of miR-4516 109 . Mechanistically, MSCs promote angiogenesis through paracrine secretion of some bioactive substances related to angiogenesis [such as VEGF, hypoxia-inducible factor 1-alpha (HIF-1α), platelet-derived growth factor-BB (PDGF-BB), stromal cell-derived factor 1 (SDF-1), and angiogenin]34,52,110, as well as differentiation to vascular endothelial cells 111 and smooth muscle cells 112 . For example, ADMSCs transplantation significantly increased peritubular vascular density and the number of CD31- and vWF (von Willebrand factor)-positive cells in renal interstitium and peritubular area of mice with IRI injury, as well as improved blood perfusion in the kidney of mice 113 . The above studies suggest that MSCs have beneficial effects against KD progression by promoting renal angiogenesis and preventing peritubular capillary loss.
Anti-Fibrosis
Renal interstitial fibrosis is the common pathological hallmark of CKD progression, and eventually inevitably develops into end-stage KD 114 , causing a huge socioeconomic burden. Increasing evidence has confirmed that EMT of renal tubular cells is a key event in renal interstitial fibrosis, characterized by fibroblast proliferation and an imbalance between ECM production and degradation115,116, and inhibition of renal tubular EMT may be a potential therapeutic strategy for the treatment of CKD 117 . Numerous studies have shown that MSCs, as a protective mediator of renal interstitial fibrosis, can play an important regulatory role in the process of EMT through their anti-fibrotic activity and paracrine mechanisms, delaying tubular EMT and improving renal fibrosis 118 . For example, Tang et al. 119 showed that BMMSCs treatment prevents renal interstitial fibrosis by blocking the Akt/GSK3β/Snail signaling pathway in adenine-induced CKD. Another study proved that glial cell line–derived neurotrophic factor–modified ADMSCs suppressed EMT and renal fibrosis via inhibition of the PI3K/Akt pathway in CKD 120 .
As the research progresses, subsequent studies have shown that MSCs-Exo exerts anti-fibrotic and EMT-suppressive effects by delivery of genetic information to target cells, thereby alleviating renal fibrosis in CKD. For instance, Grange et al. 121 found that EVs of MSCs can inhibit and reverse the progression of glomerular and tubule-interstitial fibrosis in the DN mouse models by downregulating fibrosis-related genes (eg, Serpia1a, TIMP1, MMP3, collagen I, and Snail). The MSCs-Exo inhibited the EMT process of transforming growth factor (TGF)-β1–treated renal tubular epithelial cells and renal fibrosis in a unilateral ureteric obstruction (UUO)–induced renal fibrosis mouse model via delivery of miRNA-122a 73 and miR-186-5p 82 . Liu et al. 79 found that UCMSCs-Exo exhibits anti-fibrotic effects in CKD through the inactivation of the reactive oxygen species (ROS)–mediated p38 mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK) pathway.
Anti-Oxidative Stress
Oxidative stress is involved in the development and progression of KD, including AKI 122 and CKD 123 . Several studies have confirmed that oxidative stress induces renal tubular inflammation, fibrosis, and renal tubular epithelial cell apoptosis, and resulted in promoting the progression of KD124,125. Meanwhile, the kidney acts as an essential organ for the production of reactive oxygen species (ROS), and oxidative stress is a mediator of CKD progression 126 . In recent years, numerous studies have proved that MSCs were reported to be used as an antioxidant therapeutic drug in the treatment of KD98,127,128. Therefore, the regulation of oxidative stress is the essential mechanism of MSCs-based treatment in KD. Recently, Song et al. 129 showed that MSCs alleviated adriamycin-induced nephropathy by inhibiting oxidative stress and NF-κB-mediated inflammation. Another study showed that valsartan- and melatonin-modified MSCs improved renal architecture and function in CKD by diminishing oxidative stress 130 .
In addition, several preclinical studies demonstrate that MSCs-derived EVs promote tissue repair and reduce oxidative stress in KD 131 . For instance, Zhang et al. 132 revealed that human Wharton’s jelly MSCs-EVs could protect the kidney against IRI by mitigating oxidative stress. Another study confirmed that exosomes derived from UCMSCs prevented AKI progression via suppressing renal oxidative stress and inflammation as well as improving kidney function of kidney failure 48 . Cao et al. 133 showed that human placenta MSCs-Exo reduced oxidative stress and mitochondrial fragmentation in a renal IRI model through activation of the Nrf2/keap1 pathway.
Regulating Autophagy
Autophagy is a type II programmed cell death 134 that can be activated to promote cell survival 135 or resulted in cell death 136 by stimulating various physiological and pathological factors. A basal level of autophagy occurs as a self-eating cellular process to degrade cytosolic proteins and subcellular organelles in lysosomes, recycle the cytoplasmic components, and regenerate cellular building blocks and energy, thus maintaining cellular and tissue homeostasis in all eukaryotic cells137,138. Recently, transplantation of MSCs has emerged as an effective strategy in regenerative medicine to repair injured organ function via regulating autophagy 139 . For instance, hypoxic MSCs alleviate AKI progression by promoting renal tubular autophagy 32 . Feng et al. 140 found that transplantation of sirtuin3-overexpression amniotic fluid stem cells serves as promoting therapeutic strategies for DN through activation of mitophagy and inhibition of apoptosis. Other studies confirmed that UCMSCs enhanced autophagy in advanced oxidation protein products–treated HK-2 cells through inactivation of the PI3K/Akt/mTOR pathway141,142. Intriguingly, upregulation of autophagy remarkably increased the secretion of TGF-β1 from MSCs and suppressed the proliferation of CD4+ T lymphocytes 143 , whereas inhibition of autophagy reduced the responsiveness of T cells to mitogen IL-2 and increased the production of immunosuppressive prostaglandin E2 144 . For example, Yuan et al. 25 showed that MSCs ameliorate kidney injury in DN via eliciting macrophages into anti-inflammatory phenotype and elevating PGC-1α (peroxisome-proliferator-activated receptor-γ coactivator-1alpha)/TFEB (transcription factor EB)-mediated lysosome-autophagy. In addition, autophagy is active in the physiological state or can be activated by cellular stresses such as oxidative stress 145 . Gergin et al. 146 demonstrated that transplanted MSCs inhibited oxidative stress in colistin-induced nephrotoxicity by modulating autophagy. Autophagy and oxidative stress are correlated, and the underlying mechanisms of MSCs-based treatment have not been fully explored.
At the same time, some researchers demonstrated that MSCs-Exo has become a research focus for targeted therapy of KD 147 . Wang et al. 50 discovered that UCMSCs-Exo preprocessing can prevent cisplatin-induced AKI in vivo and in vitro by activating autophagy. Jia et al. 148 identified that UCMSCs-Exo can prevent cisplatin-induced AKI by activating autophagy. Ebrahim et al. 71 confirmed that MSCs-Exo enhances autophagy and then slows the progression of DN via activating the mTOR pathway.
Senescence
Cellular senescence is a specialized cell state of permanent cell cycle arrest caused by the accumulation of cellular damage due to a variety of stressors such as telomere shortening, DNA damage, oxidative stress, and activation of oncoproteins80,149. Senescent cells are known to be present at increased levels in KD, and accumulation of senescent cells is thought to facilitate renal fibrosis, DN, severe AKI, and decay in renal function150,151. Several studies have shown that the removal of senescent tubular cells in the kidney by transgenic or pharmaceutical approaches reduced features of tissue aging and efficiently ameliorated glomerulosclerosis, inflammation, and renal function152–154. Of note, ADMSCs transplantation can alleviate ischemia–reperfusion (I/R)-induced kidney injury through reducing renal senescence 155 . Rodrigues et al. 156 found that UCMSCs can prevent IRI-induced renal senescence in AKI.
In addition, several studies have demonstrated that MSCs-derived exosomes exhibit therapeutic effects on KD by regulating cell senescence157,158. For example, Wang et al. 159 showed that MSCs-derived exosomal let-7b-5p ameliorates cisplatin-induced AKI by reducing renal senescence and cell apoptosis. Another study confirmed that treatment with exosomes derived from MSCs efficiently reduced senescence in renal tubular epithelial cells by diminishing the transcription of senescence markers and senescence-associated secretory phenotype factors 80 . In addition, the paracrine effects of MSCs were enhanced after pretreated with metformin and inhibited MSCs senescence by suppressing SA-β-gal activity, p16Ink4a expression, and p53 and NF-κB activation, thus effectively reducing CKD inflammation and fibrosis 160 . Taken together, the above studies have proven that MSC-EVs are effective in treating KD.
