Abstract
Background:
Hepatitis C virus (HCV) infection is common among persons who inject drugs (PWID), mostly due to needle sharing. The number of new cases in PWID are steadily increasing despite the availability of effective treatments. The objective of this model is to increase uptake and compliance with HCV treatment. We developed a model to treat HCV and opioid use disorder simultaneously in a methadone maintenance program.
Methods:
Patients were screened on site for HCV at admission and then annually. Once HCV was positive, the genotypes and fibrosis scores were identified. Patients were enrolled into the treatment program after obtaining written consent. Patients either self-administered the medications at home or utilized a directly observed treatment (DOT). The sustained virologic response (SVR) was tested at 12 weeks posttreatment. We conducted a retrospective review of patients who received treatment and reviewed the demographic data, co-infections, medication administration, and SVR results at the end of study period.
Results:
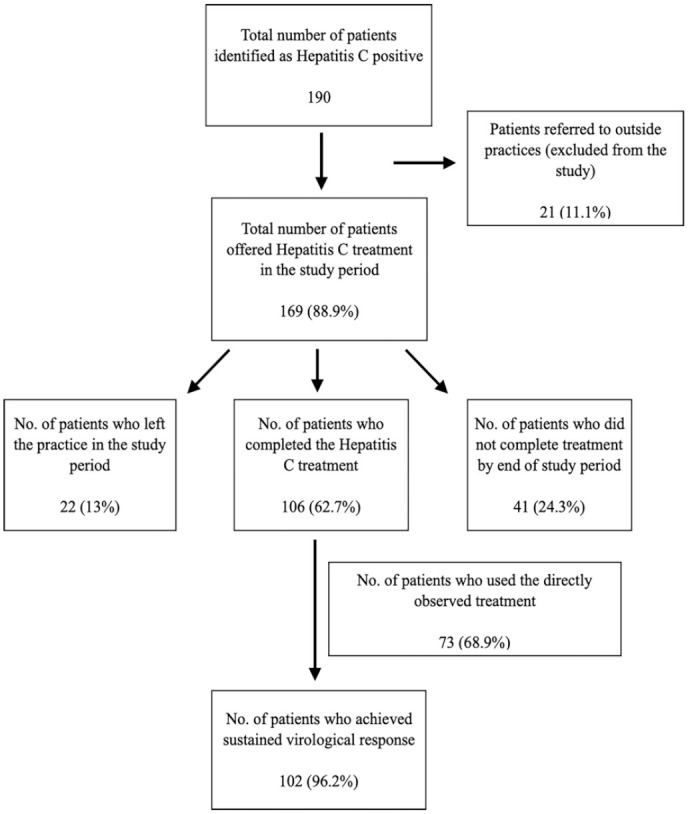
One hundred ninety patients were identified as Hepatitis C positive. 88.9% (169 patients) received HCV treatment during the study period. 62.7% (106 patients) were male and 37.3% were female (63 patients). 62.7% of them (106 patients) completed HCV treatment by the end of study period. Out of them, 96.2% (102 patients) achieved SVR. 68.9% (73 patients) utilized DOT for medication administration.
Conclusions:
Our model successfully treated HCV in our patient population, who are otherwise deprived of resources and access to health care. Replicating this model is a potential strategy to reduce the disease burden and break the transmission cycle of HCV.
