Abstract
The sex difference in the prevalence of autism spectrum disorder (ASD) may be magnified by sex differences on diagnostic measures. The current study compared autistic males and females on items on the gold-standard diagnostic measure, the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). In a sample of 8-to-17-year old autistic individuals from research (n = 229) and clinical settings (n = 238), females were less likely to show atypicalities on most items related to social-communication behaviors and on total and subscale scores. When controlling for overall intensity of symptomatology, no sex differences survived statistical corrections. Diagnostic criteria and/or gold-standard assessments may be less sensitive to female presentations of ASD and/or autistic females may exhibit fewer or less intense behaviors characteristic of ASD.
Keywords: Autism, Diagnosis, ADOS, Sex differences
Introduction
Autism spectrum disorder (ASD) is characterized by challenges in social communication and restricted, repetitive behaviors and interests (RRBs; American Psychiatric Association [APA], (2013)). The prevalence of ASD has increased dramatically over the past decades, and recent estimates show that 1 in 54 children are diagnosed with the disorder in the United States (Knopf, 2020) and estimates using national registries indicate a prevalence of 0.76% and incidence of 0.12% (Kiselev et al., 2020; Knopf, 2020). Within these estimates, there is a significant sex/gender1 difference, with a ratio of 3.5–4 males to each female (Loomes et al., 2017), even when girls’ symptoms are equally intense (Giarelli et al., 2010; Russell et al., 2011). However, these numbers vary across studies, and the male-to-female ratio in autistic children2 with intellectual disability (ID) is much smaller (Loomes et al., 2017). While there are biological explanations for the large sex/gender prevalence difference (Baron-Cohen et al., 2011), the gap is also likely to be magnified by sex/gender-related social and behavioral factors (e.g., gender socialization) that impact ascertainment and diagnosis (Halladay et al., 2015). Thus, research is needed on sex/gender presentations on ASD diagnostic assessments to account for potential differences in presentation that impact prevalence estimates.
Females are more likely than males to experience diagnostic substitution or be diagnosed with ASD later in life (Begeer et al., 2013; Kentrou et al., 2019; Shattuck et al., 2009; Trubanova et al., 2014). Prior research has found that autistic female and male children have similar overall language and social interaction (review in Rubenstein et al., 2015), but females with complex phrase speech may be diagnosed later than males with similar verbal ability (Salomone et al., 2015). Sex/gender differences in social and behavioral features that may not be captured by diagnostic criteria or assessments that are based on White cisgender males have been hypothesized as potential sources of diagnostic and ascertainment bias (Goldman, 2013; Koenig & Tsatsanis, 2005; Lai et al., 2015). For example, some studies have reported that female children use more nonverbal communication, reciprocal communication on preferred topics (Hiller et al., 2014), and pay more visual attention to faces (Harrop et al., 2019). In addition, preschool through schoolaged females’ social-communication challenges may also be relatively difficult to detect as they tend to be more socially motivated and less shy compared to autistic males (Mandy et al., 2012; Milner et al., 2019; Øien et al., 2018a, b; Sedgewick et al., 2016). Autistic female school-aged children are also more likely to initiate interactions (Hiller et al., 2014), to briefly join group activities, and to play solitary activities in close proximity to groups, similar to neurotypical (NT) males. In contrast, autistic males spend more time alone than NT males or females (Dean et al., 2017). Thus, while autistic males and females may have similar challenges in maintaining interactions or friendships, females may have enough similarities to NT males that they subvert concerns in mixed sex/gender environments at young ages (Dean et al., 2017; Harrop et al., 2019).
Females’ reduced RRBs may also contribute to decreased ascertainment as RRBs are relatively noticeable ASD characteristics. Autistic females show fewer and less intense RRBs compared to males, including fewer routines, rituals, and repetitive motor mannerisms, from age six years through adulthood (Uljarević et al., 2020; review in Rubenstein et al., 2015; Van Wijngaarden-Cremers et al., 2014). Their restricted interests are also more likely to be similar to their NT peers’ interests in content (e.g., baby dolls, animals), although not in quality or intensity (Gould & Ashton-Smith, 2011; Mandy et al., 2012). In the absence of these more obvious atypicalities, females may be less likely than males to be referred for evaluations and/or diagnosed with ASD.
Clinical referrals are also impacted by sex/gender differences in co-occurring behaviors. Among 18-month olds who had a false negative screen for ASD, males showed more social inhibition and females showed less social inhibition, in comparison to true-negative screens (Øien et al., 2018a, b). Later in development, from elementary school through adolescence, autistic and non-autistic males are more likely to show externalizing problems, and females are more likely to show internalizing ones (Lai et al., 2019; May et al., 2014; Posserud et al., 2018; Rynkiewicz & Łucka, 2018; Solomon et al., 2012). Internalizing problems are relatively difficult to detect in childhood (compared to adolescence) and cause less distress for others, resulting in lower rates of diagnostic referrals (Mandy et al., 2012). In sum, females often require a greater symptom intensity, developmental difference, or intellectual disability load to meet criteria for ASD, which likely relates both to a genetically-driven female protective factor (Robinson et al., 2013), as well as to differences in presentation from males that negatively impact the timeliness and/or accuracy of diagnosis in females (Dworzynski et al., 2012).
Further complicating sex/gender ASD phenotypic differences, females may also be better able to “camouflage” or “mask” atypicalities. There is an ongoing debate about the construct validity of camouflaging as well as the role of camouflaging in late diagnosis and female presentation of ASD (Fombonne, 2020; Lai et al., 2020). However, there is generally a consensus that camouflaging may be one coping strategy for autistic individuals (Fombonne, 2020; Hull et al., 2020). There is also research demonstrating that autistic females show relatively more awareness of their social differences, social understanding (Lai et al., 2011), and social motivation (Sedgewick et al., 2016; Young et al., 2018) compared to autistic males. This may help autistic youth and adults employ camouflaging and similar compensatory strategies (e.g., mimicking socially successful peers, memorizing conversational response) to hide their symptoms and/or form some friendships (Corbett et al., 2021; Dean et al., 2017; Livingston et al., 2019; Milner et al., 2019; Sedgewick et al., 2016). Female ASD-related challenges may become more pronounced and salient for females when their social demands increase in intensity and complexity in adolescence (Kopp & Gillberg, 2011; Mandy et al., 2018). For example, adolescent autistic females may have more difficulty than NT peers in identifying and managing the sophisticated, relational aggression that increases in adolescence (Björkqvist, 2018; Sedgewick et al., 2016). Further, while some females have enough NT-appearing behaviors to delay functional impairments and detection of challenges, many of their ASD-related traits do become more notably impairing over time (Lai et al., 2015, 2019; Mandy et al., 2018). Given the aforementioned constellation of sex/gender differences in presentation, it is important to investigate sex/gender differences in diagnostic measures so as to promote early detection and diagnosis that may optimize females’ outcomes.
Sex/Gender Differences on the ADOS
Several studies have investigated sex/gender differences on diagnostic measures such as the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999, 2012), now in its second edition. The ADOS is generally considered the gold standard behavioral measure in both research and clinical practice, and thus has been a primary focus of much of this research. On the ADOS-G (based on DMS-IV criteria), no differences emerged on total or subscale scores in studies with a wide range of ages (ages 4–20 years in Holtmann et al., 2007; ages 2–56 years in Mussey et al., 2017). Similarly, and consistent with previously mentioned similarities among males and autistic females in social-communication abilities, few studies found social interaction or communication differences on the ADOS-G (review in Van Wijngaarden-Cremers et al., 2014). However, some studies reported that female toddlers (ages 1.5–3 years; Hartley & Sikora, 2009; Carter et al., 2007) through adolescents (ages 4–18 years) in research settings (Frazier et al., 2014) showed more communication challenges compared to males, controlling for IQ. In contrast, research in community settings indicated that in toddlers and children administered modules 2 or 3 of the ADOS, females showed less social interaction atypicalities than males (with no differences in modules 1 or 4; Lawson et al., 2018; Mussey et al., 2017). Taken together, there are inconsistencies in social interaction and communication findings that may be impacted by age range, setting, small sample sizes, or the small number of females included.