Clinical Trials of MSCs Therapy in KD
In the last decade, the beneficial efficacy of MSCs in the treatment of KD has been confirmed in multiple cellular and animal experimental models. For example, MSCs-base therapy was first shown to promote renal tubular regeneration and improve renal function in cisplatin-induced AKI mice models in 2004 161 . Subsequent studies have also confirmed that MSCs alleviate other animal models of AKI induced by ischemia–reperfusion, glycerol, sepsis, cecal ligation and puncture162,163, and so on. In addition, MSCs transplantation significantly alleviated CKD progression by inhibiting renal tubular epithelial cell apoptosis, EMT process, and inducing cell autophagy15,164. For example, Liu et al. 165 showed that treatment with BMMSCs restricted inflammation and renal damage in the IRI model. Of note, several clinical studies are completed or ongoing to evaluate the safety and efficacy of MSCs for the treatment of KD according to ClinicalTrials.gov (Table 2). For example, a phase I/II clinical trial by Swaminathan et al. 166 showed that BMMSCs alleviated inflammatory response in patients with AKI by secreting anti-inflammatory factors. Two other clinical trials (NCT00698191 and NCT01741857) confirmed that UCMSCs transplantation for refractory systemic lupus erythematosus improved disease activity and renal function, and reduced proteinuria, as well as no adverse events occurred. Currently, a total of seven and nine clinical trials are ongoing to evaluate the safety and efficacy of MSCs in patients with DN and CKD. However, MSC-based therapy is limited by the low survival rate of MSCs when used to treat severe KD 167 . Several factors such as poor control of the disease, cellular microenvironment, anoikis, ischemia, inflammation, and ROS production reduce the efficacy of MSC-based therapies168,169. Some preclinical studies have suggested that the preconditioning or cotreatment of MSCs protects them from the harmful environment at the site of damage and improves their function 23 , including cytokines or natural/chemical compounds. In addition, as several clinical trials are in recruiting status, it is worthwhile to further consider and explore whether there are safety issues and insignificant efficacy of MSCs for the treatment of KD. Therefore, we will further explore multiple treatment strategies based on MSCs for KD after obtaining the results of the existing clinical trials to prolong the survival of patients and delay the progression of KD.
Table 2.
The Ongoing Clinical Trials of MSCs Therapy in KD.
| No. | Estimated enrollment | Phase | MSCs source | Status | Sponsor | Clinical trial ID |
|---|---|---|---|---|---|---|
| AKI | ||||||
| 1 | 80 | I/II | UCMSCs | Not recruiting | Chinese PLA General Hospital, China | NCT04194671 |
| 2 | 15 | I | ADMSCs | Recruiting | Tambi Jarmi, USA | NCT04388761 |
| 3 | 15 | I | BMMSCs | Completed | AlloCure Inc., USA | NCT00733876 |
| 4 | 24 | I/II | MSCs | Not recruiting | Sentien Biotechnologies, Inc. | NCT03015623 |
| CKD | ||||||
| 1 | 7 | I | BMMSCs | Completed | Royan Institute, Iran | NCT02195323 |
| 2 | 44 | I/II | UCMSCs | Recruiting | Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, China | NCT05512988 |
| 3 | 20 | I | BMMSCs | Recruiting | Mayo Clinic Florida, USA | NCT05362786 |
| 4 | 31 | I/II | ADMSCs | Recruiting | Bangladesh Laser & Cell Surgery Institute & Hospital, Bangladesh | NCT03939741 |
| 5 | 116 | II | UCMSCs | Unknown | Zhujiang Hospital, China | NCT02966717 |
| 6 | 40 | I | ADMSCs | Recruiting | Mayo Clinic Florida, USA | NCT04869761 |
| 7 | 6 | I | BMMSCs | Completed | Royan Institute, Iran | NCT02166489 |
| 8 | 10 | I | BMMSCs | Recruiting | Pharmicell Co., Ltd., Korea | NCT05042206 |
| 9 | 20 | I | UCMSCs | Recruiting | The Foundation for Orthopaedics and Regenerative Medicine, Antigua, and Barbuda | NCT05018845 |
| 10 | 7 | I | BMMSCs | Completed | Royan Institute, Iran | NCT02195323 |
| 11 | 20 | I/II | MSCs | Unknown | Fuzhou General Hospital, China | NCT00659620 |
| 12 | 30 | II | ADMSCs | Recruiting | Mayo Clinic in Rochester, USA | NCT03325322 |
| 13 | 31 | I/II | ADMSCs | Recruiting | Bangladesh Laser & Cell Surgery Institute & Hospital, Bangladesh | NCT03939741 |
| 14 | 42 | I | ADMSCs | Completed | University of Alabama, USA | NCT02266394 |
| 15 | 60 | Urinary MSCs | Not recruiting | Hospices Civils de Lyon, France | NCT04998461 | |
| 16 | 100 | Not applicable | ADMSCs | Unknown | The Affiliated Hospital of Xuzhou Medical University, China | NCT03321942 |
| 17 | 30 | I/II | MSCs | Unknown | Nanjing Medical University, China | NCT03460223 |
| 18 | 100 | Not applicable | ADMSCs | Unknown | The Affiliated Hospital of Xuzhou Medical University, China | NCT03321942 |
| DN | ||||||
| 1 | 30 | I | ADMSCs | Recruiting | Mayo Clinic in Rochester, USA | NCT03840343 |
| 2 | 54 | I/II | UCMSCs | Unknown | Shanghai East Hospital, China | NCT04216849 |
| 3 | 15 | Early I | UCMSCs | Recruiting | Yan’an Affiliated Hospital of Kunming Medical University, China | NCT04125329 |
| 4 | 38 | Not applicable | UCMSCs | Unknown | Renmin Hospital of Wuhan University, China | NCT04562025 |
| 5 | 48 | I/II | BMMSCs | Recruiting | Mario Negri Institute for Pharmacological Research, Ireland | NCT02585622 |
| 6 | 20 | I/II | Wharton Jelly MSCs | Unknown | University of Jordan, Jordan | NCT03288571 |
| 7 | 15 | I | ADMSCs | Recruiting | Albert Hakaim, USA | NCT04392206 |
| Lupus nephritis | ||||||
| 1 | 16 | I | Human amniotic MSCs | Completed | Yan’an Affiliated Hospital of Kunming Medical University, China | NCT04318600 |
| 2 | 230 | II | UCMSCs | Unknown | The Affiliated Drum Tower Hospital of Nanjing University Medical School, China | NCT03580291 |
| 3 | 20 | I/II | BMMSCs | Unknown | Fuzhou General Hospital, China | NCT00659217 |
| 4 | 30 | Not applicable | UCMSCs | Unknown | The First Affiliated Hospital of Dalian Medical University, China | NCT03458156 |
| 5 | 36 | II | BMMSCs | Not recruiting | Hanyang University Hospital, Korea | NCT03673748 |
| 6 | 7 | I | BMMSCs | Completed | Corestem, Inc., Korea | NCT03174587 |
| 7 | 25 | II | UCMSCs | Unknown | Second Affiliated Hospital & SLE Research Centre, Kunming Medical University, China | NCT01539902 |
| 8 | 7 | I | BMMSCs | Completed | Hanyang university hospital, Korea | NCT03174587 |
ADMSCs: adipose-derived mesenchymal stem cells; AKI: acute kidney injury; BMMSCs: bone marrow mesenchymal stem cells; CKD: chronic kidney disease; DN: diabetic nephropathy; KD: kidney disease; MSCs: mesenchymal stem cells; UCMSCs: umbilical cord mesenchymal stem cells.
Current Challenges of MSCs Therapy in KD
Selection of MSCs Source
Interestingly, the results of animal models and clinical trials have confirmed that MSCs have shown positive results for the treatment of various KD, and no adverse effects or serious adverse complications have been observed. Currently, MSCs are widely available in clinical trials, but the ultimate goal is to use MSCs to delay KD progression and avoid its progression to end-stage renal disease. Therefore, the choice of autologous or allogeneic MSCs for transplantation should be considered in clinical applications. Autologous MSCs have low immunogenicity and no risk of infection, but the longer time required for autologous cell preparation may limit their practical application in clinical treatment. Allogeneic MSCs can be selectively derived from young healthy donors and have the potential to be produced rapidly and in large quantities in vitro culture, significantly reducing costs 118 , while the use of allogeneic MSCs includes a higher risk of immunological reactions and shorter cell survival times following injection 170 . Although transplantation with autologous MSCs is safer and more ethical than allogeneic MSCs, there are still some problems in clinical applications. First, after autologous MSCs are extracted, the in vitro culture cycle is long, which may not fully meet the needs of the body. Second, there is a significant difference between the secretion and immune regulation of autologous MSCs 171 . However, a clinical study reported that injections of allogeneic or autologous BMMSCs were both associated with low rates of treatment-emergent serious adverse events (such as immunologic reactions) in patients with ischemic cardiomyopathy 172 . Further studies to overcome the immune rejection caused by allogeneic MSCs during the treatment process are necessary. Owing to the many sources of allogeneic MSCs and the high efficiency of in vitro culture, the treatment of immune rejection caused by allogeneic MSCs is still receiving widespread attention 173 . On the contrary, an obvious solution is to immediately use autologous MSCs as a ready-made product. In addition, new products such as acellular exosomes and MSCs derived from human pluripotent stem cells are exciting developments that are attracting significant attention 174 .