Keywords: hepatitis C virus, persons who inject drugs, opiate use, healthcare access
Introduction
Hepatitis C virus (HCV) infection is common among persons who inject drugs (PWID). The Centers for Disease Control (CDC) estimates that this group is at highest risk of acquiring HCV, mostly due to needle sharing. 1 The incidence of HCV infections steadily increased every year from 2013 and the estimated number of new infections in 2019 was 57 500. 2 The number of new cases in PWID has shown to increase steadily despite the availability of effective treatments. From 2010 to 2019, the number of estimated annual acute HCV infections increased by 387% and latest reports indicate at least 2.8 cases per 100 000 population. 3 The increase in the number of HCV cases are both due to true increase in incidence and, to some extent due to increased screening. 4 Intravenous drug use is estimated as the most common reason for increased incidence of new HCV infections. 5
Some reports estimate a large percentage (up to 59%) of PWID attending medication assisted treatment (MAT) programs have HCV infection. 5 Many programs refer such patients to other practices and facilities for treatment but unfortunately only a small percentage follow through and receive treatment. 6 It is a well studied fact that chronic opioid abuse disrupts the brain’s normal neural-circuitry areas that are associated with decision-making, risk evaluation, and impulse control.7,8 These disruptions limit the patient’s ability to recognize the importance of routine primary care, preventive care, and also HCV treatment. Patients with opioid and other substance use disorders have limited access to healthcare due to a multitude of reasons like lack of insurance, lack of housing and transportation, living conditions, guilt, and the fear of being stigmatized.9,10
To increase compliance with HCV treatment, we developed a model to treat HCV infection and opioid use disorder simultaneously in our MAT program in Philadelphia, Pennsylvania. Due to the significant prevalence of untreated HCV among the PWID in MAT programs, our model aims to improve access to HCV treatment for patients with opioid use disorder while attending the MAT program. This model aims to simplify the treatment so that minimal or no effort is required of the patients. The goal of this model is to minimize opportunities for patients to decline treatment. The treatment modalities we offered in this model are based on the guidelines designed by the American Association for the Study of Liver Diseases (AASLD) and Infectious Disease Society of America (IDSA). 11
This manuscript aims at explaining the model we developed to treat HCV infection in our MAT program. We also described the efficacy of the model by doing a retrospective review of the model at the end of a 2 years period.
Methods
Study Site
The study model was implemented at a single site MAT program in Philadelphia, Pennsylvania. During the study period, the program had a census of 610 patients and offered treatment with methadone, buprenorphine, and long-acting injectable Buprenorphine and Naltrexone. 12
Treatment Model
In our model, treatment was offered to both opioid use disorder and HCV infection simultaneously at the same site; to both new and established patients starting upon admission and simultaneously during routine appointments. Patients receiving either methadone or buprenorphine were included in the model. The model also included the patients that previously completed HCV treatment and did not achieve a sustained virologic response (SVR) or became re-infected. 13
The program excluded patients with decompensated cirrhosis, compensated cirrhosis with multiple comorbidities, and patients with medical conditions that were outside the practice’s scope of practice. Excluded patients received a referral to an outside practice.
Our program implemented this model in December 2019 but temporarily stopped within its first few months due to the onset of COVID-19 pandemic. After putting safety procedures in place, the program resumed treating HCV in June 2020. 14
The new patients enrolled into the program were screened on site for hepatitis A, B, C and HIV at admission. The previously enrolled patients into the program were screened for the same as a part of their annual screening. The admission and annual laboratory screening included a complete blood count (CBC), comprehensive metabolic panel, hepatitis A antibody, hepatitis B Ag screen, hepatitis B surface and core antibodies, HIV-1/HIV-2 antibodies, and HIV-1 p24 antigen. If HCV antibody resulted positive, the HCV viral load was quantified through the same blood sample. The program had an on site phlebotomist for blood draws. The samples were sent to a nearby laboratory for analysis and results.
Once HCV was identified as positive, patients were enrolled into the treatment program after obtaining written consent. These patients had a second set of blood draws for necroinflammatory activity score, fibrosis scores, apolipoprotein A-1 level, haptoglobin level, and alpha-2 macroglobulin level to identify the fibrosis stage and the HCV genotype. 15
The treatment plan was then decided by the physicians based on the results and discussed with them in the subsequent visit. Either Mavyret® (Glecaprevir-pibrentasvir) for a duration of 8 weeks, Epclusa® (Sofosbuvir-velpatasvir) for 12 weeks, or Vosevi® (Sofosbuvir-velpatasvir-voxilaprevir) for 12 weeks were the medications used for management of HCV in this model.16-18 The medications were chosen based on the patient’s genotype, fibrosis score, patient’s insurance plan, and medication availability.16-18 The medications were then prescribed to the program’s affiliated pharmacy. Prior authorization was obtained by the staff if needed. Specialty pharmacies sent the HCV treatment medication directly to the MAT program. Patients either self-administered the medications at home or utilized a directly observed treatment (DOT) format, when they were receiving methadone. Patients were allowed to make their own choice if they wanted to get treated under DOT or take the medications home. Patients were monitored in their subsequent visits about the adherence and tolerance of the medications. After completing the treatment, the sustained virologic response (SVR) was tested 12 weeks post-treatment (Figure 1).
