Abstract
Food-specific go/no-go training might reduce overeating and facilitate weight loss. In this pilot study, we examined whether a food-specific go/no-go training over five weeks, as compared to a non-food-specific training, could produce changes in behavioral and neural responses to food images and body weight. Here, we used a sample of 51 overweight participants divided into training and control groups whose brain activity and food evaluation were measured before and after the training. Compared with the control group, in the training group we found significant reductions in high-calorie food evaluation. We also found lower activations in inhibitory control- and reward-related brain regions in response to high-calorie food images. Further, activation change of the mid-insula in response to the high-calorie food images was positively associated with change in the evaluation of those images. However, we found no evidence for a significant effect of food-specific go/no-go training on body weight change. Our findings highlight that food-specific go/no-go training in overweight individuals can reduce high-calorie food evaluation, but also neural activations in inhibitory control- and reward- related brain regions.
Keywords: overweight, food-specific inhibition training, food evaluation, fMRI
Introduction
Excess weight has become a cause of increasing health care costs and accounts for over 2.8 million deaths per year (World Health Organization, 2021), highlighting the need for an effective therapy. Traditional interventions (e.g. restrictive diets) yield limited success as excess weight is partly driven by more automatic processes, such as greater reward responsivity toward high‐calorie food cues (Marteau et al., 2012; Stice and Yokum, 2016; Devoto et al., 2018; Stice and Burger, 2019; Yang et al., 2021) or deficits in inhibitory or executive control (Smith et al., 2011; Vainik et al., 2018; Yang et al., 2018, 2019b, 2020; Garcia-Garcia et al., 2019; Gunstad et al., 2020; Robinson et al., 2020).
According to the incentive sensitization theory (Berridge et al., 2010) and dynamic vulnerability model of obesity (Stice and Yokum, 2016; Stice and Burger, 2019), increased incentive salience of high-calorie food cues and activity of brain’s reward system in response to high-calorie food cues can predict overeating and weight gain. Lowe and colleagues further proposed that inhibitory control impairment can reduce individuals’ capacity to modulate the elevated reward region responsivity induced by food cues, which, in turn, can result in increased intake of appetitive yet unhealthy foods and, eventually, weight gain (Lowe et al., 2019). Therefore, it is plausible that food-specific training, e.g. a go/no-go training, aimed to reduce reward system responses to high-calorie foods and increase inhibitory control can have beneficial effects on eating behavior or body weight (Stice et al., 2016; Forcano et al., 2018; Mehl et al., 2019; Yang et al., 2019a; Luis-Ruiz et al., 2020).
In the food-specific go/no-go training, people need to quickly respond (e.g. press a button) to food items (e.g. high‐ and low‐calorie food items) displayed on a computer screen and to withhold this response when a no-go cue (e.g. a bold frame around the picture) is displayed. Critically, in a task aimed specifically at retraining particular behaviors, e.g. responses to high-calorie food cues, the no-go cue is disproportionately paired with high‐calorie food items (e.g. 90%). In contrast, in a control task, go and no-go cues are usually paired with non-food items equally (Houben and Jansen, 2015; Oomen et al., 2018).
Several meta-analyses confirmed the effectiveness of food-specific go/no-go training for short-term appetitive behavior in predominantly normal-weight samples showing a moderate effect (Allom et al., 2016; Jones et al., 2016, 2018; Yang et al., 2019a). A recent P-curve analysis suggested published food-related inhibition training (e.g. go/no-go training) studies have limited power to detect small effects, and the observed effect sizes are likely inflated (Carbine and Larson, 2019). Two updated P-curve analyses found food-related inhibition training did influence eating behavior (Veling et al., 2020; Navas et al., 2021). Fewer studies have been conducted to investigate the effect of inhibition training on weight loss. Veling et al. (2014) conducted the first study to test the effectiveness of food-specific go/no-go training to facilitate weight loss. The authors found that dieters in the active condition (i.e. four times online food-specific go/no-go training in four weeks) relative to control condition (i.e. neutral go/no-go training) showed significant weight loss. In a sample of 83 participants with a body mass index (BMI) over 18.5 kg/m2, Lawrence and colleagues demonstrated that four sessions of a food-specific go/no-go training resulted in greater directly-measured weight loss compared with controls who completed a sham training (Lawrence et al., 2015). However, this effect did not replicate in another study (Forman et al., 2019). Differences in study characteristics could influence the observed differing effects. For example, Lawrence et al. (2015) focused on targeted foods and recruited both normal weight and overweight individuals, while all participants in Forman et al. (2019) were also participating in a no-added-sugar diet. Clearly, more research is needed to examine the effect of food-specific go/no-go training on weight loss, especially in overweight and obese individuals.
Overall, it is possible that at least two mechanisms can be responsible for the effects of a food-specific go/no-go training. First, repeatedly inhibiting responses toward no‐go food cues (e.g. high-calorie foods pictures) during food-specific go/no-go training may lead to the formation of food cue‐stop reaction associations, that is, automatic inhibition (Houben and Jansen, 2015; Veling et al., 2017). Second, food cues may be devalued after repeatedly inhibiting responses to them (Chen et al., 2018a, 2018b). A combination of brain imaging and behavioral data can and should be used to assess those possible mechanisms. In line with this, three previous studies have investigated electrophysiological underpinnings of this phenomena but reached inconsistent conclusions (van de Vijver et al., 2018; Aulbach et al., 2020; Carbine et al., 2021). van de Vijver et al. (2018) found that after a go/no-go training, larger increases in theta power at frontal midline electrodes were present upon presentation of food pictures previously associated with a no-go response. However, the two remaining studies showed that neither single session nor multiple sessions of a food-specific go/no-go training affected food-related N2 event-related potential (ERP) response (an indicator of inhibitory control processes) to go- or no-go trials after training (Aulbach et al., 2020; Carbine et al., 2021). Further, Stice et al. (2017) have used functional magnetic resonance imaging (fMRI) to examine neural mechanisms of a new multi-faceted treatment that included both response inhibition training and attention training. This study found that completing a food response and attention training was associated with significant reductions in reward (e.g. mid-insula, putamen) and attention (e.g. inferior parietal lobe) regions’ response to high-calorie food images. However, this study could not separate the unique contributions of these training tasks in changing neural responses to high-calorie food cues (Stice et al., 2017).