Findings on the ADOS-G measure of RRBs are generally more consistent. While sex/gender differences in RRBs were not found in community-based samples of toddlers (Lawson et al., 2018; Reinhardt et al., 2015), most studies found that males showed higher rates of RRBs in a sample of toddlers with a high likelihood of ASD (Messinger et al., 2015), or confirmed ASD (ages 1.5–3 years; Hartley & Sikora, 2009), young children (ages 3–8 years; Lord et al., 1982), and adolescents (Bölte et al., 2011), controlling for IQ. Autistic males also showed quantitatively and qualitatively more intense RRBs in one IQ-matched sample with a wide age range (3–18 years; Mandy et al., 2012). Finally, the sex/gender difference in RRBs may be general, as children with a high-likelihood of ASD with a confirmed diagnosis, children with a high likelihood of ASD with no diagnosis, and NT control males all showed higher levels of RRBs compared to females (Messinger et al., 2015).
Research on the ADOS-2 and DSM-5 criteria is relatively limited. Ratto et al. (2018) analyzed sex differences on items of Module 3, the module for children and adolescents with fluent speech. They found that females showed less atypicality on some individual items (i.e., excessive interests, hyperactivity items) and less direction of facial expressions. However, there were no differences in overall “calibrated symptom severity” (CSS) when IQ was not controlled. Knutsen et al. (2019) focused on items on the RRB subscale in a comparison of males and females separated by age (younger than 6, or 6–18 years) and IQ (less than 70, or greater than or equal to 70). The only item with sex/gender differences was the repetitive interests/stereotyped behaviors. Younger females with an IQ greater than 70 and older females with an IQ below 70 had lower scores on this item compared to males. Finally, Kaat et al. (2021) analyzed a large multisite sample of children who completed all modules of the ADOS-2. They found significantly higher mean scores for males on the RRB CSS at ages 3, 7, and 15 years, and higher mean scores on the social affect (SA) CSS at age 7 years after accounting for nonverbal IQ and language level.
While research on sex/gender differences on the ADOS-2 is growing, gaps remain. Research is needed on sex differences on individual ADOS items, rather than just subscale or CSS scores, as it seems more likely that males and females differ on specific behaviors and the ways in which they meet diagnostic criteria, which would be obscured by comparisons of algorithm scores (Hiller et al., 2014; Lai et al., 2015; Mandy et al., 2012). Although these differences may not directly affect whether or not an individual meets diagnostic cutoff criteria on the ADOS-2, they may influence clinical decision-making and may also capture meaningful variability in the overall presentation of ASD by sex. In light of previous findings of sex/gender differences in ASD phenotype, the current study leveraged a large, multisite sample to investigate and ascertain quantified sex-differences at item level on the ADOS. First, cross-tabs were conducted to replicate Ratto et al.’s (2018) study on sex differences on items. Next, polytomous regression analyses were conducted both with and without controlling for overall ASD-traits. It was hypothesized that autistic females would show lower scores on RRB items, compared to autistic males (Rubenstein et al., 2015; Van Wijngaarden-Cremers et al., 2014). On socialcommunication items, sex differences were expected on direction of facial expressions (Ratto et al., 2018). It was hypothesized that there would not be differences in overall ASD traits, as measured by the CSS, and that controlling for CSS would account for most sex differences.
Method
Participants
The present study utilized secondary analysis of previously collected data. Participants were drawn from both the Gender Exploration of Neurogenetics and Development to Advance Autism Research (GENDAAR; NIMH100028) Consortium (n = 229) and a clinic-based sample (n = 2831). GENDAAR is a multi-site research study (Boston MA, Seattle WA, Los Angeles CA, New Haven CT) aimed at better understanding the causes and expression of ASD in females. All participants in the research sample were screened for having a clinical diagnosis of ASD, and diagnoses were confirmed using an ADOS-2. Participants from the clinic-based sample were seen either for (a) clinical evaluation by a psychologist or neuropsychologist at an autism specialty clinic within a pediatric hospital in the Washington, DC metropolitan area or (b) participation in a clinical research study run by the same clinic. See Rau et al. (2020) for additional information regarding this study sample. Children were included if they presented at the clinic for an autism evaluation and received a module 3 or 4 of the ADOS-2 and met inclusion criteria (n = 238; see Fig. 1 for Consort Diagram). All children resided in the United States at the time of the evaluation, and all testing was performed in English.
Fig. 1.
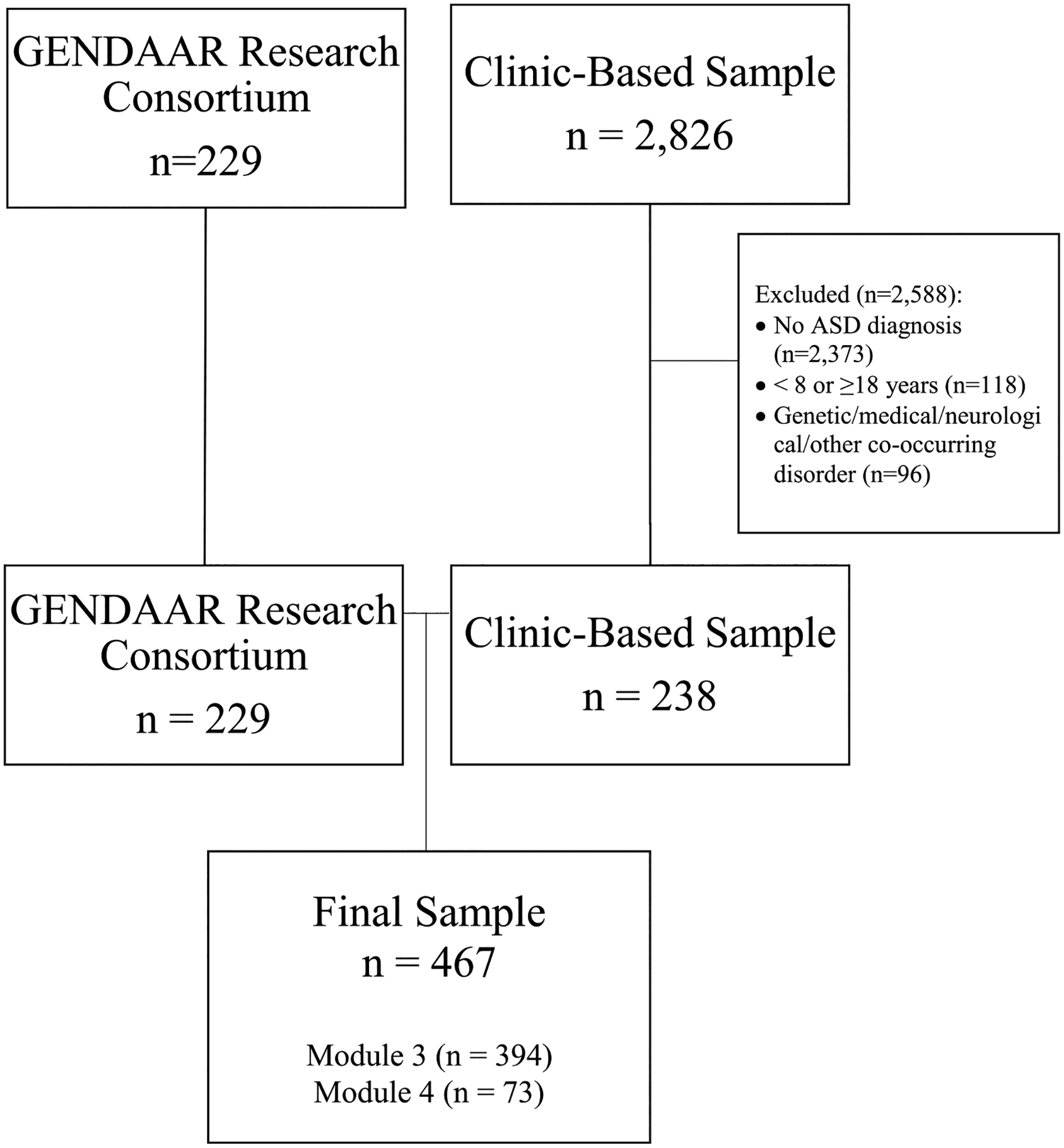
Consort diagram to indicate sample recruitment
Final Sample
From these two previously collected research and clinical samples, a smaller sample was drawn, utilizing the following eligibility criteria: aged 8–17 years 11 months, fullscale IQ ≥ 70, and of appropriate age and language skills for administration of either Module 3 (n = 395; 31.4% female) or 4 (n = 73; 34.2% female) of the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Participants were also required to have a clinical diagnosis of ASD, which was confirmed by trained clinician using the DSM-IV-TR (APA, 2010) or DSM-5 (APA, 2013) diagnostic criteria. Participants also had to meet criteria for ASD based on at least one of the following: the ADOS-2 algorithm score (Lord et al., 2012) or the Autism Diagnostic Interview, Revised (ADI-R), although most met criteria for ASD on both. Participants who did not meet ADOS-2 criteria but were nonetheless considered to have a clinical diagnosis of ASD were included to ensure variability in scores and generalizability of findings. As item level analysis of the ADOS-2 was the primary focus of this study, only participants with complete data on the ADOS-2 were included in the study to avoid the need for imputation of missing data. Participants with missing data on some other measures were included (see Statistical Analysis). Exclusion criteria were presence of a genetic disorder associated with ASD (e.g., Fragile X), medical, or neurological disorder, clinically significant visual or auditory impairment after correction, sensory motor difficulties that would preclude use of diagnostic instruments, brain damage, seizures, current use of certain or unstable medications (e.g., benzodiazepines, barbiturates, or antiepileptic medications). Due to the use of neuroimaging in GENDAAR, participants were also excluded from that study if they had metal in the body, twin status, pregnancy, a history of pre/perinatal complications, or a tic disorder that would interfere with neuroimaging. Based on inclusion/exclusion criteria, 118 children from the clinical sample were excluded based on age, five children had two evaluations so only one time point was retained, and 96 children were excluded for genetic, medical, neurological or other disorders. This resulted in a clinical sample of 238 children. All children in the research sample (n = 229) met these inclusion criteria, as well.