Transplantation Protocol of MSCs
Currently, MSCs are mostly transplanted in animal experiments by intravenous, arterial, intraperitoneal, and local injections for KD, whereas clinical transplantation of MSCs includes arterial and intravenous injections. Previous studies have shown that MSCs transplantation via arterial injection was more effective than intravenous injection in promoting renal regeneration 175 . Moreover, local injection of MSCs also plays a positive role in renal repair 176 , but this route was less commonly used in clinical practice. Importantly, different transplantation modalities have a significant impact on the survival and homing rate of MSCs, and the optimal implantation modality needs to be determined 177 . Furthermore, the timing of MSCs injection, the number of injections, the number of cells per injection, exploring the optimal strategy for MSCs migration to the damaged site, understanding the interactions between MSCs and other tissue cells, and the adverse effects of MSCs after transplantation (eg, low differentiation in vivo and tumorigenesis) 178 , all of which pose challenges for MSCs to move from basic experiments to clinical applications.
Migration and Survival of MSCs
A prerequisite for the efficacy of MSCs is the ability to migrate to damaged tissues. Previous studies have found that MSCs can localize to diseased sites 179 , but only a small fraction of MSCs 180 . Several studies found that the migration of MSCs in vivo was regulated by various surface adhesion molecules (eg, CD44, VLA-4/VCAM1, SDF-1/CXCR4, and CXCL5/CXCR2)181–183. Importantly, pretreatment with cytokines or active substances can improve the localization/migration ability of MSCs. For example, MSCs modified by CXC chemokine receptors (such as CXCR3 184 and CXCR4 185 ) exhibited better migration and localization abilities. In addition, enhanced migration and anti-inflammatory activities of MSCs mediated by the transient ectopic expression of CXCR4 and IL-10 or IL-35186,187. However, the source, culture, and amplification methods of MSCs may affect the expression of their localized surface molecules 188 , as well as the cell activity, therapeutic effects, and safety of modified MSCs were difficult to control. Meanwhile, there is a lack of effective strategies to precisely localize MSCs to damaged tissues.
Safety of MSCs Transplantation
With the gradual increase of studies on the application of MSCs in clinical practice, the safety of MSCs has received widespread attention. In the phase I clinical trial by Liu et al. 189 , no physical abnormalities were found in healthy volunteers after receiving BMMSCs infusion at a 2-month follow-up. Wang et al. 190 conducted a toxicity study of UCMSCs transplantation in 32 macaques and no adverse reactions were observed. Ra et al. 191 evaluated the safety of ADMSCs preparations using an animal model of ulcerative colitis and no toxicity or tumorigenicity was found in immunodeficient mice. Hu et al. 192 showed that no severe adverse reactions or tumorigenicity was observed in clinical trials with either autologous or allogeneic transplantations of MSCs. These results indicated that MSCs were relatively safe in the treatment of diseases. Currently, no US Food and Drug Administration (FDA)-approved MSCs on market for disease treatment, whereas some MSCs-approved products for human disease are in other countries (Table 3). Meanwhile, most clinical studies of MSCs are still in the early stage, as well as the source, isolated, purified methods, and injection route of MSCs are different. Therefore, the safety of MSCs needs to be summarized and improved with continuous clinical trials.
Table 3.
Current Approved in South Korea, Europe, Japan, and Other Countries With MSCs for Diseases.
| Sources | Clinical condition | Trade name | Approving country (year) |
|---|---|---|---|
| ADMSCs | Subcutaneous tissue defects | Queencell | South Korea (2010) |
| BMMSCs | Acute myocardial infarction | Cellgram-AMI | South Korea (2011) |
| ADMSCs | Crohn’s fistula | Cupistem | South Korea (2012) |
| UCMSCs | Knee articular cartilage defects | Cartistem | South Korea (2012) |
| BMMSCs | Graft-versus-host disease | Prochymal | Canada (2012) |
| BMMSCs | Graft-versus-host disease | Remestemcel-L | New Zealand (2012) |
| BMMSCs | Amytrophic lateral sclerosis | Neuronata-R | South Korea (2014) |
| BMMSCs | Graft-versus-host disease | Temcell HS Inj | Japan (2015) |
| BMMSCs | Critical limb ischemia | Stempeucel | India (2016) |
| BMMSCs | Spinal cord injury | Stemirac | Japan (2018) |
| ADMSCs | Complex perianal fistulas in Crohn’s disease | Darvastrocel (Alofisel) | Europe (2018) |
ADMSCs: adipose-derived mesenchymal stem cells; BMMSCs: bone marrow mesenchymal stem cells; MSCs: mesenchymal stem cells; UCMSCs: umbilical cord mesenchymal stem cells.
Others
Except for the current challenges mentioned above, clinical applications of MSCs have other limitations. For example, tissue sources and isolation methods can influence MSC proliferation and differentiation potential193,194. In addition, microenvironment, donor age, and environmental factors affect the genetic stability of MSCs. No consensus on the standard properties (eg, phenotype, differentiation potential, physiological functions, and biological properties) of MSCs has been developed 195 . Of note, MSCs can only proliferate for a limited number of passages in vitro and will eventually enter a senescence state 196 . Progressively slow growth and lack of differentiation of high-passaged MSCs have been reported in several studies197,198. Other important challenges are the isolation and culturing of MSCs using xenofree conditions 199 as cells grown in media containing fetal bovine serum and other animal or bacterial products cannot be used for clinical purposes. Thus, a better understanding of the origin, biological properties, and function of MSCs derived from different tissues could provide insight into what truly is an “MSC.”
Improvement of MSCs’ Therapeutic Effect in KD
MSC-based therapy has been widely studied for KD therapy and has been shown to result in improved renal function and the recovery of damaged renal tissues in animal studies and clinical trials 200 . However, the limited effects of the current therapy for KD drive the need for the development of novel strategies such as preconditioning, genetic modifications, and strategies for scalability. For instance, several cytokines and natural/chemical compounds have been shown to have protective effects by enhancing cell survival and proliferation 201 . Docosahexaenoic acid (DHA) is a necessary omega-3 fatty acid found in the blood and the kidney. The 14S,21R-dihydroxy-doxosa 4Z,7Z19Z,12E,16Z,19Z-hexaenoic acid (14S,21R-dHDHA) has been identified as a new DHA-derived lipid mediator, and treatment with this compound has been shown to enhance the function of MSCs. In vitro and IRI mouse models, MSCs treated with 14S,21R-dHDHA show reduced apoptosis and inflammatory responses, and improved renal function 202 . Other studies have shown that the pharmacological agent, S-nitroso N-acetyl penicillamine (SNP), a nitric oxide donor associated with cytoprotective and tissue-protective effects, promoted MSCs functionality by increasing cell proliferation and survival in renal IR model 203 . Moreover, administration of SNP-treated MSCs resulted in a significant improvement in renal function and increased the expression of pro-survival and pro-angiogenic factors in ischemic renal tissue. Darbepoetin-α is an erythropoietic agent that shows similar protective and hematopoietic effects and reduces kidney damage in an animal model of renal IRI 204 . In a mice model of renal ischemia, the administration of melatonin-pretreated MSCs increased the secretion of angiogenic cytokines and the survival of engrafted MSCs in CKD-associated ischemic sites. Moreover, miRNAs (eg, miR-146a-5p 205 , miR-19a-3p 206 , miR-374a-5p 207 , and miR-34a 208 )-modified MSCs ameliorated KD progression via reducing inflammation, oxidative stress, renal fibrosis, cell apoptosis, and so on.
Conclusion
Numerous studies have confirmed the safety and tolerability of MSCs transplantation for the treatment of KD209–212. Given the increasing incidence of KD worldwide, MSCs-based therapy appears to be an innovative intervention approach with tremendous potential for the management of KD, but there is still much work to be done before MSCs can be used for clinical treatment on a large scale. First, the issues of donor heterogeneity, mass production, immunogenicity, and cryopreservation of MSCs need to be addressed. Second, how to make MSCs with more efficient targeting ability, more precise immunomodulatory function, and safer application effect by artificial means need to be studied. Existing studies have provided several strategies, including genetic engineering, microparticle engineering, and preculture, which theoretically improve the efficiency of MSCs application. Third, the detailed underlying mechanisms of MSCs for the treatment of KD and the functional role of targeting kidney-related injury need to be further explored. Fourth, the therapeutic safety of MSCs (eg, carcinogenic) remains controversial. With the advancement of novel biotechnology, there are many strategies to enhance the efficacy and safety of MSCs (such as drug conjugation, hypoxia condition, cytokine pretreatment, and genetic modification), but long-term efficacy has not been proven and standardized clinical trial protocols are still needed. Currently, the treatment of KD is limited to drug therapy, dialysis, and renal transplantation, whereas MSCs transplantation has emerged as a promising alternative therapy and has been supported by evidence from relevant clinical studies213,214. From the perspective of functional improvement and clinical parameters, the results of clinical trials are more favorable, and the development of new technologies is expected to overcome current barriers to the clinical application of MSCs therapy. In conclusion, with the continuous innovation of treatment protocols and more and larger scale clinical trials in the future, MSCs-based therapy is expected to become a major “tool” for the treatment of KD.