In our pilot study, a sample of overweight individuals divided into training (food-specific go/no-go training) and control (non-food-specific go/no-go training) groups performed a passive food picture viewing task in an fMRI scanner and rated the pictures used in the task before and after a five-week-long training. Based on the findings of previous relevant studies (e.g. Chen et al., 2018a,b) and the aforementioned mechanisms of food-specific go/no-go training (e.g. Stice et al., 2016; Veling et al., 2017), we hypothesized that food-specific go/no-go training would be associated with changes in the activity of inhibitory control- and reward-related brain regions, food valuation and weight.
Methods
Participants
Fifty-two overweight male and female undergraduate students from Southwest University (93% female; mean BMI = 25.6; 27% participants with obesity), aged 17–23 years, participated in this study. Subjects were recruited via on-campus advertisements. Inclusion criteria required that participants had weight concerns, were willing to participate in the current weight control trials and had a BMI of 23 or greater (e.g. cut-off value of BMI in Asian adults) (Zeng et al., 2014). Exclusion criteria were self-reported current eating disorders (e.g. binge eating), mental disorders (e.g. depression) or head injuries. Because one participant dropped out of the experiment, the final sample consisted of 51 participants. In addition, three participants refused to participate in the fMRI scanning, six were excluded from the imaging analyses due to excessive in-scanner head motion (i.e. mean frame wise displacement ≥2 standard deviation above the sample mean) (Di Martino et al., 2014). Therefore, the sample size for the imaging data analysis is 42 participants.
Power analyses were conducted in G*Power (Version 3.1) (Faul et al., 2007) with the following parameters1: (i) repeated measures ANOVA, power = 0.80, α = 0.05 and small (η2 = 0.01), medium (η2 = 0.06) or large (η2 = 0.14) effect sizes (e.g. Cohen, 1988); (ii) correlation, power = 0.80, α = 0.05 and small (r = 0.1), medium (r = 0.3) or large (r = 0.5) effect sizes. Power analysis for repeated measures ANOVA yielded sample sizes of 198, 34 and 16 for small, medium and large effect sizes, respectively. Power analysis for correlation yielded sample sizes of 782, 84 and 29 for small, medium and large effect sizes, respectively. Therefore, readers should keep in mind that the sample size of current pilot study was only able to detect medium-to-large effects.
Measurements
fMRI data acquisition and food image exposure paradigm
MRI images were acquired using a Siemens TRIO 3-Tesla scanner. High-resolution T1-weighted 3D fast-field echo sequences were obtained for anatomical reference (TR = 1900 ms; TE = 2.52 ms; flip angle = 9°; FOV = 250 × 250 mm2; slices =176; thickness = 1.0 mm; voxel size = 1 × 1 ×1 mm3). During the functional task, blood oxygen level dependent (BOLD) imaging was performed using a single shot echo-planar imaging (EPI) sequence (TR = 2000 ms; TE = 30 ms; flip angle = 90°; FOV = 220 × 220 mm2; matrix size = 64 × 64; voxel size = 3.4 × 3.4 × 3.0 mm3; interslice skip = 0.99 mm; Slices = 33; thickness = 3.0 mm).
During the fMRI scan, 40 images of high-calorie foods and 40 images of low-calorie foods were presented to participants using an event-related design in a random order to assess neural response to these food images (80 images in total). There were two runs in total, each run consisted of 20 pictures of each type (40 images in each run), and each image was presented one time. To better assess the generalizability of the training effects, half of these food images were from the food-specific go/no-go training and half were not (Stice et al., 2017). After a 2 s fixation period, a picture was presented on the screen for 4 s. A 2–4 s inter-trial interval occurred between each picture during which a blank screen with a fixation cross was presented.
Hunger rating
Participants’ current feelings of hunger were assessed with the following question: ‘How hungry do you feel right now?’ ranging from 1 (not hungry at all) to 9 (extremely hungry).
Pre-training food evaluation
Participants of both groups were randomly presented with 80 color images of high-calorie foods and 80 images of low-calorie foods that were included in the training, one by one. Images were presented in the center of the screen, with the question, ‘How attractive does this food item look to you?’ presented at the bottom of the screen (Chen et al., 2018b). For each image, participants gave a rating by clicking on a 100-point slider ranging from 0 (not at all attractive) to 100 (extremely attractive). The food image remained on the screen until participants pressed a key (‘continue’) to confirm their rating and moved on to the next question.
Food-Specific go/no-go training and control training
The training task parameters are similar to Lawrence et al. (2015). The evaluated 80 high-calorie food images and 80 low-calorie food images were used in the food-specific go/no-go training that was performed by the training group. During the task, 160 food images and 40 water glass fillers images (height = 235, width = 264) were randomly presented individually on the left or right-hand side of a computer screen for 1250 ms followed by a 500 ms inter-stimulus interval. The numbers of food and filler images are consistent with Stice et al. (2017). Participants were told to press a button as quickly and accurately as possible to indicate the side of the stimulus presentation (go-trial, ‘c’ for left and ‘m’ for right) (for an incorrect response, a red cross appeared for 500 ms; for a missed response, the message ‘too slow!’ appeared for 500 ms). On half of the trials, a rectangular frame surrounding the picture was bold, which was a signal for the participants to withhold their response (no-go trial). High-calorie food images were always paired with no-go trials, whereas low-calorie foods were always paired with go-trials. The water glass filler images were equally often associated with go and no-go trials. Training session consisted of one block of 200 trials (e.g. 100 go trails and 100 no-go trails) and took approximately 10 min to complete.