Figure 1.
Fibrosis stage of the patients at the beginning of treatment of hepatitis C.
Model Evaluation
The model was developed in 2019 and we decided to evaluate the efficacy of the model in 2021. We performed a literature review at that time but did not find any similar models that treated HCV infection in a methadone maintenance program. The institution’s approval was taken to conduct the HCV treatment in this particular model and the approval was also obtained to evaluate the model. To evaluate this model, we conducted a retrospective review of patients that received HCV treatment between December 2019 through November 2021. We reviewed the demographic data (age, gender, and ethnicity), co-infections (hepatitis A, B and HIV), viral load at beginning of the treatment, genotype, fibrosis score, medication administration records, and sustained virologic testing results at the end of the study period. We reviewed SVR testing results until November 30, 2021. We evaluated the performance of the model based on the adherence with the treatment and SVR results at the end of the treatment. The efficacy of the model was determined based on achievement of SVR at the end of treatment period. Ability to screen the patients for HCV and initiating the patients on treatment were also taken as the benchmarks to evaluate the efficacy of the model.
Results
In the entire study period, 190 patients were identified as HCV positive. Twenty-one patients were referred to an outside facility as they met the exclusion criteria. A total of 169 patients received the HCV treatment during the study period. Out of the 169 patients, 62.7% (106 patients) were male and 37.3% were female (63 patients). 64.5% of the patients were Caucasian, 21.3% African-American and 14.2% Hispanic. The average age of the sample was 42.8 years. 4.2% (6 patients) were co-infected with HIV and 2.1% (3 patients) were co-infected with hepatitis B (Table 1).
Table 1.
Demographics of Patients Who Received Treatment for Hepatitis C Virus in the Medication Assisted Treatment Program.
| Demographic | |
|---|---|
| Age, years | 42.8 (6.5) |
| Mean (SD) | |
| Gender, n (%) (n = 169) | |
| Female | 63 (37.3) |
| Male | 106 (62.7) |
| Ethnicity, n (%) (n = 169) | |
| Caucasian | 109 (64.5) |
| African American | 36 (21.3) |
| Hispanic | 24 (14.2) |
| Co-infections, n (%) (n = 169) | |
| Human immunodeficiency virus (HIV) | 6 (4.2) |
| Hepatitis B virus | 3 (2.1) |
| Hepatitis C genotype, n (%) (n = 169) | |
| Genotype 1a | 101 (59.9) |
| Genotype 1b | 13 (7.7) |
| Genotype 2 | 11 (6.3) |
| Genotype 3 | 42 (24.6) |
| Genotype 4 | 2 (1.4) |
| Genotype 5 | 0 (0) |
| Medication prescribed for hepatitis C treatment, n (%) (n = 169) | |
| Mavyret® (Glecaprevir-pibrentasvir) | 131 (76) |
| Epclusa® (Sofosbuvir-velpatasvir) | 34 (20.4) |
| Vosevi® (Sofosbuvir-velpatasvir-voxilaprevir) | 4 (3.6) |
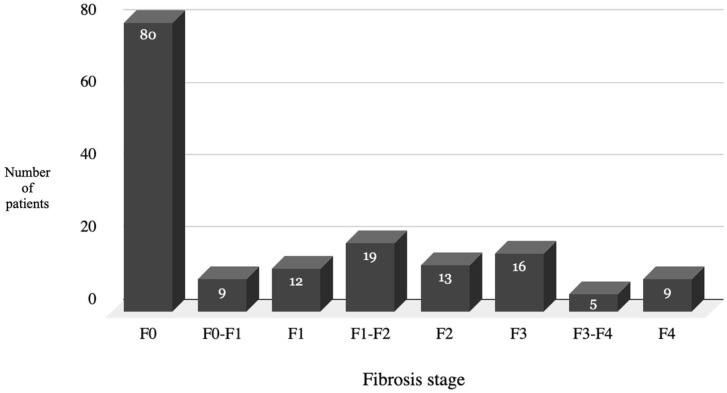
The fibrosis score of the patients is illustrated in Figure 2. 47.3% of the patients (80 patients) were in the F0 (no fibrosis) stage, 34.9% (59 patients) were in the F1 (mild fibrosis) or F2 (moderate fibrosis) stage. The remaining 17.8% patients (30 patients) were in the advanced F3 or F4 stage.