The final sample included 467 children and adolescents (ages 8–18; M = 12.20, SD = 2.85). There was a difference in average age between the clinical (M = 11.81, SD = 2.70) and research (M = 12.62, SD = 2.95) samples t(457) = − 3.11, p = .002). Demographics for the full sample are presented in Table 1. IQ was assessed using a variety of measures, including the Differential Ability Scales-Second Edition (DAS-II; Elliot, 2007; n = 228), the Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II; Wechsler, 2011; n = 98), the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV; Wechsler, 2003; n = 23), the Wechsler Intelligence Scale for Children-Fifth Edition (WISC-V; Wechsler, 2014; n = 94), the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV; Wechsler, 2008; n = 19). There was no difference between the clinical (M = 102.12, SD = 17.42) and research (M = 102.01, SD = 19.01) samples in terms of IQ (t(454) = .07, p = .948).
Table 1.
Participant characteristics
| Demographic | Combined N = 468 | Males N = 321 | Females N = 147 | Statistic, p value |
|---|---|---|---|---|
| Age Myears (SDyears) | 12.20 (2.85) | 12.17 (2.91) | 12.28 (2.71) | 0.402 (0.688) |
| IQ Mean (SD) | 102.08 (18.18) | 102.34 (18.06) | 101.50 (18.49) | − 0.459 (0.647) |
| Race N (%) | χ2 = 4.27 (0.234) | |||
| White | 313 (66.9%) | 213 (66.4%) | 100 (68.0%) | |
| Black/African American | 11 (2.4%) | 6 (1.9%) | 5 (3.4%) | |
| More than one race | 57 (12.2%) | 35 (10.9%) | 22 (15.0%) | |
| Other | 57 (12.2%) | 44 (13.7%) | 13 (8.8%) | |
| Unknown | 30 (6.4%) | 23 (7.2%) | 7 (4.8%) | |
| Ethnicity N (%) | χ2 = 0.29 (0.590) | |||
| Non-Hispanic | 340 (72.6%) | 230 (71.6%) | 110 (75.8%) | |
| Hispanic | 73 (15.6%) | 47 (14.6%) | 26 (17.7%) | |
| Unknown | 55 (11.7%) | 44 (13.7%) | 11 (7.5%) | |
| Maternal education N (%) | χ2 = 1.56 (0.458) | |||
| Less than or some college | 104 (22.2%) | 66 (20.6%) | 38 (25.9%) | |
| Bachelor’s or associate’s degree | 125 (26.7%) | 89 (27.7%) | 36 (24.5%) | |
| More than bachelor’s or associate’s degree | 150 (32.1%) | 102 (31.8%) | 48 (32.7%) | |
| Unknown | 89 (19.0%) | 64 (19.9%) | 25 (17.0%) | |
| Paternal education N (%) | χ2 = 2.87 (0.24) | |||
| Less than or some college | 127 (27.1%) | 77 (24.3%) | 49 (33.3%) | |
| Bachelor’s or associate’s degree | 107 (22.9%) | 76 (23.7%) | 31 (21.1%) | |
| More than bachelor’s or associate’s degree | 130 (27.8%) | 90 (28.0%) | 40 (27.2%) | |
| Unknown | 104 (22.2%) | 77 (24.0%) | 27 (18.4%) |
p < 0.05
Measures
Autism Diagnostic Observation Schedule, Second Edition (ADOS-2)
Participants were assessed using the ADOS-2, a standardized, semi-structured observational assessment with five potential modules, administered depending on the individual’s age and expressive language level. Based on interactions during the assessment, clinicians rate individuals on items related to social affect (SA) and restricted and repetitive behaviors (RRB), with higher scores indicating greater intensity. There are also three items related to commonly cooccurring behaviors: anxiety, overactivity/agitation, and tantrums/aggression/negative or disruptive behavior. Selected items contribute to algorithms to calculate social-affect (SA) and restricted repetitive behavior (RRB) subscale scores, as well as a total score, with a cut-off score to determine if a participant meets diagnostic criteria for ASD. The subscale and total algorithm scores can also be converted to a Calibrated Severity Score (CSS), a measure of “overall symptom severity” allowing for comparisons across modules (Hus & Lord, 2014; Hus et al., 2012). The CSS utilizes a rating from 1 to 10 (1: minimal-to-no evidence of ASD, 10: high evidence) to allow for comparisons across modules. All ADOS-2 were administered and scored live. For the research sample, scoring was done by a research reliable licensed clinical psychologists or clinical psychology trainee. In the clinical setting, all clinicians had undergone clinical training in the ADOS-2 and meet monthly for ADOS-2 reliability reviews.
Of the available modules, module 3 is designed for children and adolescents with fluent speech and module 4 is for older adolescents and adults with fluent speech. Modules 3 and 4 include 12 identical tasks. Module 3 includes 2 additional make-believe play tasks with action figures. As those tasks are not developmentally appropriate for adults, mod 4 includes 3 additional brief interviews about work/school, daily living, and plans/hopes that would not be developmentally appropriate for younger adolescents. Scoring is also almost identical, with module 4 only including two additional items as compared to module 3. Only scoring items that overlapped between modules 3 and 4 were included. Items are rated on ordinal scales, where 0 indicates no evidence of ASD-related behaviors, 1 indicates mild ASD-related behaviors, and 2–3 indicates significant ASD-related behaviors. There are some differences in scoring among items. For example, the item related to atypical eye contact is rated on a two-point scale (0 or 2). Some items also include scores of 7 for atypicalities that are not related to ASD (e.g., stutter or stammer or other fluency disorder that results in speech atypicalities) or are extreme behaviors (e.g., unusually frequent, intense, or excessive demands for attention) and some include scores of 8 to indicate a behavior is not applicable (e.g., due to physical disability).