In the control group, participants completed an identical task except that non-food images replaced the food images (Houben and Jansen, 2015; Stice et al., 2017). In total, 80 flower images were paired with no-go trials and 80 household items images were paired with go-trials. The filler images were 40 animal pictures.
Post-training food evaluation
After the five-week-long training, participants rated the 160 food images that were included in the training again, using the same procedure as in the pre-training evaluation.
Procedure
Participants were randomly assigned to a training group (n = 25, fMRI sample n = 21) or a control group (n = 27, fMRI sample n = 21). Participants visited our lab seven times. During the first visit (visit #1), participants completed demographic surveys and weight measurement. After the removal of shoes and coats, weight was assessed using a digital scale. Afterwards, participants completed an fMRI scan. During visit #2, one to two days after visit #1, participants rated the attractiveness of 80 color images of high-calorie foods (e.g. crisps) and 80 images of low-calorie foods (e.g. vegetables) and completed the first of their training sessions. Food images were selected from a food picture database (Blechert et al., 2014) and an unpublished database from our group that was modified to match the size, resolution and luminance of the first set of food pictures. During visits #3–#5, participants returned to the lab to complete their go/no-go training sessions. During visit #6, immediately after their fifth and last training session, participants rated the attractiveness of the same 160 food pictures again. One-two days after visit #6, participants completed their second fMRI scan and weight measurement (visit #7). In addition, participants’ hunger level was also measured during the first, second, sixth and final visits. The go/no-go trainings were repeated once per week during visits #2–#6. The same images were used in the training during each visit. The interval time between each visit was six days, and participants completed 50 min of training in total over five weeks (see Figure 1 for the depiction of study procedure). All images that were used in both the fMRI task and the trainings are posted to the Open Science Framework (https://osf.io/x38v6/).
Fig. 1.

Depiction of study procedure.
Statistical analysis
Behavioral data analysis
Behavioral data were analyzed using SPSS Software 24.0. Independent samples t-tests or chi-square tests were conducted to test between‐group differences in age, sex, weight and food evaluation pre-training. Training‐by‐time effects were tested with several repeated measures ANOVAs. Specifically, the effects of training on ratings of food images were tested with a 2 (training or control) × 2 (pre-training on visit #2 or post-training on visit #6) design; weight change and neural responses to food images were tested with a 2 (training or control) × 2 (pre-training on visit #1 or post-training on visit #7) design. When analyzing the effect of training on food pictures ratings, hunger difference between the pre-training on visit #2 and post-training on visit #6 was added into the ANOVA model as a covariate of no interest, given the influence of hunger on food evaluation (Chen et al., 2018b). For all variables, cases with more than 2.5 standard deviations from their group mean were deemed to be outliers and excluded from the analysis (Houben and Jansen, 2015).
Functional image data analyses
fMRI data preprocessing was conducted using the Data Processing Assistant for Resting-State fMRI (Chao-Gan and Yu-Feng, 2010) in the following manner: first, slice timing and spatial realignment to the first image to correct for head motion was performed. Subsequently, each participant’s functional images were normalized to EPI templates based on the Montreal Neurological Institute space (resampling voxel size was 3 × 3 ×3 mm3) and then spatially smoothed with a Gaussian kernel of 8 mm at full width half-maximum.
Neuroimaging data were analyzed using SPM12 software. At the subject level, in the first-level analysis, the three conditions (high-calorie food images, low-calorie food images, and filler images) and six motion regressors were modeled using the general linear model, with delta functions convolved with a canonical hemodynamic response function. The critical contrast of interest was within-subject activations during high-calorie food images vs low-calorie food images pre-training and post-training (e.g. pre-training high-calorie food images> low-calorie food images”). A two-way 2 (training, control) × 2 (pre, post) repeated measures ANOVA with hunger as a covariate of no interest was then conducted at the group level using contrast images from the subject level. This was done to examine group differences in change in neural response to high-calorie and low-calorie food images. Significant clusters were identified using the cluster-forming threshold of P < 0.001 (uncorrected) and a cluster-level family wise error rate-corrected threshold P < 0.05. Effect sizes (r) were derived from the Z-values (Z/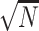 ).
).
Finally, we addressed the fact that 50% of food pictures presented in our fMRI task were used in the inhibitory training for the training group. This could mean that our fMRI results were not due to the training itself, but rather due to repeated exposure of the training group to these stimuli. Hence, we also analyzed the data using only pictures that were not presented in the inhibitory training. Here, the analytical steps were identical to the ones described above.
Ethics
All participants provided written informed consent to participate in the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committees.
Results
Behavioral results
Training task performance
We found no group differences in terms of demographic factors or study variables at baseline (Table 1). Participants in all training sessions made little errors (e.g. average accuracy > 95%), demonstrating that they were engaged in the training (Supplementary material Table S1). Further, as demonstrated by the higher accuracy rates to the 100% vs 50% associated images, both groups showed similar learning of stimulus-specific go and no-go associations (see the Supplementary material for more details).
Table 1.