Footnotes
Author Contributions: F.C. and N.C. are responsible for the acquisition, analysis and interpretation of the data, and drafting of the manuscript. Others contributed to the critical revision of the manuscript.
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Natural Science Foundation of China (Grant No. 81860144).
ORCID iD: Chen Zhou  https://orcid.org/0000-0002-2092-8442
https://orcid.org/0000-0002-2092-8442
References
- 1.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, Fox CS, Gansevoort RT, Heerspink HJL, Jardine M, Kasiske B, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390(10105):1888–917. [DOI] [PubMed] [Google Scholar]
- 2.Rota C, Morigi M, Imberti B. Stem cell therapies in kidney diseases: progress and challenges. Int J Mol Sci. 2019;20(11):2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Kidney Int. 2017;91(2):260–62. [DOI] [PubMed] [Google Scholar]
- 4.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabrizi F, Cerutti R, Ridruejo E. Hepatitis B virus infection as a risk factor for chronic kidney disease. Expert Rev Clin Pharmacol. 2019;12(9):867–74. [DOI] [PubMed] [Google Scholar]
- 6.Wali RK, Henrich WL. Chronic kidney disease: a risk factor for cardiovascular disease. Cardiol Clin. 2005;23(3):343–62. [DOI] [PubMed] [Google Scholar]
- 7.Crews DC, Bello AK, Saadi G. Burden, access, and disparities in kidney disease. J Nephrol. 2019;4:372–79. [DOI] [PubMed] [Google Scholar]
- 8.Ralto KM, Parikh SM. Mitochondria in acute kidney injury. Semin Nephrol. 2016;36(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saran R, Pearson A, Tilea A, Shahinian V, Bragg-Gresham J, Heung M, Hutton DW, Steffick D, Zheng K, Morgenstern H, Gillespie BW, et al. Burden and cost of caring for US veterans with CKD: initial findings from the VA renal information system (VA-REINS). Am J Kidney Dis. 2021;77(3):397–405. [DOI] [PubMed] [Google Scholar]
- 10.Câmara NO, Iseki K, Kramer H, Liu ZH, Sharma K. Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol. 2017;13(3):181–90. [DOI] [PubMed] [Google Scholar]
- 11.Bastani B. The present and future of transplant organ shortage: some potential remedies. J Nephrol. 2020;33(2):277–88. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi A, Rad NK, Ezzatizadeh V, Moghadasali R. Kidney regeneration: stem cells as a new trend. Curr Stem Cell Res Ther. 2020;15(3):263–83. [DOI] [PubMed] [Google Scholar]
- 13.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20(1):5–14. [DOI] [PubMed] [Google Scholar]
- 14.Bochon B, Kozubska M, Surygała G, Witkowska A, Kuźniewicz R, Grzeszczak W, Wystrychowski G. Mesenchymal stem cells-potential applications in kidney diseases. Int J Mol Sci. 2019;20(10):2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Yang L. Mesenchymal stem cells and extracellular vesicles in therapy against kidney diseases. Stem Cell Res Ther. 2021;12(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Shan SK, Guo B, Li F, Zheng MH, Lei LM, Xu QS, Ullah MHE, Xu F, Lin X, Yuan LQ. The multi-therapeutic role of MSCs in diabetic nephropathy. Front Endocrinol. 2021;12:671566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PW, Wu BS, Yang CY, Lee OK. Molecular mechanisms of mesenchymal stem cell-based therapy in acute kidney injury. Int J Mol Sci. 2021;22(21):11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maqsood M, Kang M, Wu X, Chen J, Teng L, Qiu L. Adult mesenchymal stem cells and their exosomes: sources, characteristics, and application in regenerative medicine. Life Sci. 2020;256:118002. [DOI] [PubMed] [Google Scholar]
- 19.Han Q, Wang X, Ding X, He J, Cai G, Zhu H. Immunomodulatory effects of mesenchymal stem cells on drug-induced acute kidney injury. Front Immunol. 2021;12:683003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13(9):1738–55. [DOI] [PubMed] [Google Scholar]
- 21.Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37(1):115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazeli Z, Abedindo A, Omrani MD, Ghaderian SMH. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: a systematic review. Stem Cell Rev Rep. 2018;14(1):1–12. [DOI] [PubMed] [Google Scholar]
- 23.Yun CW, Lee SH. Potential and therapeutic efficacy of cell-based therapy using mesenchymal stem cells for acute/chronic kidney disease. Int J Mol Sci. 2019;20(7):1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickson LJ, Eirin A, Conley SM, Taner T, Bian X, Saad A, Herrmann SM, Mehta RA, McKenzie TJ, Kellogg TA, Kirkland JL, et al. Diabetic kidney disease alters the transcriptome and function of human adipose-derived mesenchymal stromal cells but maintains immunomodulatory and paracrine activities important for renal repair. Diabetes. 2021;70(7):1561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Y, Yuan L, Li L, Liu F, Liu J, Chen Y, Cheng J, Lu Y. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1α activation. Stem Cells. 2021;39(7):913–28. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–16. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y, Zhou Y, Li HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong CY. Current advances of stem cell-based therapy for kidney diseases. World J Stem Cells. 2021;13(7):914–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monsel A, Zhu YG, Gennai S, Hao Q, Liu J, Lee JW. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014;121(5):1099–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliva J. Therapeutic properties of mesenchymal stem cell on organ ischemia-reperfusion injury. Int J Mol Sci. 2019;20(21):5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu N, Liu J, Li X. Therapeutic role of mesenchymal stem cells (MSCs) in diabetic kidney disease (DKD). Endocr J. 2022;69(10):1159–72. [DOI] [PubMed] [Google Scholar]
- 32.Tseng WC, Lee PY, Tsai MT, Chang FP, Chen NJ, Chien CT, Hung SC, Tarng DC. Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy. Stem Cell Res Ther. 2021;12(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Wang R, Liu D. Bone marrow-derived mesenchymal stem cells ameliorate sepsis-induced acute kidney injury by promoting mitophagy of renal tubular epithelial cells via the SIRT1/Parkin axis. Front Endocrinol. 2021;12:639165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishiuchi N, Nakashima A, Doi S, Yoshida K, Maeda S, Kanai R, Yamada Y, Ike T, Doi T, Kato Y, Masaki T. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res Ther. 2020;11(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Cai S, Zhang W, Liu X, Li Y, Zhang C, Zeng Y, Xu M, Rong R, Yang T, Shi B, et al. High-mobility group box 1 protein antagonizes the immunosuppressive capacity and therapeutic effect of mesenchymal stem cells in acute kidney injury. J Transl Med. 2020;18(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang M, Zhang K, Li Y, He QH, Li GQ, Zheng QY, Zhang KQ. Mesenchymal stem cells alleviate acute kidney injury by down-regulating C5a/C5aR pathway activation. Int Urol Nephrol. 2018;50(8):1545–53. [DOI] [PubMed] [Google Scholar]
- 37.Aslam R, Hussain A, Cheng K, Kumar V, Malhotra A, Gupta S, Singhal PC. Transplantation of mesenchymal stem cells preserves podocyte homeostasis through modulation of parietal epithelial cell activation in adriamycin-induced mouse kidney injury model. Histol Histopathol. 2020;35(12):1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He W, Qin D, Li B, Zhang H, Cheng X, Sun J, Hua J, Peng S. Immortalized canine adipose-derived mesenchymal stem cells alleviate gentamicin-induced acute kidney injury by inhibiting endoplasmic reticulum stress in mice and dogs. Res Vet Sci. 2021;136:39–50. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JB, Wang XQ, Lu GL, Huang HS, Xu SY. Adipose-derived mesenchymal stem cells therapy for acute kidney injury induced by ischemia-reperfusion in a rat model. Clin Exp Pharmacol Physiol. 2017;44(12):1232–40. [DOI] [PubMed] [Google Scholar]
- 40.Begum S, Ahmed N, Mubarak M, Mateen SM, Khalid N, Rizvi SAH. Modulation of renal parenchyma in response to allogeneic adipose-derived mesenchymal stem cells transplantation in acute kidney injury. Int J Stem Cells. 2019;12(1):125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao F, Zuo B, Wang Y, Li S, Yang J, Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. [DOI] [PubMed] [Google Scholar]
- 42.Cao S, Huang Y, Dai Z, Liao Y, Zhang J, Wang L, Hao Z, Wang F, Wang D, Liu L. Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis. Bioengineered. 2022;13(3):6323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Zhang J, Huang H. Exosomes from adipose-derived stem cells inhibit inflammation and oxidative stress in LPS-acute kidney injury. Exp Cell Res. 2022;420(1):113332. [DOI] [PubMed] [Google Scholar]
- 44.Zhu G, Pei L, Lin F, Yin H, Li X, He W, Liu N, Gou X. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol. 2019;234(12):23736–49. [DOI] [PubMed] [Google Scholar]