Statistical Analysis
In accordance with the ADOS-2 algorithm scoring protocol, scores of 3 were collapsed to 2. In contrast to algorithm scoring protocol of converting 7 and 8 scores to a 0, 7’s and 8’s were entered as missing data given the infrequency of those values and the description of those values as implying some level of atypicality that is not commensurate with 0 scores. To decrease the number of analyses, items that are not focused on ASD symptomatology were omitted (i.e., overall non-echoed language, overactivity, tantrums, and anxiety). Additionally, the item “Language Production and Linked Nonverbal Communication” was omitted, as scoring for this item is linked to the scoring of other items (i.e., that is automatically scored an 8 for individuals with significant challenges on items rating gestures, eye contact, and/or facial expressions resulting in a high rate of “8” scores (n = 332 of 468). T-tests were used to assess sex differences in the ADOS CSS for total ASD-related behaviors, social affect, and RRB. For preliminary analyses, Pearson Chi Squares were conducted to evaluate sex differences in rates of meeting ASD criteria on the ADOS-2 items (see Supplementary Table 1). Next, analysis of statistical differences between levels on items were conducted using a polytomous regression, first without and second with controlling for overall CSS. Males served as the reference group. Thus, positive beta values indicate more atypicalities for males, negative beta values indicate more atypicalities for females. The false discovery rate procedure was used to control for Type I error in all analyses (Benjamini & Hochberg, 1995).
Results
Comparisons of Overall and Subscale Scores
On average, males (M = 7.46, SE = 0.12) had a significantly higher total CSS score than females (M = 6.26, SE = 0.18), indicating higher levels of overall ASD-related behaviors for males (p < .001, Cohen’s D = .50). In contrast to hypotheses, the Social Affect CSS was significantly different between males (M = 7.48, SE = 0.12) and females (M = 6.38, SE = 0.18; p < .001, Cohen’s D = .48). There was also a difference on the RRB CSS between males (M = 7.19, SE = 0.12) and females (M = 6.66, SE = 0.21; p = .029, Cohen’s D = .25). Descriptive statistics for each item are presented in Table 2. Supplemental Table 1 presents cross tabs of each item to replicate findings by Ratto et al. (2018). Findings on the direction of a range of facial expressions were in the opposite direction as reported in Ratto et al. (2018). Specifically, females in the current sample were more likely to direct a range of facial expressions, compared to males. Females were also more likely to identify and share emotions in others. There were also sex differences in offering information, reporting events, conversation, gestures, shared enjoyment, insight, amount of reciprocal social communication, quality of rapport, and imagination.
Table 2.
Descriptive statistics of ADOS-2 Items
| Item | Overall mean (SD) | Male mean (SD) | Female mean (SD) |
|---|---|---|---|
| Speech abnormalities | 1.23 (0.69) | 1.25 (0.69) | 1.19 (0.71) |
| Echoed language | 0.08 (0.30) | 0.08 (0.30) | 0.07 (0.31) |
| Stereotyped language | 0.94 (0.67) | 0.98 (0.68) | 0.87 (0.64) |
| Offers information | 0.41 (0.66) | 0.47 (0.70) | 0.27 (0.53) |
| Asks for information | 1.07 (0.76) | 1.05 (0.75) | 1.11 (0.76) |
| Reporting of events | 0.62 (0.65) | 0.68 (0.65) | 0.48 (0.61) |
| Conversation | 1.14 (0.66) | 1.22 (0.67) | 0.98 (0.62) |
| Gestures | 0.51 (0.59) | 0.58 (0.60) | 0.37 (0.55) |
| Unusual eye contact | 1.47 (0.88) | 1.53 (0.85) | 1.33 (0.95) |
| Directed facial expressions | 0.93 (0.60) | 1.02 (0.61) | 0.72 (0.55) |
| Shared enjoyment | 0.75 (0.75) | 0.85 (0.78) | 0.54 (0.65) |
| Empathy | 0.81 (0.67) | 0.86 (0.67) | 0.69 (0.66) |
| Insight | 1.42 (0.67) | 1.49 (0.62) | 1.27 (0.74) |
| Quality of social overtures | 1.12 (0.55) | 1.17 (0.55) | 1.03 (0.51) |
| Amount of social overtures | 0.83 (0.82) | 0.88 (0.82) | 0.70 (0.79) |
| Quality of social response | 1.12 (0.55) | 1.16 (0.53) | 1.03 (0.51) |
| Amount of reciprocal social communication | 0.96 (0.67) | 1.07 (0.65) | 0.73 (0.66) |
| Quality of rapport | 1.01 (0.66) | 1.04 (0.69) | 0.93 (0.60) |
| Imagination | 0.84 (0.69) | 0.92 (0.70) | 0.66 (0.64) |
| Unusual sensory interests | 0.61 (0.72) | 0.65 (0.72) | 0.52 (0.71) |
| Hand finger mannerisms | 0.59 (0.80) | 0.64 (0.81) | 0.48 (0.76) |
| Self-injurious behaviors | 0.32 (0.65) | 0.36 (0.69) | 0.22 (0.54) |
| Excessive interests | 0.43 (0.70) | 0.40 (0.69) | 0.50 (0.72) |
| Compulsions | 0.55 (0.70) | 0.60 (0.74) | 0.45 (0.61) |
Ordinal Regression without Covariates
Contrary to hypotheses, there were only sex differences on social communication items, and not on RRB items (Table 3). An ordinal regression analysis revealed that on items pertaining to verbal language and social communication, males were 1.91 times more likely to have greater challenges with spontaneously offering information (95% CI 0.20, 1.09, p = .042), were 1.84 times more likely to be rated as impaired in their ability to independently report nonroutine events (95% CI 0.22, 0.99, p = .002), and were 2.04 times more likely to be impaired in reciprocal conversation skills (95% CI 0.33, 1.10, p < .001).
Table 3.
Regression analyses without and without CSS as a covariate indicating which sex has more difficulties (positive β means more difficulties for males) and odds ratio of scoring higher
| Item | Polytomous regression | Polytomous regression controlling for overall CSS | ||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | OR | p value | β | SE | OR | p value | |
| Speech abnormalities | 0.163 | 0.189 | 1.177 | 0.387 | − 0.542 | 0.208 | 0.58 | 0.009 |
| Echoed language | 0.118 | 0.408 | 1.252 | 0.772 | − 0.299 | 0.426 | 0.74 | 0.482 |
| Stereotyped language | 0.301 | 0.192 | 1.351 | 0.117 | 0.058 | 0.201 | 1.06 | 0.771 |
| Offers information | 0.647 | 0.227 | 1.910 | 0.004* | 0.248 | 0.242 | 1.28 | 0.306 |
| Asks for information | − 0.154 | 0.185 | 0.857 | 0.404 | − 0.469 | 0.194 | 0.63 | 0.016 |
| Reporting of events | 0.605 | 0.196 | 1.831 | 0.002* | 0.256 | 0.212 | 1.29 | 0.227 |
| Conversation | 0.712 | 0.196 | 2.048 | < 0.001* | 0.184 | 0.214 | 1.20 | 0.391 |
| Gestures | 0.734 | 0.205 | 2.083 | < 0.001* | 0.413 | 0.218 | 1.51 | 0.058 |
| Unusual eye contact | 0.483 | 0.219 | 1.621 | 0.027* | − 0.055 | 0.260 | 0.95 | 0.833 |
| Directed facial Expressions | 1.001 | 0.207 | 2.721 | < 0.001* | 0.556 | 0.232 | 1.74 | 0.017 |
| Shared enjoyment | 0.738 | 0.193 | 2.092 | < 0.001* | 0.392 | 0.211 | 1.48 | 0.062 |
| Empathy | 0.480 | 0.192 | 1.616 | 0.012* | 0.278 | 0.199 | 1.32 | 0.162 |
| Insight | 0.583 | 0.192 | 1.791 | 0.002* | 0.270 | 0.203 | 1.31 | 0.184 |
| Quality of social overtures | 0.552 | 0.218 | 1.737 | 0.011* | − 0.126 | 0.244 | 0.88 | 0.606 |
| Amount of social overtures | 0.417 | 0.204 | 1.517 | 0.041 | 0.050 | 0.212 | 1.05 | 0.815 |
| Quality of social response | 0.564 | 0.225 | 1.758 | 0.012* | − 0.140 | 0.250 | 0.87 | 0.575 |
| Amount of reciprocal Social communication | 1.015 | 0.199 | 2.759 | < 0.001* | 0.567 | 0.219 | 1.76 | 0.010 |
| Quality of rapport | 0.340 | 0.194 | 1.405 | 0.079 | − 0.282 | 0.215 | 0.75 | 0.190 |
| Imagination | 0.701 | 0.192 | 2.016 | < 0.001* | 0.336 | 0.208 | 1.40 | 0.107 |
| Unusual sensory interests | 0.416 | 0.196 | 1.516 | 0.034 | 0.095 | 0.210 | 1.10 | 0.650 |
| Hand finger mannerisms | 0.429 | 0.205 | 1.536 | 0.036 | 0.092 | 0.217 | 1.10 | 0.672 |
| Self-injurious behaviors | 0.530 | 0.262 | 1.699 | 0.043 | 0.187 | 0.274 | 1.21 | 0.494 |
| Excessive interests | − 0.359 | 0.208 | 0.698 | 0.085 | − 0.488 | 0.216 | 0.61 | 0.024 |
| Compulsions | 0.338 | 0.199 | 1.402 | 0.089 | 0.050 | 0.212 | 1.05 | 0.815 |
Significant with Hochberg false discovery rate
Positive β indicates more difficulties for males; negative β indicates more difficulties for females
OR Odds ratios, or likelihood of having a higher score on the item
Males also showed challenges in nonverbal communication, including being 2.08 times more likely to be impaired in their use of gestures (95% CI 0.33, 1.134, p < .001), 1.62 times more likely to show unusual eye contact (95% CI 0.05, 0.91, p = .027), and 2.72 times more likely to be impaired in direction of a range of facial expressions to the examiner (95% CI 0.60, 1.41, p < .001).