Test of group differences on demographic characteristics and baseline measures
| Training group (n = 24) | Control group (n = 27) | Test statistics | ||
|---|---|---|---|---|
| Women (n) | 23 | 25 | x 2(1, 51) = − 0.01 | P > 0.05 |
| Age | 19.24 (1.30) | 19.70 (1.79) | t(49) = −1.02 | P > 0.05 |
| Weight (in kg) | 69.50 (12.27) | 70.07 (12.48) | t(49) = −0.17 | P > 0.05 |
| High-calorie foods evaluation | 62.37 (14.18)a | 54.82 (17.70)b | t(45) = 1.59 | P > 0.05 |
| Low-calorie foods evaluation | 33.67 (16.35)c | 36.00 (15.61) | t(47) = −0.51 | P > 0.05 |
Note: n = sample size; mean and standard deviations are presented for continuous variables. aOne outlier and two missing values in this group; bone outlier in this group; ctwo missing values in this group.
Training effects on food evaluation
For high-calorie food evaluation, results revealed a significant interaction effect between group and time (F (1, 44) = 6.62, P = 0.01, partial η2 = 0.13), but no main effect of time (F (1, 44) = 3.17, P > 0.05) or group (F (1, 44) = 0.95, P > 0.05) (Figure 2). Decomposing this interaction, we found that high-calorie food evaluation significantly decreased from pre-training (M = 62.37, SD = 14.18) to post-training (M = 58.20, SD = 14.64) in the training group (mean difference = 4.75, F(1, 44) = 5.59, P = 0.02, partial η2 = 0.11), but not in the control group (F (1, 44) =1.56, P > 0.05). No significant effects were found for low-calorie food evaluation (Table 2).
Fig. 2.

Pre- to post-evaluation of high-calorie food images in the food-specific go/no-go training vs control condition. Error bars represent ±1 SEM. *P < 0.05. ns: none significant.
Table 2.
Tested outcomes before and after the training
| Pre-training | Post-training | |||
|---|---|---|---|---|
| Control group | Training group | Control group | Training group | |
| Weight (in kg) | 70.07 (12.48) | 69.50 (12.27) | 70.72 (12.87) | 69.63 (11.18) |
| High-calorie foods evaluation | 54.82 (17.70) | 62.37 (14.18) | 56.60 (17.03) | 58.20 (14.64) |
| Low-calorie foods evaluation | 36.00 (15.61) | 33.67 (16.35) | 38.05 (15.60) | 32.99 (15.91) |
Note: mean and standard deviations are presented.
Training effects on weight change
Results showed that weight did not change over time, did not differ as a function of group, and did not show a significant group × time interaction (all Ps > 0.05) (Table 2).
Training effects on neural response to high-calorie vs low-calorie food pictures
Whole brain between-group analysis revealed significant group × time interactions in the left middle frontal gyrus (MFG; r = 0.68; Figure 3A) and right inferior frontal gyrus (IFG)/right mid-insula (r’s = 0.66 and 0.58; Figure 3B and C; Table 3) in response to high-calorie food images > low-calorie food images. Specifically, the training group showed decreases in BOLD activity in these brain regions and the control group showed minor increases in BOLD activity. Post-hoc analyses tested whether changes in these regions were significant within groups. The parameter estimates at the individual level were extracted from the peak coordinates of significant clusters (i.e. MFG [MNI coordinates: −36, 51, 15], IFG [MNI coordinates: 48, 12, 15], mid-insula [MNI coordinates: 42, 3, 3]). Bonferroni tests were used to correct for the number of tests (P < 0.05/3 peaks = 0.017). The results showed that the training group showed significant decreases in the left MFG (mean difference = 0.34, F (1, 40) = 12.94, P = 0.001, partial η2 = 0.24), right IFG (mean difference = 0.26, F (1, 40) = 11.04, P = 0.002, partial η2 = 0.22) and the right mid-insula (mean difference = 0.23, F (1, 40) = 7.27, P = 0.010, Partial η2 = 0.15), while the control group showed significant increase in the IFG (mean difference = 0.21, F (1, 40) = 11.04, P = 0.008, partial η2 = 0.16).
Fig. 3.

Pre- to post-BOLD response decreases in the (A) left middle frontal gyrus (MNI: −36, 51, 15), (B) right inferior frontal gyrus (MNI: 48, 12, 15) and (C) right mid-insula (MNI: 42, 3, 3) in response to high-calorie food images > low-calorie food images in the food-specific go/no-go training vs control condition. Error bars represent ±1 SEM. **P < 0.01. ns: none significant.
Table 3.
ANOVA interaction in brain activation during exposure to food images
| Contrasts and regions | k | Z-value | MNI coordinates | r |
|---|---|---|---|---|
| Training > control | ||||
| High-calorie > low-calorie | ||||
| Pre > post: | ||||
| Left middle frontal gyrus | 93 | 4.38 | −36, 51, 15 | 0.68 |
| 3.89 | −30, 48, 24 | 0.60 | ||
| 3.35 | −48, 45, 15 | 0.52 | ||
| Right inferior frontal gyrus | 93 | 4.29 | 48, 12, 15 | 0.66 |
| Right mid-insula | 3.75 | 42, 3, 3 | 0.58 |
Notes: Peaks within the regions were considered significant at P < 0.001 and corrected for multiple comparisons at cluster level, P < 0.05.
In addition, our results showed no significant group × time interaction in BOLD signal in response to low-calorie food images > high-calorie food images.
Next, we tested whether pre–post changes in BOLD response of the identified brain regions correlated with pre–post changes in the high-calorie food evaluation across all participants using hunger as a covariate of no interest. The results showed significant correlation between pre–post changes in the high-calorie food evaluation and pre–post changes in BOLD activity in the right mid-insula (r= 0.44, P = 0.006; MNI coordinates: 42, 3, 3) (Figure 4).
Fig. 4.