- 45.Xie X, Yang X, Wu J, Tang S, Yang L, Fei X, Wang M. Exosome from indoleamine 2,3-dioxygenase-overexpressing bone marrow mesenchymal stem cells accelerates repair process of ischemia/reperfusion-induced acute kidney injury by regulating macrophages polarization. Stem Cell Res Ther. 2022;13(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao JY, Wang B, Tang TT, Wen Y, Li ZL, Feng ST, Wu M, Liu D, Yin D, Ma KL, Tang RN, et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11(11):5248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Cao H, Cui B, Ma X, Gao L, Yu C, Shen F, Yang X, Liu N, Qiu A, Cai G, et al. Mesenchymal stem cells-derived exosomes ameliorate ischemia/reperfusion induced acute kidney injury in a porcine model. Front Cell Dev Biol. 2022;10:899869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Wang C, Bai Z, Li P. Umbilical cord mesenchymal stem cell exosomes alleviate the progression of kidney failure by modulating inflammatory responses and oxidative stress in an ischemia-reperfusion mice model. J Biomed Nanotechnol. 2021;17(9):1874–81. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R, Zhu Y, Li Y, Liu W, Yin L, Yin S, Ji C, Hu Y, Wang Q, Zhou X, Chen J, et al. Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol Lett. 2020;42(4):669–79. [DOI] [PubMed] [Google Scholar]
- 50.Wang B, Jia H, Zhang B, Wang J, Ji C, Zhu X, Yan Y, Yin L, Yu J, Qian H, Xu W. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res Ther. 2017;8(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Wang R, Jia Y, Rong R, Xu M, Zhu T. Exosomes derived from mesenchymal stem cells ameliorate renal ischemic-reperfusion injury through inhibiting inflammation and cell apoptosis. Front Med. 2019;6:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang E, Han B, Zhang Q, Rao W, Wang Z, Chang C, Zhang Y, Tu C, Li C, Wu D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 2020;11(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.An X, Liao G, Chen Y, Luo A, Liu J, Yuan Y, Li L, Yang L, Wang H, Liu F, Yang G, et al. Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem Cell Res Ther. 2019;10(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SE, Jang JE, Kim HS, Jung MK, Ko MS, Kim MO, Park HS, Oh W, Choi SJ, Jin HJ, Kim SY, et al. Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells. Exp Mol Med. 2019;51(7):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, Xiang E, Li C, Han B, Zhang Q, Rao W, Xiao C, Wu D. Umbilical cord-derived mesenchymal stem cells ameliorate nephrocyte injury and proteinuria in a diabetic nephropathy rat model. J Diabetes Res. 2020;2020:8035853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takemura S, Shimizu T, Oka M, Sekiya S, Babazono T. Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model. J Diabetes Investig. 2020;11(3):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Liu J, Liao G, Zhang J, Chen Y, Li L, Li L, Liu F, Chen B, Guo G, Wang C, et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41(5):2629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sávio-Silva C, Soinski-Sousa PE, Simplício-Filho A, Bastos RMC, Beyerstedt S, Rangel ÉB. Therapeutic potential of mesenchymal stem cells in a pre-clinical model of diabetic kidney disease and obesity. Int J Mol Sci. 2021;22(4):1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan Y, Li L, Zhu L, Liu F, Tang X, Liao G, Liu J, Cheng J, Chen Y, Lu Y. Mesenchymal stem cells elicit macrophages into M2 phenotype via improving transcription factor EB-mediated autophagy to alleviate diabetic nephropathy. Stem Cells. 2020;38(5):639–52. [DOI] [PubMed] [Google Scholar]
- 60.Bai Y, Wang J, He Z, Yang M, Li L, Jiang H. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor β (TGF-β)/smad pathway and pro-inflammatory cytokines. Med Sci Monit. 2019;25:3069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mao R, Shen J, Hu X. BMSCs-derived exosomal microRNA-let-7a plays a protective role in diabetic nephropathy via inhibition of USP22 expression. Life Sci. 2021;268:118937. [DOI] [PubMed] [Google Scholar]
- 62.Xing L, Song E, Yu CY, Jia XB, Ma J, Sui MS, Wang MA, Gao X. Bone marrow-derived mesenchymal stem cells attenuate tubulointerstitial injury through multiple mechanisms in UUO model. J Cell Biochem. 2019;120(6):9737–46. [DOI] [PubMed] [Google Scholar]
- 63.Saberi K, Pasbakhsh P, Omidi A, Borhani-Haghighi M, Nekoonam S, Omidi N, Ghasemi S, Kashani IR. Melatonin preconditioning of bone marrow-derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. J Mol Histol. 2019;50(2):129–40. [DOI] [PubMed] [Google Scholar]
- 64.Li H, Rong P, Ma X, Nie W, Chen Y, Zhang J, Dong Q, Yang M, Wang W. Mouse umbilical cord mesenchymal stem cell paracrine alleviates renal fibrosis in diabetic nephropathy by reducing myofibroblast transdifferentiation and cell proliferation and upregulating MMPs in mesangial cells. J Diabetes Res. 2020;2020:3847171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L, Wang Y, Li S, Zuo B, Zhang X, Wang F, Sun D. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics. 2020;10(20):9425–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin J, Shi Y, Gong J, Zhao L, Li Y, He Q, Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yea JH, Yoon YM, Lee JH, Yun CW, Lee SH. Exosomes isolated from melatonin-stimulated mesenchymal stem cells improve kidney function by regulating inflammation and fibrosis in a chronic kidney disease mouse model. J Tissue Eng. 2021;12:20417314211059624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin S, Zhou S, Ren D, Zhang J, Xin H, He X, Gao H, Hou J, Zeng F, Lu Y, Zhang X, et al. Mesenchymal stem cell-derived exosomes attenuate epithelial-mesenchymal transition of HK-2 cells. Tissue Eng Part A. 2022;28(13–14):651–9. [DOI] [PubMed] [Google Scholar]
- 69.Alasmari WA, El-Shetry ES, Ibrahim D, ElSawy NA, Eldoumani H, Metwally AS, Saleh AA, Mona MM, Abd-Elsalam MM, Hendam BM, Essawi WM, et al. Mesenchymal stem-cells’ exosomes are renoprotective in postmenopausal chronic kidney injury via reducing inflammation and degeneration. Free Radic Biol Med. 2022;182:150–9. [DOI] [PubMed] [Google Scholar]
- 70.Wu X, Wang Z, Wang J, Tian X, Cao G, Gu Y, Shao F, Yan T. Exosomes secreted by mesenchymal stem cells induce immune tolerance to mouse kidney transplantation via transporting LncRNA DANCR. Inflammation. 2022;45(1):460–75. [DOI] [PubMed] [Google Scholar]
- 71.Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, et al. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells. 2018;7(12):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin J, Qian F, Zheng D, He W, Gong J, He Q. Mesenchymal stem cells attenuate renal fibrosis via exosomes-mediated delivery of microRNA let-7i-5p antagomir. Int J Nanomedicine. 2021;16:3565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li D, Qu J, Yuan X, Zhuang S, Wu H, Chen R, Wu J, Zhang M, Ying L. Mesenchymal stem cells alleviate renal fibrosis and inhibit autophagy via exosome transfer of miRNA-122a. Stem Cells Int. 2022;2022:1981798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Guo Y, Bao S, Huang H, Liu W, Guo W. Bone marrow mesenchymal stem cell-derived exosomal microRNA-381-3p alleviates vascular calcification in chronic kidney disease by targeting NFAT5. Cell Death Dis. 2022;13(3):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Guo W, Guo Y, Chen X, Liu W. Bone marrow mesenchymal stem cell-derived exosomes improve renal fibrosis via regulating Smurf 2/Smad 7. Front Biosci. 2022;27(1):17. [DOI] [PubMed] [Google Scholar]
- 76.Lu Y, Yang L, Chen X, Liu J, Nie A, Chen X. Bone marrow mesenchymal stem cell-derived exosomes improve renal fibrosis by reducing the polarisation of M1 and M2 macrophages through the activation of EP2 receptors. IET Nanobiotechnol. 2022;16(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alasmari WA, Abdelfattah-Hassan A, El-Ghazali HM, Abdo SA, Ibrahim D, ElSawy NA, El-Shetry ES, Saleh AA, Abourehab MAS, Mahfouz H. Exosomes derived from BM-MSCs mitigate the development of chronic kidney damage post-menopause via interfering with fibrosis and apoptosis. Biomolecules. 2022;12(5):663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu S, Cheuk YC, Jia Y, Chen T, Chen J, Luo Y, Cao Y, Guo J, Dong L, Zhang Y, Shi Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-21a-5p alleviates renal fibrosis by attenuating glycolysis by targeting PFKM. Cell Death Dis. 2022;13(10):876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu B, Hu D, Zhou Y, Yu Y, Shen L, Long C, Butnaru D, Timashev P, He D, Lin T, Xu T, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am J Transl Res. 2020;12(9):4998–5014. [PMC free article] [PubMed] [Google Scholar]
- 80.Liao CM, Luo T, von der Ohe J, de Juan Mora B, Schmitt R, Hass R. Human MSC-derived exosomes reduce cellular senescence in renal epithelial cells. Int J Mol Sci. 2021;22(24):13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui C, Zang N, Song J, Guo X, He Q, Hu H, Yang M, Wang Y, Yang J, Zou Y, Gao J, et al. Exosomes derived from mesenchymal stem cells attenuate diabetic kidney disease by inhibiting cell apoptosis and epithelial-to-mesenchymal transition via miR-424-5p. FASEB J. 2022;36(10):e22517. [DOI] [PubMed] [Google Scholar]