Males showed more atypicalities in understanding of as well as their ability to initiate and maintain social interactions: They were 2.09 times more likely to be impaired in their ability to indicated shared enjoyment in interaction (95% CI 0.36, 1.11, p< .001), 1.62 times more likely to be impaired in their ability to show understanding of others’ emotions (95% CI 0.10, 0.86, p = .012), 1.79 times more likely to show limited insight into typical social situations and relationships (95% CI .21, .96, p = .002), 1.75 times more likely to show reduced quality of social overtures (i.e., 95% CI 0.13, 0.98, p = .110) and social responses (95% CI 0.12, 1.01, p = .012), and 2.76 times more likely to show a reduced amount of reciprocal social communication (95% CI 0.63, 1.40, p < .001). Finally, males were 2.02 times more likely to show limited imaginative play and creative thinking skills (95% CI 0.32, 0.11, p < .001).
Ordinal Regression Controlling for Overall ASD-Related Behaviors
When “overall symptom severity” (CSS) was entered as a covariate in the regression, no items survived Hochberg FDR correction.
Discussion
The current study explored sex differences on ADOS-2 items in a large multi-site, multi-study sample of verbal adolescents with average or higher cognitive functioning. This study leveraged large research and clinical samples to increase the variability in scores and generalizability of findings. When interpreting the results, it is important to remember that lower scores on the ADOS-2 indicate behaviors that are less characteristic of ASD. Contrary to our hypotheses and inconsistent with much of the previous literature on the ADOS-G and ADOS-2, there were sex differences on the total CSS, social affect subscale score, and many social affect items. Interestingly, there was a difference on the RRB subscale but no differences on most RRB items. Specifically, when overall ASD-related behaviors were not taken into consideration, females appeared to have lower scores, indicating behavior less characteristic of ASD, in all ADOS domains of social communication, including nonverbal communication (Rynkiewicz & Łucka, 2018), verbal reciprocity (Hiller et al., 2014), and quality of initiation. While others have similarly found that restricting the sample to a limited age range yields sex differences in total and subscale scores on the ADOS-G and ADOS-2 (e.g., Frazier et al., 2014; Lawson et al., 2018; Mussey et al., 2017), the current study’s age range likely does not fully explain discrepancies with past research (e.g., Ratto et al., 2018). This finding is also likely not due to IQ, which was not different between males and females.
The current findings of robust sex differences in social communication items do align with several existing theories. For example, the “extreme male brain theory” argues that autism is an extreme of the “typical” cisgender male cognitive profile characterized by relative strengths in systematizing over empathizing, while females show the exact opposite, on average (Baron-Cohen, 2002). Thus, according to this theory, males would be expected to show higher levels of autistic traits, consistent with findings in the current sample. Similarly, the female protective effect theory suggests that females may require a higher genetic load to meet criteria for autism. Thus, as found in this study, autistic females may show reduced autistic behaviors and characteristics (Zhang et al., 2020).
Additionally, the current findings suggest that autistic females may show relatively less social-communication and RRBs traits that are characteristic of ASD, relative to autistic males; although these females still exhibited differences compared to the larger population. One reason females’ autistic behaviors may not be as evident on the ADOS-2 is that females may camouflage more effectively and frequently (Dean et al., 2017). For example, it may be easier for females to imitate ADOS-2 clinicians during evaluations because clinicians are often cisgender females.
Another possibility is that female autistic behaviors may manifest in ways that are not captured by current criteria or scoring definitions. In support of this notion, almost all items that contribute to the diagnostic algorithm for Social Affect for modules 3 and 4 were different between males and females in the current study. Using the existing algorithms, verbal females with average or higher cognitive functioning may have autistic behaviors that are under-reported and under-diagnosed. It is particularly notable that autistic females scored lower on the ADOS-2 in the current samples as the clinicians (both in the research and clinical settings) had specific interests, expertise, and training in autistic female diagnoses. As actuarial procedures are more reliable than clinical judgement in most diagnoses (Dawes et al., 1989), we may need to modify diagnostic measures to better capture females’ autistic behaviors. Modifications may entail adding items that are more relevant for females and/or adding tasks that better elicit ASD behaviors in females. Clinicians may also need to consider overall presentation and different presentations of symptoms when interpreting the ADOS-2 for females.
In analyses that accounted for CSS, no items survived multiple comparison correction, indicating that overall ASD-related behavior differences appear to be driving sex differences. Given that these females were diagnosed with autism, it is worth identifying the areas in which they show behaviors that are more characteristic of autism that contributed to a diagnosis. Examination of confidence intervals on estimated group differences as well as uncorrected significance suggests that females may show less challenges with shared enjoyment and amount of reciprocal social communication; as well as relatively more speech atypicalities in intonation, volume, rhythm, and rate; more difficulty asking for information; and a higher presence of excessive interests. Future research should examine the generalizability and replicability of these female-specific patterns of behavior.
The potential impacts of clinician perception and cohort effects may also play a role in present findings. The data collection sites’ specific interests and specialties in the role of sex/gender on the manifestation of ASD may make them more likely to identify autism in females who have social behaviors that are not represented by the ADOS, but who nonetheless meet diagnostic criteria for ASD. Additionally, all of the present data were collected after the shift to DSM-5, which may have enabled the identification of more autistic females, due to shifts in diagnostic criteria (e.g., inclusion of sensory processing differences in the RRB category). Although prior literature has not consistently captured these social differences, it is possible that contemporary samples include a more diverse range of ASD manifestations as our understanding of ASD as a field continues to advance. Relatedly, the lack of significant sex differences on RRB items may suggest some adjustments by clinicians to account for female differences in phenotype. It is possible that clinicians are interpreting female RRBs based on the relatively large body of research that indicates sex differences in presentation. Further assessment of clinician practices in this area will be important for codifying and standardizing procedures. As the ADOS is only one tool that contributes to an ASD diagnosis, it may also be important to consider individuals who score high on the ADOS but do not meet diagnostic criteria for ASD.