Correlation between pre to post change of high-calorie food valuation and pre to post change of mid-insula activation in the full sample.
Finally, we tested whether our main results would replicate when only using food pictures not shown during the inhibitory training. Indeed, we found clusters in the MFG and IFG resembling the ones in our main analysis, suggesting that our main results were not merely due to repeated exposure of the training group to some of the food stimuli (see the Supplementary Materials).
Discussion
In sum, our study investigated the efficacy of a food-specific go/no-go training compared to a control training, completed one time per week over five weeks in individuals with overweight. Behaviorally, compared to a control group, our training group exhibited significant reductions in high-calorie, but not low-calorie foods valuation. On a neural level, in the training group we observed significant reduced neural activations in inhibitory control- and reward-related brain regions. Finally, we did not observe an effect of training on weight change.
Our results suggest that food-specific go/no-go training was effective at reducing the valuation of high-calorie foods. Several meta-analyses have already confirmed the effectiveness of similar interventions on eating behaviors (Allom et al., 2016; Jones et al., 2016; Veling et al., 2020; Yang et al., 2019a, but see Carbine and Larson, 2019). However, fewer studies have examined the mechanisms underpinning this training effect. Houben and Jansen (2015) found that repeated pairing of chocolate stimuli with a stop response reduced automatic chocolate-go associations (i.e. decreased implicit association test score) (Houben and Jansen, 2015). Chen et al. (2018b) observed a devaluation of high-calorie pictures after a similar go/no-go training (216 trials in total), however, only in hungry obese and normal-weight individuals (Chen et al., 2018b). Our results expand on these previous findings by showing that a five-week-long food-specific go/no-go training could reduce the evaluation of high-calorie foods in overweight individuals.
To the best of our knowledge, current study is the first to use fMRI to investigate the neural mechanism underpinning the effect of food-specific go/no-go training. Three ERP studies have investigated the electrophysiological mechanisms underpinning a similar training (van de Vijver et al., 2018; Aulbach et al., 2020; Carbine et al., 2021). van de Vijver et al. (2018) found that after a go/no-go training, larger increases in theta power at frontal midline electrodes were present upon presentation of food pictures previously associated with a no-go response. However, two studies showed that neither single session nor multiple sessions of a food-specific go/no-go training affected food-related N2 ERP response (an indicator of inhibitory control processes) to go- or no-go trials after training (Aulbach et al., 2020; Carbine et al., 2021). It should be noted, however, that ERP response was recorded during passive viewing of food images in van de Vijver et al. (2018) and during the completion of the go/no-go task in Aulbach et al. (2020) and Carbine et al. (2021). This difference in task characteristic might explain the observed differing effects. So far, one study has used fMRI to examine the neural mechanisms of a multi-faceted treatment that included both response inhibition training (food-specific go/no-go training) and attention training (Stice et al., 2017). However, this study could not separate the unique contributions of these training tasks to overall training effects. Our results expand on these previous findings by showing that completing multiple sessions of a food-specific go/no-go training is associated with significant pre–post reductions in neural responsivity to high-calorie vs low-calorie food images in IFG, MFG and mid-insula.
IFG and MFG activations have been linked to inhibitory control (Batterink et al., 2010; Zhang et al., 2017). Counterintuitively, these inhibitory control regions showed decreased, rather than increased, activations to high-calorie vs low-calorie food images after our training. This could be explained from at least two perspectives. First, decreased evaluation of high-calorie images may be related to decreased activation in inhibitory control regions because these images were rated as less attractive post- as compared to pre-training. Second, recent work suggested that food-specific go/no-go training could not strengthen top-down inhibitory control toward foods and should not be viewed as an inhibitory control training (e.g. Veling et al., 2017; Johannes et al., 2021). Instead, researchers proposed an automatic inhibition hypothesis (Verbruggen and Logan, 2008; Veling et al., 2014; Verbruggen et al., 2014; Best et al., 2016). According to this, repeated inhibition toward specific stimuli (e.g. high-calorie food cues) during go/no-go training may create automatic stimulus-stop associations, which may reduce the need for top-down recruitment of executive inhibitory control when these no-go stimuli are encountered outside the training. To sum up, decreased inhibitory control region responses to high-calorie vs low-calorie food images may be related to a training-induced devaluation of high-calorie images and/or the development of automatic high-calorie food cues-stop associations.
This potential development of automatic stimulus-stop association may represent a key advantage of go/no-go training over behavioral weight loss interventions. Specifically, some behavioral interventions involved prolonged caloric deprivation which could increase the reward value of high-calorie foods/food cues (e.g. Stice et al., 2013). In contrast, go/no-go training relies on implicit training and creates a bottom-up association (e.g. high-calorie foods-stop), which might be more effective for individuals with an automatic attentional or approach response to high‐calorie foods and/or weak executive control. In addition, given some primary evidence suggested that trained automatic stimulus-stop associations might generalize to untrained stimuli when training focused on a category level (e.g. healthy food = go; unhealthy food = no-go) (Chen et al., 2016; Folkvord et al., 2016; Adams et al., 2017; Veling et al., 2017), this potential generalization effect might be another advantage of food-specific go/no-go training.
Next, the food-specific go/no-go training in our study was also associated with greater reductions in mid-insula activities in response to high-calorie foods in the training vs control group. The mid-insula plays an important role in (food) reward processing (Liu et al., 2011; Sescousse et al., 2013) and sensory-based food craving (e.g. Pelchat et al., 2004; Zhou et al., 2019). For instance, a meta-analysis of functional neuroimaging studies showed that mid-insula was activated by positive reward (Liu et al., 2011). Sescousse et al. (2013) found mid-insula activation was involved in processing taste reward outcomes. In addition, Zhou et al. (2019) found that mid-insula activation was positively associated with subjective food craving. Therefore, current findings could suggest that the food-specific go/no-go training may reduce brain activations to high-calorie food images in the areas related to food reward processing. Importantly, reductions in responsivity in the mid-insula were positively associated with the decrease in the evaluation of the high-calorie ‘inhibited’ food items. These findings suggest that the reductions in the mid-insula could have contributed to the decrease in the evaluation of the high-calorie foods and thus provide a potential neural mechanism for the devaluation effect within our food-specific go/no-go training.