Figure 2.
Outcome of patients enrolled in the hepatitis C treatment model.
Out of the 169 patients, 59.9% (101 patients) were identified with genotype 1a, 7.7% patients (13 patients) had genotype 1b, 6.3% had genotype 2 (11 patients), 24.6% had genotype 3 (42 patients) and 1.4% had genotype 4 (2 patients) (Table 1).
Among the 169 patients, 62.7% (106 patients) completed HCV treatment and had SVR testing. Out of these 169 patients, 13% (22 patients) left the MAT program during the study period and lost to follow up. 24.2% (41 patients) started HCV treatment but did not complete the treatment or did not get the SVR testing by the end of study period. Out of the 106 patients who completed the HCV treatment, 96.2% (102 patients) of the patients achieved SVR at the end of the study period (Figure 1).
76% (131 patients) were treated with Mavyret® (Glecaprevir-pibrentasvir) for a duration of 8 weeks, 20.4% were prescribed Epclusa® (Sofosbuvir-velpatasvir) for 12 weeks (34 patients) and 3.6% (4 patients) were treated with Vosevi® (Sofosbuvir-velpatasvir-voxilaprevir) for 12 weeks (Table 1).
68.9% (73 out of 106 patients) that completed the HCV treatment utilized directly observed therapy (DOT) for medication administration. The other 31.1% (33 out of 106 patients) took the medications at home.
Discussion
With a very high prevalence of HCV infection in the people who inject drugs and the persistent increase in the number of new cases of HCV, we developed a model to treat the infection in our MAT program in Philadelphia. The goal was to make the treatment as convenient as possible so that the opportunities to decline or stop treatment are minimal. In our model, we could successfully treat both the opioid use disorder and HCV infection concurrently and the entire process occurred on-site at the program including blood sample collection, care coordination, prior authorization, and medication administration. The patients were not required to go to a laboratory for testing or to a pharmacy to pick up the medications.
The CDC proposed a goal to reduce the number of new HCV infections to an estimated 35 000 in 2025. A 39.1% reduction from 2019 is needed to meet this 2025 goal.19,20 The patients suffering from opioid use disorder are otherwise deprived of any other access to healthcare and a lot of other resources like shelter, transportation, and community support. Our model successfully overcomes a lot of these barriers and offers management by avoiding additional appointments with another practice and commuting to a laboratory for testing.
Out of the census of 610 patients in the practice, we could screen and identify 190 patients infected with HCV (31.1%), showing a very high prevalence of HCV infection in our patient population. The high prevalence also highlights the importance of screening this set of patient populations and is in line with the recommendations from CDC. 20
68.9% of the patients who received treatment for HCV opted for the directly observed treatment (Figure 1). We attributed this to factors like homelessness and lack of proper shelter and not being able to safely store the medication. Our model addresses this concern by offering to store their treatment medications on-site and dispense them directly to the patient daily with their dose of methadone. Patients’ regular attendance for methadone provides an opportunity to simultaneously treat the HCV infection. Only 31.1% (33 patients) opted to take the medications home.
The demographics of the HCV in the sample was quite similar to the prevalence of HCV in the country with the most common genotype being 1a. 21 47.3% of the patients screened were identified in the F0 (no fibrosis) stage and 34.9% were in the early fibrosis stages. Only 18% of the patients were identified in the late fibrosis stages. This early screening gave us the opportunity to successfully treat the infection before the patients went on to develop complications like cirrhosis and hepatocellular carcinoma. These results suggest the significance of early screening and treatment of the virus in the opiate dependent population to prevent further morbidity and mortality in the future.