- 82.Yang Y, Wang J, Zhang Y, Hu X, Li L, Chen P. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Hum Cell. 2022;35(1):83–97. [DOI] [PubMed] [Google Scholar]
- 83.Wen Y, Yan HR, Wang B, Liu BC. Macrophage heterogeneity in kidney injury and fibrosis. Front Immunol. 2021;12:681748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heerspink HJL, Perco P, Mulder S, Leierer J, Hansen MK, Heinzel A, Mayer G. Canagliflozin reduces inflammation and fibrosis biomarkers: a potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia. 2019;62(7):1154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martos-Rus C, Katz-Greenberg G, Lin Z, Serrano E, Whitaker-Menezes D, Domingo-Vidal M, Roche M, Ramaswamy K, Hooper DC, Falkner B, Martinez Cantarin MP. Macrophage and adipocyte interaction as a source of inflammation in kidney disease. Sci Rep. 2021;11(1):2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viehmann SF, Böhner AMC, Kurts C, Brähler S. The multifaceted role of the renal mononuclear phagocyte system. Cell Immunol. 2018;330:97–104. [DOI] [PubMed] [Google Scholar]
- 87.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, IJpelaar DHT. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2017;32(8):1322–9. [DOI] [PubMed] [Google Scholar]
- 88.Nikolic-Paterson DJ, Wang S, Lan HY. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int Suppl. 2014;4(1):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wise AF, Ricardo SD. Mesenchymal stem cells in kidney inflammation and repair. Nephrology. 2012;17(1):1–10. [DOI] [PubMed] [Google Scholar]
- 90.Song T, Eirin A, Zhu X, Zhao Y, Krier JD, Tang H, Jordan KL, Woollard JR, Taner T, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles induce regulatory t cells to ameliorate chronic kidney injury. Hypertension. 2020;75(5):1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao L, Hu C, Zhang P, Jiang H, Chen J. Genetic communication by extracellular vesicles is an important mechanism underlying stem cell-based therapy-mediated protection against acute kidney injury. Stem Cell Res Ther. 2019;10(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang SY, Hong Q, Zhang CY, Yang YJ, Cai GY, Chen XM. miRNAs in stem cell-derived extracellular vesicles for acute kidney injury treatment: comprehensive review of preclinical studies. Stem Cell Res Ther. 2019;10(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao L, Zhong X, Jin J, Li J, Meng XM. Potential targeted therapy and diagnosis based on novel insight into growth factors, receptors, and downstream effectors in acute kidney injury and acute kidney injury-chronic kidney disease progression. Signal Transduct Target Ther. 2020;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou X, Zhang G, Cheng Z, Yin D, Du T, Ju G, Miao S, Liu G, Lu M, Zhu Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5(2):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, Qiu J, Fan Y. CCR2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016;2016:1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu N, Tian J, Cheng J, Zhang J. Migration of CXCR4 gene-modified bone marrow-derived mesenchymal stem cells to the acute injured kidney. J Cell Biochem. 2013;114(12):2677–89. [DOI] [PubMed] [Google Scholar]
- 97.Zheng J, Wang Q, Leng W, Sun X, Peng J. Bone marrow-derived mesenchymal stem cell-conditioned medium attenuates tubulointerstitial fibrosis by inhibiting monocyte mobilization in an irreversible model of unilateral ureteral obstruction. Mol Med Rep. 2018;17(6):7701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee KH, Tseng WC, Yang CY, Tarng DC. The anti-inflammatory, anti-oxidative, and anti-apoptotic benefits of stem cells in acute ischemic kidney injury. Int J Mol Sci. 2019;20(14):3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imberti B, Morigi M, Benigni A. Potential of mesenchymal stem cells in the repair of tubular injury. Kidney Int Suppl. 2011;1(3):90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birtwistle L, Chen XM, Pollock C. Mesenchymal stem cell-derived extracellular vesicles to the rescue of renal injury. Int J Mol Sci. 2021;22(12):6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tseng WC, Lee PY, Tsai MT, Chang FP, Chen NJ, Chien CT, Hung SC, Tarng DC. Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy. Stem Cell Res Ther. 2021;12(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xing L, Cui R, Peng L, Ma J, Chen X, Xie RJ, Li B. Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res Ther. 2014;5(4):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen J, Park HC, Addabbo F, Ni J, Pelger E, Li H, Plotkin M, Goligorsky MS. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74(7):879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villanueva S, Carreño JE, Salazar L, Vergara C, Strodthoff R, Fajre F, Céspedes C, Sáez PJ, Irarrázabal C, Bartolucci J, Figueroa F, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci. 2013;125(4):199–210. [DOI] [PubMed] [Google Scholar]
- 107.Zou X, Gu D, Xing X, Cheng Z, Gong D, Zhang G, Zhu Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8(10):4289–99. [PMC free article] [PubMed] [Google Scholar]
- 108.Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant. 2018;27(7):1080–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yoon YM, Lee JH, Song KH, Noh H, Lee SH. Melatonin-stimulated exosomes enhance the regenerative potential of chronic kidney disease-derived mesenchymal stem/stromal cells via cellular prion proteins. J Pineal Res. 2020;68(3):e12632. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia D, Fang S, Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2):e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang WY, Chen LC, Jhuang YT, Lin YJ, Hung PY, Ko YC, Tsai MY, Lee YW, Hsu LW, Yeh CK, Hsu HH, et al. Injection of hybrid 3D spheroids composed of podocytes, mesenchymal stem cells, and vascular endothelial cells into the renal cortex improves kidney function and replenishes glomerular podocytes. Bioeng Transl Med. 2021;6(2):e10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen L, Fukuda N, Shimizu S, Kobayashi H, Tanaka S, Nakamura Y, Matsumoto T, Abe M. Role of complement 3 in renin generation during the differentiation of mesenchymal stem cells to smooth muscle cells. Am J Physiol Cell Physiol. 2020;318(5):C981–90. [DOI] [PubMed] [Google Scholar]
- 113.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–26. [DOI] [PubMed] [Google Scholar]
- 115.Xie M, Wan J, Zhang F, Zhang R, Zhou Z, You D. Influence of hepatocyte growth factor-transfected bone marrow-derived mesenchymal stem cells towards renal fibrosis in rats. Indian J Med Res. 2019;149(4):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin S, Yu L, Ni Y, He L, Weng X, Lu X, Zhang C. Fibroblast growth factor 21 attenuates diabetes-induced renal fibrosis by negatively regulating TGF-β-p53-Smad2/3-mediated epithelial-to-mesenchymal transition via activation of AKT. Diabetes Metab J. 2020;44(1):158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee M, Kim SH, Jhee JH, Kim TY, Choi HY, Kim HJ, Park HC. Microparticles derived from human erythropoietin mRNA-transfected mesenchymal stem cells inhibit epithelial-to-mesenchymal transition and ameliorate renal interstitial fibrosis. Stem Cell Res Ther. 2020;11(1):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhuang Q, Ma R, Yin Y, Lan T, Yu M, Ming Y. Mesenchymal stem cells in renal fibrosis: the flame of cytotherapy. Stem Cells Int. 2019;2019:8387350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang H, Zhang P, Zeng L, Zhao Y, Xie L, Chen B. Mesenchymal stem cells ameliorate renal fibrosis by galectin-3/Akt/GSK3β/Snail signaling pathway in adenine-induced nephropathy rat. Stem Cell Res Ther. 2021;12(1):409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Li S, Wang Y, Wang Z, Chen L, Zuo B, Liu C, Sun D. Enhanced renoprotective effect of GDNF-modified adipose-derived mesenchymal stem cells on renal interstitial fibrosis. Stem Cell Res Ther. 2021;12(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, Brizzi MF. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9(1):4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andrianova NV, Zorov DB, Plotnikov EY. Targeting inflammation and oxidative stress as a therapy for ischemic kidney injury. Biochemistry. 2020;85(12):1591–602. [DOI] [PubMed] [Google Scholar]
- 123.Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34(6):975–91. [DOI] [PubMed] [Google Scholar]
- 124.Aranda-Rivera AK, Cruz-Gregorio A, Aparicio-Trejo OE, Ortega-Lozano AJ, Pedraza-Chaverri J. Redox signaling pathways in unilateral ureteral obstruction (UUO)-induced renal fibrosis. Free Radic Biol Med. 2021;172:65–81. [DOI] [PubMed] [Google Scholar]