Our findings of significant pre–post reductions in inhibitory control-related regions vary from the results of some previous related studies (e.g. Stice et al., 2017; Aulbach et al., 2020; Carbine et al., 2021). Although both Stice et al. (2017) and current study found a training effect on reward-related regions (e.g. mid-insula), Stice et al. (2017) did not report any changes in the regions that have been implicated in inhibitory control. It should be noted that Stice et al. (2017) utilized a multi-faceted food response and attention training, while we were specifically interested in the effects of food-specific go/no-go training. It is possible that the use of inhibition training alone in current study may be more sensitive to activation changes in inhibitory control-related regions. In addition, our findings contradict two previous ERP studies, which reported that performing go/no-go training did not alter N2 component amplitude toward high-calorie foods (Aulbach et al., 2020; Carbine et al., 2021). This result difference could be explained from at least three perspectives. First, given the N2 response did not change over training irrespective of the task-irrelevant food images in their study, Aulbach et al. (2020) proposed that that ‘N2 component is not a good index of behavioral training effects in the go/no-go paradigm’ (p. 9, paragraph 4). Second, both Aulbach et al. (2020) and Carbine et al. (2021) examined neural changes during the completion of the go/no-go task, while current study investigated the influence of go/no-go training on the perception of food images during a food viewing task. Third, following Stice et al. (2017), current study assessed neural changes to the food pictures that were both used and not used in the training, while Carbine et al. (2021) used different food images in the go/no-go training and the ERP go/no-go task. This last point is related to a potential limitation of our study design, whereby repeated exposure to food stimuli in the go/no-go task in the training group, but not the inhibitory training itself, could affect group differences in brain responses to food images in the post-training fMRI task. To address this, we reanalyzed the fMRI data using only trials with pictures that were not used in the inhibitory training and found similar results to our main analyses.
It should be noted that the control group showed a significant increase in activity in the IFG. Similarly, Stice et al. (2017) also showed significant increase in some brain regions (e.g. superior temporal gyrus) in a control group. Different from the training group, after the pre-training rating, participants in the control group were not exposed to high-calorie food images during the go/no-go training. Thus, this lack of presentation of high-calorie images in the control group may cause brain activity increase in response to these highly appetitive food items.
Finally, consistent with two recent studies (Forman et al., 2019; Carbine et al., 2021), but different than in Lawrence et al. (2015) and in Veling et al. (2014), we did not find a significant effect of food-specific go/no-go training on weight change. Several study design differences might explain the discrepancy between the results. For example, Lawrence et al. (2015) required that upon recruitment participants also exhibited loss of control over eating. Their training was also focused on only three high‐calorie foods relevant to the participants. Therefore, future inhibition training research might benefit from using target foods in the target population (Yang et al., 2019a).
Our study has several limitations. First, because of the relatively small sample size, readers should keep in mind that the current pilot study was only able to detect medium-to-large effects. Future preregistration studies with larger samples and sufficient power (e.g. power to detect small effects) are needed to examine whether current results are replicable. Second, we did not investigate long-term effect of the food-specific go/no-go training. Third, the sample was predominantly female, which may limit generalizability to male samples. In addition, we have not assessed the exercise levels and dieting status of the participants, which might confound weight loss results. Finally, there were no ratings of control images used in the go/no-go training.
To conclude, our findings highlighted that a five-week-long food-specific go/no-go training in overweight individuals was related to a reduction in food evaluation and reduced neural activations in inhibitory control- and reward-related brain regions in response to food cues. However, because the current pilot study focused on immediate training effects in an overweight sample, long-duration studies with samples including individuals with obesity are needed to examine the generalizability of these results.
Supplementary Material
Acknowledgements
The authors would like to thank the reviewers for their time spent on reviewing our manuscript and their comments helping us improving the article.
Footnotes
Power analyses were conducted based on reviewer comments and not before conducting the study.
Contributor Information
Yingkai Yang, Faculty of Psychology, Southwest University, Chongqing 400715, China.
Filip Morys, Montreal Neurological Institute, McGill University, Montreal, QC H3A 2B4, Canada.
Qian Wu, The Lab of Mental Health and Social Adaptation, Faculty of Psychology, Research Center of Mental Health Education, Southwest University, Chongqing 400715, China.
Jiwen Li, Faculty of Psychology, Southwest University, Chongqing 400715, China.
Hong Chen, Faculty of Psychology, Southwest University, Chongqing 400715, China; Key Laboratory of Cognition and Personality (Ministry of Education), Southwest University, Chongqing 400715, China.
Funding
This work was supported by China Postdoctoral Science Foundation, 7810100287, and Fundamental Research Funds for the Central Universities, SWU 7110200685.
Conflict of interest
The authors declared that they had no conflict of interest with respect to their authorship or the publication of this article.
Supplementary data
Supplementary data is available at SCAN online.
Data availability
The study data are available on request from the corresponding author.