While this study is one of the larger comparisons of autistic males and females on ADOS-2 items, this study is not without limitations. Replication will be needed with individuals with lower IQ and at younger ages. Additionally, research settings routinely use more stringent scoring criteria and cut-offs, as compared to clinicians, which may limit generalizability (Lai et al., 2015). As we have limited information on the ADOS-2 administrators, we are unable to calculate reliability between raters or between sites. By restricting the sample to individuals who have an autism diagnosis and by including a large research sample, we may have some ascertainment bias in our sample. Research is also needed in more sociodemographically diverse samples to better understand the intersection of race, gender, and autism. We currently only have information on sex assigned at birth, but, as the difference in presentation is likely driven in part by (socialized) gender differences, future research should consider gender differences in items. Non-autistic, neurodiverse, and/or clinical samples with overlapping disorders should be used as comparison groups in future research. Such comparisons would allow us to understand if the observed differences are unique to autism, or if they reflect broader sex/gender differences.
Summary and Conclusions
Our large research and clinical sample of children through early adolescents (8–14 years) with average and higher cognitive functioning indicated significant sex differences on most social-communication items of the ADOS-2 modules 3 and 4. Notably, most of the item differences (with females showing a less severe presentation) would account for the lower CSS for autistic females as most algorithm items were different between males and females. Thus, algorithm items in particular may be an area wherein females show fewer behaviors characteristic of ASD and other items may potentially be better assessments of clinical concerns for autistic females. Findings support previous research on sex differences in total and subscale scores on the ADOS-G, that overreliance on the ADOS-2 may result in underdiagnosis for females, and that diagnostic tools likely contribute to estimated prevalence differences among sexes (Adamou et al., 2018). Some clinicians have proposed alternative formats for assessments for females to account for sex/gender differences. For example, some have reported anecdotally that autistic females are more likely to present with social-communication behaviors that are not characteristic of ASD during initial visits, but that it becomes apparent in follow-up appointments that those social-communication behaviors are repetitive and are signs of scripting, camouflaging, and/or a routinized need for sameness (J. Gerdts, personal communication, May 12, 2021). Alternatively, clinicians report that females may camouflage less over time either due to fatigue within one long visit or due to comfort with the environment (Group discussion during Rounds, personal communication, August 26, 2021. Therefore, there would be benefits to conducting more shorter visits to account for stereotyped greetings/speech and increasing comfort with the clinician, as well as at least one longer visit to assess for reductions in camouflaging with fatigue. Clinicians may need to be mindful of biases based on initial impressions compared to interactions at the end of a session or in follow-up visits.
It may also be important to include self-report of the inner experience of the autistic person undergoing assessment. While autistic individuals’ experiences are often elicited in intake interviews and somewhat through the ADOS, most of our semi-structured assessments for ASD rely on clinician judgement and parent report. However, many autistic individuals, as well as their families, feel strongly about giving the person being evaluated more of a voice. Semi-structured or structured self-report interviews and questionnaires that acknowledge and utilize autistic individuals’ self-awareness and expressiveness would be more inclusive and informative. Relatedly, and likely particularly important for females, masking or social compensation behaviors may not always be apparent to the clinician. It has been reported that there may be a “cost” to these compensatory behaviors—autistic people who frequently camouflage or socially compensate may experience elevated levels of internalizing symptoms (Cage & Troxell-Whitman, 2019; Cassidy et al., 2018; Livingston et al., 2019), which has important implications for treatment.
Additionally, clinicians need feedback about diagnostic outcomes for females, particularly when provisional diagnoses are given. Clinicians should prioritize follow-up visits with families with females to provide continued learning about their diagnostic accuracy and developmental trajectory. Clinics should also prioritize ongoing care for families as another method for providing diagnosticians with feedback. Finally, future research should explore changes to ASD diagnostic instruments that may account for differences in female presentation, including consideration of the role of self-report instruments.
Supplementary Material
Acknowledgments
We wish to thank the families, parents and children, who participated in the GENDAAR study at the four data collection sites, as well as those who participated through Children’s National Hospital. Funding was provided by the NIMH R01 MH10028 ACE Network (Pelphrey). This publication was also supported by Award Number 1U54HD090257 from the NIH, District of Columbia Intellectual and Developmental Disabilities Research Center Award (DCIDDRC) program, and by a private grant to Children’s National through the Isadore and Bertha Gudelsky Family Foundation.
The ACE GENDAAR Consortium includes contributions from: Kevin Pelphrey PhD (PI), Katy Ankeman MSW, Elizabeth Aylward PhD, Raphael Bernier PhD, Susan Bookheimer PhD, Sarah Corrigan MA, Mirella Dapretto PhD, Daniel Geschwind MD, Abha Gupta PhD, Allison Jack PhD, Zack Jacokes, Erin Libsack, Jennifer Lowe PhD, Desiree Lussier PhD, Shafali Jeste MD, Anna Kresse MPH, James McPartland PhD, Adam Naples PhD, Charles Nelson PhD, Emily Neuhaus PhD, Megha Santhosh MHE, Matthew State MD, Catherine Sullivan, Carina Torgenson, Jack Van Horn PhD, Pamela Ventola PhD, Julie Wolf PhD.
Funding
The authors did not receive support from any organization for the submitted work.
Footnotes
Conflict of interest Hannah M. Rea, Roald A. Øien, Sara Jane Webb, and Allison Ratto report no affiliations with or involvement in any organization or entity with any financial interest in the outcome of this project. Frederick Shic is a paid consultant for Roche and Janssen pharmaceutical companies.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10803-022-05566-3.
Ethical Approval Ethical oversight was provided by the Yale Institutional Board (Yale, SCRI), the UCLA Office of Human Research Protection Program (UCLA), Boston Children’s Hospital Institutional Review Board (BCH), and USC Office for the Protection of Research Subjects. All procedures performed were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all parents of children participating in the study; children provided written assent.
Informed Consent Informed consent was obtained from legal guardians.
Sex is defined as biological and physiological characteristics related to being male, female, or intersex. Gender refers to the socially constructed characteristics associated with sex (World Health Organization, 2002). As gendered social constructs begin early in development, and gender identity is rarely assessed to appropriately distinguish between the effect of sex or gender on presentation, the term sex/gender will be used in reviews of extant literature (e.g., Lai et al., 2015). Additionally, while we recognize the increased prevalence of gender diversity (e.g., transgender, nonbinary, etc.) among autistic individuals who may not conform to “traditional” gender norms (Janssen et al., 2016; Øien et al., 2018a, b; Strang et al., 2014), the present study focuses on ASD diagnostic assessments based on sex as data was not collected on gender identity.
Additionally, we are using “identity-first language” based on consultation with self-advocates, preferences by autistic people (Kenny et al., 2016), and reports that this language is less stigmatizing than person-first language (Bottema-Beutel et al., 2021).
Data Availability
Patients signed informed consent regarding publishing their data.