Non-compliance and non-adherence to the treatment was projected as a significant hurdle to the model at the beginning of the study but over the entire duration of the study period, only 13% of the patients left the practice. Majority of the sample (62.7%) of the patients did in fact complete the HCV treatment. Our sample had a small subset of patients co-infected with HIV and hepatitis B and evidence based management was provided to them in association with treatment of HCV under the same model.22,23
96.2% of the patients achieved a sustained virologic response and were deemed as cured from the virus. The medications used for treating the virus were no different from any traditional practices to treat HCV infection but the 96% SVR suggests the importance of just having access to the treatment and medications. Patients’ regular attendance at programs for methadone provides an opportunity to easily treat the HCV infection. Most patients receiving methadone maintenance attended the program daily to receive their methadone dose, which permits the program’s staff to assess and triage any issues as needed.
The model involves coordinated care from multiple disciplines; including motivated physicians to formulate a treatment plan, support staff to guide the patients into following the treatment protocols, an on site phlebotomist to draw the blood samples at the treatment site, a dedicated HCV navigator and care coordinator, support from the institution, collaboration with a pharmacy to deliver medications to the site and a strategic partnership with a local laboratory (Figure 3). This model also reinforced the findings from previous studies that a minimal monitoring approach by checking SVR at the end of treatment period is effective for the treatment of HCV infection. 24
Figure 3.
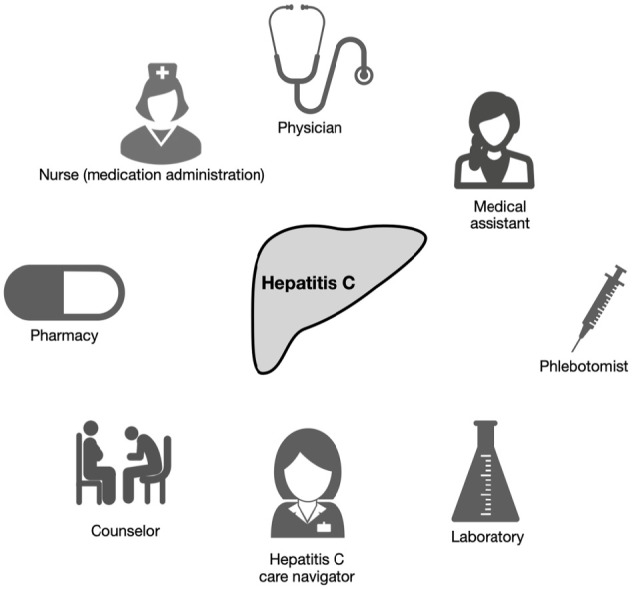
The key stakeholders of the integrated model to treat hepatitis C infection at a methadone maintenance program.
Losing 13% of the patients from the study population was a major limitation of our model. We were not able to identify the reasons for these patients to leave the practice and we assume it was multifactorial, including relocation to a different practice or city or completely stopping methadone therapy. We also have limitations in the data collection methods of the sample as we were not able to obtain the status of their mental health conditions, major medical comorbidities that might have affected the management. We also do not have data like MELD score, Child Pugh score, and the presence or absence of hepatocellular carcinoma that might affect the mortality of the patients. 25 Overall, we were able to measure the SVR testing results at the end of treatment period but we were not able to comment on the overall mortality benefit of the model.