References
- Adams R.C., Lawrence N.S., Verbruggen F., Chambers C.D. (2017). Training response inhibition to reduce food consumption: mechanisms, stimulus specificity and appropriate training protocols. Appetite, 109, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allom V., Mullan B., Hagger M. (2016). Does inhibitory control training improve health behaviour? A meta-analysis. Health Psychology Review, 10(2), 168–86. [DOI] [PubMed] [Google Scholar]
- Aulbach M.B., Harjunen V.J., Spape M., Knittle K., Haukkala A., Ravaja N. (2020). No evidence of calorie-related modulation of N2 in food-related Go/No-Go training: a preregistered ERP study. Psychophysiology, 57(4), e13518. [DOI] [PubMed] [Google Scholar]
- Batterink L., Yokum S., Stice E. (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage, 52(4), 1696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Ho C.Y., Richard J.M., DiFeliceantonio A.G. (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best M., Lawrence N.S., Logan G.D., McLaren I.P., Verbruggen F. (2016). Should I stop or should I go? The role of associations and expectancies. Journal of Experimental Psychology. Human Perception and Performance, 42(1), 115–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J., Meule A., Busch N.A., Ohla K. (2014). Food-pics: an image database for experimental research on eating and appetite. Frontiers in Psychology, 5, 617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbine K.A., Muir A.M., Allen W.D., et al. (2021). Does inhibitory control training reduce weight and caloric intake in adults with overweight and obesity? A pre-registered, randomized controlled event-related potential (ERP) study. Behaviour Research and Therapy, 136, 103784. [DOI] [PubMed] [Google Scholar]
- Carbine K.A., Larson M.J. (2019). Quantifying the presence of evidential value and selective reporting in food-related inhibitory control training: a p-curve analysis. Health Psychology Review, 13(3), 318–43. [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Veling H., Dijksterhuis A., Holland R.W. (2016). How does not responding to appetitive stimuli cause devaluation: evaluative conditioning or response inhibition? Journal of Experimental Psychology. General, 145(12), 1687–701. [DOI] [PubMed] [Google Scholar]
- Chen Z., Veling H., Dijksterhuis A., Holland R.W. (2018a). Do impulsive individuals benefit more from food go/no-go training? Testing the role of inhibition capacity in the no-go devaluation effect. Appetite, 124, 99–110. [DOI] [PubMed] [Google Scholar]
- Chen Z., Veling H., de Vries S.P., et al. (2018b). Go/no-go training changes food evaluation in both morbidly obese and normal-weight individuals. Journal of Consulting and Clinical Psychology, 86(12), 980–90. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic. [Google Scholar]
- Devoto F., Zapparoli L., Bonandrini R., et al. (2018). Hungry brains: a meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals. Neuroscience and Biobehavioral Reviews, 94, 271–85. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Yan C.G., Li Q., et al. (2014). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. MolecularPsychiatry, 19(6), 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007). G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–91. [DOI] [PubMed] [Google Scholar]
- Folkvord F., Veling H., Hoeken H. (2016). Targeting implicit approach reactions to snack food in children: effects on intake. Health Psychology, 35(8), 919–22. [DOI] [PubMed] [Google Scholar]
- Forcano L., Mata F., de La Torre R., Verdejo-Garcia A. (2018). Cognitive and neuromodulation strategies for unhealthy eating and obesity: systematic review and discussion of neurocognitive mechanisms. Neuroscience and Biobehavioral Reviews, 87, 161–91. [DOI] [PubMed] [Google Scholar]
- Forman E.M., Manasse S.M., Dallal D.H., et al. (2019). Computerized neurocognitive training for improving dietary health and facilitating weight loss. Journal of Behavioral Medicine, 42(6), 1029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia I., Michaud A., Dadar M., et al. (2019). Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. International Journal of Obesity, 43(5), 943–51. [DOI] [PubMed] [Google Scholar]
- Gunstad J., Sanborn V., Hawkins M. (2020). Cognitive dysfunction is a risk factor for overeating and obesity. American Psychologist, 75(2), 219–34. [DOI] [PubMed] [Google Scholar]
- Houben K., Jansen A. (2015). Chocolate equals stop. Chocolate-specific inhibition training reduces chocolate intake and go associations with chocolate. Appetite, 87, 318–23. [DOI] [PubMed] [Google Scholar]
- Johannes N., Buijzen M., Veling H. (2021). Beyond inhibitory control training: inactions and actions influence smartphone app use through changes in explicit liking. Journal of Experimental Psychology. General, 150(3), 431–45. [DOI] [PubMed] [Google Scholar]
- Jones A., Di Lemma L.C., Robinson E., et al. (2016). Inhibitory control training for appetitive behaviour change: a meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite, 97, 16–28. [DOI] [PubMed] [Google Scholar]
- Jones A., Hardman C.A., Lawrence N., Field M. (2018). Cognitive training as a potential treatment for overweight and obesity: a critical review of the evidence. Appetite, 124, 50–67. [DOI] [PubMed] [Google Scholar]
- Lawrence N.S., O’Sullivan J., Parslow D., et al. (2015). Training response inhibition to food is associated with weight loss and reduced energy intake. Appetite, 95, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 35(5), 1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe C.J., Reichelt A.C., Hall P.A. (2019). The prefrontal cortex and obesity: a health neuroscience perspective. Trends in Cognitive Sciences, 23(4), 349–61. [DOI] [PubMed] [Google Scholar]
- Luis-Ruiz S., Caldu X., Sanchez-Castaneda C., Pueyo R., Garolera M., Jurado M.A. (2020). Is cognitive training an effective tool for improving cognitive function and real-life behaviour in healthy children and adolescents? A systematic review. Neuroscience and Biobehavioral Reviews, 116, 268–82. [DOI] [PubMed] [Google Scholar]