References
- Adamou M, Johnson M, & Alty B (2018). Autism diagnostic observation schedule (ADOS) scores in males and females diagnosed with autism: A naturalistic study. Advances in Autism, 4(2), 49–55. 10.1108/AIA-01-2018-0003 [DOI] [Google Scholar]
- American Psychiatric Association (2010). Diagnostic and statistical manual of mental disorders (DSM-IV-TR). American Psychiatric Association [Google Scholar]
- American Psychiatric Association (2013). DSM 5. American Psychiatric Association [Google Scholar]
- Baron-Cohen S (2002). The extreme male brain theory of autism. Trends in Cognitive Sciences, 6(6), 248–254. 10.1016/S1364-6613(02)01904-6 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, & Knickmeyer R (2011). Why are autism spectrum conditions more prevalent in males? PLoS Biology, 9(6), e1001081. 10.1371/journal.pbio.1001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, & Koot HM (2013). Sex differences in the timing of identification among children and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(5), 1151–1156. 10.1007/s10803-012-1656-z [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (methodological), 57(1), 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Björkqvist K (2018). Gender differences in aggression. Current Opinion in Psychology, 19, 39–42. 10.1016/j.copsyc.2017.03.030 [DOI] [PubMed] [Google Scholar]
- Bölte S, Westerwald E, Holtmann M, Freitag C, & Poustka F (2011). Autistic traits and autism spectrum disorders: The clinical validity of two measures presuming a continuum of social communication skills. Journal of Autism and Developmental Disorders, 41(1), 66–72. 10.1007/s10803-010-1024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottema-Beutel K, Kapp SK, Lester JN, Sasson NJ, & Hand BN (2021). Avoiding ableist language: Suggestions for autism researchers. Autism in Adulthood, 3(1), 18–29. 10.1089/aut.2020.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cage E, & Troxell-Whitman Z (2019). Understanding the reasons, contexts and costs of camouflaging for autistic adults. Journal of Autism and Developmental Disorders, 49(5), 1899–1911. 10.1007/s10803-018-03878-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, & Tager-Flusberg H (2007). Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 37(1), 86–97. 10.1007/s10803-006-0331-7 [DOI] [PubMed] [Google Scholar]
- Cassidy S, Bradley L, Shaw R, & Baron-Cohen S (2018). Risk markers for suicidality in autistic adults. Molecular Autism, 9(1), 1–14. 10.1186/s13229-018-0226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schwartzman JM, Libsack EJ, Muscatello RA, Lerner MD, Simmons GL, & White SW (2021). Camouflaging in autism: Examining sex-based and compensatory models in social cognition and communication. Autism Research, 14(1), 127–142. 10.1002/aur.2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes RM, Faust D, & Meehl PE (1989). Clinical versus actuarial judgement. Science, 243(4899), 168–1674. 10.1126/science.2648573 [DOI] [PubMed] [Google Scholar]
- Dean M, Harwood R, & Kasari C (2017). The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism, 21(6), 678–689. 10.1177/1362361316671845 [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, & Happé F (2012). How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Child & Adolescent Psychiatry, 51(8), 788–797. 10.1016/j.jaac.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Elliot C (2007). Differential abilities scale—2nd edition (DAS-II) manual. Harcourt Assessment Inc. [Google Scholar]
- Fombonne E (2020). Camouflage and autism. Journal of Child Psychology and Psychiatry, 61(7), 735–738. 10.1111/jcpp.13296 [DOI] [PubMed] [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, & Hardan AY (2014). Behavioral and cognitive characteristics of females and males with autism in the Simons simplex collection. Journal of the American Academy of Child & Adolescent Psychiatry, 53(3), 329–340. 10.1016/j.jaac.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, & Mandell D (2010). Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disability and Health Journal, 3(2), 107–116. 10.1016/j.dhjo.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S (2013). Opinion: Sex, gender and the diagnosis of autism—A biosocial view of the male preponderance. Research in Autism Spectrum Disorders, 7(6), 675–679. 10.1016/j.rasd.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J, & Ashton-Smith J (2011). Missed diagnosis or misdiagnosis? Girls and women on the autism spectrum. Good Autism Practice (GAP), 12(1), 34–41. [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, Messinger D, Pelphrey K, Sanders SJ, & Singer AT (2015). Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Molecular Autism, 6(1), 1–5. 10.1186/s13229-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop C, Jones D, Zheng S, Nowell S, Schultz R, & Parish-Morris J (2019). Visual attention to faces in children with autism spectrum disorder: Are there sex differences? Molecular Autism, 10(1), 1–10. 10.1186/s13229-019-0276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, & Sikora DM (2009). Which DSM-IV-TR criteria best differentiate high-functioning autism spectrum disorder from ADHD and anxiety disorders in older children? Autism, 13(5), 485–509. 10.1177/1362361309335717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2014). Sex differences in autism spectrum disorder based on DSM-5 criteria: Evidence from clinician and teacher reporting. Journal of Abnormal Child Psychology, 42(8), 1381–1393. 10.1007/s10802-014-9881-x [DOI] [PubMed] [Google Scholar]
- Holtmann M, Bölte S, & Poustka F (2007). Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Developmental Medicine & Child Neurology, 49(5), 361–366. 10.1111/j.1469-8749.2007.00361.x [DOI] [PubMed] [Google Scholar]
- Hull L, Petrides KV, & Mandy W (2020). The female autism phenotype and camouflaging: A narrative review. Review Journal of Autism and Developmental Disorders, 7, 306–317. 10.1007/s40489-020-00197-9 [DOI] [Google Scholar]
- Hus V, Gotham K, & Lord C (2012). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44, 2400–2412. 10.1007/s10803-012-1719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, & Lord C (2014). The autism diagnostic observation schedule, module 4: Revised algorithm and standardized severity scores. Journal of Autism and Developmental Disorders, 44(8), 1996–2012. 10.1007/s10803-014-2080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A, Huang H, & Duncan C (2016). Gender variance among youth with autism spectrum disorders: A retrospective chart review. Transgender Health, 1(1), 63–68. 10.1089/trgh.2015.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat AJ, Shui AM, Ghods SS, Farmer CA, Esler AN, Thurm A, Georgiades S, Kanne SM, Lord C, & Kim YS (2021). Sex differences in scores on standardized measures of autism symptoms: A multisite integrative data analysis. Journal of Child Psychology and Psychiatry, 62(1), 97–106. 10.1111/jcpp.13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny L, Hattersley C, Molins B, Buckley C, Povey C, & Pellicano E (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. 10.1177/1362361315588200 [DOI] [PubMed] [Google Scholar]
- Kentrou V, de Veld DM, Mataw KJ, & Begeer S (2019). Delayed autism spectrum disorder recognition in children and adolescents previously diagnosed with attention-deficit/hyperactivity disorder. Autism, 23(4), 1065–1072. 10.1177/1362361318785171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev Y, Handal M, Hjellvik V, Reichborn-Kjennerud T, Stoltenberg C, Suren P, Havdahl A, & Skurtveit S (2020). Nationwide study of neuropsychiatric comorbidity and medicines use in children with autism spectrum disorder in Norway. Frontiers in Psychiatry, 11, 1–8. 10.3389/fpsyt.2020.596032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf A (2020). Autism prevalence increases from 1 in 60 to 1 in 54: CDC. The Brown University Child and Adolescent Behavior Letter, 36(6), 6–7. 10.1002/cpu.30499 [DOI] [Google Scholar]
- Knutsen J, Crossman M, Perrin J, Shui A, & Kuhlthau K (2019). Sex differences in restricted repetitive behaviors and interests in children with autism spectrum disorder: An Autism Treatment Network study. Autism, 23(4), 858–868. 10.1177/1362361318786490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig K, & Tsatsanis KD (2005). Pervasive developmental disorders in girls. In Bell DJ, Foster SL, & Mash EJ (Eds.), Handbook of behavioral and emotional problems in girls. Issues in clinical child psychology Springer. [Google Scholar]
- Kopp S, & Gillberg C (2011). The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): An instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Research in Developmental Disabilities, 32(6), 2875–2888. 10.1016/j.ridd.2011.05.017 [DOI] [PubMed] [Google Scholar]
- Lai MC, Baron-Cohen S, & Buxbaum JD (2015). Understanding autism in the light of sex/gender. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Hull L, Mandy W, Chakrabarti B, Nordahl CW, Lombardo MV, Ameis SH, Szatmari P, Baron-Cohen S, Happé F, & Livingston LA (2020). Commentary: ‘Camouflaging’ in autistic people-reflection on Fombonne. Journal of Child Psychology and Psychiatry, 62(8), 1037–1041. 10.1111/jcpp.13344 [DOI] [PubMed] [Google Scholar]
- Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, Szatmari P, & Ameis SH (2019). Prevalence of co-occurring mental health diagnoses in the autism population: A systematic review and meta-analysis. The Lancet Psychiatry, 6(10), 819–829. 10.1186/s13229-015-0021-4 [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Pasco G, Ruigrok AN, Wheelwright SJ, Sadek SA, Chakrabarti B, MRC AIMS Consortium, & Baron-Cohen S (2011). A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS ONE, 6(6), e20835. 10.1371/journal.pone.0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LP, Joshi R, Barbaro J, & Dissanayake C (2018). Gender differences during toddlerhood in autism spectrum disorder: A prospective community-based longitudinal follow-up study. Journal of Autism and Developmental Disorders, 48(8), 2619–2628. 10.1007/s10803-018-3516-y [DOI] [PubMed] [Google Scholar]
- Livingston LA, Colvert E, Social Relationships Study Team, Bolton P, & Happé F (2019a). Good social skills despite poor theory of mind: Exploring compensation in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 60(1), 102–110. 10.1111/jcpp.12886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston LA, Shah P, & Happé F (2019b). Compensatory strategies below the behavioural surface in autism: A qualitative study. The Lancet Psychiatry, 6(9), 766–777. 10.1016/S2215-0366(19)30224-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(6), 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (1999). ADOS. Autism diagnostic observation schedule. Manual WPS. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism diagnostic observation schedule, (ADOS-2) modules 1–4. Western Psychological Services. [Google Scholar]
- Lord C, Schopler E, & Revicki D (1982). Sex differences in autism. Journal of Autism and Developmental Disorders, 12(4), 317–330. 10.1007/BF01538320 [DOI] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, & Skuse D (2012). Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders, 42(7), 1304–1313. 10.1007/s10803-011-1356-0 [DOI] [PubMed] [Google Scholar]
- Mandy W, Pellicano L, St Pourcain B, Skuse D, & Heron J (2018). The development of autistic social traits across childhood and adolescence in males and females. Journal of Child Psychology and Psychiatry, 59(11), 1143–1151. 10.1111/jcpp.12913 [DOI] [PubMed] [Google Scholar]
- May T, Cornish K, & Rinehart N (2014). Does gender matter? A one year follow-up of autistic, attention and anxiety symptoms in high-functioning children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(5), 1077–1086. 10.1007/s10803-013-1964-y [DOI] [PubMed] [Google Scholar]
- Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, Carver L, Charman T, Chawarska K, & Curtin S (2015). Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Molecular Autism, 6(1), 1–12. 10.1186/s13229-015-0027-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner V, McIntosh H, Colvert E, & Happé F (2019). A qualitative exploration of the female experience of autism spectrum disorder (ASD). Journal of Autism and Developmental Disorders, 49(6), 2389–2402. 10.1007/s10803-019-03906-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussey JL, Ginn NC, & Klinger LG (2017). Are males and females with autism spectrum disorder more similar than we thought? Autism, 21(6), 733–737. 10.1177/1362361316682621 [DOI] [PubMed] [Google Scholar]
- Øien RA, Cicchetti DV, & Nordahl-Hansen A (2018a). Gender dysphoria, sexuality and autism spectrum disorders: A systematic map review. Journal of Autism and Developmental Disorders, 48(12), 4028–4037. 10.1007/s10803-018-3686-7 [DOI] [PubMed] [Google Scholar]
- Øien RA, Schjølberg S, Volkmar FR, Shic F, Cicchetti DV, Nordahl-Hansen A, Stenberg N, Hornig M, Havdahl A, & Øyen A-S (2018b). Clinical features of children with autism who passed 18-month screening. Pediatrics. 10.1542/peds.2017-3596 [DOI] [PubMed] [Google Scholar]
- Posserud M, Hysing M, Helland W, Gillberg C, & Lundervold AJ (2018). Autism traits: The importance of “co-morbid” problems for impairment and contact with services. Data from the Bergen Child Study. Research in Developmental Disabilities, 72, 275–283. 10.1016/j.ridd.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Ratto AB, Kenworthy L, Yerys BE, Bascom J, Wieckowski AT, White SW, Wallace GL, Pugliese C, Schultz RT, & Ollendick TH (2018). What about the girls? Sex-based differences in autistic traits and adaptive skills. Journal of Autism and Developmental Disorders, 48(5), 1698–1711. 10.1007/s10803-017-3413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau S, Skapek MF, Tiplady K, Seese S, Burns A, Armour AC, & Kenworthy L (2020). Identifying comorbid ADHD in autism: Attending to the inattentive presentation. Research in Autism Spectrum Disorders, 69, 101468–101480. 10.1016/j.rasd.2019.101468 [DOI] [Google Scholar]
- Reinhardt VP, Wetherby AM, Schatschneider C, & Lord C (2015). Examination of sex differences in a large sample of young children with autism spectrum disorder and typical development. Journal of Autism and Developmental Disorders, 45(3), 697–706. 10.1007/s10803-014-2223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, & Ronald A (2013). Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences, 110(13), 5258–5262. 10.1073/pnas.1211070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein E, Wiggins LD, & Lee L-C (2015). A review of the differences in developmental, psychiatric, and medical endophenotypes between males and females with autism spectrum disorder. Journal of Developmental and Physical Disabilities, 27(1), 119–139. 10.1007/s10882-014-9397-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G, Steer C, & Golding J (2011). Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Social Psychiatry and Psychiatric Epidemiology, 46(12), 1283–1293. 10.1007/s00127-010-0294-z [DOI] [PubMed] [Google Scholar]
- Rynkiewicz A, & Łucka I (2018). Autism spectrum disorder (ASD) in girls. Co-occurring psychopathology. Sex differences in clinical manifestation. Psychiatria Polska, 52(4), 629–639. 10.12740/PP/OnlineFirst/58837 [DOI] [PubMed] [Google Scholar]
- Salomone E, Charman T, McConachie H, & Warreyn P (2015). Prevalence and correlates of use of complementary and alternative medicine in children with autism spectrum disorder in Europe. European Journal of Pediatrics, 174(10), 1277–1285. 10.1007/s00431-015-2531-7 [DOI] [PubMed] [Google Scholar]
- Sedgewick F, Hill V, Yates R, Pickering L, & Pellicano E (2016). Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. Journal of Autism and Developmental Disorders, 46(4), 1297–1306. 10.1007/s10803-015-2669-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, Lee L-C, Rice C, Giarelli E, & Kirby R (2009). Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. Journal of the American Academy of Child & Adolescent Psychiatry, 48(5), 474–483. 10.1097/CHI.0b013e31819b3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Miller M, Taylor SL, Hinshaw SP, & Carter CS (2012). Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(1), 48–59. 10.1007/s10803-011-1215-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang JF, Kenworthy L, Dominska A, Sokoloff J, Kenealy LE, Berl M, Walsh K, Menvielle E, Slesaransky-Poe G, Kim K-E, Luong-Tran C, Meagher H, & Wallace GL (2014). Increased gender variance in autism spectrum disorders and attention deficit hyperactivity disorder. Archives of Sexual Behavior, 43(8), 1525–1533. 10.1007/s10508-014-0285-3 [DOI] [PubMed] [Google Scholar]
- Trubanova A, Donlon K, Kreiser NL, Ollendick TH, & White SW (2014). Underidentification of autism spectrum disorder in females: A case series illustrating the unique presentation of this disorder in young women. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology, 2(2), 66–76. 10.21307/sjcapp-2014-010 [DOI] [Google Scholar]
- Uljarević M, Hedley D, Rose-Foley K, Magiati I, Cai RY, Dissanayake C, Richdale A, & Trollor J (2020). Anxiety and depression from adolescence to old age in autism spectrum disorder. Journal of Autism and Developmental Disorders, 50(9), 3155–3165. 10.1007/s10803-019-04084-z [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, & Van der Gaag RJ (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders, 44(3), 627–635. 10.1007/s10803-013-1913-9 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2003). Wechsler intelligence scale for children–Fourth edition (WISC-IV). The Psychological Corporation. [Google Scholar]
- Wechsler D (2008). Wechsler adult intelligence scale–Fourth Edition (WAIS–IV) (Vol. 22, pp. 816–827). NCS Pearson. [Google Scholar]
- Wechsler D (2011). Wechsler abbreviated scale of intelligence–second edition. NCS Pearson. [Google Scholar]
- Wechsler D (2014). Wechsler Intelligence Scale for Children-(WISCV). Pearson Education. [Google Scholar]
- World Health Organization (2002). Report of a technical consultation on sexual health. Geneva, pp 28–31 [Google Scholar]
- Young H, Oreve M-J, & Speranza M (2018). Clinical characteristics and problems diagnosing autism spectrum disorder in girls. Archives De Pédiatrie, 25(6), 399–403. 10.1016/j.arcped.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li N, Li C, Zhang Z, Teng H, Wang Y, Zhao T, Shi L, Zhang K, Xia K, Li J, & Sun Z (2020). Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Translational Psychiatry, 10(1), 1–10. 10.1038/s41398-020-0699-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patients signed informed consent regarding publishing their data.