- 125.Ueda N. A rheostat of ceramide and sphingosine-1-phosphate as a determinant of oxidative stress-mediated kidney injury. Int J Mol Sci. 2022;23(7):4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coppolino G, Leonardi G, Andreucci M, Bolignano D. Oxidative stress and kidney function: a brief update. Curr Pharm Des. 2018;24(40):4794–99. [DOI] [PubMed] [Google Scholar]
- 127.Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2011;86(2):191–96. [DOI] [PubMed] [Google Scholar]
- 128.Zhao L, Hu C, Zhang P, Jiang H, Chen J. Melatonin preconditioning is an effective strategy for mesenchymal stem cell-based therapy for kidney disease. J Cell Mol Med. 2020;24(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song IH, Jung KJ, Lee TJ, Kim JY, Sung EG, Bae YC, Park YH. Mesenchymal stem cells attenuate adriamycin-induced nephropathy by diminishing oxidative stress and inflammation via downregulation of the NF-kB. Nephrology. 2018;23(5):483–92. [DOI] [PubMed] [Google Scholar]
- 130.Yang CC, Sung PH, Chen KH, Chai HT, Chiang JY, Ko SF, Lee FY, Yip HK. Valsartan- and melatonin-supported adipose-derived mesenchymal stem cells preserve renal function in chronic kidney disease rat through upregulation of prion protein participated in promoting PI3K-Akt-mTOR signaling and cell proliferation. Biomed Pharmacother. 2022;146:112551. [DOI] [PubMed] [Google Scholar]
- 131.Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014;127(1–4):75–80. [DOI] [PubMed] [Google Scholar]
- 132.Zhang G, Zou X, Miao S, Chen J, Du T, Zhong L, Ju G, Liu G, Zhu Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9(3):e92129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao H, Cheng Y, Gao H, Zhuang J, Zhang W, Bian Q, Wang F, Du Y, Li Z, Kong D, Ding D, et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano. 2020;14(4):4014–26. [DOI] [PubMed] [Google Scholar]
- 134.Uchiyama Y. Autophagic cell death and its execution by lysosomal cathepsins. Arch Histol Cytol. 2001;64(3):233–46. [DOI] [PubMed] [Google Scholar]
- 135.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26(7):1749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8(6):569–81. [DOI] [PubMed] [Google Scholar]
- 137.Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22(7–8):830–44. [DOI] [PubMed] [Google Scholar]
- 138.Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263(1):55–72. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Z, Yang C, Shen M, Yang M, Jin Z, Ding L, Jiang W, Yang J, Chen H, Cao F, Hu T. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell Res Ther. 2017;8(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feng J, Lu C, Dai Q, Sheng J, Xu M. SIRT3 facilitates amniotic fluid stem cells to repair diabetic nephropathy through protecting mitochondrial homeostasis by modulation of mitophagy. Cell Physiol Biochem. 2018;46(4):1508–24. [DOI] [PubMed] [Google Scholar]
- 141.Li M, Jiang T, Zhang W, Xie W, Guo T, Tang X, Zhang J. Human umbilical cord MSC-derived hepatocyte growth factor enhances autophagy in AOPP-treated HK-2 cells. Exp Ther Med. 2020;20(3):2765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiang J, Jiang T, Zhang W, Xie W, Tang X, Zhang J. Human umbilical cord-derived mesenchymal stem cells enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Cell Res. 2019;378(2):198–205. [DOI] [PubMed] [Google Scholar]
- 143.Gao L, Cen S, Wang P, Xie Z, Liu Z, Deng W, Su H, Wu X, Wang S, Li J, Ouyang Y, et al. Autophagy improves the immunosuppression of CD4+ T cells by mesenchymal stem cells through transforming growth factor-β1. Stem Cells Transl Med. 2016;5(11):1496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Volarevic V, Gazdic M, Simovic Markovic B, Jovicic N, Djonov V, Arsenijevic N. Mesenchymal stem cell-derived factors: immuno-modulatory effects and therapeutic potential. Biofactors. 2017;43(5):633–44. [DOI] [PubMed] [Google Scholar]
- 145.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gergin ÖÖ, Pehlivan SS, Ulger M, Mat OC, Bayram A, Gönen ZB, Gökdemir NS, Biçer C, Yildiz K, Yay AH. Efficacy of stem cell-based therapies for colistin-induced nephrotoxicity. Environ Toxicol Pharmacol. 2022;94:103933. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Lu X, He J, Zhao W. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res Ther. 2015;6(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jia H, Liu W, Zhang B, Wang J, Wu P, Tandra N, Liang Z, Ji C, Yin L, Hu X, Yan Y, et al. HucMSC exosomes-delivered 14-3-3ζ enhanced autophagy via modulation of ATG16L in preventing cisplatin-induced acute kidney injury. Am J Transl Res. 2018;10(1):101–13. [PMC free article] [PubMed] [Google Scholar]
- 149.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, et al. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–27. [DOI] [PubMed] [Google Scholar]
- 150.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol. 2017;13(2):77–89. [DOI] [PubMed] [Google Scholar]
- 151.Docherty MH, O’Sullivan ED, Bonventre JV, Ferenbach DA. Cellular senescence in the kidney. J Am Soc Nephrol. 2019;30(5):726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169(1):132–147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim SR, Puranik AS, Jiang K, Chen X, Zhu XY, Taylor I, Khodadadi-Jamayran A, Lerman A, Hickson LJ, Childs BG, Textor SC, et al. Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J Am Soc Nephrol. 2021;32(8):1987–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johmura Y, Yamanaka T, Omori S, Wang TW, Sugiura Y, Matsumoto M, Suzuki N, Kumamoto S, Yamaguchi K, Hatakeyama S, Takami T, et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science. 2021;371(6526):265–70. [DOI] [PubMed] [Google Scholar]
- 155.Kim SR, Zou X, Tang H, Puranik AS, Abumoawad AM, Zhu XY, Hickson LJ, Tchkonia T, Textor SC, Kirkland JL, Lerman LO. Increased cellular senescence in the murine and human stenotic kidney: effect of mesenchymal stem cells. J Cell Physiol. 2021;236(2):1332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rodrigues CE, Capcha JM, de Bragança AC, Sanches TR, Gouveia PQ, de Oliveira PA, Malheiros DM, Volpini RA, Santinho MA, Santana BA, Calado RD, et al. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res Ther. 2017;8(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsuji K, Kitamura S, Wada J. Secretomes from mesenchymal stem cells against acute kidney injury: possible heterogeneity. Stem Cells Int. 2018;2018:8693137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Racchetti G, Meldolesi J. Extracellular vesicles of mesenchymal stem cells: therapeutic properties discovered with extraordinary success. Biomedicines. 2021;9(6):667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang SY, Xu Y, Hong Q, Chen XM, Cai GY. Mesenchymal stem cells ameliorate cisplatin-induced acute kidney injury via let-7b-5p [published online ahead of print December 22, 2022]. Cell Tissue Res. doi: 10.1007/s00441-022-03729-3. [DOI] [PubMed] [Google Scholar]
- 160.Kim H, Yu MR, Lee H, Kwon SH, Jeon JS, Han DC, Noh H. Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell. 2021;20(2):e13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–804. [DOI] [PubMed] [Google Scholar]
- 162.Tögel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6(3):179–83. [DOI] [PubMed] [Google Scholar]
- 163.Selim RE, Ahmed HH, Abd-Allah SH, Sabry GM, Hassan RE, Khalil WKB, Abouhashem NS. Mesenchymal stem cells: a promising therapeutic tool for acute kidney injury. Appl Biochem Biotechnol. 2019;189(1):284–304. [DOI] [PubMed] [Google Scholar]
- 164.Cao Q, Huang C, Chen XM, Pollock CA. Mesenchymal stem cell-derived exosomes: toward cell-free therapeutic strategies in chronic kidney disease. Front Med. 2022;9:816656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu X, Cai J, Jiao X, Yu X, Ding X. Therapeutic potential of mesenchymal stem cells in acute kidney injury is affected by administration timing. Acta Biochim Biophys Sin. 2017;49(4):338–48. [DOI] [PubMed] [Google Scholar]
- 166.Swaminathan M, Kopyt N, Atta MG, Radhakrishnan J, Umanath K, Nguyen S, O’Rourke B, Allen A, Vaninov N, Tilles A, LaPointe E, et al. Pharmacological effects of ex vivo mesenchymal stem cell immunotherapy in patients with acute kidney injury and underlying systemic inflammation. Stem Cells Transl Med. 2021;10(12):1588–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, Müller-Ehmsen J, Schenk K, Fries JW, Baldamus CA, Benzing T. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114(3):e107–16. [DOI] [PubMed] [Google Scholar]
- 168.He N, Zhang L, Cui J, Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014;2014:128436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mias C, Trouche E, Seguelas MH, Calcagno F, Dignat-George F, Sabatier F, Piercecchi-Marti MD, Daniel L, Bianchi P, Calise D, Bourin P, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26(7):1749–57. [DOI] [PubMed] [Google Scholar]