Conclusion
This real-world model demonstrates the practicality of integrating HCV treatment into the routine services provided at a MAT program. Integrating HCV treatment into MAT programs is a strategy to treat HCV in a very high risk population like PWID. Replicating this model is a potential strategy to reduce the disease burden and break the transmission cycle of HCV.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Srikrishna Varun Malayala  https://orcid.org/0000-0002-9923-8638
https://orcid.org/0000-0002-9923-8638
References
- 1.Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2019. US Department of Health and Human Services, Centers for Disease Control and Prevention. 2020. Accessed January 4, 2023. https://www.cdc.gov/hepatitis/statistics/2019surveillance/index.htm [Google Scholar]
- 2.Seo S, Silverberg MJ, Hurley LB, et al. Prevalence of spontaneous clearance of hepatitis C virus infection doubled from 1998 to 2017. Clin Gastroenterol Hepatol. 2020;18: 511-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology. 2019;69:1020-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karimi SE, Bayani A, Higgs P, et al. Prevalence and high risk behaviours associated with HCV testing among people who inject drugs: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy. 2020;15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan AE, Cleland CM, Wyka K, Schackman BR, Perlman DC, Nash D.Hepatitis C virus incidence in a cohort in medication-assisted treatment for opioid use disorder in New York City. J Infect Dis. 2020;222(Supplement_5):S322-S334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katchman H, Adelson M, Avitan O, et al. Hepatitis C virus elimination in methadone-treated patients: implementation of hepatology clinic in a methadone treatment program. J Addict Med. 2022;16:e350-e355. [DOI] [PubMed] [Google Scholar]
- 7.Uhl GR, Koob GF, Cable J.The neurobiology of addiction. Ann N Y Acad Sci. 2019;1451:5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley-Quon LI, Cho J, Strong DR, et al. Association of nonmedical prescription opioid use with subsequent heroin use initiation in adolescents. JAMA Pediatr. 2019;173(9): e191750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McVicar D, Moschion J, van Ours JC.Early illicit drug use and the age of onset of homelessness. J R Stat Soc Ser A Stat Soc. 2019;182:345-372. [Google Scholar]
- 10.Motavalli D, Taylor JL, Childs E, et al. Health is on the back burner: multilevel barriers and facilitators to primary care among people who inject drugs. J Gen Intern Med. 2021;36: 129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Association for the Study of Liver Diseases. Initial treatment of adults with HCV infection. Accessed January 14, 2022. https://www.hcvguidelines.org/treatment-%20naive
- 12.Malayala SV, Papudesi BN, Bobb R, Wimbush A.Xylazine-induced skin ulcers in a person who injects drugs in Philadelphia, Pennsylvania, USA. Cureus. 2022;14(8): e28160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pockros PJ.Management of patients who have achieved sustained virologic response for hepatitis C virus infection. Gastroenterol Hepatol. 2018;14(5):305-307. [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M, Adil SF, Alkhathlan HZ, et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules. 2020;26(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghany MG, Strader DB, Thomas DL, Seeff LB.Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aschenbrenner DS.Hepatitis C and compensated cirrhosis drug approved for eight-week treatment. Am J Nurs. 2020; 120(1):24-25. [DOI] [PubMed] [Google Scholar]
- 17.Sokol R.Sofosbuvir/velpatasvir (Epclusa) for hepatitis C. Am Fam Physician. 2017;95(10):664-666. [PubMed] [Google Scholar]
- 18.Childs-Kean LM, Brumwell NA, Lodl EF.Profile of sofosbuvir/velpatasvir/voxilaprevir in the treatment of hepatitis C. Infect Drug Resist. 2019;12:2259-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics. Multiple cause of death 1999–2018. Data are from the multiple cause of death files, 1999–2018, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. 2020. Accessed January 22, 2023. http://wonder.cdc.gov/mcd-icd10.html
- 20.Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2018. US Department of Health and Human Services, Centers for Disease Control and Prevention. 2020. Accessed January 22, 2023. https://www.cdc.gov/hepatitis/statistics/2018surveillance/pdfs/2018HepSurveillanceRpt.pdf [Google Scholar]
- 21.Dickey BL, Coghill AE, Rathwell JA, et al. Hepatitis C virus (HCV) seroprevalence, RNA detection, and genotype distribution across Florida, 2015–2018. Prev Med. 2022;161:107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mavilia MG, Wu GY.HBV-HCV coinfection: viral interactions, management, and viral reactivation. J Clin Transl Hepatol. 2018;6(3):296-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Association for the Study of Liver Diseases. Patients with HIV/HCV coinfection. Accessed January 14, 2023. https://www.hcvguidelines.org/unique-populations/hiv-hcv
- 24.Solomon SS, Wagner-Cardoso S, Smeaton L, et al. A minimal monitoring approach for the treatment of hepatitis C virus infection (ACTG A5360 [MINMON]): a phase 4, open-label, single-arm trial. Lancet Gastroenterol Hepatol. 2022;7(4): 307-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Axley P, Ahmed Z, Ravi S, Singal AK.Hepatitis C virus and hepatocellular carcinoma: a narrative review. J Clin Transl Hepatol. 2018;6(1):79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]