- Marteau T.M., Hollands G.J., Fletcher P.C. (2012). Changing human behavior to prevent disease: the importance of targeting automatic processes. Science, 337(6101), 1492–5. [DOI] [PubMed] [Google Scholar]
- Mehl N., Morys F., Villringer A., Horstmann A. (2019). Unhealthy yet avoidable-how cognitive bias modification alters behavioral and brain responses to food cues in individuals with obesity. Nutrients, 11, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas J.F., Verdejo-Garcia A., Vadillo M.A. (2021). The evidential value of research on cognitive training to change food-related biases and unhealthy eating behavior: a systematic review and p-curve analysis. Obesity Reviews, 22, e13338.doi: 10.1111/obr.13338. [DOI] [PubMed] [Google Scholar]
- Oomen D., Grol M., Spronk D., Booth C., Fox E. (2018). Beating uncontrolled eating: training inhibitory control to reduce food intake and food cue sensitivity. Appetite, 131, 73–83. [DOI] [PubMed] [Google Scholar]
- Pelchat M.L., Johnson A., Chan R., Valdez J., Ragland J.D. (2004). Images of desire: food-craving activation during fMRI. Neuroimage, 23(4), 1486–93. [DOI] [PubMed] [Google Scholar]
- Robinson E., Roberts C., Vainik U., Jones A. (2020). The psychology of obesity: an umbrella review and evidence-based map of the psychological correlates of heavier body weight. Neuroscience and Biobehavioral Reviews, 119, 468–80. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Caldu X., Segura B., Dreher J.C. (2013). Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(4), 681–96. [DOI] [PubMed] [Google Scholar]
- Smith E., Hay P., Campbell L., Trollor J.N. (2011). A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obesity Reviews, 12(9), 740–55. [DOI] [PubMed] [Google Scholar]
- Stice E., Burger K., Yokum S. (2013). Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage, 67, 322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Lawrence N.S., Kemps E., Veling H. (2016). Training motor responses to food: a novel treatment for obesity targeting implicit processes. Clinical Psychology Review, 49, 16–27. [DOI] [PubMed] [Google Scholar]
- Stice E., Yokum S., Veling H., Kemps E., Lawrence N.S. (2017). Pilot test of a novel food response and attention training treatment for obesity: brain imaging data suggest actions shape valuation. Behaviour Research and Therapy, 94, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Burger K. (2019). Neural vulnerability factors for obesity. Clinical Psychology Review, 68, 38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S. (2016). Neural vulnerability factors that increase risk for future weight gain. Psychological Bulletin, 142(5), 447–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainik U., Baker T.E., Dadar M., et al. (2018). Neurobehavioral correlates of obesity are largely heritable. Proceedings of the National Academy of Sciences, 115(37), 9312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver I., van Schie H.T., Veling H., van Dooren R., Holland R.W. (2018). Go/no-go training affects frontal midline theta and mu oscillations to passively observed food stimuli. Neuropsychologia, 119, 280–91. [DOI] [PubMed] [Google Scholar]
- Veling H., van Koningsbruggen G.M., Aarts H., Stroebe W. (2014). Targeting impulsive processes of eating behavior via the internet. Effects on body weight. Appetite, 78, 102–9. [DOI] [PubMed] [Google Scholar]
- Veling H., Lawrence N.S., Chen Z., van Koningsbruggen G.M., Holland R.W. (2017). What is trained during food go/no-go training? A review focusing on mechanisms and a research agenda. Current Addiction Reports, 4(1), 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veling H., Chen Z., Liu H., Quandt J., Holland R.W. (2020). Updating the p-curve analysis of Carbine and Larson with results from preregistered experiments. Health Psychology Review, 14(2), 215–9. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Best M., Bowditch W.A., Stevens T., McLaren I.P. (2014). The inhibitory control reflex. Neuropsychologia, 65, 263–78. [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Logan G.D. (2008). Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. Journal of Experimental Psychology. General, 137(4), 649–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2021). Obesity. Available: https://www.who.int/news-room/facts-in-pictures/detail/6-facts-on-obesity [June 9, 2021].
- Yang Y., Shields G.S., Guo C., Liu Y. (2018). Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neuroscience and Biobehavioral Reviews, 84, 225–44. [DOI] [PubMed] [Google Scholar]
- Yang Y., Shields G.S., Wu Q., Liu Y., Chen H., Guo C. (2019a). Cognitive training on eating behaviour and weight loss: a meta-analysis and systematic review. Obesity Reviews, 20(11), 1628–41. [DOI] [PubMed] [Google Scholar]
- Yang Y., Shields G.S., Wu Q., Liu Y., Guo C. (2019b). Obesity is associated with poor working memory in women, not men: findings from a nationally representative dataset of U.S. adults. Eating Behaviors, 35, 101338. [DOI] [PubMed] [Google Scholar]
- Yang Y., Shields G.S., Wu Q., Liu Y., Chen H., Guo C. (2020). The association between obesity and lower working memory is mediated by inflammation: findings from a nationally representative dataset of U.S. adults. Brain, Behavior, and Immunity, 84, 173–9. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wu Q., Morys F. (2021). Brain responses to high-calorie visual food cues in individuals with normal-weight or obesity: an activation likelihood estimation meta-analysis. Brain Sciences, 11(12), 1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q., He Y., Dong S., et al. (2014). Optimal cut-off values of BMI, waist circumference and waist:height ratio for defining obesity in Chinese adults. British Journal of Nutrition, 112(10), 1735–44. [DOI] [PubMed] [Google Scholar]
- Zhang R., Geng X., Lee T.M.C. (2017). Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Structure & Function, 222(9), 3973–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Gao X., Small D.M., Chen H. (2019). Extreme spicy food cravers displayed increased brain activity in response to pictures of foods containing chili peppers: an fMRI study. Appetite, 142, 104379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study data are available on request from the corresponding author.