- 170.Colbath AC, Dow SW, McIlwraith CW, Goodrich LR. Mesenchymal stem cells for treatment of musculoskeletal disease in horses: relative merits of allogeneic versus autologous stem cells. Equine Vet J. 2020;52(5):654–63. [DOI] [PubMed] [Google Scholar]
- 171.Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2(6):455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama. 2012;308(22):2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, van Kooten C, Fibbe WE, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med. 2013;2(2):107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang LT, Liu KJ, Sytwu HK, Yen ML, Yen BL. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10(9):1288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Y, He J, Pei X, Zhao W. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology. 2013;18(3):201–8. [DOI] [PubMed] [Google Scholar]
- 176.Zhen-Qiang F, Bing-Wei Y, Yong-Liang L, Xiang-Wei W, Shan-Hong Y, Yuan-Ning Z, Wei-Sheng J, Wei C, Ye G. Localized expression of human BMP-7 by BM-MSCs enhances renal repair in an in vivo model of ischemia-reperfusion injury. Genes Cells. 2012;17(1):53–64. [DOI] [PubMed] [Google Scholar]
- 177.Berebichez-Fridman R, Montero-Olvera PR. Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos Univ Med J. 2018;18(3):e264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pool M, Leuvenink H, Moers C. Reparative and regenerative effects of mesenchymal stromal cells-promising potential for kidney transplantation? Int J Mol Sci. 2019;20(18):4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5(12):1028–38. [DOI] [PubMed] [Google Scholar]
- 180.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001. [DOI] [PubMed] [Google Scholar]
- 181.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2011;108(6):2258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li Q, Zhang A, Tao C, Li X, Jin P. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2013;441(3):675–80. [DOI] [PubMed] [Google Scholar]
- 184.Lin Y, Zhou HC, Chen N, Ren Y, Gao R, Li Q, Deng Y, Han X, Zhang X, Xiang AP, Guo B, et al. Unveiling the improved targeting migration of mesenchymal stem cells with CXC chemokine receptor 3-modification using intravital NIR-II photoacoustic imaging. J Nanobiotechnology. 2022;20(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kalimuthu S, Oh JM, Gangadaran P, Zhu L, Lee HW, Rajendran RL, Baek SH, Jeon YH, Jeong SY, Lee SW, Lee J, et al. In vivo tracking of chemokine receptor CXCR4-engineered mesenchymal stem cell migration by optical molecular imaging. Stem Cells Int. 2017;2017:8085637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hervás-Salcedo R, Fernández-García M, Hernando-Rodríguez M, Quintana-Bustamante O, Segovia JC, Alvarez-Silva M, García-Arranz M, Minguez P, Del Pozo V, de Alba MR, García-Olmo D, et al. Enhanced anti-inflammatory effects of mesenchymal stromal cells mediated by the transient ectopic expression of CXCR4 and IL10. Stem Cell Res Ther. 2021;12(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tan C, Tan S, Zhang H, Zhang M, Fan H, Nan Z, Liu X, Wang W, Zhang L, Deng S, Zuo D, et al. Enhanced migration and immunoregulatory capacity of BMSCs mediated by overexpression of CXCR4 and IL-35. Mol Immunol. 2022;150:1–8. [DOI] [PubMed] [Google Scholar]
- 188.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8(3):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu L, Sun Z, Chen B, Han Q, Liao L, Jia M, Cao Y, Ma J, Sun Q, Guo M, Liu Z, et al. Ex vivo expansion and in vivo infusion of bone marrow-derived Flk-1+CD31-CD34- mesenchymal stem cells: feasibility and safety from monkey to human. Stem Cells Dev. 2006;15(3):349–57. [DOI] [PubMed] [Google Scholar]
- 190.Wang Y, Han ZB, Ma J, Zuo C, Geng J, Gong W, Sun Y, Li H, Wang B, Zhang L, He Y, et al. A toxicity study of multiple-administration human umbilical cord mesenchymal stem cells in cynomolgus monkeys. Stem Cells Dev. 2012;21(9):1401–8. [DOI] [PubMed] [Google Scholar]
- 191.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20(8):1297–308. [DOI] [PubMed] [Google Scholar]
- 192.Hu ZB, Wang LS, Cui CP, Chen J, Wu ZZ, Xie JX, Wu CH. Stem cell clinical application safety assessment report. Chin Med Biotechnol. 2013;8(5):349–61. [Google Scholar]
- 193.Beeravolu N, Khan I, McKee C, Dinda S, Thibodeau B, Wilson G, Perez-Cruet M, Bahado-Singh R, Chaudhry GR. Isolation and comparative analysis of potential stem/progenitor cells from different regions of human umbilical cord. Stem Cell Res. 2016;16(3):696–711. [DOI] [PubMed] [Google Scholar]
- 194.Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, Chaudhry GR. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017;122:55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, Tenedini E, et al. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016;6(6):897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Legzdina D, Romanauska A, Nikulshin S, Kozlovska T, Berzins U. Characterization of senescence of culture-expanded human adipose-derived mesenchymal stem cells. Int J Stem Cells. 2016;9(1):124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Turinetto V, Vitale E, Giachino C. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 2016;17(7):1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Laitinen A, Oja S, Kilpinen L, Kaartinen T, Möller J, Laitinen S, Korhonen M, Nystedt J. A robust and reproducible animal serum-free culture method for clinical-grade bone marrow-derived mesenchymal stromal cells. Cytotechnology. 2016;68(4):891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges. Stem Cell Res Ther. 2017;8(1):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tian H, Lu Y, Shah SP, Wang Q, Hong S. 14S,21R-dihydroxy-docosahexaenoic acid treatment enhances mesenchymal stem cell amelioration of renal ischemia/reperfusion injury. Stem Cells Dev. 2012;21(7):1187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Masoud MS, Anwar SS, Afzal MZ, Mehmood A, Khan SN, Riazuddin S. Pre-conditioned mesenchymal stem cells ameliorate renal ischemic injury in rats by augmented survival and engraftment. J Transl Med. 2012;10:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Altun B, Yilmaz R, Aki T, Akoglu H, Zeybek D, Piskinpasa S, Uckan D, Purali N, Korkusuz P, Turgan C. Use of mesenchymal stem cells and darbepoetin improve ischemia-induced acute kidney injury outcomes. Am J Nephrol. 2012;35(6):531–9. [DOI] [PubMed] [Google Scholar]
- 205.Zhang Y, Le X, Zheng S, Zhang K, He J, Liu M, Tu C, Rao W, Du H, Ouyang Y, Li C, et al. MicroRNA-146a-5p-modified human umbilical cord mesenchymal stem cells enhance protection against diabetic nephropathy in rats through facilitating M2 macrophage polarization. Stem Cell Res Ther. 2022;13(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee MS, Yip HK, Yang CC, Chiang JY, Huang TH, Li YC, Chen KH, Sung PH. Overexpression of miR-19a and miR-20a in iPS-MSCs preserves renal function of chronic kidney disease with acute ischaemia-reperfusion injury in rat. J Cell Mol Med. 2021;25(16):7675–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liang M, Zhang D, Zheng D, He W, Jin J. Exosomes from miR-374a-5p-modified mesenchymal stem cells inhibit the progression of renal fibrosis by regulating MAPK6/MK5/YAP axis. Bioengineered. 2022;13(2):4517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.He J, Jiang YL, Wang Y, Tian XJ, Sun SR. Micro-vesicles from mesenchymal stem cells over-expressing miR-34a inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition in renal tubular epithelial cells in vitro. Chin Med J. 2020;133(7):800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mohr A, Chu T, Clarkson CT, Brooke GN, Teif VB, Zwacka RM. Fas-threshold signalling in MSCs promotes pancreatic cancer progression and metastasis. Cancer Lett. 2021;519:63–77. [DOI] [PubMed] [Google Scholar]
- 210.Kim HS, Lee JS, Lee HK, Park EJ, Jeon HW, Kang YJ, Lee TY, Kim KS, Bae SC, Park JH, Han SB. Mesenchymal stem cells ameliorate renal inflammation in adriamycin-induced nephropathy. Immune Netw. 2019;19(5):e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu D, Cheng F, Pan S, Liu Z. Stem cells: a potential treatment option for kidney diseases. Stem Cell Res Ther. 2020;11(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fernández-Santos ME, Garcia-Arranz M, Andreu EJ, García-Hernández AM, López-Parra M, Villarón E, Sepúlveda P, Fernández-Avilés F, García-Olmo D, Prosper F, Sánchez-Guijo F, et al. Optimization of mesenchymal stromal cell (MSC) manufacturing processes for a better therapeutic outcome. Front Immunol. 2022;13:918565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Westenfelder C, Togel FE. Protective actions of administered mesenchymal stem cells in acute kidney injury: relevance to clinical trials. Kidney Int Suppl. 2011;1(3):103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Peired AJ, Sisti A, Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016;2016:4798639. [DOI] [PMC free article] [PubMed] [Google Scholar]